13.3
Impact Factor
Theranostics 2022; 12(5):2063-2079. doi:10.7150/thno.69198 This issue Cite
Research Paper
Heterogeneity of tyrosine-based melanin anabolism regulates pulmonary and cerebral organotropic colonization microenvironment of melanoma cells
1. The Third Affiliated Hospital of Soochow University and State Key Laboratory of Radiation Medicine and Protection, Institutes for Translational Medicine, Soochow University, 199 Renai Road, Suzhou, Jiangsu 215123, China.
2. CAS Key Laboratory of Tissue Microenvironment and Tumor, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 320 Yueyang Road, Shanghai 200031, China.
3. Fujian Provincial Key Laboratory of Translational Cancer Medicine, Cancer Bio-immunotherapy Center, Fujian Medical University Cancer Hospital, 420 Fuma Road, Fuzhou 350014, China.
4. Shanghai Jiao Tong University School of Medicine, Shanghai Center for Systems Biomedicine Research, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China.
5. Department of Experimental Medicine, TOR, University of Rome Tor Vergata, Rome 00133, Italy.
Abstract
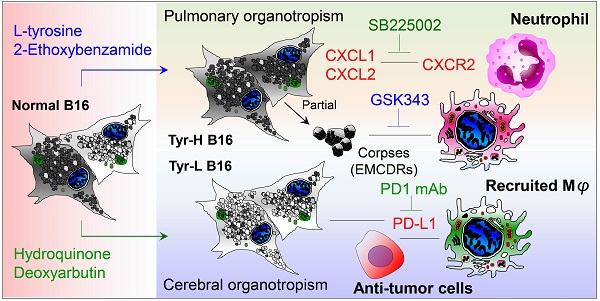
Background: Dietary tyrosine regulating melanoma progression has been well-recognized. However, whether tyrosine-based melanin anabolism contributes to pulmonary and cerebral organotropic colonization of melanoma remains elusive. Furthermore, approaches based on targeting tyrosinase activity to inhibiting multi-organ metastasis of melanoma cells need to be designed and validated.
Methods: Patients derived melanoma cells and mouse B16 melanoma cells with different pigmentation were employed in this investigation. Tyrosine content dynamics in tumors and multiple organs during the melanoma progression was monitored, and tyrosine-based melanin synthesis of melanoma cells derived from multi-organ was determined. Additionally, we also adopted RNA-seq, flow cytometry, real-time PCR and composite metastasis mouse model to analyze organotropic colonization and to validate designed therapeutic strategies.
Results: B16 melanoma cells with high activity of tyrosinase and sensitivity of tyrosine utilization for melanin synthesis (Tyr-H cells) easily colonized in the lung, while B16 melanoma cells lacking above characteristics (Tyr-L cells) exhibited potent proliferation in the brain. Mechanistically, Tyr-H cells recruited and trained neutrophils and macrophages to establish pulmonary metastatic niche dependent on highly secreted CXCL1 and CXCL2 and an excessive melanosome accumulation-induced cell death. Tyr-L cells enhanced PD-L1 expression in tumor-infiltrated macrophages when they are progressing in the brain. Accordingly, intervention of tyrosinase activity (2-Ethoxybenzamide or hydroquinone) in combination with inhibitors of phagocytosis (GSK343) or chemotaxis (SB225002) suppressed organotropic colonization and significantly improved the survival of melanoma- bearing mice treated with immune checkpoint blockade (PD1 antibody).
Conclusions: The heterogeneity of melanoma cells in utilization of tyrosine is associated with organotropic colonization, providing the basis for developing new strategies to combat melanoma.
Keywords: Melanoma, organotropic colonization, tumor heterogeneity, melanin anabolism, immune checkpoint
 Global reach, higher impact
Global reach, higher impact