13.3
Impact Factor
Theranostics 2022; 12(11):5015-5033. doi:10.7150/thno.74785 This issue Cite
Review
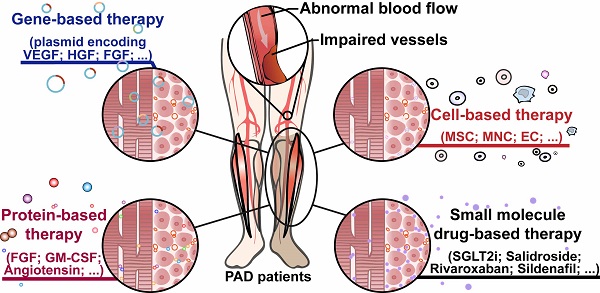
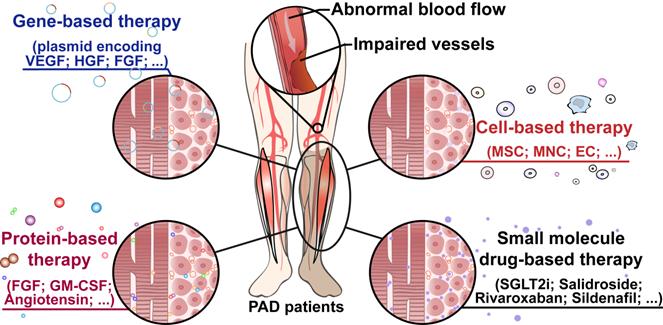
Therapeutic angiogenesis-based strategy for peripheral artery disease
1. The Key Laboratory of Biorheological Science and Technology, Ministry of Education, College of Bioengineering, Chongqing University, Chongqing 400044, China.
2. State and Local Joint Engineering Laboratory for Vascular Implants, Chongqing 400044, China.
3. The 111 Project Laboratory of Biomechanics and Tissue Repair, College of Bioengineering, Chongqing University, Chongqing 400044, China.
#These authors contributed equally to this manuscript.
Received 2022-5-6; Accepted 2022-6-14; Published 2022-6-27
Abstract
Peripheral artery disease (PAD) poses a great challenge to society, with a growing prevalence in the upcoming years. Patients in the severe stages of PAD are prone to amputation and death, leading to poor quality of life and a great socioeconomic burden. Furthermore, PAD is one of the major complications of diabetic patients, who have higher risk to develop critical limb ischemia, the most severe manifestation of PAD, and thus have a poor prognosis. Hence, there is an urgent need to develop an effective therapeutic strategy to treat this disease. Therapeutic angiogenesis has raised concerns for more than two decades as a potential strategy for treating PAD, especially in patients without option for surgery-based therapies. Since the discovery of gene-based therapy for therapeutic angiogenesis, several approaches have been developed, including cell-, protein-, and small molecule drug-based therapeutic strategies, some of which have progressed into the clinical trial phase. Despite its promising potential, efforts are still needed to improve the efficacy of this strategy, reduce its cost, and promote its worldwide application. In this review, we highlight the current progress of therapeutic angiogenesis and the issues that need to be overcome prior to its clinical application.
Keywords: therapeutic angiogenesis, peripheral artery disease (PAD), critical limb ischemia (CLI), vascular regeneration, angiogenesis factors
Introduction
Peripheral artery disease (PAD) has always been a global health issue affecting people's lives and quality of life [1]. PAD is indicated by the presence of stenosis or occlusion in the peripheral artery, mainly in the lower extremities [2]. In 2015, more than 235 million people aged 25 years or older were estimated to suffer from PAD [3]. The prevalence of PAD increases along with age and the presence of several important risk factors, including smoking, diabetes, hypertension, and dyslipidemia. As the number of projected individuals with the aforementioned risk factors increases, the number of PAD patients is expected to rise in the coming years [1].
Critical limb ischemia (CLI) is the most severe clinical manifestation of PAD. Limb ischemia stands for the pathological changes in blood vessels in patients' feet, resulting in insufficient blood supply, especially in the lower extremity [4]. In severe cases, CLI might lead to tissue necrosis, amputation, and even mortality [5-7]. Previous clinical studies have revealed that 20% of patients with chronic CLI would die within the first year of symptom onset, while 22% of them would experience major amputation [5, 8]. Mortality rates increased from 50% to 65% within five years, exceeding the five-year mortality rate of other diseases such as myeloma and colon cancer [9, 10]. Furthermore, patients with PAD have an increased risk of coronary and cerebrovascular disease-related morbidity and mortality [11]. However, despite its high prevalence and serious implications, PAD remains relatively underdiagnosed [3, 12, 13].
Surgical-based revascularizations that aim to provide a blood flow in the ischemic tissue through direct interventions in the affected vasculature, such as implantation of the catheter, vascular stent, or balloon, are currently the first choice and the standard strategy for restoring blood perfusion in PAD patients [14, 15]. While these strategies could reach 60% to 80% efficacy in correctly selected patients [16], there are several limitations to their application: (1) the optimal timing and indications remain controversial; (2) some patients may suffer from severe postoperative complications; and (3) some patients are not suitable for revascularization [16-19]. Indeed, 20 to 40% of patients with CLI could not receive surgical-based revascularization treatments due to already severely damaged vessels, and many of these “no-option” patients required amputation to prevent the generation of other complications [19, 20]. Furthermore, even after revascularization, 10% of patients are expected to be hospitalized again due to major adverse limb events (MALE) within one year [21], leading to a poor prognosis [22]. Amputation of the lower extremities is not only associated with lower survival time, but it also has a significant impact on patients' quality of life, disturbing their mobility and daily activities [23].
Therapeutic angiogenesis, which aims to induce endogenous vessel formation and blood perfusion recovery in the ischemic sites, is considered one of the most promising methods for treating no-option PAD patients [24, 25]. Utilizations of gene-, cell-, protein-based therapy, small molecule drugs, and combinatorial therapies have been studied for more than two decades; however, due to the lack of benefit and efficiency in clinical trial results, these approaches have not been established as a widely used strategy for PAD patients [24, 26]. Currently, there are only two commercially available therapeutic strategies for PAD, both of which are gene-based. One of them uses non-viral supercoiled plasmid encoding vascular endothelial growth factor (VEGF), which was approved in Russia in 2011 and then in Ukraine in 2013 [27, 28]. In contrast, the other uses a viral vector encoding hepatocyte growth factor (HGF), which was recently approved in Japan in 2019 [29]. However, their expensive costs hinder their use worldwide. Hence, low-cost, effective agents for inducing effective therapeutic angiogenesis are urgently needed. In this review, we will discuss the molecular mechanism underlying PAD and the current progress in therapeutic angiogenesis using different approaches.
Peripheral artery disease
In the human vascular system, a single blood flow of the aorta is bifurcated into two main arteries to further provide the bloodstream to each leg. From this point, the artery from each leg is branched into several arteries in the tibial area, with one main artery serving blood flow to the distal leg. Peripheral artery disease is a pathological condition caused by an obstruction in blood flow, mainly caused by the formation of plague and/or damage to blood vessels. Formation of plaque, for example, due to the accumulation of low-density lipoprotein or dead cells inside blood vessels, could lead to platelets and eventually atherosclerosis, resulting in vessel restriction or occlusion [30]. Dysfunction or even cell death of blood vessel-forming cells, such as endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) is also a significant cause of PAD. This could be caused by the increase of reactive oxygen species (ROS) and advanced glycation end-products (AGEs) as a result of risk factors such as smoking, diabetes, hypertension, and aging. Occlusion in the vessel disrupts the blood flow and interrupts the deliveries of oxygen and nutrients inside the blood vessel, further impairing ECs and VSMCs survival and functions by causing oxidative stress [31-34]. Besides ECs and VSMCs, PAD is also associated with the damage of other cells involved in angiogenesis [35]. Macrophage, especially its anti-inflammatory state (M2 phenotype), is crucial for angiogenesis, as it could induce wound healing by secreting profibrotic factors that contribute to tissue repair. However, damage-associated molecular signals released during the progression of PAD induce macrophages polarization into the pro-inflammatory state (M1 phenotype), further contributing to cellular damages [36]. PAD could also lead to fibrosis in red blood cells, the most abundant cells in the circulating blood, causing them to collide with the arterial wall, inducing local retention of lipid from the red blood cells membrane and local hemolysis. Ruptured red blood cells could also release heme-ferrous ions with high toxicity for ECs and VSMCs, further contributing to ECs and VSMCs dysfunction and death [37, 38]. Furthermore, the accumulation of these dead cells could also induce plaque formation, thus further contributing to advanced PAD. As the total occlusion of this single continuous vessel will lead to PAD, the blood supply will be dependent on collateral vessels [24].
PAD is classified as asymptomatic or symptomatic, and both can be diagnosed using lower ankle-brachial index (ABI) measurement as an indicator [12, 39]. However, since patients with asymptomatic PAD have not yet experienced symptoms such as pain or claudication, the lack of noticeable symptoms hinders early intervention of PAD clinically. Furthermore, the relationship between ABI screening and improved health outcomes in asymptomatic adults is limited [39, 40], putting patients with asymptomatic PAD at risk of morbidity and mortality [41-43]. Although asymptomatic PAD is mostly unnoticed and draws less attention in most studies, its occurrence contributes to the most among PAD prevalence. Moreover, it is associated with a significantly poorer prognosis than vascular diseases in other territories [12, 44]. As the risk of disease progression from the milder manifestation of PAD to the more severe state has been underestimated in previous studies [45], new tools or investigations should be adopted to screen for asymptomatic PAD [39-41, 43].
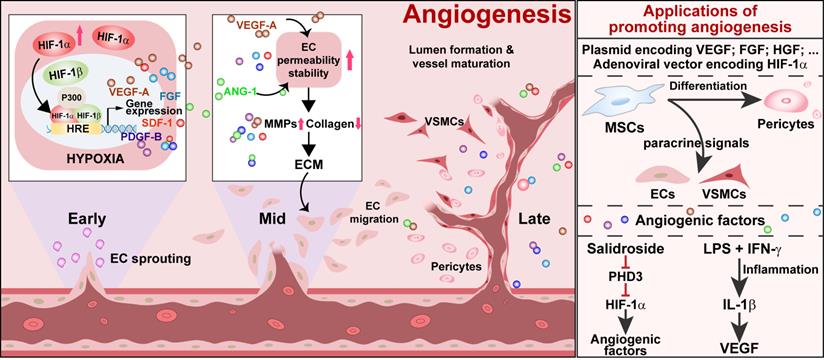
Schematic diagram of ischemic/hypoxic-triggered angiogenesis and its applications. Accumulated HIF-1α triggers the expression of various angiogenic factors by binding with the HIF-1 response element (HRE) on their promoters. These angiogenic factors promote endothelial cells permeability as well as stability, thus enhancing the process of endothelial sprouting from the existing vessel. Vascular smooth muscle cells and pericytes assist in new blood vessel formation and maturation to promote angiogenesis. ECs: endothelial cells; VSMCs: vascular smooth muscle cells.
In symptomatic PAD, the degree of manifestation ranges from intermittent claudication (IC) to CLI. IC, which is indicated by exercise-induced limb discomforts, such as pain, fatigue, and cramping, is primarily a quality-of-life disease that could be relieved by resting. Meanwhile, people with CLI suffer from limb discomfort, even at rest, with or without the presence of ulcers or gangrene [2, 6]. While amputation occurs in 4 to 27% of IC patients [45], 30% of CLI patients will undergo alive amputation within one year. Moreover, 50% of CLI patients will eventually die within five years of their diagnosis, increasing the burden of CLI treatment [8, 20].
