13.3
Impact Factor
Theranostics 2022; 12(9):4067-4080. doi:10.7150/thno.72948 This issue Cite
Review
Current update on theranostic roles of cyclophilin A in kidney diseases
Medical Proteomics Unit, Office for Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Received 2022-3-16; Accepted 2022-5-1; Published 2022-5-13
Abstract
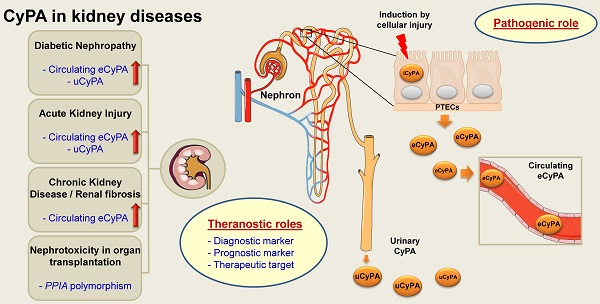
Cyclophilin A (CyPA) or peptidylprolyl isomerase A (PPIA), an immunophilin with peptidyl-prolyl cis/trans isomerase (PPIase) activity, is an abundant cellular protein widely expressed across various cell types and tissues, including the kidney. Expression of CyPA in the kidney is relatively higher in proximal tubular epithelial cells than others along the nephron. Alterations in expression and secretion of CyPA play important roles in physiological processes and pathophysiology of several diseases affecting the kidney. Herein, we provide a brief overview of CyPA structural biology and present the current update on its theranostic roles in various kidney diseases, including diabetic nephropathy, acute kidney injury, chronic kidney disease, renal fibrosis, and nephrotoxicity associated with organ transplantation. Notably, the diagnostic/prognostic role for urinary CyPA in several of these kidney diseases is promising. Finally, future perspectives on the CyPA research, especially targeting CyPA for therapeutics, are discussed. A comprehensive understanding of the theranostic roles of CyPA in kidney diseases is expected to provide novel insights into the design of new therapeutic interventions and prevention strategies.
Keywords: AKI, Biomarker, CKD, Diabetic nephropathy, Nephrotoxicity, PPIA, Renal fibrosis, Therapeutics
1. Introduction
Cyclophilin A (CyPA), also known as peptidylprolyl isomerase A (PPIA), is one among several proteins in a family with peptidyl-prolyl cis/trans isomerase (PPIase) activity. The PPIase activity was first identified by Fischer et al. [1] in 1984 as a catalytic enzyme for conversion of cis and trans isomers of proline imidic peptide bonds. In the same year, Handschumacher et al. [2] firstly reported that an 18-kDa protein from bovine thymocytes serves as an intracellular receptor for an immunosuppressive agent, cyclosporin A (CsA). Therefore, this CsA-binding protein has been named as CyPA [2]. By the end of the 1980s, PPIase and cyclophilin were found to be the same molecule [3, 4]. However, it has been documented that the immunosuppressive effect of CsA is unrelated to the isomerase activity of PPIase but rather occurs via formation of the CsA-CyPA complex, which inhibits calcineurin activity, leading to suppression of T-cell activation through nuclear factor of the activated T-cells (NFAT) pathway [5-7]. CyPA can be intracellularly and extracellularly expressed in many cell types, e.g., vascular smooth muscle cells (VSMCs), endothelial cells, macrophages, and kidney cells [8, 9]. As a result, CyPA serves as a multifunctional protein [8, 9]. Although the roles for CyPA have been well documented in several diseases and conditions, its roles in kidney diseases remained not well understood and under-investigated. In this article, we therefore provide a brief overview of the CyPA structural biology and update the current knowledge on its theranostic roles in various kidney diseases.
2. A brief overview of CyPA structural biology
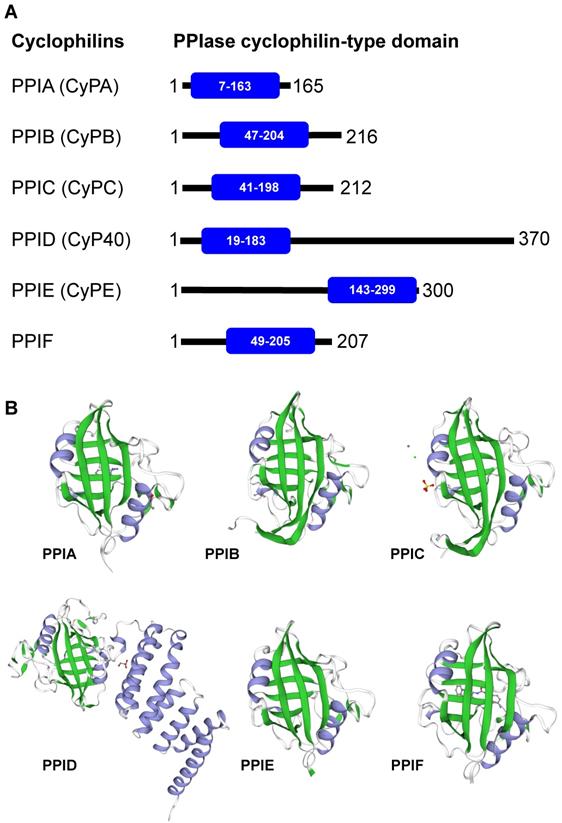
Cyclophilins are members of the immunophilin protein family, which is characterized by a signature domain containing PPIase activity [10]. They are ubiquitously expressed in prokaryotes and eukaryotes, ranging from bacteria, fungi, insects, plants and mammals [11]. Additionally, cyclophilins are widely expressed across many different organs [10, 12]. The major forms of cyclophilins reported in humans include CyPA (PPIA), CyPB (PPIB), CyPC (PPIC), CyP40 (PPID), CyPE (PPIE), and PPIF [3]. Herein, we avoid using an acronym CyPD, which is somewhat confusing as it can refer to PPID (encoded by the PPID gene on chromosome 4) and/or PPIF (encoded by the PPIF gene on chromosome 10). Mapping of the PPIase cyclophilin-type domain in these cyclophilins and their 3D structures are illustrated in Figures 1A and 1B, respectively. Most of the cyclophilins (CyPA, CyPB and CyPC) are found in cytoplasm and extracellularly, while CyPE is found in nuclear compartment and PPIF is identified as a mitochondrial cyclophilin [3]. Among them, CyPA is the most abundant cyclophilin accounting for about 0.1-0.6% of total protein in the cytoplasmic compartment [12].
Human cyclophilins. Amino acid sequences, protein name, and/or description of human cyclophilins were obtained from The UniProt Knowledgebase (UniProtKB) database (https://www.uniprot.org/). (A): Alignment of human cyclophilins together with the PPIase cyclophilin-type domain in each cyclophilin. (B): The 3D structure of individual cyclophilins generated using SWISS-MODEL template library (https://swissmodel.expasy.org/templates/), including PPIA:PDB (4n1s), PPIB:PDB (1cyn), PPIC:PDB (2esl), PPID:PDB (1ihg), PPIE:PDB (2r99), and PPIF:PDB (5ccs). Their β-sheet and α-helix are labelled with green and greyish purple, respectively. Note that the unique structure of the cyclophilin protein family consists of 8 β-strands and 2 α-helices.
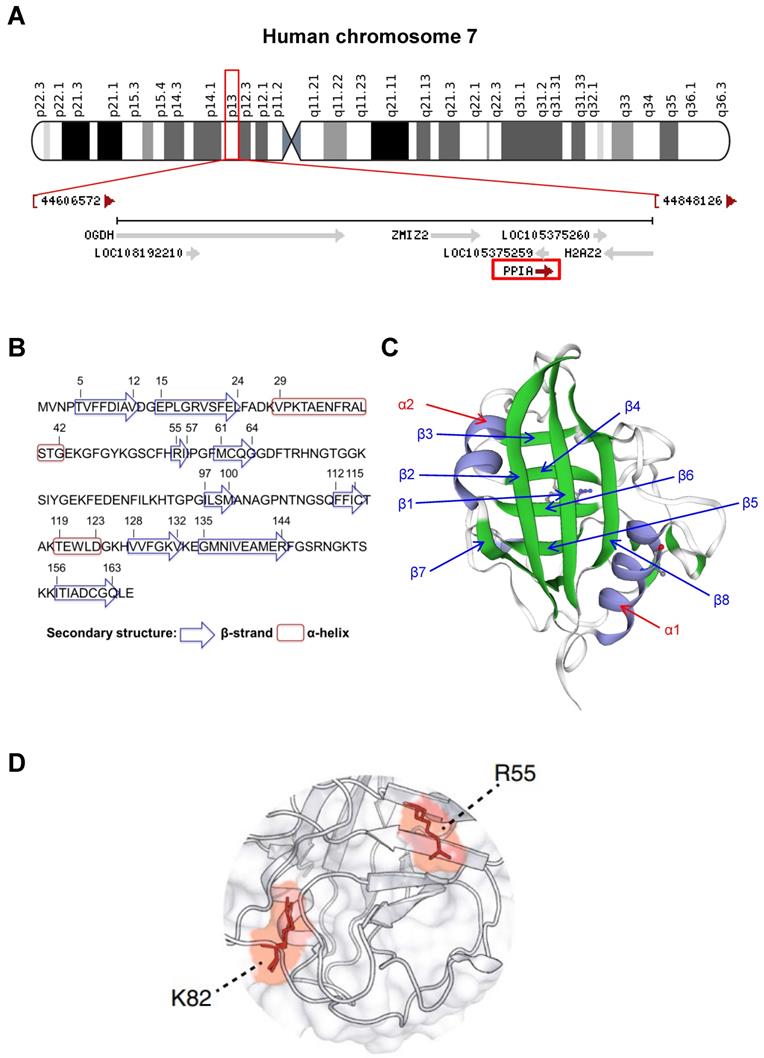
CyPA is encoded by PPIA gene on chromosome 7 at location 7p13 (NC_000007.14: 44,795,960-44,803,117) (Figure 2A). This protein belongs to the cyclophilin-peptidyl prolyl isomerase-like family and comprises 165 amino acids with 8 β-strands and 2 α-helices (Figures 2B and 2C). The 3D structure of the CyPA PPIase isomerization active site has been elucidated elsewhere, and the key residues have been characterized (Figure 2D). The study has shown that arginine at the 55th residue (R55) and lysine at the 82nd residue (K82) are important for catalytic activity of CyPA-mediated cis/trans-isomerization [13]. This has been confirmed by alanine (A) substitutions at these two positions that lead to demolition of the catalytic function of CyPA [13].
