13.3
Impact Factor
Theranostics 2023; 13(14):4781-4801. doi:10.7150/thno.88002 This issue Cite
Review
Advanced hitchhiking nanomaterials for biomedical applications
1. School of Medical Imaging, Tianjin Medical University, Tianjin 300203, China.
2. Tianjin Key Laboratory of Retinal Functions and Diseases, Tianjin Branch of National Clinical Research Center for Ocular Disease, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin 300384, China.
3. Key Laboratory of Functional Polymer Materials of Ministry of Education, College of Chemistry, Nankai University, Tianjin 300071, China.
Received 2023-7-11; Accepted 2023-8-9; Published 2023-8-28
Abstract
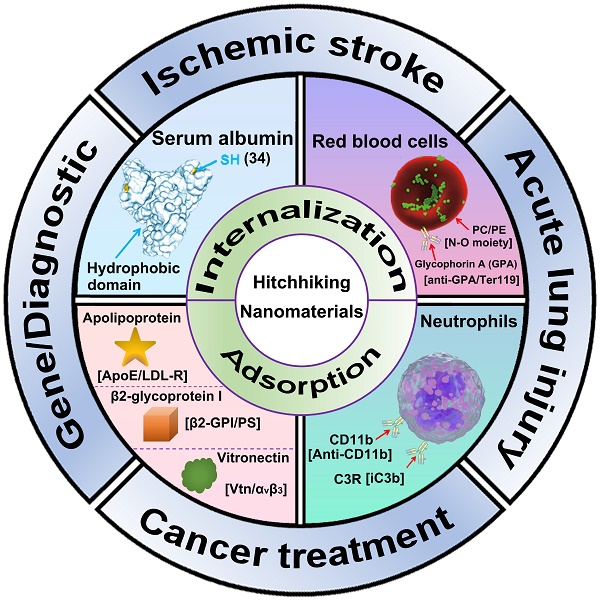
Hitchhiking, a recently developed bio-inspired cargo delivery system, has been harnessed for diverse applications. By leveraging the interactions between nanoparticles and circulatory cells or proteins, hitchhiking enables efficient navigation through the vasculature while evading immune system clearance. Moreover, it allows for targeted delivery of nutrients to tissues, surveillance of the immune system, and pathogen elimination. Various synthetic nanomaterials have been developed to facilitate hitchhiking with circulatory cells or proteins. By combining the advantages of synthetic nanomaterials and circulatory cells or proteins, hitchhiking nanomaterials demonstrate several advantages over conventional vectors, including enhanced circulatory stability and optimized therapeutic efficacy. This review provides an overview of general strategies for hitchhiking, choices of cells and proteins, and recent advances of hitchhiking nanomaterials for biomedical applications.
Keywords: hitchhiking, nanomaterials, circulatory cells, navigation, targeted delivery
Introduction
With the rapid development of nanotechnology, drug delivery systems (DDS) based on engineered nanoparticles have demonstrated remarkable efficacy in various biomedical applications [1-3]. Nanomaterials employed in drug delivery can be derived from diverse sources, offering versatility in their composition. In addition, their physical properties can be readily customized by manipulating factors such as size, shape, composition, surface characteristics, structure, and morphology [4-6]. More importantly, nanomedicine offers several notable advantages over free drugs, including improved stability and solubility, enhanced therapeutic efficacy, and reduced toxic side effects [7-9]. Capitalizing on these distinctive attributes, several types of nanomedicines have received approval from the Food and Drug Administration (FDA) for cancer treatment. Notable examples include Doxil® for Kaposi sarcoma and Lipolatin for non-small cell lung cancer [10, 11]. These nanomedicines have significantly enhanced the clinical benefits of anticancer drugs, leading to a considerable extension in the survival of cancer patients. However, traditional nanomedicines still face several challenges, such as high systemic toxicity, low response rates, and limited durability, which hinder their widespread adoption in clinical settings [12]. Therefore, addressing the means to further enhance the effectiveness and safety of nanomedicine becomes a crucial matter for future advancements in nanomaterials.
Cell-based therapies have a long-standing history, with the first recorded human blood transfusion dating back to the mid-17th century [13]. In the era of modern medicine, significant progress has been achieved in harnessing the inherent capabilities of cells for targeted therapeutic interventions. These advancements encompass diverse applications, such as transfusions of specific blood components (such as platelets, plasma, white blood cells (WBC), or red blood cells (RBC)) [14, 15], mesenchymal stem cells for tissue regeneration [16-18], and immunotherapies for cancer treatment [19-21]. Remarkably, the circulatory cells and proteins in the bloodstream, including RBC, macrophages, monocytes, lymphocytes, and serum albumin (SA), serve as nature's own “delivery vehicles”, has evolved to carry out delivery functions within living organisms efficiently [22-25]. These unique properties make circulating cells/proteins promising candidates for the development of therapeutics that can overcome the limitations often associated with traditional nanomedicines.
Hitchhiking, in the context of cargo delivery, refers to using external carriers to transport cargo to its desired destination [26]. Some organisms have evolved hitchhiking behavior in nature, where smaller organisms attach to larger ones and get transported to new locations [27, 28]. Drawing Inspiration from this natural phenomenon, hitchhiking has recently been adapted as a bio-inspired cargo delivery system for various applications [29]. The versatility of nanoparticles allows for their interaction with circulatory cells or proteins, thereby facilitating hitchhiking [3]. In this system, circulatory cells or proteins play a pivotal role as innate “moving gears” that facilitate the transport of hitchhiking nanoparticles throughout the body, until reaching their desired target site [29, 30]. Hitchhiking nanoparticles leverage the natural ability of circulatory cells or proteins to (i) navigate the vasculature while evading immune system clearance and (ii) perform specific tasks such as delivering nutrients to tissues, eliminating pathogens, and surveilling the immune system [22, 31-38]. Additionally, hitchhiking nanoparticles can be used to genetically engineer and label specific cell types, such as macrophages, T cells, and B cells [37, 39, 40]. This allows further customization and manipulation of these cells, enhancing their capabilities for targeted drug delivery and other therapeutic applications.
This review introduces the general strategies for nanoparticle to hitchhiking circulatory cells or proteins, including internalization-mediated hitchhiking and adsorption-medicated hitchhiking (Figure 1). Next, the cell and protein choices for cellular hitchhiking (RBC, neutrophils, and SA) are demonstrated. Finally, we summarize the recent advances of hitchhiking nanomaterials in biomedical applications, including ischemic stroke, acute lung injury, cancer treatment, gene therapy, and diagnostic imaging.
Schematic illustration of Advanced hitchhiking nanomaterials for biomedical applications. Advanced hitchhiking nanomaterials employ internalization-mediated or adsorption-medicated strategy to hitchhike circulatory cells and proteins for various biomedical applications, including ischemic stroke, acute lung injury, cancer treatment, gene therapy, and diagnostic imaging. Adapted with permission from [121], copyright 2023 American Chemical Society; [31], copyright 2018 Springer Nature.
General strategies for hitchhiking
Circulatory cells are biological entities composed of biomolecules including polysaccharides, lipids, and proteins that provide a variety of functional groups and surface properties [41, 42]. These characteristics allow for the utilization of various techniques to hitchhike these cells. The delivery of nanomaterials for cell-specific targeting represents a crucial step in achieving efficient cell hitchhiking. However, successfully and effectively implementing this strategy requires addressing several challenges. Notably, two major hurdles are associated with the stability and half-life of nanomaterials. One promising approach to improve nanoparticle storage and circulation stability is by encapsulating them within protective coatings or matrices, such as PEG and zwitterionic molecules [43, 44]. These protective layers shield the nanomaterials from potential degradation and immune recognition, enhancing their overall stability during transport. Additionally, the design of intelligent release mechanisms offers a solution to ensure controlled and targeted payload release. These mechanisms can be engineered to respond to specific triggers, such as changes in pH or enzymatic activity, allowing for precise release of the payload at the desired target site [8, 45, 46]. With these strategies, the stability of the nanomaterials can be further augmented, leading to an efficient and successful cell-specific targeting approach. In this section, we will briefly describe two major strategies for cell hitchhiking: (1) internalization-mediated hitchhiking and (2) adsorption-mediated hitchhiking. Furthermore, we will discuss the advantages and disadvantages of these two hitchhiking strategies.