PAD is also one of the major complications in diabetes, as hyperglycemia could induce systematic impairment in cellular functions, including paracrine function, proliferation, and migration potential [46, 47]. Hyperglycemia-induced AGEs impair cell functions and, subsequently, angiogenic potentials in diabetic PAD patients, leading to severe outcomes and a poor prognosis, with a wider damaged area and a higher reoccurrence rate [48]. Hyperglycemia also causes severe damage to skeletal muscle tissues, which are the largest secretory organ in the human body that could secrete a myriad of factors, including cytokines and angiogenic growth factors, involved in the neovascularization process [49-51]. Indeed, a previous study has revealed a correlation between the poor prognosis of limb ischemia disease with the loss of skeletal muscle capillary density [52]. Furthermore, AGEs reduce mitochondrial efficiency in myoblasts and the expression of myogenic regulatory factors, thus increasing skeletal muscle cell death while reducing muscular strength in diabetic patients [53-55]. These factors, taken together, constitute the main obstacles to inducing effective therapeutic angiogenesis in diabetic PAD patients [49, 56, 57].
Molecular mechanism of vascular regeneration in PAD
Angiogenesis is the formation of new blood vessels from an existing vascular structure. It is a complex process involving multiple angiogenic factors and various types of cells (Figure 1). In the early stage of angiogenesis, the expression of VEGF-A, fibroblast growth factor-2 (FGF-2), and HGF are stimulated. VEGF-A promotes ECs permeability. At the same time, it stimulates pericytes to express matrix metalloproteinases (MMPs), which degrades collagen, vascular basement membrane, and extracellular matrix (ECM). FGF-2 stimulates ECs to produce both MMPs and VEGF-A and ECs proliferation, pericyte attraction, and ECM deposition in the ischemic site [58]. HGF further stimulates VEGF-A expression, ECs growth, and proliferation [59, 60]. These events lead to the extravasation of plasma proteins from ECs to generate a provisional ECM, which, in turn, supports the migration of ECs to sprout from the existing vessel and form a vessel lumen [61, 62]. However, up to this stage, vessels are formed merely by ECs and are immature and leaky. Coverage by VSMCs and pericytes is needed for the maturation and stabilization of vessels. Angiopoietin-1 (ANG-1) produced by mural cells assists in this process, as it could activate its endothelial-specific tyrosine kinase receptor Tie2. Activated Tie2 promotes vessel assembly and maturation by mediating survival signals for ECs and regulating the recruitment of mural cells [63], thus stabilizing blood vessels and promoting their leak resistance by tightening endothelial junctions [64]. Platelet-derived growth factor-BB (PDGF-BB) stimulates the stabilization of newly formed vessels by inducing the alteration, proliferation, migration, and recruitment of pericytes and VSMCs associated with ECs-induced sprouts [62, 65, 66]. Subsequently, these angiogenic factors will trigger the proliferation and migration of mural cells, consisting of VSMCs and pericytes [67]. These cells are recruited to cover the EC-formed vessel lumen, leading to the formation and stabilization of a functional blood vessel.
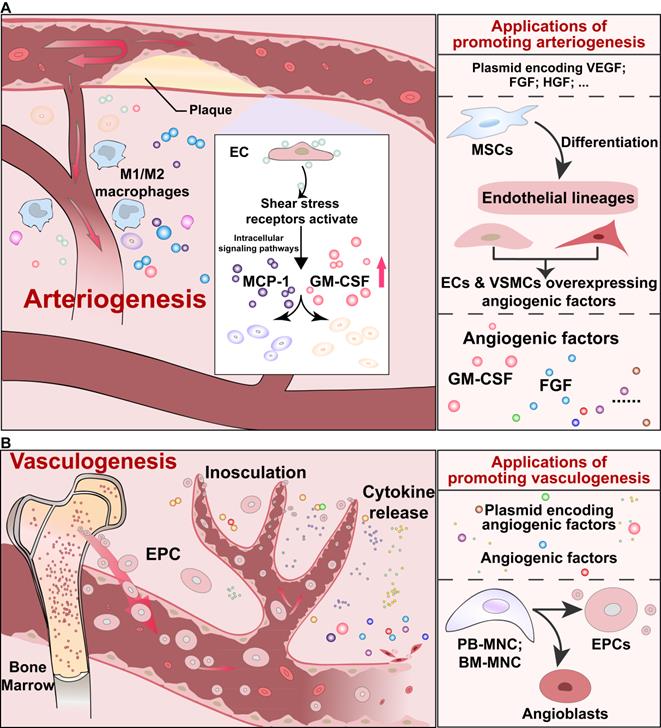
The arteriogenesis process will also be induced in response to arterial blockage irrespective of the ischemic condition. As shown in Figure 2A, in the arteriogenesis process, increased shear stress and inflammation due to arterial occlusion leads to the formation of collateral vessels. Shear stress receptors on ECs activate the downstream intracellular signaling pathways and release several important cytokines, such as monocyte chemoattractant protein 1 (MCP-1), granulocyte-macrophage colony-stimulating factor (GM-CSF), and cell adhesion molecules. Subsequently, monocytes, which produce proteases, and other white blood cells, which produce growth factors, such as FGFs, will be recruited to mediate vessel remodeling [24, 68].
In addition to angiogenesis and arteriogenesis, vasculogenesis, which is activated by endothelial progenitor cells (EPCs), is another process of neovascularization (Figure 2B) [69, 70]. Vasculogenesis was previously known to occur only during the embryonic developmental stage, as EPCs could physically adhere to the endothelium, integrate into blood vessels, and support angiogenesis via their paracrine actions. However, Asahara et al. and Shi et al. showed the presence of EPCs in adult peripheral blood and bone marrow, respectively [71, 72]. Mainly circulated from bone marrow, EPCs could reach the affected vessels and stimulate neovascularization through paracrine function [24].
While the interplay of angiogenesis, arteriogenesis, and vasculogenesis is important for restoring limb function, these processes are disrupted in PAD patients due to microvascular and endothelial dysfunction [73]. Generally, obstructive lesion in the lower limb artery is generated by atherosclerosis and it is worsened by the concurring state of the patient itself, such as hyperglycemia, hypertension, and dyslipidemia.
PAD also leads to the ischemic condition, causing systemic damage to blood vessels as well as other cells that support angiogenesis, for example, skeletal muscle cells, which secrete various angiogenic factors, such as ANG-1, VEGF-A, and PDGF-BB, all of which are crucial in inducing angiogenesis [49, 51]. However, while the damage caused by PAD is usually severe, previous studies using hindlimb ischemia (HLI) model mice have shown that blood perfusion in mild HLI could recover [56, 74, 75], indicating the presence of revascularization and self-recovery innate potential. Indeed, hypoxic condition triggers the activation of hypoxia-inducible factor-1 (HIF-1) signaling, which is responsible for the cellular response to acute hypoxic stress [76, 77]. Under the normoxic condition, HIF-1α, one of the two subunits forming HIF-1, is hydroxylated by the prolyl-hydroxylase domain (PHD) family, especially PHD-2 and PHD-3, using oxygen as its substrate [78, 79]. This hydroxylation leads to ubiquitination and, subsequently, the proteasomal degradation of the HIF-1α protein [80, 81]. When cells are exposed to hypoxic conditions, the PHD family fails to hydroxylate HIF-1α due to the lack of oxygen, resulting in its accumulation [82, 83]. This, in turn, enhances the HIF-1α heterodimer formation with another HIF-1 subunit, HIF-1β, and subsequently stimulates the transcription of HIF-1 target genes. More than 160 genes have been identified as HIF-1 targets [84, 85], including those involved in angiogenesis such as VEGF-A, PDGF-BB, stromal cell-derived factor-1 (SDF-1), and endothelial nitric oxide synthase (eNOS) [86-89], as well as those involved in cell survival such as heme oxygenase-1 (HO-1) [90, 91]. However, this innate potential is frequently insufficient to cover the damage induced by PAD, especially in patients with severe blood vessel damage, such as those with CLI or diabetes [24, 25, 57, 92].
Schematic diagrams of arteriogenesis and vasculogenesis, and their applications. (A) Signaling pathways of arteriogenesis that contribute to vessel remodeling, and applications to induce arteriogenesis. (B) Signaling pathways of endothelial precursor cells-mediated vasculogenesis, and applications to promote vasculogenesis. EPC: endothelial precursor cell.
Hence, promoting innate angiogenic potential, which leads to increased expression of angiogenic factors, has emerged as an attractive strategy for enhancing the neovascularization process in PAD patients. Evidently, as discussed below, attempts using different approaches have been made to establish potential strategies for treating PAD by promoting patients' innate angiogenic potential.
Trends in therapeutic angiogenesis for PAD
For approximately two decades, “therapeutic angiogenesis” has been studied as an investigational approach to treat symptomatic PAD, especially for “no-option” patients [24-26]. Therapeutic angiogenesis aims to stimulate vessel formation and blood perfusion to alleviate hypoxic damages caused by insufficient oxygen supply into organs and tissues due to ischemia [24]. Since the concept of therapeutic angiogenesis first appeared in 1994, multiple approaches to ischemic diseases have been investigated [61, 93]. The first attempt at therapeutic angiogenesis was performed by Takeshita et al. in 1994. They intraarterially administered VEGF in HLI rabbits and observed the development of angiographically visible collateral arteries [61]. The first human trial of therapeutic angiogenesis for limb ischemia was conducted by Isner et al. in 1996. They used a hydrogel-coated balloon-angioplasty-catheter to intraarterially transfer a plasmid encoding a soluble 165-amino acids isoform of human VEGF-A protein (VEGF-A165) into a patient with an ischemic right leg and observed an increase in collateral vessels and blood flow, suggesting that the treatment promoted angiogenesis in the ischemic limb [94]. Following this clinical study, exploration studies into therapeutic angiogenesis have been carried out through different approaches, including gene-, cell-, and protein-based therapy, as well as small molecule compound therapy (Figure 3).
Therapeutic angiogenesis-based strategy for peripheral artery disease. Schematic diagram of regenerative approaches for treating peripheral artery disease and their applications. PAD: peripheral artery disease.
Gene-based therapy
Gene-based therapy is a method of increasing revascularization by injecting genetic materials encoding target genes into ischemic sites in patients. Generally, the introduction of genetic material into a host can be achieved by using non-viral vectors, such as plasmid DNA or viral vectors, such as adenovirus and Sendai virus [95, 96]. Since the first clinical trial for VEGF was conducted in 1996, extensive studies have been performed to assess the effectiveness of other pro-angiogenic factors, such as HGF and FGF (Table 1) [29, 97-101]. Furthermore, many studies using other pro-angiogenic factors, such as angiogenic factor with G-patch and Forkhead-associated domain 1 (AGGF1), human telomerase reverse transcriptase (hTERT), and some microRNAs (miRNAs), also showed promising results in promoting angiogenesis in animal models [102-105].
VEGF-A is one of the most studied factors in angiogenesis-related diseases due to its critical role in initiating the angiogenesis process [68]. A phase I clinical trial established by Rajagopalan et al. demonstrated that intramuscular injection of an adenoviral vector encoding the 121-amino acid isoform of VEGF-A into PAD patients with IC or rest pain (RP) may favorably influence lower-extremity endothelial function [106, 107]. A drug containing VEGF-A165-expressing plasmid called Neovasculgen was approved in Russia in 2011 and Ukraine in 2013. Neovasculgen targets a spectrum of patients with mild to severe claudication due to CLI, yielding a significant increase in pain-free walking distance in a five-year follow-up study and five-year post-marketing surveillance [27, 28]. While Deev et al. claimed that no gene transfer-related adverse events or side effects were observed, other information about this drug is limited, as it is currently only approved in Russia and Ukraine [27, 28]. However, a clinical trial established by Isner et al. found that angiogenesis in patients' ischemic sites promoted by VEGF-A failed to prevent the need for limb amputation five months after the gene transfer, probably because the blood vessel lumen induced by VEGF-A is immature and leaky [62, 94]. Attention should also be paid to the adverse events of this strategy, as the development of spider angiomata and edema was observed in the patient's limb distal to the site of VEGF-A gene transfer, possibly due to the effect of VEGF on vascular permeability [106, 108, 109].
