13.3
Impact Factor
Theranostics 2022; 12(15):6576-6594. doi:10.7150/thno.78034 This issue Cite
Review
Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy
1. Institute of Translational Medicine, Shanghai University, Shanghai, 200444, China.
2. Musculoskeletal Organoid Research Center, Shanghai University, Shanghai, 200444, China.
#These authors contributed equally to this work.
Received 2022-8-15; Accepted 2022-9-2; Published 2022-9-11
Abstract
Bone and soft tissue tumors are complex mesenchymal neoplasms that seriously endanger human health. Over the past decade, the relationship between microorganisms and human health and diseases is getting more attention. The extracellular vesicles derived from bacteria have been shown to regulate bacterial-host cell communication by transferring their contents, including nucleic acids, proteins, metabolites, lipopolysaccharides, and peptidoglycans. Bacteria extracellular vesicles (BEVs) are promising lipid-bilayer nanocarriers for the treatment of many diseases due to their low toxicity, drug loading capacity, ease of modification and industrialization. Specially, BEVs-based cancer therapy has attracted much attention because of their ability to effectively stimulate immune responses. In this review, we provide an overview of the biogenesis, composition, isolation, classification, and internalization of BEVs. We then comprehensively summarize the sources of BEVs in cancer therapy and the BEVs-related cancer treatment strategies. We further highlight the great potential of BEVs in bone and soft tissue tumors. Finally, we conclude the major advantages and challenges of BEVs-based cancer therapy. We believe that the comprehensive understanding of BEVs in the field of cancer therapy will generate innovative solutions to bone and soft tissue tumors and achieve clinical applications.
Keywords: Bone and soft tissue tumors, Bacteria extracellular vesicles, Immunotherapy, Synergistic therapy, Nanotechnology
Introduction
Bone and soft tissue tumors (BSTTs) account for approximately 1% of adult malignancies and about 20% of pediatric neoplasms [1]. Nearly 200,000 people are diagnosed with sarcoma each year in the world [2]. As a diverse and heterogeneous group, these tumors comprise more than 50 subtypes, of which approximately half are musculoskeletal tumors occurring in the extremities [3, 4]. The distribution of BSTTs sites, mainly including head and neck, skin, trunk, limbs, and other sites. Currently, the main treatment for BSTTs is a combination of surgery and chemotherapy [5]. In addition, some therapies such as gene therapy and immunotherapy have achieved certain results [6, 7]. However, in the past 30 years, the progress in primary malignancies treatment has remained slow and clinical outcomes have not been significantly improved. Therefore, it is urgent to explore innovative treatment strategies for BSTTs.
The human body is a complex ecosystem inhabited by trillions of microorganisms, such as bacteria, fungi, and viruses [8]. The Human Microbiome Project (HMP) supported by National Institutes of Health (NIH) has greatly expanded our understanding of the relationship between the human microbiome and human health and disease. It has been reported that more than 1000 species of microorganisms inhabit in a healthy human body [9]. These microorganisms are widely parasitic in the oral cavity, genitourinary tract, skin, and gastrointestinal tract, and affect the human health and disease in a subtle and complex way [10]. Recently, increasing evidence has shown a strong link between intestinal dysbiosis and BSTTs [11, 12]. Although the mechanism exploration [13-15] and bioactive material development [16-19] of bone and soft tissue diseases have been well developed, the mechanism of commensal bacteria affecting disease still needs further exploration.
Extracellular vesicles (EVs) are particles with lipid bilayer released by all domains of life, including eukaryotes, bacteria, and archaea [20, 21]. The relationship between EVs and BSTTs has also been focused [22-24]. The growing understanding of human microbial communities in health and disease has led to insights into EVs derived from microorganisms, especially the bacterial EVs (BEVs), and their roles in microbiota-host communication [25, 26]. BEVs are thought to modulate intercellular communications by transferring their contents including nucleic acids (DNA, RNA, miRNA), proteins (cytoplasmic and periplasmic proteins), metabolites, lipopolysaccharide (LPS), and peptidoglycan [27, 28]. The intersection of microorganisms and EVs is emerging as attractive research in the biomedical field. In recent years, the roles of BEVs in promoting health and causing pathologies are becoming increasingly obvious [29, 30]. The growing recognition that BEVs can enter the systemic circulation and be detected in human body fluids, where it may stimulate the progress of microbiome research, liquid biopsies technology, and BEV-based therapies [31]. Importantly, bacteria have the advantages of rapid proliferation and mature fed-batch culture technology [32-34], BEVs-based therapy is a promising strategy to overcome large-scale production problems associated with mammalian EVs (MEVs) and other synthetic nanomaterials [35, 36]. In addition, advance in synthetic biology have also made it possible to use bioengineered BEVs to precisely deliver effective agents to cancer cells or tissue [37, 38]. BEVs have emerged as a new drug delivery vehicle and crucial signaling mediators with great potential for clinical application due to the advantages of nanosized structure, safety, stable loading capacity, good biocompatibility, ease of modification and production [39-41]. In conclusion, the development of BEVs and their applications in the treatment of BSTTs is of great significance (Figure 1).
BEVs-based cancer therapy is of great significance. BEVs are derived from Gram-positive and Gram-negative bacteria and can be designed as functionalized BEVs for tumor therapy by engineering approaches. The resulting BEVs have shown great promise against various tumors, including bone and soft tissue tumors. Figure was created with https://app.biorender.com/.
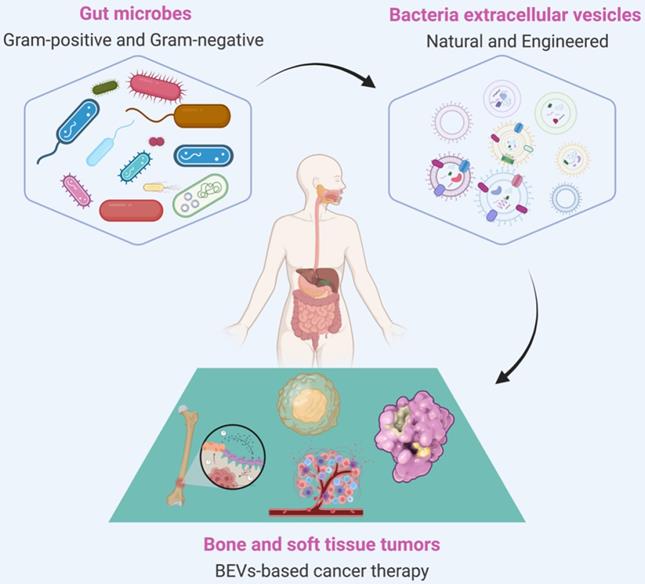
Overview of biogenesis, composition, and classification of BEVs. The Gram-positive bacteria can generate CMVs by the mechanism of bubbling cell death. In contrast, the Gram-negative bacteria have two kinds of mechanisms to generate BEVs. The OMVs are produced by the mechanism of blebbing of the outer membrane; the OIMVs and EOMVs are resulted from explosive lysis. In general, BEVs contain many inclusions such as nucleic acids (DNA/RNA), proteins, and metabolites, etc. IM: Inner membrane, OM: other membrane, PG: peptidoglycan, LPS: lipopolysaccharide.
In this review, an overview of the biogenesis, composition, isolation, classification, and internalization of BEVs is summarized. Then, special attention is focused on the sources of BEVs in cancer therapy and the BEVs-based cancer treatment strategies. Moreover, the potential role of BEVs in BSTTs is highlighted. Finally, the major advantages and challenges of BEVs in the treatment of cancer are comprehensively discussed.
Overview of BEVs
In the past decade, BEVs-based cancer therapy has attracted much attention in the biomedical field [31]. BEVs are a promising platform for treating and preventing many diseases due to the ability to deliver virulence, transmit genetic material, and regulate signaling pathway [42]. For better understand the applications of BEVs in cancer, we summarize the biogenesis, composition, classification, isolation, and internalization of BEVs.
Biogenesis, composition, and classification of BEVs
Both commensal and pathogenic bacteria could secrete EVs, a spherical membrane particles with a size of 20~400 nm in diameter [27]. Since bacteria can be divided into Gram-positive and Gram-negative bacteria, BEVs are also divided into two categories according to the source of the parental strains [39]. The Gram-positive bacteria, such as lactobacillus rhamnosus, staphylococcus aureus, diplococcus pneumoniae, and Bacillus spp, were considered incapable of producing BEVs due to its thick peptidoglycan (or thick cell wall) [43]. However, growing evidence suggests that Gram-positive bacteria can secrete cytoplasmic membrane vesicles, named CMVs, through a mechanism of bubbling cell death (Right of Figure 2) [44]. CMVs contain a lot of cargoes, including DNA, RNA, plasmic membrane proteins, virulence factors, and endolysins [45]. On the other hand, the Gram-negative bacteria, such as Escherichia coli, Salmonella sp., Helicobacter pylori, and Akkermansia muciniphila, have two kinds of mechanisms to generate outer membrane vesicles (OMVs), outer-inner membrane vesicles (OIMVs), and explosive outer-membrane vesicles (EOMVs) [27] (Left of Figure 2). OMVs were generated by blebbing of the outer membrane and thus contain large amounts of outer membrane proteins and lipids [27, 39]. The OIMVs and EOMVs were produced by explosive cell lysis and are rich in outer membrane proteins, cytoplasmic (or inner) membrane proteins, plasmids, RNA, DNA, endolysins, virulence factors, and phages [39, 46]. Generally, BEVs derived from Gram-negative always contain the innate immune response activator LPS, while Gram-positive BEVs do not contain [47]. It's worth pointing out that there are exceptions. Escherichia coli Nissle 1917 (EcN) is a very special Gram-negative bacteria that does not contain intact LPS and is often used as a probiotic for the treatment of inflammatory gastrointestinal dysfunction [48]. Naturally, ECN-derived EVs do not contain complete LPS. Furthermore, the msbB mutant Gram-negative bacteria derived EVs do not contain intact LPS [38, 49].
A set of isolation method applicable to the vast majority of bacteria. After proper culture, the isolation of BEVs is generally divided into three steps: 1) Removal of bacteria and their debris; 2) Removal of non-BEVs proteins and concentration; 3) Isolation and purification. Finally, the collected BEVs are characterized by TEM, NTA, and WB, if necessary.
