13.3
Impact Factor
Theranostics 2021; 11(13):6138-6153. doi:10.7150/thno.54999 This issue Cite
Research Paper
Progressive stretch enhances growth and maturation of 3D stem-cell-derived myocardium
1. Walter-Brendel-Centre of Experimental Medicine, University Hospital, Ludwig-Maximilians-University München, Munich, Germany.
2. Institute of Cellular and Molecular Physiology, Friedrich-Alexander University Erlangen-Nürnberg, Erlangen, Germany.
3. First Department of Medicine, Cardiology, Klinikum rechts der Isar, Technical University München, Munich, Germany.
4. Institute for Medical Information Processing Biometry and Epidemiology, Ludwig-Maximilians-University München, Munich, Germany.
5. DIFUTURE (Data Integration for Future Medicine), Ludwig-Maximilians-University München, Munich, Germany.
6. DZHK (German Center for Cardiovascular Research), Partner site Munich Heart Alliance, Munich, Germany.
7. Clinic of Anaesthesiology, University Hospital, Ludwig-Maximilians-University München, Munich, Germany.
Abstract
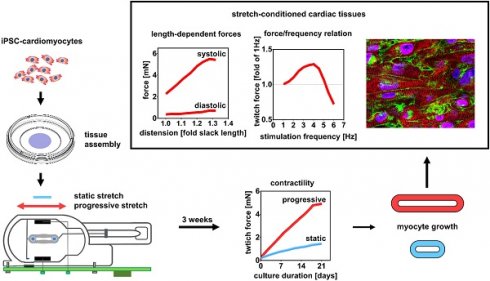
Bio-engineered myocardium has great potential to substitute damaged myocardium and for studies of myocardial physiology and disease, but structural and functional immaturity still implies limitations. Current protocols of engineered heart tissue (EHT) generation fall short of simulating the conditions of postnatal myocardial growth, which are characterized by tissue expansion and increased mechanical load. To investigate whether these two parameters can improve EHT maturation, we developed a new approach for the generation of cardiac tissues based on biomimetic stimulation under application of continuously increasing stretch.
Methods: EHTs were generated by assembling cardiomyocytes derived from human induced pluripotent stem cells (hiPSC-CM) at high cell density in a low collagen hydrogel. Maturation and growth of the EHTs were induced in a custom-made biomimetic tissue culture system that provided continuous electrical stimulation and medium agitation along with progressive stretch at four different increments. Tissues were characterized after a three week conditioning period.
Results: The highest rate of stretch (S3 = 0.32 mm/day) increased force development by 5.1-fold compared to tissue with a fixed length, reaching contractility of 11.28 mN/mm². Importantly, intensely stretched EHTs developed physiological length-dependencies of active and passive forces (systolic/diastolic ratio = 9.47 ± 0.84), and a positive force-frequency relationship (1.25-fold contractility at 180 min-1). Functional markers of stretch-dependent maturation included enhanced and more rapid Ca2+ transients, higher amplitude and upstroke velocity of action potentials, and pronounced adrenergic responses. Stretch conditioned hiPSC-CMs displayed structural improvements in cellular volume, linear alignment, and sarcomere length (2.19 ± 0.1 µm), and an overall upregulation of genes that are specifically expressed in adult cardiomyocytes.
Conclusions: With the intention to simulate postnatal heart development, we have established techniques of tissue assembly and biomimetic culture that avoid tissue shrinkage and yield muscle fibers with contractility and compliance approaching the properties of adult myocardium. This study demonstrates that cultivation under progressive stretch is a feasible way to induce growth and maturation of stem cell-derived myocardium. The novel tissue-engineering approach fulfills important requirements of disease modelling and therapeutic tissue replacement.
Keywords: engineered heart tissue, progressive stretch, stem-cell-derived myocardium, maturation, biomechanics
 Global reach, higher impact
Global reach, higher impact