Besides the PPIase isomerization function, CyPA can be secreted to the extracellular compartment to mediate chemotactic effects [14-16]. This action occurs via specific binding of CyPA to a type I integral membrane glycoprotein, CD147 [14-16]. Proline and glycine residues at positions 180 and 181 (P180 and G181) of the CD147 extracellular domain are the key amino acids that mediate CyPA-CD147 interaction [17]. Nonetheless, another later study has reported that P211 (instead of P180) of CD147 transmembrane domain is a critical residue for such binding [18]. This has been supported by evidence demonstrating that P211A mutation drastically reduces CD147-derived peptide and CyPA interaction [19]. Even though the results obtained from these studies are contradictory, CD147 evidently binds CyPA, while the precision of key residues responsible for such CyPA-CD147 interaction still needs further elucidations. On the CyPA side, its three amino acids, including R69, H70 and T107, have been identified as the key residues that play important role in CD147 binding [18].
3. Physiologic and pathophysiologic roles of CyPA in general
The physiologic function of CyPA-PPIase isomerase activity has been documented as a molecular chaperone that regulates protein folding, trafficking and activities [11, 20, 21]. Several studies have also reported that CyPA is a multifunctional molecule with known major roles in cellular signaling, gene regulation, inflammation and apoptosis [21-23]. The data obtained from VSMCs study has shown that reactive oxygen species (ROS) activate vesicle-associated membrane protein, resulting in secretion of CyPA via Rho-associate protein kinase (ROCK) pathway [24]. ROCK is one of the serine/threonine kinases that serves as a key downstream effector of the small GTP-binding protein, RhoA, which regulates actin cytoskeleton organization and activates myosin II phosphorylation essential for transportation of CyPA toward plasma membranes [24].
CyPA undergoes various post-translational modifications [25, 26]. CXCR4 (C-X-C motif chemokine receptor 4) signaling induces phosphorylation, while oxidative stress and angiotensin II promote acetylation of intracellular CyPA (iCyPA) [27, 28]. The acetylated form of iCyPA is then secreted from the cells to extracellular space [28]. Interestingly, acetylated extracellular CyPA (eCyPA), in turn, can display an autocrine effect to activate cellular functions [28]. Moreover, acetylation at different lysine (K) residues in eCyPA can determine differential activities of eCyPA [28, 29]. Generally, eCyPA has similar roles as of iCyPA (e.g., to induce inflammatory response and cell proliferation) [30, 31]. Nevertheless, eCyPA also has some unique functions in activating apoptosis, cell migration, extracellular matrix (ECM) degradation, and ROS production [25, 30, 32, 33].
For pathophysiologic function, it has been documented that HK-2 renal cells under hyperglycemic condition have increased secretion of eCyPA, which in turn stimulates p38 mitogen-activated protein kinase (MAPK) pathway [34]. Another study has also reported that eCyPA binds CD147, resulting in activation of ERK1/2 and p38 MAPK signaling pathways and cell proliferation [35]. In concordance, an adhesion molecule secreted from Mycoplasma genitalium can induce secretion of eCyPA and its interaction with CD147 on urothelial cells, resulting in activation of extracellular signal-regulated kinase (ERK)/nuclear factor (NF)-κB pathway [36]. As a result, cellular inflammatory response occurs together with production and release of proinflammatory cytokines and mediators, e.g., interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), and matrix metalloproteinase-9 (MMP-9) [36]. Altogether, the accumulated evidence strengthens the role of eCyPA to evoke the inflammatory response.
CyPA has been reported to serve as a key factor in viral infections, including human immunodeficiency virus-1 (HIV-1) [37], hepatitis B virus (HBV) [38], hepatitis C virus (HCV) [39], and severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) [40]. The precise pathogenic roles for CyPA depend on type of the viral infection. For example, CyPA serves as an important intermediate proinflammatory cytokine that advocates the pathogenesis of SARS-CoV-2 infection through the CD147-dependent MAPK pathway [41]. Also, plasma eCyPA level is greater in patients with severe coronavirus disease 2019 (COVID-19) as compared with those with milder forms of COVID-19 and healthy individuals [40].
General structural biology of CyPA. (A): Mapping of location of PPIA gene on chromosome 7 at location 7p13 (NC_000007.14: 44,795,960-44,803,117) as indicated in the red box. (B): Amino acid sequence of CyPA. The residues that form the secondary structure (β-strand and α-helix) are labeled. (C): The 3D structure of CyPA with 8 β-strands and 2 α-helices. (D): The key amino acids, R55 and K82, that play crucial role in CyPA-mediated cis/trans isomerization (adopted from Ref. [13] that is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format).
Furthermore, many other diseases have been reported to be associated with the pleiotropic functions of CyPA. For example, elevation of eCyPA level correlates with cardiovascular diseases [42], rheumatoid arthritis [43, 44], and liver diseases [45]. For kidney diseases, much greater details are provided in the following sections.
4. Roles of CyPA in kidney diseases
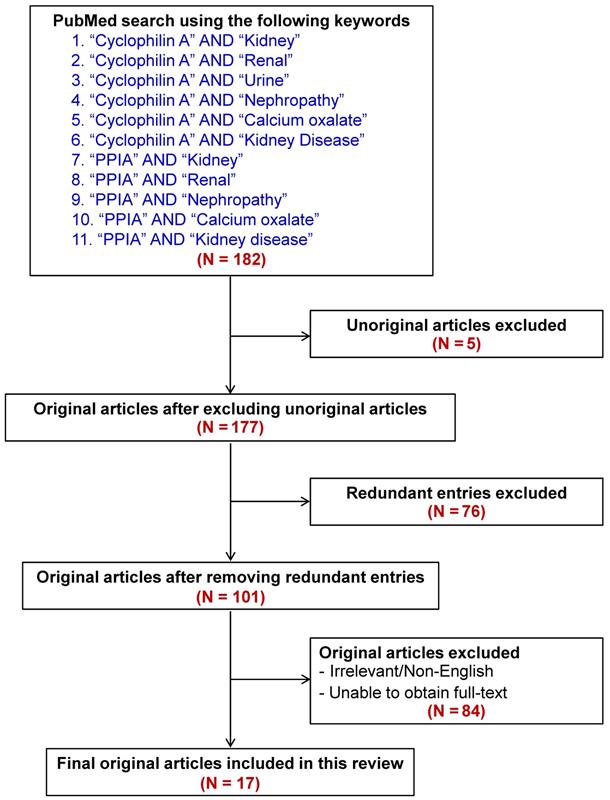
By using a pairwise searching method with the keywords indicated in Figure 3, a total of 182 articles were initially retrieved from PubMed literature search. The unoriginal, redundant, irrelevant, non-English, and fulltext-inaccessible articles were then excluded. Finally, there were a total of 17 articles qualified for discussion in this review (also summarized in Table 1). In addition, other articles that were not within the search criteria but their contents were related to our topic were also included for thorough discussion.