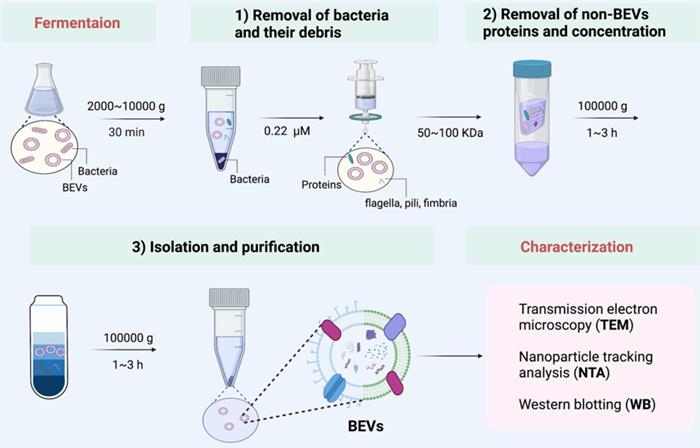
Isolation of BEVs
Efficient isolation methods of BEVs determines their further application [50, 51]. Many isolation and purification techniques have been developed to obtain high quality BEVs from culture broth. Traditional isolation methods include ultracentrifugation, ultrafiltration, precipitation, affinity isolation, size exclusion chromatography, and density gradient centrifugation [52, 53]. The major advantages and disadvantages of these isolation methods have been summarized in Table 1. In general, these methods can obtain great efficiency and purity of BEVs. However, the combination of these methods may achieve better results. For example, Liu et al. [54] collected high purity BEVs derived from Akkermansia muciniphila through the combination of ultracentrifugation, ultrafiltration, and density gradient centrifugation.
The major advantages and disadvantages of different BEVs isolation methods
| Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Ultracentrifugation | Simple process; Great homogeneity; Lower costs. | Limited efficiency; Limited purity; Time-consuming. | [39] |
| Ultrafiltration | Simple process; Great homogeneity; High recovery rate. | Limited efficiency; Limited purity; Product loss. | [55] |
| Precipitation | Simple process; Lower costs; Suitable for large sample | Limited efficiency; Limited purity; Time-consuming. | [56] |
| Affinity isolation | High purity | High cost; Low availability. | [56] |
| Size exclusion chromatography | High purity; High biological properties | Time-consuming; Not suitable for large sample. | [55] |
| Density gradient centrifugation | High purity | High cost; Time-consuming. | [40] |
Here, we summarized a set of isolation method applicable to the vast majority of bacteria (Figure 3). We used this method to successfully collect BEVs from a variety of bacteria, such as Gram-negative bacteria (such as E. coli Nissle 1917 and Akkermansia muciniphila), and Gram-positive bacteria (such as Lactobacillus rhamnosus GG). Generally, the bacteria and their debris in fermentation broth are completely removed by low-speed centrifugation (2000 g ~ 1000 g) and 0.22 μm sterile filter. Then, the non-BEVs associated proteins are eliminate by 100 kDa ultrafiltration membrane. Further, the BEVs are isolated and purification by ultracentrifugation (100000 g) and iodixanol gradient centrifugation. The physicochemical properties of collected BEVs can be characterized by transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), and western blotting (WB), if necessary [57].
The internalization of BEVs. Three major internalization pathways for BEVs internalization have been proposed: 1) Receptor-mediated signaling; 2) Endocytosis via endocytosis via phagocytosis, macropinocytosis, lipid raft, and caveolae; 3) Membrane fusion. Figures were created with https://app.biorender.com/.
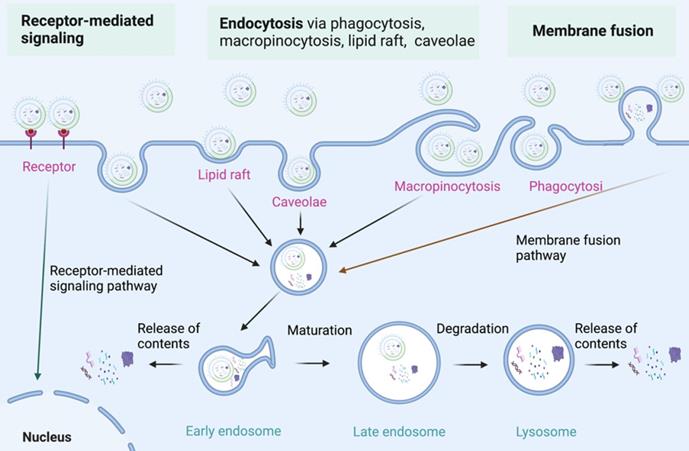
Internalization of BEVs
As nanoparticles with phospholipid bimolecular membranes, BEVs can be internalized by host cells, which is similar to that of MEVs [58-62]. The communication of BEVs and host cells includes the interaction of BEVs with cell receptors, the delivery of agents into cells by BEVs, and the complete entry of BEVs into cells [63, 64]. The specific molecular mechanism of BEVs internalization by host cells need further study [65]. Currently, three major internalization pathways for BEVs internalization have been proposed (Figure 4) [66, 67]: 1) Endocytosis; 2) Membrane fusion; 3) Receptor mediated signalling. Endocytosis is the primary mode of BEVs internalization by host cells. Endocytosis and membrane fusion allow BEVs enter into early endosomes, thereby releasing their contents. Moreover, BEVs can also communicate with host cells through toll-like receptors (such as TLR1, TLR2, TLR4, and TLR6)-mediated signaling. The internalization of BEVs initiates a series of responses of host cells, which lays the foundation for the use of BEVs in cancer therapy.
Sources of BEVs in cancer therapy
Bacteria-based cancer treatment has received many attentions due to the high immunogenicity, which can recruit more immune cells to kill tumor [68-70]. However, the application of intact bacteria may introduce safety concerns, such as excessive infection and sepsis [71, 72]. Therefore, the BEVs, which have many pathogen-associated molecular patterns (PAMPs), LPS, PG, and bacterial nucleic acids, for instance, have been another superior option for cancer treatment [38, 73, 74].
The PAMPs of BEVs can be engage with host pattern recognition receptors (PRRs) in immune and nonimmune cells to activate immunomodulatory [75]. It's worth noting that the immune response elicited by BEVs is mainly dependent on the parental strain and the relationship between parental strain and its host [72, 76]. For example, the BEVs derived from pathogenic bacteria, such as Escherichia coli (E. coli), can mediate the activation of caspase-11 [66] or intrinsic apoptosis [77]. In contrast, the BEVs produced by symbiotic bacteria, such as Lactobacillus rhamnosus GG, E. coli Nissle 1917, and Akkermansia muciniphila can protect the intestinal epithelium and activate the immune and defense responses [78-80]. In addition to these probiotics, many researchers have utilized msbB mutant strains as starting strains for subsequent applications [35, 38, 81]. The deletion of msbB results in under-acylated LPS and exhibits reduced endotoxicity and immunogenicity to human cells [49, 82]. Moreover, similar attenuated strains could be obtained by using lysozyme to remove the cell wall [83, 84]. Here, we comprehensively summarize the most commonly used bacterial sources for BEVs-based tumor therapy, mainly including pathogenic strains, attenuated strains, and probiotics (Table 2). The summary of sources of BEVs in cancer therapy lays a good foundation for BEVs-based therapy for BSTTs.
BEVs-based cancer treatment strategies
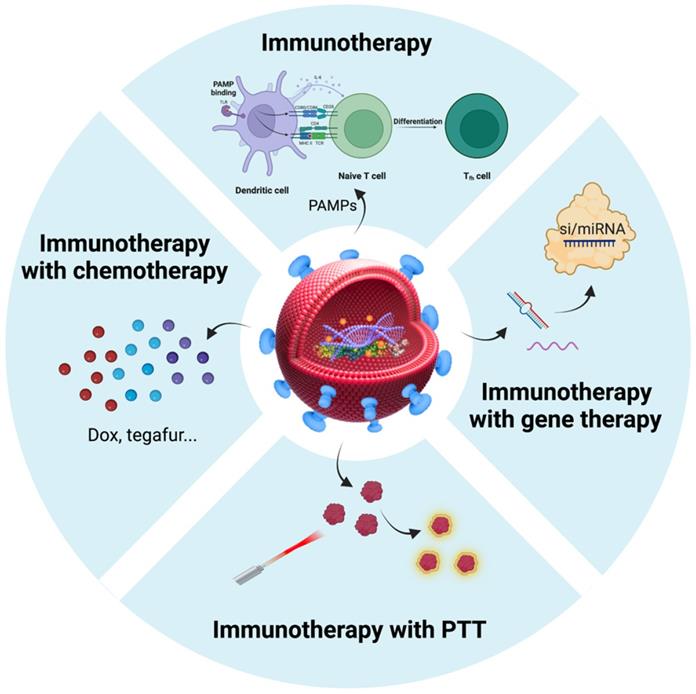
After considering the source of BEVs in cancer therapy, we focused on BEVs-based cancer treatment strategies. The properties of BEVs of immunogenicity, cell-free system, safety, and nanoscale structure endow them with the potential to treat cancer [103]. Nanostructured BEVs enable efficient lymphatic drainage when injected subcutaneously and enhance localization to solid tumors through passive targeting effect when injected systemically [104]. Importantly, another major advantage of BEVs in cancer applications is the ease of genetic engineering editing that can confer various functions on BEVs [39, 40, 105]. Therefore, in addition to immunotherapy, BEVs have been applied to the combination with other types of therapies (such as chemotherapy, gene therapy, and photothermal therapy) to synergistically amplify antitumor efficacy (Table 2). Here, we summarized the BEVs-based cancer treatment strategies (Figure 5), which may provide constructive guidance for the treatment of BSTTs.
Summarization of BEVs-based cancer treatment strategies. Immunotherapy is an important therapy in the field of BEV-based cancer therapy. In addition to immunotherapy, BEVs have been applied to the combination with chemotherapy, gene therapy, and photothermal therapy to amplify antitumor efficacy. Nanostructured BEVs enable efficient lymphatic drainage when injected subcutaneously and enhance localization to solid tumors through passive targeting effect when injected systemically. More importantly, the targeting ability of BEVs can be enhanced by displaying specific proteins on the membrane surface, which can greatly enhance local drug concentration and reduce side effects. Figure was created with https://app.biorender.com/.
Summary of the sources of BEVs and different therapies in cancer therapy
| Therapeutic strategy | Cancer cells | BEVs source | References |
|---|---|---|---|
| Immunotherapy | B16-F10 and CT26 | Attenuated E. coli W3110△msbB | [74] |
| Immunotherapy | B16-BL6, CT26, 4T1, and MC38 | Attenuated E. coli W3110△msbB | [81] |
| Immunotherapy | B16-F10 and B16-OVA | E. coli Rosetta (DE3) | [85, 86] |
| Immunotherapy | 4T1, Panc1, and MC38 | Probiotics E. coli Nissle 1917△nlpIa | [87] |
| Immunotherapy | MDA-MB-468 | Attenuated E. coli W3110△msbB△pagPb | [88] |
| Immunotherapy | B16-F10 | Attenuated E. coli DH5α | [84] |
| Immunotherapy | TC-1 | Attenuated E. coli DH5α | [89] |
| Immunotherapy | B16-F10, 4T1, EMT6, and CT26 | Attenuated E. coli DH5α | [83] |
| Immunotherapy | HTC116, MCF-7, and HepG2 | Attenuated Salmonella Typhimurium | [90] |
| Immunotherapy | RM1, DU145, and PC-3 | Probiotics Akkermansia muciniphila | [91] |
| Immunotherapy | HepG2 | Probiotics Lactobacillus rhamnosus GG | [92] |
| Immunotherapy | B16-F10, MC38, and CT26 | E. coli DH5α | [93] |
| Immunotherapy | B16-F10 and CT26 | E. coli BL21 (DE3) | [94] |
| Immunotherapy | B16-F10 and B16-OVA | E. coli TOP 10 | [95] |
| Immunotherapy with chemotherapy | A549 | Attenuated Klebsiella pneumonia | [96] |
| Immunotherapy with chemotherapy | B16-F10 and 4T1 | Attenuated Salmonella | [97] |
| Immunotherapy with gene therapy | SKOV3, BT474, and HCC-1954 | Attenuated E. coli W3110△msbB | [38] |
| Immunotherapy with gene therapy | B16-OVA | Attenuated E. coli W3110△msbB | [98] |
| Immunotherapy with PTTd | 4T1 | Attenuated E. coli W3110△msbB | [35] |
| Immunotherapy with PTT | B16-F10 | E. coli DH5α | [99] |
| Immunotherapy with PTT | CT26 and CT26-luc | E. coli BL21 (DE3) | [100] |
| Immunotherapy with PTT | 4T1 | Attenuated Salmonella | [101] |
| Immunotherapy with PTT | CT26 and 4T1 | Attenuated Salmonella typhimurium △ppGppc | [102] |
anlpI, encoding lipoprotein NlpI, the deletion of nlpI increases the amount of BEVs.
bpagP, encoding Lipid A palmitoyltransferase, which is important for virulence in E. coli.
cppGpp, encoding guanosine 5′-diphosphate-3′-diphosphate.
dPTT, photothermal therapy.
