13.3
Impact Factor
Theranostics 2021; 11(20):9937-9952. doi:10.7150/thno.65480 This issue Cite
Review
Targeting ferroptosis-based cancer therapy using nanomaterials: strategies and applications
1. The Marine Biomedical Research Institute, Guangdong Medical University, Zhanjiang, Guangdong, 524023, China.
2. The Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, Guangdong, 524023, China.
3. Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang), Zhanjiang, Guangdong, 524023, China.
4. The First Clinical College, Guangdong Medical University, Zhanjiang, Guangdong, 524023, China.
5. Experimental Animal Center, Guangdong Medical University, Zhanjiang, Guangdong, 524023, China.
6. Affiliations Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
7. Guangdong Provincial Key Laboratory of Food Quality and Safety, College of Food Science, South China Agricultural University, Guangzhou, 510642, China.
Received 2021-7-28; Accepted 2021-10-10; Published 2021-10-22
Abstract
As an iron-dependent mode of programmed cell death induced by lipid peroxidation, ferroptosis plays an important role in cancer therapy. The metabolic reprogramming in tumor microenvironment allows the possibility of targeting ferroptosis in cancer treatment. Recent studies reveal that nanomaterials targeting ferroptosis have prospects for the development of new cancer treatments. However, the design ideas of nanomaterials targeting ferroptosis sometimes vary. Therefore, in addition to the need for a systematic summary of these ideas, new ideas and insights are needed to make possible the construction of nanomaterials for effectively targeting this cell death pathway. At the same time, further optimization of nanomaterials design is required to make them appropriate for clinical treatment. In this context, we summarize this cross-cutting research area covering from the known mechanism of ferroptosis to providing feasible ideas for nanomaterials design as well as their clinical application. We aim to provide new insights and enlightenment for the next step in developing new nanomaterials for cancer treatment.
Keywords: Ferroptosis, Nanomaterials, Cancer therapy, Tumor microenvironment, Clinical strategy
1. Introduction
Ferroptosis is a type of programmed cell death mediated by iron-dependent peroxidation proposed by Dixon et al. in 2012 [1]. Although its mechanism has not been fully elucidated, the collapse of cell membranes caused by overwhelming peroxidation is considered as a key step in ferroptosis. This process is mediated by an iron-dependent Fenton-like reaction [2]. The occurrence of this overwhelming lipid peroxidation alters to some extent the morphological structure of the cell and represents a signature of ferroptosis distinct from those of other types of programmed cell death. The most remarkable difference between ferroptosis and other types of programmed cell death lies in the changes in mitochondrial morphology. The specific manifestations are shrinkage of mitochondria and disappearance of cristae under the electron microscope. Biochemically, on the other hand, it is mainly characterized by the absence of intracellular antioxidant systems, the increase of labile iron, and the enrichment of lipid substrates. Ferroptosis can be induced by various small molecule compounds referred to asferroptosis inducers (FINs) [3], and even some approved chemotherapeutic drugs have been found to induce ferroptosis.
From the perspective of cellular metabolism, it can be concluded that ferroptosis is closely associated with cellular metabolism [4]. Given that ferroptosis is an iron-dependent process [2], regulating iron metabolism and/or lipid metabolism affects the sensitivity of cells to ferroptosis. At the same time, the composition of the antioxidant system depends on amino acid metabolism and the mevalonate pathway, as well as on the uptake of trace elements. Thus, the targets of ferroptosis are abundant and diverse. However, it is usually difficult to achieve adequate efficacy in the treatment of patients with small molecule compounds, which is why most drug clinical trials to date have failed. Therefore, the development of effective nanomaterials carriers is critical to improve the delivery, release, and targeting efficiency of drugs. The strategy of using nanomaterials for the delivery of drugs targeting ferroptosis has been widely studied in recent years. However, little work has been done to systematically examine the targeting mechanisms of ferroptosis, as well as ideas for constructing related nanomaterials. In this review, the mechanism of ferroptosis and various ideas for drug design and development are examined, and innovative possibilities are proposed in order to summarize previous findings and expand the horizon of drug delivery systems.
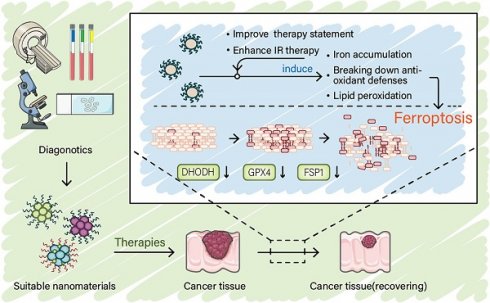
2. The trilogy of inducing ferroptosis in cancer
The process of ferroptosis in cells involves three key steps: depletion of antioxidant defenses, accumulation of iron, and lipid peroxidation. The accumulation of iron leads to the continuous induction of lipid peroxidation [5]. In the absence of antioxidant systems to scavenge reactive oxygen species (ROS) and protect membrane lipid, overwhelming lipid peroxidation leads to the collapse of the cell membrane, and eventually cell death. Accordingly, understanding the process of ferroptosis can help researchers better develop drugs as well as cancer treatment strategies (Figure 1).
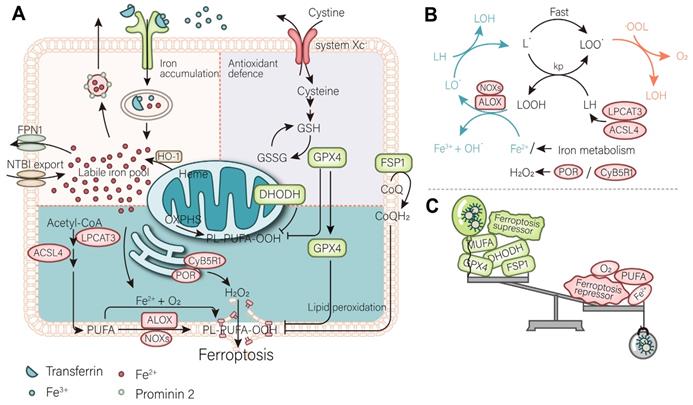
Mechanism of ferroptosis. (A) A mutually independent antioxidant defense axis composed of xCT-GPX4, FSP1, DHODH, protects cells against ferroptosis by antagonizing lipid peroxidation. At the same time, the entry and exit of intracellular iron as well as changes in availability regulate the sensitivity of cancer cells to ferroptosis. Lipid metabolism and PUFA synthesis provide substrates for the occurrence of ferroptosis. A series of oxidases become promoters of lipid peroxidation and the Fenton reaction. (B) An illustration of the role of these proteins in the detailed mechanism of lipid peroxidation is partially shown. (C) In general, the imbalance of intracellular ferroptosis-inducing and -inhibiting factors leads to ferroptosis, while the purpose of nanomaterials is to increase the weight of ferroptosis-inducing factors and reduce that of ferroptosis-inhibiting factors (such as antioxidant defenses).
2.1. The depletion of antioxidant defense
To date, three antioxidant defense systems, namely GPX4, FSP1, and DHODH, which localize to different parts of the cell and function independent of each other, have also been found to play a complementary role in inhibiting ferroptosis. Since each antioxidant defense system is independent, their status needs to be considered comprehensively according to the cancer type when developing drug and clinical treatment strategies.
GPX4, which localizes in the cytoplasm, is generally considered the predominant ferroptosis resistance factor [1, 6]. It forms an antioxidant defense system with the membrane protein system xc-. GPX4 is a selenoprotein whose active site contains a selenocysteine [7], which converts toxic lipid peroxides (L-OOH) into a non-toxic lipid (L-OH) form by catalyzing them, thereby curbing continuous lipid peroxidation. GPX4 is indispensable to perform this function, together with its substrate GSH [8, 9]. During peroxidation, GSH is converted into its oxidized form GSSG and regenerated into GSH by GSH-disulfide reductase (GSR) using flavin adenine dinucleotide (FAD) as coennezyme and reduced nicotinamide adenine dinucleotide phosphate (NADPH) as cofactor, which continuously provides power for GPX4. Conceivably, the abundance of GSH determines the catalytic efficiency of GPX4 and the sensitivity of cancer cells to ferroptosis. Downregulation of GSH has been observed in cells undergoing ferroptosis. The rate-limiting step in the synthesis of GSH is the ligation of cysteine to glutamate, which is catalyzed by GCLC. In contrast, the extracellular intake of cystine, a precursor of cysteine, is the most important source of intracellular cysteine [10]. System xc- is a key cystine-glutamate transporter, consisting of two subunits, namely SLC7A11 and SLC3A2 [11], which transports cystine into the cell in exchange for glutamate in a 1:1 ratio. Since it regulates the production of GSH, its activity is essential for the anti-ferroptosis effect of GPX4. P53, an important transcription factor in cancer cells, triggers ferroptosis and suppresses cancer development by inhibiting the expression of SLC7A11 [12]. Conversely, Nrf2 upregulates SLC7A11 and GPX4, thereby inhibiting ferroptosis through its anti-oxidative stress activity [13].
However, it turns out that GPX4 is not the only antioxidant defense system within cancer cells. The discovery of two other known antioxidant pathways not only indicates that GPX4 is not the only ferroptosis suppressor but also partially reveals the ferroptosis resistance of some cancer cell lines to the targeted system xc--GPX4 axis. Two independent laboratories simultaneously identified FSP1 as an antioxidant system localized on the cell membrane [14, 15]. FSP1 exerts its activity as an antioxidant defense system parallel to that of the GPX4 system. In addition to being a member of the electron transport chain, CoQ10 also plays a role in ferroptosis. Its reducing mode, ubiquitin, can inhibit the peroxidation of lipids and suppress ferroptosis. However, FSP1 suppresses ferroptosis by catalyzing the continuous regeneration of CoQ10 [14, 15]. It is well established that the peroxidation of mitochondria and cell membranes is the initial step of ferroptosis. Since there is an antioxidant system specialized in cell membranes, there is naturally an antioxidant system specialized in mitochondria. DHODH is an antioxidant defense system recently identified in mitochondria, where it localizes to the inner mitochondrial membrane [16]. DHODH indirectly promotes the regeneration of CoQH2 by reducing FMN and inhibits lipid peroxidation in mitochondria. The increase in DHODH activity increased the resistance of cancer cells to ferroptosis in cell lines overexpressing GPX4 and FSP1.
