13.3
Impact Factor
Theranostics 2022; 12(8):3862-3881. doi:10.7150/thno.70951 This issue Cite
Research Paper
Extracellular vesicles derived from astrocyte-treated with haFGF14-154 attenuate Alzheimer phenotype in AD mice
1. Department of Cell Biology & Institute of Biomedicine, College of Life Science and Technology, Jinan University, Guangzhou 510632, China.
2. Guangdong Provincial Key Laboratory of Bioengineering Medicine, Jinan University, Guangzhou 510632, China.
3. Guangzhou Biopharmaceutical R&D Center of Jinan University Co., Ltd, Guangzhou 510632, China.
4. Department of Laboratory Medicine, Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, China.
5. Department of Psychiatry, First Affiliated Hospital of Jinan University, Guangzhou 510630, China.
Abstract
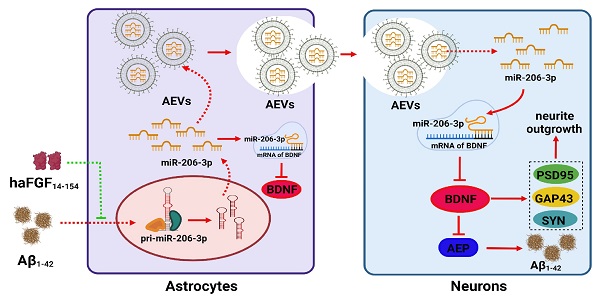
Background: aFGF content in serum and cerebrospinal fluid is increased in Alzheimer's disease (AD) patients and attenuates the activation of astrocytes. Extracellular vesicles (EVs) are a major mediator in astrocyte-neuron communications. Since excessive or persistent reactive astrocytes lead to chronic inflammation and neuronal dysfunction, and the activation of astrocytes can be inhibited by aFGF, we proposed that the cargoes of astrocyte-derived EVs (AEVs) might be modified by aFGF stimulation, playing an important role in AD progression. However, the mechanisms underlying the role of aFGF remain unclear.
Methods: AEVs were isolated from damaged astrocytes, treated with or without aFGF in Aβ-loading condition, and were intranasally administered to AD mice. We determined the ability of AEVs to enter the brain, ameliorate cognitive behavior deficits, alleviate the Aβ burden in the brain, and improve synapse ultrastructure. Subsequently, the miRNAs enriched in AEVs were sequenced to identify the key molecules specifically modified by aFGF. Finally, we explored the protective effects of miR-206-3p inhibition on cognitive deficiency and its regulatory mechanism and determined its role as a specific biomarker for potential AD diagnosis.
Results: AEVs stimulated by aFGF (defined as AEVs-Aβ+H) had favorable neuroprotection in AD pathology by enhancing neurite growth and reduction of Aβ loading on neurons in vitro. Following intranasal administration, AEVs-Aβ+H ameliorated cognitive behavior deficits, promoted synaptic plasticity, and alleviated brain Aβ burden in the APP/PS1 and Aβ brain-injected mice. AEVs-Aβ+H showed beneficial effects on AD similar to AEVs produced in normal situations (AEVs-Ctrl). aFGF stimulation modified the cargoes in EVs derived from Aβ damaged astrocytes, the most significant of which being the down-regulation of miR-206-3p. The miR-206-3p level was specifically high in the plasma of AD mice and patients, and miR-206-3p antagomir reversed the Alzheimer phenotype in AD mice. The brain-derived neurotrophic factor (BDNF) gene was negatively regulated by miR-206-3p and upregulated by AEVs-Aβ+H and miR-206-3p antagomir in AD mice. AEVs-Aβ+H inhibited δ-secretase (Asparagine endopeptidase, AEP) activation via the miR-206-3p/BDNF axis to alleviate Aβ burden in the AD brain.
Conclusion: Our findings highlight the role of aFGF in the modification of AEVs cargoes, especially miR-206-3p that can potentially serve as a biomarker for AD diagnosis and therapeutic target.
Keywords: Alzheimer's disease, aFGF, AEVs, miR-206-3p, BDNF
 Global reach, higher impact
Global reach, higher impact