13.3
Impact Factor
Theranostics 2022; 12(18):7668-7680. doi:10.7150/thno.75965 This issue Cite
Research Paper
Adoptive therapy with amyloid-β specific regulatory T cells alleviates Alzheimer's disease
1. Department of Physiology, College of Korean Medicine, Kyung Hee University, 26-6 Kyungheedae-ro, Dongdaemoon-gu, Seoul 02453, Korea
2. Cancer Immunology Branch, National Cancer Center, 323 Ilsan-ro, Ilsandong-gu, Goyang 10408, Korea
3. Department of Chemistry, Pohang University of Science and Technology, Pohang 37673, Korea
4. Institute of Life Science & Biotechnology, VT Bio. Co., Ltd. 3 rd FL, 16 Samseong-ro 76-gil, Gangnam-gu, Seoul 06185, Korea
5. Department of Anatomy and Acupoint, College of Korean Medicine, Gachon University, Seongnam 13120, Korea
6. Department of Health Sciences, The Graduate School of Dong-A University, 840 Hadan-dong, Saha-gu, Busan 49315, Korea
7. Division of RI Application, Korea Institute Radiological and Medical Sciences, 75 Nowon-ro, Nowon-Gu, Seoul 01812, Korea
8. Center for Self-assembly and Complexity, Institute for Basic Science (IBS), Pohang 37673, Korea
9. Stroke center, Kyung Hee University, 26-6 Kyungheedae-ro, Dongdaemoon-gu, Seoul 02453, Korea
†These authors contributed equally to this work.
Abstract
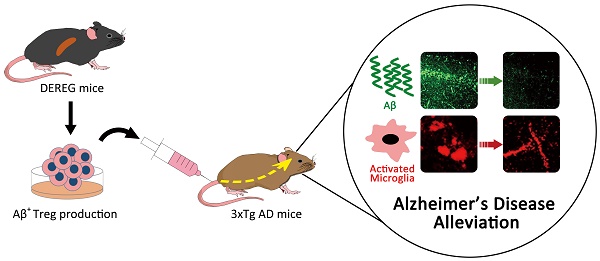
Rationale: Neuroinflammation is a primary feature of Alzheimer's disease (AD), for which an increasing number of drugs have been specifically developed. The present study aimed to define the therapeutic impact of a specific subpopulation of T cells that can suppress excessive inflammation in various immune and inflammatory disorders, namely, CD4+CD25+Foxp3+ regulatory T cells (Tregs).
Methods: To generate Aβ antigen-specific Tregs (Aβ+ Tregs), Aβ 1-42 peptide was applied in vivo and subsequent in vitro splenocyte culture. After isolating Tregs by magnetic bead based purification method, Aβ+ Tregs were adoptively transferred into 3xTg-AD mice via tail vein injection. Therapeutic efficacy was confirmed with behavior test, Western blot, quantitative real-time PCR (qRT-PCR), enzyme-linked immunosorbent assay (ELISA), and immunohistochemistry staining (IHC). In vitro suppression assay was performed to evaluate the suppressive activity of Aβ+ Tregs using flow cytometry. Thy1.1+ Treg trafficking and distribution was analyzed to explore the infused Tregs migration into specific organs in an antigen-driven manner in AD mice. We further assessed cerebral glucose metabolism using 18F-FDG-PET, an imaging approach for AD biological definition. Subsequently, we evaluated the migration of Aβ+ Tregs toward Aβ activated microglia using live cell imaging, chemotaxis, antibody blocking and migration assay.
Results: We showed that Aβ-stimulated Tregs inhibited microglial proinflammatory activity and modulated the microglial phenotype via bystander suppression. Single adoptive transfer of Aβ+ Tregs was enough to induce amelioration of cognitive impairments, Aβ accumulation, hyper-phosphorylation of tau, and neuroinflammation during AD pathology. Moreover, Aβ-specific Tregs effectively inhibited inflammation in primary microglia induced by Aβ exposure. It may indicate bystander suppression in which Aβ-specific Tregs promote immune tolerance by secreting cytokines to modulate immune responses during neurodegeneration.
Conclusions: The administration of Aβ antigen-specific regulatory T cells may represent a new cellular therapeutic strategy for AD that acts by modulating the inflammatory status in AD.
Keywords: Neuroinflammation, antigen-specific Tregs, adoptive transfer, microglia, bystander suppression
 Global reach, higher impact
Global reach, higher impact