Current studies on ferroptosis-targeted cancer therapy have focused on antioxidant defenses, particularly the system xc- - GPX4 axis. Available clinical drugs, such as erastin and sorafenib, have been shown to inhibit system xc- [17, 18]. Alternatively, FIN-like compounds (e.g., RSL3) are the most widely used GPX4 inhibitors to significantly induce ferroptosis. However, targeting the other two systems in parallel to GPX4 cannot be overlooked. It has been experimentally demonstrated that iFSP, an inhibitor of FSP1, and BQR, a DHODH inhibitor, can reverse part of the resistance to ferroptosis in targeted GPX4-tolerant cell lines. Therefore, these pathways should be considered comprehensively in future drug development. Personal consideration of the patient's phenotype for personalized targeted therapy is required in theranostics.
2.2. The accumulation of iron
Accumulation of iron is a prerequisite for ferroptosis. Ferroptosis is triggered by divalent iron ions, whose abundance determines the sensitivity of cells to ferroptosis. Instead, only free iron can participate in the triggering of ferroptosis (which will be discussed in the following subsection) [2]. In this subsesction, we describe various ways in which the abundance of divalent iron ions in cells is affected. Both exogenous input of iron and intracellular iron have value for use with targeted nanomaterials.
Transferrin is a protein that binds extracellular ferric ions and is transported intracellularly by binding to TfR on cancer cells [19, 20]. The ferric ions released from transferrin are subsequently reduced to ferrous ions that drain into the labile iron pool (LIP). The TfR is upregulated during ferroptosis, thus it is recognized as a biomarker of ferroptosis [21]. In fact, due to their metabolic peculiarity, cancer cells themselves have a higher iron requirement than normal tissues. Therefore, the expression level of TfR is generally maintained at a relatively high level in cancer cells [22]. These properties enable the possibility to induce ferroptosis in cancer cells and suggest that TfR can be used as a specific target for enrichment of nanomaterials. In addition, several membrane proteins (SLC39A14, SLC11A2, SLC39A8) that mediate non-transferrin-bound iron (NTBI) [23] trafficking also mediate iron ion entry and increase the LIP and, together with the TfR, control the import of exogenous iron to regulate ferroptosis [24]. Ferritin controls the size of the LIP by binding free iron. Ferritinophagy is a ferritin-specific type of autophagy that is mainly triggered by NCOA4 and mediates the degradation of ferritin, releasing free iron to expand the LIP [25]. Undoubtedly, ferritinophagy enhances cellular sensitivity to ferroptosis and serves as a key way of regulating ferroptosis. In mitochondria, heme oxygenease 1 (HMOX1) breaks down heme to release free iron [26]. Meanwhile, FXN regulates ferroptosis sensitivity by limiting iron availability by synthesising iron-sulfur clusters. Downregulation of FXN has been found to sensitize cancer cells to ferroptosis [27]. In contrast, iron export decreases the LIP and sensitizes cancer cells to ferroptosis. Ferroportin 1 (FPN1, also known as SLC40A1), is the only known iron transporter mediating iron transport from the inside of the cell to the outside of the cell in humans. High expression of FPN1 can sensitize cells to ferroptosis [28]. At the same time, prominin 2 mediates the formation of ferritin-containing vesicular bodies and exosomes that transport iron out of the cell by exocytosis, similarly reducing the sensitivity of cancer cells to ferroptosis [29].
Measurement of the levels of iron, in vitro, has confirmed that ferroptosis occurs when iron overload occurs, while measurement of in vivo levels have also confirmed that a high iron diet can increase the efficiency of cancer therapy targeting ferroptosis. This has led to the idea of developing nanomaterials to regulate ferroptosis based on iron overload. While development of drugs targeting ferritinophagy is worth tapping into, negative regulators targeting ferritinophagy increase intracellular free iron levels.
2.3. The occurrence of lipid peroxidation
The ultimate effect of free iron accumulation is to induce lipid peroxidation. In general, the Fenton-like reaction of ferrous ions with membrane phospholipids is the beginning of the entire lipid peroxidation chain reaction. On the one hand, polyunsaturated fatty acids (PUFAs) are excellent substrates for this reaction because the C-H bonds of the methylene groups on either side of the C-C double bond are the weakest known C-H bonds (about 76 kcal/mol). Arachidonic acid (C20:4) and epinephrine (C22:4), meanwhile, are considered the most predominant reaction substrates and are enriched at the beginning of ferroptosis. Iron and oxygen are involved in the Fenton reaction in lipid peroxidation to generate hydroxyl radical, seizing hydrogen atoms from the substrate to generate carbon-centered radicals, then react with O2 to generate peroxyl groups, through the peroxyl group generated through the reaction with another molecule of substrate, continue to perform Fenton-like reaction with divalent iron to create the chain reaction (Figure 1). The termination reaction competes with this process to generate carbonyl compounds, alcohols, and oxygen. Hydrogen peroxide and other oxidases (ALOXs, NOXs) can also initiate the above reaction by producing peroxyl groups [30, 31]. POR and CYB5R1, which located in the endoplasmic reticulum (ER), are essential sources of hydrogen peroxide and have been shown to induce ferroptosis [32]. On the other hand, monounsaturated fatty acids (MUFAs) and ether phospholipids block lipid peroxidation to prevent ferroptosis [33, 34]. Regulation of membrane phospholipid synthesis pathways regulates sensitivity to ferroptosis. ACSL4 with LPCAT3 is a key regulatory enzyme in the formation of PUFA phospholipids, while ACSL4 is enriched during ferroptosis and can serve as a biomarker of ferroptosis [35].Instead, ACSL3, a key enzyme catalyzing MUFA biosynthesis, is a negative regulator of ferroptosis [33].
Recently, it has been reported that beneficial small-molecule oxidants trigger ferroptosis by direct oxidation of lipids [36], which can be combined with nanocarriers to enable their precise targeted delivery to tumour tissues only. Therapeutic strategies that additionally induce the production of oxidants within cancer cells, such as hydrogen peroxide, are also feasible. They can additionally make it more suitable for targeted ferroptosis therapy by promoting the enrichment of PUFAs within cancer tissues.
3. Ideas for the development of nanomaterials to target ferroptosis
The nature of the nanocarrier itself and the active ingredients it carries, its applicability to cancer, and type of patient determine the quality of the clinical treatment. The development strategies of drugs targeting ferroptosis are therefore particularly important. Besides targeting strategies that trigger ferroptosis directly, synergy with radiation therapy and immunotherapy also needs to be considered.
3.1. Targeting antioxidant defense and lipid peroxidation
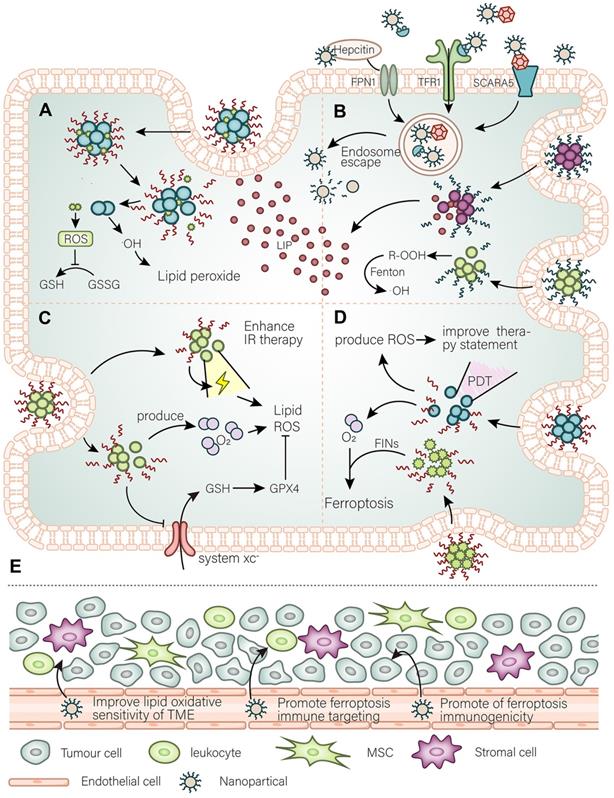
Breaking down antioxidant defenses means breaking down the defense that cancer cells have for protection against ferroptosis. However, some types of cancer cells possess an active antioxidant defense system, which renders them resistant to ferroptosis. The impairment of the antioxidant defense system sensitizes cancer cells to ferroptosis (Figure 2). There have been many studies on the induction of ferroptosis in cancer cells by small molecules against antioxidant defense system targets. For example, the traditional anticancer drug sorafenib targets system xc- in solid tumors and induces ferroptosis [17]. In addition, sulfasalazine was demonstrated to target ferroptosis in prostate cancer, and lymphoma [37, 38]. In experimental studies, the well-characterized erastin [39], RSL3 [40], iFSP, and BQR have been shown to target various antioxidant defense systems leading to ferroptosis. It is worth mentioning that traditional Chinese medicines, such as artemisinin and its derivatives, have good effect against antioxidant activity and deserve to be considered as drug development candidates. To develop nanomaterials against an antioxidant defense system, it is necessary to comprehensively consider the parallel antioxidant defense systems and select compounds with good selectivity and kinetics. Given the heterogeneity of tumor antioxidant defense systems in different patients, their suitable nanomaterials can be ascertained by clinical diagnosis for precise personalized medical treatment.
The main purpose of nanomaterials to target ferroptosis. (A) A general simple strategy for the development of nanomaterials is to improve the targeting of ferroptosis inhibitors to cancer tissues and modify the intracellular oxidative environment. (B) At the same time, the high demand for iron by cancer cells is the basis for the development of nanomaterials to target cancer cells, such as using Tfr to further expand or utilize the abundant unstable iron pool in the cancer cells. (C) By developing a strategy for releasing O2 as well as weakening of antioxidant defense systems to promote the damaging effect of ionizing radiation (IR) and further promote IR-induced ferroptosis. (D) Photodynamic triggered nanomaterials improve the treatment quality by adding an oxygen-releasing strategy combined with FINs. (E) In the TME, nanomaterials promote cancer cell immunogenic ferroptosis, promote infiltration of immune cells, and adjust the lipid balance of the immune microenvironment to curb the invasion and spread of cancer cells.