Summary of main findings in previous studies on roles of CyPA in kidney diseases.
| Kidney disease | Reference* | Publication date | Main findings | Type of sample(s) | CyPA role in diagnostics/ prognostics | CyPA role in therapeutics |
|---|---|---|---|---|---|---|
| Diabetic nephropathy (DN) | Ramachandran S, et al. [46] | 2014 | Level of circulating extracellular CyPA (eCyPA) significantly increases in diabetic patients. | Human plasma. | Yes | - |
| Tsai SF, et al. [55] | 2015 | The first report demonstrating that urinary CyPA (uCyPA) serves as a new biomarker for early detection of DN. | Human urine. MES-13 cells. HK-2 cells. | Yes | - | |
| Tsai SF, et al. [34] | 2016 | uCyPA is a sensitive marker for early detection of DN. | Mouse urine. MES-13 cells. HK-2 cells. | Yes | - | |
| Chiu PF, et al. [15] | 2018 | Plasma eCyPA and CD147 levels correlate with progression of DN in Type 2 diabetic patients. | Human plasma. | Yes | - | |
| Zhang X, et al. [47] | 2020 | Overexpression of STAT3 and CyPA leads to podocyte injury induced by high glucose. | Mouse podocytes. | Yes | - | |
| El-Ebidi AM, et al. [56] | 2020 | uCyPA level in 24-h urine is significantly higher in diabetic rats compared with non-diabetic controls. | Rat urine. | Yes | - | |
| Salem NA, et al. [57] | 2020 | uCyPA and uCyPA/Cr ratio increase in Type 1 DM pediatric patients with microalbuminuria. | Human urine. | Yes | - | |
| Acute kidney injury (AKI) | Lee CC, et al. [66] | 2019 | eCyPA can be used for postoperative AKI detection in cardiac surgery patients. Circulating eCyPA and uCyPA levels markedly increase in AKI compared with non-AKI group. | Human serum. Human urine. | Yes | - |
| Leong KG, et al. [64] | 2020 | CyPA increases in the kidney during renal ischemia/reperfusion-induced AKI. Knockdown of its gene (PPIA) reduces tissue inflammation. | Mouse kidney. Mouse primary tubular cells. | Yes | - | |
| Leong KG, et al. [62] # | 2020 | CyPA inhibitor (GS-642362) has a protective effect against acute renal failure in the renal ischemia/reperfusion-induced AKI model in a dose-dependent manner. It decreases tubular cell death via reduction of neutrophil infiltration. | Mouse kidney. Mouse plasma. Mouse primary tubular cells. | - | Yes | |
| Cabello R, et al. [63] | 2021 | Renal tubular cells secrete greater amount of eCyPA during different cell death pathways. Increasing uCyPA serves as the biomarker of ischemia/reperfusion-induced AKI independent from other parameters of kidney function. | HK-2 cells. MCT cells. Human urine. | Yes | - | |
| Chronic kidney disease (CKD) and renal fibrosis | Liu MC, et al. [81] | 2015 | The level of serum eCyPA may be associated with impaired renal function in CKD. eCyPA released from kidney cells or other cell types under the CKD environment promotes atherosclerosis. | Human serum. | Yes | - |
| Chen YH, et al. [84] | 2016 | CyPA is a mediator for ROS production. Caveolin-1 inhibits CyPA-induced ROS overproduction. | Rabbit kidney. Rabbit serum. | - | Yes | |
| Jin K., et al. [85] | 2017 | Markedly elevated plasma eCyPA positively correlates with systemic inflammation markers, such as high-sensitivity C-reactive protein, IL-6 and TNF-α in ESRD patients. | Human plasma. | Yes | - | |
| Dihazi GH., et al. [87] | 2020 | Secretion of eCyPA is associated with renal fibrosis. Inhibition of CyPA activity decreases ECM protein production and accumulation. | Mouse kidney. TK173 cells. TK188 cells. | Yes | Yes | |
| Leong KG, et al. [62] # | 2020 | CyPA inhibitor (GS-642362) also inhibits PPIF. GS-642362 decreases tubular cell death, macrophage infiltration, and renal fibrosis in unilateral ureteric obstruction (UUO) animal model. | Mouse kidney. Mouse plasma. Mouse primary tubular cells. | - | Yes | |
| Nephrotoxicity associated with organ transplantation | Moscoso-Solorzano GT, et al. [97] | 2008 | Polymorphism (-11 G/C) on PPIA promoter is associated with nephrotoxicity after renal transplantation. | Human (EDTA-preserved) blood. | Yes | - |
| Yilmaz DE, et al. [96] | 2022 | PPIA knockdown increases the unfolded protein response (UPR) similar to the effects of CsA treatment. Modifying CyPA and/or UPR may help to reduce nephrotoxicity associated with CsA in renal transplantation. | HEK-293 cells. HRPTEpCs primary cells. Rat proximal tubules. | - | Yes |
* See details of search parameters and criteria in Figure 3.
# Both AKI and CKD/renal fibrosis were investigated in this study.
Flow chart representing inclusion and exclusion literature search criteria to retrieve the articles for this review. From a total of 182 articles initially retrieved using the well-defined keywords, 17 relevant articles were included for discussion in this review. Main findings reported in these articles are summarized in Table 1.
4.1. CyPA and diabetic nephropathy (DN)
Various cell types can secrete eCyPA under different conditions [21, 22]. In diabetic patients, hyperglycemia and oxidative stress can stimulate the secretion of eCyPA from peripheral blood monocytes, resulting in a rise of plasma eCyPA level [46]. Furthermore, a positive correlation has been found between plasma eCyPA level and age, blood sugar level and hemoglobin A1c (HbA1c) level [46]. High glucose also activates signal transducer and activator of transcription 3 (STAT3)-CyPA interaction, which induces inflammation, oxidative stress, and apoptosis in podocytes [47]. IL-37 is an anti-inflammatory and anti-immune response molecule. A recent study has shown that overexpression of STAT3 and CyPA leads to inhibition of the IL-37-mediated protective effects against podocyte injury induced by high glucose [47]. These data on kidney cells are consistent with those obtained from human umbilical endothelial cells demonstrating that STAT3 promotes CyPA expression via binding to the STAT3-responsive element (SRE), a specific region in the CyPA gene (PPIA) promoter [48]. Mechanistically, STAT3 forms a complex with co-factors and other transcription factors to regulate CyPA expression [48].
For almost two decades, we have known that CD147 is a membrane-bound glycoprotein that serves as a principal signaling receptor for circulating eCyPA [15, 19]. It has been documented that eCyPA-CD147 binding can activate ERK/NF-κB pathway, leading to proinflammatory cytokines/chemokines release, leukocyte recruitment, and matrix overproduction [14, 49]. Additional evidence has demonstrated that the complex of CyPA-CD147 is associated with inflammatory lesions [50]. In the normal kidney, expression of CD147 is predominant in proximal and distal tubular cells. The increased expression of CD147 is found at the locales with infiltrating inflammatory cells as observed in kidney injury and lupus nephritis [51]. Interestingly, plasma eCyPA and CD147 levels correlate with progression of DN in Type 2 diabetic patients [15].
In DN, which is a common disease worldwide [52], albuminuria is a traditional marker used for its clinical detection [53]. However, albumin is absent in some DN patients who had already developed end-stage renal disease (ESRD) [54]. Therefore, a higher sensitive biomarker for DN is urgently required. Tsai et al. [55] have reported, for the first time, the potential role of urinary CyPA (uCyPA) as a novel biomarker for early detection of DN. Their investigations in kidney cell lines, mesangial (MES-13) and tubular (HK-2) cells, have also shown that eCyPA can be secreted from both cell types. Furthermore, measurement of uCyPA level in DN outpatients has revealed 90% sensitivity and 72.7% specificity for its use to diagnose Stage 2 DN with a moderate to high ROC (receiver operating characteristic) curve for diagnostic power (AUC = 0.85) [55]. However, coefficient of determination of uCyPA with urinary albumin/creatinine (ACR) ratio is very low (R2 = 0.054) [55]. A study in an animal model has also shown that uCyPA level in 24-h urine is significantly higher in diabetic rats compared with the non-diabetic controls with 77.8% sensitivity, 67% specificity, 70% positive predictive value (PPV), 75% negative predictive value (NPV), and 0.778 AUC of ROC curve [56]. Coefficient of correlation of uCyPA with ACR and 24-h urinary protein are both statistically significant (R = 0.426, p = 0.011 and R = 0.456, p = 0.043, respectively) [56]. This result is consistent with the findings in another study by Tsai et al. [34], who have reported that uCyPA concentration increases 12.7-fold in the diabetic mice at eighth week. Moreover, uCyPA (68.3% sensitivity, 53.3% specificity, 74.5% PPV, 45.7% NPV, 0.856 AUC of ROC curve) and uCyPA/creatinine ratio (62.5% sensitivity, 93.3% specificity, 90.8% PPV, 70.4% NPV, and 0.830 AUC of ROC curve) both increase in Type 1 DM pediatric patients with microalbuminuria [57]. These studies have concluded that uCyPA may serve as an effective biomarker for early DN detection due to its high sensitivity, high specificity and non-invasiveness [55-57].
4.2. CyPA and acute kidney injury (AKI)
AKI is a renal disorder recognized by the rapid loss of kidney function or kidney damage that rapidly develops within hours or days. The most common causes of AKI include acute tubular necrosis and prerenal azotemia [58]. Kidney cells, particularly renal tubular epithelial cells, are prone to the damage and susceptible to intrinsic oxidative stress such as excessive inflammatory response and ischemia [59]. Under such conditions, eCyPA serves as a key oxidative stress-induced secretory factor [60]. eCyPA also serves as a chemotactic factor to induce leukocyte infiltration at the inflammatory locales [21, 61]. The increase of eCyPA secreted from human proximal tubular epithelial cells (PTECs) is found after the cells are exposed to harmful agents [62]. PTECs also secrete greater amount of eCyPA during cell death and/or kidney injury [63].