Immunotherapy
Immunotherapy, an important therapy in the field of BEV-based cancer therapy, has revolutionized the clinical treatment of cancer [106]. The breakthroughs of immunotherapy have shown great potentials over the last decade [107]. As mentioned above, the PAMPs can coffer excellent intrinsic immunomodulatory properties on BEVs, which are often used as pathogen mimicking adjuvants [47, 108]. BEVs-based immunotherapeutic, such as Bexsero and MeNZB, have been approved for clinical treatment of meningococcal group B infections [47]. It has been reported that intravenous injection of attenuated E. coli derived BEVs elicited a strong and long-term IFN-γ and T cell mediated antitumor immune response, which could completely eradicate established tumors without significant side effects [81]. However, the IFN-γ could upregulate the expression of immune checkpoint programmed death 1 ligand 1(PD-L1), which may induce the dysfunction and apoptosis of T cell by interacting with programmed death 1 (PD1) on its surface, and hence, limit the effectiveness of immunotherapy [109, 110]. In turn, inhibiting the interaction of PD-L1 and PD1 could enhance the immune response to the cancer cells. Recognition of tumor antigens by the immune system has been shown to be critical to the success of therapy [111].
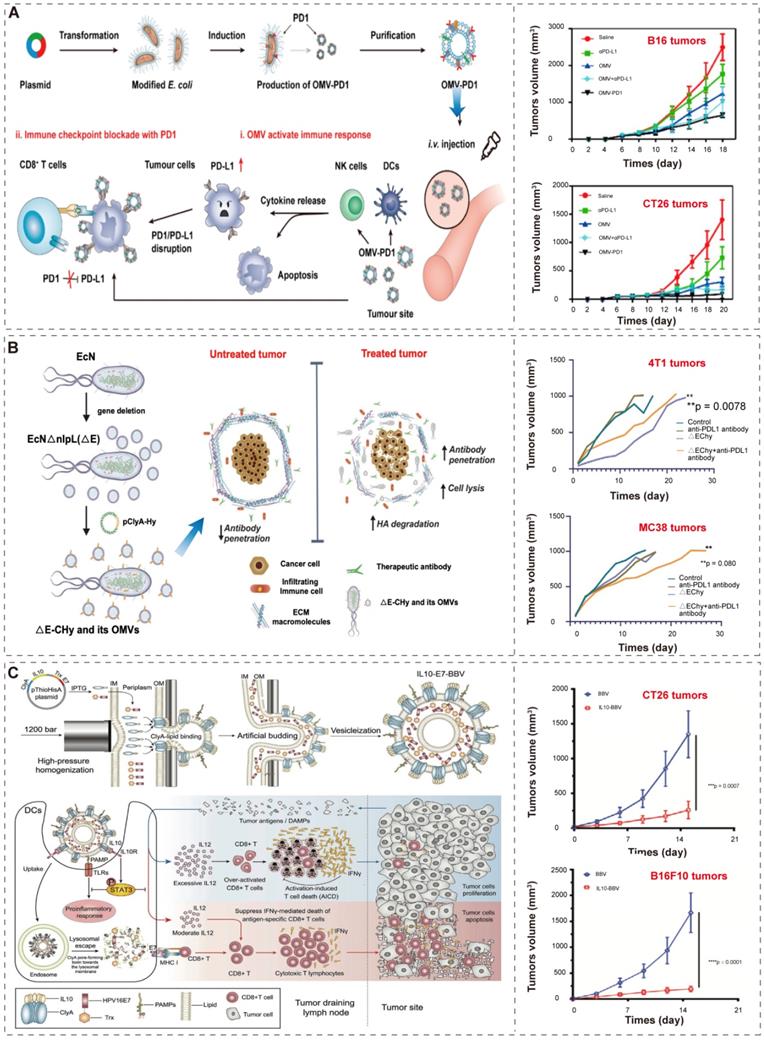
Recently, Li et al. [74] and Cheng et al. [86] developed an efficient “Plug and display” system for displaying exogenous proteins on BEVs. They applied ClyA, a secreted, pore-forming protein [112, 113], to promote the localization of exogenous proteins (such as luciferase, Luc) or antigen (such as PD1) on the outer membrane of bacteria and their secreted BEVs. The bioengineered BEVs-PD1 retain the ability of immune activation. Moreover, the co-incubation of BMVs-PD1 and bone marrow dendritic cells (BMDCs) show great biocompatibility. Further, the BEVs-PD1 can promote the combination of PD-L1 on the surface of tumor cells and protect T cells from the PD1/PD-L1 immunosuppressive axis (Figure 6A). In conclusion, the “Plug and display” system based on BEVs offer a broad prospect for tumor immunotherapy.
Furthermore, since BEVs have the excellent intrinsic immunomodulatory properties, different synthetic BEVs have also been developed [84, 94]. Considering that desmoplastic solid tumors are characterized by the accumulation of hyaluronic acid (HA), which prevents the infiltration of immune cells [114], Thomas et al.[87] used “Plug and display” system to display hyaluronidase (Hy) instead of antigen on probiotic E. coli Nissle 1917 derived BEVs (Figure 6B). BEVs-Hy can reconstruct the tumor microenvironment, enabling immune checkpoint antibodies and tyrosine kinase inhibitors work together to exert immunotherapy effects (Figure 6B).
Moreover, Hua et al. [94] utilized high-pressure homogenization to produce biomimetic BEVs with immunomodulator IL-10 (Figure 6C). These biomimetic BEVs platform have a promising potential in cancer immunotherapy (Figure 6C). Similarly, Park et al. [84] applied high lysozyme and high pH treatment of bacteria to generate synthetic BEVs, which contained few cytosolic ingredients and nucleic acid (such as RNA and DNA). Compared to conventional BEVs, these BEVs were safer and did not induce systemic pro-inflammatory factors in vivo, and successfully induce tumor regression in melanoma mice.
BEVs-based immunotherapy. (A) Schematic illustration of the antitumor mechanism and characterization of BEVs-PD1 for cancer immunotherapy. Adapted with permission from [74], copyright 2020, American Chemical Society. (B) Schematic illustration of the construction of EcN into ΔE-CHy and its application. Adapted with permission from [87], copyright 2022, Wiley-VCH GmbH. EcN, E. coli Nissle 1917; ΔE, EcN with the deletion of nlpI; CHy, ClyA-Hy. (C) Schematic illustration of the construction and the mechanism of multifunctional modified biomimetic BEVs. Adapted with permission from [94], copyright 2021, Wiley-VCH GmbH. It is worth noting that the author uses OMV to represent the extracellular vesicles produced by bacteria, which is not exactly the same as the OMVs described in “2.1 Biogenesis, composition, and classification of BEVs”. In order to maintain the consistency of the article, we use BEVs to represent the extracellular vesicles produced by bacteria, and the explanation will not be repeated later.
BEVs-based immunotherapy and chemotherapy. (A) Schematic illustration of the construction of bioengineered BEVs-coated polymeric micelles and the effect of combination of immunotherapy and chemotherapy. Adapted with permission from [97], copyright 2020, American Chemical Society.
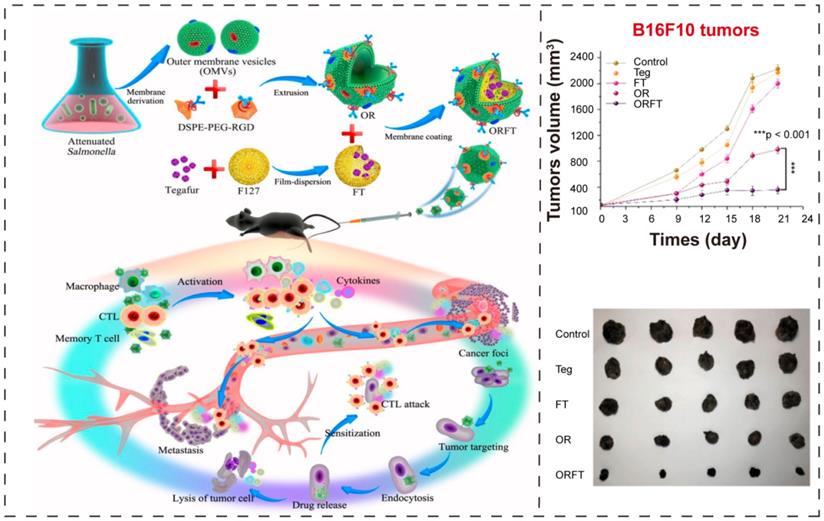
The combination of immunotherapy and chemotherapy
Broad-spectrum antitumor drugs, such as doxorubicin (DOX) and tegafur, are often used to treat tumors [115]. However, chemotherapy drugs have no specific targeting and have obvious side effects, which often lead to poor therapeutic effects [40]. Therefore, there is a need to develop safer and more effective drug delivery systems to alleviate toxicity and enhance the antitumor effect of drugs.
In a recent report, Chen et al. [97] proposed an ingenious combinational strategy where attenuated Salmonella derived BEVs coated polymeric nanomedicine to improve the immunotherapeutic efficacy (Figure 7A). Here, they exploited BEVs as initiator of antitumor immune response, and utilized RGD (Arg-Gly-Asp) [116] as targeting ligand to enhance the tumor-targeting ability, and used tegafur, a prodrug of fluorouracil [117, 118], as chemotherapeutic drug to amplify the immunotherapeutic potential of BEVs. The BEVs-coated hybrid nanoparticles could simultaneously show chemotherapeutic and immunological efficacy, sensitizing melanoma cells to cytotoxic T lymphocytes (CTLs), further achieving noticeable inhibition of cancer metastasis.
