13.3
Impact Factor
Theranostics 2023; 13(5):1545-1570. doi:10.7150/thno.82790 This issue Cite
Review
Nanoparticle formulations for therapeutic delivery, pathogen imaging and theranostic applications in bacterial infections
State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics & Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, 361102, China.
# Contribute equally
Received 2023-1-19; Accepted 2023-2-27; Published 2023-3-5
Abstract
Pathogenic bacterial infections represent an ever-growing crisis, now significantly threatening life expectancy across the worldwide population and thus novel approaches to tackle this issue are urgently needed. The application of nanotechnology in recent years has opened up new horizons in the selective or specific delivery of drugs or imaging agents to infectious sites. In particular, the development of nanoparticles for both delivery of active substances and imaging of infection sites is now gathering much interest. Although still in its infancy, the field of antibacterial nanomedicines provides exciting new possibilities to combat multi-resistant bacterial infections and shows great promise for personalized medicine in antibacterial stewardship. This review examines nanoparticle-based formulations used for therapeutic delivery, pathogen tracking in diagnosis, and combined “theranostic” approaches to more effectively treating bacterial infections.
Keywords: Nanoparticles, Therapeutic delivery, Diagnosis, Theranostic, Bacterial Infection
Introduction
Infectious diseases caused by a causative agent, a bacterium, parasite, or virus, represent a growing international health risk, as over 50% of global deaths each year can be ascribed to infectious agents [1, 2]. However, the incidence of microorganisms with drug resistance has grown due to the widespread usage of antibiotics over the past 70 years, which is now a worldwide severe health concern [3, 4]. Inefficiencies further exacerbate this problem with antibiotics, including low bioavailability, short half-life, side effects, and poor permeability of cellular barriers and biofilms [5, 6]. Therefore, researchers have increasingly evaluated drug delivery systems and other antimicrobial materials to improve the shortcomings associated with current antibiotics to enhance their effectiveness and prevent the development of resistance in patients [7-11].
The bio-application of nanotechnology has been extensively examined to develop more effective therapeutic delivery platforms [12, 13], including formulations such as liposomes [14-17], dendrimers [18-20], nano-emulsions [21, 22], polymeric [23-26] and metallic nanoparticles [27, 28]. These modalities, coupled with the ability to manipulate their features, excipients, and size, provide the ability to develop formulations that can be optimized to efficiently encapsulate drugs of different solubilities, tailor/control their release, and generate more targeted effects. The apparent superiority of nanoparticle delivery technology in antimicrobial therapy is its ability to tackle existing drug resistance, avoiding further development and adverse effects. Moreover, by inhibiting the biofilm formation and targeting intracellular microbes, nanoparticle delivery system also presents their unique advantages. More importantly, some nanoparticles also “deliver” therapeutic effects through their antimicrobial properties, such as antimicrobial polymeric nanoparticles [29], reactive oxygen species-producing metallic nanoparticles [30], and antimicrobial photothermal therapy-inducing carbon nanotubes [31, 32].
In addition to their obvious application as therapeutic delivery systems, nanoparticles are promising platforms for developing imaging modalities. Magnetic nanoparticles [33-35], gold nanoparticles [36-38], and fluorescent nanoparticles have all been employed for microbiological diagnostics. In contrast to small molecule probes, which are likely to be inactivated by non-specific binding of substances in vivo, nanoparticle-based probes or imaging modules can significantly extend the retention duration of imaging moieties in vivo and thus improve imaging quality with time. In addition, nanoparticles may be easily absorbed by cells, for example showing a very pronounced EPR (enhanced permeability and retention) effect, whereas small molecule probes virtually ever do. More critically, with the advancement of nanoparticle surface engineering, sensitive and specific detection for pathogenic bacteria could be easily achieved through binding nanoparticles with affinity probes, such as antibodies. In the recent ten years, particularly, there has been significant advancement in the development of nanotechnology-based diagnostics, resulting in the production of goods like Feraheme® [39].
The ultimate aim of many researchers in the nanotechnology arena is to develop nanomaterials that have both therapeutic and diagnostic potential; the coined “theranostic” modality represents the holy grail [40-42]. Conceptually, a theranostic combines diagnostic and therapeutic utility as a single formulation/modality (Figure 1). There are several well-established, effective, and safe theranostic anti-cancer therapies, such as lutetium octreotate [43] for somatostatin-positive tumour cells and Iodine-131 therapy [44] for thyrotoxicosis and thyroid cancer. However, given the complexity required to develop such formulations, proposed theranostic nanoparticle anti-infection strategies are currently in incipient stages. The next sections will go through the several recent nano-formulations that have successfully completed approaches for the treating and diagnostic imaging of microbial infections as well as the potential for integrating theranostic nanoparticles.
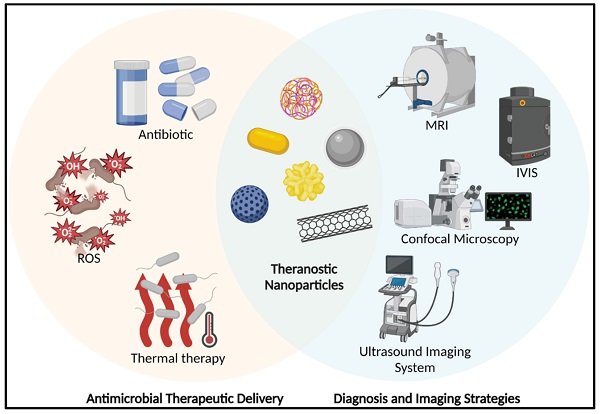
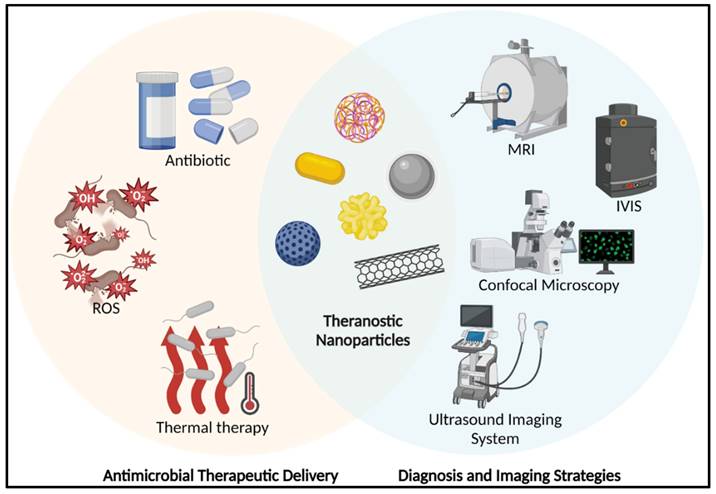
A schematic represents the theranostic nanoparticles in anti-bacterial applications. (MRI: Magnetic Resonance Imaging; IVIS: In Vivo Imaging System; ROS: Reactive Oxygen Species). Created with BioRender.com.
Lipid-based Nano-formulations
Lipid-based nano-formulations as antimicrobial deliver systems
Liposomes represent the most developed and widely applicable drug delivery platform. They are composed of phospholipid bilayers that combine to create a spherical vesicle with an aqueous centre. Liposomes have an amphiphilic structure that allows them to transport hydrophobic substances into their bilayers or encapsulate hydrophilic molecules in their core. As the most developed drug delivery system, numerous liposome formulations have successfully completed clinical evaluation and many liposome formulations have also been commercialized successfully. For example, Arikayce®, composed of DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) and cholesterol, is an inhaled liposomal amikacin formulation, which is the first and only therapy approved in the U.S. specifically for patients with MAC (mycobacterium avium complex) lung illness [45, 46].
Recent studies have investigated liposomal DDS (drug delivery system) for treating various bacterial infections, suggesting that liposomal formulations that would deliver antibiotics to the site of action with a controlled release could significantly improve therapeutic utility [47-49]. An example of this is the addition of molecules such as oleic acid derived quaternary lipid, whose structural conformation changes under acidic conditions, causes the liposomes to disintegrate and release the contained drug [50]. Such acid-responsive formulations are well adapted to the weakly acidic inflammatory microenvironment in which bacterial infections occurred. Then there are some thermosensitive liposomal molecules (e.g. DPPC, DSPC), which maintain a stable liposomal structure under physiological conditions and can trigger drug release in association with topical thermotherapy which can also inactivate bacteria through thermal effects, making them applicable to the treatment of wound infections in superficial tissues [51-53].
Liposome-based formulations provide an expansive range of opportunities to deliver therapies to generate synergistic effects. However, some drawbacks, such as short circulation half-life, low encapsulation efficacy of water-soluble drugs, and uncontrollable fusion, have plagued liposomal vesicles. To solve these pharmacological challenges, liposomes can be modified with a surface coat of PEG (polyethylene glycol) [54], gangliosides [55-57] and sialic acid derivatives [58, 59] to limit opsonization by the reticuloendothelial system and attenuate this rapid clearance from blood circulation. Drug encapsulation can be improved by introducing new steps, such as the dehydration-rehydration technique in the liposome formulation process [60]. As for improving the stability against uncontrollable fusion among liposomes, some studies reported that cationic and/or anionic nanoparticles could be decorated onto liposomes to enhance stability effectively [61]. Pornpattananangkul et al. synthesized a liposome stabilized by gold nanoparticles (modified with chitosan) [62]. They demonstrated that the attaching of gold nanoparticles to liposomes may successfully block fusion between liposomes to prevent undesirable vancomycin release. Moreover, the triggered release was achieved by inserting toxins secreted by S. aureus (Staphylococcus aureus) into liposomal layers and forming pores to release the encapsulated vancomycin. This novel approach has been proven to be applied in treating infection caused by toxin-secreting bacteria.
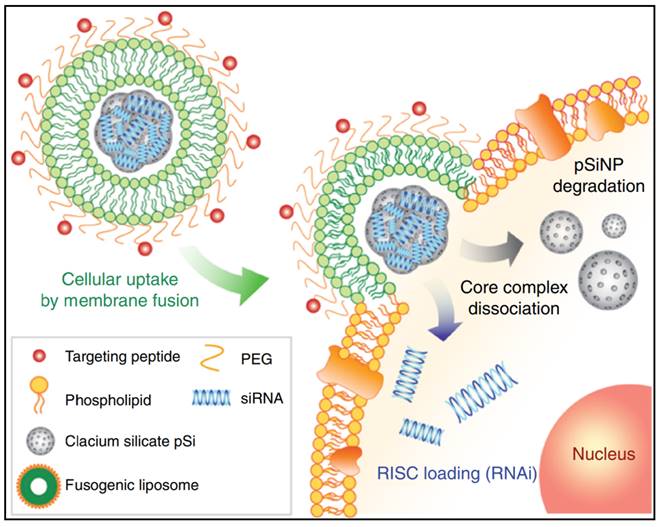
siRNA (small interfering RNA) is an alternative approach to fight bacterial infections by enhancing phagocytosis and bacterial clearance of macrophages. For instance, Niu et al. created liposomes loaded with siRNA against TGF-β1 (transforming growth factor-β1) that could target M. tuberculosis (Mycobacterium tuberculosis)-infected host THP-1 macrophages and enhance their autophagy [63]. In such siRNA therapies, the effectiveness of RNA interference is crucial. However, in conventional methods, such as nanoparticles loaded with siRNA, most of the nanoparticles are taken up via endocytosis of cells and then remain in the low pH endosomal compartment, which is later fused by lysosomes to form late endosomes, where they are degraded. Furthermore, lysosomes contain nucleases that degrade siRNA, thus no RNA interference can occur if the loaded siRNA cannot be leaked and excreted into the cytoplasm during early endosome uptake [64-66]. This is the most significant problem faced by RNA delivery systems for intracellular transport. To overcome this deficiency and improve gene therapy, Kim et al. developed a combinatorial system, nanoporous particles incorporated with fusogenic liposomes and peptides in the outer layer (Figure 2) [67]. In this nano-system, the high colocalization of the DiI and FAM signals in the fusogenic formulation suggests that CRV binds the nanoparticles to the particular macrophage membrane receptors to facilitate localized membrane fusion. By transferring hydrophobic molecules from the lipid bilayer to the cellular membranes, this technique enables the hydrophilic payload (siRNA) to be released immediately from the silica NP through into cell cytoplasm. This efficient delivery system was shown to be successful in enhancing clearance and macrophage survival in an S. aureus infection model.
A schematic presents the mechanism of the fusogenic pSiNP. Copyright © 2018 Springer Nature
SLNs (solid lipid nanoparticles) have attracted increasing interest because these formulations have the opportunity to achieve excellent drug loading. For instance, in a formulation created by Vieira et al., the encapsulation efficacy of rifampicin was approximately 90% [68]. In addition, these anhydrous formulations may offer advantages in terms of long-term stability and biocompatibility. A particular property of SLN technology is the ability to prepare combination drug formulations more easily and uniformly than other drug delivery platforms. This is particularly important for infections such as M. tuberculosis that require combination therapy. Compared to conventional antibiotic administration, the creation of SLNs packed with isoniazid, pyrazinamide, and rifampicin via emulsion solvent diffusion approach showed a considerable improvement in antibiotic residence duration and dosage [69]. No M. tuberculosis was detected in organs after daily nebulized administration of the multidrug SLNs over seven days; this is an astounding result, considering that regular oral administration of these drugs would require 46 daily doses to achieve equivalent therapeutic effects.
Lipid-based nano-formulations as bacteria-labelling platforms
The accurate determination of bacterial load and infection foci is of significant importance. This is particularly relevant considering bacterial and sterile inflammation symptoms can appear very similar; thus, it is critically important to distinguish these to make appropriate clinical decisions. Targeting the leukocytes that migrate and localize at the infected site is widely considered a gold standard for imaging infections [70]. However, there are limitations for labelled leukocytes in diagnostic accuracy, and the technique is time-consuming and can be hazardous to the patient. Therefore, more effective bacteria-imaging strategies are needed, which could directly label the invading pathogen instead of targeting surrogate micro-environmental factors. One promising approach is to attempt to tag the bacteria with fluorescent probes [71]. Such a route may provide a convenient approach to visualizing infection in real-time. With these aspirations in mind, nanotechnology has brought a new perspective to labelling specific molecules or biomarkers. For example, liposomes have been exploited and utilized in bacteria-related bio-labelling [72-74]. Fluorescence-based liposomes made from cholesterol, DPPC, DPPE, DPPG, and SRB were synthesized and functionalized with rabbit anti-C. muytjensii (Cronobacter muytjensii) IgG on its surface. This liposomal immunoassay provided directly proportional signal intensity to the number of C. muytjensii, suggesting this approach could offer a rapid and effective tool for C. muytjensii diagnosis [75].
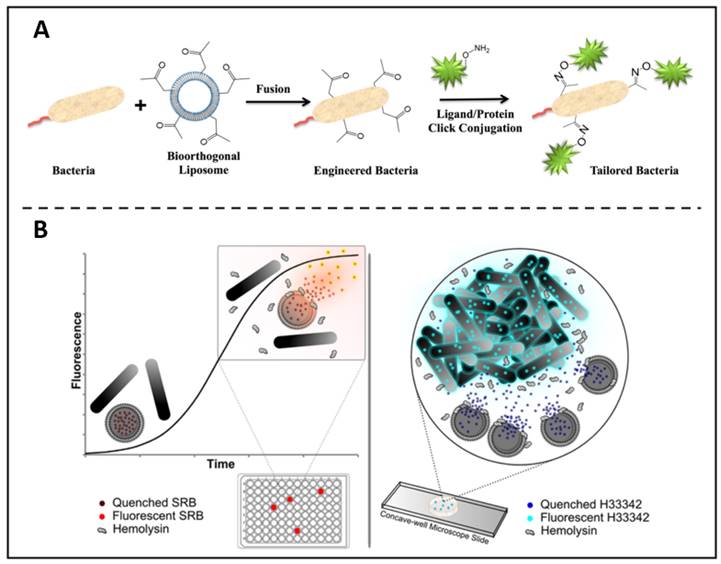
In addition to being a fluorescent material that can directly label bacteria, liposomes have a number of properties that can be used as a precursor step to biolabeling. For example, based on the liposome-cell membrane fusion, Elahipanah et al. presented a bacteria-labelling strategy through the bio-orthogonal chemistry (Figure 3A) [76]. In this work, by incubating with ketone-functionalized liposomes, in the presence of membrane fusion, ketone moieties could be presented on the bacterial membrane surface, which can subsequently be anchored by bio-orthogonal reactions with oxyamine tailored fluorescent molecules, proteins and probes that act as bio-markers. In another work, Sum et al. found that certain specific components of liposomes could be decomposed by β-hemolysin [77]. They then loaded fluorophores within the liposomes and co-incubated with bacteria. In contrast to α- and γ-haemolytic bacteria, it was shown that β-haemolytic pathogens could lyse and release fluorophores that were enclosed in liposomes. This enables the detection and separation of certain bacteria with extreme sensitivity and accuracy (Figure 3B).