By the rise of eCyPA level, accumulation of leukocytes to the injured site is promoted and tissue damage is amplified. CyPA has been demonstrated to increase in the kidney of ischemia/reperfusion-induced AKI animal model, whereas knockdown of its gene (PPIA) reduces tissue inflammation [64]. To address the pathogenic role of eCyPA in AKI, the effect of a cyclophilin inhibitor (GS-642362, which was derived from sanglifehrin A macrocycle [65]) has been examined. The data have shown that GS-642362 has a protective effect against acute renal failure in the renal ischemia/reperfusion-induced AKI model in a dose-dependent manner [62]. Such protective effect correlates with the decrease of tubular cell death via the reduction of neutrophil infiltration [62]. These findings highlight the pathogenic roles of eCyPA in AKI and its potential to be used as a novel biomarker for early detection of AKI.
eCyPA is secreted from the damaged cells to the extracellular environment [63]. Moreover, circulating eCyPA can filter freely through the glomerulus in the nephron. Therefore, a high level of uCyPA may reflect elevation of eCyPA secretion from the toxic renal cells and/or its increased glomerular filtration or leakage [63, 66]. The increase of uCyPA in ischemia/reperfusion-induced AKI can thus serve as the biomarker independent from other functional and clinical parameters [63, 66]. Also, it is evident that uCyPA may be used in complement with serum creatinine and other classical markers for AKI, e.g., neutrophil gelatinase-associated lipocalin (NGAL). Moreover, it can be used for the diagnosis of AKI in patients who do not match the AKI functional impact-based diagnostic criteria [59, 66].
In addition to CyPA, other cyclophilins/immunophilins are also involved in AKI and deserve discussion here. PPIF is a mitochondrial protein that acts as an essential regulator of the mitochondrial permeability transition pore (mPTP) [67, 68]. Apoptotic stimuli can induce PPIF to form a complex with p53, leading to mPTP opening, mitochondrial swelling, leakage of cytochrome c into cytoplasm, and tubular cell death [69, 70]. A recent study has proven for the first time that PPIF contributes to acute tubular necrosis and AKI induced by high dose of plant-derived nephrotoxic agent, aristolochic acid [70]. By contrast, the loss of renal functions, tubular cell damage and death, and neutrophil infiltration are improved in the PPIF-/- mice [70]. These findings are consistent with those reported in an earlier study showing that proximal tubule-specific PPIF-knockout mice are protective from cisplatin-induced fatty acid β-oxidation (FAO) and AKI [71]. Such protective mechanism is mediated by PPIF-peroxisome proliferator-activated receptor-α (PPARα) complex formation within mitochondria. This complex suppresses nuclear transcription of PPARα-regulated FAO genes during cisplatin-induced AKI [71]. Taken together, although CyPA and PPIF promote AKI by different mechanisms, both of them serve as the promising therapeutic targets for management of AKI.
FK506-binding protein 12 (FKBP12) is another immunophilin being a primary target for two structurally related drugs, FK506 and rapamycin [72]. Binding of FKBP12 with FK506 inhibits bone morphogenetic proteins (BMPs)-related signaling pathway [73]. Among several BMPs, BMP7 is necessary for development and homeostasis of the kidney [74]. In contrast to CyPA and PPIF, BMP7 preserves kidney function in an animal model of AKI by restoring PTECs function and inflammatory response [75, 76]. The binding of FKBP12 to FK506 can be inhibited by using a FK506 analog, oxtFK, thereby promoting the BMP7 activity to prevent AKI induced by ischemia/reperfusion [73]. These data highlight the promising role of FKBP12 as a new therapeutic target for treatment of AKI.
4.3. CyPA in chronic kidney disease (CKD) and renal fibrosis
CKD is characterized by the presence of chronic morphological and functional disorders in the kidney that can progress to ESRD [77-79]. General pathological processes of CKD and many other diseases are frequently associated with inflammation. Both local and systemic inflammatory responses are involved in these CKD processes. The presence of the cardiovascular mesh system allows the inflammatory mediators to travel throughout the body, from one organ to another. It has been shown that CKD is associated with peripheral arterial occlusive disease (PAOD) with a high incidence [80]. PAOD is an atherosclerotic disease with lesions in the lower extremities and intermittent claudication as the major and classical clinical manifestations. A previous study has shown that CyPA plays role in the pathogenesis and progression of PAOD [81]. CyPA is secreted by VSMCs in response to oxidative stress and promotes the development of atherosclerosis in many ways [81]. CyPA can also facilitate migration and proliferation of VSMCs, stimulate proinflammatory cytokine pathways in endothelial cells, exert chemotactic effects, and enhance ROS production [81-84]. The inflammatory mediators generated in the vascular system can definitely enter into the kidney as well as other organs and affect the cells that are exposed to them. It has been documented that a high serum level of eCyPA may be related to the decline of renal function [81]. Furthermore, markedly elevated plasma eCyPA positively correlates with systemic inflammation markers, such as high-sensitivity C-reactive protein, IL-6 and TNF-α, in ESRD patients undergoing hemodialysis and peritoneal dialysis [85].
It is interesting that PTECs also serve as the non-professional antigen-presenting cells (APCs) in the kidney tissue that play role in modulation of immune responses [86]. This gives rise to the question that whether the immune modulation in kidney injury occurs via the effect of the non-professional APCs function of PTECs or the effect of CyPA. Alternatively, both induction pathways may occur and cooperate. The general pathological processes of CKD normally involve both local and systemic inflammatory responses [79]. Under the CKD environment, eCyPA affects the impairment of renal function through the vascular mesh networks. The limitation of tissue regeneration after cellular injury in the kidney can lead to the progressive decline of renal function and ultimately ESRD [79].
Renal fibrosis is a key determinant and prognostic marker for the chronic progression of kidney failure. The molecular mechanisms of CyPA in promoting tubulointerstitial fibrosis after kidney injury have been investigated [62]. Following tissue injury, eCyPA promotes leukocyte recruitment and inflammatory cascade [62]. These data underline the important role of CyPA in the pathogenesis of renal fibrosis. Moreover, the eCyPA level is associated well with the degree of renal fibrosis [87]. Therefore, eCyPA also serves as a potential biomarker for CKD and renal fibrosis.
There is in vitro evidence suggesting that inhibition of CyPA activity significantly decreases ECM protein production and accumulation that may exert a therapeutic effect on renal fibrosis [87]. Although convincing, validation in clinical setting is required. Since fibrosis is not limited to just one organ, a common fibrotic pathway has been thought to exist [88]. Interestingly, caveolin-1 (a vesicular transport regulator) has been shown to reduce CyPA-induced ROS overproduction in an animal model of hypercholesterolemia-associated atherosclerosis and renal damage [84]. Similar effects of CyPA inhibition have been found also in cardiac diseases. For example, inhibiting the eCyPA activity significantly reduces inflammation and myocardial fibrosis [89]. In addition, a recent study has also reported the pathogenic role of PPIF in the development of renal fibrosis, as PPIF gene deletion minimizes tubular cell apoptosis, protects peritubular capillary loss, and reduces kidney inflammation in the obstructive kidney [90]. Interestingly, GS-642362 (a potent CyPA inhibitor as mentioned above) also inhibits PPIF [62]. In addition to ischemia/reperfusion-induced AKI, GS-642362 also reduces cell death, macrophage infiltration, and fibrotic development in the unilateral ureteric obstruction (UUO) model of renal fibrosis [62]. However, the degree of such protection in the UUO model is less than that in the ischemia/reperfusion-induced AKI model [62]. Therefore, both eCyPA and PPIF are involved in the pathogenesis of renal fibrosis and serve as the new therapeutic targets for treatment of CKD and renal fibrosis.
4.4. CyPA in nephrotoxicity associated with organ transplantation
In organ transplantation, CsA usually serves as a major immunosuppressive drug. It is widely used in tissue and organ transplantation to prevent rejection and acute graft-versus-host disease (aGVHD) [7]. This first-line therapy involves the ability of the CsA-CyPA complex to bind calcineurin, resulting in immune suppression by reduced production of IL-2 and other cytokines, as well as inhibition of T-cell activation [7, 91]. Nonetheless, clinical use of CsA has many adverse events, including nephrotoxicity, neurotoxicity, malignancy risk and infection [7, 92, 93]. It has been reported that long-term exposure to CsA induces renal tubular cell atrophy and interstitial fibrosis [94, 95]. CsA promotes interstitial ECM accumulation by a combination of suppressed activity of matrix degradation enzyme, enhanced synthesis of collagen from renal cortical fibroblasts, induction of autocrine insulin-like growth factor-I (IGF-I) secretion and function, and increased secretion of transforming growth factor-β1 (TGF-β1) from PTECs. Modulation of the cytokine networks has been shown to play an important role in the tubulointerstitial pathology [94, 95].