Kuerban et al. [96] obtained engineered BEVs derived from attenuated K. pneumonia, and then loaded with DOX, one of the most widely used antineoplastic drugs [119], by incubated at 37 °C for 4 h. In addition to the appropriate immunogenicity, BEVs-DOX also increased the expression of cleavage caspase-3, cleavage PARP, and F4/80 protein, thereby achieving the purpose of inhibiting tumor growth. Importantly, BEVs-DOX had a better biosafety than free DOX and no side effects of toxicity to major organs. In general, BEVs can not only serve as efficient drug delivery vehicles for chemotherapeutic agents, but also introduce appropriate immune responses.
The combination of immunotherapy and gene therapy
Gene therapy, a method to compensate or correct mutant genes in tumor cells by delivering gene regulators, such as miRNA, siRNA etc., has shown significant therapeutic effects and great safety record and has proven to be an extremely promising approach to cancer treatment [120-122]. Generally, miRNAs or siRNAs are well known for their poor stability, short half-life, and poor penetration capacity [123, 124]. Therefore, the efficient delivery for customized gene regulator is still a challenge. Currently, lipid nanoparticles are the major carriers for RNA delivery in clinical practice [125, 126]. However, in tumor treatment, these lipid-based nanoparticles often require the addition of immune adjuvants to activate adaptive immune, which complicates the material preparation process [127, 128]. As mentioned above, the BEVs have the excellent properties of intrinsic immunoregulation and drug delivery. Therefore, the BEVs are the ideal nanocarriers for the combination of immunotherapy with gene therapy.
Gujrati et al. [38] constructed bioengineered BEVs to deliver kinesin spindle protein (KSP) siRNA (Figure 8A). KSP is abundantly overexpressed in tumor tissues to regulate cell cycle progression; the silencing of KSP mRNA expression results in the arrest of cell cycle and the induction of apoptosis [129, 130]. The bioengineered BEVs, derived from attenuated E. coli W3110△msbB, display human epidermal growth factor receptor 2 (HER2) affibody in the outer membrane to specifically target tumors through the “Plug and display” system (ClyA-Luc). Then, KSP siRNA was loaded into BEVs-HER2 by conventional electroporation method [131]. Finally, the bioengineered BEVs-HER2-KSP siRNA induced obvious tumor growth regression without nonspecific side effects.
In a recent study, Li et al. [98] developed BEVs-based box C/D mRNA delivery platform by ClyA-L7Ae (L7Ae, an archaeal RNA-binding protein [132, 133]) and CylA-LLO (LLO, a lysosomal escape protein [134]). The BEVs-L7Ae-LLO can combine box C/D mRNA through L7Ae binding, resulting in BEVs-L7Ae-LLO-mRNA. Then, BEVs-L7Ae-LLO-mRNA deliver the mRNA into dendritic cells for the purpose of antigen presentation and innate immune stimulation (Figure 8B). The mRNA vaccines can encode one or more tumor-specific antigens (TSA), and undergo protein translation and antigen processing in cells, and bind to major histocompatibility antigen complex I (MHCI) in antigen-presenting cells, and finally presented to T cells to induce a strong tumor-specific T cell response to kill tumor cells [135, 136]. In addition, the innate immunity by BEVs enhanced the activation of antigen-specific T cells, which in turn significantly inhibited the tumor progression. The BEVs based mRNA delivery indicates the great potential of BEVs as a vehicle for combined immunotherapy and gene therapy in the treatment of tumors.
BEVs-based immunotherapy and gene therapy. (A) Schematic representation of the BEVs-based siRNA delivery system, which displays HER2 affibody in the outer membrane to specifically target tumors. Adapted with permission from [38], copyright 2014, American Chemical Society. (B) Schematic representation of the BEV-based mRNA delivery system and innate immunity activation and antigen presentation. Adapted with permission from [98], copyright 2022, Wiley-VCH GmbH.
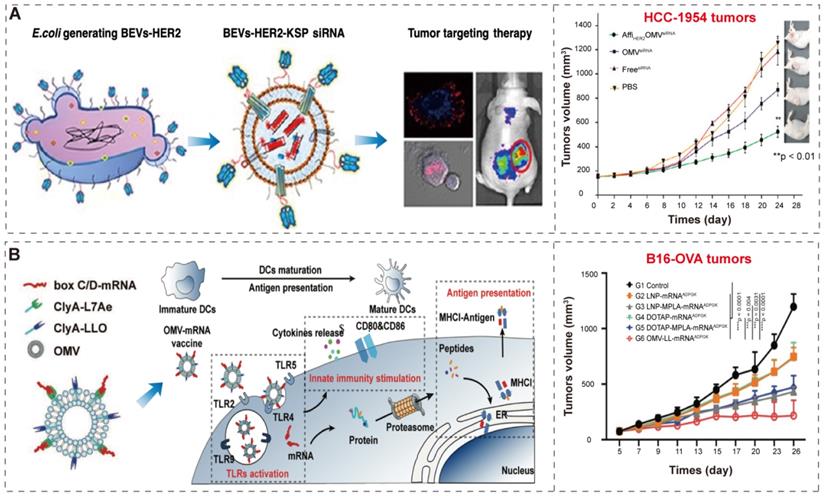
BEVs-based immunotherapy and photothermal therapy. (A-B) Schematic illustration of BEV-Mel production. Adapted with permission from [35], copyright 2019, Springer Nature. (C-D) Schematic illustration of the construction of HPDA@BEV-CC NPs and their antitumor immune responses after PTT. Adapted with permission from [99], copyright 2020, American Chemical Society.
The combination of immunotherapy and photothermal therapy
Photothermal therapy (PTT) is one of the latest tumor treatment options that applies photothermal transduction agents (PTAs) with photothermal conversion efficiency and converts light energy into heat energy under the near-infrared (NIR) laser to kill cancer cells [137-139]. The tumor-specific cytotoxic T cells are activated by tumor antigens released by heat-damaged tumor cells [140]. Generally, compared with traditional surgery, chemotherapy, and gene therapy, PTT is a more low-toxic and minimally invasive tumor-targeted therapy. In the past three years, there have been numerous success cases in the treatment of tumors through the combination of photothermal therapy and BEVs-based immunotherapy [35, 99-102].
Gujrati et al. [35] overexpressed biopolymer-melanin (Mel) in the attenuated E. coli W3110△msbB and obtained the bioengineered BEVs-Mel, which could produce appropriate optoacoustic signals for imaging applications in cancer photothermal therapy (Figure 9A-B). Although the exact mechanism for Mel encapsulation is not known, the entire process is a natural event that does not require any complex synthetic skills, and BEVs-Mel can be obtained in large quantities through cost-effectively, well-established large-scale bacterial culture [32-34]. Importantly, the BEVs-Mel platform did not induce chronic systemic toxicity and side effects despite repeated injections into mouse tumor models. Except for Mel, the hemoglobin can also be used in PTT cancer treatment [141]. Zhuang et al. [102] proposed a new strategy, which applying BEVs to induce extravasation of red blood cells (RBCs) in tumors, thereby accumulating hemoglobin (one of PTAs). Compared with previous BEVs-based cancer treatments [38, 81] that required high-dose injection, the combination of BEVs with PTT enables better efficacy with only low-dose injection, which significantly reduces side effects and toxicity concerns caused by BEVs injection.
Given that the efficiency of tumor antigen recognition and operation by the immune system is also important for successful immune activation after PTT [142, 143]. Therefore, Li et al. [100] presented the BEVs-based multifunctional NPs, which have the ability of antigen capture and immunomodulation. The native BEVs was first modified with maleimide (Mal), which can bind proteins/antigens via thioether bonds. Then, they loaded them with 1-methyl-tryptophan (1-MT, an inhibitor of indoleamine 2, 3-dioxygenase [144]), producing the 1-MT@BEV-Mal NPs. The cancer cells were incubated with 20 μg/mL indocyanine green (ICG, one of PTAs) for PTT. These BEVs-based NPs possesses antigen capture and immunomodulation to promote immune-mediated tumor clearance after PTT.
In addition, the hybrid membrane nanoplatforms provide a promising biomimetic strategy in cancer treatment. Wang et al. [99] constructed hybrid nanoparticles (NPs) by using attenuated BEVs and B16-F10 cancer cells (CCs) membrane and then coated them onto hollow polydopamine (HPDA, one of PTAs [145]) (Figure 9C-D). They used these nanoparticles to target melanoma by combining immunotherapy with BEVs and HPDA-mediated photothermal therapy, and finally successfully eradicated melanoma without significant side effects. Chen et al. [101] also designed a hybrid membrane eukaryotic-prokaryotic vesicles (EPVs) with tumor-specific antigenic by fusing eukaryotic cancer cell membrane vesicles (CCMVs) and prokaryotic BEVs. Subsequently, they conferred these EPVs with PTT module by coating with poly (lactic-co-glycolic acid)-ICG nanoparticles [146, 147] (PLGA-ICG NPs, PI), resulting in PI@EPV NPs. The PI@EPV NPs obtained two immunological functions from their parent membranes and the PTT module, which could enhance the immunotherapeutic effects and destroy the solid tumor.
Potential role of BEVs in BSTTs
Osteosarcoma, chondrosarcoma, and Ewing sarcoma are the three most common primary malignant bone tumors. The pathological types of soft tissue tumors are complex, the most common of which are undifferentiated pleomorphic sarcoma, liposarcoma and leiomyosarcoma [148]. Conventional BSTTs treatment approaches include radiation therapy, surgery, and chemotherapy [149]. With the understanding of the pathogenesis and progression of BSTTs, disrupting the receptors of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PGDF) that control angiogenesis, hindering the formation of the mitotic spindle, disturbing the late S-phase and G2 phase of the cell cycle, and inhibiting the association of DNA-binding proteins are considered to be essential for the management of these tumors [148]. Therefore, many targeted drugs such as Pazopanib [150], Sorafenib [151], Eribulin [152], and Trabectedin [153] have been developed for the treatment of the diseases. In addition, recent basic and clinical studies have confirmed the relationship between immune checkpoints and malignant tumor progression, as well as the efficacy of immune checkpoint inhibitors on various malignant tumors [154-156]. However, these antitumor drugs have not achieved satisfactory efficacy due to the rarity and diversity of BSTTs.
In recent years, it has been confirmed that the metabolites derived from disturbed gut microbiota affects the progression of BSTTs [11, 12]. For example, compared with healthy subjects, the gut microbiota of multiple myeloma (MM, also known as plasma cell myeloma, an incurable tumor that accumulate in the bone marrow [157]) patients is rich in opportunistic nitrogen-cycling bacteria and produces more available L-glutamine, which accelerates MM progress [158]. In conclusion, there is a strong link between commensal bacteria and these tumors. Therefore, it is possible to apply fecal microbiota transplantation (FMT) to the treatment of BSTTs [159]. FMT is an emerging therapeutic approach that affects the course of a variety of chronic diseases, including metabolic syndrome, autoimmune, and cancer [160, 161]. However, manipulating the gut microbiome also carries certain risks [162]. BEVs are cell-free nanocarriers that carry a variety of key bioactive contents and PAMPs derived from parental strain. From a therapeutic perspective, direct systemic administration of BEVs derived from symbiotic bacteria in healthy hosts to tumor-bearing hosts may be a better alternative than of FMT [159].