HGF, another well-known potent mitogen for endothelial cells, is also an attractive target for inducing angiogenesis. In a phase III clinical trial conducted in 2010, intramuscular-injected plasmid DNA expressing human HGF maintained limb perfusion, promoted complete ulcer healing rate and decreased RP in patients with CLI [98, 99]. Furthermore, clinical trials conducted in Japan also demonstrated that intramuscular injection of plasmid encoding HGF tended to improve RP and decreased the size of the ischemic ulcer in patients with CLI [29, 110, 111], and this gene-based therapy has attained marketing approval for CLI patients in Japan in March 2019. In China, another clinical trial for CLI was conducted using an intramuscular injection of plasmid encoding human HGF, pUDK-HGF. The results of its phase II trial in 2014 showed complete pain relief or pain reduction in patients with CLI [97], prompting the recruitment of phase III clinical trial participants (China Clinical Trial Registry, unique identifier: CTR20181274). Several clinical trials involving the combination of human HGF isoforms are also being held. For the treatment of PAD using intramuscularly-injected VM202, a plasmid encoding two human HGF isoforms HGF723 and HGF728, phase II clinical trial recruitment is currently underway (NCT03363165). Meanwhile, Pyun et al. conducted a preclinical study in rabbit HLI models using a plasmid encoding the 728 and 723 amino acids isoforms of HGF, namely HGF728 and HGF723, respectively, and discovered that intramuscular injection of HGF efficiently increased the number of angiographically recognizable collateral vessels and blood flow [112]. Currently, NL003, a plasmid containing novel genomic cDNA hybrid human HGF encoding HGF728 and HGF723, has already passed the phase II clinical trial and is in the process of recruiting CLI patients for the phase III clinical trial (NCT04274049).
Clinical trials using gene-based therapy
| Administered drug | Phase | Administration | Target patient | Reference |
|---|---|---|---|---|
| Adenoviral vector encoding VEGF-A121 | I | Intramuscular | PAD patients with IC or RP | [106] |
| Plasmid encoding VEGF-A165 (Neovasculgen) | III | Intramuscular | Limb ischemia of atherosclerotic genesis | [28] |
| Plasmid encoding human HGF | I/II | Intramuscular | CLI | [98, 99] |
| Plasmid encoding human HGF (pUDK-HGF) | II | Intramuscular | CLI | [97] |
| Plasmid encoding human HGF (pUDK-HGF) | III | Intramuscular | CLI | CTR20181274 |
| Plasmid encoding human HGF728 and HGF723 (VM202) | II | Intramuscular | PAD | NCT03363165 |
| Plasmid encoding HGF728 and HGF723 (NL003) | III | Intramuscular | CLI | NCT04274049 |
| Plasmid encoding human FGF (NV1FGF) | II | Intramuscular | CLI | [113] |
| Recombinant Sendai virus encoding human FGF-2 (SeV-hFGF2/dF) | I | Intramuscular | Peripheral arterial occlusive disease | NCT03668353 |
| Adeno-associated virus encoding human hTERT (AAV-hTERT) | I | Intravenous | CLI | NCT04110964 |
| Plasmids encoding human VEGF-A165 and HGF | I | Intramuscular | CLI patients with diabetes | [119] |
| Adenoviral vector encoding human HIF-1α | I | Intramuscular | Advanced atherosclerosis and tissue ischemia | [89, 119] |
| Adenoviral vector encoding human HIF-1α | II | Intramuscular | PAD patients with IC | [131] |
Abbreviation: CLI: critical limb ischemia; IC: intermittent claudication; PAD: peripheral artery disease; RP: rest pain.
Other clinical trials based on gene therapy using angiogenic factors have been conducted using plasmids encoding FGFs [113]. Nikol et al. found that intramuscular injection of plasmid encoding human FGF-1, namely NV1FGF, into CLI patients reduced the risk of all amputations, major amputations, and death [113]. SeV-hFGF2/dF, a recombinant Sendai virus encoding the human FGF-2 gene, has currently advanced into phase I clinical trials and is recruiting patients with peripheral arterial occlusive disease (NCT03668353).
While still in preclinical studies, AGGF1 has gained attention as a new angiogenic factor that could effectively promote angiogenesis in HLI animal models [102, 103]. Lu et al. found that intramuscular injection of plasmid encoding human AGGF1 increased the blood flow and the density of ECs-induced vessels in the ischemic hindlimb of HLI mice [114]. Yao et al. revealed that AGGF1 activated the antioxidative processes in EPCs to inhibit reactive oxygen species generation, thus promoting EPCs function in diabetic HLI model mice [115]. Moreover, Wang et al. reported integrin α5β1 as a receptor for AGGF1 in ECs could promote ECs adhesion, migration, capillary tube formation, and therapeutic angiogenesis in the HLI mouse model [116].
While the abovementioned studies focus on angiogenic factors, hTERT is also being assessed in clinical trials for its efficacy in PAD. hTERT is an important component of telomere elongation and thus is expected to ameliorate telomere dysfunction in aging patients in whom PAD mostly occurs. Previous studies have shown that hTERT is one of the VEGF-A downstream effectors and is essential in mediating the angiogenic process in vivo. Early preclinical studies demonstrated that overexpression of hTERT could enhance the regenerative properties of EPCs [117] and capillaries formation in the HLI rat model [104]. Recruitment of CLI patients for a phase I clinical trial using an adeno-associated virus expressing hTERT was conducted in 2019 (NCT04110964).
Several gene-based therapies have emerged into clinical trials or even have been approved; however, many of them did not significantly decrease mortality or amputation rates in patients with PAD [118]. Along with the insufficient efficacy of gene transfer, the complexity of the neovascularization process, which involves various angiogenic factors and cell types, is also considered one of the main underlying reasons for this failure. For this reason, inducing the expression of multiple angiogenic factors simultaneously has become an attractive strategy. This leads to the development of a second-generation therapeutic angiogenesis strategy, in which multiple angiogenic factors are combined. In a phase I trial for diabetic CLI patients, Barc et al. used a plasmid encoding VEGF-A165/HGF genes, and the results showed that intramuscular injection of this plasmid significantly decreased patients' RP and improved vascularization [119].
Apart from the overexpression of certain genes, the regulation of certain miRNAs has emerged as another potential approach. Numerous miRNAs have been linked to PAD development, suggesting their potential roles for PAD diagnostic and therapeutic targets [120]. A preclinical study showed that inhibiting miR-15a/16 with an adenovirus-based decoy system improved angiogenesis in the HLI mouse model through tyrosine kinase receptor upregulation [105]. Another preclinical study reported that injecting let-7g intramuscularly caused neovascularization and blood perfusion in HLI mice [121]. Meanwhile, overexpression of miR-210 enhanced angiogenic ability in the HLI mice model [122]. Another interesting gene delivery approach using mRNA was done by Gan et al. Although the phase I clinical trial was not specifically conducted in PAD-related patients, intradermal administration of modified mRNA encoding VEGF-A165 in men with type 2 diabetes mellitus could induce VEGF-A protein levels and skin blood flow [123].
Given that HIF-1α could regulate the transcription of multiple angiogenic factors while enhancing cell survival under hypoxia, attempts have been made to stabilize its protein and increase its accumulation. We previously designed shRNA expression vectors targeting murine PHD2 (shPHD2) and found that subcutaneously-injected matrigel-encapsulated shPHD2 plasmid induced the expressions of multiple angiogenic growth factors by stabilizing HIF-1α protein in mice models [124]. We also discovered that hydrodynamic limb vein injection of naked shPHD2 plasmid significantly promoted the formation of mature and functional blood vessels in the ischemic site of the HLI mice model, resulting in enhanced blood perfusion recovery [125]. Intramuscular injection of adenoviral vector encoding HIF-1α (Ad2/HIF-1α/VP16) efficiently increased collateral blood vessel formation and blood flow in rabbit HLI and mice diabetic HLI models [126, 127]. In a phase I clinical trial, intramuscular injection of Ad2/HIF-1α/VP16 reduced RP and promoted ulcer healing in some patients [89]; however, in a phase III clinical trial, an increase in edema rate was observed, probably due to continuously activated HIF-1α [89]. Furthermore, no differences in ABI and quality-of-life measurements were found in IC patients who received Ad2/HIF-1α/VP16 injections. Several reasons have been proposed for these unsatisfactory results, including low expression levels of the coxsackie-adenovirus receptor in skeletal muscle in patients [95]. The age of patients recruited in the phase III trial, which ranged from 40 to 80 years old, might also affect the outcome of the clinical trial, as a previous study revealed that aging is associated with reduced HIF-1α activity and decreased expression of angiogenic growth factors, as well as attenuated angiogenesis [128]. Moreover, the selection of model animals is also assumed to be a critical factor underlying the failures of not only HIF-1α clinical study but also other early therapeutic angiogenesis studies. In early preclinical therapeutic angiogenesis studies, New Zealand white rabbit and C57BL/6 mice, which possess strong self-recovery abilities, were used as the HLI animal models to examine the efficacy of Ad2/HIF-1α/VP16 in inducing therapeutic angiogenesis, leading to a gap between animal and clinical studies [89, 95]. Thus, to narrow the gap between preclinical and clinical studies, selecting appropriate model animals, for example, Balb/c mice with poor angiogenic potential, is necessary. Moreover, the fact that Ad2/HIF-1α/VP16 demonstrated positive effects only in part of CLI of patients in phase I clinical trial suggested that the genetic background of the patients, as well as the stage of disease progression, are also critical in the clinical outcome of this approach [89]. Thus, while further preclinical and clinical studies are still necessary, the determination of specific criteria of the patient's genetic background and the disease progression might be crucial for determining the appropriate therapeutic angiogenesis strategy for each patient.
Together, gene-based therapies using single factor or multi-factors demonstrate positive effects in promoting therapeutic angiogenesis in preclinical experiments and early stages of clinical trials. However, most gene-based therapies have not progressed into larger-scale clinical trials, and for those that have entered phase II or III clinical trials, many of the results did not meet the previous expectations, suggesting that improvement of delivery efficacy, as well as criteria for selecting appropriate patients according to their genetic background and disease progression, are needed. Meanwhile, although two drugs based on gene therapy have already been approved to treat PAD clinically, their effect on severe CLI remains to be investigated in a larger clinical trial or post-marketing surveillance. Furthermore, the socioeconomic burdens due to their high costs might hinder their global application. Thus, efforts should also be made to reduce the cost of this approach.
Cell-based therapy
Cell-based therapy requires the administration of viable stem or progenitor cells into a host to induce angiogenesis, either directly through their roles in vessel formation or through the paracrine signaling exerted by the respective cells [129]. Since the first clinical trial of this therapeutic strategy using autologous bone marrow-derived mononuclear cell (BM-MNC) in 2002 [130], several studies have been conducted in preclinical and clinical settings using various types of cells, such as mesenchymal stem cells (MSCs), MNCs, marker-specific cells, and bone marrow aspirate, to treat PAD [131], and some of them have emerged into clinical trials (Table 2). Cell-based therapy could be classified into two types based on the source of the cells used: allogeneic, which uses cells from the healthy donor, and autologous, which uses cells from the corresponding patient directly. The advantages and disadvantages of allogeneic and autologous cell therapies are shown in Table 3 [132, 133].
Clinical trials using cell-based therapy
| Administered drug | Phase | Administration | Target patient | Reference |
|---|---|---|---|---|
| Allogeneic BM-MSC | II | Intramuscular | CLI (Rutherford 4-5) | [137] |
| Allogeneic placental-derived mesenchymal-like cells | III | Intramuscular | CLI due to atherosclerosis (Rutherford 5) | [138] |
| GM-CSF-mobilized PB-MNC | - | Intramuscular | CLI patients with diabetes | [139] |
| Autologous BM-MNC | III | Intramuscular | CLI (Rutherford 4-5) | [140] |
| Autologous BM-MNC (REX-001) | III | Intraarterial | CLI patients with diabetes (Rutherford 4) | NCT03111238 |
| Autologous BM-MNC (RvEX-001) | III | Intraarterial | CLI patients with diabetes (Rutherford 5) | NCT03174522 |
| BM-MNC | III | Intramuscular | Non-reconstructable PAD | [131] |
| ECs expressing ANG-1, combined with autologous smooth muscle cells expressing VEGF-A165 | I | Intraarterial | CLI | [141] |
| Autologous blood-derived angiogenic cell precursor (ACP-01) | II | Intramuscular | CLI (except advanced CLI) | NCT02551679 |
Abbreviations: BM-MNC: bone marrow-derived mononuclear cells; BM-MSC: bone marrow-derived mesenchymal stem cells; CLI: critical limb ischemia; ECs: endothelial cells; GM-CSF: granulocyte-macrophage colony-stimulating factor; PAD: peripheral artery disease; PB-MNC: peripheral blood-derived mononuclear cell.