Internalization-mediated hitchhiking
One promising strategy for hitchhiking circulatory cells involves leveraging the natural ability of specific cells to phagocytose foreign nanomaterials, commonly referred to as the “Trojan Horse” method [47-49]. In this strategy, the phagocytic capacity of cells is crucial for successful hitchhiking. Typically, this is achieved by incubating nanoparticles with cells capable of phagocytosis in a culture medium, allowing the nanoparticles to adsorption on cells' surface and subsequently cellular internalization. Using this strategy, various nanomaterials, including liposomes, polymeric nanoparticles, nanozymes, and metal-based nanomaterials, have been successfully incorporated into neutrophils and macrophages [48, 50-52]. Importantly, this method preserves the integrity of the cell membrane and avoids the off-target effect of the nanomaterials in vivo. However, it is important to recognize that the origins of the “Trojan Horse” method lie in targeted drug delivery approaches that are used to kill cells, especially in the context of cancer nanomedicine [53]. The premature release of loaded drugs during hitchhiking may disrupt the integrity of the cell membrane, thus impairing the migration, homing, and other functions of hitchhiked cells/proteins, resulting in unpredictable therapeutic efficacy. Therefore, optimize the release behaviors of loaded drugs to ensure the precisely controlled release of the drug only in the target is a prerequisite for this internalization-mediated hitchhiking. Besides, despite the high efficiency and specificity of this in vitro culture method, common issues associated with complex steps, such as cell extraction and purification, limit the generalizability of this strategy [54, 55]. To overcome these limitations, direct in vivo hitchhiking strategies have been developed, primarily by utilizing the scavenging capability of macrophages and monocytes. For example, Wang et al. discovered that activated neutrophils could specifically endocytose denatured BSA nanoparticles via Fcγ receptor (FcγR) III in vivo [56]. Building on this finding, they subsequently developed a series of denatured BSA-based nanomaterials for in vivo neutrophil hitchhiking. Similarly, Wang et al. demonstrated that a specific lipid called DOCP could rapidly accumulate complement fragment iC3b upon intravenous injection, triggering neutrophil hitchhiking through C3 receptor-mediated phagocytosis [57].
In addition to exploiting the scavenging capability of circulatory cells, ligand-receptor interaction is another way for nanomaterials to hitchhike circulatory cells. The ligand-receptor interaction refers to highly selective and complementary interactions between a ligand molecule and its corresponding receptor protein within a living organism [58, 59]. Ligands are signaling molecules that can bind to specific receptors, usually present on the surface of cells, to trigger a cellular response [60, 61]. This interaction is analogous to a lock and key mechanism, where only a specific ligand (the key) can fit into its corresponding receptor (the lock) to activate a particular biological pathway or process. This specificity prevents unwanted crosstalk between different signaling pathways and helps in achieving accurate cellular responses. For example, Stephan et al. presented an anti-CD3e f(ab')2-decorated polymeric nanoparticle and successfully hitchhiked and programmed leukemia-specific T cells through the CD3/anti-CD3 interaction [62]. Similarly, Stavrou et al. reported an anti-Ly6G-intergrated liposome and effectively hitchhikes activated neutrophils through the Ly6G/anti-Ly6G interaction [63]. This ligand-receptor attachment strategy offers several advantages, including reproducibility, reliability, specificity, and the potential for translation into in vivo hitchhiking when specific ligand-receptor pairs are involved. Furthermore, this approach allows for designing a platform technology that can attach to various cell types by modifying the attachment ligand on the nanoparticle surface, making it ideal for scalable particle technology. These innovative approaches offer promising alternatives for hitchhiking circulatory cells in vivo, circumventing the complexities associated with in vitro methods. However, it is worth noting that this strategy is easier to implement in cell types capable of phagocytosis, such as monocytes and macrophages. Furthermore, biodegradable nanoparticles may undergo intracellular degradation and release drugs after phagocytosis, thereby affecting the migratory function of the cells [64-66].
Adsorption-mediated hitchhiking
Adsorption-mediated hitchhiking mainly occurs in circulatory cells that are non-endocytic or poorly endocytic, such as T cells, B cells, and RBCs. According to the different interaction modes between nanomaterials and cells, adsorption-mediated hitchhiking can be roughly divided into two categories: (1) non-specific adsorption driven by electrostatic interaction, van Der Waals forces, and hydrogen bonding; and (2) site-specific adsorption driven by ligand-receptor interactions. It is important to note that when nanoparticles are tethered on the cell surface, it becomes paramount to ensure stability and controlled dissociation from the cells. As circulatory cells transport nanoparticles through the bloodstream, they encounter significant shear forces [67, 68]. Proper stability of the nanoparticle-cell complex is crucial for preventing the premature release of nanoparticles during the hitchhiking process. Therefore, an ideal nanoparticle that relies on adsorption for in vivo hitchhiking should consider the following aspects when designing: 1) The binding affinity between nanoparticles and cells should be strong enough to withstand shear forces during blood circulation, and 2) controlled release from hitchhiked cells upon reaching targets for optimized therapeutic efficacy and minimized toxic effect. However, the current hitchhiking nanomaterials only consider enhancing the interaction with the cells while ignoring the controlled release. This may be because the side effects caused by the destruction of a small number of hitchhiking cells are largely ignored. In addition, long-term interactions with the cell surface may promote the cellular internalization of nanoparticles, which may affect the original function of nanoparticles. Therefore, resistance to cellular endocytosis should also be an important property of these nanomaterials.
Non-specific adsorption
One of the simplest methods for attaching nanoparticles to cells is passive adsorption on the cell surface. The surface charge, shape, and size of nanoparticles are the three important parameters that affecting their non-specific adsorption onto cells. (1) Surface charge. The mammalian cells usually possess a negative-charged surface due to negatively charged groups such as sialic acid, carboxylate, and phosphate on biomolecules [69]. This inherent characteristic of cells offers a straightforward approach to attaching cationic nanoparticles to their surface through electrostatic interactions. For example, Manshian et al. discovered that polyethyleneimine-coated poly (lactic-co-glycolic acid) nanoparticles (PEI-PLGA, 17.3 ± 0.4 mV) demonstrated a twofold higher binding efficacy to RBCs than that of plain PLGA NPs (-25.9 ± 0.2 mV) [70]. Similarly, Nikitin et al. presented that chitosan-coated nanoparticles (42.1 ± 8.8 mV) strongly bind to RBCs even after multiple washes [71]. However, it is essential to note that while these positively charged nanoparticles exhibit strong adsorption to cell membranes, excessive positive charges may lead to cellular internalization, cell membrane dissolution and potential toxicity [72, 73]. (2) Particle size. Nanoparticle size is a crucial factor influencing cellular adsorption efficiency. Smaller nanoparticles have been demonstrated to exhibit higher cellular uptake efficiency [74, 75]. For instance, Liu et al. conducted a study wherein nano-sized aggregates with diameters of 132.6 nm (CA-5) displayed slower uptake kinetics compared to smaller-sized particles such as CA-1 (46.9 nm) and CA-3 (37.59 nm) [76]. Consequently, nanoparticles with suitable sizes, generally larger than 100 nm, are expected to yield benefits in adsorption on the cell surface. (3) Particle shape. Nanoparticles of different shapes possess distinct surface areas relative to their volumes. Those with larger surface areas tend to establish stronger interactions with cell surfaces due to increased contact points [77, 78]. However, such strong interactions might also lead to enhanced cellular internalization. For instance, cubes exhibit the lowest cellular uptake efficacy among various shapes, followed by cylinders, spheres, and rods. In light of these findings, achieving a delicate balance between enhancing cell interactions and avoiding excessive endocytosis becomes crucial for successfully applying non-specific adsorption-mediated hitchhiking. In addition, van Der Waals forces and hydrogen bonds also contribute greatly to non-specific adsorption [79, 80]. For example, Shen et al. found that N-oxide moiety can reversibly bind to RBCs membranes through interaction with the hydrophilic groups of phosphatidylethanolamines (PE) and phosphatidylcholine (PC) [81]. In addition, this zwitterion feature of N-oxide moiety effectively avoided undesired RBC internalization. With this discovery, they successfully delivered 7-Ethyl-10-hydroxycamptothecin (SN38) to tumor tissues by hitchhiking RBCs and significantly enhanced tumor suppression in HepG2 tumor-bearing mice models [82].