Furthermore, the combination of immunotherapy with other types of therapy is a powerful strategy for enhancing antitumor responses [163, 164]. Three schemes can be used to enrich the functions of BEVs for BSTTs (Figure 10). The first approach is to display proteins and antigens on the membranes to enhance the immunotherapy or targeted ability through the “Plug and display” system. The second solution is to apply the drug loading capacity of BEVs to achieve synergistic therapy with other therapeutic approaches by delivering therapeutic agents such as Doxorubicin, Pazopanib, miRNA, and PTAs to BSTTs cells. The third method is to hybridize other functionalized biological membranes such as lipopolymers and tumor membranes with BEVs to obtain new functions.
The potential role of BEVs in BSTTs. Displaying proteins or antigens on the membranes, loading therapeutic agents (such as chemotherapeutics, miRNA, siRNA, and PTAs), and hybridizing other functionalized biological membranes (such as lipopolymers and tumor membranes) can be used to enrich the therapeutic and targeting functions of BEVs for BSTTs. Moreover, oral administration of BEVs or BEVs-based symbiotic bacteria will be one of the promising directions of BEVs-based cancer therapy. In addition, another important application area of BEVs is the diagnostic biomarker in tumor liquid biopsies. Figure was created with https://app.biorender.com/.
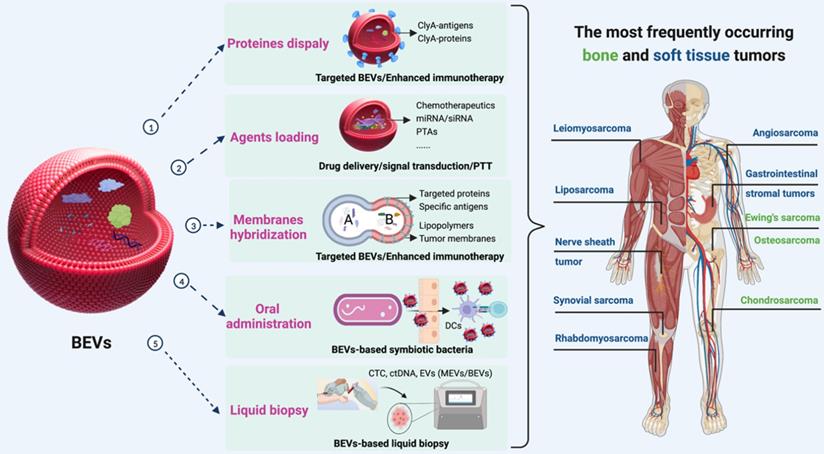
Currently, BEVs-based cancer therapies are administered either subcutaneous injection or intramuscular injection, where the immune activation is controlled by limited draining lymph nodes, and thus resulting in insufficient immunogenicity [165]. In addition to enhancing tumor antigen display and the combination of multiple therapies, oral administration of BEVs or BEVs-based symbiotic bacteria will be the future direction of BEVs-based cancer therapy (Figure 10). Lately, Yue et al. [95] proposed a BEVs-based oral tumor vaccine for specific immune activation. Compared with subcutaneous or intramuscular injection, oral administration may offer better safety, better patient compliance, and lower healthcare cost to BSTTs patients [166].
The major advantages and challenges of BEVs in cancer therapy
| Advantages of BEVs | Challenges of BEVs |
|---|---|
| Intrinsic immunomodulatory properties; Ease of industrialization; Ease of customization; Higher safety. | Lack of standardization; Potential off-target effects; Potential biosafety; Ambiguous contents. |
In addition, another important application area of BEVs is the diagnostic biomarker in tumor liquid biopsies (Figure 10) [167-169]. Tumor EVs are gradually developing as a third liquid biopsy marker besides circulating tumor cell (CTC) and circulating tumor DNA (ctDNA) [170, 171]. In recent years, studies have shown that microbial colonization also exists in tumors [172], and these bacteria can also affect host behavior such as metastasis [173]. With the advancement of omics, important proteins, nucleic acids, and lipids, potential liquid biopsy markers, can be identified in BEVs isolated from BSTTs.
Major advantages and challenges of BEVs
BEVs have been regarded as a source of revolutionary nanotechnology therapeutics, which have a number of advantages that make them attractive for cancer therapy [30]. Compared with MEVs, the unique advantages of BEVs in cancer therapy are intrinsic immunomodulatory properties, ease of industrialization, and ease of customization. Although numerous reports have demonstrated that the obvious therapeutic effects of BEVs in cancer treatment, there is still various challenges to be conducted to move them from the laboratory to clinical applications. Here, we summarize the major advantages and challenges of BEVs in cancer therapy (Table 3).
Advantages
Intrinsic immunomodulatory properties
Compared with other therapeutical nanoparticles, such as exosomes (or MEVs) [174-176], lipidosome [177], and metal nanoparticles [178, 179], one of the unique advantages of applying BEVs into cancer therapy is that the intrinsic immunomodulatory properties elicited by PAMPs. Therefore, in addition to immunotherapy, BEVs-based therapies can synergize with a variety of other therapies such as chemotherapy, gene therapy, and photothermal therapy to enhance antitumor effects.
Ease of industrialization
Despite the considerable success of synthetic nanomaterials in the treatment of diseases such as cancer in preclinical trials, only a few synthetic drugs have entered clinical trials [180, 181]. The factors that limiting the clinical application of most synthetic nanomaterials include challenges involving material-related toxicity, low biocompatibility, and high cost of large-scale production [182, 183]. Bacteria-based fermentation has always been an economical, scalable, and environmentally friendly technology [32-34]. The rapid proliferation and large-scale fermentation processes of bacteria provide a scalable and powerful platform to produce a large number of cell-free BEVs to meet commercial and clinical needs.
Ease of customization
The rapid development of synthetic biology brings infinite possibilities for engineering editing of bacteria [184, 185]. The therapeutic components of interest can be easily loaded inside or outside the BEVs by manipulating their parent bacteria. Importantly, the biogenesis mechanism of BEVs such as explosive cell lysis for Gram-negative bacteria and bubbling cell death for Gram-positive bacteria make customization of BEVs possible. Moreover, in addition to customized modification in bacteria, we can also perform personalized modification after isolation of BEVs through engineering techniques such as membrane fusion [186], membrane coating [187], covalent reactions [188], and noncovalent reactions [189].
Higher safety
Bacteria, especially the attenuated bacteria, based cancer immunotherapies have attracted much attention due to their unique ability to trigger host antitumor immunity [69, 190]. Although the virulence and the risk of septic shock have been reduced, the safety of attenuated bacteria still needs to be improved to meet the requirements of clinical application. BEVs, cell-free and non-replicable nanoparticles [191], are considered to be safer than parental strains. Importantly, BEVs contain most of the immunogenic membrane-associated components of their parental strains, which can activate and modulate the immune response even in small injections, thereby improving their safety in vivo studies [81, 192].
Challenges
Lack of standardization
A definite standard for BEVs production, isolation, and characterization has still not been achieved, which seriously hinders the commercial and clinical application of BEVs. In the process of fermentation, especially at large-scale, changes in culture medium (such as carbon source, nitrogen source, trace elements), pH, temperature, and regulation modes etc. will affect the metabolic process of bacteria, thereby affecting the production, size, and composition of BEVs [65]. Actually, the expression of some heterologous proteins, such as PD1, needs to be induced under low temperature conditions (such as 18°C [74]), but this condition may not be suitable for the mass production of BEVs. Moreover, the methods of BEVs isolation remains controversial. Although there are a variety of widely recognized purification schemes [39], most of them require repeated and time-consuming ultracentrifugation and ultrafiltration. The existing commercial purification kits are faced with high price and low purity. Furthermore, natural MEVs have abundant and clear specific biomarkers in the membrane, such as CD81, CD63, CD9, and TSG101 [20]. However, BEVs are lack of definite individual markers, which is not conducive to the characterization of BEVs [193]. In conclusion, it is urgent to determine a definite standard for BEVs production, isolation, and characterization.
Potential off-target effects
In addition to being an activator of immune response, BEVs can also be used as drug delivery systems to deliver chemotherapeutic drugs and gene disruptors in BEVs-based cancer therapy. These exogenous cargoes loaded in BEVs may interfere or interact with endogenous cargoes, that is off-target effects [58]. The heterogeneity, including size, content, functional and source heterogeneity, of BEVs will become a prominent obstacle if off-target effects are excessive, especially in cancer gene therapy [20, 58]. For example, single miRNA may not completely cure the disease when the off-target effects exceed the therapeutic goal, and thus, a family of miRNAs may be needed to ensure that upstream and downstream targets are modulated [58]. Therefore, reducing the off-target effects is of great concern when constructing therapeutic BEVs.
Potential biosafety
It must be pointed out that “Potential biosafety” in the challenges does not conflict with “Higher safety” in the advantages. Although many BEVs-based cancer therapies have confirmed that appropriate injection of BEVs does not cause any significant side effects [83-91], we still need to evaluate the safest bacteria source for obtaining therapeutic BEVs that do not deliver unwanted toxic substances and cause incalculable problems [194]. In addition, in vivo experiments are still needed to clarify the distribution, dose, and clearance rate of BEVs to comprehensively evaluate the safety of BEVs [95].
Ambiguous contents
The intrinsic composition of BEVs is plentiful, for example, natural E. coli DH5α derived BEVs have been identified 141 proteins by global proteomic profiling [195], which may contain the unwanted toxic substances or off-target effects substances. Therefore, Park et al. [84] obtained synthetic BEVs with few cytosolic ingredients and nucleic acid by applying high lysozyme and high pH treatment of bacteria. Although many BEVs-based cancer therapeutic strategies have successfully induced significant regression of tumor growth without significant side effects, the content of these BEVs remains ambiguous [69, 190]. Fortunately, with the development of high-throughput sequencing and omics, a clear and complete map of contents of BEVs will be presented to us, so that BEVs-based cancer treatment can be more refined.
Conclusions and perspectives
In this review, we outlined the biogenesis, composition, isolation, classification, and internalization of BEVs. Gram-negative bacteria derived BEVs are generated by explosive cell lysis and blebbing of the outer membrane, while the biogenesis mechanism of Gram-positive bacteria is bubbling cell death. According to the different biogenesis mechanisms, BEVs can be divided into four categories, OIMVs, EOMVs, OMVs, and CMVs. For composition, OMVs mainly contain outer membrane proteins, and other BEVs contain a large number of proteins, nucleic acids, metabolites, etc. Moreover, an efficient BEVs isolation strategy based on ultracentrifugation and density gradient centrifugation is provided. When considering the interaction of BEVs with cells, three main internalization mechanisms, including endocytosis, receptor mediated signalling, and membrane fusion, have been identified.
