13.3
Impact Factor
Theranostics 2023; 13(6):1892-1905. doi:10.7150/thno.78078 This issue Cite
Research Paper
HSF1 promotes CD69+ Treg differentiation to inhibit colitis progression
1. Laboratory of Cancer Biology, Key Lab of Biotherapy in Zhejiang Province, Cancer Center of Zhejiang University, Sir Run Run Shaw hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
2. Department of Medical Oncology, Sir Run Run Shaw hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
3. Department of Pathology, Sir Run Run Shaw hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
4. Department of respiratory medicine, The First People's Hospital of Xiaoshan District, Xiaoshan First Affiliated Hospital of Wenzhou Medical University, Hangzhou, 311200, Zhejiang, China
5. Institute of Immunology, and Department of Orthopedics of the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
6. Institute of Immunology, and Bone Marrow Transplantation Center of the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
7. Institute of Hematology, Zhejiang University & Zhejiang Engineering Laboratory for Stem Cell and Immunotherapy, Hangzhou, China
# Equal contribution
Abstract
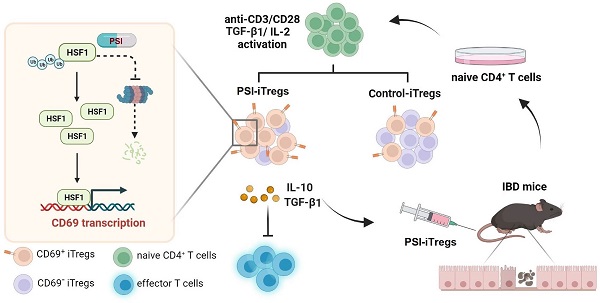
Regulatory T cells (Tregs) are critical for generating and maintaining peripheral tolerance. Treg-based immunotherapy is valuable for the clinical management of diseases resulting from dysregulation of immune tolerance. However, the lack of potency is a potential limitation of Treg therapy. In addition, CD69 positive-Treg (CD69+ Treg) represent a newly identified subset of Tregs with potent immune suppressive capability.
Methods: Foxp3YFP-CreCD69fl/fl and CD4CreCD69fl/fl mice were generated to determine the relevance of CD69 to Treg. Chromatin Immunoprecipitation Assay (ChIP) and luciferase Assay were performed to detect the regulation of CD69 transcription by heat shock transcription factor 1(HSF1). Gene expression was measured by western blotting and qRT-PCR. The differentiation of naive T cells to CD69+Foxp3+ iTregs was determined by flow cytometry. The immunosuppressive ability of Tregs was analyzed by ELISA and flow cytometry. Colon inflammation in mice was reflected by changes in body weight and colon length, the disease activity index (DAI), and H&E staining of colon tissues.
Results: Induced Tregs (iTregs) from CD4CreCD69fl/fl mice failed to alleviate colitis. The transcription factor HSF1 interacted with the promoter of the CD69 gene to prompt its transcription during Treg differentiation. Genetic and chemical inhibition of HSF1 impaired CD69+ Treg differentiation and promoted the pathogenesis of colitis in mice. In contrast, HSF1 protein stabilized by inhibiting its proteasomal degradation promoted CD69+ Treg differentiation and alleviated colitis in mice. Moreover, adoptive transfer of iTregs with HSF1 stabilization by proteasome inhibitor (PSI) dramatically prevented the development of colitis in mice and was accompanied by decreased production of pro-inflammatory cytokines and reduced accumulation of pro-inflammatory lymphocytes in colitis tissue, whereas Tregs induced in the absence of PSI were less stable and ineffective in suppressing colitis.
Conclusions: HSF1 promotes CD69+ Tregs differentiation by activating the CD69 transcription, which is critical for the immunosuppressive function of Tregs. Stabilization of HSF1 by PSIs results in the efficient generation of Tregs with high potency to treat colitis and probably other autoimmune diseases involving Tregs deficiency.
Keywords: Treg, CD69, HSF1, IBD, proteasome inhibitor
 Global reach, higher impact
Global reach, higher impact