13.3
Impact Factor
Theranostics 2022; 12(5):2383-2405. doi:10.7150/thno.67296 This issue Cite
Review
Recent advances in nanotechnology for eradicating bacterial biofilm
1. Université de Paris, CNRS, ITODYS, F-75013 Paris, France.
2. Centro de Investigación Cooperativa en Biomateriales (CIC BiomaGUNE), 20009 Donostia-San Sebastián, Guipúzcoa, Spain.
3. Université de Paris, CNRS, Laboratoire de Chimie et Biochimie Pharmacologiques et Toxicologiques, F-75006 Paris, France.
4. Université Paris-Saclay, INRAE, AgroParisTech, Micalis Institute, Jouy-en-Josas, France.
Received 2021-9-20; Accepted 2021-12-1; Published 2022-2-28
Abstract
Microorganisms grouped together into spatially-organized communities called biofilms, are the cause of dramatic chronic infections in plants, animals and humans. In this review, the characteristics of biofilms and their interactions with antimicrobials are first described. Limitations of antibiotic treatments are discussed, and state-of-the-art alternative approaches based on the use of polymer, lipid, organic, inorganic and hybrid nanoparticles are presented, highlighting recent achievements in the application of nanomaterials to the field of theranostics for the eradication of biofilm. The aim of this review is to present a complete vision of nanobiotechnology-based approaches for eradicating bacterial biofilms and fighting antimicrobial tolerance.
Keywords: biofilms, bacteria, antibiotics, antimicrobials, nanotechnology, nanoparticles, therapy, diagnosis.
Introduction
The year 2020 was for the whole world the year of the Coronavirus, more precisely of the SARS-CoV-2 virus which causes an illness known as Covid-19. The on-going pandemic has highlighted the role of viruses as vectors of infectious diseases. The layman generally does not distinguish between a virus and a bacterium, even less the other classes of micro-organisms, but in the 1980s the general public, perhaps for the first time, learnt what a virus looks like, thanks to illustrations of the human immunodeficiency virus (HIV). The HIV is associated with sexual behaviour and intravenous drug use, whereas the latest coronavirus is for everybody. Other new viral diseases, notably SERS, MERS and Ebola, have tended to be geographically circumscribed, and the present coronavirus is by far the most “successful” in its ability to travel and to infect very large populations. However, this recent surge of viral diseases should not lead us to ignore the fact that many of the great pandemics, or plagues, of the past were caused by bacterial infections. Medieval plagues, such as the Black Death, which decimated populations over the greater part of Europe and Africa, were caused by a bacterium, Yersinia pestis. Cholera and typhus, which have been responsible for pandemics in recent years, are also bacterial diseases. Bacteria are everywhere, some benevolent, such as those in our digestive system, but many of them are responsible for chronic infections, and are becoming increasingly resistant to treatment. An imbalance in the microbiota can lead to many pathologies involving biofilms, the most important of which are listed in Table 1. Pathogenic bacteria lead to severe illnesses worldwide, thus increasing mortality in the short and long run.
Infections caused by bacteria highly resistant to antibiotics have been classified by the World Health Organization (WHO) among its top 10 research priorities. Globally, the number of deaths associated with antimicrobial resistance is estimated at 7 million per year. Mortality could rise to 10 million deaths per year in 2050 if effective therapies are not found [1]. Besides mortality, the spread of bacterial diseases is expected to increase the costs of health care to more than 1.5 billion euros / year in Europe [2].
Selection pressure has led to the development of certain phenotypes of bacteria, the variations of which give an advantage in survival and reproduction. Only resistant bacteria survive, the others being killed by antibiotics. ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) are bacteria capable of escaping most common antibiotics by the acquisition of antimicrobial-resistant genes [3]. This resistance results from the misuse of antibiotics, in particular broad-spectrum antibiotics, in the treatment of human diseases as well as in the agricultural sector.
Bacterial antibiotic resistance is a scourge that appeared with the introduction of the first antibiotics. Many antibiotics discovered in the 1940s, such as penicillin, introduced in 1941, encountered resistance shortly after their distribution to the general public. Resistance appears earlier and earlier, notably because antibiotics in clinical use focus on a limited number of biological targets. There is now a race between the production of new antibiotics and the appearance of bacterial resistance.
In addition to this phenomenon, a very common strategy used by bacteria for their survival is grouping together into spatially organized communities called biofilms. Biofilms are involved in more than 65% of nosocomial infections [4], related to implanted equipment such as joint prostheses and heart valves [4], and also associated with chronic infections such as those involved in cystic fibrosis [5]. Chronic wounds or ulcers have increased significantly during recent years in correlation with the increase of pathologies such as cancer, diabetes and obesity. Indeed, bacterial biofilm-associated infections are an important cause of death in victims of cystic fibrosis and viral infections. Thus, the complexity and severity of biofilm infections has urged researchers to study the molecular mechanisms involved in the formation and growth of biofilms, in order to identify the key steps that could be exploited to eradicate them.
Current control strategies for biofilms are dominated by chemical biocides and antiseptic solutions, which produce harmful by-products, and antibiotics with limited efficacy on resistant and slow-growing, persistent bacteria. Biofilms are hot-spots for the emergence of resistant mutants, genetic transfer of mobile elements and a source of antimicrobial tolerance. The advent of nanotechnology and especially nanomedicine has opened up new possibilities for diagnosis and treatment of many diseases. Due to their size and surface properties, nanomaterials have the potential to overcome physiological barriers. Nanoparticles (NPs) have been studied extensively in the last decade as drug carriers in many different fields, including oncology, immunotherapy, and neuroscience [6,7]. They also offer many possibilities for eradicating bacteria, especially if combined with active molecules [8].
Scientific and medical studies on antibacterial treatments for biofilms constitute a major research field of the utmost importance. This research has led to many treatments that are expected to enhance the activity of currently used antibiotics or inhibitors either present in their free state or incorporated into a formulation. In this review, we first present the specificities of microorganisms living as biofilms, followed by an overview of the strategies used to treat bacterial biofilm communities and their drawbacks. Alternative therapies, effective ways of delivering antibiotics into the biofilm, and means for localized antibacterial action at a surface will be discussed. Finally, the contribution of nanotechnology, new advances in the field, and developments of theranostics will be described.
Diseases and infections associated with biofilms
| Location | Bacteria | Consequences | Refs |
|---|---|---|---|
| Oral cavity | Streptococcus mutans, Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans, P. gingivalis, P. intermedia, E. corrodens, and oral spirochetes | Development of periodontal infections near the gums, with emergence of highly pathogenic biofilms that induce acute inflammatory reaction, leading to breakdown of periodontal tissues and possibly to loosening of teeth. Also, the structure of the tongue promotes the formation of a unique and complex bacterial biofilm, in which odor-producing periodontal pathogens are frequently found, resulting in halitosis. | [33,34] |
| Otitis media | Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis | Complex set of infectious and inflammatory conditions affecting middle ear | [35] |
| Musculoskeletal system | S. aureus, coagulase-negative staphylococci, Enterococcus spp. and Streptococcus spp. | Bacteria aggregate on dead bones (sequestering), or on implants leading to biofilm infections | [36] |
| Necrotizing fasciitis | Group A streptococci, but other bacteria can be the cause (especially those that grow in water, such as Vibrio spp.) | Necrotizing fasciitis: serious infection that causes death of skin tissues and those underneath (subcutaneous, muscles). Causes tissue necrosis, often requires amputations or major surgical operations. Can also lead to heart disease, systolic shock (blood pressures <90 mm Hg) and clostridial infections | [37] |
| Cystic fibrosis | P. aeruginosa and S. aureus | Also called mucoviscidosis, affecting the lungs, kidneys, and digestive system. If severe form develops, lung transplant may be only solution. Build-up of mucus in lungs causes lung infections. Most common symptoms are: persistent cough with thick discharge from mucus, inflamed nasal passages, shortness of breath, wheezing and salty-tasting skin | [5] |
2. Microorganisms living within biofilms
This section constitutes an introduction to biofilms, which represent a common life-style for bacteria, one which gives them many advantages, particularly much higher tolerance to antimicrobial agents than free planktonic bacteria. We describe their life cycle, their architecture, how they function, and how the bacteria communicate and protect themselves from environmental stresses.
2.1 Formation and characteristics of microbial biofilms
Confronted by hostile environmental changes, the genetic richness of microorganisms allows them to survive and to multiply as well as to develop the host genome. There are between 15 000 and 30 000 different species of bacteria in the human body, about 38 trillion bacteria in a 70 kg "reference" man, between 20 and 30 years old [9]. A large number of bacteria live on the skin, in the digestive tract as well as in the ear, nose and throat sphere. Most are either harmless or beneficial, when a balance between bacteria and host is established [10]. The balance between pathogenic and beneficial bacteria in the intestine is a criterion determining good health and sickness. If an imbalance is present, called dysbiosis, the host-microorganism relationship is altered, and conditions such as inflammatory bowel disease, colon cancer, diabetes and obesity can emerge. The formation and accumulation of bacterial biofilms disrupts this balance and is one of the major causes of chronic infections [11].
Biofilms represent a much higher level of social and spatial organization than free single bacteria [5,12]. The term “biofilm” refers to surface-associated microbial communities surrounded by a self-produced polymer matrix made of extracellular polymeric substances (EPS), typically composed of a mixture of exopolysaccharides, extracellular DNA (eDNA), and proteins [13]. It is important to distinguish between sessile (adhering) bacteria and planktonic (free-swimming) organisms. Bacteria in biofilms are clearly different from their planktonic counterparts. The biofilm mode of growth is the predominant life-mode of most bacterial species. It is estimated that 40 to 80% of the microbial biomass on earth is associated with a biofilm [12]. Microbial biofilms have been compared to human cities in which individuals choose a city, select the neighborhood that best suits their needs, set up their homes amongst others, and occasionally leave when life conditions deteriorate [14].
The matrix occupies most of the biofilm volume (typically 85%) and strengthens its structure while retaining great mechanical plasticity to the structure. Its composition depends on the microbial communities and the growth environment. These sessile biostructures are frequently vascularized by a network of water channels that allows nutrients as well as oxygen to be delivered to bacteria in the bulk, and to reject waste [15]. Within biofilms, significant gradients appear: oxygen, pH or substrates. The biofilm spatial structure depends on these gradients which generate heterogeneous local microenvironments and diversification of cell types, including slow-growth and persister subpopulations which show a very high tolerance to disinfectants and antibiotics [16].
The biofilm way of life is adopted by bacteria in stressful situations as a form of protection. For the bacteria to survive and grow even under hostile conditions, the biofilm matrix adsorbs and retains nutrients and water [17]. In order to cope with changing environmental conditions, bacteria adapt their metabolism, for example, by switching from respiration to fermentation if the amount of oxygen in the medium is limited [18].
Biofilms can form on any type of surface, biotic or abiotic. In humans, there are important sites for the formation of biofilms. Biofilms are often made up of several different bacterial species. This can result in a competition for nutrients and space, or in a synergy that allows the development of phenotypes beneficial for the survival and propagation of the biofilm. Bacteria communicate with each other through a phenomenon called quorum sensing (QS), which is an intercellular signaling pathway that allows bacteria to coordinate gene regulation and group activity in response to population density. It is a mode of communication between bacteria of the same species, but also of different species. This system is a key regulatory mechanism, based on the production of diffusible signaling molecules (autoinducers) which allow biofilms to adapt to changing circumstances [19]. In general, Gram-negative bacteria use acyl-homoserine lactones (AHL) as autoinducers, and Gram-positive bacteria use processed oligo-peptides to communicate. In some cases, QS directs the production of the essential components of the biofilm such as biosurfactants or eDNA [20]. Indeed, surfactants keep channels open by modifying cell-cell interactions and the attachment of bacteria to surfaces by adhesins. In P. aeruginosa bacteria, rhamnolipid biosurfactants are responsible for the complex 3D architecture of the biofilm [21].
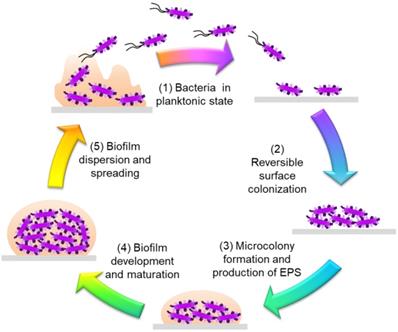
In order to understand the tolerance mechanisms of biofilms, it is important to decipher their life cycles, from their formation to their dissemination. The formation of biofilms takes place in several stages (Figure 1).
- Initially, planktonic bacteria, evolving freely in an aqueous environment, attach themselves to a biotic or an abiotic surface. For bacteria to attach, certain structures and proteins must be present on the bacterial surface, such as curli, fimbriae and pili present in E. coli or Salmonella spp. [22] [23]. Surface coating with an organic conditioning film can alter bacterial initial adhesion and promote biofilm growth [24]. Different phenomena are involved in the transport of bacteria to a surface: motility, Brownian motion, gravitation, diffusion, convective transport, etc. [25]. This first attachment is reversible and is triggered, when bacteria are between 2 and 50 nm from the surface, by non-specific interactions such as van der Waals, Lewis acid-base and electrostatic interactions [25].
- The attachment becomes irreversible mainly by the formation of adhesins and adhesive proteins, which anchor the bacteria to the surface. By modifying their environment, sensors located in the internal membrane of bacteria can activate a succession of reactions (autophosphorylation, transient protein-protein interactions, enzymatic activity) which leads to cyclic diguanosyl monophosphate (c-di-GMP) synthesis. The intracellular level of c-di-GMP directs the bacteria towards a chronic lifestyle to form biofilms (e.g. if the level is high), or towards an acute lifestyle which corresponds to the bacteria in the planktonic state (e.g. when the level is low) [26]. The bacteria change their patterns of gene expression to adopt a biofilm mode of life, for example by triggering EPS production that cements them together [27].
- The next step is biofilm maturation. This is characterized by an increase in size, thanks to the EPS synthesized by the bacteria, and a 3D structure is obtained. The shape depends on the bacterial species and the specific strain involved. The production of large quantities of EPS helps to protect the biofilm and also increases its tolerance to antimicrobial action [28]. Gene expression in bacteria present within the complex structure of the mature biofilm is extremely heterogeneous and distinct from that in planktonic bacteria.
- The last step is the dispersion of the biofilm. After the biofilm matures, some detached bacteria may adhere to a new surface and form a new biofilm: this completes the life cycle of the bacterial biofilm. There are several reasons for the return to the planktonic state: a lack of nutrients that causes bacteria to seek a better environment, or an accumulation of toxic residues [28,29]. Other reasons for the detachment of bacteria are mechanical disturbances (induced by shearing forces, by abrasion), enzymatic degradation of the polymer matrix (thanks to dispersin B, for example), the production of surfactants (e.g. rhamnolipids in P. aeruginosa), the induction of motility with a new synthesis of flagella, and the release of the EPS [29]. The c-di-GMP also plays a role in the dispersion of the biofilm, together with QS which is regulated by the agr gene in S. aureus [30].