There are now various cases of ferroptosis inducers loaded in nanomaterials. However, there are still some ideas that can be developed into effective ferroptosis-based therapy for cancer. Sulfur dioxide can regulate the balance of oxidative levels in tumors, and For instance, Shen et al. [41] developed a GSH-responsive sulfur dioxide polymer prodrug for use as a carrier for the corresponding loaded drugs. This carrier cleverly uses intracellular GSH (thiolyl) as its triggering molecule and rapidly releases sulfur dioxide from N-(3-azidopropyl)-2,4-dinitrobenzenesulfonamide (AP-DNS), a drug external to the nanocarrier, leading to an increase in intracellular ROS and a decrease in GSH. This type of carrier can be simultaneously used as a prodrug for multidrug-resistant tumors and as a prodrug for photodynamic therapy (PDT) [42], as described below. These findings demonstrate the potential for using this nanocarrier to carry ferroptosis inducers to treat tumors. Another multistage synergistic nanoplatform that achieves co-delivery of proteins with drugs is equally noteworthy. In the initial study by Zhang et al. [43], they chose an exquisitely designed three-piece copolymer, namely mPEGb-PGCA-b-PGTA. The mPEGb-PGCA-b-PGTA copolymer can achieve endosomal escape and drug release triggered by pH changes. Zhang et al. used this copolymer to carry the chemotherapeutic drug DOX with modified RNAse to induce intracellular ROS and RNA breakdown and successfully kill cancer cells. The advantage of this nanoplatform is its rich plasticity, and the expectation is to carry a variety of ferroptosis inducers to target different antioxidant defense systems and carry oxidase (or regulate peroxide production enzyme) proteins to induce ferroptosis in cancer cells by synergy. In sum, selecting the correct carrier and comprehensively considering the different antioxidant defense systems within cancer cells is the key to the development of nanomaterials to effectively target ferroptosis in cancer cells.
PDT, a therapy that uses light and photosensitizers, kills cancer cells through light-triggered chemical damage. As a popular treatment, PDT has been used in combination with nanomaterials therapy. Simultaneously, PDT can be combined with other traditional treatment modalities [44]. Most notably, PDT can increase the immunogenicity of tumors. PDT can induce cell death through a variety of cell deathtypes, and the process can be artificially guided. Due to the different properties of PCDs (programmed cell deaths), guided ferroptosis may be the best choice for cancer treatment. There have been some ingeniously conceived nano-mediated PDT approaches targeting ferroptosis (introduced in the next section). The most significant disadvantage of PDT-based strategies targeting ferroptosis is the oxygen deficiency in tumors, but such disadvantage can be circumvented. The general idea is to add oxygen to the donor or induce oxygen-independent free radical generation. In a study by Xu et al. [45], they combined hemoglobin with photodynamic nanomaterials. On the one hand, hemoglobin provides oxygen for PDT, and on the other hand essential iron for ferroptosis. In addition, Zou et al. [46] developed a simple oxygen release strategy to achieve chemical energy storage, which can be used to continuously release oxygen in a dark hypoxic tumor environment after laser triggering. Also, the BiOI@Bi2S3@BSA nanoparticles (NPs) constructed by Zhao et al. [47] can produce oxygen free radicals based on electron-hole pairs through a PDT process using X-ray irradiation, providing an additional oxygen-independent approach. In summary, besides solving the problem of hypoxia, the use of PDT for the guidance of ferroptosis in treatments targeting ferroptosis deserves to be given attention. Meanwhile, the use of PDT should take into account the directionality of ferroptosis.
3.2. Targeting iron metabolism and availability
Cancer cells meet their demand for iron by enhancing iron metabolism. Thus, on the one hand, sufficient iron enables to meet the need for vigorous biological activity of cancer cells, while on the other hand abundant iron has the potential to induce ferroptosis in cancer cells. Predictably, cancer cells upregulate various membrane proteins that control various forms of iron entry to meet their iron requirement. A variety of membrane proteins such as TfR1, FPN, SCARA5, and CD163 are upregulated in cancer cells and become possible targeting candidates. There have been several nanomaterials that target tumor cells through use of the TfR1 (NCT02340117, NCT00964080, NCT02340156). In addition, worth noting is a nanomaterial loaded with artemisinin recently reported [48], which also uses the TfR1 to target tumors, while artemisinin is loaded into fullerenes and modified with hyaluronic acid. These nanomaterials not only have good targeting ability but also are suitable for PDT. Moreover, compared to other nanomaterials, nanomaterials that use transferrin also have the ability of penetrating the blood-brain barrier and can be used to perform targeted therapy to such brain tumors. Since transferrin can cross the blood-brain barrier to deliver iron to the central nervous system and meet its demand for iron, compared to other nanomaterials, transferrin-containing NPs also have the ability to penetrate the blood-brain barrier and deliver drug molecules into the central nervous system, and thus can be used to perform targeted therapy for central nervous system brain tumors. Therefore, ferritin is also an excellent choice for the development of nanomaterials for use in targeted cancer therapy. Ferritin receptors are upregulated in cancer. In addition, the structural and physicochemical characteristics of ferritin make it suitable as a drug carrier. For instance, ferritin can form a unique nanocage structure [49], which can carry a large number of drug molecules. Moreover, the formation of this structure is pH-sensitive [50], decomposes at lower pH, and assembles at physiological pH. There are already therapeutic strategies that use this structure to target cancers, such as pancreatic cancer [50]. It is also relatively easy to fabricate ferritin nanomaterials to target ferroptosis in cancer cells. Hepcidin is a class of factors that regulate the systemic iron cycle and mediate its own endocytosis together with FPN. Also, the abundance of hepcidin itself in vivo can directly regulate ferroptosis [51]. Therefore, it is likely that the use of hepcidin for targeting ferroptosis is relatively efficient. Although there is no specific therapeutic strategy, drug development targeting the endocytosis of hepcidin and FPN to induce ferroptosis, it is worth a try. When implementing a particular clinical treatment, diagnostic means first should determine the target expression level of cancer cells. Given the increased iron levels in cancer cells, therapeutic strategies targeting unstable iron pools are feasible. Very recently, monodispersed ferrihydrite NPs, synthesized inspired by organisms that naturally synthesize ferrihydrite biominerals, were reported to release enormous amounts of free Fe2+ and mediate related lipid ROS production after being excited by blue light [52]. The advantage of this approach is that it overcomes the in vivo toxicity in humans caused by the vast majority of Fe2+ releasing particles. Similarly, only the LIP present within cancer cells can be used to induce ferroptosis to kill cancer cells without the need to consider toxicity issues—co-loaded NPs of peroxide (R 'OOH) and the ferroptosis inducer erastin [53]. R'OOH can initiate the Fenton reaction with the LIP in a rapid response and further induce lipid peroxidation. In developing such endogenous nanomaterials, targeting intracellular iron metabolism (e.g., promoting ferritinophagy) to release free iron ions to expand the endogenous LIP can be considered. It is a good option for ferritin and mitochondrial iron. This study expands the idea of using the Fenton reaction to treat cancer. Arachidonic acid and adrenal acid, the best substrates for ferroptosis, can be peroxidized and targeted enriched in cancer cells using nanomaterials as "kindlings" triggered by ferroptosis. This idea is inspired in the reasons for a similar iron overload that has been preliminarily confirmed in a recent study [54]. In addition, the scheme can synergistically weaken the antioxidant defense systems. Adequate understanding of the status of iron in cancer is important to develop precisely targeted ferroptosis nanomaterials, which must take into account both the peculiarity of iron metabolism in cancer cells and the iron requirement in normal tissues (Figure 2).
3.3. Potential strategies for collaborative radiation therapy
Radiation therapy can lead to various cellular phenomena, such as activation of ataxia-telangiectasia mutated (ATM), and p53, which promotes cell death. Recent studies have confirmed the relationship between radiation therapy and ferroptosis [3, 55]. Radiation therapy induces ferroptosis in cancer cells through multiple pathways. Through the generation of oxygen free radicals, radiation therapy also disrupts the redox balance of cells by reducing GSH, SLC7A11 [56], and reducing antioxidant defenses via ATM [57] and p53. In addition, radiation therapy induces a range of cellular effects, such as a variety of autophagic behaviors that promote ferroptosis and ACSL4 production [58]. Meanwhile, cells resistant to radiotherapy usually show resistance to ferroptosis, and the hypoxia-associated HIF pathway is activated in these cells. The hypoxic tumor microenvironment (TME) represents an essential mechanism of radiation resistance. Activation of the HIF pathway makes cancer cells resistant to ferroptosis, which on the other hand, enhances oxidative stress in cells. Therefore, radioresistant cells are highly dependent on antioxidant defense systems and can be used as a breakthrough. There are already various small molecule drugs against antioxidant defense systems that increase the effect of radiotherapy in vivo [59]. The same function of nanomaterials is not reproduced here, but in cells resistant to radiotherapy, resistance to ferroptosis is observed and the hypoxia-associated HIF pathway is activated [60]. Furthermore, cancer cells achieve radioresistance by reprogramming lipid metabolism. For example, KRAS mutant cancer cells upregulate ACSL3 to enrich MUFA, which makes it difficult to trigger ferroptosis, thereby achieving radioresistance [61]. Thus, it is possible to develop nanomaterials to enrich phosholipid-bound-PUFA (PL-PUFA) in the tumor environment and effectively cause radiosensitization (Figure 2).
In addition, a variety of radiosensitizing agents have been developed to enhance therapeutic efficacy and reduce radiation toxicity to normal tissues. Due to their physicochemical properties, nanomaterials with inorganic configurations seem to be the optimal choice for radiosensitization. Such nanomaterials usually allow cancer cells to rapidly produce ROS under radiotherapy conditions and can create a good condition to induce ferroptosis. The targeting of nanomaterials, synergy with other therapies, and timely clearance in patients need to be considered for their development. For example, a selective Cu2(OH)PO4 nanocrystals can catalyze the degradation of hydrogen peroxide into hydroxyl radicals by the corresponding X-ray stimulation, and the process can only be performed in the hypoxic TME [62]. Meanwhile, since the reaction cannot proceed in oxygen-rich normal tissues normal cells are not harmed. In summary, the combination of nano-induced ferroptosis with radiotherapy requires careful consideration of the substrates of ferroptosis, the tumor oxygen-poor environment, and strategies to target antioxidant defense systems. Targeted nano prodrugs can be developed to regulate the lipid metabolic balance of the TME, before radiotherapy, rendering them suitable for radiotherapy targeting ferroptosis, thereby preventing further development of cancer, and improving prognosis.
