13.3
Impact Factor
Theranostics 2022; 12(1):362-378. doi:10.7150/thno.65597 This issue Cite
Research Paper
Sonobiopsy for minimally invasive, spatiotemporally-controlled, and sensitive detection of glioblastoma-derived circulating tumor DNA
1. Department of Biomedical Engineering, Washington University in St. Louis, Saint Louis, MO 63130, USA.
2. Mallinckrodt Institute of Radiology, Washington University School of Medicine, Saint Louis, MO, 63110, USA.
3. Department of Neurosurgery, Washington University School of Medicine, St. Louis, MO, 63110, USA.
4. Andrew M. and Jane M. Bursky Center for Human Immunology and Immunotherapy Programs, Washington University School of Medicine, St. Louis, MO, 63110, USA.
5. Department of Radiation Oncology, Washington University School of Medicine, Saint Louis, MO 63108, USA.
6. Department of Pharmacology and Regenerative Medicine, University of Illinois at Chicago, Chicago, IL, 60612, USA.
7. University of Illinois Cancer Center, Chicago, IL, 60612, USA.
8. Division of Comparative Medicine, Washington University School of Medicine, Saint Louis, MO, 63110, USA.
9. Department of Genetics, Washington University School of Medicine, St. Louis, MO, 63110, USA.
10. Department of Computer Science and Engineering, Washington University in St. Louis, Saint Louis, MO 63130, USA.
11. Siteman Cancer Center, Washington University School of Medicine, St. Louis, MO, 63110, USA.
12. Department of Neuroscience, Washington University School of Medicine, Saint Louis, MO, 63110, USA.
13. Center for Innovation in Neuroscience and Technology, Washington University School of Medicine, Saint Louis, MO, 63110, USA.
# These two authors contributed equally to this study.
Abstract
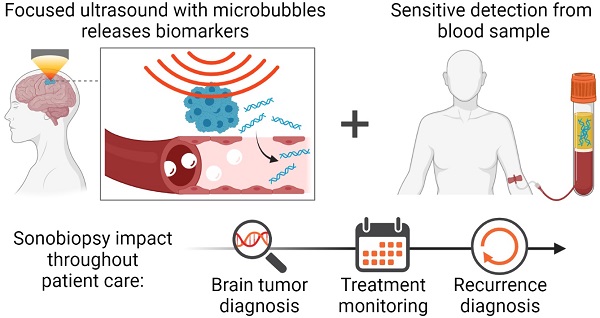
Though surgical biopsies provide direct access to tissue for genomic characterization of brain cancer, they are invasive and pose significant clinical risks. Brain cancer management via blood-based liquid biopsies is a minimally invasive alternative; however, the blood-brain barrier (BBB) restricts the release of brain tumor-derived molecular biomarkers necessary for sensitive diagnosis.
Methods: A mouse glioblastoma multiforme (GBM) model was used to demonstrate the capability of focused ultrasound (FUS)-enabled liquid biopsy (sonobiopsy) to improve the diagnostic sensitivity of brain tumor-specific genetic mutations compared with conventional blood-based liquid biopsy. Furthermore, a pig GBM model was developed to characterize the translational implications of sonobiopsy in humans. Magnetic resonance imaging (MRI)-guided FUS sonication was performed in mice and pigs to locally enhance the BBB permeability of the GBM tumor. Contrast-enhanced T1-weighted MR images were acquired to evaluate the BBB permeability change. Blood was collected immediately after FUS sonication. Droplet digital PCR was used to quantify the levels of brain tumor-specific genetic mutations in the circulating tumor DNA (ctDNA). Histological staining was performed to evaluate the potential for off-target tissue damage by sonobiopsy.
Results: Sonobiopsy improved the detection sensitivity of EGFRvIII from 7.14% to 64.71% and TERT C228T from 14.29% to 45.83% in the mouse GBM model. It also improved the diagnostic sensitivity of EGFRvIII from 28.57% to 100% and TERT C228T from 42.86% to 71.43% in the porcine GBM model.
Conclusion: Sonobiopsy disrupts the BBB at the spatially-targeted brain location, releases tumor-derived DNA into the blood circulation, and enables timely collection of ctDNA. Converging evidence from both mouse and pig GBM models strongly supports the clinical translation of sonobiopsy for the minimally invasive, spatiotemporally-controlled, and sensitive molecular characterization of brain cancer.
Keywords: Image-guided focused ultrasound, blood-brain barrier, blood-based liquid biopsy, glioblastoma mutation, droplet digital PCR
 Global reach, higher impact
Global reach, higher impact