Depending on their environment, bacterial biofilms have different growth patterns, different spatial organizations as well as different phenotypic and genetic characteristics [31]. Within a single species of bacteria, a great variety of subpopulations can be obtained due to their great genetic plasticity. Division of the biofilm into several populations is affected by environmental conditions: chemical gradients (oxygen, nutrients and electron donors), adaptation to local environmental conditions, gene expression and the genotypic variation that occurs through mutation and selection [32].
Life cycle of a bacterial biofilm. Planktonic bacteria, free in a medium (1) bind reversibly to a surface (2). They secrete adherent proteins as well as EPS resulting in irreversible attachment and form a microcolony (3). The biofilm grows and matures (4) until, after an event, the bacteria in the biofilm revert to a planktonic lifestyle (5).
Biofilms on medical equipment
| Medical equipment | Location | Bacteria | Disease | Refs |
|---|---|---|---|---|
| Catheters | Intravascular catheters (IVCs), as well as central venous catheters (CVCs) | Coagulase-negative Staphylococci, Gram-negative rods, Staphylococcus aureus (S. aureus), and Candida albicans | Catheter-related bloodstream infection (CRBSI) | [39] |
| In-dwelling catheters | E. coli, Enterococci, P. aeruginosa, Klebsiella pneumonia, Candida albicans, Enterobacter, P. mirabilis and coagulase-negative Staphylococci | Catheter-associated urinary tract infection leading to cystitis, pyelonephritis, Gram-negative bacteremia, prostatitis, epididymitis, endocarditis, vertebral osteomyelitis, septic arthritis, endophthalmitis and meningitis | [40] | |
| Heart valves | Mechanical heart valves, on surrounding tissues or on reconstructed native heart valves | S. epidermidis, S. aureus, Streptococcus spp., Gram-negative bacilli, diphtheroids, enterococci, and Candida spp | Prosthetic valve endocarditis, infection of peripheral tissues | [41] |
| Orthopedic prostheses | Joint or bone replacement | Gram-positive cocci: S. aureus, coagulase-negative staphylococci and enterococci | Infections associated with prostheses, as well as dissemination to other sites | [42] |
| Implants | Endotracheal and tympanostomy tubes, orthopaedic and breast implants, contact lenses, intrauterine devices (IUDs), sutures and vascular grafts | S. aureus (generally found on metallic implants and in acute infections), coagulase-negative staphylococci (S. epidermis), beta-hemolytic streptococci, and aerobic Gram-negative rods | Acute local inflammatory reactions, that can persist as chronic inflammation, destruction of bone tissue (osteolysis), as well as mechanical loosening (aseptic) | [38,43] |
2.2 Biofilms on artificial surfaces: Medical equipment
Medical equipment, particularly that intended for insertion into the body, provides many opportunities for bacteria to enter the body, to develop biofilms and to proliferate [38]. Catheters, implants and prostheses spread biofilms into the blood or other organs, causing inflammation and disease. Preventative treatments of the surface of medical equipment with antimicrobials, or insertion of cement and bone spacers releasing broad-spectrum antibiotics, are required. Despite very strict health protocols, biofilm formation is widespread (Table 2).
3. Failure of antimicrobial action on biofilm communities
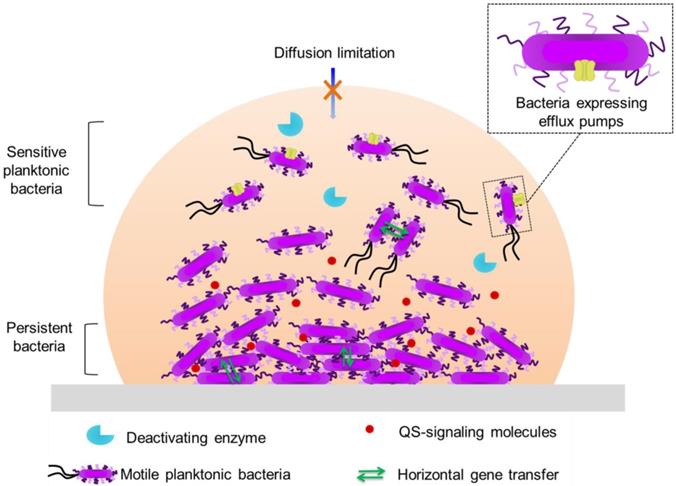
The high tolerance of biofilm bacteria to otherwise lethal conditions and to the immune system is due to different factors: i) the matrix hampers diffusion/reaction of biocides; ii) preferentially expressed genes are involved in stress response or in antibiotic efflux; iii) phenotypic variants with enhanced ability to survive adverse conditions appear [44]. However, no current consensus exists regarding the mechanisms of biofilm tolerance, though numerous competing theories are presently under investigation [45]. Biofilm-associated persistence is of particular importance in the context of antibiotic multiresistant bacteria, and powerful molecules are actively sought.
3.1 Disinfectants, antiseptics and antibiotics
A vast array of compounds, known collectively as antimicrobials, are used to fight bacteria and, more generally, microorganisms. Disinfectants, antiseptics and antibiotics are products with an antimicrobial, therefore antibacterial, antifungal and/or antiviral action. They are intended to inhibit growth (bacteriostatic, fungistatic) and/or kill microorganisms (bactericide, fungicide, virucide). Disinfectants are used on inert materials (medical equipment, floors, etc.). Conversely, antiseptics are used on living tissues, for external use only, and are designed so as not to destroy the tissues to which they are applied. Unlike disinfectants, antibiotics target only bacteria and are given orally, by ingestion or by perfusion. An antibiotic can be both bactericidal and bacteriostatic, depending on its dose. There are several families of antibiotics, with different structures and modes of action. They are grouped according to the mechanism of their action on planktonic bacteria:
- β-lactam family for the inhibition of bacterial wall synthesis.
- aminoglycosides, macrolides, phenicols, cyclins, fusidic acids and oxazolidinones for the inhibition of protein synthesis.
- quinolones and mupirocins for the inhibition of nucleic acid synthesis.
- sulfonamides for folic acid inhibition.
- other antibiotics, such as nitro derivatives and anti-tuberculosis drugs [46].
The minimum inhibitory concentration (MIC) is the standard for determining the sensitivity of planktonic bacteria to an antimicrobial. It represents the lowest concentration of an antimicrobial needed to prevent bacterial growth under specific conditions. Compared to planktonic bacteria cultured in liquid media, bacterial biofilms can survive antibiotic doses up to 1 000 times the MIC [47]. The presence of EPS, the spatial organization of the community and the physiological changes produced by biofilm growth greatly enhance the survival of bacteria. Consequently, the MIC is not directly related to the minimal biofilm eradication concentration (MBEC).
Biofilm-associated antibiotic tolerance exhibits certain characteristics that combine with those associated with planktonic bacteria [44]: low cell permeability; efflux pumps (rejection of the antibiotic); inhibitory enzymes (β-lactamase) (Figure 2).
3.2 Antimicrobial diffusion-reaction limitation within biofilms
The matrix acts as a barrier to certain toxic molecules, reducing or preventing their diffusion [44]. In P. aeruginosa the major component of the matrix is alginate, which is responsible for its anionic character. The EPS matrix of biofilms can prevent antimicrobial interaction with embedded bacteria. The delivery of antibiotics to the bacteria deepest in the biofilm can be compromised, and only the upper layers are exposed to a lethal dose. Bacteria in biofilms formed by P. aeruginosa are more tolerant than their planktonic counterparts to several antibiotics (aminoglycosides and β-lactams) partially due to their low membrane permeability and their low proportion of porins on the outer membrane of the cell wall [48].
In addition, in P. aeruginosa biofilms, tolerances have developed, with the production of a β-lactamase which lyses the β-lactam ring during its diffusion in the matrix [49]. Non-mucous strains form biofilms which have much lower tolerance towards tobramycin, one of the antibiotics most widely used to fight P. aeruginosa [50]. Nevertheless, β-lactam antibiotics can penetrate the biofilm, and it appears that biofilm tolerance is not really related to the matrix interference [51].
The matrix of S. epidermidis interferes with the sensitivity to a large number of antimicrobials [52]. Not all activities are equally reduced; glycopeptides such as vancomycin and teicoplanin are significantly affected, and one hypothesis is that this is due to their high molecular weights, 1 450 and 1 600-1 900 g/mol, respectively. Agents such as rifampicin, clindamycin and macrolides, with molecular weights from 360 to 830 g/mol, are either unchanged or little affected. However, this hypothesis is challenged, because daptomycin (MW = 1 619 g/mol) is not significantly perturbed by the matrix. Another hypothesis is that the matrix itself interferes with the local defenses of the host and creates conditions of local immunosuppression.
Several advanced fluorescence microscopic tools show that the phenomenon of diffusion in the matrix is not sufficient to explain the resistance of biofilms to antibiotics. Confocal time-lapse imaging, FLIM, FRAP, and FCS methods demonstrate that vancomycin penetrates the S. aureus biofilm matrix within 2 to 3 minutes (depending on the strain) [53]. This slow diffusion is explained by the interaction between the cationic vancomycin and the anionic biofilm components (cell surface of the bacteria, pocket of eDNA…). Only between 50 and 60% of the vancomycin is able to diffuse freely in the biofilm. This retention of antibiotics impacts bioavailability, making them less effective on biofilms.
Daptomycin is an antibiotic that works by creating large pores through the membranes of cells, breaking them down, causing potassium ions to leak, thereby killing bacteria. The failure of daptomycin to treat S. aureus biofilms was considered, in early studies, to be due to its poor diffusion within the biofilm. However, it has since been shown to diffuse into the biofilm and interact with the bacteria therein, but oligomerization allowing pore formation does not occur [54]. The explanation lies in the composition of the cell membrane, and more precisely in the nature of the fatty acids they contain. In fact, the bacteria in S. aureus biofilms produce more saturated fatty acids than planktonic bacteria, thus increasing membrane rigidity, limiting exchanges and therefore constituting a defense mechanism against foreign elements.
The action of tobramycin against Acinetobacter baumannii and S. aureus biofilms is limited by adsorption at the binding sites of the matrix surface [55]. The diffusion of five anti-pseudomonas antibiotics (ceftazidime, cefsulodin, piperacillin, gentamicin and tobramycin) through alginate gels was studied [56]. β-Lactams diffuse through the matrix faster than aminoglycosides which are hydrophilic and positively charged; these initially bind to alginates, but diffusion increases after a latency period of 80 to 100 minutes. This has inspired the use of cationic compounds such as quaternary ammonium compounds (QACs) to destabilize the biofilm; these also have bactericidal properties. However, QACs are generally not very selective and are therefore very toxic. Short alkyl chains cannot cross the surface of the biofilm. QACs with C8 and C10 alkyl chains enter water channel systems easily; this is facilitated by convective flow and there is no loss of their bactericidal activities. However, a further increase in the hydrophobicity leads to a gradual loss of activity [57]. QACs diffuse poorly in P. aeruginosa biofilms and therefore are not very effective [58]. Their effect is correlated with the composition of the biofilm matrix, as well as its thickness.
3.3 Biofilm-specific Metabolism and Physiology
Within the biofilm, several subpopulations are formed with an increased ability to survive difficult conditions. Bacteria, called persisters, are stress-tolerant microorganisms that do not die or grow in the presence of multiple antibacterials [59]. These are found in several species of bacteria such as E. coli, P. aeruginosa, S. aureus, and Gardnerella vaginalis. They are largely responsible for the persistence of chronic infections related to biofilms. These bacteria are said to be in a state of dormancy, where their metabolism is inactive and there are no genetic changes. The availability of nutrients is a key factor in determining the metabolism of the biofilm [60]. A very slow metabolism can limit the activity of many antimicrobial compounds. When an antibacterial is applied, the persisters are not sensitive and, when the level of biocide decreases, they can repopulate the biofilm. This tolerance to antibiotics is not genetic, but stems from the fact that their slow-growth state protects them. The level of persistence depends on the antibiotic used as well as the level of biofilm maturation.
The local oxygen level greatly influences the tolerance of P. aeruginosa to antibiotics [16]. This is because tobramycin and ciprofloxacin are able to penetrate the biofilm matrix, but can only kill bacteria at the air-biofilm interface. The oxygen-rich zone coincides with the region of active metabolism in the biofilm. In the absence of oxygen, these antibiotics are unable to kill bacteria embedded deep in the biofilm. This shows that limited antibiotic diffusion is not the primary protective mechanism for these biofilms, because in this case the tolerance to antibiotics is related to the oxygen levels. Results for artificial E. coli biofilms against two antibiotics, latamoxef and tobramycin, are similar [16].
3.4 Genetic plasticity
The expression of certain genes allows biofilm bacteria to survive in response to stress, to the introduction of antibiotics or to the failure of cell-to-cell communication (Figure 2). A number of genes, as well as regulatory processes, are activated during biofilm formation, particularly during the adhesion, growth and maturation stages. For P. aeruginosa, the biosynthesis of alginate is encoded by the algC gene, the expression of which is triggered by QS [61]. For S. aureus, the agr gene, which drives biofilm formation, is also activated by QS [33].
In response to the introduction of an antibiotic, multi-drug efflux pumps can be overexpressed at the surface of the exposed bacteria [62]. There are several families of pumps, the best known and studied being the resistance-nodulation-division (RND) family. This efflux pump has three components: a cytoplasmic inner-membrane pump (AcrB protein), a periplasmic adapter protein (AcrA) and an outer-membrane protein channel (TolC). The pump crosses the wall of Gram-negative bacteria, and allows the expulsion of a broad range of antibiotics. In P. aeruginosa biofilms, when an antibiotic is introduced, the efflux pumps increase their activity [63]. When the concentration of the antibiotic is below the inhibitory threshold, alginate genes are expressed [64]. This reaction in response to antibiotic stress is present in biofilms formed by E. coli, where there are genes associated with multidrug efflux pumps. In fact, the production of biofilms is significantly slowed down when certain genes are removed by mutation [65]. When these pumps are deactivated (using an inhibitor molecule), biofilm growth is reduced and the tolerance towards antibiotics is blocked [66]. This demonstrates that efflux pumps are a mechanism of bacterial biofilm resistance (Figure 2).
Generally, several species of microorganisms are present within biofilms. This allows the sharing of genetic material by horizontal gene transfer [67]. Biofilms provide the ideal conditions for such transfer, such as high local cell density, accumulation of genetic elements from different bacteria and elevated mutation frequencies. This transfer of information is conducive to the acquisition of new traits and to increased resistance. Gene transfers have been identified and described in a variety of biofilms [68]. The density and age of the biofilm have an influence on the efficiency of gene transfer.