3.4. Possible strategies for synergistic immunotherapy
The relationship between ferroptosis and immunity is partially understood. The role of immunotherapy against PD-L1/PD-1 in ferroptosis has been explained in part. CD8 + T cells have been found to act on cancer cells by releasing INFg, leading to the downregulation of antioxidant defense systems and ultimately inducing ferroptosis [63]. Also, treatment with anti-PD-L1 greatly enhanced the killing efficacy of CD8 + T cells against cancer cell-induced ferroptosis. Moreover, therapies against PD-L1 in cells resistant to ferroptosis showed a resistance effect [63]. Accordingly, the focus of the development of drugs targeting ferroptosis should be set on: (1) remodeling the sensitivity of cancer cells to ferroptosis; (2) enhancing the synergy of the immune response and PD-L1; (3) increasing the activity of T cells and intratumoral penetration. The way cancer cells regain their sensitivity to ferroptosis has been described above. However, attention needs to be paid to the selectivity of nanomaterials for targeting cancer cells to avoid causing unnecessary death of immune cells. The approach to achieve synergistic anti-PD-L1 immunotherapy has been developed. For example, the nano platform developed by Li et al., which can enlarge LIP and enhance the function of CD8 + T cells and their intratumoral infiltration as well as synergize with immunotherapy [64].
Ferroptosis is also regulated by other immune activities. For example, we now know that ferroptosis is immunogenic. Damage-associated molecular patterns (DAMPs), PGE2, and other substances released by ferroptotic cells can inhibit or activate immune cells [65], although it remains unclear whether ferroptotic cells will release specific substances to regulate the immune system. Also, oxidized phospholipids on the cell membrane act as "eat me" signals [66] when ferroptosis sets off and recruits phagocytes. Since the EMT status of cells is highly sensitive to ferroptosis, the tumor microenvironment becomes the key to their migration success rate. The abundance of different iron and fatty acid species in the immune microenvironment determines the difficulty of cancer cell invasion and metastasis and regulates cancer cells' immune evasion. For example, cancer cells in the lymphatic environment are more likely to metastasize than cancer cells in the blood vessels. For this reason, the purpose of nanomaterials is to remodel the tumor immune microenvironment: sensitize to ferroptosis, prevent its metastasis, enhance the killing effect of immune cell-induced ferroptosis, improve the inhibitory effect of cancer cell ferroptosis on immune cells, and enhance the immunogenicity of ferroptosis in tumor tissues. Just recently, the nanomaterials Fe3O4-SAS @ PLT developed by Jiang et al., consisting of dielectric magnetic NPs (Fe3O4) loaded with sulfasalazine (SAS) and camouflaged with platelet (PLT) membranes, triggered ferroptosis by inhibiting the glutamate-cystine countertransport system xc pathway. Thus, the research to date has revealed that these nanomaterials significantly transformed TME macrophages from M2 to M1 phenotype, while having a good synergistic effect with anti-PD-L1 therapy [67]. Coincidentally, another study [68] has also reported that nanomaterials targeting ferroptosis can also promote the conversion of M2 macrophaes to M1 macrophages within the TME and attenuate PD-L1 expression in the TME. Their study suggests that ferroptosis promotes antitumor immunity [68]. Another study, confirmed the promotion of the recruitment of infiltrating T cells by targeted ferroptosis nanomaterials and suggested their joint promotion with immunotherapy [69]. However, a better understanding of the relationship between ferroptosis and the TME is required to fully grasp this type of therapy (Figure 2).
Moreover, the differences in the sensitivity of immune cells to ferroptosis within the TME also illustrate difficulty of developing a nanotherapy targeting ferroptosis. For example, antitumor CD8 + T cells are similarly sensitive to ferroptosis [70]. Compared with conventional CD4 + T cells, CD8 + T cells are more sensitive to inhibitors of GPX4. Meanwhile, the CD36 lipotranslocase expressed by CD8 + T cells was found to increase ferroptosis sensitivity and impair the function of antitumor immunity [71]. Overexpression of GPX4 and treatment with ferroptosis inhibitors reversed this phenomenon. However, CD8 + T cells have a higher cysteine utilization efficiency, and they are not as sensitive to inhibitors of system xc - as cancer cells [72]. Also, Tregs that are detrimental to immunity evade ferroptosis by upregulating GPX4 [73]. These lines of evidence illustrate the need for nanomaterials targeting ferroptosis to be selective for cells, or for strategies that provide therapeutics against different immune microenvironments. Targeting the epidemic desert or promoting the immune microenvironment of tumors can directly and highly target ferroptosis and increase the infiltration of antitumor immune cells. Aiming at the microenvironment with good immune infiltration, a nanomaterials can be developed, which is only specifically enriched in tumor cells, while sparing antitumor immune cells from significant lethal effects. Options include targeting iron metabolism-related proteins on the cell membrane surface, or using different intracellular pH to control the release of drugs. In addition, CD8 + T cells can be further targeted to evade ferroptosis, for instance, by targeting the CD36 receptor to reduce their sensitivity to ferroptosis. With the increase of the understanding of ferroptosis in tumor cells and immune cells, more "differentially targeted" strategies will be proposed.
4. Progress of nanomaterials in targeting ferroptosis
With the deepening of the study on the mechanism of ferroptosis, there has been good progress in the anticancer treatments with nanomaterials targeting ferroptosis. The classification of the mechanisms of action of these nanomaterials has been discussed in more detail in other reviews [74-76]. Nanomaterials targeting ferroptosis are generally classified into iron-based and non-iron-based nanomaterials. In this section we will describe the current status of these two types of nanomaterials targeting ferroptosis. More representative nanomaterials are summarized in Table 1. In any case, the design objectives of these nanomedicines are described in the above two sections of this paper.
4.1. Iron-based nanomaterials
This type of nanomaterials has some advantages in its ability to directly trigger the Fenton reaction. As the name implies, this class of nanomaterials contains iron with the general purpose of increasing the availability of intracellular iron. This nanomaterials use the Fenton reaction to disrupt the intracellular oxidative balance. The vast majority of iron-based nanomaterials are designed to target tumors specifically and can trigger and release iron at specific times. Alternatively, the oxidative balance of cells is disrupted by the nanomaterial itself promoting the Fenton reaction.
Iron-based nanomedicines are usually classified into the following types:
Iron oxide NPs (IO NPs): is a simple nanomaterial that has been approved by the United States Food and Drug Administration (FDA) agency for the treatment of iron deficiency. The most primitive IO NPs usually simply kill cells by producing an overwhelming amount of ROS through the release of free iron. In addition, this nanomaterial can also be further expanded to achieve better ferroptosis-inducing effect or tumor targeting. In general, the purpose of this expansion is to better promote the Fenton reaction and produce ROS, or to confer imaging diagnostic function. For example, Ma et al. co-loaded cisplatin with a nanosized drug to overcome resistance to cisplatin and further induce ferroptosis [77]. Alternatively, through modification, the release of oxygen is combined with the release of iron ions. For example, Zhou et al. attached IO NPs to linoleic acid hydrogen peroxide (LAHP) and produced a triggerable IO-LAPH-NPs [78]. These NPs can produce singlet oxygen through the Russell reaction of Fe2 + with the LahP produced by IO NPs under acidic conditions at the tumor site. In addition, Li et al. further encapsulated IO NPs with H2O2 into polymers to form the structure of H2O2/fe3o4 - PLGA polymer bodies [79]. The encapsulation of H2O2 plays a crucial role in simultaneously providing O2 for echo reflection and OH as therapeutic ROS.
Iron-doped nanomaterial, or amorphous iron NPs: these nanomedicines usually refer to nanomaterials mixed with amorphous iron. This nanomaterial usually relies on the release of its mixed iron to sensitize cells to ferroptosis and achieve better killing effects. In one study, zinc (II) protoporphyrin IX (ZnP) was injected into the particle oxyurea (BFR) to replace the heme group, extending the N-terminal portion of the BFR using peptides that could target overexpressed receptors on tumor vasculature and cells. The Fenton reaction between intracellular Fe2 + and H2O2 promoted by this nanomedicine targets iron-loaded ZnP-BFR structures, produces -OH and oxygen upon light irradiation, and inhibits cancer cell development [80].
Iron-organic frameworks: these nanomedicines have ultra-high porosity (up to 90% free volume) and huge inner surface regions, as well as strong extensibility [81]. Thus, catalytic membranes can be formed with potential anticancer potency. In addition, this nanomaterial is pH responsive and can be further expanded, such as by the addition of anticancer small molecule components [82]. These nanomedicines also have the imaging ability of magnetic resonance. In summary, these nanomedicines have great potential to be developed.