(A) Bio-orthogonal chemistry is used to quantitatively characterise the surface engineering of bacteria. Copyright © 2016 American Chemical Society (B) Liposome lysis is selectedly initiated by β-haemolytic bacteria, allowing quick and accurate pathogen identification. Copyright © 2017 American Chemical Society
LIPID-based nano-formulations as antibacterial theranostics
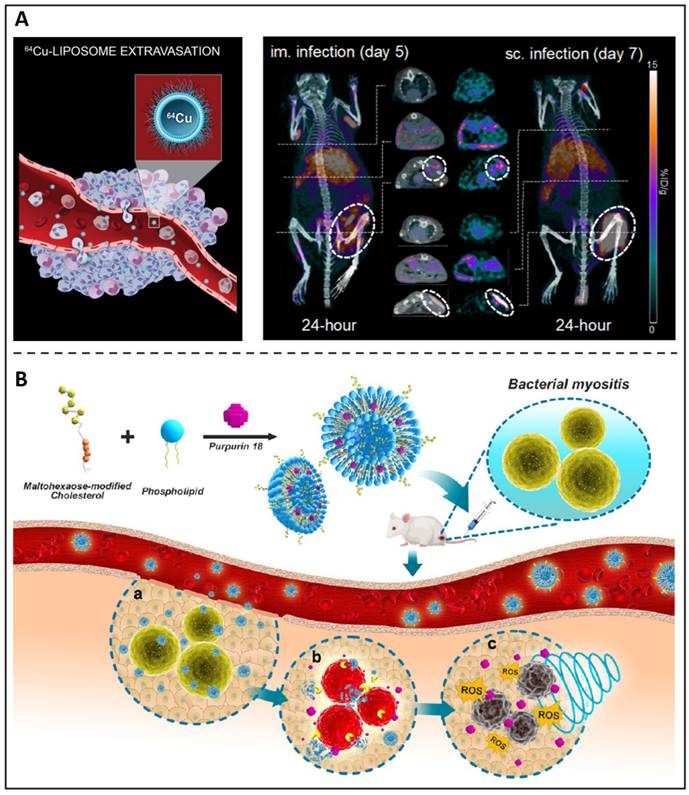
Because of their chemical versatility in labelling for imaging needs and their capacity to transport antimicrobial medicines, liposomes constitute an appealing theranostic approach for treating microbial infections. Kaul et al. synthesized a folate receptor targeting, pegylated liposomal formulation with rifampicin and ofloxacin encapsulated to improve the therapeutic window towards mycobacterial infections [78]. Additionally, the antibiotic-loaded liposomes were conjugated with a gamma-emitting radionuclide, thus providing diagnostic capabilities and presenting an effective and promising theranostic agent against tuberculosis. Clausen et al. developed an intramuscular and subcutaneous S. aureus-induced infection model using a bioluminescent strain (S. aureus Xen29) to examine the possibility of Cu-64 liposomes (Figure 4A) as a therapeutic approach to diagnose and evaluate patients who could achieve improved antibiotic therapy [79]. Images revealed a distinct luminous signal in the injection location that diminished throughout the period, suggesting that the mice were capable of mounting an immune response. On 24-hour PET images, 64Cu-liposomes showed high activity at both intramuscular and subcutaneous infection sites of the inoculum. Due to the extremely high liposome activity, infectious lesions may benefit from using liposomes as a diagnostic imaging and pharmaceutical delivery system. We previously reported a sonotheranostic strategy, based on purpurin 18-loaded and maltohexaose-decorated nanoliposome (MLP18), for bacteria-specific labelling and the visualization of sonodynamic therapy (Figure 4B) [80]. After entering the body, the prepared nano-liposomes can accurately stay at the site of bacterial infection and produce fluorescence / photoacoustic labelling to distinguish the lesions from joint inflammation or tumours. This vigorous acoustic dynamic activity was observed by in situ MRI imaging, which also showed that MLP18-mediated sonodynamic therapy effectively eliminated inflammation and abscesses in animals of microbial myositis.
Liposomes are extensively regarded and investigated as lipid-based drug delivery vehicles, not only due to the fundamental raw material for their manufacture, phospholipids, is an inherent element in human cells, making them friendly and non-immunogenic, but also since they can be manufactured as nanoscale particles, making them simpler to cross intrinsic limitations like blood vessel barriers and cellular membranes. In addition to antibacterial medication delivery, they are now employed in a variety of purposes, including as the treatment of cancer [81-83], eye illnesses [84, 85], and neurodegenerative diseases [86, 87].
Polymeric Nano-formulations
Antibiotic-loaded polymeric nanoparticles
The most common approach to preparing polymeric systems containing antibiotics is to load the antibiotics in the form of a physical encapsulation in polymeric particles. polymeric nanoparticles have the potential to be endocytosed intact by cells and bacteria, and successfully deliver the contained therapeutic agent into the cell, avoiding confinement at the cell membrane. This strategy improves both the solubility of hydrophobic antibiotics and the intracellular uptake of hydrophilic antibiotics, enhancing the antimicrobial efficacy to eliminate bacterial infections. In-depth research has been conducted on the exploitation of various biocompatible polymers, either natural or manufactured, as the building blocks for this nanosystem.
Due to their biocompatibility and biodegradability, natural polymers, including chitosan, alginate, and albumin, have been investigated as drug carriers [88-97]. For example, ceftriaxone-loaded chitosan tripolyphosphate nanoparticles exhibited enhanced antibacterial effects against intracellular S. typhimurium (Salmonella typhimurium) than the inefficient free drug [98]. Tobramycin-loaded alginate/chitosan nanoparticles engineered with DNase alfa were produced by Deacon and colleagues, which exhibited improved therapeutic effects against P. aeruginosa (Pseudomonas aeruginosa) in cystic fibrosis sputum, through at least in part, degradation of the extracellular DNA characteristically found in the sputum of these patients [99]. In addition to these natural polymer-based nanoparticles, many artificial polymers have been used as delivery system material. The most investigated polymer for the targeted administration of antimicrobials into infected cells is PLGA (poly(lactic-co-glycolic acid)) [100-107]. Radovic-Moreno et al. described the possibilities for a triblock copolymer of poly(d,l-lactic-co-glycolic acid)-b-poly(L-histidine)-b-poly(ethylene glycol) (PLGA-PLH-PEG) based on PLGA to self-assemble into nanostructures and encapsulate vancomycin in aqueous medium [108]. Other synthetic polymers used in products approved by the FDA have also been investigated as drug delivery platforms to treat bacterial infections. For example, PCL (polycaprolactone) is highly suitable for colloidal drug delivery as it can entrap various drug agents readily and has low toxicity properties [104, 109, 110]. A novel PCL-pluronic nanoparticle delivery platform was developed to improve the antibacterial effects of chloramphenicol, which further showed noticeably improved anti-MRSA (methicillin-resistant Staphylococcus aureus) effectiveness against a range of clinical specimens while dramatically reducing its toxicity [111]. Copolymer based on PCL, for example, poly(ethyleneglycol)-b-polycaprolactone-b-poly(β-amino ester) (PEG-b-PCL-b-PAE) has been reported by Chu and co-workers [112]. The bacterial infection-induced local acidity generated a charge transition in this self-assembled polymeric nano-micelle, prompting the encapsulated vancomycin to release and eradicate the bacteria at the site of the subcutaneous infection.
(A) A schematic presents 64Cu-liposome; PET/CT images of S. aureus Xen29 infection and injected with 64Cu-liposomes. Copyright © 2020 Elsevier B.V. (B) A schematic depicts MLP18 nanoliposomes for MRSA identification and elimination. Copyright © 2019 American Chemical Society.
Antibiotic-conjugated polymeric nanoparticles
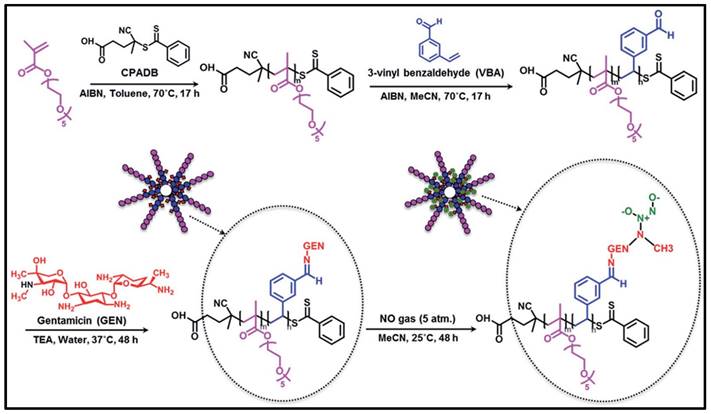
Although encapsulation of antibiotic drugs in polymeric nanomicelles and nanoparticles has been successful in reducing bacterial loads and mitigating bacterial infections, it is undesirable for loaded medications to release prematurely or in an unintended manner, particularly hydrophilic antibiotics such as β-lactams, aminoglycosides, and sulphonamides. To get around this restriction, antibiotic molecules can be chemically bonded to polymers using a variety of chemical linkages, including amides, esters, ethers and carbamates, to form antibiotic-polymer conjugates, thus forming nanoparticles. Dendrimers, as one representative of this drug-polymer conjugation, are repetitively branched polymeric molecules with spherical three-dimensional morphology. Unlike polymeric nano-systems, a particular advantage of dendrimers is that drug molecules can be engineered as an integral part of the carrier through covalent conjugation, so that the payload could be delivered in a controlled way by the linkage or through some triggering events at the desired location [113-116]. For instance, a penicillin V-conjugated PEG-PAMAM star polymer was developed in which the penicillin V was modified with PEG and then conjugated to PAMAM (polyamindoamine) dendrimer [117]. This ester-containing penicillin-conjugated dendrimer was incubated within S. aureus cultures, where the ester bonds hydrolysed and released penicillin exhibiting antimicrobial effects. Another example, Nguyen and colleagues reported a novel polymeric nanoparticle based on gentamicin-NONOate (nitric oxide donor) co-conjugated polymer, which is capable of releasing both gentamicin and nitrous oxide gas, with a synergistic effect on the elimination of biofilms and planktonic bacteria (Figure 5) [118].
Schematic approach showing the RAFT polymerization is used in the manufacture of gentamicin-NONOate nanoparticles. Copyright © 2016 The Royal Society of Chemistry
Polymeric nanoparticles-based theranostics against bacterial infection
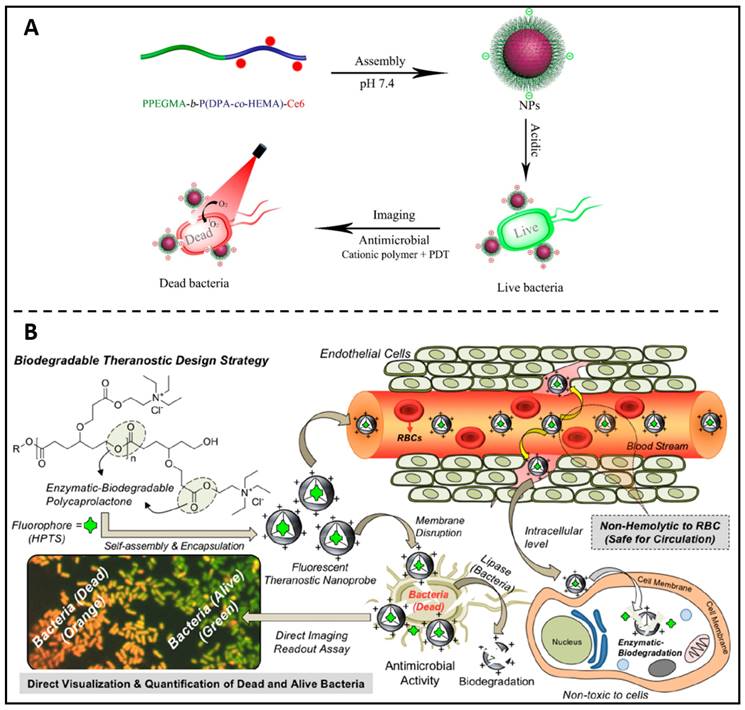
Following the concept of antibiotic-polymer conjugates, Liu et al. replaced the antibiotic portion with the photosensitiser Ce6 and in this way prepared self-assembled polymer nanoparticles with therapeutic properties [119]. The self-assembled PPEGMA-b-P(DPA-co-HEMA)-Ce6 nanoparticles were able to bind bacteria efficiently via cations and enable fluorescent bacterial imaging at acidic pH (Figure 6A). In addition, PPEGMA-b-P(DPA-co-HEMA)-Ce6 NPs can achieve good antibacterial effects by photodynamic therapy at acidic pH conditions. Ghosh and colleagues developed a biodegradable fluorescent theranostic nanoprobe based on anionic biomarker HPTS-loaded cationic-charged PCL nanoparticles, for calculating antimicrobial activity quantitatively while simultaneously visualizing (Figure 6B) [120]. This theranostic nanoprobe solely functions as an indicator to significantly increase living E. coli's (Escherichia coli) green-fluorescent emissions, with no antibacterial activities. After activation by a lipase enzyme or lysosomal esterase enzyme, the theranostic probe disrupts membranes of E. coli, allowing the co-staining of the nucleus with propidium iodide (red fluorescent). Consequently, green and orange markers, respectively, might be used to discriminate between alive and dying bacteria. These positive findings have inspired polymer chemists to develop new biocompatible polymers with advanced features, such as self-assembled cationic nanoparticles with copolymers which could destabilize in acidic biofilm microenvironments and release antimicrobial farnesol to disrupt S. mutans (Streptococcus mutans) biofilms [121], and conducting polymeric nanoparticles loaded with chloramphenicol which act as an intelligent nanotheranostic electro-responsive platform to real-time monitoring antibiotic release and suppressing the development of E. coli and S. sanguinis (Streptococcus sanguinis) [122].