4. Alternative therapies for biofilm eradication
Due to the failure of most antibiotics to treat biofilms, alternative therapies have been sought in order to eradicate or to inhibit them. Several points of intervention are possible, from the formation of the biofilm until its maturation. Many natural products hinder QS, have anti-adhesin activity, inhibit film growth or have non-specific antimicrobial properties. Other therapies use bacteriophages, viruses which specifically target certain bacteria, or enzymes which degrade the extracellular biofilm matrix.
4.1 Natural products
Some natural antibiofilm agents are able to inhibit or drastically reduce QS signaling. Among them, garlic extracts render P. aeruginosa sensitive to tobramycin as well as to phagocytosis by polymorphonuclear leukocytes, by reducing signal production [69]. By studying the critical interactions within the binding site and structural motifs in the isolated molecular components of garlic, it has been possible to design compounds that inhibit QS; examples include: citrus limonoids, isolimonic acid and hordenine, an extract from barley kernels [70]. Chamaemelum nobile is a plant widely used for its anti-inflammatory, deodorant, bacteriostatic, antimicrobial, carminative, sedative, antiseptic, anticatarrhal and spasmolytic properties. It inhibits QS in P. aeruginosa resulting in inhibition of biofilm formation and growth [71].
The ethyl acetate-soluble fraction of Cocculus trilobus has anti-adhesin effects at the adhesion stage of biofilm formation from Gram-positive bacteria [72]. Polyphenols from cranberries have effects on the formation, growth and adhesion to surfaces of Porphyromonas gingivalis biofilms [73]. They also inhibit the adhesion of E. coli, inhibit the sialic acid-specific adhesion of Helicobacter pylori [74], and prevent biofilm formation of Streptococcus mutans [75] and Streptococcus sobrinus [76]. Herba patriniae, a traditional Chinese medicinal herb, inhibits the expression of six key genes associated with P. aeruginosa biofilm formation and EPS production [77]. Other molecules such as ginkgolic acids, extracted from the Ginkgo biloba tree, have neuroprotective, antimicrobial, and antitumor properties [78], and are active against biofilm formation in E. coli and on three strains of S. aureus [79].
Manuka honey, native to New Zealand, and Sidr honey, native to Yemen, have bactericidal efficacy, as well as antibiofilm activity [80]. This use of honeys is particularly interesting in that they are natural and non-toxic products. These honeys can destroy in vitro planktonic bacterial cultures and biofilms of 11 strains of S. aureus sensitive to methicillin (MSSA), 11 strains of S. aureus resistant to methicillin (MRSA) and 11 strains of P. aeruginosa (Table 3). This is better than most commonly used antibiotics.
Bactericidal efficiency of Sidr and Manuka honeys against MRSA (Methicillin-resistant S. aureus), MSSA (Methicillin-sensitive S. aureus) and P. aeruginosa biofilms
| Sidr honey | Manuka honey | |
|---|---|---|
| MSSA biofilm | 63% | 82% |
| MRSA biofilm | 73% | 63% |
| P. aeruginosa biofilm | 91% | 91% |
Indeed, of 9 antibiotics (rifampin, cefazolin, oxacillin, vancomycin, azithromycin, fusidic acid, gentamicin and linezolid) of different classes, and used routinely against S. aureus, only rifampin eradicates MSSA or MRSA biofilms [81]. Rifampin is bactericidal for 18% of MSSA and 42% of MRSA, which is significantly poorer than for the two honeys. The active ingredient of Manuka honey is methylglyoxal, also known as pyruvaldehyde or 2-oxopropanal [82]. It is present at a concentration 100 times greater than in other honeys, where the bactericidal activity is due to in situ-generated hydrogen peroxide [83], the amount varying with the species. Being a powerful oxidant and reducing agent, H2O2 denatures the proteins of microorganisms.
The defense mechanisms of biofilms are numerous and varied: diffusion of antibiotics limited; production of persistent slow-growth bacterial subpopulations; production of enzymes that degrade antibiotics. Bacteria communicate with each other through quorum sensing (QS) and share genetic material by horizontal gene transfer.
4.2 Bacteriophages
Alongside natural products, other alternative therapies which mimic strategies observed in nature have been designed in order to destroy bacterial biofilms. Phage therapy is based on the use of bacteriophages, viruses that specifically infect bacteria. These viruses do not infect eukaryotic cells, thus reducing the risk of opportunistic infections. Their average size ranges from 25 to 200 nm, and they consist of a protein capsid protecting their genome (containing nucleic acid), most often a tail of variable length (through which the genetic material is injected) and of tail fibers ensuring recognition of the host (pili can be receptors) [84]. Bacteriophages are able to alter polysaccharides in biofilms using enzymes, called depolymerases, located at the ends of the tail fibers [85], increasing their diffusion into the matrix of the biofilm and hence their efficacy. Moreover, bacteriophages can diffuse freely through the water channels of the biofilm [86]. Bacteriophages are very specific to a species subgroup population and do not have such a broad spectrum of action as antibiotics.
In the same way as for planktonic cultures, phage-resistant subpopulations can emerge within the biofilm community [87]. Phage-resistant mutants of P. aeruginosa biofilm appear after treatment with anti-pseudomonas bacteriophages, due to the mutation of genes encoding the phage receptors [88]. QS is related to resistance against bacteriophages in biofilms: E. coli reduces the number of receptors on the cell surface in response to AHL detection signals, resulting in a two-fold reduction in the rate of phage adsorption [89].
During the 20th century, the Soviet Union (USSR) invested in bacteriophages to treat bacterial infections. Not being able to afford antibiotics, produced mainly by western countries, the USSR focused on other means of treating their populations. For this reason, in Georgia, formerly part of the USSR, the Eliava Institute now has one of the largest libraries of bacteriophages, having started its research in 1923, just a few years after their discovery [90].
4.3 Biofilm-dispersing enzymes
Due to its porous structure, as well as its exposure to the surrounding environment, the biofilm matrix is an attractive target for antibiofilm therapy. Certain enzymes are able to degrade the polymers of the biofilm matrix. This prevents their formation, loosens the matrix formed on a surface and increases the sensitivity of the bacteria in the biofilms to antibacterials.
One of these enzymes is Deoxyribonuclease I (DNase I). Its antibiofilm action was tested on Gram-positive bacteria (Enterococcus faecalis, S. aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, Streptococcus intermedius, Streptococcus mutans, Streptococcus pneumoniae and Streptococcus pyogenes), and Gram-negative bacteria (Acinetobacter baumannii, Aggregatibacter actinomycetemcomitans, Bdellovibrio bacteriovorus, Campylobacter jejuni, Comamonas denitrificans, E. coli, Haemophilus influenzae, Klebsiella pneumoniae and P. aeruginosa) [91]. It inhibits the formation of biofilms of 7 species out of the 17 tested, can partially or totally detach biofilms from 15/17; and makes 7 out of 17 species sensitive to antibacterials. This enzyme also induces biofilm dispersion in Bordetella bronchiseptica, Bordetella pertussis and Gardnerella vaginalis.
Pulmozyme® is a drug marketed in France in the form of a solution by the ROCHE laboratory [92]. Based on DNase I it is used to treat P. aeruginosa infections in patients with cystic fibrosis. This drug exhibits biofilm detachment activity against established P. aeruginosa and S. pneumoniae biofilms in vitro.
Dispersin B is produced naturally by A. actinomycetemcomitans. This enzyme hydrolyzes an extracellular polysaccharide, poly-(β-1,6)-N-acetylglucosamine (PNAG), produced by several bacteria including S. epidermidis, S. aureus and numerous members of the Gram-negative Proteobacteria family. Degradation of the biofilm matrix triggers its dispersion and resensitizes residual bacteria to the action of an antibiotic [93].
Another strategy is to use enzymes which are part of EPS synthetic pathways, such as alginate lyase and PelA. Alginate lyase hydrolyzes alginate, one of the components of the extracellular matrix of biofilms, thus disrupting its structure. It reduces the viscosity of sputum in cystic fibrosis, improves phagocytosis and increases the effectiveness of antibiotics. PelA is a protein necessary for the synthesis of the polysaccharide Pel, which is a component of the biofilm matrix [94]. Pel promotes cell-cell interaction in the biofilm structure, and plays a role in cell adhesion to a surface. It also protects bacteria against certain aminoglycoside antibiotics. PelA can hydrolyze Pel, control its length and rectify errors in the case of malformation. It can inhibit the formation of biofilms of P. aeruginosa as well as Pseudomonas spp., and in the latter, can destroy an already formed biofilm.
5. Contribution of nanotechnology against biofilms
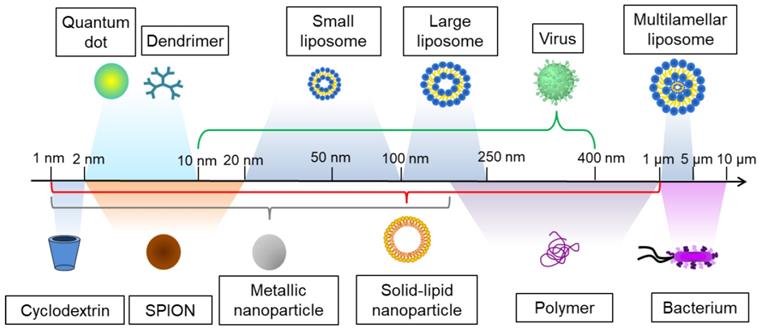
In parallel with the classic methods for treating bacterial biofilms, new technologies have emerged. Nanotechnology can be defined as the design and study of materials at the nanometer scale, typically between 1 and 1 000 nm (Figure 3). The nanoscale character results in unique properties, both physicochemical and biological. This is due to their large surface-to-volume ratios, which confer properties different from those of the bulk. The applications of nanotechnologies are very diverse and numerous, in health [95], energy [96], defense [97], environment [98], information storage [99], etc.
Nano-formulations have several advantages in the field of drug delivery in that they allow:
- administration of drugs that are poorly soluble in water;
- protection of the drug from enzymatic reactions;
- targeting of drugs to the specific organ to be treated, thereby reducing potential toxicity;
- crossing of several membranes impermeable to traditional medicines;
- intracellular and transcellular delivery of large macromolecules.
Since the pore diameter of biofilms averages about 50 nm (this value depends on the density of the biofilm) [100], nanomaterials, with sizes below this value, can diffuse through the biofilm matrix and easily reach bacteria in the inner regions of the biofilm. The biokinetics of encapsulated drugs is different from that of free drugs, focusing antibiotic action on the biofilm and minimizing exposure to human cells. Nanomedicines can increase efficiency, specificity, and biodistribution, as well as reducing dosages and thus the toxicity of the corresponding drug formulations.
The surface functionalization of nanomaterials also plays a role in diffusion within the biofilm. Positively charged nanomaterials better penetrate biofilms having a negatively charged matrix, and hydrophobic particles have a better distribution within biofilms than hydrophilic ones [101]. In addition, the physical properties of nanomaterials can be exploited to fight biofilm. The intrinsic bacterial toxicity of some inorganic nanomaterials, or the capacity of some nanomaterials to locally induce heat, can be harnessed to cause bacterial death. In the constant search to improve treatments while reducing the doses administered, the idea of a multifunctional nanomaterial combining therapy and diagnosis (theranostics) has emerged.
Diagnostic techniques using nanomaterials as biomarkers are widely used in medical imaging. Depending on the technique used, different information can be obtained. By means of nanomaterials it is possible to perform structural imaging, to obtain a 2D or 3D image of an organ, with information about its size, volume and the location of any visible abnormalities (tumors, for example). Different methods are based on:
- magnetic fields (magnetic resonance imaging (MRI), magnetoencephalography (MEG), etc.)
- X-rays (radiography, X-ray-computed tomography, two-photon X-ray absorption (DEXA))
- ultrasound
- light rays (diffuse optical tomography, near-infrared spectroscopic imaging)
- radioactivity (positron emission tomography (PET), single-photon-emission-computed tomography (SPECT))
The relative sizes of several nanomaterials are shown here ranging from 1 nm to >1 µm. Superparamagnetic iron oxide nanoparticles (SPION).
Nanomaterials with activity against biofilms.
Functional imaging gives quantitative information about biological parameters, metabolism in particular. Usually only one type of imaging is used. However, it is possible to combine them in bimodal imaging, which allows microscopic and macroscopic information to be obtained from two imaging techniques with a single probe. Among the most common are the combinations of:
- MRI and PET scans
- MRI with optical imaging (fluorescence, bioluminescence)
- MRI and photoacoustics
- PET and computed tomography (CT)
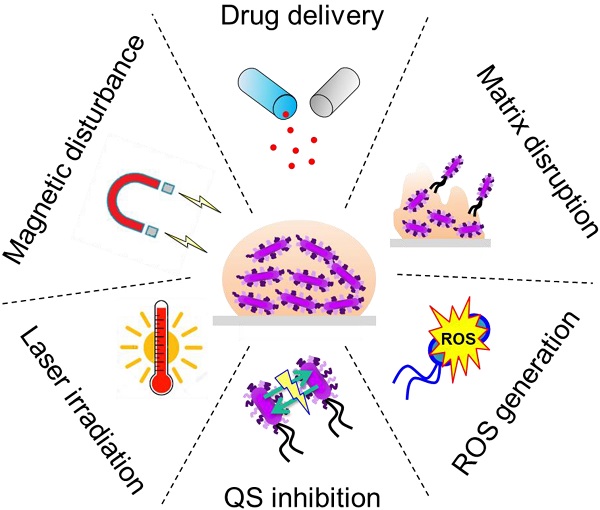
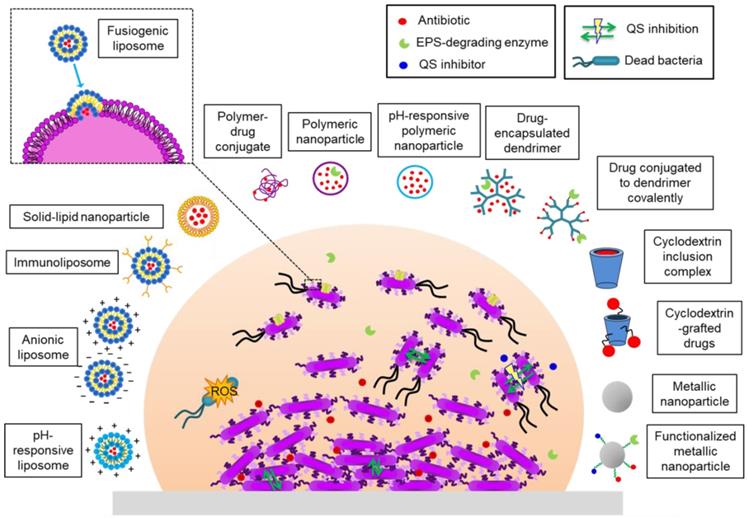
Several nanomaterials have been devised and tested in order to eradicate bacterial biofilms (Figure 4). They can be divided into two groups: (i) organic nanoparticles including liposomes, polymeric nanoparticles (NPs), dendrimers, cyclodextrins and solid-lipid NPs; (ii) inorganic NPs which include metallic NPs (gold, silver, silica, copper, etc.), metal oxides (iron oxide, aluminum oxide, etc.), quantum dots, fullerene and organic-inorganic hybrids. In some systems bactericidal activity is due to the nature of the nanomaterial or the nanocarrier. Others allow a drug to be encapsulated, protecting it from enzymatic deactivation and environmental conditions that can compromise its action (lack of oxygen, pH) and the defense mechanisms of the bacteria (Figure 5) [102-104].