Indeed, CyPA also serves as a key modulator of CsA action. A recent study has demonstrated that knockdown of gene encoding CyPA (PPIA) in renal cells increases the unfolded protein response (UPR) similar to the effects of CsA treatment, which suppresses the CyPA chaperone function, leading to ER stress, UPR and renal epithelial cell apoptosis [96]. Modifying CyPA and/or UPR may help to reduce nephrotoxicity associated with CsA in renal transplantation [96]. Another study has reported that polymorphism (-11G/C) on PPIA promoter is related to the nephrotoxicity after renal transplantation by affecting the PPIA gene expression [97]. Therefore, monitoring this PPIA gene polymorphism in organ transplant patients may help to prevent secondary nephrotoxicity before undergoing serious progression.
5. Proposed pathogenic mechanisms of CyPA in kidney diseases
ROCK pathway has been demonstrated to play roles in VSMCs functions and is hence associated with cardiovascular diseases [98, 99]. Previous studies have shown that up-regulation of CyPA at aortic aneurysm and atherosclerotic plaques is mediated by ROCK activity [99, 100]. This data is consistent with that reported from another study demonstrating that CyPA is a ROS-related protein that is secreted by VSMCs under the RhoA/Rho-kinase activation [99, 101]. This raises the possibility that other cells that express CyPA may also behave the same under similar conditions. On this basis, we have proposed herein the pathogenic mechanisms of CyPA in kidney diseases, especially at PTECs, in which CyPA expression is highly predominant. The schematic representation of CyPA-induced pathogenic mechanisms of kidney diseases is shown in Figure 4.
Proposed pathogenic mechanisms of CyPA in kidney diseases. During cellular injury, CyPA expression and secretion are induced by various stimuli, e.g., oxidative stress, hypoxia, infection, inflammation, hyperglycemia and mechanical stretch, via the Rho-dependent pathway. The downstream cascade is associated with Rho kinase and actin remodeling mediators. Intracellular CyPA (iCyPA) can be secreted (eCyPA) from the cells into the extracellular compartment and performs paracrine and autocrine functions. For the autocrine function, the binding of eCyPA to CD147 activates MAPK pathway via p38, ERK1/2 and NF-κB, leading to cell proliferation, migration and inflammatory cascade. For the paracrine function, eCyPA secreted from the injured cells promotes accumulation and activation of leukocytes, such as neutrophils, monocytes and T-cells, which subsequently mediate tubular cell necrosis, interstitial inflammation, fibrogenesis and impaired kidney function. Finally, the increase in circulating eCyPA and uCyPA serves as a promising biomarker for many kidney diseases.
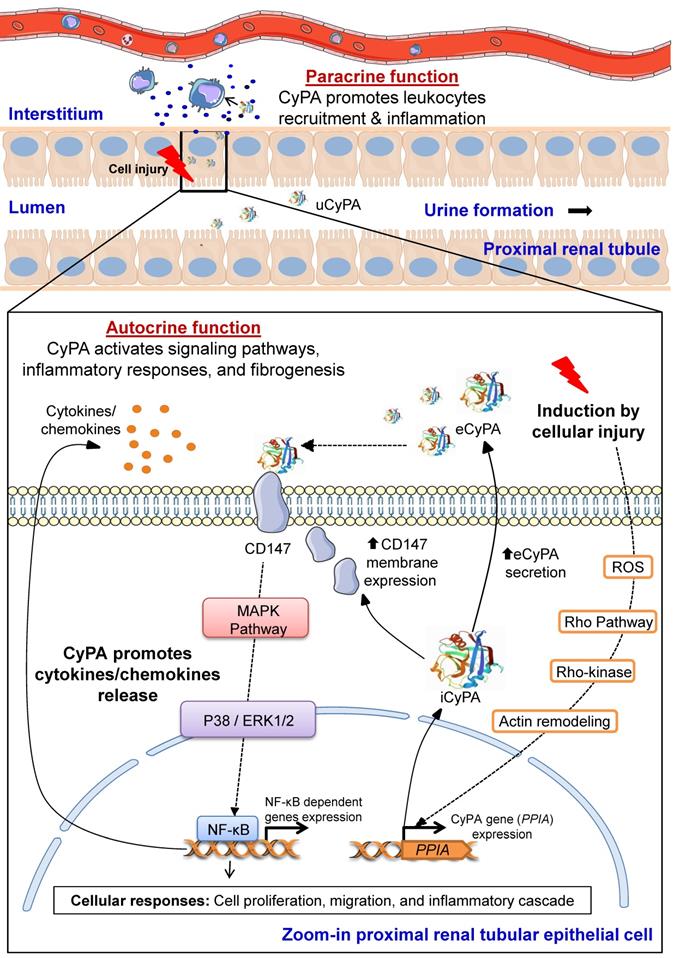
In response to stimuli such as oxidative stress, inflammation, hypoxia, infection, hyperglycemia and mechanical stretch, expression and transcription of PPIA gene encoding CyPA is induced via Rho-kinase activation. iCyPA subsequently plays a role as a chaperone and regulator for protein trafficking and activity. Additionally, iCyPA induces membrane expression of CD147 [55]. CyPA can be also secreted (eCyPA) via the vesicular secretion pathway into the extracellular space and performs both paracrine and autocrine functions [55]. The binding of eCyPA to CD147 mediates cellular signaling cascade via MAPK pathway involving p38, ERK1/2 and NF-κB, which further trigger cell proliferation, migration and inflammatory response. Subsequently, proinflammatory cytokines/chemokines are released and stimulate the downstream cellular signals that affect the progression of tubular injury, interstitial inflammation and fibrogenesis [55]. Finally, overproduction of eCyPA results in the increase of circulating eCyPA and uCyPA that can be used as promising biomarkers for early diagnosis and prognosis of several kidney diseases [55].
6. Conclusions
CyPA is a multifunctional molecule that plays important roles as a key factor in many pathological conditions. Herein, we highlight its theranostic roles in various kidney diseases, including DN, AKI, CKD, renal fibrosis, and nephrotoxicity associated with organ transplantation. eCyPA is secreted from the cells via the vesicular secretion pathway to exert paracrine and autocrine effects. eCyPA can bind to CD147 and subsequently trigger MAPK signaling pathway via downstream p38, ERK1/2 and NF-κB, resulting in cell proliferation, migration, inflammatory cascades, progression of tubular injury, interstitial inflammation and fibrogenesis. Circulating eCyPA can filter freely through the glomeruli. A high level of uCyPA may reflect an elevation of plasma eCyPA level and/or its increased secretion by the damaged renal tubular cells. Therefore, uCyPA and plasma eCyPA serve as the promising biomarkers for diagnostics and prognostics in various kidney diseases. Since CyPA function has a high impact on the pathogenesis of several kidney diseases, CyPA may serve as a new therapeutic target, and utilization of a clinically safe CyPA inhibitor can be considered as an alternative therapeutic approach for future management of these kidney diseases.
7. Future perspectives
It is evidently clear that CyPA plays significant theranostic roles in various kidney diseases. Nevertheless, its roles in kidney diseases seem to be under-investigated. In addition to DN, AKI, CKD, renal fibrosis, and nephrotoxicity associated with organ transplantation, the pathogenic and theranostic roles of CyPA should be more extensively elucidated in several other kidney diseases.
Note that most of the previous studies have reported the potential roles of uCyPA and plasma eCyPA as the new diagnostic/prognostic markers for kidney diseases. However, only few of them have compared their sensitivity, specificity, PPV, NPV, accuracy, etc. with other conventional tests. Therefore, future research of CyPA should also focus on such comparative analyses with the gold standards. Additionally, the use of different forms of CyPA (uCyPA and plasma eCyPA) as the diagnostic/prognostic markers should be validated in large patient cohorts of these kidney diseases aiming toward their clinical applications at the bedside.
Special attention for future CyPA research should be paid to its promising role as a new therapeutic target for treatment and/or prevention of kidney diseases. CyPA inhibitors exert therapeutic effects in several disease entities. In addition to GS-642362 with a potential therapeutic role in AKI and renal fibrosis as mentioned above, several other CyPA inhibitors deserve further investigations in kidney diseases. Because plasma eCyPA level correlates with kidney disease progression, eCyPA seems to be a more potent pathogenic mediator as compared with iCyPA [102]. MM218, a CsA derivative in which its chemical group was modified to be cell-impermeable, selectively inhibits eCyPA but does not affect iCyPA [103, 104]. MM218 has been shown to effectively decline inflammation by blocking eCyPA-mediated leukocyte accumulation in allergic lung inflammation in a murine model [103]. In amyotrophic lateral sclerosis (ALS), which is frequently unresponsive to treatment, MM218 has been shown to protect motor neurons and concomitantly reduces MMP-9 production [104]. MM218 also reduces NF-κB activation in the spinal cord of the ALS mice [104].
