13.3
Impact Factor
Theranostics 2022; 12(8):3946-3962. doi:10.7150/thno.73268 This issue Cite
Research Paper
The interplay between lncRNAs, RNA-binding proteins and viral genome during SARS-CoV-2 infection reveals strong connections with regulatory events involved in RNA metabolism and immune response
1. COVID-19 International Research Team (COV-IRT).
2. Instituto de Medicina Molecular João Lobo Antunes, Faculdade de Medicina, Universidade de Lisboa, 1649-028 Lisboa, Portugal.
3. MEtRICs, Department of Sciences and Technology of Biomass, NOVA School of Science and Technology,FCT NOVA, Universidade NOVA de Lisboa, 2829-516 Caparica, Portugal.
4. Department of Radiation Medicine, Georgetown University School of Medicine, Washington, DC 20007, USA.
5. Center for Translational Data Science, Biological Sciences Division, The University of Chicago, Chicago, IL 60615, USA.
6. Clever Research Lab, IL, USA.
7. Department of Biochemistry, Atal Bihari Vajpayee Institute of Medical Sciences & Dr Ram Manohar Lohia Hospital, New Delhi-110001, India.
8. Facultad de Ingeniería, Universidad Nacional de Asunción, San Lorenzo, Central, Paraguay.
9. Department of Biomedical and Health Informatics, The Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA.
10. Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
11. Bioinformatics and Computational Biology Program, Center for Metabolic Biology, Department of Genetics, Development and Cell Biology, Iowa State University, Ames, IA 50011, USA.
12. Logyx LLC, Mountain View, CA 94043, USA.
13. Space Biosciences Division, NASA Ames Research Center, Moffett Field, CA 94035, USA.
14. Johns Hopkins School of Medicine, Baltimore, MD 21287, USA.
15. Neuroscience Institute, Department of Neurobiology/ Department of Pharmacology and Toxicology, Morehouse School of Medicine, Atlanta, GA 30310, USA.
16. Department of Agricultural and Biological Engineering, Purdue University, West Lafayette, IN 47907, USA.
17. Center for Mitochondrial and Epigenomic Medicine, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA.
18. McAllister Heart Institute, Department of Pharmacology, and Department of Pathology and Lab Medicine, The University of North Carolina at Chapel Hill, NC 27599, USA.
19. Department of Physiology, Biophysics and Systems Biology, Weill Cornell Medicine, New York, NY, USA.
20. The HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute for Computational Biomedicine, Weill Cornell Medicine, New York, NY, USA.
21. New York Genome Center, New York, NY, USA.
22. The Feil Family Brain and Mind Research Institute, Weill Cornell Medicine, New York, NY, USA.
23. KBR, Space Biosciences Division, NASA Ames Research Center, Moffett Field, CA, 94035, USA.
24. Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, MA, 02142, USA.
Received 2022-3-26; Accepted 2022-4-24; Published 2022-5-9
Abstract
Rationale: Viral infections are complex processes based on an intricate network of molecular interactions. The infectious agent hijacks components of the cellular machinery for its profit, circumventing the natural defense mechanisms triggered by the infected cell. The successful completion of the replicative viral cycle within a cell depends on the function of viral components versus the cellular defenses. Non-coding RNAs (ncRNAs) are important cellular modulators, either promoting or preventing the progression of viral infections. Among these ncRNAs, the long non-coding RNA (lncRNA) family is especially relevant due to their intrinsic functional properties and ubiquitous biological roles. Specific lncRNAs have been recently characterized as modulators of the cellular response during infection of human host cells by single stranded RNA viruses. However, the role of host lncRNAs in the infection by human RNA coronaviruses such as SARS-CoV-2 remains uncharacterized.
Methods: In the present work, we have performed a transcriptomic study of a cohort of patients with different SARS-CoV-2 viral load and analyzed the involvement of lncRNAs in supporting regulatory networks based on their interaction with RNA-binding proteins (RBPs).
Results: Our results revealed the existence of a SARS-CoV-2 infection-dependent pattern of transcriptional up-regulation in which specific lncRNAs are an integral component. To determine the role of these lncRNAs, we performed a functional correlation analysis complemented with the study of the validated interactions between lncRNAs and RBPs. This combination of in silico functional association studies and experimental evidence allowed us to identify a lncRNA signature composed of six elements - NRIR, BISPR, MIR155HG, FMR1-IT1, USP30-AS1, and U62317.2 - associated with the regulation of SARS-CoV-2 infection.
Conclusions: We propose a competition mechanism between the viral RNA genome and the regulatory lncRNAs in the sequestering of specific RBPs that modulates the interferon response and the regulation of RNA surveillance by nonsense-mediated decay (NMD).
Keywords: SARS-CoV-2, long non-coding RNA, RNA-binding protein, regulatory network
Introduction
Pervasive transcription of the human genome generates a wide range of regulatory RNA molecules that control the flow of genetic information originated from the cell nucleus. Among these regulatory RNAs, long non-coding RNAs (lncRNAs), defined as those non-coding RNAs (ncRNAs) with sizes larger than 200 nucleotides and originated from specialized transcriptional units, are a very diverse class. These genes typically harbor their own promoters and regulatory sequences, many undergoing splicing and post-transcriptional modifications [1]. According to a recent update of the GENCODE database, the estimated number of lncRNA genes in the human genome is now over 18,000, a comparable number to the protein coding genes (around 20,000) [2]. LncRNA transcriptional units typically generate structured RNA molecules with regulatory functions that modulate the genomic output at different levels, including acting as scaffolds of high-molecular weight complexes as well as interacting with other biomolecules such as DNA, RNAs, and proteins [3-5]. LncRNAs have been found to have cell-state specific functions and have modulating effects on protein-coding gene transcription [6]. Transcriptomic analysis driven by next-generation sequencing applications has unveiled functional relationships between lncRNA and the pathophysiology of metabolic diseases, cancer, and infections [7-9]. The existence of a pathology is often accompanied by a dysregulation of lncRNA expression that could represent a secondary event associated with the disease or as a driving factor of the condition [10].
Viral infections are extreme cases of the interaction between two organisms in which the infectious agent strictly depends on the molecular and metabolic machinery of the infected cell to complete its replication and proliferation cycle. During the hijacking of the host cellular machinery by the virus, key molecular interactions between viral components and cellular structures are established. These interactions are responsible for the reorganization of cellular membranes to facilitate virus entry, modulation of cellular metabolism, and evasion of specific defense mechanisms [11]. Most of the knowledge about cellular and viral molecular players during infection is in the protein realm, represented by the characterization of viral-encoding polypeptides that are responsible for the progression of the infection or immune evasion, and their cellular cognate targets. However, the relevance of cellular and viral RNAs as relevant players within the context of an infection must be considered [12].
The roles of lncRNAs as mediators or drivers of viral infections were first unveiled in the last decade [13]. LncRNA mediators have been shown to play key roles in the regulation of the immune and inflammatory response against viral infections [14, 15]. These well-described examples define the regulatory role of individual lncRNAs during RNA viral infections [16-18]. For instance, a leading cause of viral gastroenteritis from the human norovirus can induce a strong lncRNA-based response in the host cells that is related to the regulation of the interferon response [19]. Strains of human hepatitis C virus (HCV) associated with long-term persistence downregulate the expression of lncPINT (p53-induced transcript long non-coding RNA) as a mechanism for circumventing the interferon defense mechanism and evading the innate immune response [20]. Following a similar strategy, the recently characterized lncRNA AP000253, provides a mechanism by which hepatitis B virus can remain occult for prolonged times within the host [21]. In many of these examples, results obtained from experimental models linked the lncRNA mediators of infection with a complex network of RNA-binding proteins (RBPs) [20, 22].
SARS-CoV-2, a respiratory RNA(+) virus with a rapid transmission pattern, was responsible for the global pandemic that started in late 2019. SARS-CoV-2 is a virus belonging to the coronaviridae family that enters the cell by specific interactions with the host ACE2 receptor [23, 24]. After internalization, cell infection is characterized by a dysregulated gene and protein expression pattern that includes an up-regulation of genes involved in the interferon response and interleukin production [25, 26]. If the virus evades host cell defenses, the replication of the genetic material is enabled by a multimeric RNA-dependent RNA polymerase. The RNA genome is translated into a polypeptide that is matured by proteolytic specific digestion with two viral proteases, the main protease (MPro) and the papain-like protease (PLPro) [27]. Whole virions are assembled and secreted by a pathway that involves the participation of the endoplasmic reticulum and Golgi complex [11, 12]. In severe cases, SARS-CoV-2 infected patients showed a striking pattern of acute inflammatory responses that has been related to the uncontrolled production of cytokines and designated as “cytokine storm” [28, 29].
Genomic SARS-CoV-2 RNA and its RNA transcripts interact with specific proteins modulating cellular responses to the infection, as revealed by high-throughput proteomic analysis [25, 30]. The multiple interactions between the viral genome/transcriptome and cellular proteins are a factor in promoting replication of the virus or, contrariwise, ensuring the success of the cell in preventing replication [31, 32]. Small non-coding RNAs (ncRNAs) have been previously described as regulatory factors in the virus-host interface [33]. However, the functions and roles of lncRNAs in the development and progression of SARS-CoV-2 infection remain uncharacterized. In this work, we determined the lncRNA dysregulation pattern induced by the SARS-CoV-2 infection and characterized the lncRNA-centered regulatory networks involving RBPs associated with RNA metabolism and interferon-mediated responses, by analysis of high-throughput transcriptomes of samples obtained from patients with and without SARS-CoV-2 infection. The detailed knowledge of the complex regulatory networks involving lncRNAs could open new perspectives for the design of targeted drugs to treat severe cases of SARS-CoV-2 infection.