5.1 Drug delivery
Different nanomaterials have been applied for the delivery of antibiotics to biofilms, aiming for a more effective delivery of the drug in the biofilm, or a targeted delivery restricted to the bacteria, reducing side-effects in human tissue. We will review the most important nanomaterials used so far for antibiotic delivery.
5.1.1 Liposomes
A liposome is an artificial spherical vesicle, made up of at least one lipid bilayer, trapping a watery compartment inside. Such a structure facilitates the encapsulation of a wide variety of drugs/active molecules: hydrophilic in the aqueous internal compartment, lipophilic within the lipid bilayer, and amphiphilic between these two regions. Its biocompatibility and its capacity to reduce drug toxicity for in vivo applications make it a great platform in the medical field for the vectorization of molecules of therapeutic interest [105]. The encapsulation of vancomycin in fusogenic liposomes (which can fuse with the bacterial membrane) increases its bactericidal activity against biofilms of S. aureus [106]. Cationic liposomal capsules have been developed in order to penetrate the anionic matrix of S. aureus biofilms [107]. These drug-containing liposomes are more effective than the drug alone, being able to better slow down and inhibit bacterial biofilm growth. The use of liposomes as drug-carriers allows the internalization of drugs by endocytosis. Liposomes being biocompatible, they facilitate the penetration of mature biofilms and enhance the interaction with the membrane of the bacteria. Modifying the liposome surface by grafting a molecule recognized by the biofilm makes a specific interaction possible. By grafting an antibody to the liposome surface, a retention immunoliposome is created, which enables release of the encapsulated drug close to the surface of the biofilm, thus increasing its efficiency [108]. A disadvantage, especially with liposomes smaller than 100 nm, is their poor physicochemical stability (spontaneous fusion), leading to a loss of payload and an increase in the size of the vesicles (therefore preventing the crossing of certain barriers). This thereby eliminates the potential therapeutic benefits of nanoscale delivery.
5.1.2 Solid-lipid nanoparticles
Solid-lipid NPs (SLN) are spherical colloidal nanomaterials, with diameters typically between 10 and 1 000 nm [109], composed of a lipid core stabilized by surfactants. SLNs are an excellent antimicrobial drug delivery platform because their small size and lipid core provide good drug protection to ensure maximum bioavailability, therapeutic targeting, better biodegradation and nontoxicity. SLNs combine the advantages of lipid emulsion and polymeric NPs while overcoming temporal stability, storage, cost and in vivo issues that hamper drug delivery [110]. They are capable of encapsulating lipophilic and hydrophilic drugs, and the carrier has no intrinsic biotoxicity, being composed of lipids similar to physiological ones. It is also possible to freeze-dry and reconstitute SNLs, and they have a high drug payload [111]. Their large-scale production reveals no problems; they can be synthesized without organic solvents and they are sterilizable (in an autoclave) [109], making them very interesting for formulations in many treatments.
A. Mode of action of Ag-NPs on cell apoptosis. Adapted with permission from [102], copyright 2020 Scientific Reports. B. AHL signals bind to the Quorum Sensing receptor and switch on vio genes, activating violacein production (B. left). The presence of Quorum Quenching-active NPs breaks down AHL signals, silencing vio genes, thus inhibiting violacein production (B. right). Adapted with permission from [103], copyright 2020 Advanced Functional Materials. C. Inhibition of E. coli biofilm formation after exposure to nanoemulsion-loaded hydrogel coatings containing eugenol-NE and methyl salicylate-NE for 7 days on solid surfaces, namely (a) glass, (b) plastic and (c) meat, measured by fluorescence microscopy, phase contrast microscopy and SEM, respectively. Adapted with permission from [104], copyright 2019 Scientific Reports. D. Differences between PTT and PDT using functionalized nanoparticles.
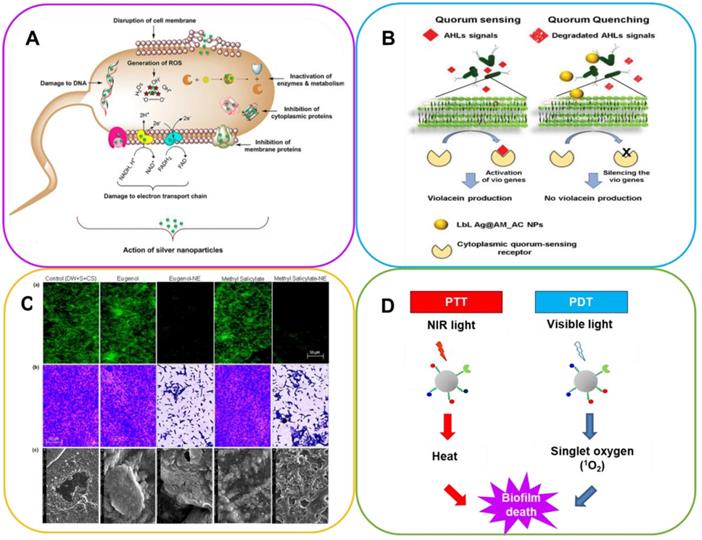
The antibacterial and antibiofilm activity of a complex composed of SLN encapsulating cefuroxime axetil (CA), a 2nd generation cephalosporin, was tested against S. aureus [112]. Encapsulation allows prolonged release: it takes 12 hours to achieve 96% release of CA encapsulated in SLN as against 99.7% in 2 hours if it is free. This complex forms an inhibition zone to biofilm formation, larger than CA alone (13 mm vs. 9 mm) and has a lower MIC against S. aureus.
Several QS-inhibitor (QSI) nanomaterials have been designed to eradicate or inhibit the formation of biofilms [113]. They have many advantages over conventional compounds: nanomaterials are able to penetrate the biofilm matrix, have better solubility, can deliver drugs efficiently and specifically, as well as maintaining the activity of the nano-inhibitor (no agglomeration or aggregation). A QSI encapsulated in an ultra-small (diameter < 100 nm) solid-lipid NP (us-SLN) shows up to seven times more activity than the free QSI alone in the eradication of P. aeruginosa biofilms [113].
5.1.3 Polymeric nanoparticles
Polymeric NPs (PNPs) constitute another type of nanotechnology developed to fight bacterial biofilms. They can be defined as solid colloidal particles with sizes less than 1000 nm. PNPs increase the solubility index of a large number of molecules, hydrophilic and hydrophobic, and can be made out of biodegradable polymers. PNPs have been applied for antibiotic delivery, especially for hydrophobic drugs. An antibiotic, levofloxacin, encapsulated in a poly(lactic-co-glycolic acid) (PLGA) nanocapsule, shows antibiofilm activity against E. coli bacteria. It destroys the biofilm and inhibits the growth of surviving bacteria [114].
5.1.4 Dendrimers
Dendrimers are 3D, branch-shaped structures with repeating molecular patterns. They are of interest for drug delivery due to their ability to encapsulate both hydrophilic and hydrophobic molecules in the free spaces between their branches [115]. It is also possible to graft molecules of therapeutic interest to these polymers, through functional groups at the surface of the dendritic structure. Some peptide-grafted dendrimers form a thermally reversible collagen-type triple helix. This allows them to act as drug transporters, with delivery capabilities under heating. Dendrimers are also widely used as vehicles for transporting DNA and anti-cancer drugs [116]. Low-molecular-weight peptide dendrimers express antimicrobial properties, without any antibiotics, against S. aureus and E. coli [117].
5.1.5 Cyclodextrins
Cyclodextrins (CD) are cyclic oligosaccharide compounds, composed of glucose units linked together by α-1,4-glycosidic bonds. There are three main types of CD, differentiated by the number of glucose units, namely α-CD (6 glucose units), β-CD (7 units) and γ-CD (8 units). They are used in various fields, in food [118], in the environment [119], in pharmaceuticals [120] as well as in drug delivery. The hydrophobic interior of CDs enables complexation with hydrophobic drugs inside the macrocycle, creating supramolecular structures. This makes them very good solubilizers and stabilizers, and allows the transport of the drugs to the target [121]. CDs increase the solubility of a drug by encapsulating it, and protect it from the degradation reactions that can occur with oral drug administration. There are more than 30 pharmaceuticals using CD-drug complexes in the world [122]. Drugs encapsulated in CDs, covalently attached to a surface, have antibiofilm activity against C. albicans. Encapsulation of anidulafungin or thymol in several CDs, attached to a gold surface, shows inhibition of C. albicans adhesion, to the extent of 64% and 75%, respectively [123]. Likewise, encapsulation of miconazole in CD, attached to polyethylene and polypropylene surfaces, reduces C. albicans biofilm formation by 87 to 96% [124].
Another example is the encapsulation of rifabutin (RFB). This drug has only a 20% biodistribution [125], the rest being eliminated through the gastro-intestinal tract. The β-CD-RFB complex eradicates biofilms of S. aureus, P. aeruginosa, E. faecalis and P. vulgaris [126]. In the absence of β-CD, with RFB alone, inhibition of the biofilm occurs but the remaining bacteria aggregate into large agglomerates. With the β-CD-RFB inclusion complex, inhibition is more efficient.
5.1.6 Hydrogels
Hydrogels are increasingly finding applications in the field of biofilms and wound dressing due to their excellent biochemical and mechanical properties. Several categories of hydrogels have been used to eliminate mature biofilms and progress has been made in related drug-delivery nanosystems. Some of them are based on polyethylene glycol dimethacrylate tethered with a dangling polyethylenimine [127], bioactive essential oil components [128], self-adapting chitosan [129], etc. Drugs, bacteriophages and other active molecules can be encapsulated inside hydrogels, and the rate of release controlled by the gel formulation. Degradation of hydrogel in the presence of infected cells leads to release of active molecules into the infection site, killing bacteria and leading to biofilm eradication, as in the case of P. aeruginosa, S. aureus, Acinetobacter baumannii, etc. Hydrogels are promising biomaterials to fight against multidrug-resistant bacterial infections. They have potential in the treatment for biofilm-associated wound infections and enhance healing [130].
5.2 Nanomaterials with intrinsic antibacterial properties and stimuli-responsive nanomaterials
5.2.1 Inorganic nanoparticles
Inorganic NPs (INPs) are particles with one or more dimensions (length, width, and thickness) in the range of 1-100 nm, composed of either pure metals (for example, gold, silver and iron), metals oxides or metals salts. The main characteristics of INPs are a large surface area-to-volume ratio compared to their bulk equivalents, large surface energies, plasmon excitation, quantum confinement, a large number of poorly coordinated sites such as corners and edges, specific chemical properties and the ability to store excess electrons. INPs are widely used in imaging as contrast agents, in diagnostics as bio-sensors using their optical properties, as well as therapeutic agents which exploit their thermal, magnetic, and drug delivery properties.
Metallic NPs (MNPs) are characterized by a phenomenon called surface plasmon resonance (SPR), which is the collective oscillation of free (valence) electrons when they are excited by light. This effect gives them excellent optical properties and induces a strong enhancement of the electric field at their surface leading to several possible applications: sensors, in vivo bio-imaging, therapeutics, etc. The most interesting metals using SPR are gold, silver and copper.
MNPs are widely used in the medical field, due to their antimicrobial effects on a large number of bacteria, viruses and fungi. Silver NPs (Ag-NPs) are widely used as a bactericide, while gold NPs (Au-NPs) have many catalytic and therapeutic applications. The main mechanisms of their toxicity involve the production of reactive oxygen species (ROS) and impairment of membrane function, for example, with dysfunctional proteins [131]. ROS include superoxide radicals, hydroxyl radicals, hydrogen peroxide and singlet oxygen, which may cause chemical damage to proteins and DNA in bacteria. An imbalance between the production of ROS and the antioxidant capacity of the cell is referred to as oxidative stress.
Gold NPs, functionalized with the enzyme proteinase-K (PK), are active against P. fluorescens biofilms [132]. PK disrupts the matrix, disperses it and destroys bacteria released from the biofilm. The combination of Au-NPs and PK shows a synergistic effect. Au-NPs functionalized with a cationic ligand also show antibiofilm activity in the disruption of the biofilm matrix of S. aureus and P. aeruginosa [133]. Au-NPs inhibit the formation of C. albicans biofilms by more than 80% [134], but this is neither due to the generation of ROS nor to the breakdown of EPS from the matrix. Au-NPs bind to the surface of bacteria by strong electrostatic interactions which interfere with those between the pathogens, which are bound by adhesins, thus disrupting and preventing the growth of the biofilm. The growth of P. aeruginosa and S. aureus biofilms is reduced by the action of Au-NPs [135]. At low Au-NP concentration, the growth of S. aureus is reduced by 13%, but that of P. aeruginosa increases by 3%. At higher concentrations, Au-NPs reduce the concentration of S. aureus by 67-70% and that of P. aeruginosa by 25-78%. Au-NPs synthesized from rhizome extracts of Rhodiola rosea inhibit the formation of P. aeruginosa and E. coli biofilms. This inhibition is particularly interesting, since Au-NPs do not have a direct bactericidal effect on P. aeruginosa and E. coli bacteria.
Ag-NPs are known for their broad-spectrum antimicrobial properties, and are also effective against multiresistant bacteria [136], HIV [137] as well as SARS-CoV and SARS-CoV-2 [138]. Their toxicity in humans limits their therapeutic use, and they also tend to aggregate. The surface functionalization of Ag-NPs improves their antimicrobial effectiveness by preventing aggregation, improving their bactericidal effect within biofilms and decreasing their cytotoxicity [139]. Smaller Ag-NPs show better antibacterial activity [140]. The shape of Ag-NPs has also an impact on toxicity: triangular NPs are more bactericidal than spherical or rod-shaped ones [141]. In aqueous environments, Ag-NPs release Ag+ cations: 12% of the silver atoms are in ionic form; thus, the antimicrobial effect cannot be attributed unambiguously to Ag-NPs or to dissolved Ag+ ions [142]. The antibacterial and antibiofilm mechanisms of action are not yet fully understood. Ag-NPs induce oxidative damage under the effect of stress resulting from the production of ROS [143]. Another explanation of the antibacterial action is the ability of Ag-NPS to influence the permeability of bacterial membranes [144] as well as to interact with their proteins, DNA and enzymes, thus leading to genetic mutations, structural alterations and cell division, triggering bacterial death [145].
