13.3
Impact Factor
Theranostics 2021; 11(8):3996-4010. doi:10.7150/thno.56035 This issue Cite
Review
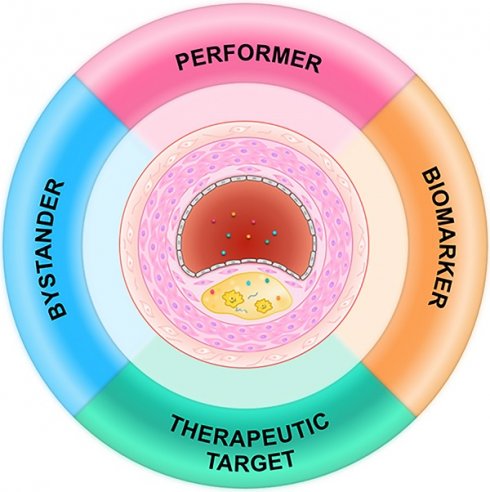
Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets
Department of Ultrasound Diagnostics, Tangdu Hospital, Fourth Military Medical University, Xi'an 710038, People's Republic of China.
#These authors contributed equally to this work.
Received 2020-11-17; Accepted 2021-1-14; Published 2021-2-15
Abstract
Exosomes are nanosized lipid vesicles originating from the endosomal system that carry many macromolecules from their parental cells and play important roles in intercellular communication. The functions and underlying mechanisms of exosomes in atherosclerosis have recently been intensively studied. In this review, we briefly introduce exosome biology and then focus on advances in the roles of exosomes in atherosclerosis, specifically exosomal changes associated with atherosclerosis, their cellular origins and potential functional cargos, and their detailed impacts on recipient cells. We also discuss the potential of exosomes as biomarkers and drug carriers for managing atherosclerosis.
Keywords: exosomes, atherosclerosis, intercellular communication, biomarker, therapy
Introduction
Atherosclerosis involves the formation of fibrofatty lesions or plaques in the artery wall. This disease causes substantial morbidity and mortality worldwide [1, 2]. The pathological process of atherosclerosis involves endothelial damage, lipid deposition, inflammatory cell infiltration, foam cell formation, and plaque formation [3, 4]. Rupture of the vulnerable plaque causes in situ thrombosis and intramural hemorrhage, which result in ischemia and stroke [1, 5, 6].
Cellular communication is essential for nearly all physiological and pathological processes, including atherosclerosis [7]. Besides their widely accepted involvement in neurotransmission and endocrine signaling, extracellular vesicles (EVs) have been recognized as new players in intercellular communication [8]. EVs are classified into exosomes, apoptotic bodies, microvesicles, ectosomes, and other vesicles. Exosomes (40-160 nm in diameter [9-11]) are secreted by nearly all cell types and carry biological molecules such as DNAs, RNAs, proteins, lipids, and metabolites [11]. The encapsulated biomolecules not only reflect the identity of the donor cell, they also have functions in the recipient cells [9]. Exosomes also display profound advantages for crossing biological barriers [12, 13], and are involved in intercellular communication over both short and long distances [14]. For these reasons, exosomes have been intensively studied as biomarkers and drug carriers for diagnostic and therapeutic applications [10, 15].
Exosomes have been found to be secreted by endothelial cells, cardiac progenitor cells, cardiac fibroblasts, and cardiomyocytes, suggesting their involvement in cardiovascular diseases [9, 16, 17]. For example, exosomes derived from endothelial cells have been found to play a central role in the phenotype switch of vascular smooth muscle cells (VSMCs) [18]. In addition, circulating exosomes released from platelets, erythrocytes, leukocytes, and endothelial cells carry biomolecules reflecting the identity of their donor cells and so can serve as biomarkers for diverse pathological states, including atherosclerosis [19, 20]. Changes in exosome levels and cargos have been reported in a variety of diseases associated with vascular injury [21-23]. It has been also suggested that extracellular vesicles, including exosomes, are involved in the microcalcification in atherosclerosis [24]. In general, significant changes of exosomes could be seen in atherosclerosis and associated risk factors. In turn, exosomes might function as performers, bystanders, biomarkers, and even therapeutic vehicle in atherosclerosis.
In this review, we summarize advances in the roles of exosomes in atherosclerosis. The potential of exosomes as diagnostic biomarkers and therapeutic drug carriers for atherosclerosis management are also discussed.
Biogenesis and composition of exosomes
Biogenesis of exosomes
Exosome biogenesis starts with invagination of the endosomal membrane, which forms a multivesicular body (MVB) inside the endosome [25]. During this process, cytosolic nucleic acids and proteins are incorporated into MVBs [26]. The encapsulated cargos are either degraded when MVBs fuse with lysosomes, or secreted in exosomes when endosomes fuse with the membrane of the parental cell [27-29]. Since endosomes result from budding of the plasma membrane, this double-invagination process produces exosomes with the same membrane protein orientation as that of the parental cell [11, 25].
Composition of exosomes
Nucleic acids
Exosomes have an aqueous core and a lipophilic shell, therefore they encapsulate hydrophilic cargos [30]. Nucleic acids in exosomes have been intensively studied, mainly focusing on their roles in mediating communication between cells and their potential as diagnostic biomarkers [31]. Among the exosomal RNAs, miRNAs are the most abundant type [32]. Exosomal miRNAs related to atherosclerosis will be discussed in more detail later in this review. Besides the intensively studied miRNAs, a broad range of lncRNAs and circRNAs have also been identified in exosomes [33, 34]. Similar to exosomal miRNAs, exosomal lncRNAs and circRNAs can also induce a series of phenotypic changes in recipient cells [35, 36].
lncRNAs are a novel group of mediators defined as long noncoding ribonucleic acids of more than 200 nucleotides. lncRNAs actively participate in biological and pathological processes [37, 38], including in cardiovascular diseases [39-41]. For example, the lncRNA NEXN-AS1 was found to regulate endothelial cell activation and monocyte adhesion via the TLR4/NF-κB pathway to deter atherogenesis [42]. In addition, the lncRNA CCL2 may contribute to human atherosclerosis via positively regulating CCL2 mRNA levels in endothelial cells [43]. Recent studies have also shown that lncRNAs carried by exosomes play critical roles in intercellular communication [44-47]. Although the involvement of exosomal lncRNA in the regulation of cardiovascular diseases has received considerable attention, their roles in vascular dysfunction and atherosclerosis still need to be explored [39, 48].
circRNAs are covalently closed biomolecules produced by precursor mRNA back-splicing with tissue-specific and cell-specific expression patterns. circRNAs have been the highlight of recent studies [49, 50]. circRNAs play regulatory roles in biological functions, such as “sponge”-like sequestration of miRNAs or proteins, and modulation of protein transcription, function, and even translation to produce polypeptides [51-53]. Moreover, circRNAs have been implicated in many diseases, especially cancer and cardiovascular diseases [54, 55]. The circRNA hsa_circ_0003575 was found to be involved in oxidized low-density lipoprotein (ox-LDL)-induced endothelial cell proliferation and angiogenesis [56]. Recently, involvement of exosomal circRNAs in cardiovascular functions and diseases has been increasingly reported [57]. For instance, plasma exosomal hsa_circ_0005540 was found to be a promising diagnostic biomarker of coronary artery disease [58]. Further, exosomal circHIPK3 was found to participate in the regulation of cardiac vascular injury and angiogenesis after myocardial infarction, suggesting a new mechanism of cellular communication in cardiovascular diseases mediated by exosomal circRNA [34, 59]. In addition, increased circ_0003204 in extracellular vesicles was found to stimulate ectopic endothelial inactivation in cerebrovascular atherogenesis [60]. lncRNAs and circRNAs associated with cardiovascular disease are listed in Table 1.
Exosomal lncRNAs and circRNAs involved in atherosclerosis
| lncRNA/circRNA | Expression/Function | Target | Implication | Isolation method | Ref. |
|---|---|---|---|---|---|
| lncRNA MALAT1 | Inhibits maturation of DCs | NRF2 | Regulates progression of atherosclerosis | miRCURY Exosome Kit | [143] |
| circHIPK3 | Regulates dysfunction of CMVECs | miR-29a/IGF-1 | Shuttles with exosomes and is a potential treatment target | Ultracentrifugation | [34] |
| Accelerates cell cycle progression and proliferation | miR-29a/VEGF-A | Cardioprotective | Ultracentrifugation | [59] | |
| circ_0003204 | Mediates endothelial phenotype | miR-370-3p/TGFβR2/phosph-SMAD3 | Novel stimulator and potential biomarker | ExoQuick | [60] |
| circ_0005540 | Elevated in patients with CAD | NA | Promising diagnostic biomarker for CAD | exoRNeasy kit | [58] |
| circ_0001445 | Downregulated in atherogenic conditions | NA | Improves the identification of coronary artery atherosclerosis | NA | [162] |
CAD: coronary artery disease; CMVEC: cardiac microvascular endothelial cell; DC: dendritic cell; IGF-1: insulin-like growth factor-1; MALAT1: metastasis-associated lung adenocarcinoma transcript 1; NA: not available; NRF2: nuclear factor erythroid 2-related factor; SMAD3: small mothers against decapentaplegic 3; TGFβR2: transforming growth factor β receptor 2; VEGF-A: vascular endothelial growth factor-A.