MM284 is another eCyPA-specific cell-impermeable inhibitor. A previous study has demonstrated that MM284 blocks eCyPA-induced migration and adhesion of monocytes and reduces MMP-9 expression in cardiac tissue of animals with myocarditis [102]. Additionally, CRV431 (another CsA derivative) potently inhibits not only CyPA but also other cyclophilins and has been demonstrated to decrease liver fibrosis and tumor masses in rodent models [105]. Furthermore, beneficial effects of many other non-immunosuppressive CsA derivatives, such as NIM811, SCY-635 and STG-175, have been reported in other diseases, particularly viral infections [106-108]. Although not yet tested, these and many other CyPA inhibitors may also exert therapeutic effects for kidney diseases and, therefore, deserve further extensive investigations.
It should be noted that the inhibitors used in several of previous studies mentioned above may not be specific only to CyPA (as they also inhibit other cyclophilins), and their chemical structures or characteristics are not well defined. Therefore, further developments of the specific CyPA inhibitors with precise information of their chemical structures, characteristics and kinetics are required. Among the cyclophilin inhibitors mentioned above, NIM811 is associated with modest increases in bilirubin and triglyceride as well as thrombocytopenia [109]. On the other hand, MM218 and CR431 seem to have safer profiles for clinical use [103, 105]. Although with potential therapeutic roles in kidney diseases, the adverse events of these CyPA inhibitors require further monitoring.
As mentioned above, a recent study has demonstrated that PPIA gene knockout can prevent ischemia/reperfusion-induced AKI, but is not protective against UUO-induced renal fibrosis [64]. A more recent study using small interring RNA (siRNA) specific to PPIA gene has demonstrated the induction of UPR by PPIA-knockdown in renal cells similar to the effects of CsA treatment to suppress CyPA function [96]. Therefore, knockout/knockdown of the PPIA gene encoding CyPA is another interesting aspect for future research on the theranostic roles of CyPA in kidney diseases to explore. Not only the specific effects on CyPA and disease outcome, their off targets should be also characterized.
Taken together, all of these data strongly indicate that CyPA serves as a promising therapeutic target for new drug development and should be more seriously concerned for its applications in treatment and prevention of kidney diseases.
Acknowledgements
This work was supported by Mahidol University.
Author Contributions
All authors (SH and VT) drafted the manuscript, read and approved the final manuscript, and are responsible for all aspects of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fischer G, Bang H, Mech C. Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed Biochim Acta. 1984;43:1101-11
2. Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544-7
3. Wang P, Heitman J. The cyclophilins. Genome Biol. 2005;6:226
4. Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid FX. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337:476-8
5. Nacev BA, Low WK, Huang Z, Su TT, Su Z, Alkuraya H. et al. A calcineurin-independent mechanism of angiogenesis inhibition by a nonimmunosuppressive cyclosporin A analog. J Pharmacol Exp Ther. 2011;338:466-75
6. Liu J, Albers MW, Wandless TJ, Luan S, Alberg DG, Belshaw PJ. et al. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992;31:3896-901
7. Otsuka S, Melis N, Gaida MM, Dutta D, Weigert R, Ashwell JD. Calcineurin inhibitors suppress acute graft-versus-host disease via NFAT-independent inhibition of T cell receptor signaling. J Clin Invest. 2021;131:e147683
8. Liao Y, Luo D, Peng K, Zeng Y. Cyclophilin A: a key player for etiological agent infection. Appl Microbiol Biotechnol. 2021;105:1365-77
9. Dawar FU, Xiong Y, Khattak MNK, Li J, Lin L, Mei J. Potential role of cyclophilin A in regulating cytokine secretion. J Leukoc Biol. 2017;102:989-92
10. Gothel SF, Marahiel MA. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol Life Sci. 1999;55:423-36
11. Daneri-Becerra C, Valeiras B, Gallo LI, Lagadari M, Galigniana MD. Cyclophilin A is a mitochondrial factor that forms complexes with p23 - correlative evidence for an anti-apoptotic action. J Cell Sci. 2021;134:jcs253401
12. Nigro P, Pompilio G, Capogrossi MC. Cyclophilin A: a key player for human disease. Cell Death Dis. 2013;4:e888
13. Favretto F, Flores D, Baker JD, Strohaker T, Andreas LB, Blair LJ. et al. Catalysis of proline isomerization and molecular chaperone activity in a tug-of-war. Nat Commun. 2020;11:6046
14. Sakamoto M, Miyagaki T, Kamijo H, Oka T, Boki H, Takahashi-Shishido N. et al. CD147-Cyclophilin a Interactions Promote Proliferation and Survival of Cutaneous T-Cell Lymphoma. Int J Mol Sci. 2021;22:7889
15. Chiu PF, Su SL, Tsai CC, Wu CL, Kuo CL, Kor CT. et al. Cyclophilin A and CD147 associate with progression of diabetic nephropathy. Free Radic Res. 2018;52:1456-63
16. Satoh K, Satoh T, Kikuchi N, Omura J, Kurosawa R, Suzuki K. et al. Basigin mediates pulmonary hypertension by promoting inflammation and vascular smooth muscle cell proliferation. Circ Res. 2014;115:738-50
17. Yurchenko V, Zybarth G, O'Connor M, Dai WW, Franchin G, Hao T. et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277:22959-65
18. Song F, Zhang X, Ren XB, Zhu P, Xu J, Wang L. et al. Cyclophilin A (CyPA) induces chemotaxis independent of its peptidylprolyl cis-trans isomerase activity: direct binding between CyPA and the ectodomain of CD147. J Biol Chem. 2011;286:8197-203
19. Yurchenko V, Pushkarsky T, Li JH, Dai WW, Sherry B, Bukrinsky M. Regulation of CD147 cell surface expression: involvement of the proline residue in the CD147 transmembrane domain. J Biol Chem. 2005;280:17013-9
20. Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989;337:473-5
21. Garimella V, McVoy JS, Oh U. The contribution of cyclophilin A to immune-mediated central nervous system inflammation. J Neuroimmunol. 2020;339:577118
22. Dawar FU, Wu J, Zhao L, Khattak MN, Mei J, Lin L. Updates in understanding the role of cyclophilin A in leukocyte chemotaxis. J Leukoc Biol. 2017;101:823-6
23. Sarro E, Duran M, Rico A, Bou-Teen D, Fernandez-Majada V, Croatt AJ. et al. Cyclophilins A and B oppositely regulate renal tubular epithelial cell phenotype. J Mol Cell Biol. 2020;12:499-514
24. Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811-7
25. Xue C, Sowden MP, Berk BC. Extracellular and Intracellular Cyclophilin A, Native and Post-Translationally Modified, Show Diverse and Specific Pathological Roles in Diseases. Arterioscler Thromb Vasc Biol. 2018;38:986-93
26. Chevalier F, Depagne J, Hem S, Chevillard S, Bensimon J, Bertrand P. et al. Accumulation of cyclophilin A isoforms in conditioned medium of irradiated breast cancer cells. Proteomics. 2012;12:1756-66
27. Pan H, Luo C, Li R, Qiao A, Zhang L, Mines M. et al. Cyclophilin A is required for CXCR4-mediated nuclear export of heterogeneous nuclear ribonucleoprotein A2, activation and nuclear translocation of ERK1/2, and chemotactic cell migration. J Biol Chem. 2008;283:623-37
28. Soe NN, Sowden M, Baskaran P, Kim Y, Nigro P, Smolock EM. et al. Acetylation of cyclophilin A is required for its secretion and vascular cell activation. Cardiovasc Res. 2014;101:444-53
29. Rosa A, Butt E, Hopper CP, Loroch S, Bender M, Schulze H. et al. Cyclophilin A Is Not Acetylated at Lysine-82 and Lysine-125 in Resting and Stimulated Platelets. Int J Mol Sci. 2022;23:1469
30. Xue C, Sowden M, Berk BC. Extracellular Cyclophilin A, Especially Acetylated, Causes Pulmonary Hypertension by Stimulating Endothelial Apoptosis, Redox Stress, and Inflammation. Arterioscler Thromb Vasc Biol. 2017;37:1138-46