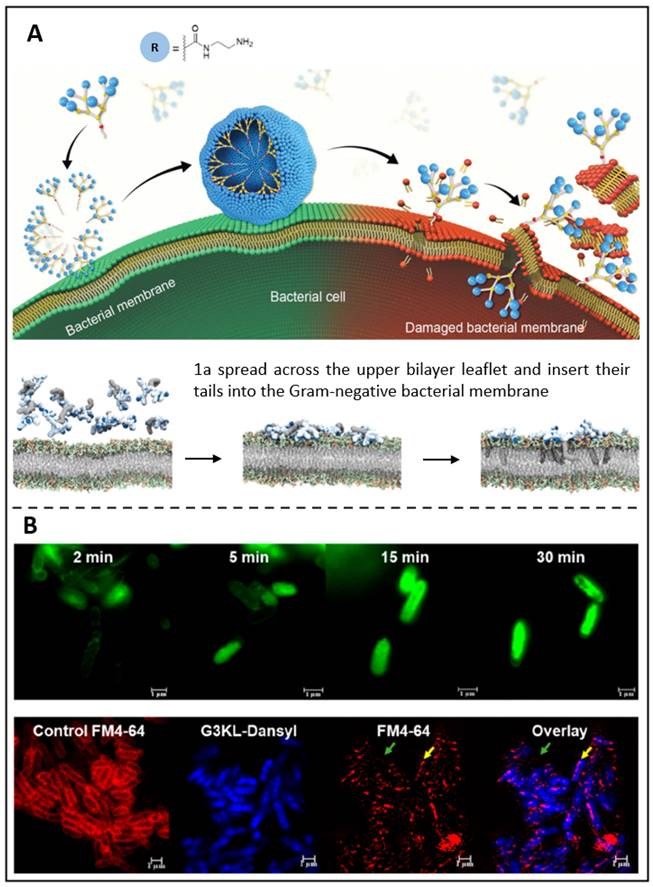
Dendrimers having various charged terminals, such as primary amine (1a), tertiary amine, guanidine, or carboxylate groups, have recently been synthesized by Dhumal et al. (Figure 7A) [228]. Among these terminals, electrostatic interactions between these terminals cause positively charged 1a dendrimers to move and adhere to oppositely charged membranes, where they enrich and dynamically self-assemble into nanoscale supramolecular components. These nanocomponents then quickly insert hydrophobic tails into the bacterial cytosol and induce cytolytic cleavage, which results in strong antibacterial activity. Such peptide dendrimers have been discovered to drastically reduce the development of Gram-negative bacteria, according to research by Gan et al. [123]. So, in their recent work, fluorescence-labelled peptide dendrimers containing fluorescein (G3KL-Fluo, Figure 7B above) or dansyl (G3KL-Dansyl, Figure 7B below) were reported. These two dendrimers bind to the bacterial surface, causing it depolarized and permeabilized while disrupting the external leaflet and inner layer. Moreover, G3KL could bind to LPS (lipopolysaccharide), preventing macrophages from releasing LPS-induced TNF-α. It was shown that G3KL is a potent membrane-disturbing antibacterial peptide that has the potential to be an antimicrobial theranostic nanoplatform.
The physicochemical properties, diameter, and shape of polymeric nanoparticles are among the most critical factors to consider when designing nanomedicine. Polymer nanoparticle characteristics can be significantly influenced by variations in the properties of polymer monomers, especially in vivo, leading to differences in performance, including circulation times, biodistribution, metabolic behaviours, and other pharmacodynamic effects. In addition, these polymeric materials' quality standards and economic effects need to be addressed during scale-up production. These considerations are fundamental to generating nanomaterials that can successfully track infections in vivo and remain the most critical issues to be addressed in the field.
(A) Schematic illustration of the PPEGMA-b-P(DPA-co-HEMA)-Ce6 nanoparticles as theranostic antimicrobial agents. Copyright © 2018 The Royal Society of Chemistry (B) Theranostic fluorescent cationic polymeric nanoprobes for assessing antibacterial activity are shown in a diagram. Copyright © 2020 American Chemical Society
(A) A schematic presents the antibacterial activity of amphiphilic dendrimers; Molecular dynamics simulations demonstrate the interaction between dendrimer 1a and bacterial membrane. Copyright © 2022 The Royal Society of Chemistry (B) Super-resolution confocal microscopy photos of P. aeruginosa exposed to G3KL-Fluo; Confocal microscopy of P. aeruginosa treated with G3KL-Dansyl. Copyright © 2019 American Chemical Society.
Metallic Nanoparticles
The antimicrobial mechanisms of metallic nanoparticles
Metallic nanoparticles possess a range of advantages such as broad antibacterial spectrum, high antibacterial activities, good biocompatibilities, durable stabilities and non-resistance. Silver [124-127], gold [127-129], copper oxide [130-132], zinc oxide [133-135], iron oxide [136-138], titanium dioxide [139-141], and magnesium oxide nanoparticles [142-144] have all been frequently documented as antibacterial substances. The antibacterial mechanism of these nanoparticles is mainly: 1. electrostatic adsorption and influences the bacterial membrane and cell wall function; 2. the destruction of bacterial enzymes, proteins, and DNA structure.
Most metallic ions, whether released from pure metallic nanoparticles or metal oxide nanoparticles, can tightly accumulate onto the bacterial surfaces due to their opposite charges, influencing the permeability of the membrane, wrecking the bacterial electron and resource transport systems; on the other hand, causing the destruction of the inherent components of the membrane, resulting in bacterial membrane rupture, cytoplasmic outflow, and ultimately the bacteria death. When the metallic ions penetrate the bacterial membrane and enter the bacterial cell, they combine with the sulfhydryl groups on the protein surface to coagulate the proteins and interfere with the action of enzymes and proteins, thereby depriving the cell of its ability to divide and proliferate. For example, the Ag+ and Zn2+ reacts with -SH to form a stable S-Ag and S-Zn bond that inactivates enzymes associated with oxygen metabolism in bacteria, which results in the disruption of ATP (adenosine triphosphate) synthesis.
In addition, metallic ions, such as Ag+, Zn2+, Cu2+, which can encourage the formation large amounts of ROS in microbial cells, leading to oxidative stress. Additionally, metal oxide like ZnO NPs and TiO2 NPs can activate electron-hole pairs to produce ROS when irradiated by light or UV light. These ROS bind to DNA and replace the hydrogen in the double helix structure of DNA molecules, thus leading to the deformation of the structure of bacterial DNA molecules, inhibiting the synthesis of DNA, RNA, and protein, and rendering the bacteria inactive.
Metallic nanoparticle-based antimicrobial strategies
There are some metallic nanoparticle-based strategies, mainly based on silver, that have been successfully marketed. AgTive® comprises central venous catheters impregnated with AgNPs to enhance bactericidal properties [145]. When in contact with bodily fluids and intravenous solutions, AgTive® releases silver ions to reduce bloodstream-borne infections. Similarly, Acticoat™ is a nanocrystalline silver wound covering that offers a powerful antimicrobial barrier to a broad spectrum of wound microorganisms [146-148]. This nano-formulation has been demonstrated to inhibit biofilm development in A. baumannii (Acinetobacter baumannii) and P. aeruginosa models by more than 90%. Salomoni and colleagues evaluated the antimicrobial efficacy of commercial 10 nm AgNP on multi-resistant P. aeruginosa strains found in hospitals [149]. The findings revealed that all strains were vulnerable to a 5 g/mL AgNPs solution, revealing the potential clinical application for nano-silver against pathogenic bacteria. These silver nanoparticles' biological effects and possible toxicity, however, might vary depending on their size and structure, as with other nanoparticles [150-153]. For example, elongated wire AgNPs elicited adverse effects on A549 cells, whereas similar concentrations of spherical particles did not produce similar adverse effects [154]. Furthermore, it has been found that AgNPs exhibit cellular cytotoxicity through the generation of ROS in eukaryotic cells, triggering caspase-1 activation that regulates pyroptosis and other forms of cell death [155, 156].
An alternative metal that has been widely examined in nanoparticulate form is gold. In addition to the enhanced safety and toxicity profile of gold over silver, it offers many adaptable features. At a mechanistic level, gold can alter the membrane potential of bacterial cells, thus abrogating adenosine triphosphate synthase activity, resulting in reduced energy production in the cell [157]. It has also been demonstrated that AuNPs can impede tRNA binding towards the ribosome, resulting in protein synthesis blockade [158, 159]. Furthermore, it is thought that the large surface area elicited by small AuNPs can enhance the surface reactivity of the particles, resulting in the promotion of charge-dependent interaction between AuNPs and bacteria, disrupting the cell wall [160]. The potential of these broad non-targeted mechanisms of action was emphasized by Li et al.; cationic AuNPs were shown to be capable of inhibiting the development of a variety of bacteria, including MDR (multidrug resistance) strains and MRSA, without inducing bacterial resistance over 20 generations.
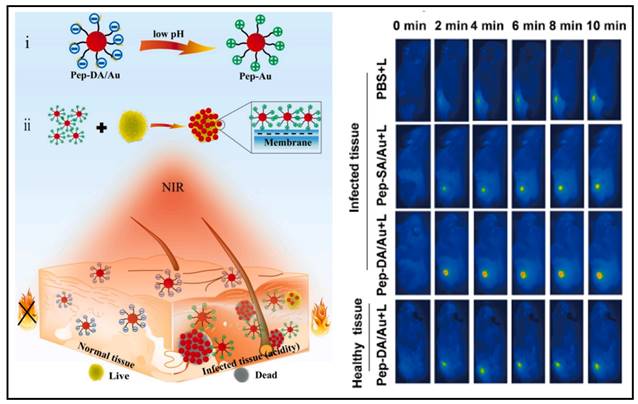
AuNPs have also been used to generate antimicrobial effects through photothermal therapy. Hu and colleagues developed a gold nanoparticle exhibiting a self-adaptive surface to target biofilms, which could inactivate the biofilm-resident MRSA upon near-infrared light irradiation [161]. Wang et al. synthesized an acidity-triggered gold nanoparticle with the charge-convertible ability (named as Pep-DA/Au) (Figure 8) [162]. In a typical physiological environment, the nanoparticles were negatively charged; however, when they reached the bacterial infection zone, the acidic environment caused DA (dimethylmaleic anhydride) to hydrolyze, exposing the amino group of Pep, causing the negatively charged NPs to become positively charged and thus accumulated onto the bacterial surface. SEM images showed that the clustered AuNPs generated heat, specifically inactivated bacteria when exposed to near-infrared light. From the infrared thermography, the abscess area injected with Pep-DA/Au exhibited a rapid temperature elevation up to > 50 ℃ under irradiation with the 808 nm laser, which was sufficient to inactivate the bacteria (Figure 8 Right). It suggested that Pep-DA/Au caused hyperthermia only in areas contaminated with bacteria and did no harm to normal organs.
Another interesting way to exploit AuNPs in antimicrobial applications is by conjugating antibiotics onto their surface. Because of the enormous surface area, physiologically relevant drug concentrations could be functionalized onto the particles, resulting in drug carrier/drug delivery systems. For example, Saha et al. functionalized AuNPs with ampicillin, streptomycin, and kanamycin [163]. These antibiotic-conjugated nanoparticles exhibited greater bactericidal activity towards three pathogenic strains, E. coli, S. aureus, and M. luteus (Micrococcus luteus), implying that AuNPs conjugated antibiotics were more potent than free drugs.
The utilization of metallic nanoparticles with broad mechanisms of action has exciting potential for the treatment of both antibiotic-resistant pathogens and preventing further development of drug resistance in the future. However, toxicity remains a significant concern, with metal particles (and by-products) linked to nephrotoxicity and immunotoxicity. Furthermore, substantial progress is needed in understanding the uptake, distribution, metabolism, excretion, and toxicology of such metallic formulations generated under appropriate production circumstances.
Metallic nanoparticle-based bacterial diagnosis strategies
Metallic nanoparticles have been investigated in the context of pathogen tagging and tracking, in addition to their potential as antibacterial treatments. Properties including a sizable particular surface area and the opportunity for external modification highlight Ag/AuNPs as potential candidates for enhancing signaling intensity, accelerating signal transduction, and providing an accurate signal readout for detecting and visualizing microorganisms [164]. An example of this is the development of mannose-Au nanodots, which have been shown in preclinical models to selectively and quantitatively label infective E. coli by virtue of mannose receptors on the bacterial membrane surface [165]. Currently, only a commercialized AuNP for diagnosis is available within the infectious diseases domain, Verigene® [166]. In approximately three hours, this in vitro test can identify the genus and species of Gram-positive microorganisms in blood, including the resistant bacterial strains. This Gram-negative blood culture nucleic acid test uses an AuNP-based hybridization mechanism to achieve amplification. Briefly, the bacterial nucleic acids are extracted from blood culture media and hybridized to specific oligonucleotides on a microarray slide. Then functionalized AuNPs further hybridize to the poly-A tail of the captured nucleic acids, followed by amplification. Finally, the scattered light of silver-amplified AuNPs is imaged and analysed by Verigene Reader.
A schematic presents AuNPs for bacterial-selective therapy. Copyright © 2020 Elsevier B.V.
Metallic nanoparticles, especially MNPs (magnetic nanoparticles) with exceptional superparamagnetic properties, have the ability to be modulated by an outside magnetic field and developed as MRI agents in the field of clinical diagnostics [167, 168]. MRI is a diagnostic technique that is well established for in vivo use and clinical adoption, delivering a high spatial resolution while avoiding hazardous radiation related to contemporary therapeutic modalities. Table 1 lists the MNP formulations that the FDA has approved as MRI contrast agents so far for clinical uses.
MNPs have now been evaluated for diagnostic purposes in a variety of inflammatory disorders. For example, in Baraki and colleagues' work, neutrophil granulocytes were firstly labelled with FeraTrackTM superparamagnetic iron oxide NPs (passively taken up through the inherent phagocytic phenotype of these cells) and then injected into S. aureus infected rats. MRI clearly demonstrated granulocyte migration to infected tissue [169]. Such an approach cannot only facilitate diagnosis of the infection site, but also offer a physiological perspective into the infection process. Prior to in vivo inoculation, Hoerr et al. used a similar technique to tag S. aureus with iron oxide nanoparticles, thereby enabling real-time surveillance of the infected tissues and, subsequently, the host inflammatory response [170].
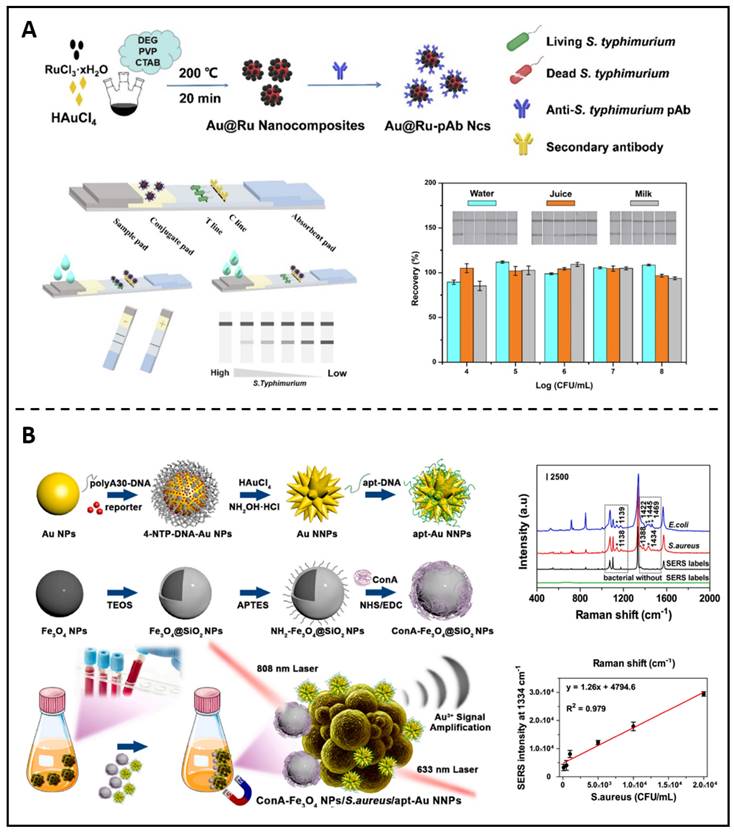
Other metallic nanoparticle-based diagnostic approaches are still in preclinical development. For example, the LFIA (lateral flow immunochromatographic assay) is a typical sensitive diagnostic technique based on antigen and antibody immune reactions because of its superior visibility, reliability, and operability. It has become a standard method in many fields, including medical assessment, environmental impact assessment, and food security requirements. Ji et al. recently described an ultra-sensitive probe based on Au@Ru Ncs linked with an anti-S. typhimurium antibody and constructed as a retinal marker for the LFIA (Figure 9A) [171]. The practicability and particularity of the presented biosensor were verified by recognizing various samples. Later investigations proved that the immunoassay method based on Au@Ru could precisely identify S. typhimurium in the target samples. SERS (Surface-enhanced Raman scattering), a potential approach based on the LSPR (localized surface plasmon resonance) of plasmonic NPs, has emerged as an attractive non-destructive option for bacterium identification. Huang et al. created a unique approach based on sandwich nanocomposites that can be used for pathogenic strains' differentiations and quantifications using SERS and ICP-MS (inductively coupled plasma mass spectrometry) (Figure 9B) [172].