Recently, exosomal mRNAs were also found, and these could be translated into proteins when exosomes are endocytosed by recipient cells [61]. Notably, although dsDNA and associated histone were found in exosomes [62], this idea was challenged in a recent study, in which the authors claim that the extracellular DNA and histones were secreted independent of exosomes [63].
Proteins
Exosomes contain abundant proteins irrespective of their cell origin, including transmembrane proteins and cytosolic proteins [64-66]. Exosomes are enriched in integrins and tetraspanins, such as CD63, CD81, CD9, and CD82 [67], and cytosolic proteins, such as RAB proteins and TSG101 [9, 68]. In addition, many proteins participating in MVBs formation can also be found in exosomes, such as ALIX and flotillin, and these proteins are categorized as non-specific exosomal proteins [9]. Additionally, heat shock proteins (HSP70 and HSP90), and cytoskeleton proteins (actin, myosin, tubulin) can also be encapsulated in exosomes [69, 70]. In contrast, exosomes are free of proteins not associated with plasma membranes or endosomes, such as protein components of the endoplasmic reticulum, Golgi, mitochondria, or nucleus [71, 72]. In addition, cytokines are also rarely seen in exosomes [73]. Appearance of these exclusive proteins might suggest impurities in the isolated exosomes [74].
Lipids
The exosomal membrane lipid components are similar but slightly different from the plasma membrane of the donor cells. Ceramides, phosphatidylethanolamines, phosphatidylserines, diacylglycerides, cholesterol, sphingomyelins, and lyso-bisphospatidic acid, have been found in exosome membranes [75, 76]. Notably, specific lipids are enriched in exosomes compared with donor cells and other types of EVs. For example, sphingolipids, cholesterol, and phosphatidylserines are enriched in exosomes. In addition, exosomes have a higher lipid order and thus are more resistant to detergents [77]. Exosomal lipids play important roles in the biology of these vesicles, modifying the phenotype of receiving cells [78]. Moreover, the lipid components might also serve as diagnostic biomarkers, with the advance of lipidomics.
Exosome isolation methods
Current conventional exosome isolation methods include differential ultracentrifugation (UC), immunoaffinity capture and microfluidics, polymer-based precipitation, ultrafiltration (UF), and size exclusion chromatography (SEC) [25, 79]. These methods have different efficiencies and purities; it is thus important to note the isolation method used when integrating data from various studies. UC can isolate exosomes from various particles, including pelleted cells, debris, and most large extracellular vesicles, by high centrifugal forces of at least 100,000 ×g [80]. But this method cannot achieve absolute separation of exosomes, meaning that clumps of EVs, protein aggregates, and even viruses are mixed together in “isolated exosomes” samples [79]. Though UC is time-consuming, labor intensive, and inefficient, it is suitable for exosomes separation of large laboratory samples [81]. However, its application is limited for clinical samples [81, 82]. Immunoaffinity capture and microfluidics, due to its higher capture efficiency and greater sensitivity, is an attractive approach for isolating exosomes. Its disadvantages include marker-dependent related omission and high cost [83]. Precipitation methods are usually based on polyethylene glycol (PEG), a nontoxic and nondenaturating water-soluble polymer [84]. This method is simple, rapid, and easy and does not require costly or specialized equipment; however, the final exosomes pellet is contaminated due to the low specificity of PEG in isolating other extracellular vesicles or proteins [85, 86]. UF is an emerging size-based isolation method that uses membrane filters of defined exclusive criterion to prepare highly pure and concentrated exosomes samples with high recovery. However, it is difficult to avoid protein contamination in the exosome pellet [81, 86, 87]. Accumulated evidence suggests that SEC is an ideal exosome isolation technique that can separate exosomes from most proteins to acquire pellets with low levels of contaminants and co-precipitates [88]. SEC is noteworthy for its superior isolation of pure exosomes from human body fluids, and is not limited by sample volume or type, indicating its great potential to generate a high yield of exosomes for clinical and commercial applications [79]. But SEC cannot distinguish exosomes from other vesicles of similar size, and it is limited by the number of samples that can be processed at one time [85, 88]. Considering sample purity, cost, efficiency, and labor, UC is still the most appropriate and standard technology for exosomes isolation [81, 82]. Notably, there is unneglectable overlap in particle size and density between exosomes and other non-vesicular contaminants, such as lipoproteins and nucleoproteins [89-91]. Therefore, the major challenge in exosomes isolation remains the need to develop simple, cheap, and rapid methods that not only maintain the viability and features of exosomes but also distinguish them from other substances [92]. Very recently, several promising methods have been developed, such as ExoTIC (exosome total isolation chip) [93], acoustofluidic platform (an integration of acoustics and microfluidics) [94], and alternating current electrokinetic microarray chip devices [95].
Exosomal changes related to atherosclerosis risk factors
Hypertension, obesity, lipid disorder, and diabetes mellitus are major risk factors for atherosclerosis [96, 97]. Accumulating studies have linked these risk factors with changes in exosome biogenesis and cargo. Cigarette smoking is also a risk factor for atherosclerosis, and future work exploring the link between smoke and exosomes are of great interest. Currently, miRNA cargos have been intensively studied, whereas exosomal lncRNAs/circRNAs are not well defined. The altered exosomal components might be important regulators of atherosclerosis, and thus the exosomal changes should be useful for predicting the risk of atherosclerosis. Moreover, therapeutic targeting these molecules might be a strategy to reduce the risk of atherosclerosis. In this section, we will focus on the relationships between atherosclerosis risk factors and exosomes.
Exosomal changes upon hypertension
Hypertension is a primary risk factor for atherosclerosis [98]. Recent studies suggest that exosomes mediate pathological processes of hypertension along with related injuries to organs [99]. Circulating exosomal miRNA was found to be altered in patients with obstructive sleep apnea and hypertension, suggesting that fluctuating high blood pressure may change plasma exosome mass and cellular exchange of information [100]. Exosomes have also been found to promote the development of hypertension. For example, Osada-Oka et al. showed that macrophage-derived exosomes at least partially contributed to inflammation of endothelial cells under hypertensive conditions [101]. In contrast, plasma exosomes were found to modestly regulate systemic blood pressure by rebuilding the structure and function of cardiovascular tissues in vivo [102]. Thus, elucidating the precise role of exosomes in hypertension might provide new therapeutics for hypertension and related cardiovascular diseases [103].
Exosomal changes upon obesity
Obesity is an independent risk factor that severely threatens human life and health. With its increasing prevalence worldwide, obesity has become a serious public health challenge [104, 105]. Adipose tissue not only stores lipids but also serves as an endocrine organ. Obesity is characterized by an imbalance in the adipose secretome, with an increase in proinflammatory adipocytokines and a decrease in anti-inflammatory adipocytokines [106, 107]. Among the secretome, exosomes secreted by adipose tissue play key roles in whole-body glucose and lipid metabolism [108]. For example, the adiponectin/T-cadherin system was found to quantitatively increase exosome biogenesis and secretion [109]. Thomou et al. observed that adipose tissue significantly modulates the plasma mass of exosomes and circulating exosomal miRNAs, which regulate the expression and translation of target mRNAs in distant recipient tissues as a novel form of adipokine [110]. Exosomal miRNAs have also shown robust changes in animal models of obesity. Treatment of lean mice with exosomes from obese mice, which mainly contained miR-122, induced metabolic dysfunction with glucose intolerance and insulin resistance [111]. It is clear that adipose-derived exosomes constitute a previously undescribed class of signaling moieties, opening an avenue to better understand the pathophysiology and treatment of obesity and associated diseases [110, 112, 113].
Exosomes in lipid disorder
Plasma lipid level is strongly associated with risk of cardiovascular disease, according to mounting prospective observational studies worldwide [3, 114-116]. Blood lipid disorder is an accepted causal risk factor for atherosclerosis, especially in plaque progression and thrombosis [96, 117]. Recently, many studies have focused on the relationship between exosomes and lipid disorder. Exosomes-mediated lipid metabolism covers the process of lipid synthesis, transportation, and degradation, which have been implicated in atherosclerosis [118]. For example, exosomes are an adequately potent source of eicosanoids such as prostaglandins and leukotrienes, both of which are active in vivo and in vitro. The biological significance and mechanism of exosomal shuttling in the eicosanoids synthesis pathway has attracted rapidly growing interest [119]. In addition to transporting lipids directly to recipient cells, exosomes can also regulate the expression of classical lipid transporters, such as reverse cholesterol transport mediated by ABCA1 [120]. Furthermore, substantial evidence suggests that brown adipose tissue (BAT)-derived exosomes can alleviate lipid accumulation and improve cardiac function, indicating that exosomes are involved in lipid degradation and adipose tissue redistribution [121]. In turn, growing evidence suggests that lipid metabolism affects the biological functions of exosomes, including bioprocesses from signal transduction by receptor-ligand interactions and exosome internalization by or fusion with recipient cells, which provides a new perspective for better understanding the occurrence and development of atherosclerosis [118].