Material and methods
Data source and group stratification
The source data for this study was generated within the framework of COV-IRT consortium (www.cov-irt.org) and deposited at the Short Read Archive (SRA) database with the project reference PRJNA671371, corresponding to a previously published study [26]. The dataset includes a shotgun metatranscriptomic (total RNA-seq) for host and viral profiling of 735 clinical specimens obtained from patients at the Weill Medical College of Cornell University, New York, USA. Patients were stratified according to the SARS-CoV-2 levels determined by qRT-PCR experiments by simultaneously using primers to amplify the E (envelope protein) and S (spike protein) genes together with the proper internal controls as previously described [26]. Patients with a cycle threshold value (Ct) less than or equal to 18 were assigned to “high viral load”, a Ct between 18 and 24 were assigned to “medium viral load”, and a Ct between 24 and 40 were assigned to “low viral load” classes, with anything above a Ct of 40 classified as “negative” [26]. These last patients were also subdivided according to the presence of other viral respiratory infections different from Covid19 and having compatible symptoms.
Analysis of RNAseq data
Raw Illumina sequence reads obtained by a pair-end sequencing strategy, were filtered, and trimmed with Trimmomatic software [34]. Filtered sequence reads were dual-aligned with the reference SARS-CoV-2 genome from Wuhan (strain reference MN908947.3) and the human genome (genome build GRCh38 and GENCODE v33) using the STAR aligner [35]. The gene counts were indexed to the different families of coding and non-coding gene transcripts by the BioMart data portal [36]. Data was normalized using the variance-stabilizing transform (vst) in the DESeq2 package [37]. Differential gene expression between working groups was determined by the Limma/Voom algorithm implemented in the iGEAK data processing platform for RNAseq data [38]. Criteria for selection of significant differentially expressed genes included an adjusted p-value < 0.05, and logFc < -1.0 or logFc > 1.0. All the gene expression data is publicly available at the Weill Cornell Medicine COVID-19 Genes Portal, an interactive repository for mining the human gene expression changes in the data from this study (covidgenes.weill.cornell.edu).
Bioinformatic analysis of lncRNA-centered regulatory networks
The functional annotation of the group of selected lncRNAs whose expression was induced by SARS-CoV-2 infection was performed by the ncFANS 2.0 platform using the ncRNA-NET module [39]. Applying this module, we determined the co-expression network involving the selected lncRNAs and protein-coding genes using data extracted from healthy tissues and compiled in the Genotype-Tissue Expression (GTEx) portal [40]. The correlated coding genes were functionally grouped by GO-term analysis, pathway enrichment, and determination of molecular signatures by the ncRNA-NET module in ncFANS. In addition to the classical GO-term enrichment analysis, the redundant ontology terms were filtered by REVIGO software [41].
The lncRNA-centered regulatory networks established between lncRNAs and RNA-binding proteins were constructed by interrogating the ENCORI database for RNA interactomes [42]. Graphical analysis and representation of lncRNA-centered regulatory networks was performed by NAViGaTOR software [43]. Functional similarity of the selected overexpressed lncRNAs in SARS-CoV-2 infection was inferred by integrating heterogeneous network data with IHNLncSim algorithm [44]. This approach integrates information from experimentally validated data at three levels of functional association: miRNA-lncRNA, disease-based correlation and GTEx expression-based networks.
Results
Host transcriptional shift induced by SARS-CoV-2 infection
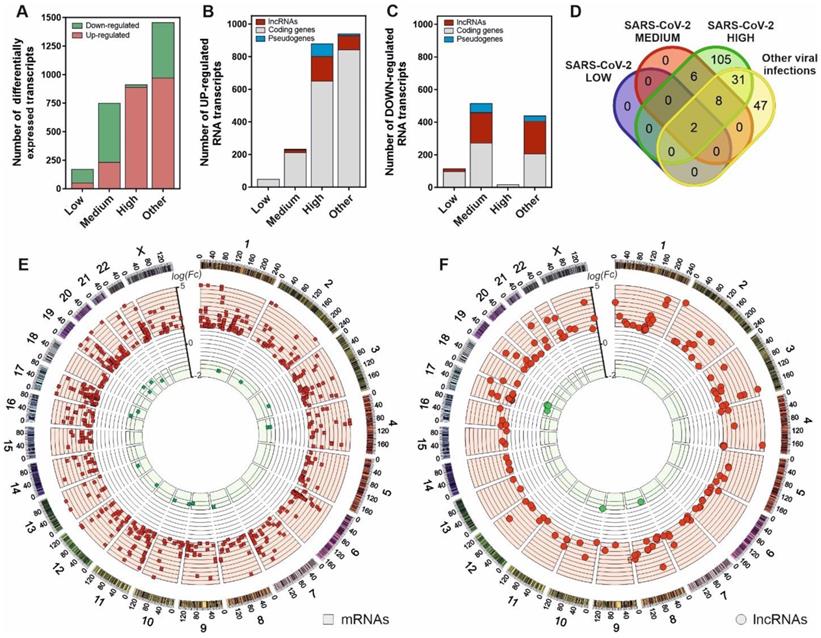
To characterize the cellular response against SARS-CoV-2 infection, we performed a transcriptomic analysis from nasopharyngeal swabs collected from patients testing for SARS-CoV-2 virus. The patients were previously stratified according to the presence or absence of positive qPCR test, the existence of other respiratory pathogens different from SARS-CoV-2 and the different virus loading depending on the amplification Ct parameters as described in the Material and Methods section. The results, depicted in Figure 1A, characterize the transcriptional dysregulation in the host cells associated with infections by SARS-CoV-2 and other respiratory viruses. In SARS-CoV-2 patients, increased viral load resulted in an increment of the number of upregulated transcripts (Figure 1B-C). Globally, the number of transcripts in infected patients with a logFC > 1 compared to uninfected control patients increased from 52 to 891 from low to high SARS-CoV-2 viral loads. Analyzing the different families of transcripts, high viral load SARS-CoV-2 infected patients together with those infected with other respiratory viruses showed a preferential upregulation pattern, where the coding RNAs were more abundant. Moreover, the patients with higher SARS-CoV-2 loads also showed greater proportion of upregulated transcripts represented by lncRNAs (Figure 1B).
Interestingly, from the 152 upregulated lncRNAs in high viral load samples, only 2 are common to all the analyzed infections. In SARS-CoV-2 infected patients, 105 upregulated lncRNAs were exclusive to the higher-level infections (Figure 1D). Positional gene enrichment analysis [46] of the upregulated lncRNA loci in high level SARS-CoV-2 infection showed two genomic regions enriched in overexpressed transcriptional units in response to the virus, comprising chr1: 148290889-155324176 and chr17: 32127595-62552121. The remaining overexpressed lncRNAs and coding mRNAs were evenly distributed across the different chromosomal loci with no evident spatial enrichment pattern (Figure 1E-F). The complete list of differentially expressed genes in all the comparisons is available as supplementary table (Table S1).
Functional analysis of upregulated lncRNAs in SARS-CoV-2 infection
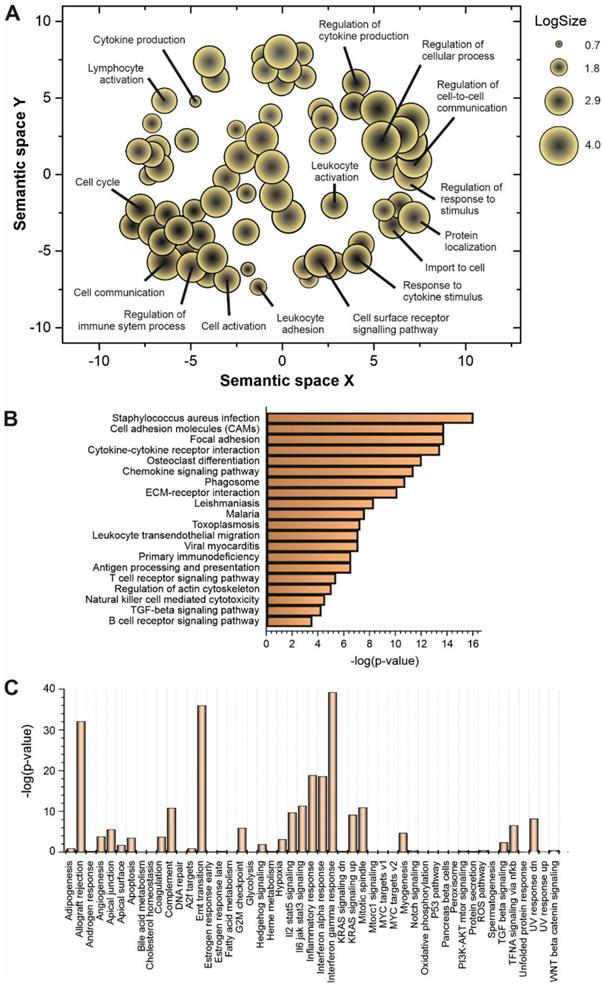
Some of the upregulated lncRNAs detected in patients with high viral load have been already characterized in different biological contexts (Table 1). However, to understand the global role of lncRNAs during SARS-CoV-2 infection, a transcriptome-wide analysis should be required. Prediction of lncRNA functions using the principles of systems biology is a challenging task due to the lack of supporting experimental evidence and the complexity of interactions established among lncRNAs and other functional players. Among the computer-based strategies available, we selected ncFANs 2.0 as a functional classifier [39]. The ncFANs-NET module was used to predict the functions of the upregulated lncRNAs in high-viral load infections by using the “guilty by association” approach. A correlation network between the differentially overexpressed lncRNAs and coding genes was constructed by ncFANs using data extracted from GTEx project database [47] and enrichment analyzed using terms from the Gene Ontology (GO) [48, 49] and KEGG databases [50]. The results of the functional analysis of the resultant co-expression network by GO-term enrichment with redundant term filtering, pathway analysis and molecular signature determination are depicted in Figure 2. GO-term enrichment within the category of molecular function resulted in the selection of terms related with cell-to-cell communication, and the general processes of lymphocyte activation and cytokine production (Figure 2A). The KEGG-pathway enrichment analysis resulted in a list of pathways also related with cytokine response and regulation, T-cell signaling and infections by viruses, bacteria, Trypanosoma and Apicomplexa parasites (Figure 2B), suggesting the common lncRNA-related regulatory responses exerted by the host cells against different infectious agents. Interestingly, the analysis of the molecular signatures in the regulatory lncRNA network revealed the existence of genes related with the interleukin signaling pathways, the interferon gamma response and the epithelial to mesenchymal transition phenomena, as more significant functions (Figure 2C).