Site-specific adsorption
The presence of abundant receptors on the cell surface provides excellent opportunities for nanomaterials to hitchhike circulatory cells through ligand-receptor interactions [52, 83, 84]. The CD44, which is widely expressed on macrophages, T cells, B cells, and tumor cells, has been extensively utilized in facilitating the attachment of nanomaterials to various cell types [85-87]. The interaction between hyaluronic acid (HA) and CD44 has been particularly effective in this regard, as demonstrated by Rubner et al., who successfully anchored HA-decorated patches to T cells and B cells through the HA-CD44 interaction [88]. Similarly, glycophorin A (GPA), a sialo-glycoprotein found on the surface of red blood cells (RBCs), has been targeted for hitchhiking purposes. For example, Brenner et al. achieved efficient hitchhiking of RBCs by utilizing anti-GPA antibodies and the GPA receptor for specific binding [89]. In addition to ligand-receptor interactions, a positive targeting strategy can also be employed to hitchhike circulatory cells. This approach initially anchors affinity ligands such as biotin, aptamers, antibodies, or peptides onto the cell membranes [90]. Subsequently, nanomaterials can be site-specifically adsorbed onto the cells by interacting with these ligands and their corresponding receptors [91]. This strategy allows for calculating the extent of conjugation based on the surface densities of membrane proteins, ensuring precise control over the hitchhiking process while minimizing the risk of compromising the surface properties of the hitchhiking cells.
Advanced hitchhiking nanomaterials for biomedical applications.
| Nanomaterials | Cells/Proteins | Hitchhiking way | Signaling pathway | Cargoes | Applications | Ref |
|---|---|---|---|---|---|---|
| Nano-integrated cascade enzymes | Neutrophils | Internalization | Fcγ receptor (FcγR) III | SOD/CAT | Ischemic stroke treatment | 135 |
| PLGA-PEG-based nanoparticle | Neutrophils | Internalization | CFLFLF peptide | Ligustrazine | Ischemic stroke treatment | 52 |
| Bacteria-derived outer-membrane vesicle | Neutrophils | Internalization | Fcγ receptor (FcγR) III | Pioglitazone | Ischemic stroke treatment | 84 |
| Denatured BSA nanoparticles | Neutrophils | Internalization | Fcγ receptor (FcγR) III | Doxorubicin | Ischemic stroke treatment | 103 |
| Denatured BSA nanoparticles | Neutrophils | Internalization | Fcγ receptor (FcγR) III | TPCA-1 (NF-κB inhibitor) | Acute lung injury treatment | 102 |
| DOCP lipid nanoparticle | Neutrophils | Internalization | C3/C3R interaction | Dexamethasone/ampicillin | Acute lung injury treatment | 57 |
| HA-decorated multistage nano-micelles | Macrophages and neutrophils | Internalization | CD44/HA interaction | KIRA6 and Dexamethasone | Acute lung injury treatment | 140 |
| Lipid-conjugated floxuridine oligonucleotide | Serum albumin | Adsorption | Hydrophobic interaction | LFU20 | Cancer treatment | 123 |
| Hitchhiking hybrid micelles (POR/DT) | Serum albumin | Adsorption | Hydrophobic interaction | OXA/TRE | Cancer treatment | 144 |
| OPDEA-PSN38 | Red blood cells | Adsorption | Interaction with the PE and PC | SN38 | Cancer treatment | 82 |
| N-oxide moiety-containing liposomes | Red blood cells | Adsorption | Interaction with the PE and PC | SN38 | Cancer treatment | 81 |
| 5-HT equipped nanoparticles | Neutrophils | Internalization | 5-HT/MPO interaction | HPPH/Zileuton | Cancer treatment | 145 |
| Anti-CD11b BSA nanoparticle | Neutrophils | Internalization | Anti-CD11b/CD11b interaction | Decitabine/IR820 | Cancer immunotherapy | 146 |
| Anti-CD3e f(ab')2-decorated nanoparticle | T cells | Internalization | CD3/anti-CD3 interaction | pDNA | Cancer immunotherapy | 62 |
| Liver SORT lipid | Apolipoprotein E (ApoE) | Adsorption | ApoE/LDL-R interaction | mRNA | Gene therapy | 152,153 |
| Spleen SORT lipid | β2-glycoprotein I (β2-GPI) | Adsorption | β2-GPI/Phosphatidyl serine | mRNA | Gene therapy | 152,153 |
| Lung SORT lipid | Vitronectin (Vtn) | Adsorption | Vtn/αvβ3 integrin | mRNA | Gene therapy | 152,153 |
| O-series LNPs | Apolipoprotein E (ApoE) | Adsorption | ApoE/LDL-R interaction | mRNA | Gene therapy | 154,155 |
| N-series LNPs | Fibrinogen | Adsorption | - | Tsc3 mRNA | Gene therapy | 154,155 |
| DOPG lipid nanoparticle | Neutrophils | Internalization | C3/C3R interaction | NIR-IIb immunotracer | Diagnostic imaging | 105 |
| T-cell-targeting fusogenic liposomes | T cells | Internalization | Anti-CD3/CD3 interaction | TEMP | Diagnostic imaging | 39 |
The adsorption of nanoparticles to cells holds great promise for targeted drug delivery and imaging in nanomedicine applications. However, to ensure the safe and effective use of this approach, it is crucial to thoroughly investigate its impact on cellular functions, particularly cell migration [22, 92]. Several concerns regarding cellular hitchhiking need to be addressed: (1) Cellular functionality: the attachment of materials or nanoparticles may affect the functionality of surface proteins on the cell membrane, potentially altering the cell's ability to interact with its environment and respond to external stimuli. This could have unintended consequences on cell behavior, potentially compromising the intended applications of hitchhiked cells; (2) Immune response: modified cells may provoke an immune response from the body, leading to potential rejection or inflammatory reactions. Even if the attached materials are designed to be biocompatible, the immune system might still recognize the hitchhiked cells as foreign entities; (3) Long-term effects: the long-term consequences of cellular hitchhiking are not yet fully understood. It is vital to investigate whether the attached materials degrade over time or accumulate on the cell surface, which could potentially lead to adverse effects on cell health. To make cellular hitchhiking a viable and safe approach, researchers must carefully design and characterize the materials used for hitchhiking. Thorough biocompatibility testing, in vitro and in vivo studies, and comprehensive analysis of cellular responses are essential steps to understand the impacts on cell function. Biodegradable and non-toxic materials are preferred to ensure that they do not accumulate in the body and cause harm over time. In this regard, nanoparticles with surface coatings that enhance biocompatibility and reduce immune responses are being explored to address these issues. Moreover, researchers must establish clear guidelines and safety standards for the clinical use of hitchhiked cells. Thorough preclinical studies and rigorous risk evaluation are essential prerequisites before translating cellular hitchhiking techniques into human therapies. In conclusion, cellular hitchhiking offers exciting possibilities in biomedical applications, but the biocompatibility of attached materials and nanoparticles must be carefully evaluated. Understanding the tradeoffs and potential risks associated with cellular hitchhiking is essential to harness its potential benefits while ensuring the safety and effectiveness of this innovative approach.
Cell/protein choices for in vivo hitchhiking
The circulatory cells/proteins exhibit several unique properties, including unrivaled systemic circulation capabilities, inherent stealth properties, natural delivery mechanisms, and the ability to navigate and traverse biologically impermeable barriers, which make circulatory cells/proteins ideal vehicles for targeted drug delivery. These features arise from the mechanical properties, distinctive structure, and surface ligands characteristic of each specific cell type. Therefore, a comprehensive understanding of these cellular functions is a prerequisite for the development of successful cell hitchhiker formulations. In this section, we will briefly describe the structure and functions of three major cells/proteins for in vivo hitchhiking, including neutrophiles, RBCs, and serum albumin.