Ag-NPs in combination with antibiotics have better antibiofilm activity than either alone. Different antibiotics (ampicillin and vancomycin) coupled to Ag-NPs have antibiofilm activity on Gram-negative bacteria (P. aeruginosa and Shigella flexneri) and on Gram-positive bacteria (S. aureus and Streptococcus pneumoniae) [146]. Synergy is also found between Ag-NPs and polymyxin B, an antibiotic, against P. aeruginosa biofilms [147], of which 83% of the samples tested come from multidrug-resistant strains. This combination inhibits the formation of biofilms three times more effectively than the components alone. Of six conventional antibiotics (ampicillin, clindamycin, gentamicin, cephalexin, vancomycin, erythromycin) tested in combination with Ag-NPs against biofilms formed by MRSA [148], the greatest synergistic effect is with clindamycin. In contrast, ampicillin shows no synergistic effect, the inhibition of the formation of MRSA biofilms being the same as for Ag-NPs alone (58%); for the others the inhibition ranges from 62-69%.
Iron atoms have 4 unpaired electrons in the 3d shell; Fe2+ ions have also 4 and Fe3+ have 5. Therefore, when they form crystals they have a strong magnetic moment, and can be ferromagnetic, antiferromagnetic or ferrimagnetic. Magnetic NPs, between 2 and 20 nm in diameter, display superparamagnetism [149]. The two main forms of superparamagnetic iron oxide NPs (SPIONs) are magnetite (Fe3O4) and its oxidized form, maghemite (γ-Fe2O3). In the absence of an external magnetic field, their magnetic properties are not displayed. Magnetic NPs in solution can absorb the energy of an alternating magnetic field and convert it into heat [150]. These properties make them ideal candidates as:
- vectors for drug delivery [151],
- contrast agents for magnetic resonance imaging (MRI) [152],
- platforms for theranostics (by combining drug delivery and MRI) [153],
- therapeutic agents for magnetic and optical hyperthermia in anti-cancer therapy [154],
- agents for regenerative medicine [155],
- magnetic biosensors [156].
Their toxicity is highly dependent on the coating, e.g. 10 nm IONPs coated with PEG are non-toxic while IONPs coated with polyethylenimine (PEI) exhibit dose-dependent lethal toxicity. The size of the IONPs is another parameter that affects toxicity, and biodegradation and clearance also depend on it. IONPs are usually distributed in the liver and spleen, with rather less in other organs such as lungs, heart and kidneys. Their main mechanism of bactericidal action is through the generation of ROS leading to cell death by necrosis or apoptosis [157]. Hydroxyl radicals are generated inside lysosomes by the Fenton reaction of free iron, Fe2+ and hydrogen peroxide. α-Fe2O3 increases the growth of P. aeruginosa biofilms [158]. This is because the smallest NPs release Fe3+ ions which interact with antimicrobial proteins, such as lactoferrin, believed to prevent colonization [159]. Lactoferrin is an iron-binding glycoprotein that sequesters free iron. It can be bactericidal by binding lipopolysaccharide of bacteria walls and results in cell breakdown (lysis) by interacting with the oxidized iron part of the bacteria.
IONPs are used as contrast agents in MRI to visualize the sites of bacterial infections of several species, such as S. aureus, H. pylori and Mycobacterium tuberculosis [160]. MRI can visualize anatomical abnormalities in 3D with high resolution. Superparamagnetic IONPs reduce the longitudinal and transverse relaxation times of surrounding protons.
5.2.2 pH-Responsiveness
A pH-dependent liposomal NP, coated with a quaternary ammonium, chitosan (a QAC), was designed to attack Porphyromonas gingivalis biofilms [161]. The environment of dental biofilms in humans has a low pH, around 4.5 or less, and the liposome NPs are soluble under both acidic and basic conditions thanks to the positive charges on chitosan, a strong polyelectrolyte. These charged species target the anionic matrix of some biofilms by strong electrostatic interactions [162]. Under acidic conditions, the amines of the QAC are protonated, and act as pH-responsive domains, thus destabilizing the liposomal NP, which releases an encapsulated drug, doxycycline hydrochloride, effective against Gram-positive and Gram-negative bacteria.
Likewise, other pH-responsive PNPs have been designed for drug delivery in oral biofilms of S. mutans. pH-Responsive p(DMAEMA)-b-p(DMAEMA-co-BMA-co-PAA) block copolymer micelles are cationic, have a high affinity for dental biofilms, and encapsulate farnesol, an antibacterial drug [163]. Another pH-responsive PNP, composed of poly(ethylene glycol)-block-poly(2-(((2-aminoethyl)carbamoyl)oxy)ethylmethacrylate) (PEG-b-PAECOEMA) encapsulating an antibacterial, chlorhexidine, eradicates biofilms formed by S. mutans [164].
Au-NPs functionalized by zwitterionic pH-sensitive ligands consisting of 11-mercapto-undecanoic acid (HS-C10-COOH) and (10-mercaptodecyl) trimethylammonium (HS-C10-NMe3) bromide are effective against MRSA biofilms [165]. The zwitterionic compound adheres strongly to the surfaces of negatively charged (pH ∼5.5 vs. 7.4 in healthy tissue) bacteria in MRSA. Au-NPs aggregate in biofilms, but their photothermal properties make this an advantage. Under near-infrared (NIR) irradiation, MRSA biofilm is destroyed but surrounding healthy tissues are undamaged, with the dispersed Au-NPs having no photothermal effect. In the acidic environment of biofilms, a nanohybrid of SiO2-PCe6-IL becomes positively charged and, by interacting with negatively charged EPS, creates holes in MRSA biofilms [166]. This nanohybrid is also a photosensitizer, and in combination with the holes created within the biofilm, significantly improves photodynamic therapy (PDT) against MRSA.
5.2.3 Matrix disruption
Certain polymer NPs containing drugs disrupt bacterial biofilm, but also act on planktonic bacteria [167]. Polymer NPs break bacterial biofilms either by delivering therapeutic molecules or by modifying their surface with antibacterial components, like alkyl-pyrimidines or QACs. Cationic functions interacting with the anionic matrix of biofilms have bactericidal activity, by destructuring cell membranes [168]. PLGA-coated NPs, functionalized with the enzyme DNase I, and encapsulating ciprofloxacin, have been designed for the eradication of P. aeruginosa biofilms. They penetrate and disrupt the matrix structure of the biofilms, locally releasing ciprofloxacin which then acts even on the bacteria most deeply buried in the biofilm. Based on the structure of antimicrobial peptides, synthetic semi-rigid polymers have been designed, with hydrophobic and cationic domains [169]. A library of quaternary ammonium-poly(oxanorborneneimides) with different degrees of hydrophobicity shows excellent therapeutic indices as well as very low toxicity to red blood cells. These polymeric NPs (PNPs) penetrate and eradicate biofilms of P. aeruginosa, En. cloacae and S. aureus. Due to the highly cationic and hydrophobic nature of PNPs, it has been suggested that their activity arises from the disruption of bacterial cell membranes. Even after several treatments, the bacteria do not develop any resistance towards this nanomaterial.
Another example: under UV irradiation thin-film composite membranes (TFCs) infused with TiO2 NPs have a photocatalytic bactericidal effect on E. coli [170], by disrupting the bacterial membrane and inhibiting attachment to its surface.
5.3 Chemical effects
5.3.1 Generation of ROS
Nanomaterial-based catalysts are heterogeneous materials mainly based on metallic NPs whose high specific surface area increases their catalytic activity. A catalytic NP (CAT-NP) with peroxidase-like activity has been designed to destroy dental S. mutans biofilms [171]. It contains biocompatible Fe3O4, which has been developed to catalyze the decomposition of H2O2 at low pH, in order to generate free radicals in situ, which simultaneously degrade the biofilm matrix and rapidly decrease bacterial proliferation. Likewise, dextran-coated IONPs, termed nanozymes (Dex-NZM), display strong peroxidase-like catalytic activity at low pH [172]. This nanocomposite targets biofilms with high specificity, penetrating the matrix of S. mutans biofilms, and locally killing bacteria by disruption of the structure. Ferumoxytol (Rienso® in Europe) is an intravenous treatment for iron deficiency. This superparamagnetic IONP disrupts intractable oral biofilms of S. mutans and prevents tooth decay in the same way [173]. Ferumoxytol binds to the biofilm matrix and generates free radicals from H2O2, causing bacterial death by rupture of the cell membrane and degradation of the EPS matrix.
Ag-NPs capable of diffusing into the biofilm (diameter about 40 nm) completely inhibit the formation of S. mutans and E. coli biofilms by the generation of ROS [174]. Very recent work shows that they inhibit the formation of biofilms (> 99%) of P. aeruginosa, E. coli and S. aureus [175] and the formation of MRSA biofilms by producing ROS. Also, they decrease the production of EPS of K. pneumoniae biofilms by 44-45% (dry weight) as well as inhibiting biofilm formation by 23-86% [176].
MgF2 NPs show antibiofilm activity against S. aureus and E. coli [177]. They attach to the surface and penetrate into the biofilm, thus disrupting the structure by inducing bacterial membrane lipid peroxidation. ROS are produced and damage unsaturated acids in cell membranes, lipoproteins or other molecules. These NPs inhibit the formation of biofilm for three days, and at the end of this period, the formation of colonies of isolated bacteria resumes.
Nitric oxide (NO)-releasing silica NPs show antibiofilm effects against P. aeruginosa, E. coli, S. aureus and S. epidermidis [178]. NO is a reactive free radical produced by neutrophils and macrophages to fight infection during inflammation. Due to its antimicrobial capacity, its small size and fast diffusion, NO destroys bacteria embedded in the matrix, and kills ≥ 99% of the bacteria in the biofilms, with the highest mortality for those of P. aeruginosa and E. coli. In addition to killing them, NO disperses bacteria in P. aeruginosa biofilms. These NPs are as toxic to fibroblasts as, or less toxic than, the antiseptics currently used, and they have advantages for healing wounds.
5.3.2 Inhibition of QS
Silencing communication between the bacteria of the biofilms, QS, is a therapeutic strategy. Silicon dioxide NPs (Si-NPs) [179], functionalized with β-CD, emit luminosity proportional to the concentration of acylated homoserine lactones (AHLs) of QS of Vibrio fischeri biofilms. These bacteria use AHL as a means of coordinating their activities, but also for bioluminescence [180]. β-CD binds non-specifically to AHL, reducing the luminosity; the bacteria are therefore no longer able to communicate and find themselves isolated.
A herbal metallic colloidal nano-formulation (Uh-Au@Nano-CF), containing Swarna (gold) NPs and polyphenols from a Usnea longissima extract (a medicinal lichen), has anti-QS properties [181]. It degrades the structure of biofilms, as well as inhibiting their formation. U. longissima also contain usnic acid, which inhibits biofilm formation or eradicates preformed biofilms at higher concentration. There is a synergistic effect between the lichen and the biogenic Au-NPs.
Silver (Ag-NP), Selenium (Se-NP) and tellurium (Te-NP) NPs are active against P. aeruginosa biofilms [182]. Se-NPs have very interesting antioxidant, anticarcinogenic and antimicrobial properties [183]. Compounds based on tellurium are used in semiconductors, rechargeable batteries and in glasses, but they also have anti-inflammatory, antimicrobial and anticarcinogenic properties [184]. Se-NPs inhibit 60-70% of P. aeruginosa biofilm formation, by disrupting QS signals so that the biofilm can no longer develop. However, they are less efficient in removing an already formed biofilm (only 15%). For Te-NPs, the efficiency is 80% for the inhibition of biofilm formation, but only 30% for the removal of preformed biofilm.
5.3.3 Localized antibacterial action
Nanomaterials offer as well several possibilities for creating antibacterial coatings on implants or medical devices that allow for a localized antibacterial action and decrease risk of biofilm formation. A localized antibacterial action at the site of an implant or on the surface of the medical device can be effective in preventing the initial steps of biofilm formation. In the case of implants the greatest risk is at the moment of the operation when the body is opened. However, the risks remain until the body is closed. A localized antibacterial action can be achieved by encapsulating antibiotics or other active molecules on the surface of implant/device or by incorporating an antibacterial material. For example, nanoscale features on surfaces offer a means of reducing bacterial proliferation, as shown by surface deposition of Ti nanotubes [185]. Incorporating graphene nanomaterials into a titania matrix increases their conductivity, enhancing transfer of extruded electrons from the bacterial cell membrane to the composite and subsequent electron enrichment at the Schottky-like interface, which in turn leads to bactericidal action [186]. Nanostructured mesoporous films of titania can be used for on-top implant loading of antibiotics, which are then released at the implant site [186]. Mesoporous titania films have been loaded with gentamicin. In physiological media there is an initial burst release of gentamicin followed by a prolonged release that lasts weeks. This slow release is explained by the interaction of OH groups from gentamicin with the walls of the titania pores. Such a release profile is highly appealing for bone implants where a high concentration of antibiotics is necessary during surgery, while a lower concentration is needed until tissue is regenerated [187].
Nanoscale polymer coatings have been extensively used to confer antibacterial properties on implants and medical devices. Polyelectrolyte multilayers fabricated by the Layer-by-Layer (LbL) technique deserve a special mention. This technique is based on the alternating assembly of oppositely charged polyelectrolytes by electrostatic interactions, and can be applied to the non-covalent modification of multiple substrates, including medical implants, provided that these are charged or can be charged. LbL assembly results in nanostructured layered films, with polymer layers a few nanometers thick, in which other charged molecules or NPs can be assembled or complexed [188]. Very interesting strategies have been developed for the loading of LbL films with antibiotics [186], antibacterial peptides, and bactericidal NPs. Moskowitz et al. [189] proposed a LbL-based antibacterial coating, consisting of a tetra-layer unit containing gentamicin sulfate, polyacrylic acid and a synthetic poly(β-amino ester). The entire film comprised up to 200 tetra-layers, fabricated over 5 days by an automated procedure. It gave an initial burst release of gentamicin followed by slow release. Coatings with 100 tetra-layers had a bactericidal effect against S. aureus. In a similar fashion Escobar et al. fabricated LbL multilayers from complexes of polyacrylic acid, gentamicin and polylysine. The complexes formed in slightly acid solution hold large amounts of gentamicin, which is released after LbL assembly at neutral pH without compromising the stability of the film [188]. LbL coatings can also restrict bacterial adhesion to surfaces. Moreover, the sequential assembly of building blocks allows the combination of antibiotics, peptides and NPs all in one, enhancing antibacterial action.