Metallic nanoparticle-based anti-bacterial theranostics
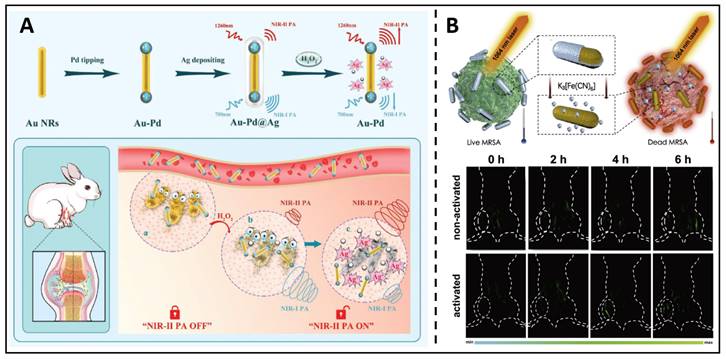
From another indirect perspective, excessive ROS generation, such as H2O2, is the pathological underpinning of inflammation caused by bacterial infection. Therefore, in situ H2O2 levels are a reliable indicator, and the real-time monitoring of H2O2 levels can be used to evaluate the progression of the disease and improve therapeutic outcomes. Therefore, theranostic NRs were developed by Ye et al., which utilised Pd-tipped gold nanorods incorporated in an Ag shell [173]. This outer silver shell shifts the maximum absorption peak of these Au-palladium nanorods. It extends to 1260 nm, allowing PA imaging at 700/1260 nm to correctly measure H2O2 in a bacterial infection mouse model (Figure 10A). Moreover, the formation of Ag ions from the selective etching of the nanorod shell by H2O2 at the infection site served as a broad-spectrum antibacterial agent, eradicating microorganisms with no resistance. Non-invasive diagnostic strategies, such as PA (photoacoustic) imaging, are always combined with the antimicrobial effects of metal NPs, such as antimicrobial ion release and phototherapeutic effects, acting as an integrated diagnosis and treatment. Mei et al. developed a therapeutic system using an activable near-infrared light source, which uses Au/Ag nanorods combined with photochemistry to realize in situ dynamic observation of MRSA infection [174]. Au/Ag NRs can be triggered rapidly, and the nanorods can constantly separate silver ions for a while after triggering, thus eliminating MRSA and gradually increasing the photothermal and PA capacity of the NIR II region. After cooperative treatment, the signal difference between tissues can be up to 20 times, which helps monitor the silver ions' release process in real-time (Figure 10B). Under 1064 nm laser irradiation, free Ag+ and near infrared-II PTT (photothermal therapy) can exert the effect of "1+1>2". After activating Au/Ag, the temperature rose dramatically from 36.0 ℃ to 51.0 ℃, successfully eliminating the MRSA; inactivated and other control groups, on the other hand, showed a limited temperature considerably underneath the thermal criterion for killing MRSA. Such alloyed nanoparticles showed great potential for an antimicrobial nanotheranostic platform.
List of magnetic nanoparticles commercialized for MRI contrast agent
| Compound | Trade Name | Type | Surface Modification | Indication |
|---|---|---|---|---|
| Ferristene | Abdoscan® | SPIO | Sulfonated styrene-DVB copolymer | Gastrointestinal |
| Ferumoxsil | Umirem® Lumirem® | SPIO | Siloxane | Gastrointestinal |
| Ferrixan | Resovist® Cliavist™ | SPIO | Dextran | Liver imaging; Cellular labelling |
| Ferumoxide | Feridex® Endorem® | SPIO | Dextran T10 | Liver imaging; Cellular labelling |
| Ferumoxytol-7228 | Feraheme® | USPIO | Carboxymethyl-dextran | Macrophage imaging; Cellular labelling |
| Feruglose | Clariscan™ | USPIO | PEGylated starch | Blood pool agent; Lymph node angiography |
SPIO: superparamagnetic iron oxide; USPIO: ultrasmall superparamagnetic iron oxide
(A) A diagram showing the manufactory process of Au@Ru-pAb nanocomposites; The detection of S. typhimurium in different samples. Copyright © 2022 Elsevier Inc. (B) A schematic presents the synthetic procedures of apt-Au NNPs and the SERS spectra of Au NNPs labels for distinguishing different bacteria strains. Copyright © 2022 Elsevier B.V.
While metallic nanoparticles are desirable candidates for theranostics due to their various broad-spectrum antimicrobial mechanisms and their own imaging properties, as well as the large-scale production capacity of metallic nanomaterials, which can be standardized in terms of quality, the in vivo distribution and metabolism of metallic nanoparticles and metallic materials has remained unclear, which leads to further difficulties in clinical trials and commercialization.
(A) A schematic presents the Au-Pd@Ag nanoprobe applied in bacterial infection. Copyright © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Schematic diagram and the NIR-II triggered PA images for in situ Ag+ release tracking. Copyright © 2020 Elsevier Ltd.
Quantum Dots
Quantum dots as antimicrobial therapeutics delivery systems
With controllable properties such as surface modification, extensive surface area, high molecular loading ability, and capability to cross physiological barriers, QDs (quantum dots), a diverse chemical semiconductor, have become promising materials in DDS for intracellular delivery of micro molecular compounds, peptides, and nucleic acids. For example, a Zn-Rif (zinc-rifampicin) complex was synthesized and loaded into transferrin-conjugated QDs to achieve an efficient DDS for improved anti-tuberculosis therapy [175]. Compared with free RIF and Zn-RIF, these drug-loaded QDs exhibited higher anti-mycobacterial activity, where it was discovered that infected dendritic cells absorbed the QDs effectively, effectively to the site of infection. Given the absence of heavy metals in the structure, ZnS QDs have a long emission lifespan, improved water solubility, and low toxicity, which allows them to be widely used in biological systems. A pH- triggered QDs drug delivery system, described by Mandani et al., was proposed for the antimicrobial application of gentamicin [176]. In this study, MSNs (mesoporous silica nanoparticles) were chosen as gentamicin carriers, and MPA-ZnS QDs were used to encapsulate the nanopores and prevent the premature release of the drug. Under acidic microbiological surroundings, MPA-ZnS QDs dissociated, MSN pores opened, and gentamicin dispersed to the outside.
Aside from the capacity to obtain high drug loading, researches have indicated that the customizable nature of semiconductor nanomaterial electrical characteristics provides an avenue for eliciting particular redox disturbances. Courtney et al. showed that small QDs, tuned through size-dependent quantum confinement (2-4 nm), generated specific LARS (light-activated redox species), to trigger pathogen cell death and eliminate various clinical MDR isolates [177]. In their theory, CdTe-2.4 has a lower band gap than other metallic oxide materials but has superior oxidation and reduction potentials to produce redox-active species in the presence of light. Reflected in the experiments, it was shown that in the system of co-incubation of MDR clinical isolates with CdTe QDs, a robust growth inhibition effect was obtained for each clinical MDR strain when there was light. In subsequent validation experiments, they found that CdTe-2.4 (non-excited state) showed no phototoxic effect in co-cultures of E. coli and HEK293T; instead, under light, CdTe-2.4 suppressed E. coli growth without causing damage to HEK293T cells, giving evidence of concept regarding the application of bacteria-specific phototoxic nanomaterials in therapeutic strategies.
Quantum dots as bacterial labelling tools and their potential as theranostic platforms
For the purpose of diagnosing bacterial infections, ensuring an early diagnosis, and monitoring the development of the illness over time, in vivo bacteria surveillance is crucial. A NIR-II surveillance technique based on fluorescent PbS QDs for real-time diagnosis of bacterial infection in vitro and in vivo was developed by Feng et al. [178]. The imaging potential of PbS QDs was investigated in a bacteria-infected nude mouse. The fluorescence signal recorded at the location of bacterial infection was typically more significant following the injection of PbS QDs than the control group. PbS QDs were found to quickly tag bacteria at the point of bacterial infection, displaying an instantaneous highly fluorescent signal.
Both Gram-positive and -negative bacterial surfaces contain charged moieties (e.g., teichoic acids and LPS, respectively) which result in a net negative charge on the bacteria's surface. Therefore, the application of cationic QDs has been examined for their ability to label pathogens [179]. For example, Arshad et al. developed CdSe/ZnS QDs to identify pathogenic V. harveyi (Vibrio harveyi) using fluorescence microscopy. The electrostatic interaction between QDs and microbial surface successfully contributed to the recognition of bacteria and bacteria-host fibroblast L929 cells [180]. In addition to this “passive” electrostatic interaction, an “active” binding approach using high-specificity peptide targeting the bacteria has also been explored. In this method, SiO2 stabilized ZnO QDs were conjugated with a potent S. aureus targeting peptide UBI29-41 and further modified with a NIR dye MPA to detect pathogenic infection. This nanoparticle was subsequently functionalized with methicillin, transforming it into a theranostic nanoparticle for identifying and treating S. aureus infection.
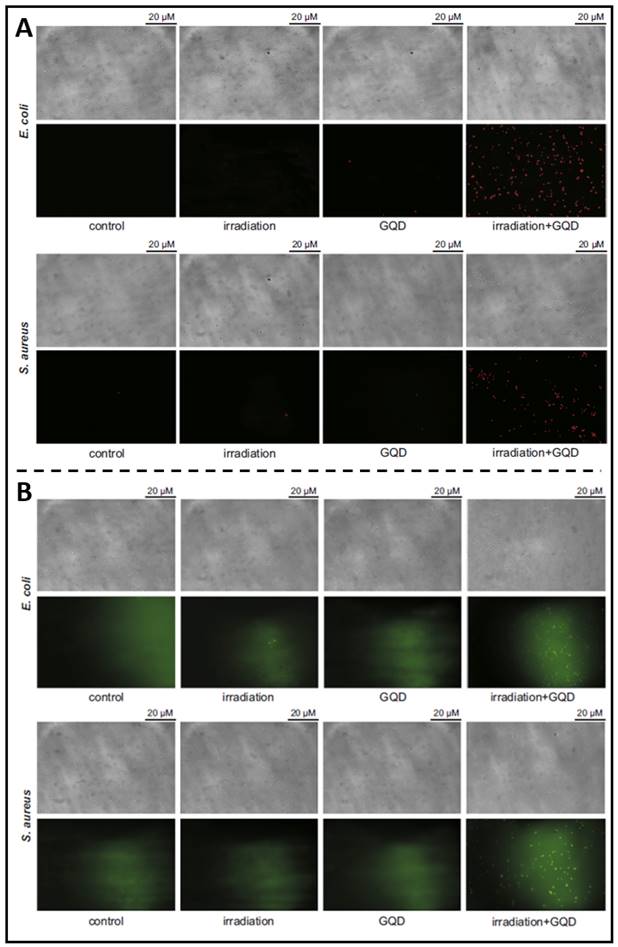
The development of carbon nanomaterials has led to a surge in interest in C-QDs (carbon quantum dots). Among carbon materials, graphene is a honeycomb-like single layer of carbon atoms. Therefore, G-QDs (graphene-based quantum dots) possess a vast surface area and outstanding thermal stability with uncomplicated production, high fluorescence activity, resistance to photobleaching, good solubility, and biocompatibility. Due to these favourable characteristics, G-QD is more suitable as a candidate material for non-toxic bioimaging or biosensing agents. For example, as reported in Ristic et al. previous article, suspended G-QDs in liquid could be excited by blue light (465-475 nm) to produce ROS through energy and electron transfer to molecular oxygen, thereby killing cancer cells [181]. They further prepared G-QDs and examined their photodynamic antibacterial effects under the irradiation of blue light on MRSA and E. coli, demonstrating photodynamical disruption on bacterial cell membrane integrity (Figure 11A) and indicating that G-QDs entered the damaged bacteria to produce additional ROS amounts (Figure 11B) [182]. Moreover, Sattarahmady et al. assessed the photothermal antibacterial action of C-QDs on the survival of methicillin-resistant and wild S. aureus in vitro [183]. PTT based on C-QDs demonstrated a targeted thermal ablation approach that induces irreversible bacterial death. These findings demonstrate the advantages of carbon-based QDs for pathogen identification and treatment, highlighting their use as candidate nanoparticulate theranostics.
In recent years, studies have shown that quantum dot materials have excellent properties as antimicrobial materials, but it is worth noting the toxicity of QDs made from inorganic materials, semiconductor materials, and carbon materials in living organisms and the potential for this toxicity being further amplified by quantum dotting, thus limiting the biological applications of these materials.
Carbon Nanotubes
Carbon nanotubes as delivery platforms and its broad-spectrum antibacterial property
When it comes to carbon nanomaterials, CNTs (carbon nanotubes) are being researched as possible antibacterial DDS platforms. CNTs with positively charged ends on their surface have been found to bind to G- bacteria, such as E. coli, preferentially [184]. Based on this, Jose Rojas-Chapana et al. developed water-dispersible multi-walled CNTs which could form temporary nano-channels across the cell wall, facilitating plasmid delivery into E. coli, paving the way for further development of targeted and controlled delivery of therapeutics towards pathogenic bacteria [184]. When CNTs come into contact with bacterium cells, they cause cellular membrane disruption, which results in high antibacterial action. For example, the fullerenes nanoparticles induce cell membrane breakdown and/or DNA cleavage due to the particle's hydrophobicity, which can easily interface with cellular membrane lipids [185]. Moreover, because of their robust ROS generation, CNTs display broad-spectrum efficacy against pathogenic organisms upon illuminating, despite medication resistance status.
Carbon nanotubes as potential deep-tissue bacteria-specific imaging materials
Due to their good Stokes' shift and flexibility of targeting agents, CNTs can be used as superior targeting materials to realize fluorescence imaging of organisms, lesions, and vascular pathways [186, 187]. Compared with the traditional dye and the first-window near-infrared imaging, as the potential fluorescence carrier of the second-window near-infrared imaging, SWCNTs (single-walled carbon nanotubes) can achieve better penetration depth in biological tissues [188]. To differentiate between F'-negative (DH5α) and F'-positive (JM109) E. coli strains, Bardhan et al. created a functional SWCNTs complex (M13-SWNTs) utilizing transgenic M13 bacteriophage [189]. After further modification, M13-SWCNT successfully combined with antimicrobial antibodies to achieve the active targeting of bacterial infection. In the detection of S. aureus muscle infection and endocarditis infection, the fluorescence signal increased by several orders of magnitude, which effectively proved its possibility in deep tissue detection.
(A) Fluorescence microscopy showing the viabilities of E. coli and S. aureus treated with G-QDs; (B) Fluorescence microscopy showing that photoexcited G-QDs induce oxidative stress in bacterial cells. Copyright © 2014 Elsevier Ltd.
Carbon nanotubes as potential antimicrobial theranostic materials
Since the SWNTs have therapeutic and imaging properties, the antimicrobial theranostic potential was examined by Kotagiri and colleagues [190]. They synthesized stealth DS (dextran sulfate) coated SWCNTs that functioned with an antibody targeting S. aureus. The DS-SWCNTs were shown to selectively target the pathogenic bacteria, as well as non-invasively purge the pathogens after NIR laser irradiation.
The three-dimensional structure formed by the unique alignment of carbon atoms provides a strong guarantee of drug delivery, while the photothermal and imaging properties generated by the special alignment make carbon nanotubes a powerful potential for theranostics materials.
Metal Organic Frameworks and Covalent Organic Frameworks
Metal organic frameworks (MOFs) and covalent organic frameworks (COFs) are innovative medically relevant nanomaterials that have lately piqued the curiosity of researchers worldwide. MOFs and COFs have been proposed for a variety of applications, including isolation, catalysis, sensors, and drug carriers.
MOFs and COFs as potential antimicrobial theranostics based on deliver properties
They are appropriate for designing as nanocarriers because of their qualities, such as vast surface areas, biodegradable and biocompatible architectures, and remarkable possibilities, such as cell-specific targeted delivery and transportation across obstacles. In addition, due to their high porosity, MOFs and COFs exhibit remarkably high loading capacity when used as carriers for antimicrobial drug delivery platforms (Table 2).