SARS-CoV-2 infection is characterized by a gene expression pattern enriched in up-regulated mRNA and lncRNA transcripts that can be correlated with the viral load observed in patients. A, number of differentially expressed transcripts observed in patients with different SARS-CoV-2 viral loads (Low, Medium and High) and those infected with different respiratory viruses (Other) in comparison with the uninfected patients; B, number of the different families of up-regulated transcripts in SARS-CoV-2 patients and infected with other respiratory viruses in comparison with the control group; C, number of the different families of down-regulated transcripts in SARS-CoV-2 patients and infected with other respiratory viruses in comparison with the control group; D, Venn diagram representing the number of up-regulated lncRNA transcripts observed in each group of study referred to the uninfected control group; E, CIRCOS plot [45] showing the genomic location and fold changes of the differentially expressed coding transcripts in the group of SARS-CoV-2 patients infected with higher viral loads in comparison with the uninfected controls (red squares, up-regulated mRNAs; green squares, down-regulated mRNAs); F, CIRCOS plot [45] depicting the genomic locations and fold changes of the differentially expressed lncRNA transcripts in the group of SARS-CoV-2 patients infected with higher viral loads in comparison with the uninfected controls (red circles, up-regulated lncRNAs; green circles, down-regulated lncRNAs).
Expression of lncRNAs involved in the regulation of interferon response is correlated with SARS-CoV-2 viral load
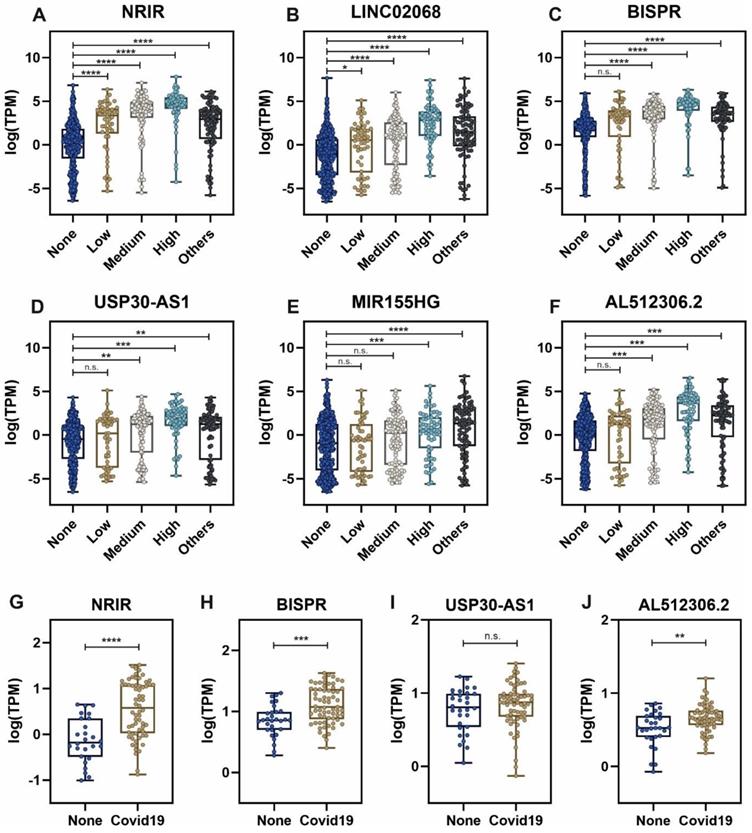
Considering the group of sample patients with higher SARS-CoV-2 viral loads, the top list of upregulated lncRNAs includes important non-coding transcripts previously described as regulators of the interferon-mediated immune responses (Table 1). The expression of these lncRNAs across the different groups of patients is depicted in Figure 3. NRIR, a driver of the interferon response [53], showed an upregulation pattern in patients infected with SARS-CoV-2 and other respiratory viruses (Figure 3A). A similar pattern can be observed in BISPR (Figure 3C), an interferon-induced lncRNA [55] and MIR155HG (Figure 3E), a lncRNA related to cell proliferation and the regulation of innate immune response against specific viral infections [69, 70]. The USP30-AS1 lncRNA is an antisense transcript to the USP30 gene that has been implicated in mitochondrial quality control in some cancers and also in the progression of virus-induced cancers such as malignant cervical tumors [61, 73], and is mainly upregulated in those SARS-CoV-2 patients with higher viral loads (Figure 3D). Interestingly, the careful analysis of COVIDOME database [74] allowed us to determine that NRIR and BISPR lncRNAs are also upregulated in the blood of Covid19 patients (Figure 3G-H). LINC02068 and AL512306.2 also showed an upregulation pattern which is dependent on the SARS-CoV-2 viral load in our patient cohort (Figure 3B-F) that can be also observed in the COVIDOME dataset for AL512306.2 transcript (Figure 3J).
upregulated lncRNAs detected in nasopharyngeal samples from patients with high SARS-CoV-2 viral loads that have been functionally characterized in different cellular processes or pathologies.
| Symbol | ENSEMBL gene | Location | Comments | References |
|---|---|---|---|---|
| NRIR | ENSG00000225964 | chr2:6968685 -6980595 | Negative regulator of interferon response. Experimental evidence linked this lncRNA to the control of the cellular immunity against viral infections. | [51-53] |
| BISPR | ENSG00000282851 | chr19:17516495 -17526545 | Interferon-stimulated positive regulator. This lncRNA belongs to a specific transcriptomic fingerprint developed in response to viral infections. | [54-56] |
| LINC02068 | ENSG00000223387 | chr3:172278691 -172313397 | This lncRNA has been described as a part of a molecular signature that predicts the outcome of endometrial cancer. | [57] |
| LINC01208 | ENSG00000223715 | chr3:176321936 -176353320 | Member of a molecular biomarker signature determined in breast cancer that can independently predict the patient survival rate. | [58] |
| USP30-AS1 | ENSG00000256262 | chr12:109489846 -109491770 | Antisense transcript to the USP30 gene. It has been characterized as an enhancer of cell proliferation in myeloid leukemia, colon, and cervical cancers. Its regulatory mechanisms involve the direct control of the expression of USP30 gene and the sponging of several miRNAs. | [59-61] |
| U62317.2 | ENSG00000272666 | chr22: 50604217 -50640354 | In bladder cancer, this lncRNA has been described as an important player in the regulation of the epithelial-to-mesenchymal transition, and directly related with the overall disease prognosis. | [62] |
| DLGAP1-AS5 | ENSG00000261520 | chr18:4264602 -4296000 | Antisense transcript to the DLGAP1 gene. In gastric cancer, its overexpression has been related with an increased endogenous immune response against the tumor and a better prognosis. | [63] |
| FMR1-IT1 | ENSG00000236337 | chrX:147028461-147029103 | Internal transcript to FMR1 gene. In head and neck carcinomas, this lncRNAs has been described as an independent prognosis biomarker. | [64] |
| MIR155HG | ENSG00000234883 | chr21:26934457-26947480 | Host gene for miR-155. This lncRNAs has been characterized as an important regulatory factor of innate immune response against viral infections and inflammation. Its regulatory actions are exerted via direct transcriptional control, epigenetic activation, miRNA sponging and the production of micropeptides. | [65-70] |
| C5orf56 | ENSG00000197536 | chr5:131746621-131811736 | Chromosome 5, open reading frame 56. Described in genetic associations with autoimmune diseases, but also as a prognosis factor of bladder cancer by its involvement in the regulation of the epithelial to mesenchymal transition. | [62, 71, 72] |
Functional prediction analysis by ncFANs 2.0 algorithm [39] of the upregulated lncRNAs observed in SARS-CoV-2 patients with high viral loads. A, GO-term enrichment analysis performed with ncFANs and filtered by removal of the redundant terms with REVIGO [41]. The filtered GO-terms are classified according to their two-dimensional arbitrary semantic space and represented by symbols with dimensions proportional to the LogSize, showing the most relevant GO-terms for the context of viral infections. B, pathway enrichment analysis by ncFANs using the KEGG database. C, molecular signature analysis by ncFANs using the MSigDB database.
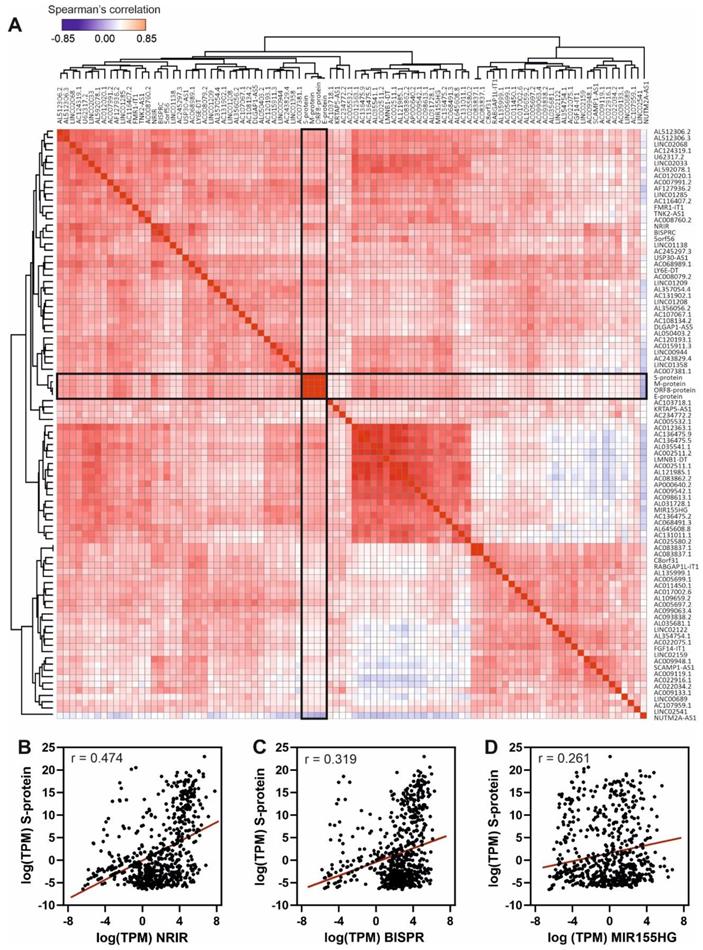
The results, depicted in Figure 4, show the Spearman's correlation analysis of the 90 most upregulated lncRNAs in high-load SARS-CoV-2 infections across all the studied samples. Correlation matrix (Figure 4A) clearly demonstrates the presence of clusters of lncRNAs with high correlation values that could respond to the existence of regulatory blocks defined by these lncRNAs. Next-generation sequencing data allowed us to detect SARS-CoV-2 transcripts in the patients' samples, the mRNA encoding for the Spike protein being the most abundant. Analyzing the correlations of S-protein mRNA with the overexpressed lncRNAs, we found moderate positive correlation with NRIR (Figure 4B) and BISPR (Figure 4C) lncRNAs and low positive correlation with MIR155HG (Figure 4D).