We then comprehensively summarize the sources of BEVs in cancer therapy. The sources of BEVs used for cancer treatment are widely distributed, ranging from pathogenic bacteria (such as E. coli Rosetta, E. coli DH5α, and E. coli TOP10) to attenuated bacteria (E. coli W3110△msbB), and to probiotics (such as E. coli Nissle 1917, Akkermansia muciniphila, and Lactobacillus rhamnosus GG). Subsequently, the BEVs-related cancer treatment strategies are comprehensively summarized. In addition to immunotherapy, BEVs have been applied to the combination with chemotherapy, gene therapy, and photothermal therapy to synergistically amplify antitumor efficacy. Based on it, we propose the potential role of BEVs in BSTTs. After a comprehensive discussion in the above sections, we highlight the major advantages and challenges of BEV in cancer therapy. The BEVs of cancer treatment display various characteristics such as intrinsic immunomodulatory properties, ease of industrialization, ease of customization, and higher safety. However, there are still challenges, including lack of standardization, potential off-target effects, potential biosafety, and ambiguous contents, to move BEVs from lab to clinic.
In the past research, our team has accumulated a lot of basic and clinical experiences in bone and soft tissue diseases [196-199]. Although the application of BEVs in BSTTs is not as prosperous as MEVs [200], as the relationship between the microbiome and human health becomes clearer, the unique properties of BEVs will make them become another promising approach for these tumors. Furthermore, with the advancement of synthetic biology and molecular biology technology, an increasing number of research have focused on BEVs-based subjects, including BEVs-mediated inflammatory responses, BEVs-based adjuvant, vaccine, and antitumor applications. Moreover, the topic of “nonmammalian EVs, especially BEVs” is considered to be one of the hottest directions [191]. Importantly, engineered BEVs will further enhance the efficacy of tumors therapy by increasing the local concentration of the therapeutic agent and minimizing side effects. In conclusion, technical advances and clinical regulatory approvals will further drive the development of BEVs-based cancer therpay including BSTTs. Despite the constant challenges, significant progresses of BEVs in cancer therapy have been made in recent years. Continued study on BEVs-based BSTTs will undoubtedly lead to more innovative solutions to current challenges and achieve clinical applications.
Abbreviations
OMVs: Outer membrane vesicles; OIMVs: Outer-inner membrane vesicles; EOMVs: Explosive outer-membrane vesicles; EcN: Escherichia coli Nissle 1917; TEM: Transmission electron microscopy; NTA: Nanoparticle tracking analysis; WB: Western blotting; PAMPs: Pathogen-associated molecular patterns; PRRs: Pattern recognition receptors; E. coli: Escherichia coli; PD-L1: Programmed death 1 ligand 1; PD1: Programmed death 1; HA: Hyaluronic acid; Hy: Hyaluronidase; DOX: Doxorubicin; CTLs: Cytotoxic T lymphocytes; KSP: Kinesin spindle protein; HER2: Human epidermal growth factor receptor 2; TSA: Tumor-specific antigens; MHCI: Major histocompatibility antigen complex I; PTT: Photothermal therapy; PTAs: Photothermal transduction agents; NIR: Near-infrared; Mel: Melanin; RBCs: Red blood cells; Mal: Maleimide; 1-MT: 1-methyl-tryptophan; ICG: Indocyanine green; NPs: Nanoparticles; CCs: Cancer cells; HPDA: Hollow polydopamine; EPVs: Eukaryotic-prokaryotic vesicles; CCMVs: Cancer cell membrane vesicles; PLGA: Poly (lactic-co-glycolic acid); FMT: Fecal microbiota transplantation; ctDNA: Circulating tumor cell (CTC), Circulating tumor DNA.
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC2001500); the National Natural Science Foundation of China (82172098, 32101084); and the Shanghai Super Postdoctoral Incentive Program (2021457).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30
2. Li X, Seebacher NA, Hornicek FJ, Xiao T, Duan Z. Application of liquid biopsy in bone and soft tissue sarcomas: Present and future. Cancer Lett. 2018;439:66-77
3. Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization classification of tumors of soft tissue and bone. Cancer. 2014;120:1763-74
4. Steffner RJ, Jang ES. Staging of bone and soft-tissue sarcomas. J Am Acad Orthop Surg. 2018;26:e269-e78
5. Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18:39-50
6. D'Angelo SP, Shoushtari AN, Keohan ML, Dickson MA, Gounder MM, Chi P. et al. Combined KIT and CTLA-4 Blockade in patients with refractory GIST and other advanced sarcomas: A phase Ib study of dasatinib plus ipilimumab. Clin Cancer Res. 2017;23:2972-80
7. Ben-Ami E, Barysauskas CM, Solomon S, Tahlil K, Malley R, Hohos M. et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer. 2017;123:3285-90
8. DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: Disentangling cause from consequence. Cell Host Microbe. 2020;28:180-9
9. Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24:392-400
10. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55-71
11. Brevi A, Cogrossi LL, Lorenzoni M, Mattorre B, Bellone M. The insider: Impact of the gut microbiota on cancer immunity and response to therapies in multiple myeloma. Front Immunol. 2022;13:845422
12. Jasiński M, Biliński J, Basak GW. The role of the crosstalk between gut microbiota and immune cells in the pathogenesis and treatment of multiple myeloma. Front Immunol. 2022;13:853540
13. Hu Y, Li X, Zhi X, Cong W, Huang B, Chen H. et al. RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss. EMBO Rep. 2021;22:e52481
14. Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9:20
15. Li X, Wang L, Huang B, Gu Y, Luo Y, Zhi X. et al. Targeting actin-bundling protein L-plastin as an anabolic therapy for bone loss. Sci Adv. 2020 6
16. Zou Y, Huang B, Cao L, Deng Y, Su J. Tailored mesoporous inorganic biomaterials: Assembly, functionalization, and drug delivery engineering. Adv Mater. 2021;33:e2005215
17. Chen S, Chen X, Geng Z, Su J. The horizon of bone organoid: A perspective on construction and application. Bioact Mater. 2022;18:15-25
18. Zhou Z, Cui J, Wu S, Geng Z, Su J. Silk fibroin-based biomaterials for cartilage/osteochondral repair. Theranostics. 2022;12:5103-24
19. Ren X, Chen X, Geng Z, Su J. Bone-targeted biomaterials: strategies and applications. Chem Eng J. 2022: 137133.
20. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 367
21. Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J. et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145
22. Li S. The basic characteristics of extracellular vesicles and their potential application in bone sarcomas. J Nanobiotechnology. 2021;19:277
23. Dai J, Shupp AB, Bussard KM, Keller ET. Extracellular vesicles and bone-associated cancer. Curr Osteoporos Rep. 2021;19:223-9
24. Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. 2020;5:242
25. Díaz-Garrido N, Badia J, Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10:e12161
26. Bittel M, Reichert P, Sarfati I, Dressel A, Leikam S, Uderhardt S. et al. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo. J Extracell Vesicles. 2021;10:e12159
27. Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17:13-24
28. Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13:620-30
29. Krishnan N, Kubiatowicz LJ, Holay M, Zhou J, Fang RH, Zhang L. Bacterial membrane vesicles for vaccine applications. Adv Drug Deliv Rev. 2022: 114294.
30. Aytar Çelik P, Derkuş B, Erdoğan K, Barut D, Blaise Manga E, Yıldırım Y. et al. Bacterial membrane vesicle functions, laboratory methods, and applications. Biotechnol Adv. 2022;54:107869
31. Chronopoulos A, Kalluri R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene. 2020;39:6951-60
32. Liu H, Fang G, Wu H, Li Z, Ye Q. L-cysteine production in Escherichia coli based on rational metabolic engineering and modular strategy. Biotechnol J. 2018 No. e1700695
33. Liu H, Hou Y, Wang Y, Li Z. Enhancement of sulfur conversion rate in the production of L-cysteine by engineered Escherichia coli. J Agric Food Chem. 2020;68:250-7
34. Liu H, Wang Y, Hou Y, Li Z. Fitness of chassis cells and metabolic pathways for L-cysteine overproduction in Escherichia coli. J Agric Food Chem. 2020;68:14928-37
35. Gujrati V, Prakash J, Malekzadeh-Najafabadi J, Stiel A, Klemm U, Mettenleiter G. et al. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat Commun. 2019;10:1114
36. Gujrati V, Ntziachristos V. Bioengineered bacterial vesicles for optoacoustics-guided phototherapy. Methods Enzymol. 2021;657:349-64
37. Gujrati V, Lee M, Ko YJ, Lee S, Kim D, Kim H. et al. Bioengineered yeast-derived vacuoles with enhanced tissue-penetrating ability for targeted cancer therapy. Proc Natl Acad Sci U S A. 2016;113:710-5
38. Gujrati V, Kim S, Kim SH, Min JJ, Choy HE, Kim SC. et al. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8:1525-37
39. Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact Mater. 2022;14:169-81
40. Liu H, Geng Z, Su J. Engineered mammalian and bacterial extracellular vesicles as promising nanocarriers for targeted therapy. Extracell Vesicles Circ Nucleic Acids. 2022;3:63-86
41. Liu H, Li M, Zhang T, Liu X, Zhang H, Geng Z. et al. Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem Eng J. 2022: 138309.
42. Caruana JC, Walper SA. Bacterial membrane vesicles as mediators of microbe - microbe and microbe - host community interactions. Front Microbiol. 2020;11:432
43. Shockman GD, Barrett JF. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol. 1983;37:501-27
44. Hong SW, Kim MR, Lee EY, Kim JH, Kim YS, Jeon SG. et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy. 2011;66:351-9
45. De Maeyer RPH, van de Merwe RC, Louie R, Bracken OV, Devine OP, Goldstein DR. et al. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol. 2020;21:615-25
46. Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK. et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun. 2016;7:11220
47. Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375-87
48. Praveschotinunt P, Duraj-Thatte AM, Gelfat I, Bahl F, Chou DB, Joshi NS. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat Commun. 2019;10:5580
49. Somerville JE Jr, Cassiano L, Darveau RP. Escherichia coli msbB gene as a virulence factor and a therapeutic target. Infect Immun. 1999;67:6583-90
50. Zhao Z WH, Godwin AK, Soper SA. Isolation and analysis methods of extracellular vesicles (EVs). Extracell Vesicles Circ Nucleic Acids. 2021;2:80-103
51. Zhang M, Ke J, Liang G, Zhang Z, Fang L, Zhou F. et al. Methods and Technologies for Exosome Isolation and Characterization. Small Methods. 2018: 1800021-.