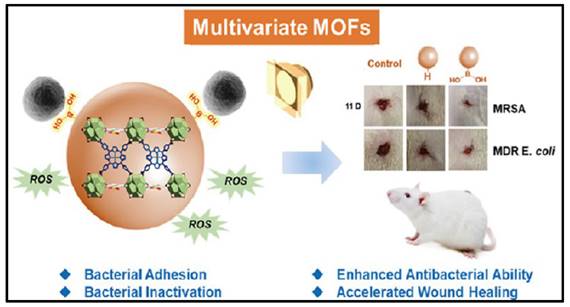
Porphyrins and metalloporphyrin dyes can be activated by visible light in the presence of O2 to generate O2- via photocatalysis. When such photosensitizers are solubilized with the substrate to be oxidized, the reactive oxygen produced is found for many biological applications. MOFs are insoluble in solvents, and when they are stabilized as suspensions, they can adsorb large amounts of molecular oxygen and efficiently produce singlet oxygen in a heterogeneous process. MOFs appear to be suitable candidates for such applications as a result of their extensive surface region and high gas adsorption capability. For example, Chen et al. induced photosensitized porphyrin Cu(II) into Zr-MOF and surface modified with bacterial-binding boronic acid ligand (Figure 12) [208]. TCPPCu-BBDC has been shown to successfully suppress the growth of four different bacterial strains. The colony counts of G+/- bacteria, and their MDR strains, were significantly reduced after 30 minutes of light exposure. In a rat dorsal skin injury model, during the same time span, the TCPPCu-BBDC-treated group with light irradiation outperformed the control group in wound healing. As efficient photosensitizers, COF variants with reticular structures that rely on covalent bonding instead of ligand interactions have recently been created. There are also examples of COFs with excellent photodynamic properties for the inactivation of microbes. Hynek et al. reported porphyrin-based COFs with three-dimensional and diamond-like structures [209]. The photophysical and photochemical characteristics of the three-dimensional COFs revealed that they were effective in producing O2 with visible light irradiation and had robust antimicrobial properties against P. aeruginosa and E. faecalis (Enterococcus faecalis) biofilms. Zhang et al. investigated the utilization of a boron-based COF for antibacterial activity [210]. The results demonstrated that COF-1, the first boron oxygen compound, could generate considerable quantities of ROS when exposed to visible light and had strong antimicrobial activities against E. coli and S. aureus. Based to the plate coating findings, all bacteria were eradicated after 120 minutes of co-incubation with COF-1 under daylight. Notably, photodynamic MOFs and COFs are powerful against G+/- bacterial strains via the "delivery" of reactive oxygen species.
Carriers, Antibiotics, and Loading Capacities of MOF/COF-based composites as conventional drug delivery platforms in Antimicrobial Applications.
| Carrier | Antibiotic | Loading capacity | REF. |
|---|---|---|---|
| MOFs | |||
| ZIF-8 | Ceftazidime | 10.9% DLE | [191] |
| ZIF-8 | Chloramphenicol | 56.8% DLE | [192] |
| ZIF-8 | Tetracycline | 31.2 μg/mg | [193] |
| ZIF-8 | Physcion | 88.72% DLE | [194] |
| ZIF-8 | Ciprofloxacin | 21% DLE | [195] |
| ZIF-8 | Vancomycin | 24% DLE | [196] |
| Zn-MOF | Amoxicillin | 60.15% EE | [197] |
| Zinc-based MOF-5 | Metronidazole | 539.33 mg/g | [198] |
| Zn2(bdc)2(dabco) MOF | Gentamicin | - | [199] |
| UiO-66 MOF | Fosfomycin | - | [200] |
| UiO-66 MOF | Ciprofloxacin | 84% DLE | [201] |
| MOF-53(Fe) | Vancomycin | 20% DLE | [202] |
| chitosan coated MOF-53(Fe) | Vancomycin | 9.87% DLE | [203] |
| γ-cyclodextrin-MOF | Florfenicol & Enrofloxacin | 42.25 mg/g | [204] |
| nanoMOFs | Amoxicillin | 36% DLE | [205] |
| COFs | |||
| Pyridine-3-boronic acid COF | Trimethoprim | - | [206] |
| ENR-FM-COF-TPU | Enrofloxacin | - | [207] |
DLE: Drug Loading Efficiency; EE: Encapsulation Efficiency
A schematic presents the MOFs with bacterial-binding abilities. Copyright © 2022 American Chemical Society.
MOFs as potential antimicrobial platforms based on ligand component releasing
It is worth noting that MOFs have been underwent substantial research as reservoirs for antimicrobial agents in the area of controlled drug release. Most MOFs with moderate or weak coordination bonds are unstable in an aqueous environment and several factors in the environment (e.g. pH) can contribute to the collapse of the MOFs structure and release of components. As the basic framework element of the MOF structures, metallic ions and organic monomers are slowly released as the MOFs decompose, exhibiting active antibacterial activity.
Metallic ions, such as Ag+, Zn2+ and Co2+, exert remarkable antibacterial activities by influencing ion channels in the bacterial cell membrane and disrupting proteins and enzymes required for bacterial metabolism. This part has been discussed in the previous section and will not be repeated here. As for the other component, some antimicrobial organic monomers cause fragmentation of bacterial DNA through binding to inorganic salt ions (e.g. calcium and magnesium ions) within the bacteria, as well as through the production of ROS. As the MOFs decompose, these organic antimicrobial monomers are slowly released, thus exhibiting antimicrobial activity. For example, Tamames-Tabar et al. reported that a Zn azelate MOF named BioMIL-5 containing the azelaic acid ligand showed synergistic inhibition of S. epidermidis after decomposition [211]. Restrepo et al. reported that a Zn-based MOF containing an antimicrobial 4-hzba (4-hydrazinyl) benzoate ligand demonstrated notable inhibition of the growth and metabolic functions of S. aureus [212]. According to their report, in the degraded composition of this MOF, the Zn2+, which is supposed to have an inhibitory effect, did not exhibit antimicrobial activity due to levels that were lower than the effective inhibitory concentration, but the other ligand, 4-hzba, exerted a remarkable antimicrobial effect.
The photosensitive monomers in the structures of COFs and MOFs, and the porous three-dimensional structures they formed, as well as the antimicrobial metallic ions and organic linkers in their component, make them highly suitable drug currieries with multi-synergistic potentials, being an emerging and promising nanomaterial for theranostics. Although still in their early stages, many teams have created MRI-trackable MOF delivery systems, MRI-trackable photothermal therapy-based drug delivery systems, and core-shell theranostic MOFs. In a nutshell, COFs and MOFs have enormous biomedical promise. These nanocarriers have the potential to usher in an age of targeted drug delivery, precise detection, and theranostic uses.
Nanostructured Antibiotic/Dye Self-assembly
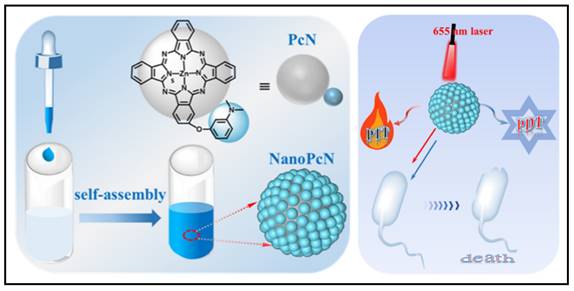
To combat issues with bacterial infections, researchers have been working to find new antimicrobial compounds, synthesize new materials, and search new approaches. One of the hottest areas of chemistry during the decade is supramolecular chemistry, which focuses on self-assemble systems produced by non-covalent electrostatic, hydrophobic bonding, hydrogen bonding, metal-ligand coordination, van der Waals interactions, and π-π stacking interactions [213]. These highly organized nano-scaled supramolecular biomaterials have the potential to create novel theranostics for the treatment of bacteria by combining therapeutics and diagnostics. For example, Xie et al. presented a new series of pure theranostic nanomedicines based on ciprofloxacin, in which they linked ciprofloxacin molecules to perfluoroaryl and aryl moieties [214]. These conjugated propeller-like ciprofloxacin derivatives, once precipitated into aqueous solution, were easily self-assembled into stable nanoaggregates, which converted these derivative compounds into activated AIE (aggregation-induced emission) luminophores, which were successfully exploited for bioimaging of bacteria; simultaneously, these aggregated ciprofloxacin nanostructures demonstrated strengthened antibacterial activities against both susceptible and drug-resistant E. coli. Similar strategies have been adapted to photosensitive nanomaterials for aPDT (antimicrobial photodynamic therapy) and aPTT (antimicrobial photothermal therapy). For example, Li et al. synthesized a novel compound 2,4,6-tris-(N,N-dimethylaminomethyl)phenoxy substituted zinc(II) phthalocyanine, named PcA [215]. In aqueous solution, PcA undergoes self-assembly to form a nanostructured phthalocyanine, called NanoPcA, which can undergo highly productive ROS generation through the Type I photoreaction, thereby exhibiting potent antibacterial activities. Based on the same conception, recently, Wang and colleagues have proposed another nanostructured modified zinc(II) phthalocyanine, called PcN (Figure 13) [216]. Self-assembled NanoPcN exhibited remarkable bimodal antimicrobial properties by yielding massive ROS and elevating temperatures upon irradiation.
Fabrication and characterization of NanoPcN; Schematic illustration of the antibacterial mechanism of NanoPcN. Copyright © 2022 American Chemical Society.
The potential for treating bacterial infections with supramolecular antimicrobial agents, such as those based on cationic chemical compounds, photodynamic, and photothermal compounds, is enormous. These materials can serve as multifunctional platforms for the unique demands of antimicrobial treatment owing to the incorporation of versatile elements into them. However, the preclinical development of nearly all of these supramolecular antibacterial compounds is still in its early phases. The effectiveness of these compounds against clinically latent bacteria remains to be thoroughly evaluated.
Challenges and Prospects
Challenges in large-scale manufacturing
Some important reasons for the challenges in clinical translation of nano-formulations are the structural and physicochemical complexity of the nano-formulations, and the need for a set of manufacturing methods and platforms that can produce scalable nano-formulations and that can consistently and reproducibly yield the same qualitative level of nano-formulation in large quantities. Several basic nanomedicine platforms (liposomes) have been successfully produced at industrial scale utilizing techniques that have been developed that do not require complicated manufacturing procedures or the usage of organic solvents. However, challenges arise when nano-formulations become more complex. Examples include surface alterations that include coatings or ligands, various targeting components, or by encapsulating several therapeutic compounds. These require the incorporation of several ingredients into a single nanocarrier, so numerous phases in the manufacturing process are required, which eventually causes issues with cGMP (current good manufacturing practice), increases production costs and makes QA and QC (quality assurance and quality control) assessment more difficult. Characterisation and validation of more complex nano-formulations are likely to be extremely difficult because of the overwhelming amount of characteristics that need to be addressed, including size, morphology, surface charge, drug encapsulation efficiency, surface coating efficiency, and surface ligands density. Moreover, the batch-to-batch stability of nano-formulations during the production process might lead to considerable differences in their physicochemical characteristics, pharmacokinetic parameters, and pharmacodynamic interactions.
Biological challenges and perspectives
As previously noted, nanoparticles have demonstrated the most significant therapeutic and diagnostic uses in the battle against bacterial infections by overcoming the pharmacokinetic limits of traditional drug treatments or by merging new technologies and modalities. However, nanoparticle-based drug delivery systems all face a series of problems posed by complex biological barriers that limit the bioavailability and therapeutic efficacy of nanodrugs at specific sites, such as the MPS (mononuclear phagocyte system), nonspecific distribution, cellular internalization, and drug efflux pumps [217-219]. The MPS, a phagocytic system mostly composed of monocytes and macrophages in the spleen, lymph nodes, and liver, is one of the most critical components of the nanodrug delivery system [220]. The MPS eliminates nanoparticles by phagocytosis, which starts with the opsonization of the nanomaterials, which involves the adhesion of serum proteins. Following protein adsorption, nanoparticles are quickly identified by phagocytes and bind to receptors located on the cell surface, before being ingested, transported to the phagosome, merged with lysosomes, and eventually destroyed.
Consequently, in the case of traditional bacterial infections, several tactics have been used in developing nanoparticles to make them less sensitive to being detected by MPS to perpetuate the retention duration at the infection site. The most straightforward and extensively used method is to modify the surface with hydrophilic polymers, like coating nanoparticles with PEG, to prevent the nanoparticles from aggregation and to hinder protein adsorption and removal by MPS for long-term circulation in vivo. Another strategy is to use self-identifying markers to conceal the surface of alien nanoparticles. For example, researchers have used sialic acid from certain pathogens to cover nanoparticles with the expectation that these particles will promote inhibition of immune activation, allowing for increased blood circulation and decreased MPS absorption [221]. For example, CD47 peptide is a potential “do not eat-me” marker of “self”, which is observed on certain pathogens that they are able to over-express CD47 on some viruses such as smallpox to evade recognition, strategies based on CD47 marker to modification NPs have been widely investigated in cancer research [222-226]. Then based on such phenomena, in the case of bacteraemia and sepsis, could we explore more natural markers and use them as surface modification ligands to reduce phagocytosis of the organism and substantially increase the cycle time in vivo?
However, as for specific intracellular pathogens, such as M. tuberculosis, S. aureus, and K. pneumoniae (Klebsiella pneumoniae), they are supposed to be recognized, opsonized, and eliminated by the MPS, but developed the mechanisms to keep alive in the MPS, which turns out that we can promisingly employ the opsonisation mechanism onto nanoparticles surface modification and use the difference in marker on the surface of infected MPS cells to explore targeted drug delivery strategies for this class of intracellular diseases.
Furthermore, new biotechnologies for eradicating harmful microorganisms are emerging due to the fast emergence of antibiotic resistance and the lengthy research period for an innovative drug. For example, in 2016, Lehar et al. developed an AAC (antibody-antibiotic conjugation) against S. aureus (THIOMAB™) that rapidly identifies and labels S. aureus circulating in the bloodstream [227]. The labelled bacterial-AAC complex is internalized by ADCP (antibody-dependent cellular phagocytosis), which releases a potent antibiotic to inactivate the intracellular pathogen through lysosomal protease-mediated cleavage. Following this work, ACNPs (antibody-conjugated nanoparticles) and genetically engineered AMVs (antibody-presented membrane vesicles) might represent a relatively novel class of strategies based on the combination of bacterial-specific antibodies and nano-DDS to enhance antimicrobial delivery and efficacy. On the one hand, the efficient drug-loading capacity of nanoparticles and surface-enriched antibodies may offer a relatively high DAR (drug-to-antibody ratio) to reduce the toxicity induced by the low DAR of conventional AACs and may enhance the ADCP-mediated internalization between nanoparticles and target infected cells due to the antibody enrichment effect; on the other hand, compared to simple AACs, thanks to the long-standing development of nano-DDS development, antibody nano-DDS can achieve a more precisely controlled release through in vivo microenvironmental stimulations and in vitro stimulations. Furthermore, in combination with some of the novel antimicrobial ingredients mentioned in the previous sections, such as photosensitizers and sonosensitizers, specific aPDTs targeting certain specific strains of bacteria can be achieved. Another example is the CRISPR-Cas (clustered regularly interspaced short palindromic repeats - CRISPR associated) technology, which has received much attention; by targeting specific bacterial DNA sequences, the RNA-guided nuclease Cas9 is able to destroy DNA by chromosomal cleavage, thus altering the resistance genes of drug-resistant strains and thus eliminating the pathogens. However, the lack of an efficient delivery method to turn this excellent treatment technique into an appropriate antibacterial agent significantly limits its efficacy. In combination with antibody nanodrug delivery systems, we hypothesized that this obstacle could be overcome. Cas9 as an antimicrobial agent might be directly targeted to the target pathogenic bacteria using antibody nano-DDS, leading to an extraordinarily sufficient and specific therapeutic strategy to tackle multidrug-resistant microorganisms.
Conclusion
In this review, we have outlined the most current developments and research into therapeutic, diagnostic, and theranostic nanomedicines for treating bacterial infections. Challenges for the clinical adoption of such technologies remain, but advances in the production of nanomedicines and a better knowledge of their in vivo behaviours and biodistribution will likely make this approach to drug delivery a fascinating proposition for the treatment of problematic pathogens in the future.
Acknowledgements
This work was supported by the Major State Basic Research Development Program of China (Grant No. 2017YFA0205201), the National Natural Science Foundation of China (Grant Nos. 81925019, U22A20333, and U1705281), the Fundamental Research Funds for the Central Universities (2020Y4003 and 20720200019), and the China Postdoctoral Science Foundation (Grant No. 2022M712667).