Hitchhiking on neutrophils
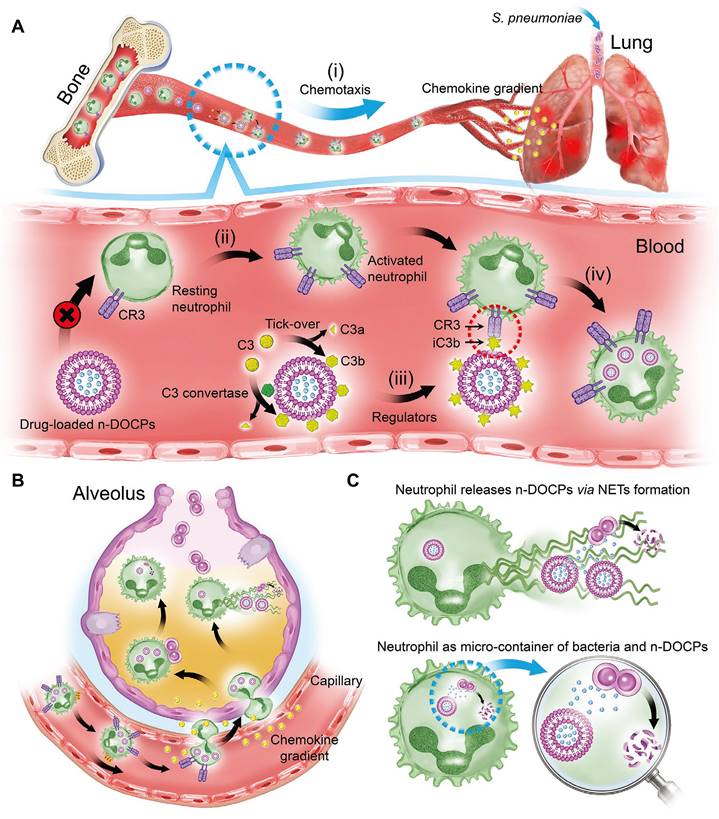
Neutrophils, also referred to as polymorphonuclear leukocytes, heterophils, or neutrocytes, represent the predominant class of granulocytes, comprising approximately 40-70 % of all WBCs in humans [93, 94]. The neutrophils play a crucial role as the frontline defense in human immunity, rapidly activating in response to inflammatory stimuli [95]. This activation triggers an up-regulation of surface receptors, such as CD11b, CD14, and TLR 4, augmenting their phagocytic capability [96-98]. Additionally, neutrophils demonstrate an impressive tropism for inflamed regions, enabling them to traverse various biological barriers, including blood vessels and the blood-brain barrier (BBB), through deformation and other mechanisms [99, 100]. Upon reaching the inflammation site, neutrophils release neutrophil extracellular traps (NETs), facilitating the traceless release of hitchhiking nanomaterials [101]. Based on these unique characteristics, several neutrophile hitchhiking nanomaterials have been developed [57, 63, 102-105]. For example, Wang et al. presented a piceatannol (spleen tyrosine kinase inhibitor)-loaded denatured BSA nanoparticles that can efficiently disrupt β2 integrin signaling in leukocytes by hitchhiking neutrophils and mitigate vascular inflammation [56]. Employing a similar approach, they successfully achieved selective elimination of inflammatory neutrophils and alleviated neurological damage in stroke through systemic administration of doxorubicin (DOX)-loaded BSA nanoparticles [103]. Another notable contribution by Wang et al. involved the presentation of a specific lipid, DOCP, which rapidly forms a protein corona containing complement fragment iC3b [57, 106, 107]. This corona facilitated neutrophil hitchhiking through C3 receptor-mediated phagocytosis. Subsequently, the hitchhiked neutrophils were able to carry liposomes to migrate across the alveolar-capillary barrier into inflamed tissues and release ampicillin-loaded liposomes via the formation of NETs, resulting in a significant alleviation in acute lung injury (ALI). Similarly, Liu et al. presented that another lipid, DOPG, exhibited a similar behavior to DOCP that can selectively adsorb complement fragment iC3b to facilitate neutrophil hitchhike [105]. By systemically administering a DOPG-modified NIR-IIb emissive immunotracer, they successfully achieved dynamic monitoring of neutrophils in inflammation-related diseases. Although promising, neutrophil hitchhiking may also cause some problems related to toxicity and bioaccumulation. Toxicity concerns: Neutrophils are immune cells that release ROS and cytotoxic enzymes to destroy pathogens. The hitchhiking process or the cargo itself might interfere with the normal function of neutrophils, affecting their ability to combat infections and potentially leading to immune-related toxicity. Bioaccumulation: After internalization, the premature release of loaded drugs in neutrophils may impair their migration, homing, and other functions, leading to unpredictable therapeutic efficacy. Therefore, precise control of the drug release profile and avoiding undesired interference with neutrophils are crucial factors in successfully hitchhiking on neutrophils.
Hitchhiking on RBCs
Red blood cells (RBCs), or erythrocytes, have been widely recognized and utilized as highly established and popular natural carriers for drug delivery since the 1970s. This is primarily due to their remarkable and diverse advantages [108]. For example, RBCs have an extremely long circulating half-life of approximately 120 days in humans, exhibit excellent biocompatibility and accessibility, and can be utterly degraded without producing toxic metabolites [109]. Moreover, RBCs possess a distinct biconcave shape with great natural markers on their surface, including glycan, sialic acid, protein, and CD47, offering numerous opportunities for efficient drug loading and in vivo hitchhiking [110, 111]. Based on these unique characteristics, several RBCs hitchhiking nanomaterials have been developed. For example, Brenner et al. presented a dual affinity to RBCs and target cells (DART) strategy for highly efficient organ and cell-type targeting [89]. DART strategy was achieved by conjugating two types of antibodies that selective binding to RBCs (anti-GPA) and endothelial epitopes (anti-PECAM) onto liposomes. After intravenous injection, the DART nanocarriers efficiently hitchhiked RBCs through GPA/anti-GPA interaction and selectively bound to the pulmonary vasculature under the guidance of RBCs. Similarly, Ranjan et al. proposed a Ter119 antibody-modified polymer nanoparticle and efficiently hitchhiked erythrocytes by targeting GPA on erythrocytes, thereby significantly prolonging the circulation time of the nanoparticles without causing any histological, hematological, and morphological complications [112]. In addition to antigen-antibody interactions, amphiphilic molecules are reported to exhibit binding affinities toward RBCs. For example, Brooks et al. demonstrated that amphiphilic polyethylene glycol (PEG)-hyperbranched polyglycerol (HPG)-polymers incorporating stearoyl chains (HPG-C18-PEG) were capable of effective adsorption onto RBCs [113]. Similarly, Shen et al. presented that zwitterionic liposomes containing tertiary amine oxide (TAO) achieved efficient hitchhiking on RBCs [82]. Furthermore, a host-guest strategy has also been employed for RBCs hitchhiking. For example, Wang et al. reported a host-guest strategy that exploits the interaction between β-cyclodextrin (β-CD) and ferrocene (Fc) to facilitate the binding of liposomes to RBCs [114]. The researchers initially modified RBCs with DSPE-PEG-CD and integrated Fc groups into liposomes, forming RBCs-CD and Lipo-Fc, respectively. Through the host-guest interactions between β-CD and Fc, Lipo-Fc effectively hitchhiked onto RBCs-CD, significantly improving circulation stability. This RBCs-hitchhiking strategy offers significant advantages regarding prolonged circulation and controlled release of therapeutic enzymes, potentially benefiting scenarios like alcohol poisoning. Moreover, it holds promise for delivering nanoparticles to body regions with high cardiac output, allowing targeted delivery of therapeutic nanoparticles to adjacent pathologies.
Despite the significant successes achieved with RBCs-hitchhiking, some limitations should be considered. Toxicity concerns: RBCs are generally considered inert and typically do not induce significant immune responses or toxicity in the body. However, safety concerns may arise, particularly hemolysis, which could occur during the hitchhiking process or following delivery to the target site. The rupture of RBCs may lead to the release of hemoglobin and other cellular contents, potentially causing adverse reactions. Bioaccumulation: RBCs have a relatively short lifespan of approximately 120 days in circulation and are eventually cleared from the body through the spleen and liver. Nonetheless, in cases where the nanoparticles remain within hitchhiked RBCs for an extended period, there exists a potential risk of bioaccumulation. This could result in the gradual build-up of cargo over time, leading to unintended consequences. Therefore, careful consideration and comprehensive evaluation of such approaches are necessary to ensure their safety and effectiveness in biomedical applications.
