13.3
Impact Factor
Theranostics 2021; 11(7):3417-3438. doi:10.7150/thno.53105 This issue Cite
Research Paper
Colon tissue-accumulating mesoporous carbon nanoparticles loaded with Musca domestica cecropin for ulcerative colitis therapy
1. Guangdong Provincial Key Laboratory of Pharmaceutical Bioactive Substances, School of Life Science and Biopharmaceutics, Guangdong Pharmaceutical University, 280 Wai Huan Dong Road, Guangzhou Higher Education Mega Center, Guangzhou 510006, People's Republic of China.
2. Intensive Care Unit, Shenzhen Second People's Hospital, the First Affiliated Hospital of Shenzhen University, Shenzhen 518031, People's Republic of China.
3. Key Laboratory of the Ministry of Health for Research on Quality and Standardization of Biotech Products, National Institutes for Food and Drug Control, Beijing 102629, People's Republic of China.
4. School of Pharmacy, Guangdong Pharmaceutical University, 280 Wai Huan Dong Road, Guangzhou Higher Education Mega Center, Guangzhou 510006, People's Republic of China.
#Co-first authors with equal contributions to this work.
Abstract
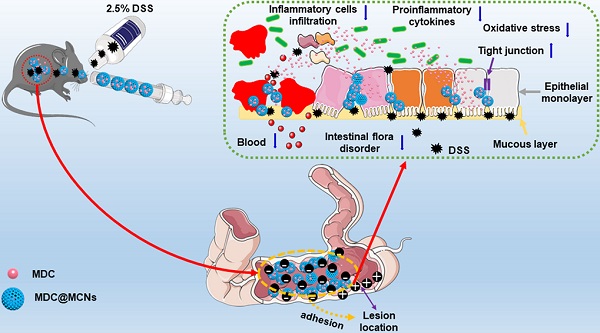
Ulcerative colitis (UC) is a modern refractory disease with steadily increasing incidence worldwide that urgently requires effective and safe therapies. Therapeutic peptides delivered using nanocarriers have shown promising developments for the treatment of UC. We developed a novel colon-accumulating oral drug delivery nanoplatform consisting of Musca domestica cecropin (MDC) and mesoporous carbon nanoparticles (MCNs) and investigated its effects and mechanism of action for the treatment of UC.
Methods: An optimized one-step soft templating method was developed to synthesize MCNs, into which MDC was loaded to fabricate MDC@MCNs. MCNs and MDC@MCNs were characterized by BET, XRD, and TEM. MDC and MDC@MCNs resistance to trypsin degradation was measured through Oxford cup antibacterial experiments using Salmonella typhimurium as the indicator. Uptake of MDC and MDC@MCNs by NCM460 cells was observed by fluorescence microscopy. The biocompatibility of MDC, MCNs, and MDC@MCNs was evaluated in three cell lines (NCM460, L02, and NIH3T3) and C57BL/6 mice. Dextran sulphate sodium was used to establish models of NCM460 cell injury and UC in mice. MTT assay, flow cytometry, and mitochondrial membrane potential assay were applied to determine the effects of MDC@MCNs on NCM460 cells injury. Additionally, a variety of biological methods such as H&E staining, TEM, ELISA, qPCR, Western blotting, and 16s rDNA sequencing were performed to explore the effects and underlying mechanism of MDC@MCN on UC in vivo. Colonic adhesion of MCNs was compared in normal and UC mice. The oral biodistributions of MDC and MDC@MCNs in the gastrointestinal tract of mice were also determined.
Results: MDC@MCNs were successfully developed and exhibited excellent ability to resist destruction by trypsin and were taken up by NCM460 cells more readily than MDC. In vitro studies showed that MDC@MCNs better inhibited DSS-induced NCM460 cells damage with lower toxicity to L02 and NIH3T3 cells compared with MDC. In vivo results indicated that MDC@MCNs have good biocompatibility and significantly improved colonic injury in UC mice by effectively inhibiting inflammation and oxidative stress, maintaining colonic tight junctions, and regulating intestinal flora. Moreover, MDC@MCNs were strongly retained in the intestines, which was attributed to intestinal adhesion and aggregation of MCNs, serving as one of the important reasons for its enhanced efficacy after oral administration compared with MDC.
Conclusion: MDC@MCNs alleviated DSS-induced UC by ameliorating colonic epithelial cells damage, inhibiting inflammation and oxidative stress, enhancing colonic tight junctions, and regulating intestinal flora. This colon-accumulating oral drug delivery nanoplatform may provide a novel and precise therapeutic strategy for UC.
Keywords: ulcerative colitis, Musca domestica cecropin, mesoporous carbon nanoparticles, colon-accumulating drug delivery, enhanced therapy
 Global reach, higher impact
Global reach, higher impact