13.3
Impact Factor
Theranostics 2022; 12(8):3637-3655. doi:10.7150/thno.72756 This issue Cite
Research Paper
Eucommiae cortex polysaccharides mitigate obesogenic diet-induced cognitive and social dysfunction via modulation of gut microbiota and tryptophan metabolism
1. College of Veterinary Medicine, Northwest A&F University, Yangling, China
2. College of Resources and Environment Sciences, Northwest A&F University, Yangling, China
3. College of Basic Medicine, Xi'an Medical University, Xi'an, China
# These authors contributed equally to this work.
Abstract
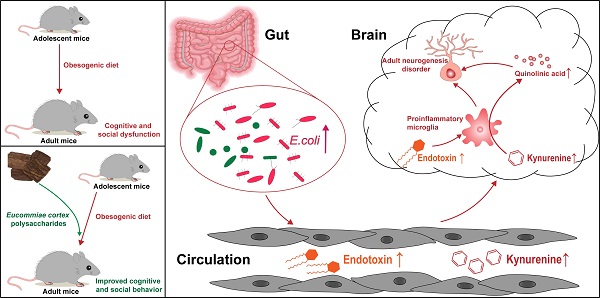
Rationale: The high fat and sucrose diet, known as the obesogenic diet (OD), has been related to low-grade chronic inflammation and neurodevelopmental disorders. Emerging evidence suggests that OD influences cognitive and social function via the gut-brain axis. However, the effects of OD during adolescence on future health have been unclear. Meanwhile, the underlying mechanisms and effective interventions are not fully understood. Polysaccharides, one of the most abundant substances in the Eucommiae cortex, exhibit potential immunomodulatory and neuroprotective effects. Here, we aimed to investigate the impact of OD on adolescents, explore the modulating roles of Eucommiae cortex polysaccharides (EPs) on OD-induced behavioral dysfunction, and elucidate the underlying molecular mechanisms.
Methods: In the present study, four-week-old mice were fed with OD for four weeks to simulate persistent OD in adolescents. The behavioral features were accessed by open field test and Morris water maze. The gut bacterial structure was identified by 16S rRNA gene amplicon sequencing. The gene and protein expression in colonic tissues and hippocampus were detected by qRT-PCR, immunoblotting, enzyme-linked immunosorbent assay, and immunofluorescence staining. Detection of biological metabolites in serum and hippocampal tissues was performed by widely targeted metabolomics and targeted metabolomics.
Results: We found that OD-fed mice showed cognitive and social-behavioral deficits accompanied by gut dysbiosis and systematic tryptophan (Trp) metabolism disorders, which increased kynurenine (Kyn) concentration in the hippocampus. Bacteria-derived lipopolysaccharide (LPS, endotoxin) induced microglia-mediated neuroinflammation, directing the metabolism of Kyn in the hippocampus toward quinolinic acid (QA), which led to glutamate-mediated hyperactivation of mossy cells (MCs) in hippocampal hilus. Furthermore, OD impaired parvalbumin (PV) interneurons-related local circuits in the hippocampal granule cell layer. These resulted in hippocampal neurogenesis deficits and related behavioral dysfunction in mice. EPs supplementation ameliorated OD-induced gut dysbiosis, as evidenced by inhibiting the expansion of Escherichia coli (E.coli) and reducing the concentration of LPS in colonic contents and serum, thereby inhibiting the subsequent neuroinflammation. In addition, oral EPs suppressed the peripheral Kyn pathway to reduce the concentration of QA and glutamic acid in the hippocampus of OD-fed mice, thereby rescuing the glutamic acid-triggered neuroexcitotoxicity. These contributed to remodeling the rhythm of hippocampal neurogenesis and mitigated behavioral dysfunction in OD-fed mice.
Conclusions: The present study addresses a gap in the understanding of neuronal dysfunction associated with OD during adolescence and provides the first evidence that EPs improved cognitive and social behavior via modulation of gut microbiota and tryptophan metabolism in adolescent mice fed with OD, which may represent novel preemptive therapy for neurodevelopmental disorders via manipulation of the tryptophan metabolite.
Keywords: Eucommiae cortex polysaccharides, obesogenic diet, gut microbiota, amino acid metabolism, adult neurogenesis
 Global reach, higher impact
Global reach, higher impact