Advantages and disadvantages of allogeneic and autologous cell-based therapy
| Allogeneic | Autologous | |
|---|---|---|
| Safety | Possible immunoreactivity and incompatibility [132] | Immunocompatible as the cells are obtained from the host itself [132] |
| Invasive cell isolation process to donors; in need of healthy and eligible donors [137] | Additional invasive cell isolation process to the patient [131] | |
| Efficacy | Robust cell availability and potency as cells are collected from healthy donors [132] | Possibility of inconsistent cell availability and potency, depends on the host's condition [133] |
Stem cell transplantation has been recognized as a potential strategy for inducing angiogenesis [134, 135]. MSCs have proliferation capabilities and differentiate into endothelial lineages. They also contribute to tissue repair and regeneration in acute and chronic skeletal muscle damage by migrating to ischemic tissues. Furthermore, they could secrete paracrine signals that affect other cells, such as ECs and VSMCs, and subsequently promote blood vessel formation and induce therapeutic angiogenesis in ischemic tissues in HLI animal models [136]. Several therapeutic angiogenesis efforts using MSCs are currently in clinical trial stages. Gupta et al. conducted a phase II clinical trial in which allogeneic MSCs were intramuscularly injected into the gastrocnemius muscle of CLI patients. The results showed that patients who received a large amount of allogeneic MSCs (2 million cells/kg) experienced ulcer healing and a significant reduction in RP [137]. Meanwhile, mesenchymal-like cells derived from the allogeneic placenta have also been tested in early clinical trials and showed promising results, such as reduced pain and increased tissue perfusion, leading its way to phase III clinical trial [138].
MNCs derived from adult peripheral blood (PB-MNCs), or bone marrow, are another promising candidate for inducing therapeutic angiogenesis, as they comprise a subset of EPCs and angioblasts, which are beneficial for vasculogenesis and neovascularization [71, 72]. A clinical trial conducted by Huang et al. subcutaneously injected recombinant human GM-CSF to mobilize PB-MNCs. They found that intramuscularly-injected mobilized PB-MNCs improved ABI and blood perfusion in diabetic CLI patients [139]. A phase I trial conducted by Murphy et al. showed that intramuscularly-injected autologous BM-MNCs increased ABI, blood perfusion, and amputation-free survival (AFS) while decreasing RP in patients with CLI [140]. Two ongoing phase III clinical trials using intraarterially-injected autologous BM-MNCs cell suspension composed of several mature cell types (REX-001) are being held to investigate its efficacy and safety in treating diabetic CLI patients with Rutherford category 4 or 5 (NCT03111238 and NCT03174522, respectively). However, Lindemen et al. claimed that there is no clinical benefit to using BM-MNCs to treat non-reconstructable PAD patients [131].
In addition to MSCs and MNCs, other cells might also favor angiogenesis. For example, in a phase I clinical trial, a cell product composed of fully differentiated ECs overexpressing ANG-1 and smooth muscle cells overexpressing VEGF165 was administered intraarterially in “no-option” CLI patients. More than 70% of Rutherford 4 and 5 patients were free from amputation at a one-year time point. In addition, complete wound healing was observed in 50% of the patients with wounds. Despite its promising phase I clinical trial results, further clinical trials are required to confirm the benefits of this cell product [141]. Another phase II clinical trial utilized intramuscularly-injected autologous blood-derived angiogenic cell precursor (ACP-01) to treat “no-option” CLI patients (NCT02551679); however, we could not find any information regarding the results at current. Meanwhile, a preclinical study conducted by Nakagami et al. transplanted autologous adipose-derived stem cells (ADSC) in a mouse HLI model and found that ADSC could favor angiogenesis in ischemic skeletal muscle tissue [142]; while another preclinical study produced by Johnson et al. demonstrated that exosomes secreted by vascular progenitor cells could stimulate angiogenesis in vivo and in vitro [143]. Furthermore, a preclinical study compared the angiogenic potential of exogenously administered ECs derived from embryonic stem cells (ESC-ECs) to ESCs, and results showed that ESC-ECs enhanced neovascularization and improved blood perfusion in the HLI mice model [144].
However, there are some obstacles to cell-based therapies that need to be overcome. First, MSCs poor retention and survival rates in the ischemic zone, and their low availability, especially in diseased states such as hyperglycemia, are major impediments to MSCs-based therapeutic angiogenesis [145, 146]. Efforts have been made in preclinical settings to overcome these problems, for example, by combining MSCs-based treatment with gene-based therapy. Compared to untreated MSCs, the injection of engineered MSCs with a lentivirus to overexpress VEGF-A resulted in enhanced autocrine and paracrine function, further increasing blood perfusion in immunodeficient HLI mice [147]. To solve the problem of MSCs poor survival rates in ischemic sites, Huang et al. tried to utilize biomaterial scaffolds mimicking ECM properties to improve MSCs adaptability. They used Nap-GFFYK-Thiol, a small molecule hydrogel, which could serve as a favorable niche for human placenta-derived MSCs to exert their paracrine function, and found that Nap-GFFYK-Thiol reduced MSCs apoptosis and maintained their undifferentiated state, resulting in significantly improved blood perfusion in HLI mice [146]. Meanwhile, to improve MSCs availability, efforts such as hypoxia treatment, drug treatment, and the use of ECM scaffold have been made to optimize the expansion condition to increase MSCs population, migratory capabilities, and paracrine function in HLI model mice [148-150]. Besides MSCs, ECs poor alignment in disturbed flow fields might lead to altered function and reduced survival. A preclinical study conducted by Huang et al. found that the alignment, function, and survival of ECs implanted in aligned nanofibrillar collagen scaffolds that mimic the structure of collagen bundles in blood vessels are improved in the ischemic sites of HLI mice [151].
Together, although preclinical studies and clinical trials claim that the efficacies of cell-based therapeutic angiogenesis are favorable; improvements in ABI and AFS only appear in selected patients [135]. Besides the availability and survival rates of the cells used to induce therapeutic angiogenesis, different administration methods and patients' comorbidities, including genetic alterations, somatic mutations, and clonal hematopoiesis, are also major factors affecting clinical outcomes of cell-based therapeutic angiogenesis [131, 152, 153]. Thus, promoting the number and quality of cells, optimizing the administration method, and analyzing individual risk factors are necessary prior to cell therapy.
Protein-based therapy
Protein-based therapy is a straightforward method using recombinant proteins delivered locally or systemically to patients to induce angiogenesis through their direct functions in regulating and/or stimulating blood vessel formation [154]. It does not involve gene transfer using virus or plasmid, or transplantation of cells into the host. Hence, compared to gene-based or cell-based therapy, protein-based therapy has been recognized as a safer and more effective method for therapeutic angiogenesis. Since the first clinical trial of a protein-based strategy using FGF-1 in 1998 [155], numerous studies based on recombinant protein as well as structurally modified protein have been conducted to test their safety and efficacy in treating PAD; however, as shown in Table 4, only a few of them have entered clinical trial phases.
Clinical trials using protein-based therapy
| Administered drug | Phase | Administration | Target patient | Reference |
|---|---|---|---|---|
| FGF-2 (bFGF) | I | Intraarterial | Atherosclerotic PAD with IC | [100] |
| Recombinant FGF-2 | II | Intraarterial | IC | [101] |
| Angiotensin 1-7 | I | Intravenous | PAD | NCT03240068 |
| GM-CSF | N/A | Subcutaneous | PAD | [156] |
Abbreviations: GM-CSF: granulocyte-macrophage colony-stimulating factor; N/A: not applicable; IC: intermittent claudication; PAD: peripheral artery disease.
Protein therapy using FGF-2 is one of the most extensively studied protein-based therapeutic angiogenesis strategies, with promising outcomes such as high tolerance and increased blood flow seen in the phase I study of intraarterially-administered FGF-2 in patients with IC [100]. The later phase II study indicated an improvement in peak walking time at 90 days, although this improvement did not last until 180 days [101]. Meanwhile, Milton et al. conducted an early phase I trial on PAD patients using intravenously-administered angiotensin-(1-7), which has been known to protect against atherosclerosis in animal models by improving blood vessel function and reducing inflammation, on PAD patients (NCT03240068); however, the results of this study are not yet available. Another clinical trial was performed using GM-CSF, which contributes to the mobilization of EPCs; however, subcutaneously-administered GM-CSF did not significantly improve the walking performance of PAD patients [156].
Despite the benefits of protein-based therapy, as described above, in many cases, a large dose injection of protein is needed to effectively induce angiogenesis. This might induce immunogenicity proteinuria, an adverse event in which urine protein excretion exceeds 300 mg in 24 hours [157]. Furthermore, their short in vivo half-lives, physical and chemical instability, as well as low bioavailability also contributed to inefficient outcomes in protein-based therapeutic angiogenesis [154, 158]. To overcome these problems, researches based on computational design have been conducted to improve their delivery efficacy by developing sustained-release forms [159]. Segers et al. added the sequences of self-assembling peptide nanofibers into recombinant SDF-1 to help the incorporation of this recombinant protein into nanofibers, thus avoiding rapid diffusion of SDF-1. This recombination strategy significantly improved blood flow and arteriogenesis in HLI mice [160].
In addition to the administration of angiogenic proteins, therapeutic angiogenesis induction using neutralization antibodies or proteins has also been reported. VEGF-A165b is an anti-angiogenic splicing form of VEGF-A165, which suppresses canonical VEGF-A signaling by competitive interference with the VEGF receptor, and thus inhibits angiogenesis. Furthermore, VEGF-A165b is upregulated in PAD, leading to impaired VEGF-A signaling revascularization in HLI mice models [52]. A preclinical study using HLI model mice showed a significant improvement in blood perfusion in the ischemic hindlimb intramuscularly administered with monoclonal antibody inhibiting VEGF-A165b [161, 162]. Meanwhile, preclinical studies also showed that polarizing primary macrophages using lipopolysaccharide and interferon-gamma increased the expression level of the inflammatory cytokine interleukin-1β (IL-1β), thus inducing angiogenesis by activating VEGF-A transcription in the HLI mouse model.
Together, while preclinical trials demonstrate the positive effects of protein-based therapy in promoting angiogenesis, clinical trials still show some drawbacks of this approach. This includes adverse events, such as immunogenicity proteinuria, inefficient outcomes due to the short half-lives, poor physical and chemical properties of the proteins, and low bioavailability. Furthermore, the discovery of the anti-angiogenic VEGF-A splicing isoform, VEGF-A165b, complicates the understanding of PAD pathogenesis and treatment approach. Hence, more studies regarding the safety, sustained delivery, and mechanism of protein-based therapy to treat PAD are needed. Moreover, computational design to optimize the structure of the protein and increase their stability, such as conducted by Sun et al. in preclinical study using modified FGF2 for wound healing [163], also could help to solve the problem of the protein-based therapeutic angiogenesis.
Small molecule drug-based therapy
Small molecule drugs offer more targeted signaling due to their small size and low molecular weight [164, 165]. Furthermore, compared to proteins, the synthesis and purification of small molecule drugs are more accessible and cost-effective, as the molecule's structure is precisely known [166]. There are three major sources of small molecule drugs: natural products, de novo synthesis of small molecule compounds or modification of previously existing small molecule compounds, and compounds that have been approved for treating other diseases. As will be discussed below, while numerous preclinical studies and clinical trials using small-molecule drugs for treating PAD have been conducted, the use of compounds that have been approved for treating other diseases might be a shortcut for developing an effective therapeutic strategy for treating PAD. Indeed, as shown in Table 5, most of the small molecule drugs that have emerged into clinical trials for treating PAD are those that have been approved for clinical use for treating other diseases.