Diabetes mellitus associated exosomal changes
Numerous studies have shown that diabetes is associated with accelerated atherosclerosis and that exosomes have pathophysiological effects on atherosclerotic plaque destabilization [122, 123]. Patients with type 1 diabetes mellitus (T1DM) have increased plasma levels of exosomes. Upregulation or downregulation of exosomal miRNAs is associated with progression of this disease [124, 125]. Karolina et al. revealed that four exosomal miRNAs (miR-17, miR-197, miR-509-5p, and miR-92a) were reduced while miR-320a was increased in patients with type 2 diabetes mellitus (T2DM) [126]. Moreover, these altered exosomes might in turn promote the development of atherosclerosis. Wang et al. determined that insulin-resistant adipocyte-derived exosomes accelerated atherosclerosis and plaque vulnerability by inducing vasa vasorum angiogenesis [127]. Moreover, insulin resistance has been reported to drive extracellular vesicles secretion, which may contribute to the quantitative alteration of plasma exosomes in diabetes, and highlights their potential as diagnostic tools of T2DM [128].
Exosomes in atherogenesis
Accumulating evidence has revealed that exosome-mediated cellular interactions play important roles in atherogenesis [19, 129]. The effects of exosomes on atherosclerosis are intensively discussed in a recent excellent review [130]. In this section, we will focus on the various origins of exosomes in atherosclerosis and the underlying mechanisms involved.
Biological functions of exosomes
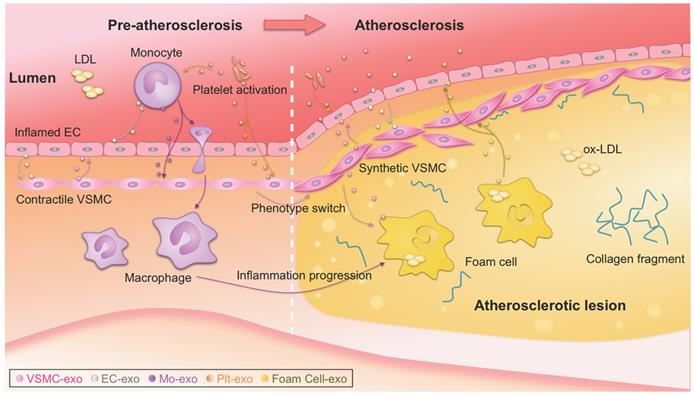
Atherosclerotic lesions are initiated by the accumulation of low-density lipoprotein (LDL) particles in the intima, adhesion of blood monocytes to the injured endothelium, migration of the monocytes into the intima, and maturation of macrophages along with the formation of lipid-filled foam cells [3]. Phenotype switching of VSMCs from contractile to synthetic type and chronic inflammation of the arterial wall also drive the progression of atherosclerosis. With the progression of atherosclerosis, a necrotic core and thrombosis ultimately form in the lesion [1, 2]. Recently, the notion that plaque healing may play a key role in the natural history of atherosclerotic disease has updated traditional theories of atherosclerosis [117]. Notably, exosomes have been reported to be actively involved in nearly all the above biological processes [19], which is a new, dynamic area of research (Figure 1) [131, 132]. It is also important to note that there is far less than one molecule of a given RNA per exosome, even for the most abundant miRNAs. This stoichiometry of miRNAs and exosomes suggests that most individual native exosomes either from pathological or physiological conditions do not carry biologically significant numbers of RNAs. Thus, individual exosome is unlikely to be functional as vehicle to transfer functional RNAs [133]. In other words, the observed pathophysiological effects might stem from that amounts of exosomes of similar function work together for a long duration.
Origins of exosomes
Endothelial cell-derived exosomes
Endothelial dysfunction is the initial step in the process of atherogenesis [134-136]. Endothelium has important functions in the regulation of inflammation, coagulation, vascular tone, and vascular wall permeability. Endothelial dysfunction triggers release of extracellular vesicles, including exosomes [137]. Moreover, cellular stress conditions are reflected in exosomal protein and RNA [22]. Endothelial cell-derived exosomes are involved in atherogenesis by transferring biological messages to other cells [138]. Endothelial cell-derived vesicles regulate VSMC phenotype via their cargos [139]. For example, miR-143/145-containing extracellular vesicles derived from KLF2-expressing endothelial cells reduced atherosclerotic lesions in ApoE-/- mice [140]. Similarly, endothelial cell-derived exosomes could inhibit the VSMC phenotype switch [141]. Moreover, exosomes of endothelial origin can modulate monocyte activation by transferring miR10a [142]. The involved exosomal miRNAs are summarized in Table 2. Furthermore, some exosomal lncRNAs and circRNAs have also been found in endothelial cell-derived exosomes. Exosomes from ox-LDL-treated endothelial cells induced dendritic cell maturation in atherosclerosis due to loss of the lncRNA MALAT1 (Table 1) [143].
Recent evidence suggests that exosomes derived from endothelial progenitor cells (EPCs) may participate in the repair of endothelial function at some stage [144]. As the precursor cells of vascular endothelial cells, EPCs are a type of stem cell from the bone marrow with limited differentiation ability and strong growth ability [145]. Exosomes derived from EPCs regulate VSMC phenotype via the ACE2/NF-κB/Ang II pathway, indicating their potential for hypertension treatment [146]. Meanwhile, it has been revealed that EPC-derived exosomes overexpressing angiotensin-converting enzyme 2 (ACE2) can protect endothelial cells by decreasing apoptosis and improving mitochondrial function [147]. Furthermore, EPC-derived exosomes significantly decreased the production of atherosclerotic plaques and inflammatory factors, and ameliorated endothelial dysfunction in a mouse model of atherosclerotic diabetes [148]. Conversely, a study showed that EPC-derived exosomes have attenuated myocardium repair properties due to enrichment of exosomal integrin-linked kinase under IL-10 deficiency or inflammation conditions, which indicates the potential of exosomal protein manipulation as an advanced therapeutic method for cardiovascular diseases [149].
VSMC-derived exosomes
VSMCs below the endothelium control vascular tension at physiological conditions [2]. VSMC-derived exosomes are novel critical regulators of vascular hemostasis [150, 151]. miR-1246, miR-182, and miR-486 in VSMC-derived exosomes play essential roles in the maintenance of vascular homeostasis [152]. Numerous studies have shown that proliferation, phenotype switching (mainly contractile to migratory state), apoptosis, and calcification of VSMCs are closely linked to the onset and progression of atherosclerosis [153-155]. In the process of atherosclerosis, VSMCs communicate with surrounding cells by secreting various factors, with exosomes emerging as a new mediator (Figure 1) [156]. Under pathological conditions, VSMCs switch to the synthetic phenotype and actively secrete exosomes to induce endothelial migration and angiogenesis, promoting the formation of atherosclerotic plaques and triggering vascular calcification [155, 157]. In addition, exosomes from calcifying VSMCs were found to accelerate calcification by propagating procalcifying signals. Moreover, proliferating VSMCs were found to release more exosomes and exosomes were found deposited in precalcified vessels, which may prime the vessel wall to calcify [150, 158, 159]. Theoretically, preventing release of exosomes from calcified VSMCs might effectively prevent vascular calcification and the formation of atherosclerotic plaques (Figure 1) [157, 160].
Exosome-mediated intercellular communication in the progression of atherosclerosis. Exosomes could be secreted by all types of the cells involved in atherosclerosis, such as monocytes, macrophages, platelet, endothelial cells (ECs), vascular smooth muscle cells (VSMCs). Intercellular communication via exosomes occurs, transmitting signal from one cell type to another, contributing to the progression of atherosclerosis. Detailed roles of the exosomes from different origins should be different, and thus the exosomes act as either bystanders or performers in the process. ox-LDL, oxidized low-density lipoprotein.