ORICD
Lai Jiang: https://orcid.org/0000-0002-1658-865X.
Linyu Ding: https://orcid.org/0009-0001-1474-4436.
Gang Liu: https://orcid.org/0000-0003-2613-7286.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat Rev Microbiol. 2017;15:453-64
2. Didelot X, Walker AS, Peto TE, Crook DW, Wilson DJ. Within-host evolution of bacterial pathogens. Nat Rev Microbiol. 2016;14:150-62
3. Larsson DGJ, Flach CF. Antibiotic resistance in the environment. Nat Rev Microbiol. 2022;20:257-69
4. Varela MF, Stephen J, Lekshmi M, Ojha M, Wenzel N, Sanford LM. et al. Bacterial Resistance to Antimicrobial Agents. Antibiotics (Basel). 2021;10:593
5. Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25-64
6. Maxson T, Mitchell DA. Targeted treatment for bacterial infections: prospects for pathogen-specific antibiotics coupled with rapid diagnostics. Tetrahedron. 2016;72:3609-24
7. Lai CKC, Ng RWY, Leung SSY, Hui M, Ip M. Overcoming the rising incidence and evolving mechanisms of antibiotic resistance by novel drug delivery approaches - An overview. Adv Drug Deliv Rev. 2022;181:114078
8. Aggarwal D, Kumar V, Sharma S. Drug-loaded biomaterials for orthopedic applications: A review. J Control Release. 2022;344:113-33
9. Briones E, Colino CI, Lanao JM. Delivery systems to increase the selectivity of antibiotics in phagocytic cells. J Control Release. 2008;125:210-27
10. Pinto-Alphandary H, Andremont A, Couvreur P. Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int J Antimicrob Agents. 2000;13:155-68
11. Eleraky NE, Allam A, Hassan SB, Omar MM. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics. 2020;12:142
12. Makabenta JMV, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19:23-36
13. Gao W, Chen Y, Zhang Y, Zhang Q, Zhang L. Nanoparticle-based local antimicrobial drug delivery. Adv Drug Deliv Rev. 2018;127:46-57
14. Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851
15. Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: Advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154-155:102-22
16. Filipczak N, Pan J, Yalamarty SSK, Torchilin VP. Recent advancements in liposome technology. Adv Drug Deliv Rev. 2020;156:4-22
17. Crommelin DJA, van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release. 2020;318:256-63
18. Santos A, Veiga F, Figueiras A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials (Basel). 2019;13:65
19. Dias AP, da Silva Santos S, da Silva JV, Parise-Filho R, Igne Ferreira E, Seoud OE. et al. Dendrimers in the context of nanomedicine. Int J Pharm. 2020;573:118814
20. Surekha B, Kommana NS, Dubey SK, Kumar AVP, Shukla R, Kesharwani P. PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloids Surf B Biointerfaces. 2021;204:111837
21. Choi SJ, McClements DJ. Nanoemulsions as delivery systems for lipophilic nutraceuticals: strategies for improving their formulation, stability, functionality and bioavailability. Food Sci Biotechnol. 2020;29:149-68
22. A N, Kovooru L, Behera AK, Kumar KPP, Srivastava P. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv Colloid Interface Sci. 2021;287:102318
23. Das SS, Bharadwaj P, Bilal M, Barani M, Rahdar A, Taboada P. et al. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers (Basel). 2020;12:1397
24. Hwang D, Ramsey JD, Kabanov AV. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv Drug Deliv Rev. 2020;156:80-118
25. Avramovic N, Mandic B, Savic-Radojevic A, Simic T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics. 2020;12:298
26. Zhang W, Mehta A, Tong Z, Esser L, Voelcker NH. Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges. Adv Sci (Weinh). 2021;8:2003937
27. Zhang D, Ma XL, Gu Y, Huang H, Zhang GW. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front Chem. 2020;8:799
28. Khursheed R, Dua K, Vishwas S, Gulati M, Jha NK, Aldhafeeri GM. et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomed Pharmacother. 2022;150:112951
29. Jiang Y, Zheng W, Tran K, Kamilar E, Bariwal J, Ma H. et al. Hydrophilic nanoparticles that kill bacteria while sparing mammalian cells reveal the antibiotic role of nanostructures. Nat Commun. 2022;13:197
30. Shi J, Wang J, Liang L, Xu Z, Chen Y, Chen S. et al. Carbothermal synthesis of biochar-supported metallic silver for enhanced photocatalytic removal of methylene blue and antimicrobial efficacy. J Hazard Mater. 2021;401:123382
31. He J, Shi M, Liang Y, Guo B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem Eng J. 2020;394:124888
32. Cuadrado CF, Diaz-Barrios A, Campana KO, Romani EC, Quiroz F, Nardecchia S. et al. Broad-Spectrum Antimicrobial ZnMintPc Encapsulated in Magnetic-Nanocomposites with Graphene Oxide/MWCNTs Based on Bimodal Action of Photodynamic and Photothermal Effects. Pharmaceutics. 2022;14:705
33. Khan AA, Khan S, Khan S, Rentschler S, Laufer S, Deigner HP. Biosynthesis of iron oxide magnetic nanoparticles using clinically isolated Pseudomonas aeruginosa. Sci Rep. 2021;11:20503
34. Han H, Sohn B, Choi J, Jeon S. Recent advances in magnetic nanoparticle-based microfluidic devices for the pretreatment of pathogenic bacteria. Biomed Eng Lett. 2021;11:297-307
35. Abafogi AT, Wu T, Lee D, Lee J, Cho G, Lee LP. et al. Vancomycin-conjugated polydopamine-coated magnetic nanoparticles for molecular diagnostics of Gram-positive bacteria in whole blood. J Nanobiotechnology. 2022;20:400
36. Dahiya B, Prasad T, Singh V, Khan A, Kamra E, Mor P. et al. Diagnosis of tuberculosis by nanoparticle-based immuno-PCR assay based on mycobacterial MPT64 and CFP-10 detection. Nanomed. 2020;15:2609-24
37. Garg N, Ahmad FJ, Kar S. Unmodified Gold Nanoparticles as Diagnostic Agents for Colorimetric Detection of Mycobacterium leprae Infection. Int J Infect Dis. 2022;116:S95-S6
38. Abdou Mohamed MA, Kozlowski HN, Kim J, Zagorovsky K, Kantor M, Feld JJ. et al. Diagnosing Antibiotic Resistance Using Nucleic Acid Enzymes and Gold Nanoparticles. ACS Nano. 2021;15:9379-90
39. Storey P, Arbini AA. Bone marrow uptake of ferumoxytol: a preliminary study in healthy human subjects. J Magn Reson Imaging. 2014;39:1401-10
40. Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48:2053-108
41. Panwar N, Soehartono AM, Chan KK, Zeng S, Xu G, Qu J. et al. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem Rev. 2019;119:9559-656
42. Choi KY, Liu G, Lee S, Chen X. Theranostic nanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nanoscale. 2012;4:330-42
43. Kam BL, Teunissen JJ, Krenning EP, de Herder WW, Khan S, van Vliet EI. et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S103-12
44. Al-Shakhrah IA. Radioprotection using iodine-131 for thyroid cancer and hyperthyroidism: a review. Clin J Oncol Nurs. 2008;12:905-12
45. Griffith DE, Thomson R, Flume PA, Aksamit TR, Field SK, Addrizzo-Harris DJ. et al. Amikacin Liposome Inhalation Suspension for Refractory Mycobacterium avium Complex Lung Disease: Sustainability and Durability of Culture Conversion and Safety of Long-term Exposure. Chest. 2021;160:831-42
46. Shirley M. Amikacin Liposome Inhalation Suspension: A Review in Mycobacterium avium Complex Lung Disease. Drugs. 2019;79:555-62
47. Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607-23
48. Nazir F, Tabish TA, Tariq F, Iftikhar S, Wasim R, Shahnaz G. Stimuli-sensitive drug delivery systems for site-specific antibiotic release. Drug Discov Today. 2022;27:1698-705
49. Sang N, Jiang L, Wang Z, Zhu Y, Lin G, Li R. et al. Bacteria-targeting liposomes for enhanced delivery of cinnamaldehyde and infection management. Int J Pharm. 2022;612:121356
50. Omolo CA, Megrab NA, Kalhapure RS, Agrawal N, Jadhav M, Mocktar C. et al. Liposomes with pH responsive 'on and off' switches for targeted and intracellular delivery of antibiotics. J Liposome Res. 2021;31:45-63
51. Zhang L, Wang Y, Wang J, Wang Y, Chen A, Wang C. et al. Photon-Responsive Antibacterial Nanoplatform for Synergistic Photothermal-/Pharmaco-Therapy of Skin Infection. ACS Appl Mater Interfaces. 2019;11:300-10
52. Abu Dayyih A, Alawak M, Ayoub AM, Amin MU, Abu Dayyih W, Engelhardt K. et al. Thermosensitive liposomes encapsulating hypericin: Characterization and photodynamic efficiency. Int J Pharm. 2021;609:121195
53. Kim MS, Lee DW, Park K, Park SJ, Choi EJ, Park ES. et al. Temperature-triggered tumor-specific delivery of anticancer agents by cRGD-conjugated thermosensitive liposomes. Colloids Surf B Biointerfaces. 2014;116:17-25
54. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28-51
55. Grad P, Gedda L, Edwards K. Effect of gangliosides on structure and integrity of polyethylene glycol (PEG)-stabilized liposomes. J Colloid Interface Sci. 2020;578:281-9
56. Hirai M, Sato S, Kimura R, Hagiwara Y, Kawai-Hirai R, Ohta N. et al. Effect of protein-encapsulation on thermal structural stability of liposome composed of glycosphingolipid/cholesterol/phospholipid. J Phys Chem B. 2015;119:3398-406
57. Wang K, Yu C, Liu Y, Zhang W, Sun Y, Chen Y. Enhanced Antiatherosclerotic Efficacy of Statin-Loaded Reconstituted High-Density Lipoprotein via Ganglioside GM1 Modification. ACS Biomater Sci Eng. 2018;4:952-62
58. Zhang T, Zhou S, Hu L, Peng B, Liu Y, Luo X. et al. Polysialic acid-modifying liposomes for efficient delivery of epirubicin, in-vitro characterization and in-vivo evaluation. Int J Pharm. 2016;515:449-59
59. She Z, Zhang T, Wang X, Li X, Song Y, Cheng X. et al. The anticancer efficacy of pixantrone-loaded liposomes decorated with sialic acid-octadecylamine conjugate. Biomaterials. 2014;35:5216-25
60. Natsaridis E, Gkartziou F, Mourtas S, Stuart MCA, Kolonitsiou F, Klepetsanis P. et al. Moxifloxacin Liposomes: Effect of Liposome Preparation Method on Physicochemical Properties and Antimicrobial Activity against Staphylococcus epidermidis. Pharmaceutics. 2022;14:370
61. Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem. 2017;409:5779-87
62. Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K. et al. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Am Chem Soc. 2011;133:4132-9
63. Niu NK, Yin JJ, Yang YX, Wang ZL, Zhou ZW, He ZX. et al. Novel targeting of PEGylated liposomes for codelivery of TGF-beta1 siRNA and four antitubercular drugs to human macrophages for the treatment of mycobacterial infection: a quantitative proteomic study. Drug Des Devel Ther. 2015;9:4441-70
64. Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151:220-8
65. Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12:492-503
66. Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci. 2010;123:1183-9
67. Kim B, Pang HB, Kang J, Park JH, Ruoslahti E, Sailor MJ. Immunogene therapy with fusogenic nanoparticles modulates macrophage response to Staphylococcus aureus. Nat Commun. 2018;9:1969
68. Vieira ACC, Chaves LL, Pinheiro M, Lima SC, Neto PJR, Ferreira D. et al. Lipid nanoparticles coated with chitosan using a one-step association method to target rifampicin to alveolar macrophages. Carbohydr Polym. 2021;252:116978
69. Pandey R, Khuller GK. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis (Edinb). 2005;85:227-34
70. Salmanoglu E, Kim S, Thakur ML. Currently Available Radiopharmaceuticals for Imaging Infection and the Holy Grail. Semin Nucl Med. 2018;48:86-99
71. Kocaoglu O, Carlson EE. Progress and prospects for small-molecule probes of bacterial imaging. Nat Chem Biol. 2016;12:472-8
72. Zhou J, Duan M, Huang D, Shao H, Zhou Y, Fan Y. Label-free visible colorimetric biosensor for detection of multiple pathogenic bacteria based on engineered polydiacetylene liposomes. J Colloid Interface Sci. 2022;606:1684-94
73. Jones MN, Kaszuba M, Reboiras MD, Lyle IG, Hill KJ, Song Y-H. et al. The targeting of phospholipid liposomes to bacteria. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1994;1196:57-64
74. Tan J, Ferdinandus, Xing B, Lee C-L. Functionalization of thermoswitchable liposomes for rapid detection of Gram-negative bacteria. Sensors and Actuators B: Chemical. 2022;367:132123
75. Song X, Shukla S, Oh S, Kim Y, Kim M. Development of fluorescence-based liposome immunoassay for detection of Cronobacter muytjensii in pure culture. Curr Microbiol. 2015;70:246-52
76. Elahipanah S, Radmanesh P, Luo W, O'Brien PJ, Rogozhnikov D, Yousaf MN. Rewiring Gram-Negative Bacteria Cell Surfaces with Bio-Orthogonal Chemistry via Liposome Fusion. Bioconjug Chem. 2016;27:1082-9
77. Sum R, Swaminathan M, Rastogi SK, Piloto O, Cheong I. Beta-Hemolytic Bacteria Selectively Trigger Liposome Lysis, Enabling Rapid and Accurate Pathogen Detection. ACS Sens. 2017;2:1441-51
78. Kaul A, Chaturvedi S, Attri A, Kalra M, Mishra AK. Targeted theranostic liposomes: rifampicin and ofloxacin loaded pegylated liposomes for theranostic application in mycobacterial infections. RSC Adv. 2016;6:28919-26
79. Clausen AS, Ostergaard DE, Holmberg P, Henriksen JR, Tham J, Damborg PP. et al. Quantitative determination of (64)Cu-liposome accumulation at inflammatory and infectious sites: Potential for future theranostic system. J Control Release. 2020;327:737-46
80. Pang X, Xiao Q, Cheng Y, Ren E, Lian L, Zhang Y. et al. Bacteria-Responsive Nanoliposomes as Smart Sonotheranostics for Multidrug Resistant Bacterial Infections. ACS Nano. 2019;13:2427-38
81. Nikolova MP, Kumar EM, Chavali MS. Updates on Responsive Drug Delivery Based on Liposome Vehicles for Cancer Treatment. Pharmaceutics. 2022 p. 2195
82. Liang W, Dong Y, Shao R, Zhang S, Wu X, Huang X. et al. Application of nanoparticles in drug delivery for the treatment of osteosarcoma: focussing on the liposomes. J Drug Target. 2022;30:463-75
83. Shaw TK, Paul P. Recent Approaches and Success of Liposome-Based Nano Drug Carriers for the Treatment of Brain Tumor. Curr Drug Deliv. 2022;19:815-29
84. Onugwu AL, Nwagwu CS, Onugwu OS, Echezona AC, Agbo CP, Ihim SA. et al. Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. J Control Release. 2023;354:465-88
85. Kailasam V, Cheruvu SS, Malani M, Sai Kameswari SM, Kesharwani P, Nirmal J. Recent advances in novel formulation approaches for tacrolimus delivery in treatment of various ocular diseases. J Drug Delivery Sci Technol. 2022;78:103945