Clinical trials using small molecule drug-based therapy
| Administered drug | Phase | Administration | Target patient | Reference |
|---|---|---|---|---|
| L-arginine (Unifuzol®) | II | Intravenous | PAD | NCT03861416 |
| L-citrulline | N/A | Oral | IC | NCT02521220 |
| Cilostazol | - | Oral | IC | [176] |
| Rivaroxaban | III | Oral | PAD | [179, 180] |
| Statin | - | Oral | PAD | [184] |
| Sildenafil | III | Oral | IC | NCT03686306 |
Abbreviations: IC: intermittent claudication; PAD: peripheral artery disease; N/A: not applicable.
Preclinical studies demonstrated that several active compounds extracted from traditional herbs and plants could promote angiogenesis. Zhang et al. found that baicalin, the major component found in the root of the traditional Chinese herb Scutellaria baicalensis, which is used to treat stroke, could promote VEGF expression in HUVECs and human fibroblast MRC-5 cell lines, suggesting the possibility of using baicalin to induce therapeutic angiogenesis in PAD [167]. Meanwhile, Pignet et al. found that orally-administered resveratrol, a nutritional polyphenol that could provide beneficial effects on skin and could be found in more than 70 different plant species such as grapes, mulberries, and blueberries, promoted wound healing by reducing oxidative stress in mice models [168]. Another promising active compound from the natural product is salidroside, which is an active compound derived from Rhodiola rosea, a plant commonly used as a traditional Chinese medicine to treat high-altitude sickness. Zhang et al. found that salidroside upregulated HIF-1α protein expression and then promoted VEGF-A, thus inhibiting hypoxia-induced cardiomyocytes necrosis and apoptosis [169]. Zheng et al. found that salidroside treatment provided an anti-hypoxia effect by stimulating HIF-1α protein accumulation, but not HIF-2α protein, in human embryonic kidney fibroblast (HEK293T) and human hepatocellular carcinoma (HepG2) cell lines [170]. Chen et al. found that intraperitoneally-administered salidroside promoted the expression of VEGF and increased skin flap angiogenesis in rat models [171]. Studies by Zhang et al. revealed that targeting skeletal muscle cells with salidroside and its metabolite, tyrosol, significantly improved their proliferation and migration potentials, as well as their paracrine function. Furthermore, they demonstrated that intramuscular injection of salidroside and tyrosol into the gastrocnemius muscle of the ischemic hindlimb of HLI mice could enhance the formation of mature and functional blood vessels in non-diabetic and diabetic HLI mice models [75, 172, 173].
Another common strategy for searching novel small molecule drugs is by synthesizing and/or modifying small molecule compounds. Despite the beneficial effects of salidroside in inducing the formation of new blood vessels and in promoting blood perfusion recovery, as mentioned above, the concentration needed to exert these effects is high, hindering its potential clinical application. Recently, a preclinical study established by Liu et al. divided the functional groups of salidroside and synthesized more than 30 salidroside analogs by modifying its structure. The efficacy of these analogs in increasing HIF-1α transcriptional activity was analyzed, and salidroside analogs with significantly better stability, angiogenic property, and drug-likeness were determined based on a structure-activity relationship (SAR) study. The most optimized compound in this study could induce better neovascularization and blood perfusion recovery than salidroside in both non-diabetic and diabetic HLI mice at a significantly lower dose [56]. Epigallocatechin-3-gallate (EGCG), the active compound extracted from green tea, has demonstrated antioxidative and anti-inflammatory functions. A preclinical study established by Duan et al. showed that by combining copper ions with EGCG into a metal-polyphenol capsule (Cu-EGCG), sustained released copper ions and EGCG from Cu-EGCG promoted blood perfusion recovery by scavenging ROS in HLI model mice [174]. Another optimization of small molecule drugs for treating PAD was exerted by Hou et al. They synthesized small molecule compounds derived from carboxyethylpyrrole (CEP) protein adducts, which have been implicated in the induction of angiogenesis in pathological conditions, such as tissue ischemia [175]. By screening the CEP analogs obtained, they found that intramuscular injection of CEP03, one of the analogs, could enhance EPCs migration and microvessel formation, thus subsequently promote angiogenesis and blood recovery rate of the HLI mice. This newly synthesized small molecule offers a more stable and easily absorbed option for the drug over CEP [166]. L-arginine improves microcirculation by activating nitrogen monoxide production and stimulating capillary blood flow, thus it can probably improve the quality of life of patients with IC; however, its biosynthesizing rate in vivo is slow, leading to the urgent need for its supplementation in bodies. Moreover, orally supplemented L-arginine could easily be metabolized, further hindering its application in treating PAD. Optimization in drug delivery method and modification of the compound administered lead to the improvement of L-arginine-based therapeutic angiogenesis. To overcome the problem of oral administration of L-arginine, a phase II clinical trial using intravenously-delivered Unifuzol®, a drug-containing L-arginine, was conducted in 2019 in patients with IC to examine its effect on improving their walking distance (NCT03861416). Meanwhile, a clinical trial using orally administered L-citrulline, a natural precursor of L-arginine, which is metabolized to a lesser degree compared to L-arginine, has also been conducted (CIPER; NCT02521220).
Furthermore, a double-blind clinical trial showed that cilostazol, an anti-platelet drug with phosphodiesterase-3A (PDE-3A) inhibitory activity in platelets and vascular smooth muscle with anti-thrombotic and vasodilatory effects, could improve limb perfusion and peak walking time in patients with IC, most plausibly by upregulating EPC mobilization and increasing the expression of HGF, VEGF-A, and ANG-1 [176, 177]. Rivaroxaban is an inhibitor of clotting factor Xa that exerts anti-thrombotic activity by selectively blocking the active site of factor Xa [178]. A phase III clinical trial showed that rivaroxaban could reduce the incidence of ischemic events in PAD patients, including acute limb ischemia [179, 180]. Anti-cholesterol drugs, statins, which decrease cholesterol synthesis by competitively inhibiting endogenous cholesterol synthesis rate-limiting enzyme reductase, are also potential candidates for treating PAD [181]. Statin administration could promote angiogenesis in HLI animal models by positively regulating eNOS-mediated vasodilation; furthermore, they could reduce the amputation rates in PAD patients [182-184]. Sildenafil is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific PDE type 5 (PDE5) and is an oral drug for treating erectile dysfunction [185]. Low concentrations of PDE5 are also found in platelets, blood vessels, visceral smooth muscle, and skeletal muscle, suggesting that sildenafil might exert beneficial effects as anti-platelet, anti-thrombotic, and dilatation drugs [186, 187]. A phase II clinical trial showed that orally administered sildenafil could efficiently improve symptoms and walking capacity in patients with stage 2 claudication. The clinical trial using this drug has now progressed into the stage of recruiting patients for the phase III trial (NCT03686306).
Preclinical studies have also revealed that small molecule drugs clinically approved for treating diabetes might also be effective in promoting therapeutic angiogenesis. Interestingly, these drugs induce therapeutic angiogenesis, not through their canonical anti-hyperglycemic mechanisms. Metformin, the first-line drug for type 2 diabetic mellitus, also has a specific regulatory effect on the process of angiogenesis. The research found that intraperitoneal injection of metformin exhibits potential for improving endothelial function and the wound-healing process in mice models without affecting their blood glucose levels [188]. Recent studies have also revealed that another FDA-approved anti-diabetic class of drugs, gliflozins, which lower blood glucose by suppressing kidney glucose reabsorption by inhibiting sodium-glucose cotransporter 2 (SGLT2) [189], are also potential small molecule drug candidate for treating PAD. Despite the absence of SGLT2 in skeletal muscle cells, intramuscular injection of two members of gliflozin, namely dapagliflozin and sotagliflozin, into the ischemic site could induce the paracrine capacity of skeletal muscle cells, resulting in a significant increase in angiogenesis and blood perfusion recovery in diabetic HLI mice [74, 190]. Similar to metformin, intramuscular administration of these drugs did not affect blood glucose levels, suggesting that their mechanism in inducing therapeutic angiogenesis is different from their mechanism for lowering blood glucose. Indeed, oral intake of dapagliflozin did not correlate with the risk of amputation, further supporting the distinct mechanism and effect induced by oral and intramuscular administration of this drug [191, 192]. Furthermore, it is noteworthy that oral administration of another member of this class of drug, canagliflozin, increased the risk of amputation in patients with type 2 diabetes and impaired BM-MSCs function in HLI mice models [193-195], indicating that structural differences in small molecule drugs might cause different effects in treating PAD. Thus, further investigations regarding the SAR and the administration method of gliflozins for treating PAD need to be conducted.
Together, small molecule-based therapies could overcome drug delivery and the high-cost problems of other therapeutic angiogenesis strategies. Furthermore, the use of drugs clinically approved for other diseases might provide a shortcut for discovering potential therapeutic angiogenic agents. However, more clinical trials should be conducted to examine their efficacy. Moreover, as many of these drugs benefit PAD most likely through mechanisms distinct from their canonical, well-known ones, detailed studies regarding the molecular mechanisms underlying their effect in inducing therapeutic angiogenesis also need to be conducted. On the other hand, while structural optimization or combination with biomaterials and intensive preclinical and clinical studies are required, traditional herbs or plants provide numerous potential active compounds for treating PAD.
Conclusion
Gene-, cell-, protein-, and small molecule drug-based therapeutic angiogenesis has emerged as an important potential strategy for treating PAD (Figure 3). Gene-based therapy uses genetic approaches to induce therapeutic angiogenesis. The first generation of gene-based therapeutic angiogenesis aims to induce the expression of single angiogenic factors, such as VEGF, HGF, and FGFs. The second generation, on the other hand, aims to induce therapeutic angiogenesis more efficiently by expressing two or more angiogenic factors simultaneously, or by expressing or suppressing upstream regulators of angiogenic factors, such as HIF-1α and the PHD family, as angiogenic is a complex process involving various vectors and cell types. Although some of the gene-based therapies have entered clinical trials, and two of them have been approved for clinical use, the concerns regarding their safety and delivery efficacy remain to be solved. Cell-based therapy has the potential to overcome the problem of the requirement of various angiogenic factors to induce efficient therapeutic angiogenesis in ischemic sites; however, it requires transplantation of viable stem or progenitor cells. These hurdles, along with the need for large amounts of cells and a lack of an appropriate niche in the transplantation sites, need to be overcome for further clinical use. Meanwhile, protein-based therapy, which introduces recombinant proteins to patients to induce angiogenesis, might benefit through their direct functions in regulating and/or stimulating blood vessel formation. However, this approach has several drawbacks, including adverse events, such as immunogenicity proteinuria, and properties of the proteins, such as short half-lives, poor physical and chemical properties, and their low bioavailability. Thus, further modifications and structure-activity relationship studies are needed to reduce their adverse effects and improve their drug-likeness. Meanwhile, due to its low-cost, small molecule drug-based therapy, which could alter the expression levels of angiogenic factors and thus improves angiogenesis in PAD patients, has attracted attention for its use in therapeutic angiogenesis. While many preclinical studies using small molecule compounds have been conducted, only a few of them have emerged into clinical trials at current, and one of the main problems to be solved is their chemical and physical characteristics as well as their drug-likeness.
To broaden the future application of therapeutic angiogenesis-based strategies in treating PAD, more sophisticated optimizations are needed to solve the problem of the gap between preclinical and clinical trial outcomes. First, delivery methods should be improved. This could be achieved by, for example, improving the specificity, stability, and biocompatibility of the gene or protein being transferred by modifying their structures or combining them with biomaterials; optimizing the gene or protein being transferred by modifying their structures or combining them with biomaterials; enhancing the viability of the cells used in cell-based therapy; and developing a sustained-release delivery system using appropriate biomaterials. Second, the availability of therapeutic angiogenesis agents should be increased to reduce costs, for example, by optimizing the extraction and purification methods of vectors and proteins or by increasing the availability of cells using conditions mimicking in vivo circumstances. In terms of availability and cost performance, small molecule drugs might offer a more economical strategy, as their synthesis and purification are easier and more cost-effective. Third, as the innate angiogenesis potential, which determines the recovery ability, differs among species and even among patients, more appropriate PAD animal models that could reflect different recovery abilities and stages of disease progression should be established. Furthermore, more appropriate criteria should also be used as standards for selecting patients appropriate for different therapeutic angiogenesis approaches.