Representative nanomaterial-mediated ferroptosis
| Name | Mechanisms | Strategies to induce ferroptosis | Ref. | |
|---|---|---|---|---|
| Iron | IO NPs | M1 macrophages release H2O2, which reacts with Fe3+ or Fe2+ to produce ROS via the Fenton reaction | Lipid peroxidation | [74] |
| Cisplatin-loaded IO NPs | Used intracellular Fe2+ released from IO NPs to enhance sensitivity to cisplatin | Lipid peroxidation | [77] | |
| IO-LAHPNPs | Fe2+ is released from the surface of IO-LAHPNPs, which triggers the formation of ROS and O2-, leading to cancer cell death | Iron accumulation | [78] | |
| Assembled IO NPs | H2O2 is released and the Fenton reaction occurs, producing -OH | Lipid peroxidation | [79] | |
| (AFeNPs) | The Fenton reaction in tumors is induced using mild acidity and excess production of H2O2 in the TME | Iron accumulation, lipid peroxidation | [87] | |
| Iron-organic Frameworks | Fe2+ is released and induces the Fenton reaction, which increases the intracellular ROS concentration | Iron accumulation | [74] | |
| FePt NPs | It releases Fe2+, which can catalyze the breakdown of intracellular H2O2 into ROS | Iron accumulation | [88] | |
| Fe (Ⅲ)-ART (Artesunate) NPs | After the release of Fe3+, it is further reduced to Fe2+ catalyzes the endoperoxides of ART to generate C-centered radicals, leading to GSH depletion | Iron accumulation, antioxidant defence: GPX4 axis | [89] | |
| FeCO-DOX@MCN | Iron loading, ROS level increase, GSH depletion, GPX4 inactivation | Iron accumulation, antioxidant defence: GPX4 axis | [90] | |
| DGU:Fe/Dox | Dox release triggered by NIR (Near infrared radiation), iron loading, ROS accumulation, downregulation of GPX4 and ACSL4 | Iron accumulation, antioxidant defence: GPX4 axis | [91] | |
| FeGd-HN@Pt@LF/ RGD2 | Increase local concentrations of Fe3+, Fe2+ and H2O2 simultaneously | Iron accumulation | [92] | |
| SPFeN | Released Fe3+ is reduced to Fe2+, and • OH is generated by the Fenton reaction under acidic conditions | Iron accumulation | [93] | |
| FePt/MoS2 | Killing of tumor cells by triggering a rapid Fenton reaction and photothermal therapy | Iron accumulation | [94] | |
| PYSNPs | Porous eggshell nanostructures of iron/Fe3O4 stabilize iron (0) and control the release of iron (0) in the TME and promote the Fenton reaction | Iron accumulation | [95] | |
| PEG-Fns | Monodisperse ferrate NPs, triggered by blue light at the tumor site generate Fe2 + | Iron accumulation | [52] | |
| SPION | Free iron species are released from the acidic environment of lysosomes, and the NIR photosensitizer Cy7-Hex anchors to the mitochondrial membrane where binding to sorafenib results in a burst of LPO (Lipid peroxidation) | Iron accumulation, antioxidant defence: GPX4 axis | [96] | |
| SRF@FeIIITA | SRFFeIIITA NPs can cause a corona dissociation reaction in response to the lysosomal acid environment, allowing the release of sorafenib to inhibit the GPX4 enzyme-triggered ferroptosis | Iron accumulation, antioxidant defence: GPX4 axis | [97] | |
| Mn-MOF | Continuously catalyzes the in situ generation of O2 from tumor-overexpressed H2O2, alleviates tumor hypoxia, decreases GSH and GPX4, and promotes the production of ROS and iron overload after ultrasound (US) irradiation in hypoxic tumors | Iron accumulation, antioxidant defence: GPX4 axis | [98] | |
| GBP@Fe3O4 | triggered bylocalized moderate heat (45 °C), leading to burst release of Fe3O4 in situ to produce potent reactive oxygen species through the Fenton reaction in the tumor microenvironment | Lipid peroxidation | [99] | |
| DOX/Fe3+/EGCG (DF) NPs | The pH-corresponding nanomicelles, promote lipid peroxidation by releasing free iron and DOX | Iron accumulation, antioxidant defence: GPX4 axis | [100] | |
| bcc-USINPs | Strong Fenton response with good immunotherapeutic synergy | Iron accumulation | [101] | |
| PCGA@FeNP | Iron-based Nanomedicines Released by Photothermal Response | Iron accumulation | [102] | |
| FePPy NP | Killing cancer cells by enrichment of free iron and photothermal effects | Iron accumulation, lipid peroxidation | [103] | |
| CoFe2O4 | Double Corresponding Fenton Reaction between sonodynamic therapy and chemodynamic therapy Triggers Nanomedicines | Lipid peroxidation | [104] | |
| Fe3O4-SAS@PLT | Platelet Membrane-Camouflaged Magnetic Nanoparticles, release iron and weaken antioxidant defenses | Iron accumulation, antioxidant defence: GPX4 axis | [67] | |
| Non-iron | BCFe@SRF | In the hypoxic environment, BSA-Ce6 is released for laser-triggered PDT, ferritin is released for iron-catalyzed Fenton reaction, and SRF is released for tumor antioxidant defense system impairment | Strategies to induce ferroptosis | [105] |
| ZnO NPs | Increases intracellular iron availability by affecting iron channels on mitochondria | Lipid peroxidation | [106] | |
| (US)-activatable nanomaterials | Impairment of antioxidant defense systems by released ferrate triggered by ultrasound overcomes the hypoxic environment | Lipid peroxidation | [107] | |
| Ce6@CMOF | Through photodynamic release, the disulfide-thiol exchange reaction leads to the depletion of intracellular GSH | Iron accumulation | [85] | |
| LDL-DHA | A low-density lipoprotein NP. The killing of cells by lipid peroxidation is triggered by the native omega-3 fatty acids | Lipid peroxidation | [86] | |
| mPEG-PLys-AA/RSL3 | Lipid peroxidation products such as ROS can induce intracellular GSH failure and indirectly enhance the inhibitory effect of RSL3 on the GPX4 enzyme | Iron accumulation, lipid peroxidation | [84] | |
| miR-101-3p nanomaterials | Intracellular delivery of miR-101-3p restores ferroptosis in tumor cells by directly targeting TBLR1. | Iron accumulation | [108] | |
| SRF@Hb-Ce6 | Photodynamic triggered nanomaterials, loaded sorafenib induces ferroptosis, and loaded heme promotes PDT and the Fenton reaction by oxygen release | Iron accumulation | [45] | |
| supramolecular Ce6-erastin nanodrug | Photodynamic triggered nanomaterials, loaded erastin leads to a decrease in system xc - and disrupt antioxidant defense systems | Iron accumulation, antioxidant defence: GPX4 axis | [109] | |
| HA-C60-Tf/AS | Targeting of Trf triggers ferroptosis in tumor cells through the loaded artemisinin | Iron accumulation, antioxidant defence: GPX4 axis | [48] | |
| FaPEG-MnMSN@SFB | Rapid clearance of GSH disrupts antioxidant defense systems by two mechanisms | Iron accumulation, antioxidant defence: GPX4 axis | [110] | |
| ZVI-NPs | Causes mitochondrial dysfunction, intracellular oxidative stress, and lipid peroxidation, promotes the degradation of Nrf2, leading to ferroptosis in cancer cells. It can also enhance macrophage M1 transformation and reduce PD-L1 expression in the TME. | Iron accumulation | [68] | |
| Pt-FMO | It has similar antitumor efficacy to cisplatin in targeting ferroptosis, but has lower toxicity. | Iron accumulation | [111] | |
| TMBF4TCNQ and TMB-TCNQ | Organic photothermal agent that absorbs near-infrared light, effectively inhibits the intracellular biosynthesis of GSH, leading to redox stress and ROS-mediated ferroptosis | Iron accumulation | [112] | |
| PBE | Ferroptosis nanomaterials, triggered by acid-base changes, release RSL3 impairs antioxidant defense systems under acidic conditions and can synergize with immunotherapy. | Iron accumulation | [69] | |
| Fe3O4-SAS @ PLT | Triggers ferroptosis through the loaded SAS and shows good synergistic immunotherapeutic effects | Iron accumulation | [67] | |
| RSL3 @ COF-Fc(2b) | Induces ferroptosis by suppressing antioxidant defense systems and generating oxygen radicals | Iron accumulation, antioxidant defence: GPX4 axis | [113] | |
| MnO2@HMCu2-xS | Photothermal triggering, release of manganese ions promotes lipid peroxidation, and mediates autophagy to aid in the development of ferroptosis | Lipid peroxidation | [114] | |
| GOx/BSO@CS PVs | Treatment of Cancer by Induction of Iron Death Synergistic Hunger Therapy | Lipid peroxidation | [115] | |
| FeOOH NSs | Imageable nanomedicines that alter the cellular oxidative environment by producing hydrogen sulfide | Lipid peroxidation | [116] | |
| amorphous calcium phosphate (ACP)-based nanoplatform | Multi-purpose combined targeted therapy nanoplatforms | Lipid peroxidation | [117] |
4.2. Non-iron-based nanomaterials
This class of nanomaterials does not contain iron and is extremely rich in diversity. The purpose of non-iron based nanomaterials is to promote the Fenton response, disrupt antioxidant defense systems, and even regulate cellular metabolism to achieve the ultimate purpose of promoting ferroptosis. These nanomaterials have the potential to carry inducers of ferroptosis to achieve better therapeutic efficiency. They also have the potential to promote the Fenton reaction by expanding PUFAs, or using intracellular LIPs. The triggering modes of these nanomaterials are also relatively diverse. Most of the PDT drugs fall into this category. In addition, nanomaterials, partially composed of other metal elements, may promote ferroptosis by disrupting the activity of iron receptors/channels on the cell membrane [83].
Non-iron-based nanomaterials are usually available in the following forms:
Simple FIN carrier system: these nanomedicines trigger ferroptosis by carrying a FIN as the main component. Among them, mPEG-PLys-AA/RSL3 releases RSL3 to trigger ferroptosis and kill cells [84]. Due to the general nature of this class of drugs, these nanomedicines usually have to improve their release, response, and enrichment mechanisms to achieve more effective therapeutic effects and reduce toxicity to normal tissues.
Photodynamic/sonodynamic nanomedicines: these nanomedicines have release mechanisms that can be actively triggered and have potential for use in diagnostic applications. Some of these nanomedicines have already been mentioned in the previous section. In addition, such nanomedicines are usually expanded, such as by carrying oxygen release mechanisms to adapt to the hypoxic environment in tumors [45, 85].
Lipid enriched nanomaterials: these nanomaterials are designed to rapidly trigger ferroptosis by promoting the enrichment of PUFAs within the tumor cell/tumor microenvironment. Such strategies to trigger ferroptosis are less noticed, but are very effective. In addition, since the substrates carried are common in the human body and can be metabolized normally, such nanomedicines may have the lowest toxicity. The “kindling" strategy was mentioned above. Among this type of nanomedicines, LDL-DHA induces ferroptosis through the omega-3 fatty acids it contains [86]. This nanomedicine can expand the oxygen release strategy to counteract the hypoxic environment within cancer cells that is not conducive to lipid peroxidation.
Nanodrugs carrying non-coding RNA (ncRNA): these nanomedicines weaken the ferroptosis defense mechanism within cancer cells by preventing the translation and gene expression of certain proteins involved in the defense against ferroptosis, which is lethal to cancer cells. Since there have been numerous studies on ncRNAs and ferroptosis, there are a large amount of materials for rfurther experimental research. However, compared with the treatment targeting ferroptosis with ncRNAs alone, gene interfered-ferroptosis therapy may be a better way to use ncRNAs to fight cancer, and this combined treatment strategy will be introduced in the next section.
5. Application and perspective in nanomaterials for targeting ferroptosis
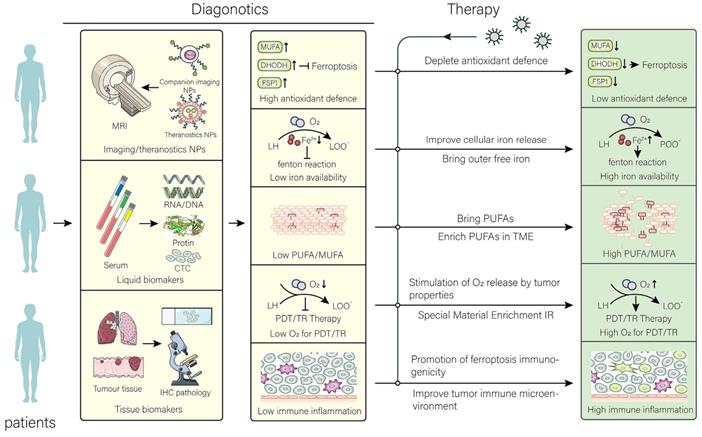
The nanomaterials used for targeting ferroptosis should be precise and effective. In this section, we elaborate on the relevant diagnostic and therapeutic strategies and discuss some problems that need to be addressed (Figure 3).