31. Feng W, Xin Y, Xiao Y, Li W, Sun D. Cyclophilin A Enhances Cell Proliferation and Xenografted Tumor Growth of Early Gastric Cancer. Dig Dis Sci. 2015;60:2700-11
32. Yang Y, Lu N, Zhou J, Chen ZN, Zhu P. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology (Oxford). 2008;47:1299-310
33. Anandan V, Thankayyan Retnabai SK, Jaleel A, Thulaseedharan T, Mullasari A, Pillai MR. et al. Cyclophilin A induces macrophage apoptosis and enhances atherosclerotic lesions in high-fat diet-fed hyperglycemic rabbits. FASEB Bioadv. 2021;3:305-22
34. Tsai SF, Hsieh CC, Wu MJ, Chen CH, Lin TH, Hsieh M. Novel findings of secreted cyclophilin A in diabetic nephropathy and its association with renal protection of dipeptidyl peptidase 4 inhibitor. Clin Chim Acta. 2016;463:181-92
35. Obchoei S, Sawanyawisuth K, Wongkham C, Kasinrerk W, Yao Q, Chen C. et al. Secreted cyclophilin A mediates G1/S phase transition of cholangiocarcinoma cells via CD147/ERK1/2 pathway. Tumour Biol. 2015;36:849-59
36. Li L, Luo D, Liao Y, Peng K, Zeng Y. Mycoplasma genitalium Protein of Adhesion Induces Inflammatory Cytokines via Cyclophilin A-CD147 Activating the ERK-NF-kappaB Pathway in Human Urothelial Cells. Front Immunol. 2020;11:2052
37. Selyutina A, Persaud M, Simons LM, Bulnes-Ramos A, Buffone C, Martinez-Lopez A. et al. Cyclophilin A Prevents HIV-1 Restriction in Lymphocytes by Blocking Human TRIM5alpha Binding to the Viral Core. Cell Rep. 2020;30:3766-77 e6
38. Phillips S, Chokshi S, Chatterji U, Riva A, Bobardt M, Williams R. et al. Alisporivir inhibition of hepatocyte cyclophilins reduces HBV replication and hepatitis B surface antigen production. Gastroenterology. 2015;148:403-14 e7
39. Colpitts CC, Ridewood S, Schneiderman B, Warne J, Tabata K, Ng CF. et al. Hepatitis C virus exploits cyclophilin A to evade PKR. Elife. 2020;9:e52237
40. Geng J, Chen L, Yuan Y, Wang K, Wang Y, Qin C. et al. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma. Signal Transduct Target Ther. 2021;6:347
41. Liu C, von Brunn A, Zhu D. Cyclophilin A and CD147: novel therapeutic targets for the treatment of COVID-19. Med Drug Discov. 2020;7:100056
42. Seizer P, Gawaz M, May AE. Cyclophilin A and EMMPRIN (CD147) in cardiovascular diseases. Cardiovasc Res. 2014;102:17-23
43. Wang L, Jia J, Wang C, Ma X, Liao C, Fu Z. et al. Inhibition of synovitis and joint destruction by a new single domain antibody specific for cyclophilin A in two different mouse models of rheumatoid arthritis. Arthritis Res Ther. 2013;15:R208
44. Wang CH, Rong MY, Wang L, Ren Z, Chen LN, Jia JF. et al. CD147 up-regulates calcium-induced chemotaxis, adhesion ability and invasiveness of human neutrophils via a TRPM-7-mediated mechanism. Rheumatology (Oxford). 2014;53:2288-96
45. Li T, Yan B, Ma Y, Weng J, Yang S, Zhao N. et al. Ubiquitin-specific protease 4 promotes hepatocellular carcinoma progression via cyclophilin A stabilization and deubiquitination. Cell Death Dis. 2018;9:148
46. Ramachandran S, Venugopal A, Kutty VR, A V, G D, Chitrasree V. et al. Plasma level of cyclophilin A is increased in patients with type 2 diabetes mellitus and suggests presence of vascular disease. Cardiovasc Diabetol. 2014;13:38
47. Zhang X, Zhu Y, Zhou Y, Fei B. Interleukin 37 (IL-37) Reduces High Glucose-Induced Inflammation, Oxidative Stress, and Apoptosis of Podocytes by Inhibiting the STAT3-Cyclophilin A (CypA) Signaling Pathway. Med Sci Monit. 2020;26:e922979
48. Xie Y, Li X, Ge J. STAT3-CyPA signaling pathway in endothelial cell apoptosis. Cell Signal. 2020;65:109413
49. Lu G, Jia Z, Zu Q, Zhang J, Zhao L, Shi H. Inhibition of the cyclophilin A-CD147 interaction attenuates right ventricular injury and dysfunction after acute pulmonary embolism in rats. J Biol Chem. 2018;293:12199-208
50. Wang YQ, Zhang J, Zhu LX, Yu JJ, Liu MW, Zhu ST. et al. Positive Correlation between Activated CypA/CD147 Signaling and MMP-9 Expression in Mice Inflammatory Periapical Lesion. Biomed Res Int. 2019;2019:8528719
51. Maeda-Hori M, Kosugi T, Kojima H, Sato W, Inaba S, Maeda K. et al. Plasma CD147 reflects histological features in patients with lupus nephritis. Lupus. 2014;23:342-52
52. Bonner R, Albajrami O, Hudspeth J, Upadhyay A. Diabetic Kidney Disease. Prim Care. 2020;47:645-59
53. Halimi JM. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab. 2012;38:291-7
54. Tramonti G, Kanwar YS. Review and discussion of tubular biomarkers in the diagnosis and management of diabetic nephropathy. Endocrine. 2013;43:494-503
55. Tsai SF, Su CW, Wu MJ, Chen CH, Fu CP, Liu CS. et al. Urinary Cyclophilin A as a New Marker for Diabetic Nephropathy: A Cross-Sectional Analysis of Diabetes Mellitus. Medicine (Baltimore). 2015;94:e1802
56. El-Ebidi AM, Saleem TH, Saadi MGE, Mahmoud HA, Mohamed Z, Sherkawy HS. Cyclophilin A (CyPA) as a Novel Biomarker for Early Detection of Diabetic Nephropathy in an Animal Model. Diabetes Metab Syndr Obes. 2020;13:3807-19
57. Salem NA, El Helaly RM, Ali IM, Ebrahim HAA, Alayooti MM, El Domiaty HA. et al. Urinary Cyclophilin A and serum Cystatin C as biomarkers for diabetic nephropathy in children with type 1 diabetes. Pediatr Diabetes. 2020;21:846-55
58. Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756-66
59. Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738-53
60. Nigro P, Satoh K, O'Dell MR, Soe NN, Cui Z, Mohan A. et al. Cyclophilin A is an inflammatory mediator that promotes atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2011;208:53-66
61. Heine SJ, Olive D, Gao JL, Murphy PM, Bukrinsky MI, Constant SL. Cyclophilin A cooperates with MIP-2 to augment neutrophil migration. J Inflamm Res. 2011;4:93-104
62. Leong KG, Ozols E, Kanellis J, Badal SS, Liles JT, Nikolic-Paterson DJ. et al. Cyclophilin Inhibition Protects Against Experimental Acute Kidney Injury and Renal Interstitial Fibrosis. Int J Mol Sci. 2020;22:271
63. Cabello R, Fontecha-Barriuso M, Martin-Sanchez D, Lopez-Diaz AM, Carrasco S, Mahillo I. et al. Urinary Cyclophilin A as Marker of Tubular Cell Death and Kidney Injury. Biomedicines. 2021;9:217
64. Leong KG, Ozols E, Kanellis J, Nikolic-Paterson DJ, Ma FY. Cyclophilin A Promotes Inflammation in Acute Kidney Injury but Not in Renal Fibrosis. Int J Mol Sci. 2020;21:3667
65. Mackman RL, Steadman VA, Dean DK, Jansa P, Poullennec KG, Appleby T. et al. Discovery of a Potent and Orally Bioavailable Cyclophilin Inhibitor Derived from the Sanglifehrin Macrocycle. J Med Chem. 2018;61:9473-99
66. Lee CC, Chang CH, Cheng YL, Kuo G, Chen SW, Li YJ. et al. Diagnostic Performance of Cyclophilin A in Cardiac Surgery-Associated Acute Kidney Injury. J Clin Med. 2019;9:108
67. Lindblom RSJ, Higgins GC, Nguyen TV, Arnstein M, Henstridge DC, Granata C. et al. Delineating a role for the mitochondrial permeability transition pore in diabetic kidney disease by targeting cyclophilin D. Clin Sci (Lond). 2020;134:239-59
68. Laker RC, Taddeo EP, Akhtar YN, Zhang M, Hoehn KL, Yan Z. The Mitochondrial Permeability Transition Pore Regulator Cyclophilin D Exhibits Tissue-Specific Control of Metabolic Homeostasis. PLoS One. 2016;11:e0167910
69. Yang H, Li R, Zhang L, Zhang S, Dong W, Chen Y. et al. p53-cyclophilin D mediates renal tubular cell apoptosis in ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol. 2019;317:F1311-F7
70. Leong KG, Ozols E, Kanellis J, Ma FY, Nikolic-Paterson DJ. Cyclophilin D Promotes Acute, but Not Chronic, Kidney Injury in a Mouse Model of Aristolochic Acid Toxicity. Toxins (Basel). 2021;13:700
71. Jang HS, Noh MR, Jung EM, Kim WY, Southekal S, Guda C. et al. Proximal tubule cyclophilin D regulates fatty acid oxidation in cisplatin-induced acute kidney injury. Kidney Int. 2020;97:327-39
72. Kasahara K. Physiological function of FKBP12, a primary target of rapamycin/FK506: a newly identified role in transcription of ribosomal protein genes in yeast. Curr Genet. 2021;67:383-8
