13.3
Impact Factor
Theranostics 2022; 12(1):277-289. doi:10.7150/thno.63441 This issue Cite
Research Paper
METTL3-mediated N6-methyladenosine modification governs pericyte dysfunction during diabetes-induced retinal vascular complication
1. Eye Institute, Eye & ENT Hospital, Shanghai Medical College, Fudan University, Shanghai, China.
2. The Affiliated Eye Hospital, Nanjing Medical University, Nanjing, China.
3. The Fourth School of Clinical Medicine, Nanjing Medical University, Nanjing, China.
4. NHC Key Laboratory of Myopia (Fudan University), Key Laboratory of Myopia, Chinese Academy of Medical Sciences, and Shanghai Key Laboratory of Visual Impairment and Restoration (Fudan University), Shanghai, China.
*These authors contributed equally to this work.
Abstract
Rationale: Microvascular complication is a major cause of morbidity and mortality among the patients with diabetes. Pericyte dysfunction is the predominant pathological manifestation of microvascular complication. N6-methyladenosine (m6A) serves as the most prevalent modification in eukaryotic mRNAs. However, the role of m6A RNA modification in pericyte dysfunction is still unclear.
Methods: Quantitative polymerase chain reactions and western blots were conducted to detect the change of m6A RNA modification in pericytes and mouse retinas following diabetic stress. MTT assay, transwell migration assay, caspase 3/7 activity assay, calcein-AM/propidium iodide (PI) staining, and TUNEL staining were conducted to determine the role of METTL3 in pericyte biology in vitro. Retinal trypsin digestion, vascular permeability assay, and IB4-NG2 double immunofluorescent staining were conducted to determine the role of METTL3 in retinal pericyte dysfunction and vascular complication. RNA sequencing, RNA pull-down assays and immunoblots were conducted to clarify the mechanism of METTL3-mediated pericyte dysfunction and vascular complication.
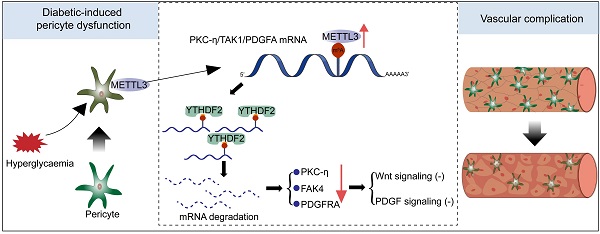
Results: The levels of m6A RNA methylation were significantly up-regulated in pericytes and mouse retinas following diabetic stress, which were caused by increased expression of METTL3. METTL3 regulated the viability, proliferation, and differentiation of pericytes in vitro. Specific depletion of METTL3 in pericytes suppressed diabetes-induced pericyte dysfunction and vascular complication in vivo. METTL3 overexpression impaired pericyte function by repressing PKC-η, FAT4, and PDGFRA expression, which was mediated by YTHDF2-dependent mRNA decay.
Conclusion: METTL3-mediated m6A methylation epigenetically regulates diabetes-induced pericyte dysfunction. METTL3-YTHDF2-PKC-η/FAT4/PDGFRA signaling axis could be therapeutically targeted for treating microvascular complications.
Keywords: Microvascular complication, Diabetic retinopathy, m6A methylation, Pericyte dysfunction
 Global reach, higher impact
Global reach, higher impact