Hitchhiking on serum albumin
Serum albumin (SA), with a molecular weight of 66.5 kDa, is the most abundant protein found in the bloodstream [115]. It possesses a remarkable systemic circulation time of approximately 19 days [116]. In addition, SA exhibits a distinctive pocket structure that enables the transportation of hydrophobic molecules, including metal ions, drugs, long-chain fatty acids, and hormones, throughout the bloodstream [117]. Furthermore, SA also exhibits remarkable capabilities in diverse physiological processes, such as evasion of renal clearance, transcytosis across endothelial cells, and efficient accumulation in rapidly growing nutrient-poor tissues [118]. These unique features make SA ideal candidate for in vivo hitchhiking, offering significant potential for optimizing therapeutic outcomes. A general strategy for in situ SA hitchhiking involves the use of maleimides for covalent attachment to thiol groups on SA cysteines. Notably, this thiol group is the most abundant free thiol in blood, while other thiol functional groups usually exist in the form of non-reactive disulfide bonds, effectively avoiding undesired off-target reactions [119]. For example, Liu et al. conjugated maleimide to paclitaxel (PTX) and effectively promoted the accumulation of the PTX prodrugs in tumor tissues through albumin receptor-mediated active targeting [120]. Similarly, Unger et al. presented a maleimide-conjugated doxorubicin for SA hitchhiking, demonstrating significantly enhanced tumor suppressions in breast carcinoma MDA-MB 435 and breast carcinoma MCF-7-bearing mice models [119]. In addition to the maleimide group, the meso-chloro is another chemical moiety capable of reacting with the thiol group of albumins. For example, Wang et al. reported a meso-chloro-integrated near-infrared window II (NIR-II) fluorescence probe, IR1080, which demonstrated precise detection of micro-metastases and facilitated fluorescence image-guided surgery through SA hitchhiking [121].
In addition to the covalent-based strategy for SA hitchhiking, the unique pocket structure also allows nanomaterials to hitchhike onto SA through hydrophobic or other interactions. For example, Irvine et al. discovered that lipo-PEG amphiphiles with two hydrophobic tails could efficiently insert into the hydrophobic core of albumin through hydrophobic interactions [122]. They further investigated the impact of hydrophobic tail length on the efficiency of albumin insertion and observed that longer diacyl tails (≥ 16 carbons) exhibited a higher affinity for albumin. With this finding, they successfully utilized SA hitchhiking to deliver lipo-CpG to lymph nodes, resulting in significantly enhanced T cell priming and cancer immunotherapy. Similarly, Tan et al. employed this strategy to achieve tumor-targeted delivery of a floxuridine homomeric oligonucleotide (LFU20) for enhanced cancer chemotherapy [123]. Another frequently used functional group for SA hitchhiking is sulfonated azophenyl, which exhibit strong binding ability towards SA. For example, Evans Blue (EB), which contains two sulfonated azophenyl groups, is a commonly used SA-specific azo dye [124, 125]. After systemic administration, EB selectively binds to circulating SA and assesses BBB permeability. Furthermore, several EB derivatives have been developed for long-acting therapeutics and diagnostic imaging. For example, diethylenetriaminepentaacetic acid-conjugated truncated EB (EB-DTPA) for gadolinium (III) complexation and magnetic resonance imaging (MRI) [126]; 1,4,7-triazacyclononane-N,N',N''-triacetic acid (NOTA) conjugated truncated EB (NEB) for isotopes labeling (68Ga, 64Cu, and Al18F) and positron emission tomography (PET) imaging [127]; EB-maleimide conjugated exendin-4 for significantly enhanced circulation stability and type 2 diabetes/obesity treatment [128]. Moreover, EB analogs have also been introduced to macrocyclic hosts. For example, Guo et al. presented a sulfonated azocalix[5]arene (SAC5A) for tumor-targeted delivery of paclitaxel (PTX) [9]. SAC5A efficiently loads PTX in vitro through host-guest interaction to form a binary supramolecular prodrug (PTX@SAC5A). Following intravenous injection, PTX@SAC5A rapidly hitchhikes with circulating SA to form a ternary formulation, PTX@SAC5A/albumin, which significantly prolongs the circulating half-life of PTX and dramatically improves its antitumor efficacy.
Serum albumin is a protein present in the bloodstream and is widely considered safe for therapeutic applications due to its natural occurrence in the body. Nevertheless, there are potential concerns regarding toxicity if the hitchhiked cargo interacts with albumin in a manner that disrupts its normal physiological function or results in unintended effects. Moreover, serum albumin exhibits a relatively extended half-life (approximately 20 days), making it an appealing carrier for sustained delivery of therapeutic payloads. However, this prolonged circulation time also raises the possibility of bioaccumulation if the hitchhiked cargo is not efficiently released from the albumin molecule. To address these challenges and optimize the therapeutic benefits, it is crucial to meticulously design and optimize the hitchhiking process and release mechanisms.
Hitchhiking on platelet
Platelets, also known as thrombocytes, are small, disc-shaped blood cells present in mammals' bloodstream, including humans. Like neutrophils, platelet can efficiently accumulate in injured or inflamed tissues and activated in response to inflammatory stimuli [129]. This activation triggers an up-regulation of surface receptors, such as P-selectin, GPIb-V-IX, and integrin aIIbb3 (GPIIb/IIIa) [130]. These receptors allow the effective binding of activated platelets on circulating tumor cells (CTCs) or injured tissues, enabling them to carry out essential functions like tumor metastasis, tissue repair, or hemostasis. Given the specific binding capabilities to these receptors, nanoparticles can be designed to adsorb on platelets and hitchhike along with them, facilitating the delivery of anticancer or antithrombosis drugs. For example, He et al. introduced a fucoidan-decorated DOX-loaded polymeric micelle for the treatment of metastatic cancer [131]. Within the bloodstream, the fucoidan groups of the micelle demonstrated exceptional adhesion to activated platelets on circulating tumor cells (CTCs) through fucoidan/P-selectin interaction [132, 133], enabling effective hitchhiking of platelets and their accumulation in tumor tissues. Consequently, the DOX efficiently induced immunogenic cell death (ICD), showcasing highly effective tumor suppression in a 4T1 tumor mice model. This study highlights the potential of platelet-mediated hitchhiking as a promising strategy for targeted drug delivery and improved cancer therapy outcomes.
Biomedical applications of hitchhiking nanomaterials
By hitchhiking on circulatory cells or proteins, these rationally designed nanomaterials exhibit multiple advantages over conventional carriers, including significantly enhanced circulation stability and half-life, active targeting, and minimized off-target effect. Benefiting from these unique properties, hitchhiking nanomaterials are increasingly used in biomedical applications. This section provides a comprehensive overview of recent advancements in hitchhiking nanomaterials for ischemic stroke, acute lung injury, cancer treatment, gene therapy, and diagnostic imaging.
Neutrophils-hitchhiking nanomaterials for ischemic stroke treatment
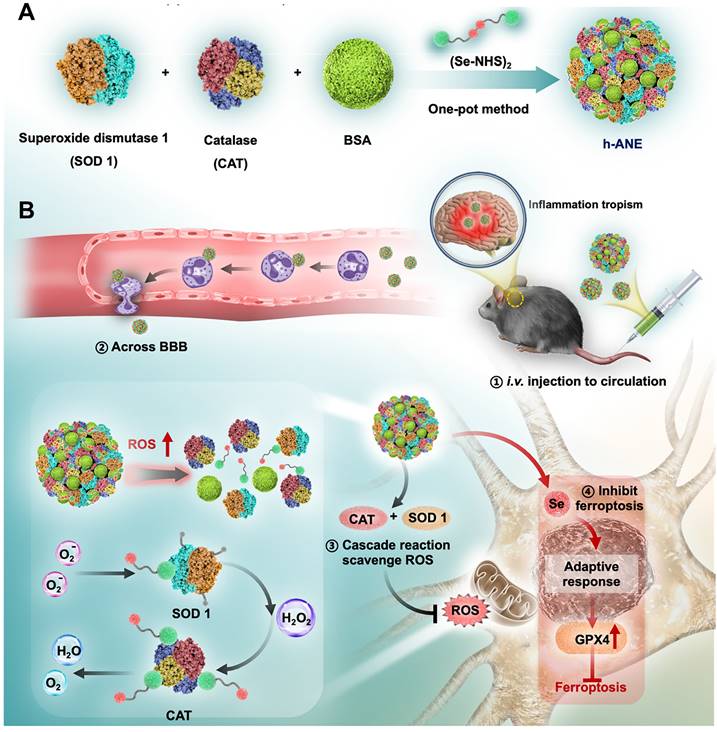
Ischemic stroke is a fatal disease caused by insufficient blood supply to the brain, often accompanied by an accumulation of toxic reactive oxygen species (ROS) in the brain. Effective treatment of ischemic stroke necessitates addressing two key aspects: (1) crossing the BBB and (2) eliminating ROS. The inflammatory tropism of neutrophils makes hitchhiking neutrophils an ideal strategy to cross the BBB and treat ischemic stroke [100, 134]. For example, Liu et al. presented nano-integrated cascade enzymes (h-ANEs) composed of superoxide dismutase (SOD), peroxidase (CAT), and BSA (Figure 2) [135]. In the bloodstream, the integrated albumin exhibits excellent adhesion to activated neutrophils, enabling effective hitchhiking of neutrophils to cross the BBB and accumulate in cerebral infarction areas. Subsequently, the SOD and CAT of h-ANEs efficiently eliminate toxic ROS, demonstrating highly efficient neuroprotection in an ischemia-reperfusion injury mouse model. Tang et al. reported a PLGA-PEG-based polymeric nanoparticle (T-TMP) to deliver ligustrazine to treat cerebral ischemia-reperfusion injury [52]. The surface of T-TMP is integrated with a neutrophils-targeting peptide (CFLFLF), which can effectively direct T-TMP to neutrophils and thus hitchhike neutrophils after intravenous injection. Upon reaching lesion tissues, T-TMP rapidly responds to high levels of ROS and release ligustrazine, resulting in significantly enhanced neuroprotection. Similarly, Zhou et al. leveraged the bacteria-scavenging capabilities of neutrophils and developed a bacteria-derived outer-membrane vesicle (OMV) for targeted delivery of pioglitazone (PGZ) to the brain [84]. In addition to eliminating ROS, inhibiting the infiltration of inflammatory cells in the brain is also an effective way to relieve ischemic stroke. For example, Wang et al. presented a DOX-conjugated BSA nanoparticle capable of selectively inducing neutrophil apoptosis, effectively preventing brain injury in cerebral ischemia/reperfusion mice model [103]. Overall, these innovative strategies that leverage neutrophils' inflammatory tropism and scavenging abilities hold great promise for developing targeted and effective therapies for ischemic stroke. By addressing both ROS accumulation and inflammatory cell infiltration, these approaches open up new possibilities for enhancing neuroprotection and improving outcomes for stroke patients. Further research and clinical studies will be essential to validate and optimize these techniques for eventual translation into clinical practice.