Application of nanomaterials in biofilm inhibition
| Nanomaterials | Mode of action | Bacteria | Biofilm impact | Refs |
|---|---|---|---|---|
| Drug delivery: | Drug carrier | |||
| Liposomes | hydrophilic, lipophilic, amphiphilic | S. aureus, P. gingivalis | Slow down, growth inhibition | [105-108] |
| SLNs | Prolonged release : hydrophilic, lipophilic, | S. aureus | Growth inhibition | [109-112] |
| QSIs | Anti-agglomeration, anti-aggregation | P. aeruginosa, V. fischeri | Eradication, growth inhibition | [113, 177-178] |
| PNPs | hydrophilic, hydrophobic | E. coli, S. mutans, S. aureus, P. aeruginosa, En. cloacae | Matrix disruption, eradication, growth inhibition | [114, 164-166] |
| Dendrimers | hydrophilic, hydrophobic | S. aureus, E. coli | Antimicrobial | [115-117] |
| Cyclodextrins | hydrophobic | C. albicans, S. aureus, P. aeruginosa, E. faecalis, P. vulgaris | Adhesion inhibition, eradication | [121-123] |
| Hydrogels | Bacteriophage, hydrophilic, hydrophobic | P. aeruginosa, S. aureus, MRSA, Acinetobacter baumannii | Biofilm eradication, wound healing | [127-130] |
| Stimuli responsive NPs: | Intrinsic properties: | |||
| SPIONs | Magnetic disturbance, ROS generation, thermal therapy, drug delivery | P. aeruginosa, H. pylori, M. tuberculosis, S. aureus, S. mutans | Oxidative stress, cell lysis, colonization prevention | [153-156, 133-135, 170] |
| Ag-NPs | ROS generation, antibacterial, drug carrier | P. aeruginosa, E. coli, S. aureus, K. pneumoniae, S. flexneri, S. mutans | Oxidative stress, inhibition, genetic mutation, structural alteration | [128-131, 135-140] |
| Au-NPs | Thermal and photodynamic therapies, photosensitizer | S. aureus, P. aeruginosa, E. coli, C. albicans | Matrix disruption, growth prevention, | [132-134, 167-169] |
| Other inorganic NPs | Photocatalysis, ROS generation, antimicrobial, antioxidant | E. coli, S. aureus, P. aeruginosa, S. epidermidis | Matrix disruption, growth inhibition | [132, 173-179] |
5.3.4 Photothermal therapy and Photodynamic therapy
Photothermal therapy (PTT) and photodynamic therapy (PDT) have received considerable attention and are recognized as viable alternatives for treating biofilm infections [190]. PTT increases the penetration of antibacterial agents into biofilm and decreases the progression of antibiotic resistance. Irradiation in the near infrared (NIR) reduces biofilm by ca. 90%, indicating the therapeutic efficacy of localized antimicrobial exposure and hyperthermia [191]. However, the high irradiation doses and photosensitizer concentrations used to ablate biofilms by PTT and PDT may cause severe tissue damage and inflammation [192].
Combined therapy can improve the therapeutic efficacy and reduce side-effects in the treatment of bacterial biofilm infections [193].
New research is now oriented towards the use of smart designs, based on hydrogels, antibacterial drugs, plasmonic nanoplatforms, etc. Moreover, synergistic methods are sought as promising alternatives for treating bacterial infections and in fighting biofilm formation.
The achievements related to the applications of nanomaterials in biofilm inhibition are summarized in Table 4.
6. New developments
In contrast to conventional treatments which consist in simply administering a drug and waiting for it to take effect, in innovative approaches an NP, which may be associated with a drug, is stimulated by a magnetic field or laser irradiation in order to produce a local temperature increase, to release the drug and/or to mechanically disrupt the biofilm. Drug efficacy is notably enhanced by association with NPs stimulated in this way.
6.1 Laser irradiation
Hybrid metal-polymer NPs enable complete destruction of S. aureus and P. aeruginosa biofilms using laser-induced direct transfer (LIFT) [194]. LIFT is a process that uses a pulsed laser beam as the driving force for depositing a thin layer of an organic or inorganic donor substrate onto an acceptor material with high spatial resolution. This transfer can be carried out in the solid or liquid phase. By LIFT S. aureus and P. aeruginosa biofilms, which act as receptors, were put into direct contact with a metal NP-polymer composite of a thin metallic film of silver, copper or gold on a polyethylene terephthalate substrate. The laser alone had no effect on the biofilms. In contrast, the silver and copper NPs completely destroyed them.
6.2 Magnetic disturbance
In a very recent article, IONPs (Fe3O4 and γ-Fe2O3) damage the matrix of MRSA biofilms when a magnetic field is applied [195]. The magnetic field controls and concentrates the IONPs at a precise point. The highest antibiofilm activity is for 11 nm IONPs (as against 8 nm and 70 nm), and the two applied magnetic fields, AC and DC, facilitate biofilm eradication more than direct contact. IONPs fail to kill the planktonic MRSA bacteria; they only act upon the physical disruption of the biofilm, thus releasing the biofilm from the surface. A rotating DC magnetic field disperses biofilms the best. A low rotation rate allows the IONPs prolonged contact with the matrix, generating significant shearing forces within the biofilm. The magnetic field and the IONPs therefore act as "shield breakers". A nanocarrier, polymersome, encapsulating IONPS and methicillin, penetrates S. epidermidis biofilms to a depth of 20 µm in a magnetic field. The IONPs partially destructure the biofilm, improving the contact of the antibiotic which, by a synergistic effect, completely destroys the sessile community [196].
The application of an AC magnetic field to IONPs to cause heating is known as magnetic hyperthermia. Local heating by IONPs is much larger than that of the medium, and the associated “hot-spots” are responsible for the detachment of the biofilm. Mild magnetic NP hyperthermia increases the sensitivity of S. aureus biofilms to conventional antibiotics [197].
Block copolymer poly((oligo(ethyleneglycol)methyl ether acrylate)-block-poly(monoacryloxyethyl phosphate))-coated IONPs disrupt the structure of P. aeruginosa biofilms by magnetic hyperthermia, [198]. The increase in the local temperature, from 23 °C to 40 °C, makes it possible to detach and disperse the biofilms. The temperature-sensitive regulatory messenger, c-di-GMP, is inactivated by hyperthermia [199]. This in turn activates the LapG protein which cleaves adhesins to trigger the detachment of the biofilm. This hybrid nanomaterial also allows improvement of antibiofilm therapy of P. aeruginosa, by combining it with gentamicin, usually used against this bacterium. It has been suggested that IONPs are able to create artificial channels, thereby improving the transport of antibiotics within the biofilm [200]. The idea is that in a magnetic field the NPs sink into the biofilm and force the creation of channels due to their passage from the surface to a point closest to the magnet. In a magnetic field, IONPs make S. aureus biofilms 4-6 times more sensitive to gentamicin.
7. Conclusions
The current state-of-the-art indicates that enhanced antimicrobial tolerance is a general trait of biofilms and is the result of different specific factors, which depend on the species of bacterium involved, the environment of the biofilm and the choice of the antimicrobial agent. There is still no strategy that allows biofilms to be consistently and efficiently eradicated: effective, easy and safe-to-use, novel antimicrobial agents are urgently required. The extracellular matrix and specific physiology (including slow-growth and membrane rigidification) play important roles in the low activity of antimicrobials on biofilms. This is why it is urgent to deepen current knowledge on biofilm formation and degradation, and on the interaction of materials with biofilms, in order to create strategies to fight them. Development of novel materials and approaches to cope with the risks of bacterial infections and pandemics in the long term, that can be applied for a large number of scenarios, regardless of the bacterial strain, is fundamentally important. At the same time, we must think about individual cases, aiming to fight specific aggressive bacterial strains and direct treatment by a more personalized medical approach. Coping with these two issues is where the big scientific challenges in the fight against biofilms and bacterial infections lie.
Acknowledgements
C.S. thanks the “Ecole Chimie Physique et Chimie Analytique de Paris Centre”, (ED388) of Université de Paris for a research grant.
All authors have given approval to the final version of this manuscript.
Competing Interests
The authors declare no competing financial interest.
References
1. WHO. Résistance aux antimicrobiens: les enjeux de la réunion des Nations Unies. Revised 27 September 2020. http://www.who.int/bulletin/volumes/94/9/16-020916/fr/
2. Ministère des Solidarités et de la Santé. L'antibiorésistance : pourquoi est-ce si grave ? 2020. Revised 14 June 2018. https://solidarites-sante.gouv.fr/prevention-en-sante/les-antibiotiques-des-medicaments-essentiels-a-preserver/des-antibiotiques-a-l-antibioresistance/article/l-antibioresistance-pourquoi-est-ce-si-grave
3. De Oliveira DMPD, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA. et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020;33:e00181-19
4. Skariyachan S, Sridhar VS, Packirisamy S, Kumargowda ST, Challapilli SB. Recent perspectives on the molecular basis of biofilm formation by pseudomonas aeruginosa and approaches for treatment and biofilm dispersal. Folia Microbiol (Praha). 2018;63:413-432
5. Høiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010;5:1663-1674
6. Yan L, Shen J, Wang J, Yang X, Dong S, Lu S. Nanoparticle-based drug delivery system: a patient-friendly chemotherapy for oncology. Dose-Response. 2020;18:1559325820936161
7. Colombo E, Feyen P, Antognazza MR, Lanzani G, Benfenati F. Nanoparticles: A challenging vehicle for neural stimulation. Front Neurosci. 2016;10:105
8. Pudlarz A, Szemraj J. Nanoparticles as carriers of proteins, peptides and other therapeutic molecules. Open Life Sci. 2018;13:285-298
9. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533
10. Blaser MJ, Kirschner D. The equilibria that allow bacterial persistence in human hosts. Nature. 2007;449:843-849
11. Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013;136:1-51
12. Flemming HC, Wuertz S. Bacteria and archaea on earth and their abundance in biofilms. Nat Rev Microbiol. 2019;17:247-260
13. Flemming HC, Baveye P, Neu TR, Stoodley P, Szewzyk U, Wingender J. et al. Who put the film in biofilm? The migration of a term from wastewater engineering to medicine and beyond. npj Biofilms Microbiomes. 2021;7:10
14. Flemming H-C. The perfect slime. Colloids Surf B. 2011;86:251-259
15. Wilking JN, Zaburdaev V, De Volder M, Losick R, Brenner MP, Weitz DA. Liquid transport facilitated by channels in bacillus subtilis biofilms. Proc Natl Acad Sci USA. 2013;110:848-852
16. Sønderholm M, Bjarnsholt T, Alhede M, Kolpen M, Jensen PØ, Kühl M. et al. The Consequences of being in an infectious biofilm: microenvironmental conditions governing antibiotic tolerance. Int J Mol Sci. 2017;18:2688
17. Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623-633
18. Bester E, Kroukamp O, Wolfaardt GM, Boonzaaier L, Liss SN. Metabolic differentiation in diofilms as indicated by carbon dioxide production rates. Appl Environ Microbiol. 2010;76:1189-1197
19. Parsek MR, Greenberg EP. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci U S A. 2000;97:8789-8793
20. Tolker-Nielsen T. Pseudomonas aeruginosa biofilm infections: from molecular biofilm biology to new treatment possibilities. APMIS Suppl. 2014;138:1-51
21. Davey ME, Caiazza NC, O'Toole GA. Rhamnolipid surfactant production affects biofilm architecture in pseudomonas aeruginosa PAO1. J Bacteriol. 2003;185:1027-1036
22. Nasr A, Olsén A, Sjöbring U, Müller-Esterl W, Björck L. Assembly of human contact phase proteins and release of bradykinin at the surface of curli-expressing escherichia coli. Mol Microbiol. 1996;20:927-935
23. Collinson SK, Emödy L, Müller KH, Trust TJ, Kay WW. Purification and characterization of thin, aggregative fimbriae from salmonella enteritidis. J Bacteriol. 1991;173:4773-4781
24. Schneider RP, Marshall KC. Retention of the Gramnegative marine bacterium SW8 on surfaces — effects of microbial physiology, substratum nature and conditioning films. Colloids Surf B. 1994;2:387-396
25. Bos R. Physico-chemistry of initial microbial adhesive interactions - its mechanisms and methods for study. FEMS Microbiol Rev. 1999;23:179-229
26. D'Argenio DA, Miller SI. Cyclic di-GMP as a bacterial second messenger. Microbiology. 2004;150:2497-2502
27. Dufrêne YF, Boonaert CJP, Rouxhet PG. Adhesion of azospirillum brasilense: role of proteins at the cell-support interface. Colloids Surf B. 1996;7:113-128
28. O'Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49-79
29. Karatan E, Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev. 2009;73:310-347
30. Boles BR, Horswill AR. agr-Mediated dispersal of staphylococcus aureus biofilms. Cossart P, Ed. PLoS Pathog. 2008;4:e1000052
31. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711-745
32. Rani SA, Pitts B, Beyenal H, Beyenal H, Veluchamy RA, Lewandowski Z, Davison WM. et al. Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. J Bacteriol. 2007;189:4223-4233
33. Colombo APV, Magalhães CB, Hartenbach FARR, do Souto RM, da Silva-Boghossian CM. Periodontal-disease-associated biofilm: a reservoir for pathogens of medical importance. Microb Pathog. 2016;94:27-34
34. Roldán S, Herrera D, Sanz M. Biofilms and the tongue: therapeutical approaches for the control of halitosis. Clin Oral Investig. 2003;7:189-197
35. Bakar MBA, McKimm J, Haque M. Otitis media and biofilm: An overview. Int J Nutr Pharmacol Neurol Dis. 2018;8:70-78
36. Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125:353-364
37. CDC. Necrotizing fasciitis: all you need to know. Revised 31 December 2019. https://www.cdc.gov/groupastrep/diseases-public/necrotizing-fasciitis.html