73. Larraufie MH, Gao X, Xia X, Devine PJ, Kallen J, Liu D. et al. Phenotypic screen identifies calcineurin-sparing FK506 analogs as BMP potentiators for treatment of acute kidney injury. Cell Chem Biol. 2021;28:1271-82 e12
74. Manson SR, Austin PF, Guo Q, Moore KH. BMP-7 Signaling and its Critical Roles in Kidney Development, the Responses to Renal Injury, and Chronic Kidney Disease. Vitam Horm. 2015;99:91-144
75. Vigolo E, Marko L, Hinze C, Muller DN, Schmidt-Ullrich R, Schmidt-Ott KM. Canonical BMP signaling in tubular cells mediates recovery after acute kidney injury. Kidney Int. 2019;95:108-22
76. Gould SE, Day M, Jones SS, Dorai H. BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int. 2002;61:51-60
77. Gutierrez-Pena M, Zuniga-Macias L, Marin-Garcia R, Ovalle-Robles I, Garcia-Diaz AL, Macias-Guzman MJ. et al. High prevalence of end-stage renal disease of unknown origin in Aguascalientes Mexico: role of the registry of chronic kidney disease and renal biopsy in its approach and future directions. Clin Kidney J. 2021;14:1197-206
78. Faria M, de Pinho MN. Challenges of reducing protein-bound uremic toxin levels in chronic kidney disease and end stage renal disease. Transl Res. 2021;229:115-34
79. Zhang R, Saredy J, Shao Y, Yao T, Liu L, Saaoud F. et al. End-stage renal disease is different from chronic kidney disease in upregulating ROS-modulated proinflammatory secretome in PBMCs - A novel multiple-hit model for disease progression. Redox Biol. 2020;34:101460
80. Hishida M, Menez S, Matsushita K. Peripheral Artery Disease in CKD: Anatomically Peripheral But Clinically Central. Am J Kidney Dis. 2020;75:687-9
81. Liu MC, Lee YW, Lee PT, Chang CS, Tai YL, Yu JR. et al. Cyclophilin A is associated with peripheral artery disease and chronic kidney disease in geriatrics: The Tianliao Old People (TOP) study. Sci Rep. 2015;5:9937
82. Jin ZG, Lungu AO, Xie L, Wang M, Wong C, Berk BC. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1186-91
83. Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J Leukoc Biol. 2007;82:613-8
84. Chen YH, Lin WW, Liu CS, Hsu LS, Lin YM, Su SL. Caveolin-1 Expression Ameliorates Nephrotic Damage in a Rabbit Model of Cholesterol-Induced Hypercholesterolemia. PLoS One. 2016;11:e0154210
85. Jin K, Vaziri ND. Elevated Plasma Cyclophillin A in Hemodialysis and Peritoneal Dialysis Patients: a Novel Link to Systemic Inflammation. Iran J Kidney Dis. 2017;11:44-9
86. Breda PC, Wiech T, Meyer-Schwesinger C, Grahammer F, Huber T, Panzer U. et al. Renal proximal tubular epithelial cells exert immunomodulatory function by driving inflammatory CD4(+) T cell responses. Am J Physiol Renal Physiol. 2019;317:F77-F89
87. Dihazi GH, Eltoweissy M, Jahn O, Tampe B, Zeisberg M, Wulfrath HS. et al. The Secretome Analysis of Activated Human Renal Fibroblasts Revealed Beneficial Effect of the Modulation of the Secreted Peptidyl-Prolyl Cis-Trans Isomerase A in Kidney Fibrosis. Cells. 2020;9:1724
88. Tampe D, Zeisberg M. Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol. 2014;10:226-37
89. Seizer P, Klingel K, Sauter M, Westermann D, Ochmann C, Schonberger T. et al. Cyclophilin A affects inflammation, virus elimination and myocardial fibrosis in coxsackievirus B3-induced myocarditis. J Mol Cell Cardiol. 2012;53:6-14
90. Hou W, Leong KG, Ozols E, Tesch GH, Nikolic-Paterson DJ, Ma FY. Cyclophilin D promotes tubular cell damage and the development of interstitial fibrosis in the obstructed kidney. Clin Exp Pharmacol Physiol. 2018;45:250-60
91. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481-508
92. Rodrigues-Diez R, Gonzalez-Guerrero C, Ocana-Salceda C, Rodrigues-Diez RR, Egido J, Ortiz A. et al. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci Rep. 2016;6:27915
93. Patocka J, Nepovimova E, Kuca K, Wu W. Cyclosporine A: Chemistry and Toxicity - A Review. Curr Med Chem. 2021;28:3925-34
94. Wu Q, Wang X, Nepovimova E, Wang Y, Yang H, Kuca K. Mechanism of cyclosporine A nephrotoxicity: Oxidative stress, autophagy, and signalings. Food Chem Toxicol. 2018;118:889-907
95. Johnson DW, Saunders HJ, Johnson FJ, Huq SO, Field MJ, Pollock CA. Fibrogenic effects of cyclosporin A on the tubulointerstitium: role of cytokines and growth factors. Exp Nephrol. 1999;7:470-8
96. Yilmaz DE, Kirschner K, Demirci H, Himmerkus N, Bachmann S, Mutig K. Immunosuppressive calcineurin inhibitor cyclosporine A induces proapoptotic endoplasmic reticulum stress in renal tubular cells. J Biol Chem. 2022;298:101589
97. Moscoso-Solorzano GT, Ortega F, Rodriguez I, Garcia-Castro M, Gomez E, Diaz-Corte C. et al. A search for cyclophilin-A gene variants in cyclosporine A-treated renal transplanted patients. Clin Transplant. 2008;22:722-9
98. Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011;301:H287-96
99. Tsuda T, Imanishi M, Oogoshi M, Goda M, Kihira Y, Horinouchi Y. et al. Rho-associated protein kinase and cyclophilin a are involved in inorganic phosphate-induced calcification signaling in vascular smooth muscle cells. J Pharmacol Sci. 2020;142:109-15
100. Su Z, Lin R, Chen Y, Shu X, Zhang H, Liang S. et al. Oxidized Low-Density Lipoprotein-Induced Cyclophilin A Secretion Requires ROCK-Dependent Diphosphorylation of Myosin Light Chain. J Vasc Res. 2016;53:206-15
101. Satoh K, Matoba T, Suzuki J, O'Dell MR, Nigro P, Cui Z. et al. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088-98
102. Heinzmann D, Bangert A, Muller AM, von Ungern-Sternberg SN, Emschermann F, Schonberger T. et al. The Novel Extracellular Cyclophilin A (CyPA) - Inhibitor MM284 Reduces Myocardial Inflammation and Remodeling in a Mouse Model of Troponin I -Induced Myocarditis. PLoS One. 2015;10:e0124606
103. Balsley MA, Malesevic M, Stemmy EJ, Gigley J, Jurjus RA, Herzog D. et al. A cell-impermeable cyclosporine A derivative reduces pathology in a mouse model of allergic lung inflammation. J Immunol. 2010;185:7663-70
104. Pasetto L, Pozzi S, Castelnovo M, Basso M, Estevez AG, Fumagalli S. et al. Targeting Extracellular Cyclophilin A Reduces Neuroinflammation and Extends Survival in a Mouse Model of Amyotrophic Lateral Sclerosis. J Neurosci. 2017;37:1413-27
105. Kuo J, Bobardt M, Chatterji U, Mayo PR, Trepanier DJ, Foster RT. et al. A Pan-Cyclophilin Inhibitor, CRV431, Decreases Fibrosis and Tumor Development in Chronic Liver Disease Models. J Pharmacol Exp Ther. 2019;371:231-41
106. Ma S, Boerner JE, TiongYip C, Weidmann B, Ryder NS, Cooreman MP. et al. NIM811, a cyclophilin inhibitor, exhibits potent in vitro activity against hepatitis C virus alone or in combination with alpha interferon. Antimicrob Agents Chemother. 2006;50:2976-82
107. Hopkins S, Scorneaux B, Huang Z, Murray MG, Wring S, Smitley C. et al. SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro. Antimicrob Agents Chemother. 2010;54:660-72
108. Gallay PA, Chatterji U, Bobardt MD, Long Z, Zhang S, Su Z. Characterization of the Anti-HCV Activities of the New Cyclophilin Inhibitor STG-175. PLoS One. 2016;11:e0152036
109. Lawitz E, Godofsky E, Rouzier R, Marbury T, Nguyen T, Ke J. et al. Safety, pharmacokinetics, and antiviral activity of the cyclophilin inhibitor NIM811 alone or in combination with pegylated interferon in HCV-infected patients receiving 14 days of therapy. Antiviral Res. 2011;89:238-45
Author contact
![]() Corresponding author: Prof. Visith Thongboonkerd, Head of Medical Proteomics Unit, Office for Research and Development, Siriraj Hospital, Mahidol University, 6th Floor - SiMR Building, 2 Wanglang Road, Bangkoknoi, Bangkok 10700, Thailand. Phone: +66-2-4192850. E-mail: thongboonkerdcom (or) vthongbocom
Corresponding author: Prof. Visith Thongboonkerd, Head of Medical Proteomics Unit, Office for Research and Development, Siriraj Hospital, Mahidol University, 6th Floor - SiMR Building, 2 Wanglang Road, Bangkoknoi, Bangkok 10700, Thailand. Phone: +66-2-4192850. E-mail: thongboonkerdcom (or) vthongbocom
 Global reach, higher impact
Global reach, higher impact