86. Khot KB, Gopan G, Bandiwadekar A, Jose J. Current advancements related to phytobioactive compounds based liposomal delivery for neurodegenerative diseases. Ageing Res Rev. 2023;83:101806
87. Hernandez C, Shukla S. Liposome based drug delivery as a potential treatment option for Alzheimer's disease. Neural Regener Res. 2022;17:1190-8
88. Rashki S, Asgarpour K, Tarrahimofrad H, Hashemipour M, Ebrahimi MS, Fathizadeh H. et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr Polym. 2021;251:117108
89. Egorov AR, Khubiev O, Rubanik VV, Rubanik VV Jr, Lobanov NN, Savilov SV. et al. The first selenium containing chitin and chitosan derivatives: Combined synthetic, catalytic and biological studies. Int J Biol Macromol. 2022;209:2175-87
90. Liu L, Ma Q, Wang S, Gao Y, Zhu C, Zhao W. et al. Efficient epidermal delivery of antibiotics by self-assembled lecithin/chitosan nanoparticles for enhanced therapy on epidermal bacterial infections. Int J Biol Macromol. 2022;218:568-79
91. Fu Y, Zhang J, Wang Y, Li J, Bao J, Xu X. et al. Reduced polydopamine nanoparticles incorporated oxidized dextran/chitosan hybrid hydrogels with enhanced antioxidative and antibacterial properties for accelerated wound healing. Carbohydr Polym. 2021;257:117598
92. Bettencourt AF, Tome C, Oliveira T, Martin V, Santos C, Goncalves L. et al. Exploring the potential of chitosan-based particles as delivery-carriers for promising antimicrobial glycolipid biosurfactants. Carbohydr Polym. 2021;254:117433
93. Bourgat Y, Mikolai C, Stiesch M, Klahn P, Menzel H. Enzyme-Responsive Nanoparticles and Coatings Made from Alginate/Peptide Ciprofloxacin Conjugates as Drug Release System. Antibiotics (Basel). 2021;10:653
94. Hazime N, Belguesmia Y, Barras A, Amiche M, Boukherroub R, Drider D. Enhanced Antibacterial Activity of Dermaseptin through Its Immobilization on Alginate Nanoparticles-Effects of Menthol and Lactic Acid on Its Potentialization. Antibiotics (Basel). 2022;11:787
95. Scutera S, Argenziano M, Sparti R, Bessone F, Bianco G, Bastiancich C. et al. Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles. Antibiotics (Basel). 2021;10:57
96. Han Y, Liu Y, Ma X, Shen A, Liu Y, Weeranoppanant N. et al. Antibiotics armed neutrophils as a potential therapy for brain fungal infection caused by chemotherapy-induced neutropenia. Biomaterials. 2021;274:120849
97. Bachmaier K, Stuart A, Singh A, Mukhopadhyay A, Chakraborty S, Hong Z. et al. Albumin Nanoparticle Endocytosing Subset of Neutrophils for Precision Therapeutic Targeting of Inflammatory Tissue Injury. ACS Nano. 2022;16:4084-101
98. Zaki NM, Hafez MM. Enhanced Antibacterial Effect of Ceftriaxone Sodium-Loaded Chitosan Nanoparticles Against Intracellular Salmonella typhimurium. AAPS PharmSciTech. 2012;13:411-21
99. Deacon J, Abdelghany SM, Quinn DJ, Schmid D, Megaw J, Donnelly RF. et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: formulation, characterisation and functionalisation with dornase alfa (DNase). J Control Release. 2015;198:55-61
100. Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505-22
101. Rubey KM, Brenner JS. Nanomedicine to fight infectious disease. Adv Drug Deliv Rev. 2021;179:113996
102. Zhang J, Sun H, Gao C, Wang Y, Cheng X, Yang Y. et al. Development of a chitosan-modified PLGA nanoparticle vaccine for protection against Escherichia coli K1 caused meningitis in mice. J Nanobiotechnology. 2021;19:69
103. Raza A, Miles JA, Sime FB, Ross BP, Roberts JA, Popat A. et al. PLGA encapsulated gamma-cyclodextrin-meropenem inclusion complex formulation for oral delivery. Int J Pharm. 2021;597:120280
104. Javaid S, Ahmad NM, Mahmood A, Nasir H, Iqbal M, Ahmad N. et al. Cefotaxime Loaded Polycaprolactone Based Polymeric Nanoparticles with Antifouling Properties for In-Vitro Drug Release Applications. Polymers (Basel). 2021;13:2180
105. Paudel S, Pena-Bahamonde J, Shakiba S, Astete CE, Louie SM, Sabliov CM. et al. Prevention of infection caused by enteropathogenic E. coli O157:H7 in intestinal cells using enrofloxacin entrapped in polymer based nanocarriers. J Hazard Mater. 2021;414:125454
106. Weng L, Wu L, Guo R, Ye J, Liang W, Wu W. et al. Lactobacillus cell envelope-coated nanoparticles for antibiotic delivery against cariogenic biofilm and dental caries. J Nanobiotechnology. 2022;20:356
107. Jiang L, Greene MK, Insua JL, Pessoa JS, Small DM, Smyth P. et al. Clearance of intracellular Klebsiella pneumoniae infection using gentamicin-loaded nanoparticles. J Control Release. 2018;279:316-25
108. Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface Charge-Switching Polymeric Nanoparticles for Bacterial Cell Wall-Targeted Delivery of Antibiotics. ACS Nano. 2012;6:4279-87
109. Li TT, Sun L, Zhong Y, Peng HK, Ren HT, Zhang Y. et al. Silk fibroin/polycaprolactone-polyvinyl alcohol directional moisture transport composite film loaded with antibacterial drug-loading microspheres for wound dressing materials. Int J Biol Macromol. 2022;207:580-91
110. Taresco V, Tulini I, Francolini I, Piozzi A. Polyglycerol Adipate-Grafted Polycaprolactone Nanoparticles as Carriers for the Antimicrobial Compound Usnic Acid. Int J Mol Sci. 2022;23:14339
111. Kalita S, Devi B, Kandimalla R, Sharma KK, Sharma A, Kalita K. et al. Chloramphenicol encapsulated in poly-ε-caprolactone-pluronic composite: nanoparticles for treatment of MRSA-infected burn wounds. Int J Nanomedicine. 2015;10:2971-84
112. Chu L, Gao H, Cheng T, Zhang Y, Liu J, Huang F. et al. A charge-adaptive nanosystem for prolonged and enhanced in vivo antibiotic delivery. Chem Commun (Camb). 2016;52:6265-8
113. Falanga A, Del Genio V, Galdiero S. Peptides and Dendrimers: How to Combat Viral and Bacterial Infections. Pharmaceutics. 2021;13:101
114. Ortega MÁ, Guzmán Merino A, Fraile-Martínez O, Recio-Ruiz J, Pekarek L, G Guijarro L. et al. Dendrimers and Dendritic Materials: From Laboratory to Medical Practice in Infectious Diseases. Pharmaceutics. 2020;12:874
115. Mignani S, Tripathi RP, Chen L, Caminade A-M, Shi X, Majoral J-P. New Ways to Treat Tuberculosis Using Dendrimers as Nanocarriers. Pharmaceutics. 2018;10:105
116. Wu LP, Ficker M, Christensen JB, Trohopoulos PN, Moghimi SM. Dendrimers in Medicine: Therapeutic Concepts and Pharmaceutical Challenges. Bioconjug Chem. 2015;26:1198-211
117. Yang H, Lopina ST. Penicillin V-conjugated PEG-PAMAM star polymers. J Biomater Sci Polym Ed. 2003;14:1043-56
118. Nguyen T-K, Selvanayagam R, Ho KKK, Chen R, Kutty SK, Rice SA. et al. Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem Sci. 2016;7:1016-27
119. Liu P, Xu LQ, Xu G, Pranantyo D, Neoh K-G, Kang E-T. pH-Sensitive Theranostic Nanoparticles for Targeting Bacteria with Fluorescence Imaging and Dual-Modal Antimicrobial Therapy. ACS Appl Nano Mater. 2018;1:6187-96
120. Ghosh R, Malhotra M, Sathe RR, Jayakannan M. Biodegradable Polymer Theranostic Fluorescent Nanoprobe for Direct Visualization and Quantitative Determination of Antimicrobial Activity. Biomacromolecules. 2020;21:2896-912
121. Horev B, Klein MI, Hwang G, Li Y, Kim D, Koo H. et al. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano. 2015;9:2390-404
122. Enshaei H, Puiggali-Jou A, Del Valle LJ, Turon P, Saperas N, Aleman C. Nanotheranostic Interface Based on Antibiotic-Loaded Conducting Polymer Nanoparticles for Real-Time Monitoring of Bacterial Growth Inhibition. Adv Healthc Mater. 2021;10:e2001636
123. Gan BH, Siriwardena TN, Javor S, Darbre T, Reymond JL. Fluorescence Imaging of Bacterial Killing by Antimicrobial Peptide Dendrimer G3KL. ACS Infect Dis. 2019;5:2164-73
124. Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front Microbiol. 2016;7:1831
125. Duran N, Duran M, de Jesus MB, Seabra AB, Favaro WJ, Nakazato G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. 2016;12:789-99
126. Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E, Mustafa N. et al. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials (Basel). 2020;10:1566
127. Kumar S, Basumatary IB, Sudhani HPK, Bajpai VK, Chen L, Shukla S. et al. Plant extract mediated silver nanoparticles and their applications as antimicrobials and in sustainable food packaging: A state-of-the-art review. Trends Food Sci Technol. 2021;112:651-66
128. Hashem AH, Shehabeldine AM, Ali OM, Salem SS. Synthesis of Chitosan-Based Gold Nanoparticles: Antimicrobial and Wound-Healing Activities. Polymers (Basel). 2022;14:2293
129. Li X, Robinson SM, Gupta A, Saha K, Jiang Z, Moyano DF. et al. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano. 2014;8:10682-6
130. Xu VW, Nizami MZI, Yin IX, Yu OY, Lung CYK, Chu CH. Application of Copper Nanoparticles in Dentistry. Nanomaterials (Basel). 2022;12:805
131. Rubilar O, Rai M, Tortella G, Diez MC, Seabra AB, Duran N. Biogenic nanoparticles: copper, copper oxides, copper sulphides, complex copper nanostructures and their applications. Biotechnol Lett. 2013;35:1365-75
132. Radetić M, Marković D. Nano-finishing of cellulose textile materials with copper and copper oxide nanoparticles. Cellulose. 2019;26:8971-91
133. Lee KS, Song Y, Kim CH, Kim YT, Kang T, Lee SJ. et al. Development of zinc oxide-based sub-micro pillar arrays for on-site capture and DNA detection of foodborne pathogen. J Colloid Interface Sci. 2020;563:54-61
134. Zhang H, Zhang X, Cao Q, Wu S, Wang X-Q, Peng N. et al. Facile fabrication of chitin/ZnO composite hydrogels for infected wound healing. Biomater Sci. 2022;10:5888-99
135. McGuffie MJ, Hong J, Bahng JH, Glynos E, Green PF, Kotov NA. et al. Zinc oxide nanoparticle suspensions and layer-by-layer coatings inhibit staphylococcal growth. Nanomed Nanotechnol Biol Med. 2016;12:33-42
136. Shatan AB, Patsula V, Dydowiczova A, Gunar K, Velychkivska N, Hromadkova J. et al. Cationic Polymer-Coated Magnetic Nanoparticles with Antibacterial Properties: Synthesis and In Vitro Characterization. Antibiotics (Basel). 2021;10:1077
137. Shah A, Tauseef I, Ali MB, Yameen MA, Mezni A, Hedfi A. et al. In-Vitro and In-Vivo Tolerance and Therapeutic Investigations of Phyto-Fabricated Iron Oxide Nanoparticles against Selected Pathogens. Toxics. 2021;9:105
138. Friedrich B, Lyer S, Janko C, Unterweger H, Brox R, Cunningham S. et al. Scavenging of bacteria or bacterial products by magnetic particles functionalized with a broad-spectrum pathogen recognition receptor motif offers diagnostic and therapeutic applications. Acta Biomater. 2022;141:418-28
139. Xu Y, Wei MT, Ou-Yang HD, Walker SG, Wang HZ, Gordon CR. et al. Exposure to TiO2 nanoparticles increases Staphylococcus aureus infection of HeLa cells. J Nanobiotechnology. 2016;14:34
140. Malmir S, Karbalaei A, Pourmadadi M, Hamedi J, Yazdian F, Navaee M. Antibacterial properties of a bacterial cellulose CQD-TiO(2) nanocomposite. Carbohydr Polym. 2020;234:115835
141. Ammendolia M, De Berardis B, Maurizi L, Longhi C. Exposure to TiO2 Nanoparticles Increases Listeria monocytogenes Infection of Intestinal Epithelial Cells. Nanomaterials. 2020;10:2196
142. Shi SF, Jia JF, Guo XK, Zhao YP, Chen DS, Guo YY. et al. Reduced Staphylococcus aureus biofilm formation in the presence of chitosan-coated iron oxide nanoparticles. Int J Nanomedicine. 2016;11:6499-506
143. Coelho CC, Araujo R, Quadros PA, Sousa SR, Monteiro FJ. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections. Mater Sci Eng C Mater Biol Appl. 2019;97:529-38
144. Liu M, Wang X, Li H, Xia C, Liu Z, Liu J. et al. Magnesium oxide-incorporated electrospun membranes inhibit bacterial infections and promote the healing process of infected wounds. J Mater Chem B. 2021;9:3727-44
145. Antonelli M, De Pascale G, Ranieri VM, Pelaia P, Tufano R, Piazza O. et al. Comparison of triple-lumen central venous catheters impregnated with silver nanoparticles (AgTive(R)) vs conventional catheters in intensive care unit patients. J Hosp Infect. 2012;82:101-7
146. Potgieter MD, Meidany P. Evaluation of the penetration of nanocrystalline silver through various wound dressing mediums: An in vitro study. Burns. 2018;44:596-602
147. Uygur F, Oncul O, Evinc R, Diktas H, Acar A, Ulkur E. Effects of three different topical antibacterial dressings on Acinetobacter baumannii-contaminated full-thickness burns in rats. Burns. 2009;35:270-3
148. Barrett JP, Raby E, Wood F, Coorey R, Ramsay JP, Dykes GA. et al. An in vitro study into the antimicrobial and cytotoxic effect of Acticoat dressings supplemented with chlorhexidine. Burns. 2022;48:941-51
149. Salomoni R, Leo P, Montemor AF, Rinaldi BG, Rodrigues M. Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. Nanotechnol Sci Appl. 2017;10:115-21
150. Akter M, Sikder MT, Rahman MM, Ullah A, Hossain KFB, Banik S. et al. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J Adv Res. 2018;9:1-16
151. Mishra AR, Zheng J, Tang X, Goering PL. Silver Nanoparticle-Induced Autophagic-Lysosomal Disruption and NLRP3-Inflammasome Activation in HepG2 Cells Is Size-Dependent. Toxicol Sci. 2016;150:473-87
152. Jaswal T, Gupta J. A review on the toxicity of silver nanoparticles on human health. Mater Today: Proc. 2021
153. Sriram M, Kalishwaralal K, BarathManiKanth S, Gurunathani S. Size-based cytotoxicity of silver nanoparticles in bovine retinal endothelial cells. Nanosci methods. 2012;1:56-77
154. Stoehr LC, Gonzalez E, Stampfl A, Casals E, Duschl A, Puntes V. et al. Shape matters: effects of silver nanospheres and wires on human alveolar epithelial cells. Part Fibre Toxicol. 2011;8:36
155. Zhang XF, Shen W, Gurunathan S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int, J Mol Sci. 2016;17:1603
156. Avalos A, Haza AI, Mateo D, Morales P. Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J Appl Toxicol. 2014;34:413-23
157. Cui Y, Zhao Y, Tian Y, Zhang W, Lu X, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33:2327-33
158. Rizvi SMD, Hussain T, Ahmed ABF, Alshammari TM, Moin A, Ahmed MQ. et al. Gold nanoparticles: A plausible tool to combat neurological bacterial infections in humans. Biomed Pharmacother. 2018;107:7-18