38. Costerton JW. Bacterial Biofilms: A common cause of persistent infections. Science. 1999;284:1318-1322
39. Lebeaux D, Fernández-Hidalgo N, Chauhan A, Lee S, Ghigo JM, Almirante B. et al. Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis. 2014;14:146-159
40. CDC. Catheter-associated Urinary Tract Infection (CAUTI). Revised 16 October 2015. https://www.cdc.gov/hai/ca_uti/uti.html
41. Donlan RM. Biofilms and device-associated infections. Medscape. 2001;7:277-281
42. Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16:397-409
43. Busscher HJ, van der Mei HC, Subbiahdoss G, Jutte PC, van den Dungen JJAM, Zaat SAJ. et al. Biomaterial-associated infection: locating the finish line in the race for the surface. Sci Transl Med. 2012;4:153rv10
44. Boles BR, Thoendel M, Singh PK. Self-generated diversity produces 'insurance effects' in biofilm communities. Proc Natl Acad Sci USA. 2004;101:16630-16635
45. Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114-122
46. Antibio-responsible. Les familles antibiotiques : en savoir plus. Revised 8 August 2020. https://www.antibio-responsable.fr/antibiotiques/familles-antibiotiques
47. Karunakaran E, Mukherjee J, Ramalingam B, Biggs CA. Biofilmology: a multidisciplinary review of the study of microbial biofilms. Appl Microbiol Biotechnol. 2011;90:1869-1881
48. Angus BL, Carey AM, Caron DA, Kropinski AM, Hancock RE. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982;21:299-309
49. Bagge N, Hentzer M, Andersen JB, Ciofu O, Givskov M, Høiby N. Dynamics and spatial distribution of beta-lactamase expression in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2004;48:1168-1174
50. Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M. et al. Alginate overproduction affects pseudomonas aeruginosa biofilm structure and function. J Bacteriol. 2001;183:5395-5401
51. Costerton J. Introduction to biofilm. Int J Antimicrob Agents. 1999;11:217-221
52. Souli M, Giamarellou H. Effects of slime produced by clinical isolates of coagulase-negative staphylococci on activities of various antimicrobial agents. Antimicrob Agents Chemother. 1998;42:939-941
53. Daddi Oubekka S, Briandet R, Fontaine-Aupart MP, Steenkeste K. Correlative time-resolved fluorescence microscopy to assess antibiotic diffusion-reaction in biofilms. Antimicrob Agents Chemother. 2012;56:3349-3358
54. Boudjemaa R, Cabriel C, Dubois-Brissonnet F, Bourg N, Dupuis G, Gruss A. et al. Impact of bacterial membrane fatty acid composition on the failure of daptomycin to kill staphylococcus aureus. Antimicrob Agents Chemother. 2018;62:e00018-e00023
55. Davenport EK, Call DR, Beyenal H. Differential protection from tobramycin by extracellular polymeric substances from acinetobacter baumannii and staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2014;58:4755-4761
56. Gordon CA, Hodges NA, Marriott C. Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J Antimicrob Chemother. 1988;22:667-674
57. McBain AJ, Ledder RG, Moore LE, Catrenich CE, Gilbert P. Effects of quaternary-ammonium-based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl Environ Microbiol. 2004;70:3449-3456
58. Bridier A, Dubois-Brissonnet F, Greub G, Thomas V, Briandet R. Dynamics of the action of biocides in pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2011;55:2648-2654
59. Lewis K. Persister cells and the riddle of biofilm survival. Biochemistry (Moscow). 2005;70:267-274
60. Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107-113
61. Zielinski NA, Maharaj R, Roychoudhury S, Danganan CE, Hendrickson W, Chakrabarty AM. Alginate synthesis in pseudomonas aeruginosa: environmental regulation of the algC promoter. J Bacteriol. 1992;174:7680-7688
62. Van Acker H, Coenye T. The role of efflux and physiological adaptation in biofilm tolerance and resistance. J Biol Chem. 2016;291:12565-12572
63. Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother. 2012;67:2069-2089
64. Bagge N, Schuster M, Hentzer M, Hentzer M, Ciofu O, Givskov M, Greenberg EP. et al. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and β-lactamase and alginate production. Antimicrob Agents Chemother. 2004;48:1175-1187
65. Matsumura K, Furukawa S, Ogihara H, Morinaga Y. Roles of multidrug efflux pumps on the biofilm formation of Escherichia coli K-12. Biocontrol Sci. 2011;16:69-72
66. Kvist M, Hancock V, Klemm P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol. 2008;74:7376-7382
67. Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34-40
68. Christensen BB, Sternberg C, Andersen JB, Eberl L, Møller S, Givskov M. et al. Establishment of new genetic traits in a microbial biofilm community. Appl Environ Microbiol. 1998;64:2247-2255
69. Bjarnsholt T, Jensen PØ, Rasmussen TB, Christophersen L, Calum H, Hentzer M. et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiol Read Engl. 2005;151:3873-3880
70. Persson T, Hansen TH, Rasmussen TB, Skindersø ME, Givskov M, Nielsen J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org Biomol Chem. 2005;3:253-262
71. Kazemian H, Ghafourian S, Heidari H, Amiri P, Yamchi JK, Shavalipour A. et al. Antibacterial, anti-swarming and anti-biofilm formation activities of chamaemelum nobile against pseudomonas aeruginosa. Rev Soc Bras Med Trop. 2015;48:432-436
72. Kim SW, Chang IM, Oh KB. Inhibition of the bacterial surface protein anchoring transpeptidase sortase by medicinal plants. Biosci Biotechnol Biochem. 2002;66:2751-2754
73. Howell AB. Cranberry proanthocyanidins and the maintenance of urinary tract health. Crit Rev Food Sci Nutr. 2002;42:273-278
74. Burger O, Ofek I, Tabak M, Weiss EI, Sharon N, Neeman I. A high molecular mass constituent of cranberry juice inhibits helicobacter pylori adhesion to human gastric mucus. FEMS Immunol Med Microbiol. 2000;29:295-301
75. Yamanaka A, Kimizuka R, Kato T, Okuda K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol Immunol. 2004;19:150-154
76. Steinberg D, Feldman M, Ofek I, Weiss EI. Cranberry high molecular weight constituents promote Streptococcus sobrinus desorption from artificial biofilm. Int J Antimicrob Agents. 2005;25:247-251
77. Gong L, Zou W, Zheng K, Shi B, Liu M. The herba patriniae (caprifoliaceae): a review on traditional uses, phytochemistry, pharmacology and quality control. J Ethnopharmacol. 2021;265:113264
78. Gerstmeier J, Seegers J, Witt F, Waltenberger B, Temml V, Rollinger JM. et al. Ginkgolic acid is a multi-target inhibitor of key enzymes in pro-inflammatory lipid mediator biosynthesis. Front Pharmacol. 2019;10:797
79. Lee JH, Kim YG, Ryu SY, Cho MH, Lee J. Ginkgolic acids and ginkgo biloba extract inhibit escherichia coli O157:H7 and staphylococcus aureus biofilm formation. Int J Food Microbiol. 2014;174:47-55
80. Alandejani T, Marsan J, Ferris W, Slinger R, Chan F. Effectiveness of honey on staphylococcus aureus and pseudomonas aeruginosa biofilms: Otolaryngol Neck Surg. 2009; 141:114-118.
81. Saginur R, StDenis M, Ferris W, Aaron SD, Chan F, Lee C. et al. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother. 2006;50:55-61
82. Mavric E, Wittmann S, Barth G, Henle T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of manuka (leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res. 2008;52:483-489
83. Brudzynski K, Abubaker K, Castle A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front Microbiol. 2011;2:213
84. Microbiology and immunology on-line. Bacteriophage. Revised 23 November 2016. https://www.microbiologybook.org/mayer/phage.htm
85. Pires DP, Oliveira H, Melo LDR, Sillankorva S, Azeredo J. Bacteriophage-encoded depolymerases: their diversity and biotechnological applications. Appl Microbiol Biotechnol. 2016;100:2141-2151
86. Sutherland IW, Hughes KA, Skillman LC, Tait K. The interaction of phage and biofilms. FEMS Microbiol Lett. 2004;232:1-6
87. Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM. Bacteriophage cocktail for the prevention of biofilm formation by pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother. 2010;54:397-404
88. Oechslin F, Piccardi P, Mancini S, Gabard J, Moreillon P, Entenza JM. et al. Synergistic interaction between phage therapy and antibiotics clears pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis. 2017;215:703-712
89. Høyland-Kroghsbo NM, Maerkedahl RB, Svenningsen SL. A quorum-sensing-induced bacteriophage defense mechanism. mBio. 2013;4:e00312
90. DW. Phages: bacterial eaters from Georgia to fight antibiotic resistance. Revised 21 November 2019. https://www.dw.com/en/phages-bacterial-eaters-from-georgia-to-fight-antibiotic-resistance/a-51350421
91. Kaplan JB. Therapeutic potential of biofilm-dispersing enzymes. Int J Artif Organs. 2009;32:545-554
92. Base de données publique des médicaments. Notice patient - PULMOZYME 2500 U/2,5 ml, solution pour inhalation par nébuliseur. Revised 2 October 2020. https://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?typedoc=N&specid=60866491
93. Izano EA, Sadovskaya I, Wang H, Vinogradov E, Ragunath C, Ramasubbu N. et al. Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in aggregatibacter actinomycetemcomitans. Microb Pathog. 2008;44:52-60
94. Szymańska M, Karakulska J, Sobolewski P, Kowalska U, Grygorcewicz B, Böttcher D. et al. Glycoside hydrolase (PelAh) immobilization prevents pseudomonas aeruginosa biofilm formation on cellulose-based wound dressing. Carbohydr Polym. 2020;246:116625
95. Khan FA. Applications of nanomaterials in human health. Springer, Singapore. 2020
96. Hussein AK. Applications of nanotechnology in renewable energies—A comprehensive overview and understanding. Renew Sustain Energy Rev. 2015;42:460-476
97. Sharon M. Nanotechnology's entry into the defense arena. In: Nanotechnology in the Defense Industry. Sharon M, Silvestre A, Rodriguez L, Sharon C, Gallardo PS, Eds: Wiley Online Library, Scrivener Publishing LLC. 2019:1-35
98. Saleem H, Zaidi SJ. Developments in the application of nanomaterials for water treatment and their impact on the environment. Nanomaterials. 2020;10:1764
99. Juang JY, Bogy DB. Nanotechnology advances and applications in information storage. Microsyst Technol. 2005;11:950-957
100. Peulen TO, Wilkinson KJ. Diffusion of nanoparticles in a biofilm. Environ Sci Technol. 2011;45:3367-3373
101. Li X, Yeh YC, Giri K, Mout R, Landis RF, Prakash YS. et al. Control of nanoparticle penetration into biofilms through surface design. Chem Commun (Cambridge). 2015;51:282-285
102. Singh P, Pandit S, Jers C, Joshi AS, Garnæs J, Mijakovic I. Silver nanoparticles produced from cedecea sp. exhibit antibiofilm activity and remarkable stability. Sci. Rep. 2020;11:12619
103. Ivanova A, Ivanova K, Tied A, Heinze T, Tzanov T. Layer-by-layer coating of aminocellulose and quorum quenching acylase on silver nanoparticles synergistically eradicate bacteria and their biofilms. Adv. Func. Mat. 2020;30:2001284
104. Prateeksha Barik SK, Singh BN. Nanoemulsion-loaded hydrogel coatings for inhibition of bacterial virulence and biofilm formation on solid surfaces. Sci. Rep. 2019;9:6520
105. Teng Y, Meng X, Sun H, Li S, Yu P, Galons H. Composition design and medical application of liposomes. Eur J Med Chem. 2019;164:640-653
106. Scriboni AB, Couto VM, de Morais Ribeiro LN, Freires IA, Groppo FC, de Pual E. et al. Fusogenic Liposomes Increase the antimicrobial activity of vancomycin against staphylococcus aureus bofilm. Front Pharmacol. 2019;10:1401
107. Wang Y. Liposome as a delivery system for the treatment of biofilm-mediated infections. J Appl Microbiol. 2021
108. Eloy JO, Petrilli R, Trevizan LNF, Chorilli M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf B. 2017;159:454-467
109. Paliwal R, Paliwal SR, Kenwat R, Kurmi BD, Sahu MK. Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin Ther Pat. 2020;30:179-194
110. Scioli Montoto S, Muraca G, Ruiz ME. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front Mol Biosci. 2020;7:319-343
111. Ekambaram P, Sathali AAH, Priyanka K. Solid lipid nanoparticles: a review. Sci Revs Chem Commun. 2012;1:80-102
112. Singh B, Vuddanda PR, Vijayakumar MR, Kumar V, Saxena PS, Singh S. Cefuroxime axetil loaded solid lipid nanoparticles for enhanced activity against s. aureus biofilm. Colloids Surf B. 2014;121:92-98
113. Nafee N, Husari A, Maurer CK, Lu C, de Rossi C, Steinbach A. et al. Antibiotic-free nanotherapeutics: ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J Control Release. 2014;192:131-140
114. Cheow WS, Chang MW, Hadinoto K. Antibacterial efficacy of inhalable antibiotic-encapsulated biodegradable polymeric nanoparticles against e. coli biofilm cells. J Biomed Nanotechnol. 2010;6:391-403
115. Svenson S, Tomalia D. Dendrimers in biomedical applications—reflections on the field. Adv Drug Deliv Rev. 2005;57:2106-2129
116. Taşkın-Tok T, Gowder SJT. Anticancer drug — friend or foe. In: Gowder SJT, Ed. Pharmacology and Therapeutics. InTech. 2014
117. Janiszewska J, Swieton J, Lipkowski AW, Urbanczyk-Lipkowska Z. Low molecular mass peptide dendrimers that express antimicrobial properties. Bioorg Med Chem Lett. 2003;13:3711-3713
118. Astray G, Gonzalez-Barreiro C, Mejuto JC, Rial-Otero R, Simal-Gándara J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009;23:1631-1640
119. S. Helal A, Mazario E, Mayoral A, Decorse P, Losno R, Lion C, et al. Highly efficient and selective extraction of uranium from aqueous solution using a magnetic device: succinyl-β-cyclodextrin-APTES@maghemite nanoparticles. Environ Sci Nano. 2018;5:158-168
120. Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1-11
121. Hedges AR. Industrial applications of cyclodextrins. Chem Rev. 1998;98:2035-2044
122. Tiwari G, Tiwari R, Rai AK. Cyclodextrins in delivery systems: applications. J Pharm Bioallied Sci. 2010;2:72-79
123. Gharbi A, Humblot V, Turpin F, Pradier CM, Imbert C, Berjeaud JM. Elaboration of antibiofilm surfaces functionalized with antifungal-cyclodextrin inclusion complexes. FEMS Immunol Med Microbiol. 2012;65:257-269
124. Nava-Ortiz CAB, Burillo G, Concheiro A, Bucio E, Matthijs N, Nelis H. et al. Cyclodextrin-functionalized biomaterials loaded with miconazole prevent candida albicans biofilm formation in vitro. Acta Biomater. 2010;6:1398-1404