Neutrophils-hitchhiking nanomaterials for acute lung injury (ALI) treatment
ALI is a diffuse heterogeneous lung injury characterized by widespread capillary leakage, low lung compliance, non-cardiogenic pulmonary edema, and hypoxemia [136, 137]. In ALI, the lungs experience a significant influx of inflammatory cells, predominantly neutrophils, into the affected areas [138]. This influx of inflammatory cells plays a critical role in exacerbating lung injury and contributing to the overall pathology of ALI. The underlying pathology of ALI is largely attributed to the cytokine storm triggered by the NF-κB pathway. The NF-κB pathway is a central regulator of inflammation and immune responses in the body [139]. Its dysregulation leads to the overproduction of pro-inflammatory cytokines and chemokines, causing an excessive lung inflammatory response. This, in turn, damages the lung capillaries, leading to the observed capillary leakage and pulmonary edema in ALI. Hypoxemia, a condition characterized by low levels of oxygen in the blood, is a prominent consequence of ALI and contributes to the severity of the disease. Due to the critical role of the NF-κB pathway in driving the cytokine storm and subsequent lung inflammation, researchers have identified it as a promising therapeutic target for ALI treatment. For example, Wang et al. developed a novel approach using denatured BSA nanoparticles (BSA-NPs) as a drug delivery system to specifically target the lungs and deliver an NF-κB inhibitor, TPCA-1 [102]. In their study, these BSA-NPs were designed to take advantage of the innate ability of neutrophils to home in on inflamed tissues. When administered intravenously, the BSA-NPs are selectively internalized by neutrophils and subsequently accumulate in the injured lung tissues. By delivering the NF-κB inhibitor directly to the site of inflammation, this targeted approach effectively reduces the activation of the NF-κB pathway. As a result, there is a significant decrease in lung inflammation, leading to alleviating ALI symptoms and improved lung function. This innovative approach of using denatured BSA nanoparticles for targeted drug delivery not only enhances the therapeutic efficacy of the NF-κB inhibitor but also minimizes off-target effects on other organs, making it a safer and more efficient strategy for ALI treatment.
Besides the NF-κB pathway, several clinical studies also suggest that endoplasmic reticulum stress (ER stress)-induced macrophage polarization contributed to the development of ALI. ER stress is a condition that occurs when the endoplasmic reticulum, a cellular organelle involved in protein synthesis and folding, becomes overwhelmed or damaged, leading to the accumulation of misfolded proteins. This stress triggers a response known as the unfolded protein response (UPR), which aims to restore ER homeostasis. However, if the stress persists or is too severe, it can lead to macrophage polarization, which contributes to the pathogenesis of ALI. Relieving ER stress to restore macrophage homeostasis represents a potential approach for ALI prevention. To this end, You et al. presented HA-decorated multistage targeted nano-micelles for delivering ER stress inhibitor (KIRA6) and anti-inflammatory drugs dexamethasone (Dex) to infected lungs [140]. In the bloodstream, the nano-micelles effectively hitchhike on myeloid macrophages and neutrophils through CD44/HA interaction, thus effectively accumulating in infected lungs. Subsequently, the drug-loaded nanoparticle quickly responds to high-level ROS to facilitate the release of KIRA6 and Dex, thereby effectively relieving ER stress, balancing macrophage homeostasis, and finally resolving ALI. Additionally, delivering anti-inflammatory drugs or antibiotics to the lung is feasible for ALI treatment. For example, Wang et al. found that liposomes comprising inverted phosphocholine lipids rapidly enriched iC3b through voluntary opsonization, facilitating neutrophil hitchhiking through C3 receptor-mediated phagocytosis (Figure 3) [57]. With this strategy, they effectively delivered Dex and antibiotics ampicillin (Amp) to the lung, leading to a significant attenuation of lung inflammation. These strategies underscore the importance of exploring innovative approaches for ALI prevention and treatment, especially those that leverage the innate properties of immune cells for targeted drug delivery. These studies offer great potential for more effective and tailored therapies that may improve patient outcomes and reduce the burden of this severe and life-threatening condition.
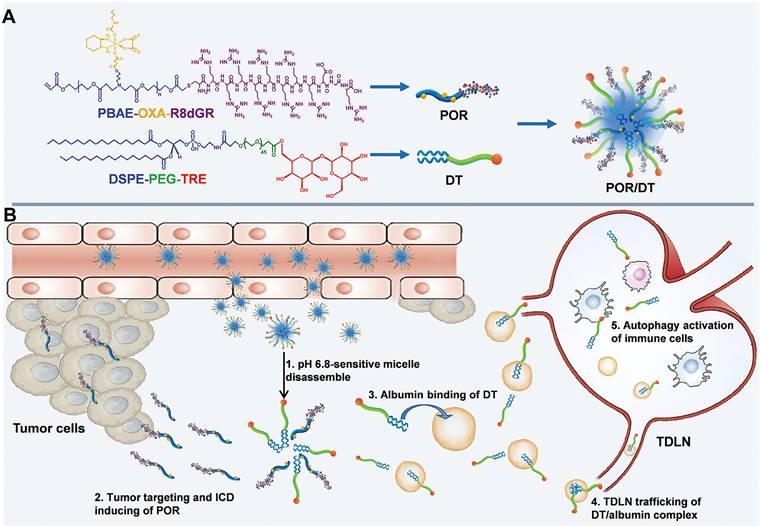
Hitchhiking nanomaterials for cancer treatment
Cancer is a leading cause of death worldwide, characterized by rapid proliferation and high invasiveness [141]. Multiple genetic mutations and alterations lead to a unique tumor microenvironment (TME) and the upregulation of tumor-specific biomarkers [142]. For example, GP60 and secreted protein acidic and rich in cysteine (SPARC) are albumin-binding proteins highly expressed in various tumors [143]. Exploiting the hitchhiking strategy on serum albumin offers a promising approach for effective cancer treatment. First, albumin is abundant in the bloodstream and is known to accumulate in the tumor due to the EPR effect. This passive targeting of the tumor by albumin carriers increases the drug's concentration at the tumor site while reducing systemic exposure and potential side effects. Additionally, the hitchhiking approach takes advantage of tumor-specific biomarkers like GP60, ensuring preferential uptake and internalization of the drug-loaded complex by cancer cells, further enhancing treatment efficacy [143]. Tan et al. presented a lipid-conjugated floxuridine oligonucleotide (LFU20) that hitchhikes SA to enhance cancer chemotherapy [123]. In blood circulation, LFU20 readily interacts with SA through hydrophobic interaction to form an LFU20/albumin complex, which then accumulates in the tumor and internalizes by cancer cells through BSA/GP60 interaction, resulting in significantly enhanced antitumor efficacy. Moreover, the small size of SA enables SA-hitchhiking materials to accumulate in lymph nodes through interstitial flow. For example, He et al. presented hitchhiking hybrid micelles (POR/DT) for the sequential delivery of oxaliplatin (OXA) and trehalose (TRE) to tumors and tumor-draining lymph nodes (TDLNs) (Figure 4) [144]. POR/DT was prepared by co-assembling poly (β-amino esters)-based pH responsive polymer (POR) and DSPE-PEG-TRE (DT). Upon reaching tumor tissue, POR/DT quickly responds to acidic TME and facilitates the disintegration of POR/DT into POR and DT. Subsequently, the POR prodrug effectively targeted tumor tissues via integrin receptor recognition, while DT hitchhiked on SA and accumulated in TDLNs through interstitial flow. Finally, these two drugs synergistically activated immunogenic cell death (ICD)-associated antitumor immunity and significantly prolonged the survival rate of CT26-bearing mice. These albumin-hitchhiking approaches hold great promise for personalized and effective cancer therapies, providing a more tailored treatment approach based on the unique characteristics of each patient's tumor microenvironment and tumor-specific biomarkers.