159. Zhao Y, Jiang X. Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale. 2013;5:8340-50
160. Rosa S, Connolly C, Schettino G, Butterworth KT, Prise KM. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017;8:2
161. Hu D, Li H, Wang B, Ye Z, Lei W, Jia F. et al. Surface-Adaptive Gold Nanoparticles with Effective Adherence and Enhanced Photothermal Ablation of Methicillin-Resistant Staphylococcus aureus Biofilm. ACS Nano. 2017;11:9330-9
162. Wang H, Zhang J, Song Z, Mu Y, Foda MF, Wu Y. et al. An intelligent platform based on acidity-triggered aggregation of gold nanoparticles for precise photothermal ablation of focal bacterial infection. Chem Eng J. 2021;407:127076
163. Saha B, Bhattacharya J, Mukherjee A, Ghosh A, Santra C, Dasgupta AK. et al. In Vitro Structural and Functional Evaluation of Gold Nanoparticles Conjugated Antibiotics. Nanoscale Res Lett. 2007;2:614
164. Zhou W, Gao X, Liu D, Chen X. Gold Nanoparticles for In Vitro Diagnostics. Chem Rev. 2015;115:10575-636
165. Tseng YT, Chang HT, Chen CT, Chen CH, Huang CC. Preparation of highly luminescent mannose-gold nanodots for detection and inhibition of growth of Escherichia coli. Biosens Bioelectron. 2011;27:95-100
166. Scott LJ. Verigene® Gram-Positive Blood Culture Nucleic Acid Test. Mol Diagn Ther. 2013;17:117-22
167. Wu K, Su D, Liu J, Saha R, Wang JP. Magnetic nanoparticles in nanomedicine: a review of recent advances. Nanotechnology. 2019;30:502003
168. Shubayev VI, Pisanic TR 2nd, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467-77
169. Baraki H, Zinne N, Wedekind D, Meier M, Bleich A, Glage S. et al. Magnetic resonance imaging of soft tissue infection with iron oxide labeled granulocytes in a rat model. PLoS ONE. 2012;7:e51770
170. Hoerr V, Tuchscherr L, Hüve J, Nippe N, Loser K, Glyvuk N. et al. Bacteria tracking by in vivo magnetic resonance imaging. BMC Biol. 2013;11:63
171. Ji L, Zhang L, Yang H, Liang S, Pan J, Zou Y. et al. Versatile Au@Ru nanocomposites for the rapid detection of Salmonella typhimurium and photothermal sterilization. J Colloid Interface Sci. 2022;621:489-98
172. Huang X, Zhang Z, Chen L, Lin Y, Zeng R, Xu J. et al. Multifunctional Au nano-bridged nanogap probes as ICP-MS/SERS dual-signal tags and signal amplifiers for bacteria discriminating, quantitative detecting and photothermal bactericidal activity. Biosens Bioelectron. 2022;212:114414
173. Ye J, Li Z, Fu Q, Li Q, Zhang X, Su L. et al. Quantitative Photoacoustic Diagnosis and Precise Treatment of Inflammation In Vivo Using Activatable Theranostic Nanoprobe. Adv Funct Mater. 2020;30:2001771
174. Mei Z, Gao D, Hu D, Zhou H, Ma T, Huang L. et al. Activatable NIR-II photoacoustic imaging and photochemical synergistic therapy of MRSA infections using miniature Au/Ag nanorods. Biomaterials. 2020;251:120092
175. Pati R, Sahu R, Panda J, Sonawane A. Encapsulation of zinc-rifampicin complex into transferrin-conjugated silver quantum-dots improves its antimycobacterial activity and stability and facilitates drug delivery into macrophages. Sci Rep. 2016;6:24184
176. Mandani S, Rezaei B, Ensafi AA, Sabzalian MR. Development of a new simple spectroscopic determination coupled acid-motivated delivery system based on fluorescence turn-off MSNs@MPA-ZnS QDs for infection. Microporous Mesoporous Mater. 2021;317:110971
177. Courtney CM, Goodman SM, McDaniel JA, Madinger NE, Chatterjee A, Nagpal P. Photoexcited quantum dots for killing multidrug-resistant bacteria. Nat Mater. 2016;15:529-34
178. Feng S, Li H, Liu C, Chen M, Sheng H, Huang M. et al. Real-Time In Vivo Detection and Monitoring of Bacterial Infection Based on NIR-II Imaging. Front Chem. 2021;9:689017
179. Zhang Y, Wang T-H. Quantum Dot Enabled Molecular Sensing and Diagnostics. Theranostics. 2012;2:631-54
180. Arshad E, Anas A, Asok A, Jasmin C, Pai SS, Bright Singh IS. et al. Fluorescence detection of the pathogenic bacteria Vibrio harveyi in solution and animal cells using semiconductor quantum dots. RSC Adv. 2016;6:15686-93
181. Markovic ZM, Ristic BZ, Arsikin KM, Klisic DG, Harhaji-Trajkovic LM, Todorovic-Markovic BM. et al. Graphene quantum dots as autophagy-inducing photodynamic agents. Biomaterials. 2012;33:7084-92
182. Ristic BZ, Milenkovic MM, Dakic IR, Todorovic-Markovic BM, Milosavljevic MS, Budimir MD. et al. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials. 2014;35:4428-35
183. Sattarahmady N, Rezaie-Yazdi M, Tondro GH, Akbari N. Bactericidal laser ablation of carbon dots: An in vitro study on wild-type and antibiotic-resistant Staphylococcus aureus. J Photochem Photobiol, B. 2017;166:323-32
184. Wang X, Zhou Z, Chen F. Surface Modification of Carbon Nanotubes with an Enhanced Antifungal Activity for the Control of Plant Fungal Pathogen. Materials (Basel). 2017;10:1375
185. Grinholc M, Nakonieczna J, Fila G, Taraszkiewicz A, Kawiak A, Szewczyk G. et al. Antimicrobial photodynamic therapy with fulleropyrrolidine: photoinactivation mechanism of Staphylococcus aureus, in vitro and in vivo studies. Appl Microbiol Biotechnol. 2015;99:4031-43
186. Zhang Y, Wu M, Wu M, Zhu J, Zhang X. Multifunctional Carbon-Based Nanomaterials: Applications in Biomolecular Imaging and Therapy. ACS Omega. 2018;3:9126-45
187. Sanginario A, Miccoli B, Demarchi D. Carbon Nanotubes as an Effective Opportunity for Cancer Diagnosis and Treatment. Biosensors. 2017;7:9
188. Ding F, Zhan Y, Lu X, Sun Y. Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging. Chem Sci. 2018;9:4370-80
189. Bardhan NM, Ghosh D, Belcher AM. Carbon nanotubes as in vivo bacterial probes. Nat Commun. 2014;5:4918
190. Kotagiri N, Lee JS, Kim JW. Selective pathogen targeting and macrophage evading carbon nanotubes through dextran sulfate coating and PEGylation for photothermal theranostics. J Biomed Nanotechnol. 2013;9:1008-16
191. Sava Gallis DF, Butler KS, Agola JO, Pearce CJ, McBride AA. Antibacterial Countermeasures via Metal-Organic Framework-Supported Sustained Therapeutic Release. ACS Appl Mater Interfaces. 2019;11:7782-91
192. Soltani S, Akhbari K. Facile and single-step entrapment of chloramphenicol in ZIF-8 and evaluation of its performance in killing infectious bacteria with high loading content and controlled release of the drug. CrystEngComm. 2022;24:1934-41
193. Zhang X, Liu L, Huang L, Zhang W, Wang R, Yue T. et al. The highly efficient elimination of intracellular bacteria via a metal organic framework (MOF)-based three-in-one delivery system. Nanoscale. 2019;11:9468-77
194. Soomro NA, Wu Q, Amur SA, Liang H, Ur Rahman A, Yuan Q. et al. Natural drug physcion encapsulated zeolitic imidazolate framework, and their application as antimicrobial agent. Colloids Surf B Biointerfaces. 2019;182:110364
195. Nabipour H, Sadr MH, Bardajee GR. Synthesis and characterization of nanoscale zeolitic imidazolate frameworks with ciprofloxacin and their applications as antimicrobial agents. New J Chem. 2017;41:7364-70
196. Chowdhuri AR, Das B, Kumar A, Tripathy S, Roy S, Sahu SK. One-pot synthesis of multifunctional nanoscale metal-organic frameworks as an effective antibacterial agent against multidrug-resistant Staphylococcus aureus. Nanotechnology. 2017;28:095102
197. Haseena Khan A, Aslam F Kanwal T, Shah MR Khalil AAK. et al. Enhanced Antibacterial Potential of Amoxicillin against Helicobacter pylori Mediated by Lactobionic Acid Coated Zn-MOFs. Antibiotics (Basel, Switzerland). 2021;10:1071
198. Kumar G, Kant A, Kumar M, Masram DT. Synthesis, characterizations and kinetic study of metal organic framework nanocomposite excipient used as extended release delivery vehicle for an antibiotic drug. Inorg Chim Acta. 2019;496:119036
199. Nabipour H, Soltani B, Ahmadi Nasab N. Gentamicin Loaded Zn2(bdc)2(dabco) Frameworks as Efficient Materials for Drug Delivery and Antibacterial Activity. J Inorg Organomet Polym Mater. 2018;28:1206-13
200. Karakecili A, Topuz B, Ersoy FS, Sahin T, Gunyakti A, Demirtas TT. UiO-66 metal-organic framework as a double actor in chitosan scaffolds: Antibiotic carrier and osteogenesis promoter. Biomater Adv. 2022;136:212757
201. Nasrabadi M, Ghasemzadeh MA, Zand Monfared MR. The preparation and characterization of UiO-66 metal-organic frameworks for the delivery of the drug ciprofloxacin and an evaluation of their antibacterial activities. New J Chem. 2019;43:16033-40
202. Lin S, Liu X, Tan L, Cui Z, Yang X, Yeung KWK. et al. Porous Iron-Carboxylate Metal-Organic Framework: A Novel Bioplatform with Sustained Antibacterial Efficacy and Nontoxicity. ACS Appl Mater Interfaces. 2017;9:19248-57
203. Ghaffar I, Imran M, Perveen S, Kanwal T, Saifullah S, Bertino MF. et al. Synthesis of chitosan coated metal organic frameworks (MOFs) for increasing vancomycin bactericidal potentials against resistant S. aureus strain. Mater Sci Eng C Mater Biol Appl. 2019;105:110111
204. Wei Y, Chen C, Zhai S, Tan M, Zhao J, Zhu X. et al. Enrofloxacin/florfenicol loaded cyclodextrin metal-organic-framework for drug delivery and controlled release. Drug Deliv. 2021;28:372-9
205. Li X, Semiramoth N, Hall S, Tafani V, Josse J, Laurent F. et al. Compartmentalized Encapsulation of Two Antibiotics in Porous Nanoparticles: an Efficient Strategy to Treat Intracellular Infections. Part Part Syst Char. 2019;36:1800360
206. Salehi A, Behpour M, Afzali D. Investigation into the antibacterial activity of covalent organic frameworks as a delivery system of trimethoprim against Escherichia coli and Staphylococcus aureus. Polym Bull. 2022;80:1447-61
207. Li C, Chen C, Zhao J, Tan M, Zhai S, Wei Y. et al. Electrospun Fibrous Membrane Containing a Cyclodextrin Covalent Organic Framework with Antibacterial Properties for Accelerating Wound Healing. ACS Biomater Sci Eng. 2021;7:3898-907
208. Chen M, Zhang J, Qi J, Dong R, Liu H, Wu D. et al. Boronic Acid-Decorated Multivariate Photosensitive Metal-Organic Frameworks for Combating Multi-Drug-Resistant Bacteria. ACS Nano. 2022;16:7732-44
209. Hynek J, Zelenka J, Rathousky J, Kubat P, Ruml T, Demel J. et al. Designing Porphyrinic Covalent Organic Frameworks for the Photodynamic Inactivation of Bacteria. ACS Appl Mater Interfaces. 2018;10:8527-35
210. Zhang Y, Xu X, Liao Q, Wang Q, Han Q, Chen P. et al. New potential of boron-based COFs: the biocompatible COF-1 for reactive oxygen generation and antimicrobial applications. J Mater Chem B. 2022;10:3285-92
211. Tamames-Tabar C, Imbuluzqueta E, Guillou N, Serre C, Miller SR, Elkaïm E. et al. A Zn azelate MOF: combining antibacterial effect. CrystEngComm. 2015;17:456-62
212. Restrepo J, Serroukh Z, Santiago-Morales J, Aguado S, Gómez-Sal P, Mosquera MEG. et al. An Antibacterial Zn-MOF with Hydrazinebenzoate Linkers. Eur J Inorg Chem. 2016;2017:574-80
213. Li X, Bai H, Yang Y, Yoon J, Wang S, Zhang X. Supramolecular Antibacterial Materials for Combatting Antibiotic Resistance. Adv Mater. 2019;31:e1805092
214. Xie S, Manuguri S, Proietti G, Romson J, Fu Y, Inge AK. et al. Design and synthesis of theranostic antibiotic nanodrugs that display enhanced antibacterial activity and luminescence. Proc Natl Acad Sci U S A. 2017;114:8464-9
215. Li X, Lee D, Huang JD, Yoon J. Phthalocyanine-Assembled Nanodots as Photosensitizers for Highly Efficient Type I Photoreactions in Photodynamic Therapy. Angew Chem Int Ed Engl. 2018;57:9885-90
216. Wang R, Kim D, Yang M, Li X, Yoon J. Phthalocyanine-Assembled "One-For-Two" Nanoparticles for Combined Photodynamic-Photothermal Therapy of Multidrug-Resistant Bacteria. ACS Appl Mater Interfaces. 2022;14:7609-16
217. Song G, Petschauer SJ, Madden JA, Zamboni CW. Nanoparticles and the Mononuclear Phagocyte System: Pharmacokinetics and Applications for Inflammatory Diseases. Curr Rheumatol Rev. 2014;10:22-34
218. Lugo-Villarino G, Cougoule C, Meunier E, Rombouts Y, Verollet C, Balboa L. Editorial: The Mononuclear Phagocyte System in Infectious Disease. Front Immunol. 2019;10:1443
219. Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin Pharmacol Ther. 2008;83:761-9
220. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941-51
221. Kim Y-H, Min KH, Wang Z, Kim J, Jacobson O, Huang P. et al. Development of Sialic Acid-coated Nanoparticles for Targeting Cancer and Efficient Evasion of the Immune System. Theranostics. 2017;7:962-73
222. Li Y, Che J, Chang L, Guo M, Bao X, Mu D. et al. CD47- and Integrin α4/β1-Comodified-Macrophage-Membrane-Coated Nanoparticles Enable Delivery of Colchicine to Atherosclerotic Plaque. Adv Healthc Mater. 2022;11:e2101788
223. Pham LM, Poudel K, Phung CD, Nguyen TT, Pandit M, Nguyen HT. et al. Preparation and evaluation of dabrafenib-loaded, CD47-conjugated human serum albumin-based nanoconstructs for chemoimmunomodulation. Colloids Surf B Biointerfaces. 2021;208:112093
224. Zhan X, Teng W, Sun K, He J, Yang J, Tian J. et al. CD47-mediated DTIC-loaded chitosan oligosaccharide-grafted nGO for synergistic chemo-photothermal therapy against malignant melanoma. Mater Sci Eng C Mater Biol Appl. 2021;123:112014
225. Vandchali NR, Moadab F, Taghizadeh E, Tajbakhsh A, Gheibihayat SM. CD47 Functionalization of Nanoparticles as a Poly(ethylene glycol) Alternative: A Novel Approach to Improve Drug Delivery. Curr Drug Targets. 2021;22:1750-9
226. Liu C, Yu D, Ge F, Yang L, Wang Q. Fluorescent and mass spectrometric evaluation of the phagocytic internalization of a CD47-peptide modified drug-nanocarrier. Anal Bioanal Chem. 2019;411:4193-202
227. Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R. et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527:323-8
228. Dhumal D, Maron B, Malach E, Lyu Z, Ding L, Marson D. et al. Dynamic self-assembling supramolecular dendrimer nanosystems as potent antibacterial candidates against drug-resistant bacteria and biofilms. Nanoscale. 2022;14:9286
Author contact
![]() Corresponding authors: Lai Jiang: laijiangedu.cn; Gang Liu: gangliu.cmitmedu.cn
Corresponding authors: Lai Jiang: laijiangedu.cn; Gang Liu: gangliu.cmitmedu.cn
 Global reach, higher impact
Global reach, higher impact