125. Blaschke TF, Skinner MH. The clinical pharmacokinetics of rifabutin. Clin Infect Dis. 1996;22:S15-S22
126. Shanmuga Priya A, Sivakamavalli J, Vaseeharan B, Stalin T. Improvement on dissolution rate of inclusion complex of rifabutin drug with β-cyclodextrin. Int J Biol Macromol. 2013;62:472-480
127. Yeo CK, Vikhe YS, Li P. et al. Hydrogel Effects Rapid Biofilm Debridement with ex situ Contact-Kill to Eliminate Multidrug Resistant Bacteria in vivo. ACS Appl Mater Interfaces. 2018;10:20356-20367
128. Prateeksha Barik SK, Singh BN. Nanoemulsion-loaded hydrogel coatings for inhibition of bacterial virulence and biofilm formation on solid surfaces. Sci Rep. 2019 9, 6520
129. Li X, Fu Y, Huang L. et al. Combating Biofilms by a Self-Adapting Drug Loading Hydrogel. ACS Appl Bio Mater. 2021;4:6219-6226
130. Liang Y, He J, Guo B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano. 2021;15:12687-12722
131. Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11:371-384
132. Giri K, Rivas Yepes L, Duncan B, Parameswaran PK, Yan B, Jiang Y. et al. Targeting bacterial biofilms via surface engineering of gold nanoparticles. RSC Adv. 2015;5:105551-105559
133. Yu Q, Li J, Zhang Y, Wang Y, Liu L, Li M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci Rep. 2016;6:26667
134. Sathyanarayanan MB, Balachandranath R, Srinivasulu YG, Kannaiyan SK, Subbiahdoss G. The effect of gold and iron-oxide nanoparticles on biofilm-forming pathogens. ISRN Microbiology. 2013;2013:272086
135. Singh P, Pandit S, Beshay M, Mokkapati VRSS, Garnaes J, Olsson ME. et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the rhodiola rosea rhizome extracts. Artif Cells Nanomedicine Biotechnol. 2018;46:S886-S899
136. Shi T, Wei Q, Wang Z, Zhang G, Sun X, He QY. Photocatalytic protein damage by silver nanoparticles circumvents bacterial stress response and multidrug resistance. mSphere. 2019;4:e00175-19
137. Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnology. 2011;9:30-38
138. Jeremiah SS, Miyakawa K, Morita T, Yamaoka Y, Ryo A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem Biophys Res Commun. 2020;533:195-200
139. Liu Y, Shi L, Su L, van der Mei HC, Jutte PC, Ren Y. et al. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem Soc Rev. 2019;48:428-446
140. Szerencsés B, Igaz N, Tóbiás Á, Prucsi Z, Rónavári A, Bélteky P. et al. Size-dependent activity of silver nanoparticles on the morphological switch and biofilm formation of opportunistic pathogenic yeasts. BMC Microbiol. 2020;20:176
141. Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium escherichia coli. Appl Environ Microbiol. 2007;73:1712-1720
142. Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H. et al. Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem. 2007;12:527-534
143. Hwang ET, Lee JH, Chae YJ, Kim YS, Kim BC, Sang BI. et al. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small. 2008;4:746-750
144. Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT. et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346-2353
145. Xu XHN, Brownlow WJ, Kyriacou SV, Wan Q, Viola JJ. Real-time probing of membrane transport in living microbial cells using single nanoparticle optics and living cell imaging. Biochemistry. 2004;43:10400-10413
146. Gurunathan S, Han JW, Kwon DN, Kim JH. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against gram-negative and gram-positive bacteria. Nanoscale Res Lett. 2014;9:373
147. Salman M, Rizwana R, Khan H, Munir I, Hamayun M, Iqbal A. et al. Synergistic effect of silver nanoparticles and polymyxin B against biofilm produced by pseudomonas aeruginosa isolates of pus samples in vitro. Artif Cells Nanomed Biotechnol. 2019;47:2465-2472
148. Rahim KAAA, Mohamed AMA. Bactericidal and antibiotic synergistic effect of nanosilver against methicillin-resistant staphylococcus aureus. Jundishapur J Microbiol. 2015 8; e25867
149. Piraux H, Hai J, Gaudisson T, Ammar S, Gazeau F, El Hage Chahine JM. et al. Transferrin-bearing maghemite nano-constructs for biomedical applications. J Appl Phys. 2015;117:17A336
150. Hai J, Piraux H, Mazarío E, Volatron J, Ha-Duong NT, Decorse P. et al. Maghemite nanoparticles coated with human serum albumin: combining targeting by the iron-acquisition pathway and potential in photothermal therapies. J Mater Chem B. 2017;5:3154-3162
151. Belkahla H, Constantinescu AA, Gharbi T, Barbault F, Chevillot-Biraud A, Decorse P. et al. Grafting TRAIL through either amino or carboxylic groups onto maghemite nanoparticles: influence on pro-apoptotic efficiency. Nanomaterials. 2021;11:502
152. Bigall NC, Dilena E, Dorfs D, Beoutis ML, Pugliese G, Wilhelm C. et al. Hollow iron oxide nanoparticles in polymer nanobeads as MRI contrast agents. J Phys Chem C. 2015;119:6246-6253
153. Di Corato R, Béalle G, Kolosnjaj-Tabi J, Espinosa A, Clément O, Silva AKA. et al. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano. 2015;9:2904-2916
154. Belkahla H, Mazarío E, Sangnier AP, Lomas JS, Gharbi T, Ammar S. et al. TRAIL acts synergistically with iron oxide nanocluster-mediated magneto- and photothermia. Theranostics. 2019;9:5924-5936
155. Van de Walle A, Perez JE, Abou-Hassan A, Hémadi M, Luciani N, Wilhelm C. Magnetic nanoparticles in regenerative medicine: what of their fate and impact in stem cells? Mater Today Nano. 2020;11:100084
156. Hasanzadeh M, Shadjou N, de la Guardia M. Iron and iron-oxide magnetic nanoparticles as signal-amplification elements in electrochemical biosensing. TrAC-Trends Anal Chem. 2015;72:1-9
157. Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep. 2018;8:2082
158. Borcherding J, Baltrusaitis J, Chen H, Stebounova L, Wu CM, Rubasinghege G. et al. Iron oxide nanoparticles induce pseudomonas aeruginosa growth, induce biofilm formation, and inhibit antimicrobial peptide function. Environ Sci Nano. 2014;1:123-132
159. Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552-555
160. Xu C, Akakuru OU, Zheng J, Wu A. Applications of iron oxide-based magnetic nanoparticles in the diagnosis and treatment of bacterial infections. Front Bioeng Biotechnol. 2019;7:141
161. Kojima C, Tsumura S, Harada A, Kono K. A collagen-mimic dendrimer capable of controlled release. J Am Chem Soc. 2009;131:6052-6053
162. Sahariah P, Gaware VS, Lieder R, Jónsdóttir S, Hjálmarsdóttir MA, Sigurjonsson OE. et al. The effect of substituent, degree of acetylation and positioning of the cationic charge on the antibacterial activity of quaternary chitosan derivatives. Mar Drugs. 2014;12:4635-4658
163. Zhou J, Horev B, Hwang G, Klein MI, Koo H, Benoit DSW. Characterization and optimization of pH-responsive polymer nanoparticles for drug delivery to oral biofilms. J Mater Chem B Mater Biol Med. 2016;4:3075-3085
164. Zhao Z, Ding C, Wang Y, Tan H, Li J. pH-Responsive polymeric nanocarriers for efficient killing of cariogenic bacteria in biofilms. Biomater Sci. 2019;7:1643-1651
165. Hu D, Li H, Wang B, Ye Z, Lei W, Jia F. et al. Surface-adaptive gold nanoparticles with effective adherence and enhanced photothermal ablation of methicillin-resistant staphylococcus aureus biofilm. ACS Nano. 2017;11:9330-9339
166. Wang C, Chen P, Qiao Y, Kang Y, Yan C, Yu Z. et al. pH responsive superporogen combined with PDT based on poly Ce6 ionic liquid grafted on SiO2 for combating MRSA biofilm infection. Theranostics. 2020;10:4795-4808
167. Lee ALZ, Ng VWL, Wang W, Hedrick JL, Yang YY. Block copolymer mixtures as antimicrobial hydrogels for biofilm eradication. Biomaterials. 2013;34:10278-10286
168. Olmo JA-D, Ruiz-Rubio L, Pérez-Alvarez L, Sáez-Martínez V, Vilas-Vilela JL. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings. 2020;10:139
169. Pham P, Oliver S, Wong EHH, Boyer C. Effect of hydrophilic groups on the bioactivity of antimicrobial polymers. Polym Chem. 2021;12:5689-5703
170. Kim SH, Kwak SY, Sohn BH, Park TH. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J Membr Sci. 2003;211:157-165
171. Gao L, Liu Y, Kim D, Li Y, Hwang G, Naha PC. et al. Nanocatalysts promote streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 2016;101:272-284
172. Naha PC, Liu Y, Hwang G, Huang Y, Gubara S, Jonnakuti V. et al. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano. 2019;13:4960-4971
173. Liu Y, Naha PC, Hwang G, Kim D, Huang Y, Simon-Soro A. et al. Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat Commun. 2018;9:2920
174. Qayyum S, Oves M, Khan AU. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLOS ONE. 2017;12:e0181363
175. Mohanta YK, Biswas K, Jena SK, Hashem A, Abd_Allah EF, Mohanta TK. Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Front Microbiol. 2020;11:1143
176. Siddique MH, Aslam B, Imran M, Ashraf A, Nadeem H, Hayat S. et al. Effect of silver nanoparticles on biofilm formation and EPS production of multidrug-resistant klebsiella pneumoniae. BioMed Research International. 2020: 6398165.
177. Lellouche J, Kahana E, Elias S, Gedanken A, Banin E. Antibiofilm activity of nanosized magnesium fluoride. Biomaterials. 2009;30:5969-5978
178. Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials. 2009;30:2782-2789
179. Miller KP, Wang L, Chen Y-P, Pellechia PJ, Benicewicz BC, Decho AW. Engineering nanoparticles to silence bacterial communication. Front Microbiol. 2015;6(189):1-7
180. Kaplan HB, Greenberg EP. Diffusion of autoinducer is involved in regulation of the vibrio fischeri luminescence system. J Bacteriol. 1985;163:1210-1214
181. Singh BN, Prateeksha, Pandey G, Jadaun V, Singh S, Bajpai R. et al. Development and characterization of a novel swarna-based herbo-metallic colloidal nano-formulation - inhibitor of streptococcus mutans quorum sensing. RSC Adv. 2015;5:5809-5822
182. Gómez-Gómez B, Arregui L, Serrano S, Santos A, Pérez-Corona T, Madrid Y. Selenium and tellurium-based nanoparticles as interfering factors in quorum sensing-regulated processes: violacein production and bacterial biofilm formation. Metallomics. 2019;11:1104-1114
183. Chhabria S, Desai K. Selenium nanoparticles and their applications. Encyclopedia of Nanoscience and Nanotehnology. 2016:1-32
184. Gómez-Gómez B, Arregui L, Serrano S, Santos A, Pérez-Corona T, Madrid Y. Selenium and tellurium-based nanoparticles as interfering factors in quorum sensing-regulated processes: violacein production and bacterial biofilm formation. Metallomics. 2019;11:1104-1114
185. Ercan B, Taylor E, Alpaslan E, Webster TJ. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology. 2011;22:295102
186. Yang M, Liu H, Qiu C, Iatsunskyi I, Coy E, Moya S. et al. Electron transfer correlated antibacterial activity of biocompatible graphene nanosheets-TiO2 coatings. Carbon. 2020;166:350-360
187. Escobar A, Muzzio N, Coy E, Liu H, Bindini E, Andreozzi P. et al. Antibacterial mesoporous titania films with embedded gentamicin and surface modified with bone morphogenetic protein 2 to promote osseointegration in bone implants. Adv Mater Interfaces. 2019;6:1801648
188. Lavalle P, Gergely C, Cuisinier FJG, Decher G, Schaaf P, Voegel JC. et al. Comparison of the structure of polyelectrolyte multilayer films exhibiting a linear and an exponential growth regime: an in situ atomic force microscopy study. Macromolecules. 2002;35:4458-4465
189. Moskowitz JS, Blaisse MR, Samuel RE, Hsu HP, Harris MB, Martin SD. et al. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of staphylococcus aureus infection in a rabbit bone model. Biomaterials. 2010;31:6019-6030
190. Xu M, Li L, Hu Q. The recent progress in photothermal-triggered bacterial eradication. Biomater Sci. 2021;9:1995-2008
191. Garcia A, Gonzalez B, Harvey C, Izquierdo-Barba I, Vallet-Regi M. Effective reduction of biofilm through photothermal therapy by gold core@shell based mesoporous silica nanoparticles. Micropor Mesopor Mat. 2021;328:111489
192. Cai X, Tian J, Zhu J. et al. Photodynamic and photothermal co-driven CO-enhanced multi-mode synergistic antibacterial nanoplatform to effectively fight against biofilm infections. Chem Eng J. 2021;426:131919
193. Li H, Gong M, Xiao J. et al. Photothermally activated multifunctional MoS2 bactericidal nanoplatform for combined chemo/photothermal/photodynamic triple-mode therapy of bacterial and biofilm infections. Chem Eng J. 2022;429:132600
194. Nastulyavichus A, Tolordava E, Rudenko A, Zazymkina D, Shakhov P, Busleev N. et al. In vitro destruction of pathogenic bacterial biofilms by bactericidal metallic nanoparticles via laser-induced forward transfer. Nanomaterials. 2020;10:2259
195. Li J, Nickel R, Wu J, Lin F, van Lierop J, Liu S. A new tool to attack biofilms: driving magnetic iron-oxide nanoparticles to disrupt the matrix. Nanoscale. 2019;11:6905-6915
196. Geilich BM, Gelfat I, Sridhar S, van de Ven AL, Webster TJ. Superparamagnetic iron oxide-encapsulating polymersome nanocarriers for biofilm eradication. Biomaterials. 2017;119:78-85
197. Alumutairi L, Yu B, Filka M, Nayfach J, Kim MH. Mild magnetic nanoparticle hyperthermia enhances the susceptibility of staphylococcus aureus biofilm to antibiotics. Int J Hyperthermia. 2020;37:66-75
198. Nguyen TK, Duong HTT, Selvanayagam R, Boyer C, Barraud N. Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy. Sci Rep. 2015;5:18385
199. Townsley L, Yildiz FH. Temperature affects c-di-GMP signalling and biofilm formation in vibrio cholerae. Environ Microbiol. 2015;17:4290-4305
200. Quan K, Zhang Z, Chen H, Ren X, Ren Y, Peterson BW. et al. Artificial channels in an infectious biofilm created by magnetic nanoparticles enhanced bacterial killing by antibiotics. Small. 2019;15:1902313
Author contact
![]() Corresponding author: Miryana Hémadi, PhD, Université de Paris, CNRS-UMR 7086, Interfaces, Traitements, Organisation et DYnamique des Systèmes (ITODYS), Paris, France. hemadifr, Tel: +33-1-55278839
Corresponding author: Miryana Hémadi, PhD, Université de Paris, CNRS-UMR 7086, Interfaces, Traitements, Organisation et DYnamique des Systèmes (ITODYS), Paris, France. hemadifr, Tel: +33-1-55278839
 Global reach, higher impact
Global reach, higher impact