13.3
Impact Factor
Theranostics 2023; 13(9):2930-2945. doi:10.7150/thno.83217 This issue Cite
Research Paper
Mechanical force drives the initial mesenchymal-epithelial interaction during skin organoid development
1. 111 Project Laboratory of Biomechanics and Tissue Repair & Key Laboratory of Biorheological Science and Technology of Ministry of Education, College of Bioengineering, Chongqing University, Chongqing 400044, China.
2. Department of Dermatology and Cosmetology, Chongqing Hospital of Traditional Chinese Medicine, Chongqing 400021, China.
3. Three Gorges Hospital, Chongqing University, Chongqing 404000, China.
4. Department of Burns and Plastic Surgery, Wuhan General Hospital of Chinese People's Liberation Army, Wuhan 430000, China.
5. Hunan Key Laboratory of Aging Biology, Xiangya Hospital, Central South University, Changsha 410008, China.
Abstract
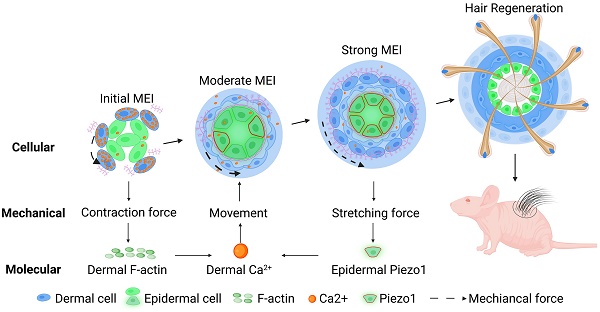
Rationale: Stem cells self-organize to form organoids that generate mini-organs that resemble the physiologically-developed ones. The mechanism by which the stem cells acquire the initial potential for generating mini-organs remains elusive. Here we used skin organoids as an example to study how mechanical force drives initial epidermal-dermal interaction which potentiates skin organoids to regenerate hair follicles.
Methods: Live imaging analysis, single-cell RNA-sequencing analysis, and immunofluorescence were used to analyze the contractile force of dermal cells in skin organoids. Bulk RNA-sequencing analysis, calcium probe detection, and functional perturbations were used to verify that calcium signaling pathways respond to the contractile force of dermal cells. In vitro mechanical loading experiment was used to prove that the stretching force triggers the epidermal Piezo1 expression which negatively regulates dermal cell attachment. Transplantation assay was used to test the regenerative ability of skin organoids.
Results: We found that dermal cell-derived contraction force drives the movement of dermal cells surrounding the epidermal aggregates to trigger initial mesenchymal-epithelial interaction (MEI). In response to dermal cell contraction force, the arrangement of the dermal cytoskeleton was negatively regulated by the calcium signaling pathway which further influences dermal-epidermal attachment. The native contraction force generated from the dermal cell movement exerts a stretching force on the adjacent epidermal cells, activating the stretching force sensor Piezo1 in the epidermal basal cells during organoid culture. Epidermal Piezo1 in turn drives strong MEI to negatively regulate dermal cell attachment. Proper initial MEI by mechanical-chemical coupling during organoid culture is required for hair regeneration upon transplantation of the skin organoids into the back of the nude mice.
Conclusion: Our study demonstrated that mechanical-chemical cascade drives the initial event of MEI during skin organoid development, which is fundamental to the organoid, developmental, and regenerative biology fields.
Keywords: Skin cyst, Dermal cell attachment, Calcium, Piezo1, Mechanical stretch
 Global reach, higher impact
Global reach, higher impact