13.3
Impact Factor
Theranostics 2022; 12(15):6437-6445. doi:10.7150/thno.77219 This issue Cite
Research Paper
Safety and efficacy of peptide receptor radionuclide therapy with 177Lu-DOTA-EB-TATE in patients with metastatic neuroendocrine tumors
1. Department of Nuclear Medicine, Beijing Key Laboratory of Molecular Targeted Diagnosis and Therapy in Nuclear Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China
2. State Key Laboratory of Complex Severe and Rare Diseases, Beijing 100730, China
3. Nanfang PET Center, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, China.
4. Department of Diagnostic Radiology, Yong Loo Lin School of Medicine and Faculty of Engineering, National University of Singapore, Singapore, 119074, Singapore
5. Clinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117599, Singapore
6. Nanomedicine Translational Research Program, NUS Center for Nanomedicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Singapore
7. Departments of Chemical and Biomolecular Engineering, and Biomedical Engineering, College of Design and Engineering, National University of Singapore, Singapore 117597, Singapore
#Contributed equally
Abstract
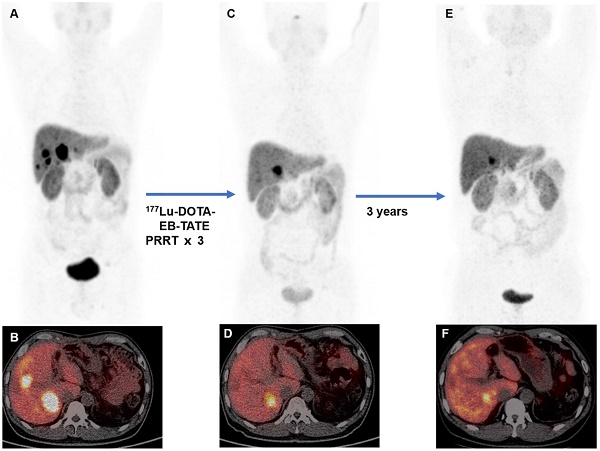
Rationale: This study aimed to assess the safety, efficacy, and survival of 177Lu-DOTA-EB-TATE in patients with metastatic neuroendocrine tumors (NETs).
Methods: Thirty patients with metastatic NETs were prospectively enrolled and treated with 177Lu-DOTA-EB-TATE (3 intended cycles at 8 to 12-week intervals, 3.7 GBq/cycle). Treatment-related adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. The treatment response was graded according to RECIST 1.1 and PERCIST 1.0 criteria. Kaplan-Meier analysis was performed to calculate progression-free survival (PFS) and overall survival (OS).
Results: Patients tolerated therapy well without acute adverse effects. During peptide receptor radionuclide therapy (PRRT), no grade 4 toxicity was observed in any of the patients; grade 3 hematotoxicity was recorded in 4 patients, including grade 3 thrombocytopenia in 4 patients (13.3%) and grade-3 anemia in 1 patient (3.3%); grade 3 hepatotoxicity was recorded in 1 (3.3%) patient, and no grade 2/3/4 nephrotoxicity was observed. On long-term follow-up, none of the patients developed grade 4 hematotoxicity or nephrotoxicity of any grade, reversible grade 3 hematotoxicity (thrombocytopenia) occurred in 1 patient. There was no incidence of leukemia or myelodysplastic syndrome for the duration of follow-up. Of 27 patients with RECIST-measurable disease, partial response and stable disease were seen in 9 and 14 patients, respectively, resulting in a response rate of 33.3% and disease control rate of 85.2%. Of 29 patients evaluable for response on 68Ga-DOTATATE PET/CT, 14 had partial response and 11 had stable disease, with a response rate of 48.3% and disease control rate of 86.2%. The follow-up period ranged from 5 to 57 months after the first 177Lu-DOTA-EB-TATE PRRT with a median follow-up of 46 months. The median PFS was 36 months, and the median OS was not reached. Ki-67 index of greater than 10% was associated with poorer PFS (P = 0.012).
Conclusions: Our results suggest that PRRT with approximately 3.7 GBq 177Lu-DOTA-EB-TATE has acceptable toxicity profile and is effective in treating metastatic NET with high disease control rate. In addition, 177Lu-DOTA-EB-TATE achieved a favorable survival outcome with encouraging PFS.
Keywords: peptide receptor radionuclide therapy (PRRT), 177Lu-DOTA-EB-TATE, 177Lu-DOTA-TATE, neuroendocrine tumor
 Global reach, higher impact
Global reach, higher impact