13.3
Impact Factor
Theranostics 2021; 11(17):8464-8479. doi:10.7150/thno.60028 This issue Cite
Research Paper
Targeting ATF4-dependent pro-survival autophagy to synergize glutaminolysis inhibition
1. Department of Medical Oncology, Sir Run Run Shaw Hospital, Medical School of Zhejiang University, Hangzhou, China
2. Laboratory of Cancer Biology, Key Lab of Biotherapy, Cancer Center of Zhejiang University, Sir Run Run Shaw Hospital, Medical School of Zhejiang University, Hangzhou, China
3. Department of Allied Health Sciences, University of Poonch Rawalakot, AJK, Pakistan
Abstract
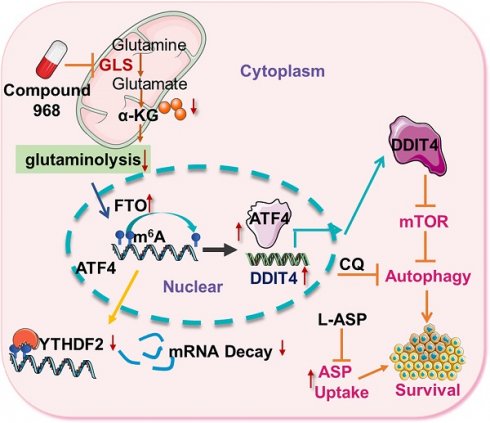
As glutamine plays a central role in cancer metabolism, inhibition of glutaminolysis has become an ideal anticancer therapeutic target. However, glutaminolysis inhibition leads to activation of autophagy, which compromises its antitumor effect. Hence, we investigated the mechanism underlying glutaminolysis inhibition-induced pro-survival autophagy.
Methods: High-throughput sequencing was performed on colorectal cancer (CRC) cells before and after glutaminolysis inhibition to identify differentially expressed genes. Activating transcription factor 4 (ATF4) pathway enrichment in glutaminolysis inhibited cells was identified through gene set enrichment analysis. ATF4 expression was assessed by quantitative real-time PCR (qRT-PCR) and western blotting. The function of ATF4 on mechanistic target of rapamycin (mTOR) regulation was assessed by western blotting. Luciferase reporter assays and chromatin immunoprecipitation were used to confirm the regulation of DNA damage inducible transcript 4 (DDIT4) by ATF4. mRNA half-life assays, RNA immunoprecipitation, qRT-PCR and western blotting were performed to determine the relationship between FTO alpha-ketoglutarate dependent dioxygenase (FTO), YTH N6-methyladenosine RNA binding protein 2 (YTHDF2), and ATF4. ATF4 regulation of pro-survival autophagy was measured by tandem monomeric red fluorescent protein-green fluorescent protein fluorescence microscopy. Finally, the synergistic effect of autophagy and glutaminolysis inhibition was analyzed in an azoxymethane/dextran sodium sulfate mouse model.
Results: The ATF4 pathway was activated in CRC cells upon glutaminolysis inhibition. Functionally, ATF4 transcriptionally upregulated DDIT4 to suppress mTOR, which induced pro-survival autophagy during glutaminolysis inhibition. Interestingly, glutaminolysis inhibition promoted ATF4 mRNA expression by abrogating N6-methyladenosine (m6A) modification and YTHDF2-mediated RNA decay. Finally, inhibition of ATF4-induced autophagy enhanced the antitumor efficacy of glutaminolysis inhibition.
Conclusion: Glutaminolysis inhibition upregulated ATF4 expression in an m6A-dependent manner to activate pro-survival autophagy through transcriptional activation of the mTOR inhibitor DDIT4. Targeting ATF4-induced autophagy is a new strategy to synergize glutaminolysis-targeting therapies for cancer treatment.
Keywords: Glutaminolysis inhibition, ATF4, DDIT4, N6-methyladenosine, metabolic synthetic lethality
 Global reach, higher impact
Global reach, higher impact