Exosomal miRNAs involved in atherosclerosis
| Origin | Cargo | Function | Target | Implication | Isolation method | Ref. |
|---|---|---|---|---|---|---|
| miR-10a | Modulates monocyte activation | NF-κB | Represses inflammatory signal in cardiovascular disease | Medium: ultracentrifugation; Plasma: ExoQuick | [142] | |
| Endothelial cell | miR-143/145 | Controls VSMC phenotypes | KLF2 | Reduces atherosclerotic lesion formation | Multi-step centrifugation | [140] |
| CD137 | Decreases anti-inflammatory effects | TET2 | Accelerates VSMC proliferation and migration and neointimal formation | Exo-spin | [141] | |
| miR-155 | Suppresses the expression of TJ proteins | ZO-1 | Impairs endothelial barrier function | Differential centrifugation | [161] | |
| noncrystalline Ca/P salt | Promotes calcification | SMPD3 | Calcifies vascular cells and enriches in calcified vasculature | Differential ultracentrifugation | [150] | |
| VSMC | miR-1246 | Inhibits EC migratory activities | NA | Maintains vascular homeostasis | ExoQuick-TC | [152] |
| miR-182 | ||||||
| miR-486 | ||||||
| Monocyte/macrophage | miR-146a | Reduces macrophage migration | IGF2BP1/HuR | Accelerates development of atherosclerosis | ExoQuick-TC, differential ultracentrifugation | [169] |
| Promotes ROS and NETs release | SOD2 | Slows atherosclerosis development | Medium: ExoQuick-TC; Serum: differential ultracentrifugation | [170] | ||
| integrins | Changes phosphorylation levels | ERK/AKT | Promotes VSMC migration and adhesion | Ultracentrifugation | [166] | |
| miR-21-3p | Promotes VSMC migration and proliferation | PTEN | Accelerates atherosclerotic plaque progression | Ultracentrifugation, sucrose density gradient centrifugation | [171] | |
| miR-99a | Fosters M2 polarization, reduces hematopoiesis, and suppresses inflammation | NF-κB/TNF-α | Reduces necrotic lesion area, stabilizes atheroma, and controls atherosclerosis | Cushioned-density gradient ultracentrifugation | [172] | |
| miR-146b | ||||||
| miR-378a | ||||||
| Platelet | HMGB1 | Initiates a cascade of platelet thrombogenesis | NA | Biomarker of platelet abnormalities | ExoQuick-TC | [178] |
| miR-223 | Inhibits ICAM-1 expression | NF-κB/MAPK | Regulates thrombosis-inflammation reaction | Ultracentrifugation | [182], [183] | |
| miR-126 | Promotes the proliferation and migration of HUVECs | NA | Contributes to intraplaque angiogenesis | ExoQuick-TC | [180] | |
| miR-25-3p | Inhibits ox-LDL-induced EC inflammation and lipid deposition | NF-κB/Adam10 | Alleviates atherosclerosis and is a potential treatment target | ExoQuick-TC | [181] |
Adam10, a disintegrin and metalloprotease 10; AKT, protein kinase B; ERK, extracellular regulated protein kinases; HMGB1, high-mobility group box 1 protein; HuR, human antigen R; ICAM-1, intercellular adhesion molecule-1; IGF2BP1, insulin-like growth factor 2 mRNA-binding protein 1; KLF2, krüppel-like factor 2; M2, M2 macrophages; MAPK, mitogen-activated protein kinase; NA, not available; NETs, neutrophil extracellular traps; NF-κB, nuclear factor-κB; PTEN, phosphatase and tension homologue; ROS, reactive oxygen species; SMPD3, sphingomyelin phosphodiesterase 3; SOD2, superoxide dismutase 2; TET2, ten-eleven translocation 2; TJ, tight junctions; TNF-α, tumor necrosis factor α; VSMC, vascular smooth muscle cell; ZO-1, zonula occludens-1.
miRNAs are considered to be the main functional cargos of VSMC-derived exosomes. For example, exosomes derived from KLF5-overexpressing VSMCs were found to transfer miR-155 to endothelial cells, which in turn inhibited endothelial cell proliferation and migration, eventually impairing tight junctions and the integrity of endothelial barriers [161]. VSMC-derived exosomal miRNAs involved in atherogenesis are summarized in Table 2. Besides miRNAs, circRNAs are also involved. For example, hsa_circ_0001445 was found to be downregulated in extracellular vesicles secreted by coronary smooth muscle cells in atherogenic conditions, which could be used as a biomarker to improve the identification of coronary artery atherosclerosis [162].
Inflammatory cell-derived exosomes
Macrophages in the subendothelial space of the artery wall, which are differentiated from monocytes, are involved in all stages of atherosclerosis, from endothelial dysfunction, to lesion expansion, and formation of the plaque [163]. Notably, the idea that macrophages have a diminished capacity to egress remains challenged [164, 165]. Exosome biogenesis is different in macrophages and the derived exosomes could play crucial roles throughout the whole process of atherosclerosis (Figure 1). Macrophage-derived foam cells release more exosomes than normal macrophages [166]. Inflamed macrophages secrete exosomes that promote cytokine production when endocytosed by recipient cells, which recruits other immune cells to inflamed sites [167]. Exosomes derived from ox-LDL‑stimulated macrophages were found to impair endothelial function [168]. Extracellular vesicles containing miR-146a secreted from macrophages in a proatherogenic environment functionally altered recipient cell function in vitro, suggesting a potential role in atherogenesis [169]. Consistently, exosomal miR-146 from atherogenic macrophages was found to deteriorate atherosclerosis development by promoting neutrophil extracellular traps [170]. Besides miR-146, other miRNAs might also be involved. For example, exosomes from nicotine-stimulated macrophages were found to at least partially contribute to nicotine-promoted atherosclerosis, in which exosomal miR-21-3p promoted VSMC migration and proliferation [171]. In addition, exosomal miR-99a/146b/378a derived from alternatively activated macrophages downregulated TNF-α/NF-κB signaling and alleviated inflammation [172]. Besides the monocyte/macrophage derived exosomes, exosomes from neutrophil and other inflammatory cells might be also involved. The detailed functions and targets of these miRNAs are summarized in Table 2.
Platelet-derived exosomes
Platelets have emerged as potent regulators of atherosclerosis by facilitating recruitment of inflammatory cells [173-175]. Heightened platelet adhesion, activation, and aggregation are pivotal pathophysiological conditions associated with the initiation and progression of atherosclerotic lesions [176, 177]. Moreover, exosomes are major mediators in the crosstalk between platelets and other cells in the pathogenesis of atherosclerosis (Figure 1) [178]. Platelet-derived exosomes are the most abundant type in the bloodstream in normal conditions [179]. Activated platelet-derived exosomes were found to promote the proliferation and migration of HUVECs, shedding new light on the effects of platelet-derived exosomes in atherosclerosis and intraplaque angiogenesis [180]. In contrast, platelet exosomal miR-25-3p was shown to inhibit ox-LDL-induced coronary vascular endothelium inflammation [181]. Platelet-derived exosomes can also be uptaken by endothelial cells, where the exosomes inhibit ICAM-1 expression at least partially via miR-223 [182, 183].
Circulating exosomes of other origins
Exosomes originating from a variety of cell types are released into the blood as circulating exosomes for long distance transport of biomolecules [179]. Exosomes associated with atherosclerosis mainly originate from platelets, leukocytes, VSMCs, and endothelial cells, and the exosomes discussed above constitute the majority of circulating exosomes [19, 62]. However, circulating exosomes can also be released from other sources. For example, adipose tissue constitutes a major source of circulating exosomes that serve as a novel form of adipokine for cellular communication and regulation [110]. As a result of mutual interactions between distribution and function, alteration of adipose tissue greatly affects circulating exosomes and their cargos [110, 184]. Additionally, adipose tissue is a well-established driver in the development of obesity, which is one of the most critical risk factors for atherosclerosis [185]. Thus, exosomes derived from adipose tissue should actively influence atherogenesis, but the specific mechanisms remain unclear. In addition, skeletal muscle with secretory activities has been suggested to be another irreplaceable source of circulating exosomes [186, 187]. The healthy state of muscle is inextricably linked to regular physical activity, which helps reduce the risk of sedentary lifestyle-induced chronic cardiovascular diseases such as atherosclerosis [188]. Collectively, muscle-derived circulating exosomes play crucial roles in atherosclerosis [189]. Studies profiling changes in circulating exosomes associated with atherosclerosis-related metabolic abnormalities, as well as identifying their mechanisms, would be highly valuable.
Exosomes in atherosclerosis diagnosis and therapy
Exosomal miRNAs as putative biomarkers
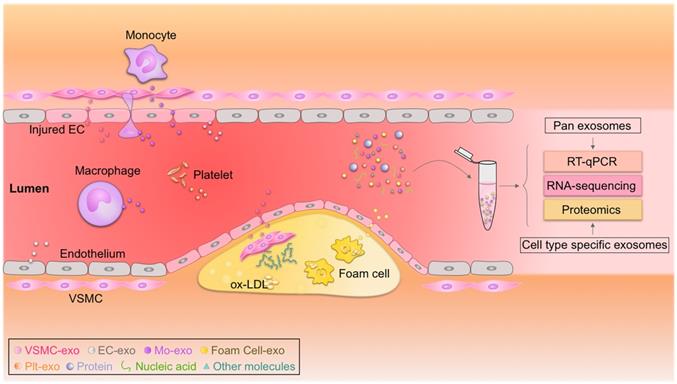
The discovery, validation, and implementation of novel biomarkers are important for improving prognosis in the clinic [190, 191]. Exosomes have emerged as rational biomarkers for various diseases as they are easily accessible, carry disease-specific cargos, and have a high degree of stability in body fluids. Exosome-derived miRNAs can be isolated from multiple fluids faultlessly, raising exciting opportunities for clinical translation (Figure 2) [190, 192]. Theoretically, exosome-derived miRNAs are a better biomarker than circulating miRNAs in plasma/serum, as exosomes from specific cell types can be purified, ensuring sensitivity and specificity [193, 194]. Jiang et al. found that a specific circulating exosomal miRNA signature (miR-122-5p, miR-27b-3p, miR-101-3p, etc.) is a novel biomarker predicting recurrent ischemic events in intracranial atherosclerotic disease [195]. Additionally, release of exosomal miR-92a-3p from endothelial cells is associated with atherogenic conditions and could serve as a potential diagnostic biomarker [196]. Furthermore, plasma exosomal miR-30e and miR-92a expressions were up-regulated in atherosclerosis and negatively correlated with plasma cholesterol and ABCA1 levels, providing a new biomarker for the clinical diagnosis and treatment of coronary atherosclerosis [197]. In addition, exosomal miRNAs involved in atherosclerotic lesion development, such as miR-133a, miR-155, miR-21, miR-210, miR-126, and miR-499, have also emerged as promising biomarkers for diagnosis, risk stratification, and prognosis prediction [194, 198, 199]. According to a recent study by Sorrentino et al., circulating exosomes and their encapsulated miRNAs correlated well with atherosclerosis severity, suggesting a potent diagnostic potential [200]. Despite these promising results, none of these biomarkers have been validated in large cohort studies. Like all other biomarkers, before exosomal biomarkers can be translated to the clinic, they must be validated and accredited by the International Organization for Standardization [201]. In addition, exosome isolation methods should also be standardized [202].