Expression levels of selected lncRNAs quantified by next-generation sequencing in nasal swabs from the working group of patients, and whole blood, obtained from the COVIDOME project database [74]. LncRNA expression in nasal swabs distributed by groups of patients: A, NRIR; B, LINC02068; C, BISPR; D, USP30-AS1; E, MIR155HG and F, AL512306.2. LncRNA levels in whole blood in SARS-CoV-2 patients (Covid19) and non-infected controls (None): G, NRIR; H, BISPR; I, USP30-AS1 and J, AL512306.2. Statistical comparisons between sample groups were made by one-way ANOVA in the case of samples from nasal swabs and by the Student's t-test in the data from the COVIDOME project (****, p-value < 0.0001; ***, p-value < 0.001; **, p-value < 0.01; *, p-value < 0.05 and n.s, non-significant).
Spearman's correlation analysis of the top 90 upregulated lncRNAs and the viral gene transcripts in the cohort of analyzed samples. A, Hierarchical clustered Spearman's correlation matrix for the overexpressed lncRNAs and the detected SARS-CoV-2 transcripts across all the samples analyzed by the BioCPR software [75]. The SARS-CoV-2 mRNA transcripts are highlighted within boxes; B, correlation analysis between NRIR lncRNA and S-protein transcript; C, correlation analysis between BISPR lncRNA and S-protein transcript; and D, correlation analysis between MIR155HG lncRNA and S-protein transcript. The correlation coefficients showed in panels b, c and d correspond to the Spearman analysis and are significant in all cases with p-values < 0.0001.
Functional links between RNA-binding proteins and lncRNAs in SARS-CoV-2 infection
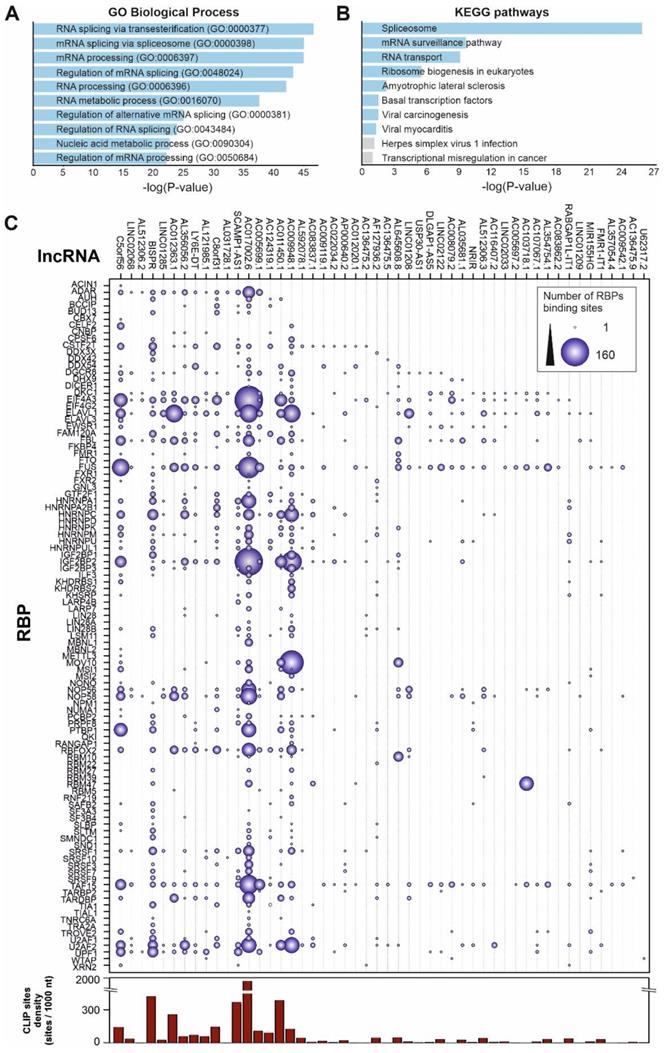
LncRNA function is exerted by their interaction with other biomolecules, namely DNA, other RNAs and proteins, and by the establishment of high-order molecular complexes where they can act as scaffolds and active regulatory players. To infer the possible role of the upregulated lncRNAs during SARS-CoV-2 infection we analyzed their interactions with RBPs to construct a network of functional relationships. We interrogated the ENCORI database of validated protein-RNA interactions [42] using the group of upregulated lncRNAs detected in patients with high SARS-CoV-2 loads and performed a functional enrichment analysis (Figure 5). The GO-term analysis of biological processes of the RBPs interacting with the upregulated lncRNAs showed an enrichment pattern of events related with RNA splicing, RNA metabolism and regulation of mRNA processing (Figure 5A). On the other hand, a pathway enrichment analysis based on KEGG database of the selected RBPs demonstrated significant enrichment in splicing, mRNA surveillance pathways, RNA transport and, interestingly, some already described viral-related processes as viral-induced carcinogenesis and viral endocarditis (Figure 5B).
To gain insights into the associations between RBPs and the Covid19-induced lncRNAs, we constructed a detailed interaction map by using the ENCORI data (Figure 5C). This connection map includes information about the specific RBPs interacting with the upregulated lncRNAs together with the density of RBP-binding sites in each lncRNA determined by CLIP experiments and extracted from the ENCORI database [42]. Considering the global number of RNA-binding sites determined by CLIP experiments, the most represented RBPs comprised the EIF4A3 helicase, core of the exon-junction complex [76], the FUS transcriptional regulator involved in DNA repair, transcription and splicing [77], the TAF15 transcriptional regulator [78], the ELAVL1 regulator of RNA stability [79], and the IGF2BP2 protein, a previously known regulatory player that can interact with several ncRNAs including miRNAs and lncRNAs [80]. Additional overrepresented RBPs include splicing factors U2AF2, FBL and CSTF2T, and the nucleolar proteins NOP56 and NOP58. We could distinguish two groups of upregulated lncRNA transcripts, depending on the density of RBP-binding sites (Figure 5C). LncRNAs AC017002.6, AC107959.1 and BISPR showed a high-density of RBP-binding sites (>500 CLIP sites per 1000 nucleotides); this is compatible with their involvement in regulatory events related with the capture of RBPs by RNA sponging [81, 82]. On the other hand, lncRNAs as NRIR, MIR155HG, U62317.2, USP30-AS1 and TNK2-AS1 contain a reduced number of RBP-binding sites (<2 CLIP sites per 1000 nucleotides), thus are likely involved in the activity of lncRNA-centered regulatory complexes [53, 70].
Interplay of RNA-binding proteins between host and viral RNAs
Experimental evidence obtained in cell and animal model systems, described the existence of lncRNA-centered regulatory networks that modulate gene expression at different levels [83]. These regulatory networks are often composed of many lncRNAs that work in a coordinated manner to exert a regulatory action over a specific pathway [6]. In the contact of an external stimuli such as an infection, cells trigger a complex response where lncRNAs are important players [8, 21, 84]. Since we determined the existence of an upregulation lncRNA pattern after SARS-CoV-2 infection, we hypothesized about the existence of a coordinated lncRNA network that could regulate the cellular response to the virus.
To evaluate the existence of putative lncRNA-based regulatory modules in Covid19 response, we computed the similarity scores of all observed upregulated lncRNAs in patients with high viral loads using the IHNLncSim algorithm [44]. The results showed the presence of a group of 6 upregulated lncRNAs with significant similarity scores computed by two of the modules within IHNLncSim, the NONCODE-net and the lncRNA-Disease-net modules. The lncRNA signature includes NRIR, BISPR, MIR155HG, USP30-AS1, FMR1-IT1 and U62317.2 non-coding transcripts (Figure 6A). NRIR and BISPR transcripts are virus-responsive lncRNAs involved in the regulation of the innate immune response and interferon signaling [51, 55]. MIR155HG has been also described as a regulator of the cellular response against influenza viruses [70] and recently characterized as upregulated in a cellular model of SARS-CoV-2 infection [85]. USP30-AS1 transcript is related to autophagy and mitochondrial quality control in the context of tumor progression [73, 86], whereas FMR1-IT1 and U62317.2 have no characterized functions.
This lncRNA signature can be complemented with two additional layers: the RBP interaction network and the connections between these RBPs and the SARS-CoV-2 genome, recently characterized in cellular models of infection [31, 32]. Regarding the lncRNA-RBP interactions, except for BISPR lncRNA, all the members of the lncRNA signature belong to the group of transcripts with low RBP-binding site density as described in 2.4. Additionally, the analysis of the functional connections between lncRNAs and the virus genome allowed to select a group of RBPs involved in direct interactions with SARS-CoV-2 genome and the members of the lncRNA network simultaneously (Figure 6A).
