13.3
Impact Factor
Theranostics 2022; 12(1):340-361. doi:10.7150/thno.65522 This issue Cite
Research Paper
Targeting protumor factor chitinase-3-like-1 secreted by Rab37 vesicles for cancer immunotherapy
1. Department of Pharmacology, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
2. Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
3. Department of Clinical Medical Research, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
4. Department of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
5. Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
6. Colorectal Division, Department of Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
7. Division of Oncology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
8. Division of General Surgery, Department of Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
* PSY, MHY, and YCH contributed equally to this work.
Abstract
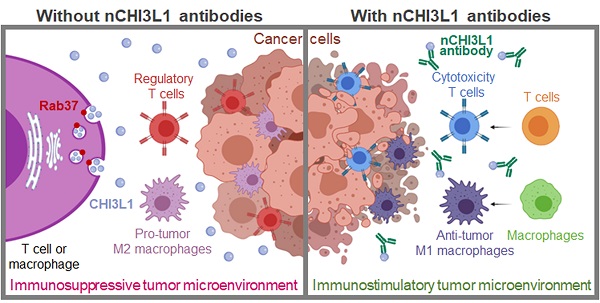
Background: Chitinase 3-like-1 (CHI3L1) is a secretion glycoprotein associated with the immunosuppressive tumor microenvironment (TME). The secretory mode of CHI3L1 makes it a promising target for cancer treatment. We have previously reported that Rab37 small GTPase mediates secretion of IL-6 in macrophages to promote cancer progression, whereas the roles of Rab37 in the intracellular trafficking and exocytosis of CHI3L1 are unclear.
Methods: We examined the concentration of CHI3L1 in the culture medium of splenocytes and bone marrow derived macrophages (BMDMs) from wild-type or Rab37 knockout mice, and macrophage or T cell lines expressing wild type, active GTP-bound or inactive GDP-bound Rab37. Vesicle isolation, total internal reflection fluorescence microscopy, and real-time confocal microscopy were conducted. We developed polyclonal neutralizing-CHI3L1 antibodies (nCHI3L1 Abs) to validate the therapeutic efficacy in orthotopic lung, pancreas and colon cancer allograft models. Multiplex fluorescence immunohistochemistry was performed to detect the protein level of Rab37 and CHI3L1, and localization of the tumor-infiltrating immune cells in allografts from mice or tumor specimens from cancer patients.
Results: We demonstrate a novel secretion mode of CHI3L1 mediated by the small GTPase Rab37 in T cells and macrophages. Rab37 mediated CHI3L1 intracellular vesicle trafficking and exocytosis in a GTP-dependent manner, which is abolished in the splenocytes and BMDMs from Rab37 knockout mice and attenuated in macrophage or T cell lines expressing the inactive Rab37. The secreted CHI3L1 activated AKT, ß-catenin and NF-κB signal pathways in cancer cells and macrophages to foster a protumor TME characterized by activating M2 macrophages and increasing the population of regulatory T cells. Our developed nCHI3L1 Abs showed the dual properties of reducing tumor growth/metastases and eliciting an immunostimulatory TME in syngeneic orthotopic lung, pancreas and colon tumor models. Clinically, high plasma level or intratumoral expression of CHI3L1 correlated with poor survival in 161 lung cancer, 155 pancreatic cancer and 180 colon cancer patients.
Conclusions: These results provide the first evidence that Rab37 mediates CHI3L1 secretion in immune cells and highlight nCHI3L1 Abs that can simultaneously target both cancer cells and tumor microenvironment.
Keywords: chitinase 3-like-1, Rab37, exocytosis, neutralizing antibody, tumor microenvironment
 Global reach, higher impact
Global reach, higher impact