Drugs clinically approved for other diseases might offer a shortcut for clinical translation of therapeutic angiogenic strategy. Although they should also undergo a complete clinical trial to re-examine their safety and efficacy in treating PAD, especially when different administration methods and/or doses are used, it will usually take a significantly shorter time than unapproved compounds. However, the molecular mechanisms underlying the therapeutic angiogenesis effects of these drugs need to be elucidated, as many of them exert beneficial effects on PAD patients, not through their known canonical pathways. Attention should also be paid to delivery methods and drugs with similar structures, as slight differences in structure and/or delivery method might result in totally different therapeutic effects. Furthermore, the combination of small molecule drugs and biomaterials might benefit future clinical applications of small molecule drug-based therapy in PAD patients.
Overall, while further preclinical investigations and more clinical trials are needed to develop safe, effective, and economic-friendly strategies, current preclinical and clinical trials have already demonstrated the promising effects of therapeutic angiogenesis, thus highlighting its importance as a potential strategy for treating PAD.
Abbreviations
ABI: ankle-brachial index; ADSC: adipose-derived stem cells; AFS: amputation-free survival; AGEs: advanced glycation end-product; AGGF1: angiogenic factor with G-patch and Forkhead-associated domain 1; ANG-1: angiopoietin-1; BM-MNC: bone marrow-derived mononuclear cell; BM-MSCs: bone marrow-derived mesenchymal stem cells; CEP: carboxyethylpyrrole; cGMP: cyclic guanosine monophosphate; CLI: critical limb ischemia; ECs: endothelial cells; ECM: extracellular matrix; EGCG: epigallocatechin-3-gallate; eNOS: endothelial nitric oxide synthase; EPCs: endothelial progenitor cells; ESC: embryonic stem cell; FGF-2: fibroblast growth factor-2; GM-CSF: granulocyte-macrophage colony-stimulating factor; HEK293T: human embryonic kidney fibroblast; HepG2: human hepatocellular carcinoma; HGF: hepatocyte growth factor; HIF-1: hypoxia-inducible factor-1; HLI: hindlimb ischemia; HO-1: heme oxygenase-1; hTERT: human telomerase reverse transcriptase; IC: intermittent claudication; IL-1β: interleukin-1β; MALE: major adverse limb events; MCP-1: monocyte chemoattractant protein 1; miRNA: micro RNA; MMP: matrix metalloproteinase; PAD: peripheral artery disease; PB-MNC: peripheral blood-derived mononuclear cell; PDE: phosphodiesterase; PDE5: PDE type 5; PDGF-BB: platelet-derived growth factor BB; PHD: prolyl-hydroxylase domain; ROS: reactive oxygen species; RP: rest pain; SAR: structure-activity relationship; SDF-1: stromal cell-derived factor-1; SGLT2: sodium-glucose cotransporter 2; VEGF: vascular endothelial growth factor; VSMCs: vascular smooth muscle cells.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31871367 and 81872273). We also thank for the support from the State and Local Joint Engineering Laboratory for Vascular Implants (Chongqing).
Our intention is to summarize the state of art. However, due to space limitations, we would like to apologize to authors whose works are not cited here. Their contributions should not be considered less important than those that are cited.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM. et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329-40
2. Creager MA, White CJ, Hiatt WR, Criqui MH, Josephs SC, Alberts MJ. et al. Atherosclerotic Peripheral Vascular Disease Symposium II: executive summary. Circulation. 2008;118:2811-25
3. Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR. et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020-e30
4. Criqui MH, Matsushita K, Aboyans V, Hess CN, Hicks CW, Kwan TW. et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation. 2021;144:e171-e91
5. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5-67
6. Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156-70
7. Farber A, Eberhardt RT. The Current State of Critical Limb Ischemia: A Systematic Review. JAMA Surg. 2016;151:1070-7
8. Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB. et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62:1642-51 e3
9. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M. et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-75
10. Society AC. Cancer facts & statistics. 2019.
11. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509-26
12. Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW. et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-24
13. Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis. 2018;275:379-81
14. Thukkani AK, Kinlay S. Endovascular intervention for peripheral artery disease. Circ Res. 2015;116:1599-613
15. Jones WS, Baumgartner I, Hiatt WR, Heizer G, Conte MS, White CJ. et al. Ticagrelor Compared With Clopidogrel in Patients With Prior Lower Extremity Revascularization for Peripheral Artery Disease. Circulation. 2017;135:241-50
16. Beckman JA, Schneider PA, Conte MS. Advances in Revascularization for Peripheral Artery Disease: Revascularization in PAD. Circ Res. 2021;128:1885-912
17. Hirsch AT, Duval S. Effective vascular therapeutics for critical limb ischemia: a role for registry-based clinical investigation. Circ Cardiovasc Interv. 2013;6:8-11
18. Althouse AD, Abbott JD, Forker AD, Bertolet M, Barinas-Mitchell E, Thurston RC. et al. Risk factors for incident peripheral arterial disease in type 2 diabetes: results from the Bypass Angioplasty Revascularization Investigation in type 2 Diabetes (BARI 2D) Trial. Diabetes Care. 2014;37:1346-52
19. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE. et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686-e725
20. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1-75
21. Hess CN, Rogers RK, Wang TY, Fu R, Gundrum J, Allen LaPointe NM. et al. Major Adverse Limb Events and 1-Year Outcomes After Peripheral Artery Revascularization. J Am Coll Cardiol. 2018;72:999-1011
22. Anand SS, Caron F, Eikelboom JW, Bosch J, Dyal L, Aboyans V. et al. Major Adverse Limb Events and Mortality in Patients With Peripheral Artery Disease: The COMPASS Trial. J Am Coll Cardiol. 2018;71:2306-15
23. Mustapha JA, Katzen BT, Neville RF, Lookstein RA, Zeller T, Miller LE. et al. Determinants of Long-Term Outcomes and Costs in the Management of Critical Limb Ischemia: A Population-Based Cohort Study. J Am Heart Assoc. 2018;7:e009724
24. Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol. 2013;10:387-96
25. Ko SH, Bandyk DF. Therapeutic angiogenesis for critical limb ischemia. Semin Vasc Surg. 2014;27:23-31
26. Iyer SR, Annex BH. Therapeutic Angiogenesis for Peripheral Artery Disease: Lessons Learned in Translational Science. JACC Basic Transl Sci. 2017;2:503-12
27. Deev R, Plaksa I, Bozo I, Isaev A. Results of an International Postmarketing Surveillance Study of pl-VEGF165 Safety and Efficacy in 210 Patients with Peripheral Arterial Disease. Am J Cardiovasc Drugs. 2017;17:235-42
28. Deev R, Plaksa I, Bozo I, Mzhavanadze N, Suchkov I, Chervyakov Y. et al. Results of 5-year follow-up study in patients with peripheral artery disease treated with PL-VEGF165 for intermittent claudication. Ther Adv Cardiovasc Dis. 2018;12:237-46
29. Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y. et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17:1152-61
30. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS. et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56
31. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852-66
32. Steven S, Daiber A, Dopheide JF, Munzel T, Espinola-Klein C. Peripheral artery disease, redox signaling, oxidative stress - Basic and clinical aspects. Redox Biol. 2017;12:787-97
33. Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018;114:540-50
34. Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR. et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114:622-34
35. Narula N, Dannenberg AJ, Olin JW, Bhatt DL, Johnson KW, Nadkarni G. et al. Pathology of Peripheral Artery Disease in Patients With Critical Limb Ischemia. J Am Coll Cardiol. 2018;72:2152-63
36. Tan RP, Ryder I, Yang N, Lam YT, Santos M, Michael PL. et al. Macrophage Polarization as a Novel Therapeutic Target for Endovascular Intervention in Peripheral Artery Disease. JACC Basic Transl Sci. 2021;6:693-704
37. Michel JB. Phylogenic Determinants of Cardiovascular Frailty, Focus on Hemodynamics and Arterial Smooth Muscle Cells. Physiol Rev. 2020;100:1779-837
38. Michel JB, Martin-Ventura JL. Red Blood Cells and Hemoglobin in Human Atherosclerosis and Related Arterial Diseases. Int J Mol Sci. 2020 21
39. Guirguis-Blake JM, Evans CV, Redmond N, Lin JS. Screening for Peripheral Artery Disease Using the Ankle-Brachial Index: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018;320:184-96
40. Polonsky TS, McDermott MM. Lower Extremity Peripheral Artery Disease Without Chronic Limb-Threatening Ischemia: A Review. JAMA. 2021;325:2188-98
41. Behroozian AA, Beckman JA. Asymptomatic peripheral artery disease: Silent but deadly. Prog Cardiovasc Dis. 2021;65:2-8