Functional analysis of the 50 top up-regulated lncRNAs by SARS-CoV-2 infection considering their validated interactions with RNA-binding proteins retrieved from ENCORI database [42]. A, GO-term enrichment analysis for biological processes of the RNA-binding proteins that interact with the selected overexpressed lncRNAs in SARS-CoV-2 patients with high viral loads; B, pathway enrichment analysis using the KEGG database and considering the RNA-binding proteins that interact with the selected overexpressed lncRNAs in SARS-CoV-2 patients with high viral loads; C, interaction map between RNA-binding proteins and the 50 top overexpressed lncRNAs in SARS-CoV-2 patients with high viral loads. The number of interactions is depicted as circles with a diameter proportional to the number of RNA-binding sites in each lncRNA. The right-hand side panel represents the density of RBP binding sites per 1000 nucleotides in each lncRNA as extracted from the ENCORI database.
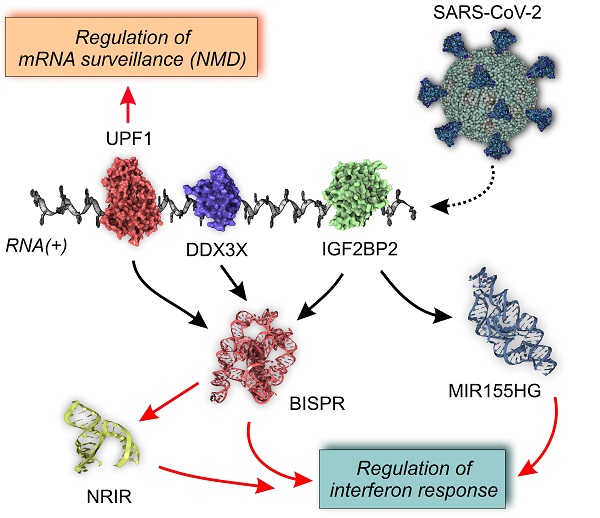
lncRNA-centered regulatory network established in SARS-CoV-2 infection involving upregulated lncRNAs, RNA-binding proteins and the viral genome. A, regulatory network built by heterogeneous network data analysis with IHNLncSim algorithm [44], the RNA-binding proteins extracted from ENCORI database [42] and the recently described interactions between host proteins and the viral genome [32]. Functional similarity among upregulated lncRNAs determined by IHNLncSim are represented by connecting continuous lines with thickness proportional to the value of the similarity coefficient value. Validated RNA-protein interactions from ENCORI database are represented by dashed grey lines. Characterized interactions between RNA-binding proteins and the SARS-CoV-2 genome are represented by dashed blue arrows. The size of the symbols representing lncRNAs (squares) and RNA-binding proteins (circles) are proportional to the number of established functional interactions. B, time course of protein expression from the selected RNA-binding proteins during SARS-CoV-2 in a cellular model, as described previously [25]. Expression data from quantitative proteomics were retrieved from the PRIDE partner repository database.
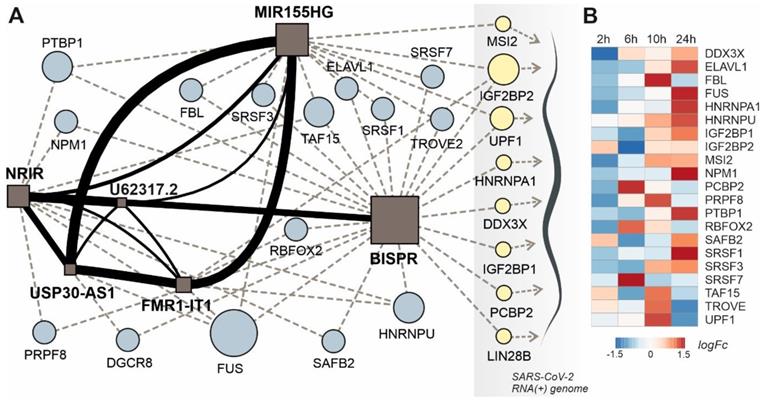
This group of RBPs comprises two helicases (UPF1 and DDX3X), three generic RBPs (IGF2BP1, IGF2BP2 and LIN28B), a poly-C-binding protein (PCBP2), a ribonucleoprotein (HNRNPA1) and a translational inhibitor (MSI2). Taking advantage of the quantitative proteomics data already available in a cellular model of SARS-CoV-2 infection [25] we analyze the time course of the selected RBPs expression during Covid19 infection (Figure 6B). During an infection time frame of 24h, the protein levels of the selected RBPs increased following the progression of the virus, showing maximum values at 10 or 24 hours of infection depending on the protein.
Discussion
Viruses interact with cellular factors to complete their replicative cycles, avoiding the cell antiviral mechanisms. The dissection of the tangled network of interactions established during viral infections is essential not only to understand viral biology but also to develop targeted antiviral therapeutics. Among host factors regulating the cellular responses against viral infections, lncRNAs have been recently described as important players in host-viral interactions [87]. LncRNAs are a diverse family of ncRNAs with regulatory potential exerted mainly by their presence in functional complexes integrated by proteins and other RNA molecules [88].
Within the framework of the COVID-19 International Team (COV-IRT), we used an integrative approach to dissect the SARS-CoV-2 infection mechanisms and their physiological consequences. As part of our multidisciplinary research efforts, we studied the role of host lncRNAs in the cellular response against the virus by using transcriptomic data obtained from nasopharyngeal swabs in a cohort of patients with different SARS-CoV-2 viral loads. We determined the existence of a transcriptomic dysregulation pattern in SARS-CoV-2 infected patients that is mainly represented by upregulated genes. In those patients with higher viral loads, a significant proportion of the upregulated transcriptome is composed by lncRNA transcripts.
To decipher the possible role of the upregulated lncRNAs during SARS-CoV-2 infection, we used a systems biology-based approach. In absence of biological validation, the “guilty by association” principle can be applied to predict the function of a group of lncRNAs [89, 90]. Using the ncFANS platform, the embedded NET algorithm and a subsequent enrichment for GO-terms and metabolic pathways, we revealed a striking molecular fingerprint of the differentially upregulated lncRNAs that pointed to their functional correlation with lymphocyte activation and cytokine signaling (Figure 2A-B). The intertwined relationship between cytokine signaling and lncRNA regulation provides a feedforward/feedback regulatory mechanism in the control of cellular responses to cytokines [91]. Moreover, the molecular signature of the upregulated lncRNAs during SARS-CoV-2 infection (Figure 2C), suggested their involvement in the regulation interferon-regulated inflammatory response.
Viral-induced lncRNA upregulation has been observed in respiratory viruses such as SARS-CoV [92], influenza [93], and SARS-CoV-2 [94, 95], using cellular or animal models of infection. Notably, in primary normal human bronchial epithelial cells (NHBE) infected with SARS-CoV-2, the transcriptomic analysis revealed the overexpression of interferon-responsive genes, namely IRF9, IFIT1, IFIT2, IFIT3, IFITM1, MX1, OAS2, OAS3, IFI44 and IFI44L, together with the induction of an acute inflammatory response and activation of tumor necrosis factor (TNF) [95]. In this model, the interferon response was also linked to the overexpression of at least 18 different lncRNAs. However, the lncRNAs induced in NHBE cells after viral infection are distinct from the signature we determined in the analyzed human nasopharyngeal samples, probably due to the cell and tissue specificity of lncRNA expression [95]. Involvement of interferon-responsive lncRNAs in the regulation of viral infections is a widespread phenomenon where lncRNAs usually have a detrimental effect over viral infections. For instance, the interferon-stimulated lncRNA (ISR) is actively induced after influenza virus infection in animal models and it is involved in the control of viral replication [15]. Interestingly, interferon-independent lncRNAs are frequently hijacked by viruses like influenza to promote their replication using different molecular mechanisms that involve the stabilization of the viral genome and its replication machinery [93]. In particular, Epstein Barr Virus is known to utilize IncRNAs in both lytic and latent phase infections [96] providing further evidence for the idea that many of these viral systems may be hijacking the same mechanism through LncRNAs. For EBV this is particularly interesting because of the role that EBV seems to play in long Covid [97], and how SARS-CoV-2 infection can induce latent phase activation of EBV itself.
The lncRNAs components of the non-coding transcriptional signature induced in nasopharyngeal samples during SARS-CoV-2 infection, NRIR, LINC02068, BISPR, USP30-AS1, MIR155HG and AL512306.2, are significantly upregulated (Figure 3). Interestingly, NRIR, BISPR, USP30-AS1 and AL512306.2 lncRNAs were also detectable at higher levels in blood samples of SARS-CoV-2 patients. NRIR, BISPR and MIR155HG levels in nasopharyngeal samples are correlated with the viral load, quantified as the expression of the S-protein coding gene (Figure 4). LncRNA NRIR, formerly designated as lncRNA-CMPK2, was first characterized as an interferon-responsive transcript that exerts a negative regulatory effect over the interferon defensive pathway. Using in vitro models of hepatitis C virus (HCV) infection, the knockdown of NRIR gene resulted in a marked reduction in HCV replication in interferon-stimulated hepatocytes, suggesting that it could affect the antiviral role of interferon [98]. In other viral infections such the Crimean-Congo hemorrhagic fever, NRIR has been also shown to be upregulated [51]. The molecular partners associated with NRIR regulatory action are still not characterized, however preliminary evidence suggested that NRIR could act via recruitment of chromatin-remodeling enzymes [53, 98]. In contrast, BISPR lncRNA is a divergent non-coding transcript generated from the promoter of the BST2 gene, that acts as a transcriptional enhancer of the BST2 gene [56]. BST2 protein, also known as Tetherin, is an interferon-induced transmembrane protein that has been involved in the inhibition of the replication of RNA viruses by controlling the release of the viral particles [99, 100] or by inducing the apoptosis of the host cells [101]. MIR155HG lncRNA has been also characterized as a positive regulator of the cellular immune response in influenza infections. In cellular models, MIR155HG showed an inhibitory effect on the expression of protein tyrosine phosphatase 1B (PTP1B) during the infection with influenza A virus, that could be directly related with the increase in the expression of interferon-beta [70]. Taken together, our data showed that the lncRNA signature induced by SARS-CoV-2 infection in the host includes counteracting regulators of cellular immunity: NRIR that could act as a promoter of viral replication by a negative regulation of interferon response, and BISPR and MIR155HG, that could act as antiviral lncRNAs. Understanding the balance between promoters and inhibitors of the viral progression will be essential to derive better effective therapeutics specially for severe cases of infection.
