13.3
Impact Factor
Theranostics 2022; 12(11):5155-5171. doi:10.7150/thno.73039 This issue Cite
Research Paper
Tumor microenvironment-activated single-atom platinum nanozyme with H2O2 self-supplement and O2-evolving for tumor-specific cascade catalysis chemodynamic and chemoradiotherapy
1. Key Laboratory of Pollution Control Chemistry and Environmental Functional Materials for Qinghai-Tibet Plateau of the National Ethnic Affairs Commission, School of Chemistry and Environment, Southwest Minzu University, Chengdu 610041, China.
2. Key Laboratory of General Chemistry of the National Ethnic Affairs Commission, School of Chemistry and Environment, Southwest Minzu University, Chengdu 610041, China.
3. CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing100040, China.
4. College of Pharmacy and Biological Engineering, Chengdu University, Chengdu, 610106, China.
5. College of Pharmacy, Southwest Minzu University, Chengdu 610041, China.
6. Department of Neurology, Affiliated Hospital of North Sichuan Medical College, Nanchong 637000, China.
*These authors contributed equally.
Abstract
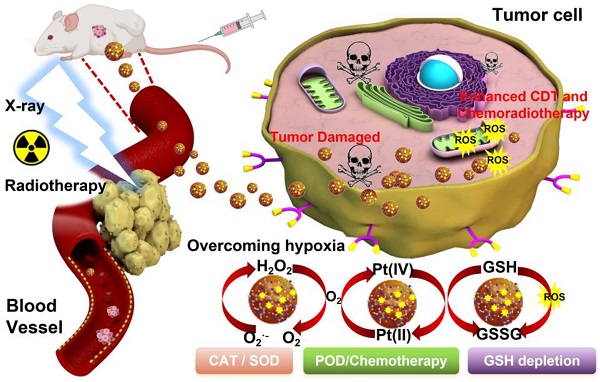
Nanozyme-based tumor collaborative catalytic therapy has attracted a great deal of attention in recent years. However, their cooperative outcome remains a great challenge due to the unique characteristics of tumor microenvironment (TME), such as insufficient endogenous hydrogen peroxide (H2O2) level, hypoxia, and overexpressed intracellular glutathione (GSH).
Methods: Herein, a TME-activated atomic-level engineered PtN4C single-atom nanozyme (PtN4C-SAzyme) is fabricated to induce the “butterfly effect” of reactive oxygen species (ROS) through facilitating intracellular H2O2 cycle accumulation and GSH deprivation as well as X-ray deposition for ROS-involving CDT and O2-dependent chemoradiotherapy.
Results: In the paradigm, the SAzyme could boost substantial ∙OH generation by their admirable peroxidase-like activity as well as X-ray deposition capacity. Simultaneously, O2 self-sufficiency, GSH elimination and elevated Pt2+ release can be achieved through the self-cyclic valence alteration of Pt (IV) and Pt (II) for alleviating tumor hypoxia, overwhelming the anti-oxidation defense effect and overcoming drug-resistance. More importantly, the PtN4C-SAzyme could also convert O2·- into H2O2 by their superior superoxide dismutase-like activity and achieve the sustainable replenishment of endogenous H2O2, and H2O2 can further react with the PtN4C-SAzyme for realizing the cyclic accumulation of ∙OH and O2 at tumor site, thereby generating a “key” to unlock the multi enzymes-like properties of SAzymes for tumor-specific self-reinforcing CDT and chemoradiotherapy.
Conclusions: This work not only provides a promising TME-activated SAzyme-based paradigm with H2O2 self-supplement and O2-evolving capacity for intensive CDT and chemoradiotherapy but also opens new horizons for the construction and tumor catalytic therapy of other SAzymes.
Keywords: Single-atom nanozyme, tumor microenvironment-activated, enzyme-like activity, tumor catalytic therapy, chemodynamic and chemoradiotherapy
 Global reach, higher impact
Global reach, higher impact