Schematic illustrating the “neutrophil piggybacking” h-ANEs for antioxidant and anti-ferroptosis therapy of cerebral I/R injury. A, Schematic illustration of the preparation of h-ANE. B, The biological process of h-ANEs for antioxidant and anti-ferroptosis therapy of cerebral I/R injury. Adapted with permission from [135], copyright 2022 Wiley-VCH.
Schematic illustration of complement fragments tagging on the nanoparticles mediated rapid neutrophilic entry for inflammation targeting. A, In vivo hitchhiking process of n-DOCPs on activated neutrophils. B, The hitchhiked neutrophils move to inflammation area under the motivation of chemokine gradient. C, Neutrophils release n-DOCPs for enhanced anti-inflammation treatment. Adapted with permission from [57], copyright 2021 Wiley-VCH.
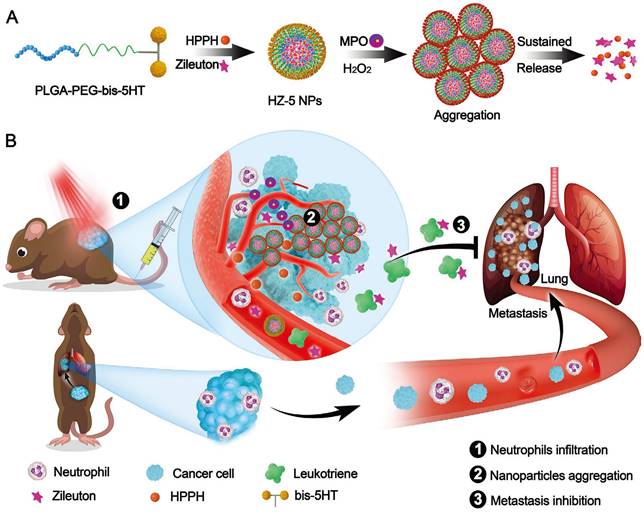
In addition, hitchhiking on red blood cells presents significant opportunities for enhancing the circulation half-life and stability of therapeutic agents. For example, Shen et al. presented a poly(2-(N-oxide-N,N-diethylamino)ethyl methacrylate) (OPDEA)-based SN38 conjugate for effective tumor extravasation and penetration [81]. During blood circulations, the N-oxide moiety of OPDEA binds reversibly to the hydrophilic head of phosphatidylethanolamine and phosphatidylcholine on the RBCs membrane. Upon reaching the tumor tissues, OPDEA disassociated from RBCs, efficiently binds to tumor capillary endothelial cells (tECs) and facilitates adsorption-mediated transcytosis. This well-designed hitchhiking strategy enables the successful delivery of SN38 to deeper tumor tissues, resulting in significantly enhanced antitumor efficacy of SN38 in HepG2 tumor-bearing mice. Similarly, they also developed a series of tertiary amine oxide (TAO)-integrated liposomes and effectively delivered the SN38/CPT-11 to deeper tumors through hitchhiking on RBCs and adsorption-mediated transcytosis [82]. Moreover, the inflammatory TME enables nanomaterials to hitchhike on neutrophils and macrophages for tumor-targeted drug delivery. For example, Chen et al. presented 5-hydroxytryptamine (5-HT) equipped nanoparticles (HZ-5 NPs) for the tumor-targeted delivery of photosensitizers (HPPH) and leukotriene inhibitor (Zileuton) to suppress tumor growth and inhibit lung metastasis (Figure 5) [145]. In the bloodstream, the 5-HT of HZ-5 NPs efficiently targets neutrophil myeloperoxidase (MPO) and accumulates in tumor tissues through neutrophil hitchhiking. Consequently, Zileuton is released from HZ-5 NPs to inhibit leukotriene release, effectively preventing the recruitment of neutrophils into the lungs and finally inhibiting lung metastasis. Similarly, Lv et al. presented an anti-CD11b conjugated BSA nanoparticle (ANP) to hitchhike activated neutrophils through CD11b/anti-CD11b interaction [146]. After hitchhiking, this rationally designed ANP effectively delivered decitabine (DAC) and IR820 to tumor tissues, thereby effective activating pyroptosis and significantly enhancing cancer immunotherapy. These studies represent a paradigm shift in drug delivery approaches, providing personalized and efficient cancer therapies based on the unique characteristics of each patient's tumor microenvironment and immune response.
Moreover, leveraging hitchhiking to engineer immune cells holds great promise for significantly enhancing cancer immunotherapy. For example, T cells are at the forefront of this approach, which is important in recognizing and eliminating various “enemies” such as infections, tumors, and foreign bodies [147]. Nevertheless, the reduced expression or absence of T cell-targeted ligands on the tumor surface can lead to immune escape and impair antitumor efficacy. To address these issues, a promising strategy involves genetically engineering T cells with cancer-specific chimeric antigen receptors (CARs). A seminal example presented by Stephan et al. is that they developed an anti-CD3e f(ab')2-decorated synthetic DNA nanocarrier to in situ programming leukemia-specific T cells [62]. This nanocarrier was loaded with plasmid DNA (pDNA) encoding leukemia-specific 194-1BBz CAR. After intravenous injection, the DNA nanocarrier efficiently targets to T cells through the CD3/anti-CD3 interaction. Subsequently, the leukemia-targeting CAR antibodies were expressed and transmembrane transported to T cells' surface, resulting in the effective recognition of leukemia cells and significantly enhanced antitumor efficacy. In addition to T cells, tumor-associated macrophages (TAMs) are another essential immune cell predominantly found in tumor tissues. They significantly contribute to the formation of immunosuppressive TME and play an essential role in promoting tumor recurrence. Jiang et al. assume that genetically engineering luminal TAMs with tumor-specific CARs can redirect their phagocytic ability toward tumor cells [148]. By acting as APCs, these modified TAMs stimulate adaptive immune responses to prevent postoperative tumor recurrence. As a proof of concept, Jiang et al. presented a nanoporter to engineering macrophages/microglia for postoperative glioblastoma therapy. The nanoporter has a core-shell structure in which pDNA encoding glioma stem cells (GSCs)-specific antibody (CD133) was encapsulated inside the core with a citraconic anhydride-modified dextran (CA-dextran)-based network layer. Similar to the DNA nanocarrier reported by Stephan et al., this nanoporter efficiently targets TAMs through dextran/CD206 interaction in tumor tissues, leading to the generation of CD133 overexpressed CAR-M. Subsequently, the newly generated CAR-M effectively seeks and engulfs GSCs, clearing residual GSCs and significantly enhancing antitumor efficacy. Consequently, this therapeutic approach greatly inhibits postoperative glioma recurrence, showing promise for future cancer treatments.