We performed additional analysis to infer the molecular consequences that might be associated with the lncRNA signature induced by SARS-CoV-2 infection. Considering that the regulatory lncRNA action is exerted by their contribution to the stability and function of macromolecular complexes, we applied a functional association method based on the IHNLncSim algorithm [44]. This strategy allowed us to characterize additional lncRNAs that could be participating in the host response to SARS-CoV-2 infection. Moreover, we combined protein-lncRNA interaction data to give insights about the possible involvement of the lncRNA-RBPs axis in the overall cellular response against infection. Globally, this functional analysis of the experimentally validated events involving RBPs and the virus-induced lncRNA signature showed a striking pattern related with the regulation of mRNA metabolism, stability, and processing by splicing (Figure 5A-B). Such connection between viral infections, lncRNAs and splicing has been demonstrated for other RNA viruses. Using Zika-infected human neural progenitor cells, Hu and coworkers observed that the transcriptional lncRNA shift induced by the virus was accompanied by a specific pattern of splicing events that affected genes involved in cell proliferation, apoptosis, and differentiation [102]. Similarly, proteomics and transcriptomic data obtained in cellular models of SARS-CoV-2 infection determined that the viral NSP16 protein binds to the mRNA recognition domains of the U1 and U2 spliceosomal RNAs, suppressing the canonical splicing events [103].
Among the viral-induced lncRNAs, we selected a specific signature composed by six elements (NRIR, BISPR, MIR155HG, USP30-AS1, FMR1-IT1, and U62317.2), for functional association studies [44]. Except for BISPR lncRNA, the functional lncRNA signature is composed of lncRNAs with low-density of RBP-binding sites, suggesting their involvement in specific regulatory events instead of being involved in the capture of RBPs by sponging (Figure 5C). Despite the previous functional characterization of some of the lncRNAs induced by SARS-CoV-2 infection [104-106], this regulatory network is not complete, and the presence of the viral RNA genome cannot be neglected. To support this idea, Schmidt and coworkers recently applied an antisense-based purification protocol coupled to mass spectrometry to determine the protein host-cell interactome in SARS-CoV-2 infection models [32]. The results presented a detailed RBP-virus interactome, showing how the external modulation of the levels of specific host proteins such LARP1 or CNBP could be used as therapeutic targets to restrict the viral replication [32].
The rationale of our proposed model is based on the integration of RBP-lncRNA interaction data combined with an additional layer of complexity established by the presence of the viral RNA genome. Analyzing the validated protein interactome of the lncRNA signature induced by SARS-CoV-2 infection and the proteomic data of the host-interacting proteins, we were able to determine the presence of RBPs that are simultaneously interacting with the SARS-CoV-2 genome and with the overexpressed lncRNAs (Figure 6A). This group of RBPs comprises MSI2, IGF2BP1, IGF2BP2, UPF1, HNRNPA1, DDX3X, PCBP2 and LIN28B proteins. Except for PCBP2, UPF1 and LIN28B proteins, the RBPs interacting with the viral genome and the upregulated lncRNAs, followed an expression time course that also accompanied the SARS-CoV-2 infection (Figure 6b) [25, 107]. This evidence suggested that the competition between host lncRNAs and the viral genome for the binding of specific RBPs could control the cellular response and the viral replicative cycle [108]. Our working hypothesis is supported by research on the roles of selected RBPs in other viral infections. For instance, UPF1, a highly processive helicase required for non-sense mediated decay (NMD) [109], has been described as an essential factor for the completion of the replicative cycle of HIV [110]. Moreover, DDX3X, another RNA helicase, could be probably the most relevant element of this lncRNA-RBP interaction network. In hepatitis B virus, DDX3X helicase is an essential factor since it can restrict the replicative cycle of the virus at the transcriptional level [111]. Additional evidence obtained in influenza virus infections also characterize DDX3X helicase as an essential and driving factor for the host cell response against influenza A virus (IAV) infection [112]. DDX3X is critical to orchestrate a multilayer antiviral innate response during infection, coordinating the activation of the inflammasome, assembly of stress granules, and type I interferon (IFN) responses. The loss of DDX3X expression in myeloid cells caused an increase of susceptibility to pulmonary infections and morbidity in IAV-infected mice [112]. However, the roles of DDX3X helicase as a promoting or preventing factor for viral infections appear to be virus-specific, as exemplified by the comparison of hepatitis B and C viruses [113]. In SARS-CoV-2 infection, the DDX3X protein levels are upregulated during infection (Figure 6B) suggesting its involvement in the cellular response against the virus. Also, among RBPs able to crosstalk with the viral genome and the upregulated lncRNAs is IGF2BP2, a multifaceted RBP able to control multiple metabolic processes [114]. There is no current evidence of a direct role of IGF2BP2 in the response against viral infections, but recent data connected its function to the lncRNAs. In thyroid cancer, IGF2BP2 is an oncogenic factor since it enhances the expression of the HAGLR lncRNA and the cellular proliferation [115]. Also, in non-small-cell lung cancer, IGF2BP2 enhances the proliferation of tumor cells by binding to the oncogenic MALAT1 lncRNA and increasing its stability [116]. In our model, IGF2BP2 can interact with BISPR and MIR155HG lncRNAs, two of the core elements of the functional signature induced by SARS-CoV-2 infection that are involved in the host cell innate immune response.
Conclusion
In summary, SARS-CoV-2 infection induces an upregulation of specific lncRNAs that are functionally correlated and involved in the innate immune response. These lncRNAs form part of an intricate network of interactions that involve specific RBPs, represented by helicases such as UPF1 and DDX3X, both regulators of RNA stability and surveillance. The presence of the viral RNA(+) genome introduces another layer of complexity since it can compete with lncRNA for the binding of the RBPs, being possibly involved in the modulation of the cellular response against infection and the viral progression. The specific functions of these interactions would require further biological validation and could open new possibilities for understanding the host components required for SARS-CoV-2 progression and designing new targeted antiviral therapeutics.
Abbreviations
IAV: Influenza A virus; HIV: human immunodeficiency virus; lncRNA: long non-coding RNA; mRNA: messenger RNA; RBP: RNA-binding protein; RNA: ribonucleic acid.
Supplementary Material
Supplementary table.
Acknowledgements
The authors would like to thank Francisco Enguita Jr. for his valuable insight and help in the design and conceptualization of the manuscript.
Author Contributions
FJE, ALL, ASB, CEM, RM and AB were responsible for the experimental design, data generation and data analysis. FJE, ALL, RM and AB designed the manuscript concept and wrote the text. FJE and ALL generated the figures and all the graphical material accompanying the manuscript. JTM, VZ, SD, DG, DT, ESW, ASB, SBB, DMP, DCW and JCS were involved in data interpretation and in technical and material support for the work. All authors critically revised the manuscript for scientific and intellectual content.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Koch L. Functional genomics: Screening for lncRNA function. Nat Rev Genet. 2017;18:70
2. Frankish A, Diekhans M, Jungreis I, Lagarde J, Loveland JE, Mudge JM. et al. Gencode 2021. Nucleic Acids Res. 2021;49:D916-D23
3. Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47-62
4. Yang Y, Wen L, Zhu H. Unveiling the hidden function of long non-coding RNA by identifying its major partner-protein. Cell Biosci. 2015;5:59
5. Zampetaki A, Albrecht A, Steinhofel K. Long Non-coding RNA Structure and Function: Is There a Link? Front Physiol. 2018;9:1201
6. Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96-118
7. Li Y, Li M, Luo H, Bai J, Zhang J, Zhong X. et al. Expression profile of lncRNA in human bronchial epithelial cells response to Talaromyces marneffei infection: A microarray analysis. Microb Pathog. 2017;104:155-60
8. Yin Z, Guan D, Fan Q, Su J, Zheng W, Ma W. et al. lncRNA expression signatures in response to enterovirus 71 infection. Biochem Biophys Res Commun. 2013;430:629-33
9. Zhang X, Ho TT. Computational Analysis of lncRNA Function in Cancer. Methods Mol Biol. 2019;1878:139-55
10. Chattopadhyay P, Srinivasa Vasudevan J, Pandey R. Noncoding RNAs: modulators and modulatable players during infection-induced stress response. Brief Funct Genomics. 2021;20:28-41