42. Andras A, Ferket B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014: CD010835.
43. McDermott MM, Guralnik JM, Ferrucci L, Tian L, Liu K, Liao Y. et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117:2484-91
44. Banerjee A, Fowkes FG, Rothwell PM. Associations between peripheral artery disease and ischemic stroke: implications for primary and secondary prevention. Stroke. 2010;41:2102-7
45. Sigvant B, Lundin F, Wahlberg E. The Risk of Disease Progression in Peripheral Arterial Disease is Higher than Expected: A Meta-Analysis of Mortality and Disease Progression in Peripheral Arterial Disease. Eur J Vasc Endovasc Surg. 2016;51:395-403
46. Cryer PE. Preventing hypoglycaemia: what is the appropriate glucose alert value? Diabetologia. 2009;52:35-7
47. Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10:711-22
48. Malmstedt J, Karvestedt L, Swedenborg J, Brismar K. The receptor for advanced glycation end products and risk of peripheral arterial disease, amputation or death in type 2 diabetes: a population-based cohort study. Cardiovasc Diabetol. 2015;14:93
49. Tateno K, Minamino T, Toko H, Akazawa H, Shimizu N, Takeda S. et al. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization. Circ Res. 2006;98:1194-202
50. Perez-Ilzarbe M, Agbulut O, Pelacho B, Ciorba C, San Jose-Eneriz E, Desnos M. et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail. 2008;10:1065-72
51. Giudice J, Taylor JM. Muscle as a paracrine and endocrine organ. Curr Opin Pharmacol. 2017;34:49-55
52. Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ. et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20:1464-71
53. Chiu CY, Yang RS, Sheu ML, Chan DC, Yang TH, Tsai KS. et al. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J Pathol. 2016;238:470-82
54. Rai AK, Jaiswal N, Maurya CK, Sharma A, Ahmad I, Ahmad S. et al. Fructose-induced AGEs-RAGE signaling in skeletal muscle contributes to impairment of glucose homeostasis. J Nutr Biochem. 2019;71:35-44
55. Snow LM, Lynner CB, Nielsen EM, Neu HS, Thompson LV. Advanced glycation end product in diabetic rat skeletal muscle in vivo. Pathobiology. 2006;73:244-51
56. Liu C, Han J, Marcelina O, Nugrahaningrum DA, Huang S, Zou M. et al. Discovery of Salidroside-Derivated Glycoside Analogues as Novel Angiogenesis Agents to Treat Diabetic Hind Limb Ischemia. J Med Chem. 2022;65:135-62
57. Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes-a review. Diabet Med. 2010;27:4-14
58. Nillesen ST, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123-31
59. Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T. et al. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379-84
60. Tomita N, Morishita R, Taniyama Y, Koike H, Aoki M, Shimizu H. et al. Angiogenic property of hepatocyte growth factor is dependent on upregulation of essential transcription factor for angiogenesis, ets-1. Circulation. 2003;107:1411-7
61. Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S. et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662-70
62. Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials. 2013;34:9201-9
63. Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575-85
64. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873-87
65. Yao Q, Renault MA, Chapouly C, Vandierdonck S, Belloc I, Jaspard-Vinassa B. et al. Sonic hedgehog mediates a novel pathway of PDGF-BB-dependent vessel maturation. Blood. 2014;123:2429-37
66. Lu QB, Wan MY, Wang PY, Zhang CX, Xu DY, Liao X. et al. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFkappaB/mTOR/P70S6K signaling cascade. Redox Biol. 2018;14:656-68
67. Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685-93
68. Wahlberg E. Angiogenesis and arteriogenesis in limb ischemia. J Vasc Surg. 2003;38:198-203
69. Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR. et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781-6
70. Fadini GP, Avogaro A. Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes Obes Metab. 2010;12:570-83
71. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-7
72. Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A. et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362-7
73. Brevetti G, Silvestro A, Di Giacomo S, Bucur R, Di Donato A, Schiano V. et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374-9
74. Luo LL, Han JX, Wu SR, Kasim V. Intramuscular injection of sotagliflozin promotes neovascularization in diabetic mice through enhancing skeletal muscle cells paracrine function. Acta Pharmacol Sin. 2022
75. Zhang J, Kasim V, Xie YD, Huang C, Sisjayawan J, Dwi Ariyanti A. et al. Inhibition of PHD3 by salidroside promotes neovascularization through cell-cell communications mediated by muscle-secreted angiogenic factors. Sci Rep. 2017;7:43935
76. Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167-71
77. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677-84
78. Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43-54
79. Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW. et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458-65
80. Maxwell PH, Eckardt KU. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol. 2016;12:157-68
81. Voit RA, Sankaran VG. Stabilizing HIF to Ameliorate Anemia. Cell. 2020;180:6
82. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510-4
83. Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M. et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485-90
84. Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37:4587-602
85. Ortiz-Barahona A, Villar D, Pescador N, Amigo J, del Peso L. Genome-wide identification of hypoxia-inducible factor binding sites and target genes by a probabilistic model integrating transcription-profiling data and in silico binding site prediction. Nucleic Acids Res. 2010;38:2332-45
86. Semenza GL. Regulation of vascularization by hypoxia-inducible factor 1. Ann N Y Acad Sci. 2009;1177:2-8
87. Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E. et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604-13
88. Jain T, Nikolopoulou EA, Xu Q, Qu A. Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharmacol Ther. 2018;183:22-33
89. Rajagopalan S, Olin J, Deitcher S, Pieczek A, Laird J, Grossman PM. et al. Use of a constitutively active hypoxia-inducible factor-1alpha transgene as a therapeutic strategy in no-option critical limb ischemia patients: phase I dose-escalation experience. Circulation. 2007;115:1234-43
90. Kuan CY, Chen HR, Gao N, Kuo YM, Chen CW, Yang D. et al. Brain-targeted hypoxia-inducible factor stabilization reduces neonatal hypoxic-ischemic brain injury. Neurobiol Dis. 2021;148:105200
91. Nakashima M, Watanabe M, Nakano K, Uchimaru K, Horie R. Differentiation of Hodgkin lymphoma cells by reactive oxygen species and regulation by heme oxygenase-1 through HIF-1alpha. Cancer Sci. 2021;112:2542-55
92. Bowling FL, Rashid ST, Boulton AJ. Preventing and treating foot complications associated with diabetes mellitus. Nat Rev Endocrinol. 2015;11:606-16
93. Annex BH, Cooke JP. New Directions in Therapeutic Angiogenesis and Arteriogenesis in Peripheral Arterial Disease. Circ Res. 2021;128:1944-57
94. Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T. et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348:370-4
95. Creager MA, Olin JW, Belch JJ, Moneta GL, Henry TD, Rajagopalan S. et al. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124:1765-73
96. Yonemitsu Y, Matsumoto T, Itoh H, Okazaki J, Uchiyama M, Yoshida K. et al. DVC1-0101 to treat peripheral arterial disease: a Phase I/IIa open-label dose-escalation clinical trial. Mol Ther. 2013;21:707-14
97. Gu Y, Cui S, Liu C, Zhao J, Li M, Li Y. et al. pUDK-HGF Gene Therapy to Relieve CLI Rest Pain and Ulcer: A Phase II, Double-Blind, Randomized Placebo-Controlled Trial. Hum Gene Ther. 2021;32:839-49
98. Powell RJ, Goodney P, Mendelsohn FO, Moen EK, Annex BH, Investigators HGFT. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF-0205 trial. J Vasc Surg. 2010;52:1525-30
99. Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M. et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58-65
100. Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY. et al. Basic fibroblast growth factor in patients with intermittent claudication: results of a phase I trial. J Am Coll Cardiol. 2000;36:1239-44
101. Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB. et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053-8
102. Tian XL, Kadaba R, You SA, Liu M, Timur AA, Yang L. et al. Identification of an angiogenic factor that when mutated causes susceptibility to Klippel-Trenaunay syndrome. Nature. 2004;427:640-5
103. Fan C, Ouyang P, Timur AA, He P, You SA, Hu Y. et al. Novel roles of GATA1 in regulation of angiogenic factor AGGF1 and endothelial cell function. J Biol Chem. 2009;284:23331-43
104. Zaccagnini G, Gaetano C, Della Pietra L, Nanni S, Grasselli A, Mangoni A. et al. Telomerase mediates vascular endothelial growth factor-dependent responsiveness in a rat model of hind limb ischemia. J Biol Chem. 2005;280:14790-8
105. Besnier M, Shantikumar S, Anwar M, Dixit P, Chamorro-Jorganes A, Sweaad W. et al. miR-15a/-16 Inhibit Angiogenesis by Targeting the Tie2 Coding Sequence: Therapeutic Potential of a miR-15a/16 Decoy System in Limb Ischemia. Mol Ther Nucleic Acids. 2019;17:49-62
106. Rajagopalan S, Shah M, Luciano A, Crystal R, Nabel EG. Adenovirus-mediated gene transfer of VEGF(121) improves lower-extremity endothelial function and flow reserve. Circulation. 2001;104:753-5
107. Mohler ER 3rd, Rajagopalan S, Olin JW, Trachtenberg JD, Rasmussen H, Pak R. et al. Adenoviral-mediated gene transfer of vascular endothelial growth factor in critical limb ischemia: safety results from a phase I trial. Vasc Med. 2003;8:9-13
108. Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST. et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480-7
109. Rajagopalan S, Mohler ER 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK. et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933-8
110. Morishita R, Shimamura M, Takeya Y, Nakagami H, Chujo M, Ishihama T. et al. Combined Analysis of Clinical Data on HGF Gene Therapy to Treat Critical Limb Ischemia in Japan. Curr Gene Ther. 2020;20:25-35
111. Sanada F, Fujikawa T, Shibata K, Taniyama Y, Rakugi H, Morishita R. Therapeutic Angiogenesis Using HGF Plasmid. Ann Vasc Dis. 2020;13:109-15
112. Pyun WB, Hahn W, Kim DS, Yoo WS, Lee SD, Won JH. et al. Naked DNA expressing two isoforms of hepatocyte growth factor induces collateral artery augmentation in a rabbit model of limb ischemia. Gene Ther. 2010;17:1442-52
113. Nikol S, Baumgartner I, Van Belle E, Diehm C, Visona A, Capogrossi MC. et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16:972-8
114. Lu Q, Yao Y, Yao Y, Liu S, Huang Y, Lu S. et al. Angiogenic factor AGGF1 promotes therapeutic angiogenesis in a mouse limb ischemia model. PLoS One. 2012;7:e46998
115. Yao Y, Li Y, Song Q, Hu C, Xie W, Xu C. et al. Angiogenic Factor AGGF1-Primed Endothelial Progenitor Cells Repair Vascular Defect in Diabetic Mice. Diabetes. 2019;68:1635-48
116. Wang J, Peng H, Timur AA, Pasupuleti V, Yao Y, Zhang T. et al. Receptor and Molecular Mechanism of AGGF1 Signaling in Endothelial Cell Functions and Angiogenesis. Arterioscler Thromb Vasc Biol. 2021;41:2756-69
117. Murasawa S, Llevadot J, Silver M, Isner JM, Losordo DW, Asahara T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;106:1133-9
118. Forster R, Liew A, Bhattacharya V, Shaw J, Stansby G. Gene therapy for peripheral arterial disease. Cochrane Database Syst Rev. 2018;10:CD012058
119. Barc P, Antkiewicz M, Sliwa B, Fraczkowska K, Guzinski M, Dawiskiba T. et al. Double VEGF/HGF Gene Therapy in Critical Limb Ischemia Complicated by Diabetes Mellitus. J Cardiovasc Transl Res. 2021;14:409-15
120. Stather PW, Sylvius N, Sidloff DA, Dattani N, Verissimo A, Wild JB. et al. Identification of microRNAs associated with abdominal aortic aneurysms and peripheral arterial disease. Br J Surg. 2015;102:755-66
121. Hsu PY, Hsi E, Wang TM, Lin RT, Liao YC, Juo SH. MicroRNA let-7g possesses a therapeutic potential for peripheral artery disease. J Cell Mol Med. 2017;21:519-29
122. Besnier M, Gasparino S, Vono R, Sangalli E, Facoetti A, Bollati V. et al. miR-210 Enhances the Therapeutic Potential of Bone-Marrow-Derived Circulating Proangiogenic Cells in the Setting of Limb Ischemia. Mol Ther. 2018;26:1694-705
123. Gan LM, Lagerstrom-Fermer M, Carlsson LG, Arfvidsson C, Egnell AC, Rudvik A. et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat Commun. 2019;10:871
124. Wu S, Nishiyama N, Kano MR, Morishita Y, Miyazono K, Itaka K. et al. Enhancement of angiogenesis through stabilization of hypoxia-inducible factor-1 by silencing prolyl hydroxylase domain-2 gene. Mol Ther. 2008;16:1227-34
125. Wu S, Zhang J, Huang C, Jia H, Wang Y, Xu Z. et al. Prolyl hydroxylase domain-2 silencing induced by hydrodynamic limb vein injection enhances vascular regeneration in critical limb ischemia mice through activation of multiple genes. Curr Gene Ther. 2015;15:313-25
126. Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA, Jiang C. et al. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1alpha/VP16 hybrid transcription factor. Circulation. 2000;102:2255-61
127. Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marce M, Semenza GL. Adenoviral transfer of HIF-1alpha enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci U S A. 2009;106:18769-74
128. Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV. et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310-8
129. Inampudi C, Akintoye E, Ando T, Briasoulis A. Angiogenesis in peripheral arterial disease. Curr Opin Pharmacol. 2018;39:60-7
130. Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H. et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427-35
131. Lindeman JHN, Zwaginga JJ, Kallenberg-Lantrua G, van Wissen RC, Schepers A, van Bockel HJ. et al. No Clinical Benefit of Intramuscular Delivery of Bone Marrow-derived Mononuclear Cells in Nonreconstructable Peripheral Arterial Disease: Results of a Phase-III Randomized-controlled Trial. Ann Surg. 2018;268:756-61
132. Karantalis V, Schulman IH, Balkan W, Hare JM. Allogeneic cell therapy: a new paradigm in therapeutics. Circ Res. 2015;116:12-5
133. Kibbe MR, Hirsch AT, Mendelsohn FO, Davies MG, Pham H, Saucedo J. et al. Safety and efficacy of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with critical limb ischemia. Gene Ther. 2016;23:399
134. Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8:125
135. Rigato M, Monami M, Fadini GP. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ Res. 2017;120:1326-40
136. Hedhli J, Konopka CJ, Schuh S, Bouvin H, Cole JA, Huntsman HD. et al. Multimodal Assessment of Mesenchymal Stem Cell Therapy for Diabetic Vascular Complications. Theranostics. 2017;7:3876-88
137. Gupta PK, Krishna M, Chullikana A, Desai S, Murugesan R, Dutta S. et al. Administration of Adult Human Bone Marrow-Derived, Cultured, Pooled, Allogeneic Mesenchymal Stromal Cells in Critical Limb Ischemia Due to Buerger's Disease: Phase II Study Report Suggests Clinical Efficacy. Stem Cells Transl Med. 2017;6:689-99
138. Norgren L, Weiss N, Nikol S, Hinchliffe RJ, Lantis JC, Patel MR. et al. PLX-PAD Cell Treatment of Critical Limb Ischaemia: Rationale and Design of the PACE Trial. Eur J Vasc Endovasc Surg. 2019;57:538-45
139. Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155-60
140. Murphy MP, Lawson JH, Rapp BM, Dalsing MC, Klein J, Wilson MG. et al. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg. 2011;53:1565-74 e1
141. Flugelman MY, Halak M, Yoffe B, Schneiderman J, Rubinstein C, Bloom AI. et al. Phase Ib Safety, Two-Dose Study of MultiGeneAngio in Patients with Chronic Critical Limb Ischemia. Mol Ther. 2017;25:816-25
142. Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y. et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542-7
143. Johnson TK, Zhao L, Zhu D, Wang Y, Xiao Y, Oguljahan B. et al. Exosomes derived from induced vascular progenitor cells promote angiogenesis in vitro and in an in vivo rat hindlimb ischemia model. Am J Physiol Heart Circ Physiol. 2019;317:H765-H76
144. Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F. et al. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol. 2010;30:984-91
145. Mohamed SA, Howard L, McInerney V, Hayat A, Krawczyk J, Naughton S. et al. Autologous bone marrow mesenchymal stromal cell therapy for "no-option" critical limb ischemia is limited by karyotype abnormalities. Cytotherapy. 2020;22:313-21
146. Huang A, Liu D, Qi X, Yue Z, Cao H, Zhang K. et al. Self-assembled GFFYK peptide hydrogel enhances the therapeutic efficacy of mesenchymal stem cells in a mouse hindlimb ischemia model. Acta Biomater. 2019;85:94-105
147. Beegle JR, Magner NL, Kalomoiris S, Harding A, Zhou P, Nacey C. et al. Preclinical evaluation of mesenchymal stem cells overexpressing VEGF to treat critical limb ischemia. Mol Ther Methods Clin Dev. 2016;3:16053
148. Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C. et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. 2010;18:1545-52
149. Ariyanti AD, Zhang J, Marcelina O, Nugrahaningrum DA, Wang G, Kasim V. et al. Salidroside-Pretreated Mesenchymal Stem Cells Enhance Diabetic Wound Healing by Promoting Paracrine Function and Survival of Mesenchymal Stem Cells Under Hyperglycemia. Stem Cells Transl Med. 2019;8:404-14
150. Wei HJ, Chen CH, Lee WY, Chiu I, Hwang SM, Lin WW. et al. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29:3547-56
151. Zhuo Y, Li SH, Chen MS, Wu J, Kinkaid HY, Fazel S. et al. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. J Thorac Cardiovasc Surg. 2010;139:1286-94 94 e1-2
152. Teraa M, Sprengers RW, Schutgens RE, Slaper-Cortenbach IC, van der Graaf Y, Algra A. et al. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial. Circulation. 2015;131:851-60
153. Qadura M, Terenzi DC, Verma S, Al-Omran M, Hess DA. Concise Review: Cell Therapy for Critical Limb Ischemia: An Integrated Review of Preclinical and Clinical Studies. Stem Cells. 2018;36:161-71
154. Annex BH, Simons M. Growth factor-induced therapeutic angiogenesis in the heart: protein therapy. Cardiovasc Res. 2005;65:649-55
155. Schumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998;97:645-50
156. McDermott MM, Ferrucci L, Tian L, Guralnik JM, Lloyd-Jones D, Kibbe MR. et al. Effect of Granulocyte-Macrophage Colony-Stimulating Factor With or Without Supervised Exercise on Walking Performance in Patients With Peripheral Artery Disease: The PROPEL Randomized Clinical Trial. JAMA. 2017;318:2089-98
157. Agrawal V, Marinescu V, Agarwal M, McCullough PA. Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol. 2009;6:301-11
158. Putney SD, Burke PA. Improving protein therapeutics with sustained-release formulations. Nat Biotechnol. 1998;16:153-7
159. Pakulska MM, Miersch S, Shoichet MS. Designer protein delivery: From natural to engineered affinity-controlled release systems. Science. 2016;351:aac4750
160. Segers VF, Revin V, Wu W, Qiu H, Yan Z, Lee RT. et al. Protease-resistant stromal cell-derived factor-1 for the treatment of experimental peripheral artery disease. Circulation. 2011;123:1306-15
161. Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683-92
162. Ganta VC, Choi M, Kutateladze A, Annex BH. VEGF165b Modulates Endothelial VEGFR1-STAT3 Signaling Pathway and Angiogenesis in Human and Experimental Peripheral Arterial Disease. Circ Res. 2017;120:282-95
163. Sun J, Wu J, Jin H, Ying T, Jin W, Fan M. et al. Structure-guided design, generation, and biofunction of PEGylated fibroblast growth factor 2 variants for wound healing. Nanoscale. 2020;12:18200-13
164. Chen F, Long Q, Fu D, Zhu D, Ji Y, Han L. et al. Targeting SPINK1 in the damaged tumour microenvironment alleviates therapeutic resistance. Nat Commun. 2018;9:4315
165. Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16:101-14
166. Hou L, Yang G, Tang S, Alcazar C, Joshi P, Strassberg Z. et al. Small Molecule Derived From Carboxyethylpyrrole Protein Adducts Promotes Angiogenesis in a Mouse Model of Peripheral Arterial Disease. J Am Heart Assoc. 2018;7:e009234
167. Zhang K, Lu J, Mori T, Smith-Powell L, Synold TW, Chen S. et al. Baicalin increases VEGF expression and angiogenesis by activating the ERR{alpha}/PGC-1{alpha} pathway. Cardiovasc Res. 2011;89:426-35
168. Pignet AL, Schellnegger M, Hecker A, Kohlhauser M, Kotzbeck P, Kamolz LP. Resveratrol-Induced Signal Transduction in Wound Healing. Int J Mol Sci. 2021 22
169. Zhang J, Liu A, Hou R, Zhang J, Jia X, Jiang W. et al. Salidroside protects cardiomyocyte against hypoxia-induced death: a HIF-1alpha-activated and VEGF-mediated pathway. Eur J Pharmacol. 2009;607:6-14
170. Zheng KY, Zhang ZX, Guo AJ, Bi CW, Zhu KY, Xu SL. et al. Salidroside stimulates the accumulation of HIF-1alpha protein resulted in the induction of EPO expression: a signaling via blocking the degradation pathway in kidney and liver cells. Eur J Pharmacol. 2012;679:34-9
171. Deheng C, Kailiang Z, Weidong W, Haiming J, Daoliang X, Ningyu C. et al. Salidroside Promotes Random Skin Flap Survival in Rats by Enhancing Angiogenesis and Inhibiting Apoptosis. J Reconstr Microsurg. 2016;32:580-6
172. Zhang J, Nugrahaningrum DA, Marcelina O, Ariyanti AD, Wang G, Liu C. et al. Tyrosol Facilitates Neovascularization by Enhancing Skeletal Muscle Cells Viability and Paracrine Function in Diabetic Hindlimb Ischemia Mice. Front Pharmacol. 2019;10:909
173. Ariyanti AD, Sisjayawan J, Zhang J, Zhang JQ, Wang GX, Miyagishi M. et al. Elevating VEGF-A and PDGF-BB secretion by salidroside enhances neoangiogenesis in diabetic hind-limb ischemia. Oncotarget. 2017;8:97187-205
174. Duan J, Chen Z, Liang X, Chen Y, Li H, Tian X. et al. Construction and application of therapeutic metal-polyphenol capsule for peripheral artery disease. Biomaterials. 2020;255:120199
175. West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC. et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972-6
176. Dawson DL, Cutler BS, Meissner MH, Strandness DE Jr. Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998;98:678-86
177. Sanada F, Kanbara Y, Taniyama Y, Otsu R, Carracedo M, Ikeda-Iwabu Y. et al. Induction of Angiogenesis by a Type III Phosphodiesterase Inhibitor, Cilostazol, Through Activation of Peroxisome Proliferator-Activated Receptor-gamma and cAMP Pathways in Vascular Cells. Arterioscler Thromb Vasc Biol. 2016;36:545-52
178. Investigators E, Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H. et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-510
179. Slomski A. Rivaroxaban Reduces Complications of PAD Revascularization. JAMA. 2020;324:327
180. Huynh K. Rivaroxaban reduces ischaemic events in patients with PAD. Nat Rev Cardiol. 2020;17:322
181. Oesterle A, Laufs U, Liao JK. Pleiotropic Effects of Statins on the Cardiovascular System. Circ Res. 2017;120:229-43
182. Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ. et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004-10
183. Fujii T, Onimaru M, Yonemitsu Y, Kuwano H, Sueishi K. Statins restore ischemic limb blood flow in diabetic microangiopathy via eNOS/NO upregulation but not via PDGF-BB expression. Am J Physiol Heart Circ Physiol. 2008;294:H2785-91
184. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL. et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180-9
185. Langtry HD, Markham A. Sildenafil: a review of its use in erectile dysfunction. Drugs. 1999;57:967-89
186. Grunwald JE, Metelitsina T, Grunwald L. Effect of sildenafil citrate (Viagra) on retinal blood vessel diameter. Am J Ophthalmol. 2002;133:809-12
187. Andersson KE. PDE5 inhibitors - pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol. 2018;175:2554-65
188. Nguyen H, Koh JY, Li H, Islas-Robles A, Meda Venkata SP, Wang JM. et al. A novel imidazolinone metformin-methylglyoxal metabolite promotes endothelial cell angiogenesis via the eNOS/HIF-1alpha pathway. FASEB J. 2021;35:e21645
189. van Raalte DH, Cherney DZI. Sodium glucose cotransporter 2 inhibition and renal ischemia: implications for future clinical trials. Kidney Int. 2018;94:459-62
190. Nugrahaningrum DA, Marcelina O, Liu C, Wu S, Kasim V. Dapagliflozin Promotes Neovascularization by Improving Paracrine Function of Skeletal Muscle Cells in Diabetic Hindlimb Ischemia Mice Through PHD2/HIF-1alpha Axis. Front Pharmacol. 2020;11:1104
191. Heyward J, Mansour O, Olson L, Singh S, Alexander GC. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: A systematic review and meta-analysis. PLoS One. 2020;15:e0234065
192. Sung J, Padmanabhan S, Gurung S, Inglis S, Vicaretti M, Begg L. et al. SGLT2 inhibitors and amputation risk: Real-world data from a diabetes foot wound clinic. J Clin Transl Endocrinol. 2018;13:46-7
193. Matthews DR, Li Q, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G. et al. Effects of canagliflozin on amputation risk in type 2 diabetes: the CANVAS Program. Diabetologia. 2019;62:926-38
194. Birkeland KI, Jorgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M. et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709-17
195. Lin Y, Nan J, Shen J, Lv X, Chen X, Lu X. et al. Canagliflozin impairs blood reperfusion of ischaemic lower limb partially by inhibiting the retention and paracrine function of bone marrow derived mesenchymal stem cells. EBioMedicine. 2020;52:102637
Author contact
![]() Corresponding authors: Vivi Kasim, College of Bioengineering, Chongqing University, Chongqing, China; Phone: +86-23-65112672, Fax: +86-23-65111802, Email: vivikasimedu.cn; Shourong Wu, College of Bioengineering, Chongqing University, Chongqing, China; Phone: +86-23-65111632, Fax: +86-23-65111802, Email: shourongwuedu.cn.
Corresponding authors: Vivi Kasim, College of Bioengineering, Chongqing University, Chongqing, China; Phone: +86-23-65112672, Fax: +86-23-65111802, Email: vivikasimedu.cn; Shourong Wu, College of Bioengineering, Chongqing University, Chongqing, China; Phone: +86-23-65111632, Fax: +86-23-65111802, Email: shourongwuedu.cn.
 Global reach, higher impact
Global reach, higher impact