5.1. Diagnostic strategy
The primary analytic for nanomaterials used for cancer treatment by targeting ferroptosis is the patient's enhanced permeability and retention (EPR) effect. The EPR effect affects the efficiency of nanomaterial therapy, and the effect has been well described in other reviews [118]. Diagnostic nanoplatforms can be selected for their detection, or biomarkers in cancer tissue and blood can be detected. In addition to identifying patients suitable for treatment with this, other means can also be used to make more patients suitable for the treatment with nanomaterials [119]. For example, remodeling blood vessels in the TME may increase drug penetration. Subsequently, the patient's diagnosis should be made according to the specific treatment strategy, as cancer cells may be diagnosed with strong ferroptosis resistance. In general, ferroptosis-resistant cancer cells widely upregulate antioxidant defense systems, balancing with more intense intracellular oxidative stress. Currently, there is an urgent need to identify human-derived tumor-characteristic ferroptosis biomarkers in blood. However, further research and understanding. The purpose of diagnosis is to design treatment strategies for the synergy of ferroptosis with other treatment modalities. This requires targeted alteration of the patient's cancer tissue oxidative balance and remodeling of the TME.
At present, theranostics nanomaterials may be a good choice for clinical practice. Compared with traditional diagnostic nanoplatform, theranostics nanomaterials can be more precise in tracking and do not depend on the patient's blood or tissue samples. Theranostics nanomaterials also have the advantage of not only reducing costs in clinical translation, but also the complexity of treatment. Some nanomaterials with integrated diagnosis and treatment have been developed. It is recommended to develop theranostics nano prodrugs that signal "appropriate" treatment after remodeling the sensitivity of cells to ferroptosis and perform the subsequent treatment or synergize with other therapeutic strategies. For example, a theranostics nanomaterial prodrug with high ROS induction can increase intracellular ROS and "light up" cancer cells when accumulated to a certain level [120]. Furthermore, theranostics nanomaterials that detect intracellular available iron levels can be developed or only target the level of lipid peroxidation. In addition, the utilization of GSH may be a good option for drugs that detect intracellular antioxidant activity.
Application and perspective in nanomaterials for targeting ferroptosis. In the clinic, the aim of nanomaterials is to overcome the limitations of different tumors and their microenvironment. After determining the limitations that are not conducive to treatment by different diagnostic tools, the targeted treatment is performed using the corresponding nanomaterials to achieve the best efficacy at the lowest cost. The common resistance of tumors to ferroptosis has been overcome by therapeutic strategies.
5.2. Therapeutic strategy
Although FINs have exhibited significant induction of ferroptotic effects in the laboratory setting, unfortunately, no small molecule FINs have been approved for clinical treatment. Nevertheless, nanomaterials developed by using FINs are promising for clinical treatment. Detailed mechanisms regarding treatment have been explained in the above sections. Since the main purpose of developing nanomaterials is to improve treatment efficiency and reduce side effects, tumor penetration strategies with drugs, such as changing drug configuration (worm-like drugs [121], etc.), are worth considering. In addition, strategies should be taken to allow nanomaterials to be released at the right time, for example, using the low pH [122] of the tumor, hypoxic environment, etc. Alternatively, interventions, such as applying light, sound [123], and magnetic field, can be considered. Some ideas for therapy are proposed here.
Combining multiple drugs can reduce single toxicity, reduce the dosage of drugs, and enhance the therapeutic effect of drug-resistant tumors. For example, in ferroptosis-resistant cancer cells combining several drugs simultaneously weakens antioxidant defense systems and aggravates free iron. However, more clinical trials are needed for evaluating the effects of multidrug combinations on ferroptosis. In addition, one drug pleiotropic or multidrug release is also feasible, but aggravates the complexity in production and clinical application. Most importantly, a set of evaluation systems for nanomaterials that induce ferroptosis should be established to evaluate the efficacy of different model animals or therapeutic strategies. In addition, the changes of ferroptosis characteristics (such as iron, PUFAs, GSH) of tumors at different time points after administration have been determined and scored by algorithms to simulate the best efficacy. Also, different treatment strategies need to be selected for different types of immune microenvironments (already mentioned above). The effects of drugs on cancer cells and immune cells should be comprehensively considered. In addition, the combination of gene therapy and other nanomedicines targeting ferroptosis has also shown superiority. A recent study has well overcome ferroptosis tolerance in tumors and achieved durable curative effects by a combination of RNA interference and ferroptosis-target nanomedicines. Perhaps the combination of nanomedicines carrying ncRNAs and iron-based nanomedicines can have good clinical results. In summary, the development of more effective nanomedicine therapeutics targeting ferroptosis requires better imagination and understanding.
6. Conclusion and perspective
Ferroptosis, as a special type of programmed cell death modality, is a promising target in cancer treatment [124]. Nanomaterial therapeutic strategies based on ferroptosis mechanism and small-molecule inhibitor construction continuously emerge. Although nanomaterials targeting ferroptosis have not yet been clinically approved, they have shown considerable promise. Meanwhile, with the increased understanding of the mechanism of ferroptosis, the diagnostic tools in this area are also continuously improving. However, researchers must fully consider the heterogeneity of the patient's TME. In the future, the general trend involves expanding new nanomaterials development ideas as well as precision-targeted ferroptosis therapy.
Although there have been exciting advances in the development of nanomaterials that induce ferroptosis, there are still problems that need to be urgently solved. The first is the problem of the limits of intracellular Fenton production. If the intracellular acidic environment is weak, the rate of ROS production dependent on hydrogen peroxide generation will be low. In terms of radiotherapy, how to achieve the efficient enrichment and clearance of nanomaterials in tumors has become the most important problem to be solved. In combination with immunotherapy, further understanding of the relationship between ferroptosis and immunity and TME is urgently needed to develop more precisely targeted nanomaterials. In addition, strategies need to be developed to guide the targeting of nanomaterials in patients. For instance, magnetic fields are used to guide the enrichment of nanomaterials that induce ferroptosis in tumors. Additionally, in the relationship between ferroptosis and antitumor immunity, more findings are needed to improve the understanding of ferroptosis in immune cells and cancer cells and further elucidate its differences [125]. The development of nanomaterials that selectively target cells through differences between cells is key to promoting both ferroptosis and immunotherapy. In addition, researchers need to devote more attention to considering the clinical manifestations of nanomaterials that induce ferroptosis and establish an effective evaluation system. In summary, nanomaterials that induce ferroptosis have great potential, but there are still many aspects that need to be improved.
Abbreviations
ACSL3: Acyl-CoA Synthetase Long Chain Family Member 3; ACSL4: Acyl-CoA Synthetase Long Chain Family Member 4; ART: Artesunate; ATM: Ataxia Telangiectasia Mutated; CYB5R1: Cytochrome B5 Reductase 1; DAMP: Damage-associated Molecular Pattern; DHODH: Dihydroorotate Dehydrogenase (Quinone); EMT: Epithelial-mesenchymal Transition; FDA: Food and Drug Administration; FMN: Flavin Mononucleotide; FPN1/SLC40A1: ferroportin 1/ solute carrier family 40 member 1; FSP1: ferroptosis suppressor protein 1; FXN: Frataxin; GCLC: Glutamate-Cysteine Ligase Catalytic Subunit; GPX4: Glutathione Peroxidase 4; GSH: Glutathione; HIFs: Hypoxia Inducible Factors; KRAS: KRAS proto-oncogene, GTPase; LIP: Labile Iron Pool; LPCAT3: Lysophosphatidylcholine Acyltransferase 3; LPO: Lipid peroxidation; NIR: Near infrared radiation; NRF2: NFE2-Related Factor 2; NTBI: non-transferrin-bound iron; PD-L1 (CD274): Programmed Death Ligand 1; PCD: Programmed cell death; POR: Cytochrome P450 Oxidoreductase; PUFA/MUFA: polyunsaturated fatty acid/ monounsaturated fatty acids; ROS: Reactive Oxygen Species; SCARA5: Scavenger Receptor Class A Member 5; SLC11A2: Solute Carrier Family 11 Member 2; SLC39A14: Solute Carrier Family 39 Member 14; SLC39A8: Solute Carrier Family 39 Member 8; SLC3A2: Solute Carrier Family 3 Member 2; SLC7A11: Solute Carrier Family 7 Member 11; TfR1: Transferrin Receptor 1; TME: Tumor microenvironment.
Acknowledgements
Funding
This project was supported by Administration of Traditional Chinese Medicine of Guangdong Province (20201180); Administration of Traditional Chinese Medicine of Guangdong Province (20211223); Science and Technology Special Project of Zhanjiang (2019A01009); Basic and Applied Basic Research Program of Guangdong Province (2019A1515110201); Program of Department of Natural Resources of Guangdong Province (GDNRC [2020]038 and [2021]53); Discipline Construction Project of Guangdong Medical University (4SG21004G); Innovation and Entrepreneurship Team Leads the Pilot Program of Zhanjing (2020LHJH005).
Author Contributions
LL Luo conceived and designed the review. H Wang, LX Luo, XL Li wrote the manuscript; W Tian helped with the drawing; Z Zhu, H Luo and RM Huang reviewed the paper and provided comments. All authors contributed to this article and approved the submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-72
2. Conrad M, Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol. 2019;15:1137-47
3. Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Research. 2020;30:146-62
4. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-82
5. Xu G, Wang H, Li X, Huang R, Luo L. Recent progress on targeting ferroptosis for cancer therapy. Biochem Pharmacol. 2021: 114584.
6. Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-31
7. Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K. et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018;172:409-22 e21
8. Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochimica Et Biophysica Acta-General Subjects. 2013;1830:3289-303
9. Harris IS, DeNicola GM. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020;30:440-51
10. Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ. et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273-85
11. Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. Journal of Biological Chemistry. 1999;274:11455-8
12. Jiang L, Kon N, Li T, Wang S-J, Su T, Hibshoosh H. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57 -+
13. Chen D, Tavana O, Chu B, Erber L, Chen Y, Baer R. et al. NRF2 Is a Major Target of ARF in p53-Independent Tumor Suppression. Mol Cell. 2017;68:224-32 e4
14. Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693-8
15. Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688 -+
16. Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586-90
17. Lachaier E, Louandre C, Godin C, Saidak Z, Baert M, Diouf M. et al. Sorafenib Induces Ferroptosis in Human Cancer Cell Lines Originating from Different Solid Tumors. Anticancer Research. 2014;34:6417-22
18. Chen L, Li X, Liu L, Yu B, Xue Y, Liu Y. Erastin sensitizes glioblastoma cells to temozolomide by restraining xCT and cystathionine-gamma-lyase function. Oncology Reports. 2015;33:1465-74
19. Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Molecular Cell. 2015;59:298-308
20. Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P. et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726-39
21. Feng H, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM. et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020;30:3411-23 e7
22. Tan Y, Liu W, Zhu Z, Lang L, Wang J, Huang M. et al. Selection and identification of transferrin receptor-specific peptides as recognition probes for cancer cells. Anal Bioanal Chem. 2018;410:1071-7
23. Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174-94
24. Knutson MD. Non-transferrin-bound iron transporters. Free Radic Biol Med. 2019;133:101-11
25. Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105 -+
26. Adedoyin O, Boddu R, Traylor A, Lever JM, Bolisetty S, George JF. et al. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am J Physiol Renal Physiol. 2018;314:F702-F14
27. Du J, Zhou Y, Li Y, Xia J, Chen Y, Chen S. et al. Identification of Frataxin as a regulator of ferroptosis. Redox Biol. 2020;32:101483
28. Ma S, Henson ES, Chen Y, Gibson SB. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307
29. Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ. et al. Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev Cell. 2019;51:575-86 e4
30. Chu B, Kon N, Chen D, Li T, Liu T, Jiang L. et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nature Cell Biology. 2019;21:579 -+
31. Yang WH, Huang Z, Wu J, Ding CC, Murphy SK, Chi JT. A TAZ-ANGPTL4-NOX2 Axis Regulates Ferroptotic Cell Death and Chemoresistance in Epithelial Ovarian Cancer. Mol Cancer Res. 2020;18:79-90
32. Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J. et al. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol Cell. 2021;81:355-69.e10
33. Magtanong L, Ko P-J, To M, Cao JY, Forcina GC, Tarangelo A. et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chemical Biology. 2019;26:420 -+
34. Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P. et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585:603-8
35. Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, IngoId I. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature Chemical Biology. 2017;13:91-8
36. Wenz C, Faust D, Linz B, Turmann C, Nikolova T, Dietrich C. Cell-cell contacts protect against t-BuOOH-induced cellular damage and ferroptosis in vitro. Arch Toxicol. 2019;93:1265-79
37. Ma S, Henson ES, Chen Y, Gibson SB. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307
38. Xia Y, Cai XY, Fan JQ, Zhang LL, Ren JH, Chen J. et al. Rho Kinase Inhibitor Fasudil Suppresses the Vasculogenic Mimicry of B16 Mouse Melanoma Cells Both In Vitro and In Vivo. Molecular cancer therapeutics. 2015;14:1582-90
39. Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM. et al. Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chemical Biology. 2019;26:623 -+
40. Garg AD, Agostinis P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126-48
41. Shen W, Liu W, Yang H, Zhang P, Xiao C, Chen X. A glutathione-responsive sulfur dioxide polymer prodrug as a nanocarrier for combating drug-resistance in cancer chemotherapy. Biomaterials. 2018;178:706-19
42. Zhang Y, Shen W, Zhang P, Chen L, Xiao C. GSH-triggered release of sulfur dioxide gas to regulate redox balance for enhanced photodynamic therapy. Chem Commun (Camb). 2020;56:5645-8
43. Zhang P, Zhang Y, Ding X, Shen W, Li M, Wagner E. et al. A Multistage Cooperative Nanoplatform Enables Intracellular Co-Delivery of Proteins and Chemotherapeutics for Cancer Therapy. Adv Mater. 2020;32:e2000013
44. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657-74
45. Xu T, Ma Y, Yuan Q, Hu H, Hu X, Qian Z. et al. Enhanced Ferroptosis by Oxygen-Boosted Phototherapy Based on a 2-in-1 Nanoplatform of Ferrous Hemoglobin for Tumor Synergistic Therapy. ACS Nano. 2020;14:3414-25
46. Zou J, Zhu J, Yang Z, Li L, Fan W, He L. et al. A Phototheranostic Strategy to Continuously Deliver Singlet Oxygen in the Dark and Hypoxic Tumor Microenvironment. Angew Chem Int Ed Engl. 2020;59:8833-8
47. Guo Z, Zhu S, Yong Y, Zhang X, Dong X, Du J. et al. Synthesis of BSA-Coated BiOI@Bi2 S3 Semiconductor Heterojunction Nanoparticles and Their Applications for Radio/Photodynamic/Photothermal Synergistic Therapy of Tumor. Adv Mater. 2017 29
48. Zhang H, Hou L, Jiao X, Ji Y, Zhu X, Zhang Z. Transferrin-mediated fullerenes nanoparticles as Fe(2+)-dependent drug vehicles for synergistic anti-tumor efficacy. Biomaterials. 2015;37:353-66
49. Jutz G, van Rijn P, Santos Miranda B, Boker A. Ferritin: a versatile building block for bionanotechnology. Chem Rev. 2015;115:1653-701
50. Lei Y, Hamada Y, Li J, Cong L, Wang N, Li Y. et al. Targeted tumor delivery and controlled release of neuronal drugs with ferritin nanoparticles to regulate pancreatic cancer progression. J Control Release. 2016;232:131-42
51. Yang L, Wang H, Yang X, Wu Q, An P, Jin X. et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct Target Ther. 2020;5:138
52. Yang Y, Tian Q, Wu S, Li Y, Yang K, Yan Y. et al. Blue light-triggered Fe(2+)-release from monodispersed ferrihydrite nanoparticles for cancer iron therapy. Biomaterials. 2021;271:120739
53. Lin L, Wang S, Deng H, Yang W, Rao L, Tian R. et al. Endogenous Labile Iron Pool-Mediated Free Radical Generation for Cancer Chemodynamic Therapy. J Am Chem Soc. 2020;142:15320-30
54. Dierge E, Debock E, Guilbaud C, Corbet C, Mignolet E, Mignard L. et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 2021;33:1701-15.e5
55. Lei G, Zhang Y, Hong T, Zhang X, Liu X, Mao C. et al. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene. 2021;40:3533-47
56. Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L. et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019;9:1673-85
57. Chen PH, Wu J, Ding CC, Lin CC, Pan S, Bossa N. et al. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 2020;27:1008-22
58. Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146-62
59. Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS. et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem Biol. 2020;15:469-84
60. Wang H, Jiang H, Van De Gucht M, De Ridder M. Hypoxic Radioresistance: Can ROS Be the Key to Overcome It? Cancers (Basel). 2019 11
61. Padanad MS, Konstantinidou G, Venkateswaran N, Melegari M, Rindhe S, Mitsche M. et al. Fatty Acid Oxidation Mediated by Acyl-CoA Synthetase Long Chain 3 Is Required for Mutant KRAS Lung Tumorigenesis. Cell Rep. 2016;16:1614-28
62. Zhang C, Yan L, Wang X, Dong X, Zhou R, Gu Z. et al. Tumor Microenvironment-Responsive Cu2(OH)PO4 Nanocrystals for Selective and Controllable Radiosentization via the X-ray-Triggered Fenton-like Reaction. Nano Lett. 2019;19:1749-57
63. Wang W, Green M, Choi JE, Gijon M, Kennedy PD, Johnson JK. et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270-4
64. Li J, Wang H, Wang Y, Gong X, Xu X, Sha X. et al. Tumor-Activated Size-Enlargeable Bioinspired Lipoproteins Access Cancer Cells in Tumor to Elicit Anti-Tumor Immune Responses. Adv Mater. 2020;32:e2002380
65. Angeli JPF, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nature Reviews Cancer. 2019;19:405-14
66. Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li ZQ. et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021;28:1971-89
67. Jiang Q, Wang K, Zhang X, Ouyang B, Liu H, Pang Z. et al. Platelet Membrane-Camouflaged Magnetic Nanoparticles for Ferroptosis-Enhanced Cancer Immunotherapy. Small. 2020;16:e2001704
68. Hsieh CH, Hsieh HC, Shih FS, Wang PW, Yang LX, Shieh DB. et al. An innovative NRF2 nano-modulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment. Theranostics. 2021;11:7072-91
69. Song R, Li T, Ye J, Sun F, Hou B, Saeed M. et al. Acidity-Activatable Dynamic Nanoparticles Boosting Ferroptotic Cell Death for Immunotherapy of Cancer. Adv Mater. 2021: e2101155.
