13.3
Impact Factor
Theranostics 2021; 11(20):9953-9966. doi:10.7150/thno.63743 This issue Cite
Research Paper
BPI overexpression suppresses Treg differentiation and induces exosome-mediated inflammation in systemic lupus erythematosus
1. Immunology Research Center, National Health Research Institutes, Zhunan, Taiwan.
2. Division of Allergy, Immunology, and Rheumatology, Taipei Veterans General Hospital, Taipei, Taiwan.
3. Division of Allergy, Immunology, and Rheumatology, Taichung Veterans General Hospital, Taichung, Taiwan.
4. Department of Medicine, Chang Gung Memorial Hospital, Taoyuan, Taiwan.
5. Department of Pathology & Immunology, Baylor College of Medicine, Houston, Texas, USA.
#These authors contributed equally to this work.
Abstract
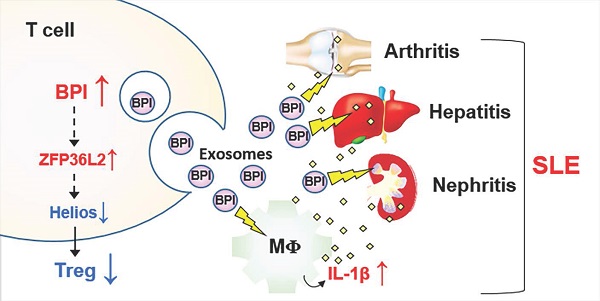
Background: Serum-derived exosomes are correlated with disease severity of human systemic lupus erythematosus (SLE). The proteins in the T-cell-derived exosomes from SLE patients could contribute to inflammation.
Methods: We characterized proteins of T cell-derived exosomes from SLE patients and healthy controls by proteomics. To study the potential pathogenic role of the identified exosomal protein, we generated and characterized T-cell-specific transgenic mice that overexpressed the identified protein in T cells using immunohistochemistry, immunoblotting, and single-cell RNA sequencing.
Results: We identified an overexpressed protein, bactericidal/permeability-increasing protein (BPI), in SLE T cells and T-cell-derived exosomes. T-cell-specific BPI transgenic (Lck-BPI Tg) mice showed multi-tissue inflammation with early induction of serum IL-1β levels, as well as serum triglyceride and creatinine levels. Interestingly, exosomes of Lck-BPI Tg T cells stimulated IL-1β expression of wild-type recipient macrophages. Remarkably, adoptive transfer of BPI-containing exosomes increased serum IL-1β and autoantibody levels in recipient mice. The transferred exosomes infiltrated into multiple tissues of recipient mice, resulting in hepatitis, nephritis, and arthritis. ScRNA-seq showed that Lck-BPI Tg T cells displayed a decrease of Treg population, which was concomitant with ZFP36L2 upregulation and Helios downregulation. Furthermore, in vitro Treg differentiation was reduced by BPI transgene and enhanced by BPI knockout.
Conclusions: BPI is a negative regulator of Treg differentiation. BPI overexpression in T-cell-derived exosomes or peripheral blood T cells may be a biomarker and pathogenic factor for human SLE nephritis, hepatitis, and arthritis.
Keywords: BPI, SLE, exosomes, Treg, T cells
 Global reach, higher impact
Global reach, higher impact