Circulating exosomes as emerging biomarkers for atherosclerosis. Circulating exosomes (exo) from different cell types associated with atherosclerosis carry cargos with identities similar to their donor cells. Real-time quantitative polymerase chain reaction (RT-qPCR), proteomic, and transcriptomic profiling of these biomarkers could be used to diagnose atherosclerosis. EC, endothelial cell; Mo, monocyte; ox-LDL, oxidized low-density lipoprotein; Plt, platelet; VSMC, vascular smooth muscle cell.
Therapeutic potential of exosomes in atherosclerosis
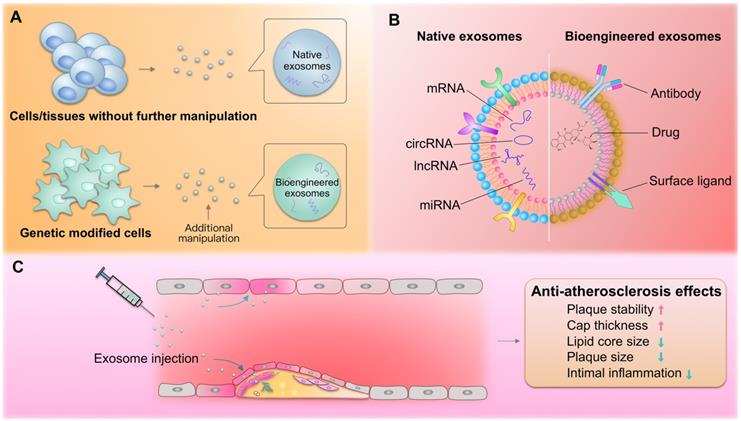
Over the last few years, exosomes have been considered as potential biotherapeutics and drug delivery vectors for various diseases. Their natural functional nucleic acid and protein cargos have raised the possibility that exosomes from specific origins may be therapeutic drugs. For example, exosomes from cardiac stem cells could regulate cellular processes in recipient cardiac cells toward better regeneration [203]. In addition, exosomes could be harnessed for the therapeutic delivery of RNAs, peptides, and synthetic drugs [204]. For example, we recently established an exosome-mediated Ldlr mRNA delivery strategy, which could effectively rebuild Ldlr expression and stabilize atherosclerotic plaques in Ldlr-/- mouse model, providing a promising therapeutic approach for atherosclerosis [205]. Numerous studies have explored the roles of exosomes in managing atherosclerosis. Compared with the commonly used nanoparticles, exosomes are of great advantage in low immunogenicity and evasion from the phagocytosis by macrophages. Exosomes derived from the native tissues/cells and gene modified cells, namely native and bioengineered exosomes, are promising for atherosclerosis therapy (Figure 3A). In addition, exosomes could be also engineered after the exosomes are isolated through click-chemistry. Generally, the exosomes could be engineered to encapsulate types of cargos with therapeutic efficacy and surface functionalized with peptides or antibodies targeting cells/tissues of interest (Figure 3B). The delivered exosomes target various cells (endothelial cells, macrophages, etc.) involved in atherosclerosis, alleviating the pathological process (Figure 3C).
Xing et al. demonstrated that exosomal miR-342-5p from adipose-derived mesenchymal stem cells protects endothelial cells against atherosclerosis [206]. Stem cell-derived exosomes have been successfully used in animal models with demonstrated efficacy and potential benefits [207]. However, the potential of stem cell-derived exosomes as drug candidates is limited by the lack of high-yield and scalable manufacturing processes for both stem cell culture and isolation [74].
Besides native exosomes, exosomes are easily manipulated to encapsulate therapeutics. For example, M2 macrophage-derived exosomes displayed effective treatment of atherosclerosis, especially when loaded with hexyl 5-aminolevulinate hydrochloride [208]. However, translation of exosomes as drug delivery vehicles has been impeded by their low loading efficiencies [209, 210]. In one approach to overcoming this limitation, large RNA cargos were encapsulated into exosomes by fusing the exosomal membrane protein CD9 and an RNA-binding protein together with the RNA of interest [211]. In addition, systemically delivered exosomes are prone to trapping in nonspecific organs, especially the liver, lung and spleen, leading to an insufficient dose in the target area [212]. Therefore, surface modifications for targeted delivery may provide opportunities to enhance or broaden the innate therapeutic capabilities of exosomes [191, 213]. And a sensitive method to label exosomes with the fusion protein makes it easier to analyze the change of exosome-mass by tracking them in vivo [214]. Surface ligand enrichment on engineered exosomes may enable the development of receptor-mediated tissue targeting, promote signaling events in recipient cells, or target exosomes to specific cell types [9, 204]. Emerging bio-nanotechnologies offer promising advances in diagnostics and therapy [215]. For example, hybrid nanosystems based on genetically engineered exosomes and thermosensitive liposomes are a novel strategy to improve delivery efficacy [216]. Additionally, drug loading and delivery efficiency can be improved through the design of exosome-like nanovesicles and membrane-camouflaged nanoparticles [217]. For example, to combine their biophysical and biomolecular advantages, gold nanoshells (which are non-cytotoxic [218]) were assembled and grown on vesicles in situ to achieve rapid and multiplexed analysis of exosomal targets, offering a novel avenue for accurate patient prognosis and therapy [219] (Figure 3). In summary, we anticipate that native and bioengineered exosomes will be translated to atherosclerosis management, and we expect that exosome-like nanoparticles will become effective strategies to address current problems.
Conclusion and outlook
Atherosclerosis and associated cardiovascular diseases are a worldwide health burden. Accumulating evidence has suggested that exosomes are important players in these diseases. Exosomes altered in the context of disease risk factors can be released and taken up by most of the known cell types in atherosclerosis [220]. These exosomes not only reflect the progress of atherosclerosis but also contribute to its development, opening avenues for diagnosis and therapy.
The methods used for exosome isolation critically impact subsequent analyses. Strategies to isolate cell-specific exosomes and methods to analyze exosomal contents with high sensitivity are needed, which in turn would broaden our understanding in the field. Currently, multiple methods with varied purity are used in different labs. It is thus strongly recommended to standardize the isolation procedure before integrating studies across different labs.
Exosomes in atherosclerosis therapy. (A) Both native and bioengineered exosomes are promising strategies for atherosclerosis therapy. The native exosomes are derived from cells/tissues without additional manipulation. The bioengineered exosomes could be either from gene modified cells or further modified with chemical or physical manipulation after isolation. (B) The native or bioengineered exosomes are of typical structure of EVs. Compared with other nanoparticles, the exosomes have advantages of high biocompatibility. The surface of exosomes could be engineered to harbor targeting moieties, such as antibodies or other ligands, while the inside could be engineered to encapsulate cargos of interest. (C) The delivered exosomes target various cells (endothelial cells, macrophages, etc.) involved in atherosclerosis, alleviating the pathological process.
There is far less than one molecule of a given RNA molecule per exosome, even for the most abundant miRNAs. Thus, the observed pathophysiological effects can only be achieved by that amounts of exosomes of similar function work together for a long duration [133]. It is thus important to load amounts of cargos for therapeutic purposes. In addition, repeat intervention should be also essential for expected effects.