52. Prados-Rosales R, Brown L, Casadevall A, Montalvo-Quirós S, Luque-Garcia JL. Isolation and identification of membrane vesicle-associated proteins in Gram-positive bacteria and mycobacteria. MethodsX. 2014;1:124-9
53. Klimentová J, Stulík J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol Res. 2015;170:1-9
54. Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, Jin L. et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci (Weinh). 2021;8:2004831
55. Tiwari S, Kumar V, Randhawa S, Verma SK. Preparation and characterization of extracellular vesicles. Am J Reprod Immunol. 2021;85:e13367
56. Li M, Zhou H, Yang C, Wu Y, Zhou X, Liu H. et al. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J Control Release. 2020;323:253-68
57. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917-50
58. Cheng L, Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022
59. Jiang Y, Li J, Xue X, Yin Z, Xu K, Su J. Engineered extracellular vesicles for bone therapy. Nano Today. 2022;44:101487
60. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-95
61. Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y. et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926-44
62. Rodrigues M, Fan J, Lyon C, Wan M, Hu Y. Role of extracellular vesicles in viral and bacterial infections: Pathogenesis, diagnostics, and therapeutics. Theranostics. 2018;8:2709-21
63. O'Donoghue EJ, Krachler AM. Mechanisms of outer membrane vesicle entry into host cells. Cell Microbiol. 2016;18:1508-17
64. Pathirana RD, Kaparakis-Liaskos M. Bacterial membrane vesicles: Biogenesis, immune regulation and pathogenesis. Cell Microbiol. 2016;18:1518-24
65. Ñahui Palomino RA, Vanpouille C, Costantini PE, Margolis L. Microbiota-host communications: Bacterial extracellular vesicles as a common language. PLoS Pathog. 2021;17:e1009508
66. Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD. et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell. 2016;165:1106-19
67. Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen AM, Asikainen S. et al. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect Immun. 2012;80:31-42
68. Yi X, Zhou H, Chao Y, Xiong S, Zhong J, Chai Z. et al. Bacteria-triggered tumor-specific thrombosis to enable potent photothermal immunotherapy of cancer. Sci Adv. 2020;6:eaba3546
69. Huang X, Pan J, Xu F, Shao B, Wang Y, Guo X. et al. Bacteria-based cancer immunotherapy. Adv Sci (Weinh). 2021;8:2003572
70. Fan J-X, Niu M-T, Qin Y-T, Sun Y-X, Zhang X-Z. Progress of engineered bacteria for tumor therapy. Adv Drug Deliv Rev. 2022;185:114296
71. Holay M, Guo Z, Pihl J, Heo J, Park JH, Fang RH. et al. Bacteria-inspired nanomedicine. ACS Appl Bio Mater. 2021;4:3830-48
72. Lee HS, Boulton IC, Reddin K, Wong H, Halliwell D, Mandelboim O. et al. Neisserial outer membrane vesicles bind the coinhibitory receptor carcinoembryonic antigen-related cellular adhesion molecule 1 and suppress CD4+ T lymphocyte function. Infect Immun. 2007;75:4449-55
73. Michalek J, Hezova R, Turanek-Knötigova P, Gabkova J, Strioga M, Lubitz W. et al. Oncolysate-loaded Escherichia coli bacterial ghosts enhance the stimulatory capacity of human dendritic cells. Cancer Immunol Immunother. 2017;66:149-59
74. Li Y, Zhao R, Cheng K, Zhang K, Wang Y, Zhang Y. et al. Bacterial outer membrane vesicles presenting programmed death 1 for improved cancer immunotherapy via immune activation and checkpoint inhibition. ACS Nano. 2020
75. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-84
76. Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509-20
77. Deo P, Chow SH, Han ML, Speir M, Huang C, Schittenhelm RB. et al. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat Microbiol. 2020;5:1418-27
78. Riehl TE, Alvarado D, Ee X, Zuckerman A, Foster L, Kapoor V. et al. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut. 2019;68:1003-13
79. Fábrega MJ, Aguilera L, Giménez R, Varela E, Alexandra Cañas M, Antolín M. et al. Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic Escherichia coli strains. Front Microbiol. 2016;7:705
80. Liu JH, Yue T, Luo ZW, Cao J, Yan ZQ, Jin L. et al. Akkermansia muciniphila promotes type H vessel formation and bone fracture healing by reducing gut permeability and inflammation. Dis Model Mech. 2020 13
81. Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH. et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 2017;8:626
82. Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354-9
83. Chen L, Qin H, Zhao R, Zhao X, Lin L, Chen Y. et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci Transl Med. 2021 13
84. Park KS, Svennerholm K, Crescitelli R, Lässer C, Gribonika I, Lötvall J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J Extracell Vesicles. 2021;10:e12120
85. Liang J, Cheng K, Li Y, Xu J, Chen Y, Ma N. et al. Personalized cancer vaccines from bacteria-derived outer membrane vesicles with antibody-mediated persistent uptake by dendritic cells. Fundamental Research. 2022;2:23-36
86. Cheng K, Zhao R, Li Y, Qi Y, Wang Y, Zhang Y. et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat Commun. 2021;12:2041
87. Thomas SC, Madaan T, Kamble NS, Siddiqui NA, Pauletti GM, Kotagiri N. Engineered bacteria enhance immunotherapy and targeted therapy through stromal remodeling of tumors. Adv Healthc Mater. 2022;11:e2101487
88. Sepahdar Z, Miroliaei M, Bouzari S, Khalaj V, Salimi M. Surface engineering of Escherichia coli-derived OMVs as promising nano-carriers to target EGFR-overexpressing breast cancer cells. Front Pharmacol. 2021;12:719289
89. Wang S, Huang W, Li K, Yao Y, Yang X, Bai H. et al. Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor. Int J Nanomedicine. 2017;12:6813-25
90. Aly RG, El-Enbaawy MI, Abd El-Rahman SS, Ata NS. Antineoplastic activity of Salmonella Typhimurium outer membrane nanovesicles. Exp Cell Res. 2021;399:112423
91. Luo ZW, Xia K, Liu YW, Liu JH, Rao SS, Hu XK. et al. Extracellular vesicles from Akkermansia muciniphila elicit antitumor immunity against prostate cancer via modulation of CD8+ T cells and macrophages. Int J Nanomedicine. 2021;16:2949-63
92. Behzadi E, Mahmoodzadeh Hosseini H, Imani Fooladi AA. The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb Pathog. 2017;110:1-6
93. Zhou J, Karshalev E, Mundaca-Uribe R, Esteban-Fernández de Ávila B, Krishnan N, Xiao C. et al. Physical disruption of solid tumors by immunostimulatory microrobots enhances antitumor immunity. Adv Mater. 2021: e2103505.
94. Hua L, Yang Z, Li W, Zhang Q, Ren Z, Ye C. et al. A novel immunomodulator delivery platform based on bacterial biomimetic vesicles for enhanced antitumor immunity. Adv Mater. 2021;33:e2103923
95. Yue Y, Xu J, Li Y, Cheng K, Feng Q, Ma X. et al. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria. Nat Biomed Eng. 2022
96. Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L. et al. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. 2020;10:1534-48
97. Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M. et al. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. 2020;20:11-21
98. Li Y, Ma X, Yue Y, Zhang K, Cheng K, Feng Q. et al. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv Mater. 2022: e2109984.
99. Wang D, Liu C, You S, Zhang K, Li M, Cao Y. et al. Bacterial vesicle-cancer cell hybrid membrane-coated nanoparticles for tumor specific immune activation and photothermal therapy. ACS Appl Mater Interfaces. 2020;12:41138-47
100. Li Y, Zhang K, Wu Y, Yue Y, Cheng K, Feng Q. et al. Antigen capture and immune modulation by bacterial outer membrane vesicles as in situ vaccine for cancer immunotherapy post-photothermal therapy. Small. 2022;18:e2107461
101. Chen Q, Huang G, Wu W, Wang J, Hu J, Mao J. et al. A hybrid eukaryotic-prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv Mater. 2020;32:e1908185
102. Zhuang Q, Xu J, Deng D, Chao T, Li J, Zhang R. et al. Bacteria-derived membrane vesicles to advance targeted photothermal tumor ablation. Biomaterials. 2021;268:120550
103. Müller L, Kuhn T, Koch M, Fuhrmann G. Stimulation of probiotic bacteria induces release of membrane vesicles with augmented anti-inflammatory activity. ACS Appl Bio Mater. 2021;4:3739-48
104. Kroll AV, Jiang Y, Zhou J, Holay M, Fang RH, Zhang L. Biomimetic nanoparticle vaccines for cancer therapy. Adv Biosyst. 2019;3:e1800219
105. Chiang CJ, Huang PH. Metabolic engineering of probiotic Escherichia coli for cytolytic therapy of tumors. Sci Rep. 2021;11:5853
106. McNutt M. Cancer immunotherapy. Science. 2013;342:1417
107. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335-7
108. Gnopo YMD, Watkins HC, Stevenson TC, DeLisa MP, Putnam D. Designer outer membrane vesicles as immunomodulatory systems-Reprogramming bacteria for vaccine delivery. Adv Drug Deliv Rev. 2017;114:132-42
109. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704
110. Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114-25
111. Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:209-22
112. Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J. et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25-35
113. Fahie M, Romano FB, Chisholm C, Heuck AP, Zbinden M, Chen M. A non-classical assembly pathway of Escherichia coli pore-forming toxin cytolysin A. J Biol Chem. 2013;288:31042-51
114. Zervantonakis IK, Poskus MD, Scott AL, Selfors LM, Lin JR, Dillon DA. et al. Fibroblast-tumor cell signaling limits HER2 kinase therapy response via activation of MTOR and antiapoptotic pathways. Proc Natl Acad Sci U S A. 2020;117:16500-8
115. Liao R, Zhang XD, Li GZ, Qin KL, Yan X. Comparison of transcatheter arterial chemoembolization with raltitrexed plus liposomal doxorubicin vs. tegafur plus pirarubicin for unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2020;11:747-59
116. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383-90
117. Broto M, McCabe R, Galve R, Marco MP. A high throughput immunoassay for the therapeutic drug monitoring of tegafur. Analyst. 2017;142:2404-10
118. Nagasaki E, Takahara A, Koido S, Sagawa Y, Aiba K, Tajiri H. et al. Combined treatment with dendritic cells and 5-fluorouracil elicits augmented NK cell-mediated antitumor activity through the tumor necrosis factor-alpha pathway. J Immunother. 2010;33:467-74
119. Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157-70
120. Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351-60
121. Song X, Liu C, Wang N, Huang H, He S, Gong C. et al. Delivery of CRISPR/Cas systems for cancer gene therapy and immunotherapy. Adv Drug Deliv Rev. 2021;168:158-80
122. Smith SN, Schubert R, Simic B, Brücher D, Schmid M, Kirk N. et al. The SHREAD gene therapy platform for paracrine delivery improves tumor localization and intratumoral effects of a clinical antibody. Proc Natl Acad Sci U S A. 2021 118
123. Kim TH, Singh RK, Kang MS, Kim JH, Kim HW. Inhibition of osteoclastogenesis through siRNA delivery with tunable mesoporous bioactive nanocarriers. Acta Biomater. 2016;29:352-64
124. Mora-Raimundo P, Lozano D, Manzano M, Vallet-Regí M. Nanoparticles to knockdown osteoporosis-related gene and promote osteogenic marker expression for osteoporosis treatment. ACS Nano. 2019;13:5451-64
125. Liu C, Feng Q, Sun J. Lipid nanovesicles by microfluidics: manipulation, synthesis, and drug delivery. Adv Mater. 2019;31:e1804788