11. Klein S, Cortese M, Winter SL, Wachsmuth-Melm M, Neufeldt CJ, Cerikan B. et al. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat Commun. 2020;11:5885
12. Murgolo N, Therien AG, Howell B, Klein D, Koeplinger K, Lieberman LA. et al. SARS-CoV-2 tropism, entry, replication, and propagation: Considerations for drug discovery and development. PLoS Pathog. 2021;17:e1009225
13. Wang J, Wang Y, Zhou R, Zhao J, Zhang Y, Yi D. et al. Host Long Noncoding RNA lncRNA-PAAN Regulates the Replication of Influenza A Virus. Viruses. 2018 10
14. Fan J, Cheng M, Chi X, Liu X, Yang W. A Human Long Non-coding RNA LncATV Promotes Virus Replication Through Restricting RIG-I-Mediated Innate Immunity. Front Immunol. 2019;10:1711
15. Pan Q, Zhao Z, Liao Y, Chiu SH, Wang S, Chen B. et al. Identification of an Interferon-Stimulated Long Noncoding RNA (LncRNA ISR) Involved in Regulation of Influenza A Virus Replication. Int J Mol Sci. 2019 20
16. Kitabayashi J, Shirasaki T, Shimakami T, Nishiyama T, Welsch C, Funaki M. et al. Upregulation of the Long Non-Coding RNA HULC by Hepatitis C Virus and its Regulation of Viral Replication. J Infect Dis. 2020
17. More S, Zhu Z, Lin K, Huang C, Pushparaj S, Liang Y. et al. Long non-coding RNA PSMB8-AS1 regulates influenza virus replication. RNA Biol. 2019;16:340-53
18. Ren F, Ren JH, Song CL, Tan M, Yu HB, Zhou YJ. et al. LncRNA HOTAIR modulates hepatitis B virus transcription and replication by enhancing SP1 transcription factor. Clin Sci (Lond). 2020;134:3007-22
19. Lin SC, Qu L, Ettayebi K, Crawford SE, Blutt SE, Robertson MJ. et al. Human norovirus exhibits strain-specific sensitivity to host interferon pathways in human intestinal enteroids. Proc Natl Acad Sci U S A. 2020;117:23782-93
20. Khatun M, Zhang J, Ray R, Ray RB. Hepatitis C Virus Evades Interferon Signaling by Suppressing Long Noncoding RNA Linc-Pint Involving C/EBP-beta. J Virol. 2021;95:e0095221
21. Hao Q, Wang Z, Wang Q, Chen B, Qian H, Liu X. et al. Identification and characterization of lncRNA AP000253 in occult hepatitis B virus infection. Virol J. 2021;18:125
22. Zuo K, Kong L, Xue D, Yang Y, Xie L. The expression and role of lncRNA AX800134 in hepatitis B virus-related hepatocellular carcinoma. Virus Genes. 2018;54:475-83
23. Datta PK, Liu F, Fischer T, Rappaport J, Qin X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10:7448-64
24. Partridge LJ, Urwin L, Nicklin MJH, James DC, Green LR, Monk PN. ACE2-Independent Interaction of SARS-CoV-2 Spike Protein with Human Epithelial Cells Is Inhibited by Unfractionated Heparin. Cells. 2021 10
25. Bojkova D, Klann K, Koch B, Widera M, Krause D, Ciesek S. et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469-72
26. Butler D, Mozsary C, Meydan C, Foox J, Rosiene J, Shaiber A. et al. Shotgun transcriptome, spatial omics, and isothermal profiling of SARS-CoV-2 infection reveals unique host responses, viral diversification, and drug interactions. Nat Commun. 2021;12:1660
27. Stevens CS, Oguntuyo KY, Lee B. Proteases and variants: context matters for SARS-CoV-2 entry assays. Curr Opin Virol. 2021;50:49-58
28. Pum A, Ennemoser M, Adage T, Kungl AJ. Cytokines and Chemokines in SARS-CoV-2 Infections-Therapeutic Strategies Targeting Cytokine Storm. Biomolecules. 2021 11
29. Thepmankorn P, Bach J, Lasfar A, Zhao X, Souayah S, Chong ZZ. et al. Cytokine storm induced by SARS-CoV-2 infection: The spectrum of its neurological manifestations. Cytokine. 2021;138:155404
30. Stukalov A, Girault V, Grass V, Karayel O, Bergant V, Urban C. et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature. 2021;594:246-52
31. Lee S, Lee YS, Choi Y, Son A, Park Y, Lee KM. et al. The SARS-CoV-2 RNA interactome. Mol Cell. 2021;81:2838-50 e6
32. Schmidt N, Lareau CA, Keshishian H, Ganskih S, Schneider C, Hennig T. et al. The SARS-CoV-2 RNA-protein interactome in infected human cells. Nat Microbiol. 2021;6:339-53
33. McDonald JT, Enguita FJ, Taylor D, Griffin RJ, Priebe W, Emmett MR. et al. Role of miR-2392 in driving SARS-CoV-2 infection. Cell Rep. 2021: 109839.
34. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114-20
35. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15-21
36. Smedley D, Haider S, Durinck S, Pandini L, Provero P, Allen J. et al. The BioMart community portal: an innovative alternative to large, centralized data repositories. Nucleic Acids Res. 2015;43:W589-98
37. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550
38. Choi K, Ratner N. iGEAK: an interactive gene expression analysis kit for seamless workflow using the R/shiny platform. BMC Genomics. 2019;20:177
39. Zhang Y, Bu D, Huo P, Wang Z, Rong H, Li Y. et al. ncFANs v2.0: an integrative platform for functional annotation of non-coding RNAs. Nucleic Acids Res. 2021;49:W459-W68
40. Consortium GT. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318-30
41. Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6:e21800
42. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92-7
43. Shirdel EA, Xie W, Mak TW, Jurisica I. NAViGaTing the micronome-using multiple microRNA prediction databases to identify signalling pathway-associated microRNAs. PLoS One. 2011;6:e17429
44. Li J, Zhao Y, Zhou S, Zhou Y, Lang L. Inferring lncRNA Functional Similarity Based on Integrating Heterogeneous Network Data. Front Bioeng Biotechnol. 2020;8:27
45. Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D. et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639-45
46. De Preter K, Barriot R, Speleman F, Vandesompele J, Moreau Y. Positional gene enrichment analysis of gene sets for high-resolution identification of overrepresented chromosomal regions. Nucleic Acids Res. 2008;36:e43
47. Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA. et al. A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project. Biopreserv Biobank. 2015;13:311-9
48. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25-9
49. Gene Ontology C. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49:D325-D34
50. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27-30
51. Bayyurt B, Bakir M, Engin A, Oksuz C, Arslan S. Investigation of NEAT1, IFNG-AS1, and NRIR expression in Crimean-Congo hemorrhagic fever. J Med Virol. 2021;93:3300-4
52. Peng Y, Luo X, Chen Y, Peng L, Deng C, Fei Y. et al. LncRNA and mRNA expression profile of peripheral blood mononuclear cells in primary Sjogren's syndrome patients. Sci Rep. 2020;10:19629
53. Mariotti B, Servaas NH, Rossato M, Tamassia N, Cassatella MA, Cossu M. et al. The Long Non-coding RNA NRIR Drives IFN-Response in Monocytes: Implication for Systemic Sclerosis. Front Immunol. 2019;10:100
54. El Samaloty NM, Shabayek MI, Ghait RS, El-Maraghy SA, Rizk SM, El-Sawalhi MM. Assessment of lncRNA GAS5, lncRNA HEIH, lncRNA BISPR and its mRNA BST2 as serum innovative non-invasive biomarkers: Recent insights into Egyptian patients with hepatitis C virus type 4. World J Gastroenterol. 2020;26:168-83
55. Paliwal D, Joshi P, Panda SK. Hepatitis E Virus (HEV) egress: Role of BST2 (Tetherin) and interferon induced long non- coding RNA (lncRNA) BISPR. PLoS One. 2017;12:e0187334
56. Kambara H, Gunawardane L, Zebrowski E, Kostadinova L, Jobava R, Krokowski D. et al. Regulation of Interferon-Stimulated Gene BST2 by a lncRNA Transcribed from a Shared Bidirectional Promoter. Front Immunol. 2014;5:676
57. Xu H, Zou R, Liu J, Zhu L. A Risk Signature with Nine Stemness Index-Associated Genes for Predicting Survival of Patients with Uterine Corpus Endometrial Carcinoma. J Oncol. 2021;2021:6653247
58. Zhou W, Pang Y, Yao Y, Qiao H. Development of a Ten-lncRNA Signature Prognostic Model for Breast Cancer Survival: A Study with the TCGA Database. Anal Cell Pathol (Amst). 2020;2020:6827057
59. Zhou W, Xu S, Deng T, Zhou R, Wang C. LncRNA USP30-AS1 promotes the survival of acute myeloid leukemia cells by cis-regulating USP30 and ANKRD13A. Hum Cell. 2022;35:360-78
60. Li C, Liang X, Liu Y. lncRNA USP30-AS1 sponges miR-765 and modulates the progression of colon cancer. World J Surg Oncol. 2022;20:73
61. Chen M, Chi Y, Chen H, Zhao L. Long non-coding RNA USP30-AS1 aggravates the malignant progression of cervical cancer by sequestering microRNA-299-3p and thereby overexpressing PTP4A1. Oncol Lett. 2021;22:505
62. Tong H, Li T, Gao S, Yin H, Cao H, He W. An epithelial-mesenchymal transition-related long noncoding RNA signature correlates with the prognosis and progression in patients with bladder cancer. Biosci Rep. 2021 41
63. Cao J, Gong J, Li X, Hu Z, Xu Y, Shi H. et al. Unsupervised Hierarchical Clustering Identifies Immune Gene Subtypes in Gastric Cancer. Front Pharmacol. 2021;12:692454
64. Wang E, Li Y, Ming R, Wei J, Du P, Zhou P. et al. The Prognostic Value and Immune Landscapes of a m(6)A/m(5)C/m(1)A-Related LncRNAs Signature in Head and Neck Squamous Cell Carcinoma. Front Cell Dev Biol. 2021;9:718974
65. Wu X, Wan Q, Wang J, Hou P, Zhang Q, Wang Q. et al. Epigenetic Activation of lncRNA MIR155HG Mediated by Promoter Hypomethylation and SP1 is Correlated with Immune Infiltration in Glioma. Onco Targets Ther. 2022;15:219-35