The various roles of exosomes from different cell types and the detailed exosomal cargos involved in atherosclerosis remain largely unknown. Beyond the commonly studied miRNAs, lncRNAs, circRNAs, and some other bioactive molecules could also be involved in the function of exosomes. For example, very long-chain acyl-CoA dehydrogenase (ACADVL), an enzyme located in mitochondria, was found to be highly enriched in exosomes derived from BAT. BAT-derived exosomes could transfer ACADVL as a functional protein into liver cells [121]. Thus, the roles of exosomal proteins and lipids in atherosclerosis are emerging research areas. Procedures to implement omics approaches to conventional biological studies should also be standardized. Before clinical translation, we urgently need to confirm which exosomal components have profound diagnostic and therapeutic value, particularly as accurate biomarkers reflecting disease, membrane moieties for targeting, and key cargos involved in disease processes. We anticipate that current and future findings from profiling and mechanism studies of exosomes in atherosclerosis could be harnessed for diagnosis and therapy.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (81671690, 81871357) and the Provincial Scientific Foundation of Shaan'xi (2020TD-038) to LJY, and the Major Clinical Renovation Project of Tangdu Hospital (NO.2013LCYJ003).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS. et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56
2. Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16:727-44
3. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317-25
4. Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med. 1999;340:115-26
5. Sturtzel C. Endothelial Cells. Adv Exp Med Biol. 2017;1003:71-91
6. Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovascular Research. 2012;95:156-64
7. Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014-8
8. Pluchino S, Smith JA. Explicating Exosomes: Reclassifying the Rising Stars of Intercellular Communication. Cell. 2019;177:225-7
9. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 367
10. Lu M, Yuan S, Li S, Li L, Liu M, Wan S. The Exosome-Derived Biomarker in Atherosclerosis and Its Clinical Application. J Cardiovasc Transl Res. 2019;12:68-74
11. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208-15
12. Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C. et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137-49
13. Aryani A, Denecke B. Exosomes as a Nanodelivery System: a Key to the Future of Neuromedicine? Mol Neurobiol. 2016;53:818-34
14. Han L, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18:59
15. Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470-6
16. Zhang Y, Hu YW, Zheng L, Wang Q. Characteristics and Roles of Exosomes in Cardiovascular Disease. DNA Cell Biol. 2017;36:202-11
17. Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052-6
18. Lao KH, Zeng L, Xu Q. Endothelial and smooth muscle cell transformation in atherosclerosis. Curr Opin Lipidol. 2015;26:449-56
19. Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259-72
20. Waldenstrom A, Ronquist G. Role of exosomes in myocardial remodeling. Circ Res. 2014;114:315-24
21. Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Reviews. 2013;27:31-9
22. de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G. et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012 1
23. Xu L, Yang BF, Ai J. MicroRNA transport: a new way in cell communication. J Cell Physiol. 2013;228:1713-9
24. Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W. et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335-43
25. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New Technologies for Analysis of Extracellular Vesicles. Chemical Reviews. 2018;118:1917-50
26. Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445-52
27. Zhang J, Li S, Li L, Li M, Guo C, Yao J. et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17-24
28. Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J. Exosomes: new molecular targets of diseases. Acta Pharmacologica Sinica. 2017;39:501-13
29. Cai J, Wu G, Jose PA, Zeng C. Functional transferred DNA within extracellular vesicles. Exp Cell Res. 2016;349:179-83
30. Conlan RS, Pisano S, Oliveira MI, Ferrari M, Mendes Pinto I. Exosomes as Reconfigurable Therapeutic Systems. Trends in Molecular Medicine. 2017;23:636-50
31. Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdisciplinary Reviews: RNA. 2017 8
32. Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M. et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319
33. Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 2019;10:393
34. Wang Y, Zhao R, Liu W, Wang Z, Rong J, Long X. et al. Exosomal circHIPK3 Released from Hypoxia-Pretreated Cardiomyocytes Regulates Oxidative Damage in Cardiac Microvascular Endothelial Cells via the miR-29a/IGF-1 Pathway. Oxid Med Cell Longev. 2019;2019:7954657
35. Ge X, Meng Q, Zhuang R, Yuan D, Liu J, Lin F. et al. Circular RNA expression alterations in extracellular vesicles isolated from murine heart post ischemia/reperfusion injury. Int J Cardiol. 2019;296:136-40
36. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617-38
37. Jandura A, Krause HM. The New RNA World: Growing Evidence for Long Noncoding RNA Functionality. Trends Genet. 2017;33:665-76
38. Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat Cell Biol. 2019;21:542-51
39. Zhang Z, Salisbury D, Sallam T. Long Noncoding RNAs in Atherosclerosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2018;72:2380-90
40. Sun HJ, Hou B, Wang X, Zhu XX, Li KX, Qiu LY. Endothelial dysfunction and cardiometabolic diseases: Role of long non-coding RNAs. Life Sci. 2016;167:6-11
41. Monteiro JP, Bennett M, Rodor J, Caudrillier A, Ulitsky I, Baker AH. Endothelial function and dysfunction in the cardiovascular system: the long non-coding road. Cardiovasc Res. 2019;115:1692-704
42. Hu YW, Guo FX, Xu YJ, Li P, Lu ZF, McVey DG. et al. Long noncoding RNA NEXN-AS1 mitigates atherosclerosis by regulating the actin-binding protein NEXN. J Clin Invest. 2019;129:1115-28
43. Khyzha N, Khor M, DiStefano PV, Wang L, Matic L, Hedin U. et al. Regulation of CCL2 expression in human vascular endothelial cells by a neighboring divergently transcribed long noncoding RNA. Proc Natl Acad Sci U S A. 2019;116:16410-9
44. Xie Y, Dang W, Zhang S, Yue W, Yang L, Zhai X. et al. The role of exosomal noncoding RNAs in cancer. Mol Cancer. 2019;18:37
45. Chang W, Wang J. Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus. Cells. 2019 8
46. Li Y, Yin Z, Fan J, Zhang S, Yang W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct Target Ther. 2019;4:47
47. Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z. et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17:82
48. Sallam T, Sandhu J, Tontonoz P. Long Noncoding RNA Discovery in Cardiovascular Disease: Decoding Form to Function. Circ Res. 2018;122:155-66
49. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675-91
50. Xiao MS, Ai Y, Wilusz JE. Biogenesis and Functions of Circular RNAs come into Focus. Trends Cell Biol. 2020;30:226-40
51. Li X, Yang L, Chen LL. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol Cell. 2018;71:428-42
52. Patop IL, Wust S, Kadener S. Past, present, and future of circRNAs. EMBO J. 2019;38:e100836
53. Ebbesen KK, Hansen TB, Kjems J. Insights into circular RNA biology. RNA Biol. 2017;14:1035-45
54. Lu D, Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol. 2019;16:661-74
55. Kristensen LS, Hansen TB, Veno MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555-65
56. Li CY, Ma L, Yu B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed Pharmacother. 2017;95:1514-9
57. Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W. et al. Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol Cancer. 2019;18:116
58. Wu WP, Pan YH, Cai MY, Cen JM, Chen C, Zheng L. et al. Plasma-derived Exosomal Circular RNA hsa_circ_0005540 as a Novel Diagnostic Biomarker for Coronary Artery Disease. Dis Markers. 2020;2020:3178642
59. Wang Y, Zhao R, Shen C, Liu W, Yuan J, Li C. et al. Exosomal CircHIPK3 Released from Hypoxia-Induced Cardiomyocytes regulates Cardiac Angiogenesis after Myocardial Infarction. Oxid Med Cell Longev. 2020;2020:8418407
60. Zhang S, Song G, Yuan J, Qiao S, Xu S, Si Z. et al. Circular RNA circ_0003204 inhibits proliferation, migration and tube formation of endothelial cell in atherosclerosis via miR-370-3p/TGFbetaR2/phosph-SMAD3 axis. J Biomed Sci. 2020;27:11
61. Zhou R, Chen KK, Zhang J, Xiao B, Huang Z, Ju C. et al. The decade of exosomal long RNA species: an emerging cancer antagonist. Mol Cancer. 2018;17:75
62. Deng W, Tang T, Hou Y, Zeng Q, Wang Y, Fan W. et al. Extracellular vesicles in atherosclerosis. Clinica Chimica Acta. 2019;495:109-17
63. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ. et al. Reassessment of Exosome Composition. Cell. 2019;177:428-45 e18
64. Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241-4
65. Li A, Zhang T, Zheng M, Liu Y, Chen Z. Exosomal proteins as potential markers of tumor diagnosis. J Hematol Oncol. 2017;10:175
66. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968-77
67. Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res. 2013;100:7-18
68. Li W, Li C, Zhou T, Liu X, Liu X, Li X. et al. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16:145
69. Mashouri L, Yousefi H, Aref AR, Ahadi Am, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Molecular Cancer. 2019 18
70. Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677-85
71. Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI. et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066
72. Henning RJ. Cardiovascular Exosomes and MicroRNAs in Cardiovascular Physiology and Pathophysiology. J Cardiovasc Transl Res. 2020
73. Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C. et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913
74. Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends in Molecular Medicine. 2018;24:242-56
75. Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2019;60:9-18
76. Skotland T, Sagini K, Sandvig K, Llorente A. An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev. 2020
77. Record M, Silvente-Poirot S, Poirot M, Wakelam MJO. Extracellular vesicles: lipids as key components of their biogenesis and functions. J Lipid Res. 2018;59:1316-24
78. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog Lipid Res. 2017;66:30-41
79. Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S. et al. Methodological Guidelines to Study Extracellular Vesicles. Circ Res. 2017;120:1632-48
80. Li P, Kaslan M, Lee SH, Yao J, Gao ZQ. Progress in Exosome Isolation Techniques. Theranostics. 2017;7:789-804
81. Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A. et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031
82. Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M. et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2020;9:1697028
83. Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G. et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46-58
84. Weng Y, Sui Z, Shan Y, Hu Y, Chen Y, Zhang L. et al. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst. 2016;141:4640-6
85. Sidhom K, Obi PO, Saleem A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int J Mol Sci. 2020 21
86. Gurunathan S, Kang M-H, Jeyaraj M, Qasim M, Kim J-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells. 2019 8
87. Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82:1024-32
88. Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750
89. Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019 8
90. Ludwig N, Whiteside TL, Reichert TE. Challenges in Exosome Isolation and Analysis in Health and Disease. Int J Mol Sci. 2019 20
91. Boriachek K, Islam MN, Moller A, Salomon C, Nguyen NT, Hossain MSA. et al. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small. 2018 14
92. Royo F, Thery C, Falcon-Perez JM, Nieuwland R, Witwer KW. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells. 2020 9
93. Liu F, Vermesh O, Mani V, Ge TJ, Madsen SJ, Sabour A. et al. The Exosome Total Isolation Chip. ACS Nano. 2017;11:10712-23
94. Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C. et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114:10584-9
95. Ibsen SD, Wright J, Lewis JM, Kim S, Ko SY, Ong J. et al. Rapid Isolation and Detection of Exosomes and Associated Biomarkers from Plasma. ACS Nano. 2017;11:6641-51
96. Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res. 2016;118:535-46
97. Back M, Yurdagul A Jr, Tabas I, Oorni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16:389-406
98. Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cifkova R, Dominiczak AF. et al. Hypertension. Nat Rev Dis Primers. 2018;4:18014
99. Martinez-Arroyo O, Ortega A, Redon J, Cortes R. Therapeutic Potential of Extracellular Vesicles in Hypertension-Associated Kidney Disease. Hypertension. 2020: HYPERTENSIONAHA12016064.