126. Dolgin E. How COVID unlocked the power of RNA vaccines. Nature. 2021;589:189-91
127. Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18:215-29
128. Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B. et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci Adv. 2020;6:eaaw6071
129. Tao W, South VJ, Zhang Y, Davide JP, Farrell L, Kohl NE. et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8:49-59
130. Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107-17
131. Ayed Z, Cuvillier L, Dobhal G, Goreham RV. Electroporation of outer membrane vesicles derived from Pseudomonas aeruginosa with gold nanoparticles. SN Applied Sciences. 2019;1:1600
132. Huang L, Lilley DM. Structure of a rare non-standard sequence k-turn bound by L7Ae protein. Nucleic Acids Res. 2014;42:4734-40
133. Moore T, Zhang Y, Fenley MO, Li H. Molecular basis of box C/D RNA-protein interactions; cocrystal structure of archaeal L7Ae and a box C/D RNA. Structure. 2004;12:807-18
134. Hamon MA, Ribet D, Stavru F, Cossart P. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol. 2012;20:360-8
135. Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics-developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759-80
136. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-79
137. Zheng L, Hu X, Wu H, Mo L, Xie S, Li J. et al. In vivo monocyte/macrophage-hitchhiked intratumoral accumulation of nanomedicines for enhanced tumor therapy. J Am Chem Soc. 2020;142:382-91
138. Huo J, Jia Q, Huang H, Zhang J, Li P, Dong X. et al. Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections. Chem Soc Rev. 2021;50:8762-89
139. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657-74
140. Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic Therapy mediated by nontoxic core-shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J Am Chem Soc. 2016;138:16686-95
141. Lee C, Lim K, Kim SS, Thien LX, Lee ES, Oh KT. et al. Near infrared light-responsive heat-emitting hemoglobin hydrogels for photothermal cancer therapy. Colloids Surf B Biointerfaces. 2019;176:156-66
142. Zhou Q, Gong N, Zhang D, Li J, Han X, Dou J. et al. Mannose-derived carbon dots amplify microwave ablation-induced antitumor immune responses by capturing and transferring "Danger Signals" to dendritic cells. ACS Nano. 2021;15:2920-32
143. Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM. et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12:877-82
144. Ou X, Cai S, Liu P, Zeng J, He Y, Wu X. et al. Enhancement of dendritic cell-tumor fusion vaccine potency by indoleamine-pyrrole 2,3-dioxygenase inhibitor, 1-MT. J Cancer Res Clin Oncol. 2008;134:525-33
145. Zhang WX, Hao YN, Gao YR, Shu Y, Wang JH. Mutual benefit between Cu(II) and polydopamine for improving photothermal-chemodynamic therapy. ACS Appl Mater Interfaces. 2021;13:38127-37
146. Chen Z, Zhao P, Luo Z, Zheng M, Tian H, Gong P. et al. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10:10049-57
147. Meng Z, Zhou X, Xu J, Han X, Dong Z, Wang H. et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv Mater. 2019;31:e1900927
148. Miwa S, Yamamoto N, Hayashi K, Takeuchi A, Igarashi K, Tsuchiya H. Therapeutic targets for bone and soft-tissue sarcomas. Int J Mol Sci. 2019 20
149. Damerell V, Pepper MS, Prince S. Molecular mechanisms underpinning sarcomas and implications for current and future therapy. Signal Transduct Target Ther. 2021;6:246
150. Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: Clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol. 2011;77:163-71
151. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H. et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-109
152. Kawano S, Asano M, Adachi Y, Matsui J. Antimitotic and non-mitotic effects of eribulin mesilate in soft tissue sarcoma. Anticancer Res. 2016;36:1553-61
153. Delaloge S, Yovine A, Taamma A, Riofrio M, Brain E, Raymond E. et al. Ecteinascidin-743: a marine-derived compound in advanced, pretreated sarcoma patients-preliminary evidence of activity. J Clin Oncol. 2001;19:1248-55
154. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61
155. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158-68
156. Zhu Z, Jin Z, Zhang M, Tang Y, Yang G, Yuan X. et al. Prognostic value of programmed death-ligand 1 in sarcoma: a meta-analysis. Oncotarget. 2017;8:59570-80
157. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-60
158. Jian X, Zhu Y, Ouyang J, Wang Y, Lei Q, Xia J. et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome. 2020;8:74
159. McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20:e77-e91
160. Avila-Calderón ED, Ruiz-Palma MdS, Aguilera-Arreola MG, Velázquez-Guadarrama N, Ruiz EA, Gomez-Lunar Z. et al. Outer membrane vesicles of gram-negative bacteria: An outlook on biogenesis. Front Microbiol. 2021 12
161. Hanssen NMJ, de Vos WM, Nieuwdorp M. Fecal microbiota transplantation in human metabolic diseases: From a murky past to a bright future? Cell Metab. 2021;33:1098-110
162. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716-29
163. Liang JL, Luo GF, Chen WH, Zhang XZ. Recent advances in engineered materials for immunotherapy-involved combination cancer therapy. Adv Mater. 2021;33:e2007630
164. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273-90
165. Qin H, Zhao R, Qin Y, Zhu J, Chen L, Di C. et al. Development of a cancer vaccine using in vivo click-chemistry-mediated active lymph node accumulation for improved immunotherapy. Adv Mater. 2021;33:e2006007
166. Vela Ramirez JE, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv Drug Deliv Rev. 2017;114:116-31
167. Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P. et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450
168. Liu C HD, Cen H, Chen H, Li L, Nie G, Zhong Z, He Q, Yang X, Guo S, Wang L, Fan Z. Nucleic acid functionalized extracellular vesicles as promising therapeutic systems for nanomedicine. Extracell Vesicles Circ Nucleic Acids. 2022;3:14-30
169. Zhou B, Xu K, Zheng X, Chen T, Wang J, Song Y. et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5:144
170. Alix-Panabières C, Pantel K. Liquid Biopsy: From discovery to clinical application. Cancer Discov. 2021;11:858-73
171. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617-38
172. Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368:973-80
173. Fu A, Yao B, Dong T, Chen Y, Yao J, Liu Y. et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185:1356-72.e26
174. Hu Y, Li X, Zhang Q, Gu Z, Luo Y, Guo J. et al. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021;6:2905-13
175. Song H, Li X, Zhao Z, Qian J, Wang Y, Cui J. et al. Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible exosomes. Nano Lett. 2019;19:3040-8
176. Sun J, Yin Z, Wang X, Su J. Exosome-Laden Hydrogels: A novel cell-free strategy for in-situ bone tissue regeneration. Front Bioeng Biotechnol. 2022;10:866208
177. Yang F, Fan R, Gou M, Yang Q, Zhang T, Dai G. et al. Research on mechanism of miR-214 packaged with lipidosome nanoparticles on prompting the apoptosis of intestinal cancer through regulating p53 pathway. J Biomed Nanotechnol. 2021;17:2391-8
178. Fan M, Han Y, Gao S, Yan H, Cao L, Li Z. et al. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics. 2020;10:4944-57
179. Medici S, Peana M, Pelucelli A, Zoroddu MA. An updated overview on metal nanoparticles toxicity. Semin Cancer Biol. 2021;76:17-26
180. Sze SK, de Kleijn DP, Lai RC, Khia Way Tan E, Zhao H, Yeo KS. et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol Cell Proteomics. 2007;6:1680-9
181. Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA. et al. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49:1248-56
182. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751-60
183. Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282-95
184. Tang T-C, An B, Huang Y, Vasikaran S, Wang Y, Jiang X. et al. Materials design by synthetic biology. Nat Rev Mater. 2021;6:332-50
185. Cubillos-Ruiz A, Guo T, Sokolovska A, Miller PF, Collins JJ, Lu TK. et al. Engineering living therapeutics with synthetic biology. Nat Rev Drug Discov. 2021;20:941-60
186. Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L. et al. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci (Weinh). 2018;5:1700611
187. Chen G, Bai Y, Li Z, Wang F, Fan X, Zhou X. Bacterial extracellular vesicle-coated multi-antigenic nanovaccines protect against drug-resistant Staphylococcus aureus infection by modulating antigen processing and presentation pathways. Theranostics. 2020;10:7131-49
188. Gao X, Ran N, Dong X, Zuo B, Yang R, Zhou Q. et al. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med. 2018 10
189. Wang J, Li W, Zhang L, Ban L, Chen P, Du W. et al. Chemically edited exosomes with dual ligand purified by microfluidic device for active targeted drug delivery to tumor cells. ACS Appl Mater Interfaces. 2017;9:27441-52
190. Pan H, Zheng M, Ma A, Liu L, Cai L. Cell/bacteria-based bioactive materials for cancer immune modulation and precision therapy. Adv Mater. 2021;33:e2100241
191. Witwer KW, Goberdhan DC, O'Driscoll L, Théry C, Welsh JA, Blenkiron C. et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. 2021;10:e12182
192. Liu Q, Liu Q, Yi J, Liang K, Liu T, Roland KL. et al. Outer membrane vesicles derived from Salmonella Typhimurium mutants with truncated LPS induce cross-protective immune responses against infection of Salmonella enterica serovars in the mouse model. Int J Med Microbiol. 2016;306:697-706
193. Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Host- and microbiota-derived extracellular vesicles, immune function, and disease development. Int J Mol Sci. 2019 21
194. Gimona M, Brizzi MF, Choo ABH, Dominici M, Davidson SM, Grillari J. et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy. 2021;23:373-80
195. Lee EY, Choi DS, Kim KP, Gho YS. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom Rev. 2008;27:535-55
196. Sun S, Liu H, Hu Y, Wang Y, Zhao M, Yuan Y. et al. Selection and identification of a novel ssDNA aptamer targeting human skeletal muscle. Bioact Mater. 2023;20:166-78
197. Xue X, Liu H, Wang S, Hu Y, Huang B, Li M. et al. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/ anti-inflammatory therapy of osteoarthritis. Compos B Eng. 2022;237:109855
198. Xue X, Hu Y, Wang S, Chen X, Jiang Y, Su J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact Mater. 2022;12:327-39
199. Xue X, Zhang H, Liu H, Wang S, Li J, Zhou Q. et al. Rational design of multifunctional CuS nanoparticle-PEG composite soft hydrogel-coated 3D hard polycaprolactone scaffolds for efficient bone regeneration. Adv Funct Mater. 2022 2202470
200. He C, Zhang M, Liu L, Han Y, Xu Z, Xiong Y. et al. Cellular membrane-based vesicles displaying a reconstructed B cell maturation antigen for multiple myeloma therapy by dual targeting APRIL and BAFF. Acta Biomater. 2022;143:406-17
Author contact
![]() Corresponding authors: E-mail: xjhuyanedu.cn (Y. Hu); nanboshan1987com (Z. Geng); drsujiacancom (J. Su).
Corresponding authors: E-mail: xjhuyanedu.cn (Y. Hu); nanboshan1987com (Z. Geng); drsujiacancom (J. Su).
 Global reach, higher impact
Global reach, higher impact