66. Zhang C, Li J, Li H, Wang G, Wang Q, Zhang X. et al. lncRNA MIR155HG Accelerates the Progression of Sepsis via Upregulating MEF2A by Sponging miR-194-5p. DNA Cell Biol. 2021;40:811-20
67. Huan Z, Mei Z, Na H, Xinxin M, Yaping W, Ling L. et al. lncRNA MIR155HG Alleviates Depression-Like Behaviors in Mice by Regulating the miR-155/BDNF Axis. Neurochem Res. 2021;46:935-44
68. Niu L, Lou F, Sun Y, Sun L, Cai X, Liu Z. et al. A micropeptide encoded by lncRNA MIR155HG suppresses autoimmune inflammation via modulating antigen presentation. Sci Adv. 2020;6:eaaz2059
69. Peng L, Chen Z, Chen Y, Wang X, Tang N. MIR155HG is a prognostic biomarker and associated with immune infiltration and immune checkpoint molecules expression in multiple cancers. Cancer Med. 2019;8:7161-73
70. Maarouf M, Chen B, Chen Y, Wang X, Rai KR, Zhao Z. et al. Identification of lncRNA-155 encoded by MIR155HG as a novel regulator of innate immunity against influenza A virus infection. Cell Microbiol. 2019;21:e13036
71. John C, Guyatt AL, Shrine N, Packer R, Olafsdottir TA, Liu J. et al. Genetic Associations and Architecture of Asthma-COPD Overlap. Chest. 2022
72. Brandt M, Kim-Hellmuth S, Ziosi M, Gokden A, Wolman A, Lam N. et al. An autoimmune disease risk variant: A trans master regulatory effect mediated by IRF1 under immune stimulation? PLoS Genet. 2021;17:e1009684
73. Wang N, Li J, Xin Q, Xu N. USP30-AS1 contributes to mitochondrial quality control in glioblastoma cells. Biochem Biophys Res Commun. 2021;581:31-7
74. Sullivan KD, Galbraith MD, Kinning KT, Bartsch KW, Levinsky NC, Araya P. et al. The COVIDome Explorer researcher portal. Cell Rep. 2021;36:109527
75. Fey V, Jambulingam D, Sara H, Heron S, Sipeky C, Schleutker J. BioCPR-A Tool for Correlation Plots. Data. 2021;6:97
76. Ryu I, Won YS, Ha H, Kim E, Park Y, Kim MK. et al. eIF4A3 Phosphorylation by CDKs Affects NMD during the Cell Cycle. Cell Rep. 2019;26:2126-39 e9
77. Morlando M, Dini Modigliani S, Torrelli G, Rosa A, Di Carlo V, Caffarelli E. et al. FUS stimulates microRNA biogenesis by facilitating co-transcriptional Drosha recruitment. EMBO J. 2012;31:4502-10
78. Law WJ, Cann KL, Hicks GG. TLS, EWS and TAF15: a model for transcriptional integration of gene expression. Brief Funct Genomic Proteomic. 2006;5:8-14
79. Rothamel K, Arcos S, Kim B, Reasoner C, Lisy S, Mukherjee N. et al. ELAVL1 primarily couples mRNA stability with the 3' UTRs of interferon-stimulated genes. Cell Rep. 2021;35:109178
80. Cai R, Zhang Q, Wang Y, Yong W, Zhao R, Pang W. Lnc-ORA interacts with microRNA-532-3p and IGF2BP2 to inhibit skeletal muscle myogenesis. J Biol Chem. 2021;296:100376
81. Kim J, Abdelmohsen K, Yang X, De S, Grammatikakis I, Noh JH. et al. LncRNA OIP5-AS1/cyrano sponges RNA-binding protein HuR. Nucleic Acids Res. 2016;44:2378-92
82. Zhang X, Zhou Y, Chen S, Li W, Chen W, Gu W. LncRNA MACC1-AS1 sponges multiple miRNAs and RNA-binding protein PTBP1. Oncogenesis. 2019;8:73
83. Gil N, Ulitsky I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat Rev Genet. 2020;21:102-17
84. Runtsch MC, O'Neill LA. GOTcha: lncRNA-ACOD1 targets metabolism during viral infection. Cell Res. 2018;28:137-8
85. Wyler E, Mosbauer K, Franke V, Diag A, Gottula LT, Arsie R. et al. Transcriptomic profiling of SARS-CoV-2 infected human cell lines identifies HSP90 as target for COVID-19 therapy. iScience. 2021;24:102151
86. Wu L, Wen Z, Song Y, Wang L. A novel autophagy-related lncRNA survival model for lung adenocarcinoma. J Cell Mol Med. 2021;25:5681-90
87. Chen L, Zhou Y, Li H. LncRNA, miRNA and lncRNA-miRNA interaction in viral infection. Virus Res. 2018;257:25-32
88. Fernandes JCR, Acuna SM, Aoki JI, Floeter-Winter LM, Muxel SM. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA. 2019 5
89. Chen X, Sun YZ, Guan NN, Qu J, Huang ZA, Zhu ZX. et al. Computational models for lncRNA function prediction and functional similarity calculation. Brief Funct Genomics. 2019;18:58-82
90. Zhu J, Fu H, Wu Y, Zheng X. Function of lncRNAs and approaches to lncRNA-protein interactions. Sci China Life Sci. 2013;56:876-85
91. Carpenter S, Fitzgerald KA. Cytokines and Long Noncoding RNAs. Cold Spring Harb Perspect Biol. 2018 10
92. Peng X, Gralinski L, Armour CD, Ferris MT, Thomas MJ, Proll S. et al. Unique signatures of long noncoding RNA expression in response to virus infection and altered innate immune signaling. mBio. 2010 1
93. Wang J, Zhang Y, Li Q, Zhao J, Yi D, Ding J. et al. Influenza Virus Exploits an Interferon-Independent lncRNA to Preserve Viral RNA Synthesis through Stabilizing Viral RNA Polymerase PB1. Cell Rep. 2019;27:3295-304 e4
94. Morenikeji OB, Bernard K, Strutton E, Wallace M, Thomas BN. Evolutionarily Conserved Long Non-coding RNA Regulates Gene Expression in Cytokine Storm During COVID-19. Front Bioeng Biotechnol. 2020;8:582953
95. Vishnubalaji R, Shaath H, Alajez NM. Protein Coding and Long Noncoding RNA (lncRNA) Transcriptional Landscape in SARS-CoV-2 Infected Bronchial Epithelial Cells Highlight a Role for Interferon and Inflammatory Response. Genes (Basel). 2020 11
96. Wang H, Liu W, Luo B. The roles of miRNAs and lncRNAs in Epstein-Barr virus associated epithelial cell tumors. Virus Res. 2021;291:198217
97. Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens. 2021 10
98. Kambara H, Niazi F, Kostadinova L, Moonka DK, Siegel CT, Post AB. et al. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic Acids Res. 2014;42:10668-80
99. Pan XB, Han JC, Cong X, Wei L. BST2/tetherin inhibits dengue virus release from human hepatoma cells. PLoS One. 2012;7:e51033
100. Pan XB, Qu XW, Jiang D, Zhao XL, Han JC, Wei L. BST2/Tetherin inhibits hepatitis C virus production in human hepatoma cells. Antiviral Res. 2013;98:54-60
101. Yi E, Oh J, Kang HR, Song MJ, Park SH. BST2 inhibits infection of influenza A virus by promoting apoptosis of infected cells. Biochem Biophys Res Commun. 2019;509:414-20
102. Hu B, Huo Y, Yang L, Chen G, Luo M, Yang J. et al. ZIKV infection effects changes in gene splicing, isoform composition and lncRNA expression in human neural progenitor cells. Virol J. 2017;14:217
103. Banerjee AK, Blanco MR, Bruce EA, Honson DD, Chen LM, Chow A. et al. SARS-CoV-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses. Cell. 2020;183:1325-39 e21
104. Huang K, Wang C, Vagts C, Raguveer V, Finn PW, Perkins DL. Long non-coding RNAs (lncRNAs) NEAT1 and MALAT1 are differentially expressed in severe COVID-19 patients: An integrated single-cell analysis. PLoS One. 2022;17:e0261242
105. Sabetian S, Castiglioni I, Jahromi BN, Mousavi P, Cava C. In Silico Identification of miRNA-lncRNA Interactions in Male Reproductive Disorder Associated with COVID-19 Infection. Cells. 2021 10
106. Taheri M, Rad LM, Hussen BM, Nicknafs F, Sayad A, Ghafouri-Fard S. Evaluation of expression of VDR-associated lncRNAs in COVID-19 patients. BMC Infect Dis. 2021;21:588
107. Kamel W, Noerenberg M, Cerikan B, Chen H, Jarvelin AI, Kammoun M. et al. Global analysis of protein-RNA interactions in SARS-CoV-2-infected cells reveals key regulators of infection. Mol Cell. 2021;81:2851-67 e7
108. Wang Y, Wang Y, Luo W, Song X, Huang L, Xiao J. et al. Roles of long non-coding RNAs and emerging RNA-binding proteins in innate antiviral responses. Theranostics. 2020;10:9407-24
109. Fiorini F, Bagchi D, Le Hir H, Croquette V. Human Upf1 is a highly processive RNA helicase and translocase with RNP remodelling activities. Nat Commun. 2015;6:7581
110. Serquina AK, Das SR, Popova E, Ojelabi OA, Roy CK, Gottlinger HG. UPF1 is crucial for the infectivity of human immunodeficiency virus type 1 progeny virions. J Virol. 2013;87:8853-61
111. Ko C, Lee S, Windisch MP, Ryu WS. DDX3 DEAD-box RNA helicase is a host factor that restricts hepatitis B virus replication at the transcriptional level. J Virol. 2014;88:13689-98
112. Kesavardhana S, Samir P, Zheng M, Malireddi RKS, Karki R, Sharma BR. et al. DDX3X coordinates host defense against influenza virus by activating the NLRP3 inflammasome and type I interferon response. J Biol Chem. 2021;296:100579
113. Chang PC, Chi CW, Chau GY, Li FY, Tsai YH, Wu JC. et al. DDX3, a DEAD box RNA helicase, is deregulated in hepatitis virus-associated hepatocellular carcinoma and is involved in cell growth control. Oncogene. 2006;25:1991-2003
114. Wang X, Ji Y, Feng P, Liu R, Li G, Zheng J. et al. The m6A Reader IGF2BP2 Regulates Macrophage Phenotypic Activation and Inflammatory Diseases by Stabilizing TSC1 and PPARgamma. Adv Sci (Weinh). 2021;8:2100209
115. Dong L, Geng Z, Liu Z, Tao M, Pan M, Lu X. IGF2BP2 knockdown suppresses thyroid cancer progression by reducing the expression of long non-coding RNA HAGLR. Pathol Res Pract. 2021;225:153550
116. Han L, Lei G, Chen Z, Zhang Y, Huang C, Chen W. IGF2BP2 Regulates MALAT1 by Serving as an N6-Methyladenosine Reader to Promote NSCLC Proliferation. Front Mol Biosci. 2021;8:780089
Author contact
![]() Corresponding authors: F.J.E., email: fenguitaulisboa.pt; A.B., email: afshin.beheshtigov
Corresponding authors: F.J.E., email: fenguitaulisboa.pt; A.B., email: afshin.beheshtigov
 Global reach, higher impact
Global reach, higher impact