70. Drijvers JM, Gillis JE, Muijlwijk T, Nguyen TH, Gaudiano EF, Harris IS. et al. Pharmacologic Screening Identifies Metabolic Vulnerabilities of CD8(+) T Cells. Cancer Immunol Res. 2021;9:184-99
71. Ma X, Xiao L, Liu L, Ye L, Su P, Bi E. et al. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001-12 e5
72. Xu S, Chaudhary O, Rodriguez-Morales P, Sun X, Chen D, Zappasodi R. et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity. 2021;54:1561-77 e7
73. Xu C, Sun S, Johnson T, Qi R, Zhang S, Zhang J. et al. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021;35:109235
74. Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv Mater. 2018;30:e1704007
75. Liang C, Zhang X, Yang M, Dong X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv Mater. 2019;31:e1904197
76. Zheng H, Jiang J, Xu S, Liu W, Xie Q, Cai X. et al. Nanoparticle-induced ferroptosis: detection methods, mechanisms and applications. Nanoscale. 2021;13:2266-85
77. Ma Pa, Xiao H, Yu C, Liu J, Cheng Z, Song H. et al. Enhanced Cisplatin Chemotherapy by Iron Oxide Nanocarrier-Mediated Generation of Highly Toxic Reactive Oxygen Species. Nano Letters. 2017;17:928-37
78. Zhou Z, Song J, Tian R, Yang Z, Yu G, Lin L. et al. Activatable Singlet Oxygen Generation from Lipid Hydroperoxide Nanoparticles for Cancer Therapy. Angew Chem Int Ed Engl. 2017;56:6492-6
79. Li WP, Su CH, Chang YC, Lin YJ, Yeh CS. Ultrasound-Induced Reactive Oxygen Species Mediated Therapy and Imaging Using a Fenton Reaction Activable Polymersome. ACS Nano. 2016;10:2017-27
80. Cioloboc D, Kennedy C, Boice EN, Clark ER, Kurtz DM Jr. Trojan Horse for Light-Triggered Bifurcated Production of Singlet Oxygen and Fenton-Reactive Iron within Cancer Cells. Biomacromolecules. 2018;19:178-87
81. Zheng DW, Lei Q, Zhu JY, Fan JX, Li CX, Li C. et al. Switching Apoptosis to Ferroptosis: Metal-Organic Network for High-Efficiency Anticancer Therapy. Nano Lett. 2017;17:284-91
82. Wang D, Zhou J, Chen R, Shi R, Wang C, Lu J. et al. Core-Shell Metal-Organic Frameworks as Fe2+ Suppliers for Fe2+-Mediated Cancer Therapy under Multimodality Imaging. Chemistry of Materials. 2017;29:3477-89
83. Herbison CE, Thorstensen K, Chua AC, Graham RM, Leedman P, Olynyk JK. et al. The role of transferrin receptor 1 and 2 in transferrin-bound iron uptake in human hepatoma cells. Am J Physiol Cell Physiol. 2009;297:C1567-75
84. Gao M, Deng J, Liu F, Fan A, Wang Y, Wu H. et al. Triggered ferroptotic polymer micelles for reversing multidrug resistance to chemotherapy. Biomaterials. 2019;223:119486
85. Meng X, Deng J, Liu F, Guo T, Liu M, Dai P. et al. Triggered All-Active Metal Organic Framework: Ferroptosis Machinery Contributes to the Apoptotic Photodynamic Antitumor Therapy. Nano Lett. 2019;19:7866-76
86. Ou W, Mulik RS, Anwar A, McDonald JG, He X, Corbin IR. Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic Biol Med. 2017;112:597-607
87. Zhang C, Bu W, Ni D, Zhang S, Li Q, Yao Z. et al. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew Chem Int Ed Engl. 2016;55:2101-6
88. Yue L, Wang J, Dai Z, Hu Z, Chen X, Qi Y. et al. pH-Responsive, Self-Sacrificial Nanotheranostic Agent for Potential In Vivo and In Vitro Dual Modal MRI/CT Imaging, Real-Time, and In Situ Monitoring of Cancer Therapy. Bioconjug Chem. 2017;28:400-9
89. Chen J, Wang X, Zhang Y, Zhang S, Liu H, Zhang J. et al. A redox-triggered C-centered free radicals nanogenerator for self-enhanced magnetic resonance imaging and chemodynamic therapy. Biomaterials. 2021;266:120457
90. Yao X, Yang P, Jin Z, Jiang Q, Guo R, Xie R. et al. Multifunctional nanoplatform for photoacoustic imaging-guided combined therapy enhanced by CO induced ferroptosis. Biomaterials. 2019;197:268-83
91. Bao W, Liu X, Lv Y, Lu GH, Li F, Zhang F. et al. Nanolongan with Multiple On-Demand Conversions for Ferroptosis-Apoptosis Combined Anticancer Therapy. ACS Nano. 2019;13:260-73
92. Shen Z, Liu T, Li Y, Lau J, Yang Z, Fan W. et al. Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. Acs Nano. 2018;12:11355-65
93. He S, Jiang Y, Li J, Pu K. Semiconducting Polycomplex Nanoparticles for Photothermal Ferrotherapy of Cancer. Angew Chem Int Ed Engl. 2020;59:10633-8
94. Zhang D, Cui P, Dai Z, Yang B, Yao X, Liu Q. et al. Tumor microenvironment responsive FePt/MoS2 nanocomposites with chemotherapy and photothermal therapy for enhancing cancer immunotherapy. Nanoscale. 2019;11:19912-22
95. Liang H, Guo J, Shi Y, Zhao G, Sun S, Sun X. Porous yolk-shell Fe/Fe3O4 nanoparticles with controlled exposure of highly active Fe(0) for cancer therapy. Biomaterials. 2021;268:120530
96. Sang M, Luo R, Bai Y, Dou J, Zhang Z, Liu F. et al. Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics. 2019;9:6209-23
97. Liu T, Liu W, Zhang M, Yu W, Gao F, Li C. et al. Ferrous-Supply-Regeneration Nanoengineering for Cancer-Cell-Specific Ferroptosis in Combination with Imaging-Guided Photodynamic Therapy. Acs Nano. 2018;12:12181-92
98. Xu Q, Zhan G, Zhang Z, Yong T, Yang X, Gan L. Manganese porphyrin-based metal-organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors. Theranostics. 2021;11:1937-52
99. Xie S, Sun W, Zhang C, Dong B, Yang J, Hou M. et al. Metabolic Control by Heat Stress Determining Cell Fate to Ferroptosis for Effective Cancer Therapy. ACS Nano. 2021;15:7179-94
100. Mu M, Wang Y, Zhao S, Li X, Fan R, Mei L. et al. Engineering a pH/Glutathione-Responsive Tea Polyphenol Nanodevice as an Apoptosis/Ferroptosis-Inducing Agent. ACS Applied Bio Materials. 2020;3:4128-38
101. Liang H, Wu X, Zhao G, Feng K, Ni K, Sun X. Renal Clearable Ultrasmall Single-Crystal Fe Nanoparticles for Highly Selective and Effective Ferroptosis Therapy and Immunotherapy. J Am Chem Soc. 2021;143:15812-23
102. Du C, Zhou L, Qian J, He M, Zhang ZG, Feng C. et al. Ultrasmall Zwitterionic Polypeptide-Coordinated Nanohybrids for Highly Efficient Cancer Photothermal Ferrotherapy. ACS Appl Mater Interfaces. 2021;13:44002-12
103. Cui X, Lu G, Fang F, Xiong Y, Tian S, Wan Y. et al. Iron Self-Boosting Polymer Nanoenzyme for Low-Temperature Photothermal-Enhanced Ferrotherapy. ACS Appl Mater Interfaces. 2021;13:30274-83
104. Fu S, Yang R, Ren J, Liu J, Zhang L, Xu Z. et al. Catalytically Active CoFe2O4 Nanoflowers for Augmented Sonodynamic and Chemodynamic Combination Therapy with Elicitation of Robust Immune Response. ACS Nano. 2021;15(7):11953-11969
105. Wang X, Wu M, Zhang X, Li F, Zeng Y, Lin X. et al. Hypoxia-responsive nanoreactors based on self-enhanced photodynamic sensitization and triggered ferroptosis for cancer synergistic therapy. J Nanobiotechnology. 2021;19:204
106. Zhang C, Liu Z, Zhang Y, Ma L, Song E, Song Y. "Iron free" zinc oxide nanoparticles with ion-leaking properties disrupt intracellular ROS and iron homeostasis to induce ferroptosis. Cell Death Dis. 2020;11:183
107. Fu J, Li T, Yang Y, Jiang L, Wang W, Fu L. et al. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials. 2021;268:120537
108. Luo Y, Niu G, Yi H, Li Q, Wu Z, Wang J. et al. Nanomedicine promotes ferroptosis to inhibit tumour proliferation in vivo. Redox Biol. 2021;42:101908
109. Zhu T, Shi L, Yu C, Dong Y, Qiu F, Shen L. et al. Ferroptosis Promotes Photodynamic Therapy: Supramolecular Photosensitizer-Inducer Nanodrug for Enhanced Cancer Treatment. Theranostics. 2019;9:3293-307
110. Tang H, Li C, Zhang Y, Zheng H, Cheng Y, Zhu J. et al. Targeted Manganese doped silica nano GSH-cleaner for treatment of Liver Cancer by destroying the intracellular redox homeostasis. Theranostics. 2020;10:9865-87
111. Cheng J, Zhu Y, Xing X, Xiao J, Chen H, Zhang H. et al. Manganese-deposited iron oxide promotes tumor-responsive ferroptosis that synergizes the apoptosis of cisplatin. Theranostics. 2021;11:5418-29
112. Ou C, Na W, Ge W, Huang H, Gao F, Zhong L. et al. Biodegradable Charge-Transfer Complexes for Glutathione Depletion Induced Ferroptosis and NIR-II Photoacoustic Imaging Guided Cancer Photothermal Therapy. Angew Chem Int Ed Engl. 2021;60:8157-63
113. Zhou LL, Guan Q, Li WY, Zhang Z, Li YA, Dong YB. A Ferrocene-Functionalized Covalent Organic Framework for Enhancing Chemodynamic Therapy via Redox Dyshomeostasis. Small. 2021: e2101368.
114. An P, Gao Z, Sun K, Gu D, Wu H, You C. et al. Photothermal-Enhanced Inactivation of Glutathione Peroxidase for Ferroptosis Sensitized by an Autophagy Promotor. ACS Appl Mater Interfaces. 2019;11:42988-97
115. Luo Y, Yan P, Li X, Hou J, Wang Y, Zhou S. pH-Sensitive Polymeric Vesicles for GOx/BSO Delivery and Synergetic Starvation-Ferroptosis Therapy of Tumor. Biomacromolecules. 2021
116. Li Y, Chen W, Qi Y, Wang S, Li L, Li W. et al. H2 S-Scavenged and Activated Iron Oxide-Hydroxide Nanospindles for MRI-Guided Photothermal Therapy and Ferroptosis in Colon Cancer. Small. 2020;16:e2001356
117. Wang X, Zhao Y, Hu Y, Fei Y, Zhao Y, Xue C. et al. Activatable Biomineralized Nanoplatform Remodels the Intracellular Environment of Multidrug-Resistant Tumors for Enhanced Ferroptosis/Apoptosis Therapy. Small. 2021: e2102269.
118. Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2-25
119. Park J, Choi Y, Chang H, Um W, Ryu JH, Kwon IC. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics. 2019;9:8073-90
120. Jiang M, Mu J, Jacobson O, Wang Z, He L, Zhang F. et al. Reactive Oxygen Species Activatable Heterodimeric Prodrug as Tumor-Selective Nanotheranostics. ACS Nano. 2020;14(12):16875-16886
121. Zeng L, Zou L, Yu H, He X, Cao H, Zhang Z. et al. Treatment of Malignant Brain Tumor by Tumor-Triggered Programmed Wormlike Micelles with Precise Targeting and Deep Penetration. Advanced Functional Materials. 2016;26:4201-12
122. Fujisaku T, Tanabe R, Onoda S, Kubota R, Segawa TF, So FT. et al. pH Nanosensor Using Electronic Spins in Diamond. ACS Nano. 2019;13:11726-32
123. Kang T, Ni JS, Li T, Wang J, Li Z, Li Y. et al. Efficient and precise delivery of microRNA by photoacoustic force generated from semiconducting polymer-based nanocarriers. Biomaterials. 2021;275:120907
124. Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv H. et al. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127-36
125. Xu S, Min J, Wang F. Ferroptosis: an emerging player in immune cells. Science Bulletin. 2021;22:2257-2260
Author contact
![]() Corresponding author: Lianxiang Luo, Email: luolianxiang321edu.cn
Corresponding author: Lianxiang Luo, Email: luolianxiang321edu.cn
 Global reach, higher impact
Global reach, higher impact