100. Khalyfa A, Gozal D, Chan WC, Andrade J, Prasad B. Circulating plasma exosomes in obstructive sleep apnoea and reverse dipping blood pressure. Eur Respir J. 2020 55
101. Osada-Oka M, Shiota M, Izumi Y, Nishiyama M, Tanaka M, Yamaguchi T. et al. Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hypertens Res. 2017;40:353-60
102. Otani K, Yokoya M, Kodama T, Hori K, Matsumoto K, Okada M. et al. Plasma exosomes regulate systemic blood pressure in rats. Biochem Biophys Res Commun. 2018;503:776-83
103. Chimenti I, Frati G. Cell-Derived Exosomes for Cardiovascular Therapies: Y (Not) RNAs? Hypertension. 2018;72:279-80
104. Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288-98
105. Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res. 2016;118:1723-35
106. Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: mechanistic insights. Can J Cardiol. 2015;31:177-83
107. Ji C, Guo X. The clinical potential of circulating microRNAs in obesity. Nat Rev Endocrinol. 2019;15:731-43
108. Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB. et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In vivo and In vitro Insulin Sensitivity. Cell. 2017;171:372-84 e12
109. Obata Y, Kita S, Koyama Y, Fukuda S, Takeda H, Takahashi M. et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight. 2018 3
110. Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450-5
111. Castano C, Kalko S, Novials A, Parrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115:12158-63
112. Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129:3990-4000
113. Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest. 2019;129:4041-9
114. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409
115. Balling M, Afzal S, Varbo A, Langsted A, Davey Smith G, Nordestgaard BG. VLDL Cholesterol Accounts for One-Half of the Risk of Myocardial Infarction Associated With apoB-Containing Lipoproteins. J Am Coll Cardiol. 2020;76:2725-35
116. O'Connor EA, Evans CV, Rushkin MC, Redmond N, Lin JS. Behavioral Counseling to Promote a Healthy Diet and Physical Activity for Cardiovascular Disease Prevention in Adults With Cardiovascular Risk Factors: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2020;324:2076-94
117. Vergallo R, Crea F. Atherosclerotic Plaque Healing. N Engl J Med. 2020;383:846-57
118. Wang W, Zhu N, Yan T, Shi YN, Chen J, Zhang CJ. et al. The crosstalk: exosomes and lipid metabolism. Cell Commun Signal. 2020;18:119
119. Boilard E. Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA. J Lipid Res. 2018;59:2037-46
120. Stamatikos A, Knight E, Vojtech L, Bi L, Wacker BK, Tang C. et al. Exosome-Mediated Transfer of Anti-miR-33a-5p from Transduced Endothelial Cells Enhances Macrophage and Vascular Smooth Muscle Cell Cholesterol Efflux. Hum Gene Ther. 2020;31:219-32
121. Zhou X, Li Z, Qi M, Zhao P, Duan Y, Yang G. et al. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics. 2020;10:8197-210
122. Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JW, Radder JK. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442-7
123. Kranendonk ME, de Kleijn DP, Kalkhoven E, Kanhai DA, Uiterwaal CS, van der Graaf Y. et al. Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc Diabetol. 2014;13:37
124. Pardo F, Villalobos-Labra R, Sobrevia B, Toledo F, Sobrevia L. Extracellular vesicles in obesity and diabetes mellitus. Mol Aspects Med. 2018;60:81-91
125. Osmai M, Osmai Y, Bang-Berthelsen CH, Pallesen EM, Vestergaard AL, Novotny GW. et al. MicroRNAs as regulators of beta-cell function and dysfunction. Diabetes Metab Res Rev. 2016;32:334-49
126. Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT. et al. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab. 2012;97:E2271-6
127. Wang F, Chen FF, Shang YY, Li Y, Wang ZH, Han L. et al. Insulin resistance adipocyte-derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE(-/-) mice. Int J Cardiol. 2018;265:181-7
128. Freeman DW, Noren Hooten N, Eitan E, Green J, Mode NA, Bodogai M. et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes. 2018;67:2377-88
129. Yuan Y, Du W, Liu J, Ma W, Zhang L, Du Z. et al. Stem Cell-Derived Exosome in Cardiovascular Diseases: Macro Roles of Micro Particles. Front Pharmacol. 2018;9:547
130. Chen YT, Yuan HX, Ou ZJ, Ou JS. Microparticles (Exosomes) and Atherosclerosis. Curr Atheroscler Rep. 2020;22:23
131. Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol. 2015;24:199-206
132. Feinberg MW, Moore KJ. MicroRNA Regulation of Atherosclerosis. Circ Res. 2016;118:703-20
133. Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM. et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111:14888-93
134. Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A. et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9:830-4
135. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285-95
136. Mannarino E, Pirro M. Endothelial injury and repair: a novel theory for atherosclerosis. Angiology. 2008;59:69S-72S
137. Lovren F, Verma S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clin Chem. 2013;59:1166-74
138. Jansen F, Li Q, Pfeifer A, Werner N. Endothelial- and Immune Cell-Derived Extracellular Vesicles in the Regulation of Cardiovascular Health and Disease. JACC Basic Transl Sci. 2017;2:790-807
139. Togliatto G, Dentelli P, Rosso A, Lombardo G, Gili M, Gallo S. et al. PDGF-BB Carried by Endothelial Cell-Derived Extracellular Vesicles Reduces Vascular Smooth Muscle Cell Apoptosis in Diabetes. Diabetes. 2018;67:704-16
140. Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM. et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature Cell Biology. 2012;14:249-56
141. Li B, Zang G, Zhong W, Chen R, Zhang Y, Yang P. et al. Activation of CD137 signaling promotes neointimal formation by attenuating TET2 and transferrring from endothelial cell-derived exosomes to vascular smooth muscle cells. Biomed Pharmacother. 2020;121:109593
142. Njock MS, Cheng HS, Dang LT, Nazari-Jahantigh M, Lau AC, Boudreau E. et al. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood. 2015;125:3202-12
143. Li H, Zhu X, Hu L, Li Q, Ma J, Yan J. Loss of exosomal MALAT1 from ox-LDL-treated vascular endothelial cells induces maturation of dendritic cells in atherosclerosis development. Cell Cycle. 2019;18:2255-67
144. Xing Z, Zhao C, Liu H, Fan Y. Endothelial Progenitor Cell-Derived Extracellular Vesicles: A Novel Candidate for Regenerative Medicine and Disease Treatment. Adv Healthc Mater. 2020;9:e2000255
145. Leone AM, Valgimigli M, Giannico MB, Zaccone V, Perfetti M, D'Amario D. et al. From bone marrow to the arterial wall: the ongoing tale of endothelial progenitor cells. Eur Heart J. 2009;30:890-9
146. Wang J, Li J, Cheng C, Liu S. Angiotensin-converting enzyme 2 augments the effects of endothelial progenitor cells-exosomes on vascular smooth muscle cell phenotype transition. Cell Tissue Res. 2020;382:509-18
147. Wang J, Chen S, Bihl J. Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell. Oxid Med Cell Longev. 2020;2020:4213541
148. Bai S, Yin Q, Dong T, Dai F, Qin Y, Ye L. et al. Endothelial progenitor cell-derived exosomes ameliorate endothelial dysfunction in a mouse model of diabetes. Biomed Pharmacother. 2020;131:110756
149. Yue Y, Wang C, Benedict C, Huang G, Truongcao M, Roy R. et al. Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ Res. 2020;126:315-29
150. Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D. et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312-23
151. Qiu H, Shi S, Wang S, Peng H, Ding SJ, Wang L. Proteomic Profiling Exosomes from Vascular Smooth Muscle Cell. Proteomics Clin Appl. 2018;12:e1700097
152. Heo J, Yang HC, Rhee WJ, Kang H. Vascular Smooth Muscle Cell-Derived Exosomal MicroRNAs Regulate Endothelial Cell Migration Under PDGF Stimulation. Cells. 2020 9
153. Comelli L, Rocchiccioli S, Smirni S, Salvetti A, Signore G, Citti L. et al. Characterization of secreted vesicles from vascular smooth muscle cells. Mol Biosyst. 2014;10:1146-52
154. Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018;114:540-50
155. Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR. et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114:622-34
156. Wang Y, Xie Y, Zhang A, Wang M, Fang Z, Zhang J. Exosomes: An emerging factor in atherosclerosis. Biomed Pharmacother. 2019;115:108951
157. Yang W, Zou B, Hou Y, Yan W, Chen T, Qu S. Extracellular vesicles in vascular calcification. Clin Chim Acta. 2019;499:118-22
158. Liberman M, Marti LC. Vascular Calcification Regulation by Exosomes in the Vascular Wall. Adv Exp Med Biol. 2017;998:151-60
159. Chen NX, O'Neill KD, Moe SM. Matrix vesicles induce calcification of recipient vascular smooth muscle cells through multiple signaling pathways. Kidney Int. 2018;93:343-54
160. Krohn JB, Hutcheson JD, Martinez-Martinez E, Aikawa E. Extracellular vesicles in cardiovascular calcification: expanding current paradigms. J Physiol. 2016;594:2895-903
161. Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang Y, Song LL. et al. Exosome-Mediated miR-155 Transfer from Smooth Muscle Cells to Endothelial Cells Induces Endothelial Injury and Promotes Atherosclerosis. Mol Ther. 2017;25:1279-94
162. Vilades D, Martinez-Camblor P, Ferrero-Gregori A, Bar C, Lu D, Xiao K. et al. Plasma circular RNA hsa_circ_0001445 and coronary artery disease: Performance as a biomarker. FASEB J. 2020;34:4403-14
163. Tabas I, Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res. 2016;118:653-67
164. Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709-21
165. Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166-72
166. Niu C, Wang X, Zhao M, Cai T, Liu P, Li J. et al. Macrophage Foam Cell-Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J Am Heart Assoc. 2016 5
167. Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234-44
168. Huang C, Huang Y, Zhou Y, Nie W, Pu X, Xu X. et al. Exosomes derived from oxidized LDL-stimulated macrophages attenuate the growth and tube formation of endothelial cells. Mol Med Rep. 2018;17:4605-10
169. Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic Matic L. et al. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler Thromb Vasc Biol. 2018;38:49-63
170. Zhang YG, Song Y, Guo XL, Miao RY, Fu YQ, Miao CF. et al. Exosomes derived from oxLDL-stimulated macrophages induce neutrophil extracellular traps to drive atherosclerosis. Cell Cycle. 2019;18:2674-84
171. Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L. et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9:6901-19
172. Bouchareychas L, Duong P, Covarrubias S, Alsop E, Phu TA, Chung A. et al. Macrophage Exosomes Resolve Atherosclerosis by Regulating Hematopoiesis and Inflammation via MicroRNA Cargo. Cell Rep. 2020;32:107881
173. Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost. 2011;106:827-38
174. Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482-94
175. Wu MD, Atkinson TM, Lindner JR. Platelets and von Willebrand factor in atherogenesis. Blood. 2017;129:1415-9
176. Tantry US, Bliden KP, Suarez TA, Kreutz RP, Dichiara J, Gurbel PA. Hypercoagulability, platelet function, inflammation and coronary artery disease acuity: results of the Thrombotic RIsk Progression (TRIP) study. Platelets. 2010;21:360-7
177. Gurbel PA, Fox KAA, Tantry US, Ten Cate H, Weitz JI. Combination Antiplatelet and Oral Anticoagulant Therapy in Patients With Coronary and Peripheral Artery Disease. Circulation. 2019;139:2170-85
178. Goetzl EJ, Goetzl L, Karliner JS, Tang N, Pulliam L. Human plasma platelet-derived exosomes: effects of aspirin. FASEB J. 2016;30:2058-63
179. Chatterjee V, Yang X, Ma Y, Wu MH, Yuan SY. Extracellular vesicles: new players in regulating vascular barrier function. Am J Physiol Heart Circ Physiol. 2020;319:H1181-H96
180. Sun Y, Liu XL, Zhang D, Liu F, Cheng YJ, Ma Y. et al. Platelet-Derived Exosomes Affect the Proliferation and Migration of Human Umbilical Vein Endothelial Cells Via miR-126. Curr Vasc Pharmacol. 2019;17:379-87
181. Yao Y, Sun W, Sun Q, Jing B, Liu S, Liu X. et al. Platelet-Derived Exosomal MicroRNA-25-3p Inhibits Coronary Vascular Endothelial Cell Inflammation Through Adam10 via the NF-kappaB Signaling Pathway in ApoE(-/-) Mice. Front Immunol. 2019;10:2205
182. Li J, Tan M, Xiang Q, Zhou Z, Yan H. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb Res. 2017;154:96-105
183. Landry P, Plante I, Ouellet DL, Perron MP, Rousseau G, Provost P. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16:961-6
184. Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol Metab. 2017;28:3-18
185. Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol. 2021;17:47-66
186. Martinez MC, Andriantsitohaina R. Extracellular Vesicles in Metabolic Syndrome. Circ Res. 2017;120:1674-86
187. Safdar A, Tarnopolsky MA. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb Perspect Med. 2018 8
188. Trovato E, Di Felice V, Barone R. Extracellular Vesicles: Delivery Vehicles of Myokines. Front Physiol. 2019;10:522
189. Estebanez B, Jimenez-Pavon D, Huang CJ, Cuevas MJ, Gonzalez-Gallego J. Effects of exercise on exosome release and cargo in in vivo and ex vivo models: A systematic review. J Cell Physiol. 2020
190. Poller W, Dimmeler S, Heymans S, Zeller T, Haas J, Karakas M. et al. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J. 2018;39:2704-16
191. He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: Biology and Translational Medicine. Theranostics. 2018;8:237-55
192. Olson EN. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci Transl Med. 2014;6:239ps3
193. Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K. et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc. 2014;3:e001249
194. Raju S, Fish JE, Howe KL. MicroRNAs as sentinels and protagonists of carotid artery thromboembolism. Clin Sci (Lond). 2020;134:169-92
195. Jiang H, Toscano JF, Song SS, Schlick KH, Dumitrascu OM, Pan J. et al. Differential expression of circulating exosomal microRNAs in refractory intracranial atherosclerosis associated with antiangiogenesis. Sci Rep. 2019;9:19429
196. Liu Y, Li Q, Hosen MR, Zietzer A, Flender A, Levermann P. et al. Atherosclerotic Conditions Promote the Packaging of Functional MicroRNA-92a-3p Into Endothelial Microvesicles. Circ Res. 2019;124:575-87
197. Wang Z, Zhang J, Zhang S, Yan S, Wang Z, Wang C. et al. MiR30e and miR92a are related to atherosclerosis by targeting ABCA1. Mol Med Rep. 2019;19:3298-304
198. Navickas R, Gal D, Laucevicius A, Taparauskaite A, Zdanyte M, Holvoet P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res. 2016;111:322-37
199. Hajibabaie F, Kouhpayeh S, Mirian M, Rahimmanesh I, Boshtam M, Sadeghian L. et al. MicroRNAs as the actors in the atherosclerosis scenario. J Physiol Biochem. 2020;76:1-12
200. Sorrentino TA, Duong P, Bouchareychas L, Chen M, Chung A, Schaller MS. et al. Circulating exosomes from patients with peripheral artery disease influence vascular cell migration and contain distinct microRNA cargo. JVS Vasc Sci. 2020;1:28-41
201. Ayers L, Pink R, Carter DRF, Nieuwland R. Clinical requirements for extracellular vesicle assays. J Extracell Vesicles. 2019;8:1593755
202. Gandham S, Su X, Wood J, Nocera AL, Alli SC, Milane L. et al. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020;38:1066-98
203. Zamani P, Fereydouni N, Butler AE, Navashenaq JG, Sahebkar A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc Med. 2019;29:313-23
204. Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63-78
205. Li Z, Zhao P, Zhang Y, Wang J, Wang C, Liu Y. et al. Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics. 2021;11:2953-65
206. Xing X, Li Z, Yang X, Li M, Liu C, Pang Y. et al. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging (Albany NY). 2020;12:3880-98
207. Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World J Stem Cells. 2016;8:297-305
208. Wu G, Zhang J, Zhao Q, Zhuang W, Ding J, Zhang C. et al. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew Chem Int Ed Engl. 2020;59:4068-74
209. Yao ZY, Chen WB, Shao SS, Ma SZ, Yang CB, Li MZ. et al. Role of exosome-associated microRNA in diagnostic and therapeutic applications to metabolic disorders. J Zhejiang Univ Sci B. 2018;19:183-98
210. Moghaddam AS, Afshari JT, Esmaeili SA, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis. 2019;285:1-9
211. Li Z, Zhou X, Wei M, Gao X, Zhao L, Shi R. et al. In vitro and in vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19:19-28
212. Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I. et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316
213. Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J. et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J Am Heart Assoc. 2018;7:e008737
214. Takahashi Y, Nishikawa M, Takakura Y. In vivo Tracking of Extracellular Vesicles in Mice Using Fusion Protein Comprising Lactadherin and Gaussia Luciferase. Methods Mol Biol. 2017;1660:245-54
215. Xu M, Yang Q, Sun X, Wang Y. Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front Bioeng Biotechnol. 2020;8:586130
216. Lv QJ, Cheng LL, Lu Y, Zhang XG, Wang YZ, Deng JF. et al. Thermosensitive Exosome-Liposome Hybrid Nanoparticle-Mediated Chemoimmunotherapy for Improved Treatment of Metastatic Peritoneal Cancer. Adv Sci. 2020 7
217. Lu M, Huang Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials. 2020;242:119925
218. Gu C, Wu H, Ge G, Li X, Guo Z, Bian Z. et al. In vitro Effects of Hollow Gold Nanoshells on Human Aortic Endothelial Cells. Nanoscale Res Lett. 2016;11:397
219. Wu X, Zhao H, Natalia A, Lim CZJ, Ho NRY, Ong CJ. et al. Exosome-templated nanoplasmonics for multiparametric molecular profiling. Sci Adv. 2020;6:eaba2556
220. Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24-30
Author contact
![]() Corresponding author: E-mail: yuanljedu.cn.
Corresponding author: E-mail: yuanljedu.cn.
 Global reach, higher impact
Global reach, higher impact