These in situ engineering strategies for immune cells hold tremendous potential in significantly enhancing cancer immunotherapy while minimizing the need for extensive purification processes and reducing the risk of undesired toxic side effects. Despite the advancements, there are still two critical directions for further improving cancer immunotherapy: i) Enhance targeting specificity to immune cells: One key aspect to address is refining the targeting specificity of the engineered therapies toward immune cells. Ensuring precise and selective targeting of immune cells will maximize the therapeutic effect while minimizing potential off-target effects on healthy cells. Research efforts should be directed towards developing more sophisticated targeting mechanisms that precisely recognize and bind to the intended immune cell populations. ii) Improve transfection efficiency in immune cells: Another vital area of improvement lies in enhancing the transfection efficiency of genetic material into immune cells. Higher transfection efficiency will result in a greater number of successfully engineered immune cells, thereby increasing the overall efficacy of the treatment. Investigating and implementing novel transfection techniques and delivery systems could lead to more efficient and reliable gene modification of immune cells. By advancing research in these two directions, cancer immunotherapy can make significant strides in achieving safer, more effective, and targeted treatments for cancer patients.
Schematic illustration of the albumin hitchhiking nanomedicine for tumor immunotherapy by autophagy activation and ICD induction. A, The preparation of POR/DT. B, The biological process of POR/DT for enhanced cancer immunotherapy. Adapted with permission from [144], copyright 2021 Wiley-VCH.
5-Hydroxytryptamine (5-HT) equipped nanoparticles (HZ-5 NPs) for enhanced cancer treatment. A, Design of HZ-5 NPs for MPO-catalyzed aggregation and sustained drug release. B, HZ-5 NPs accumulate in tumor sites and release Zileuton for enhanced cancer treatment. Adapted with permission from [145], copyright 2020 Wiely-VCH.
Hitchhiking nanomaterials for other applications
Genetic disease treatment
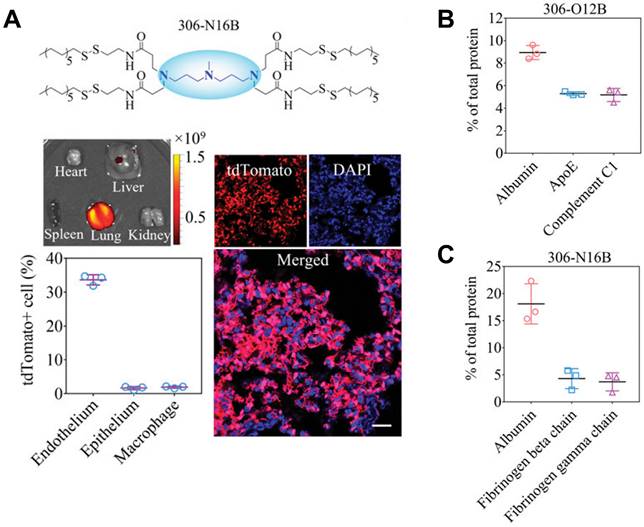
Gene therapy is a cutting-edge biotechnology that seeks to achieve therapeutic impacts by manipulating gene expression or altering the biology of living cells [149]. Depending on the duration of action, gene therapy can be broadly categorized into two main types: permanent regulation at the genome level, or temporary regulation at the protein level. Typically, these therapeutic interventions are administrated in forms of proteins, RNA, or plasmid DNA (pDNA) and require crossing multiple physiological barriers before exerting their efficacy. To this end, multiple non-virus vectors based on cationic polymer, dendrimers, micelles, and lipid nanoparticles (LNP) are developed for gene delivery [141, 150, 151]. However, these vectors are mainly distributed in the liver, and how to perform gene editing in non-liver organs remains challenging. Siegwart et al. presented a selective organ targeting (SORT) strategy for the selective delivery of message RNA (mRNA) to the liver, spleen, or lung [152, 153]. Liver SORT, spleen SORT, and lung SORT were formulated by incorporating ionizable cationic lipid, anionic lipid, and permanently cationic lipid, into initial mRNA formulation (5A2-SC8, DOPE, cholesterol, DMG-PEG; 15/15/30/3, mol/mol), respectively. Upon intravenous injection, Liver SORT efficiently adsorbs apolipoprotein E (ApoE) and hitchhiking on ApoE to accumulated in liver through ApoE/low-density lipoprotein receptor (LDL-R) interaction. Similarly, spleen SORT and lung SORT efficiently adsorbs β2-glycoprotein I (β2-GPI) and vitronectin (Vtn) and hitchhiking on these proteins to accumulated in spleen throughβ2-GPI/phosphatidyl serine interaction or lung through Vtn/αvβ3 integrin interaction, respectively. With this strategy, they effectively delivered various types of mRNA to liver, spleen, and lung, demonstrating great potential for gene editing in non-liver organs. Similarly, Xu et al. discovered that LNPs belonging to the O-series, which contain an ester bond in the tail, have a tendency to deliver mRNA specifically to the liver (Figure 6) [154, 155]. Conversely, LNPs belonging to the N-series, which contain an amide bond in the tail, demonstrate the capability to selectively deliver mRNA to the lungs of mice. This organ selectivity observed with LNPs is analogous to the behavior of SORT molecules. Specifically, O-series LNPs efficiently adsorb and hitchhike apolipoprotein E (ApoE), while N-series LNPs effectively adsorb and hitchhike fibrinogen. Using this strategy, they successfully delivered mouse tuberous sclerosis complex 2 (Tsc2) mRNA to the lungs. This led to the efficient restoration of the TSC2 tumor suppressor, resulting in a remarkable therapeutic effect by reducing tumor burden. The ability to selectively deliver therapeutic agents to specific organs offers the potential to develop organ-specific therapies and personalized medicine approaches. Despite these advancements, challenges remain in optimizing gene delivery to target organs while ensuring safety and efficacy. Continued research, advancements in vector design, and further understanding of organ-specific interactions will be essential for harnessing the full potential of gene therapy and its application in clinical settings. The development of targeted gene editing approaches for diverse organs holds the promise of transforming the landscape of medicine, paving the way for more effective and personalized treatments for a wide range of diseases.
Alzheimer's disease treatment
Alzheimer's disease (AD) is a chronic and irreversible neurodegenerative condition with prodromal phases, a prolonged preclinical period, and an average clinical duration of 8 to 10 years [156, 157]. AD pathology is marked by a progressive decline in mental, functional, and behavioral abilities, along with a loss of learning capacity. One significant hypothesis regarding AD revolves around the accumulation of cortical and cerebrovascular deposits, specifically amyloid-β (Aβ) peptide aggregates. In the pursuit of treating AD, an important strategy involves the use of nanomedicines to prevent the formation of Aβ aggregates and facilitate the removal of existing ones. In the process of AD, the circulatory serum Aβ will automatically accumulate in the brain. Hitchhiking on circulatory serum Aβ should be effective in crossing BBB and alleviating AD symptoms. For example, Gao et al. presented a KLVFF-decorated polydopamine nanoparticles (PDA@K) for AD treatment [158]. After intravenously injection, the KLVFF of PDA@K efficiently binding to serum Aβ through hydrophobic interaction, enabling it to cross the BBB via Aβ hitchhiking and accumulate in the lesion site. Subsequently, the PDA@K effectively chelated Cu2+ and Zn2+, leading to a significant suppression of aggregate formation and the restoration of behavioral and cognitive abilities. This study provides compelling evidence to consider metal dyshomeostasis and the inflammatory microenvironment as potential therapeutic targets for AD. Such a multi-pronged strategy could offer novel approaches to explore and develop treatments for this challenging neurodegenerative disease.
Lung-selective liposomes for pulmonary lymphangioleiomyomatosis treatment. A, 306-N16B specifically deliver mRNA to lung. B, The major component in protein corona formed by 306-O12B. C, The major component in protein corona formed by 306-N16B. Adapted with permission from [155], copyright 2022 National Academy of Sciences.
Non-invasive diagnostic imaging
Non-invasive diagnostic imaging holds tremendous promise in facilitating early disease detection and treatment. The commonly employed techniques for in vivo diagnostic imaging include positron emission tomography (PET), magnetic resonance imaging (MRI), computed tomography (CT), and fluorescence imaging. These techniques typically rely on the utilization of imaging probes, such as radionuclides, metal ions, and photosensitizers, to help visualize targeted structures or pathological changes. In recent years, several innovative probes capable of hitchhiking on circulatory cells or proteins have been developed and extensively utilized. This hitchhiking strategy confers two significant advantages to these imaging probes: (1) an extended half-life and (2) significantly enhanced targeting ability to lesion tissues. For example, Liu et al. presented a phospholipids DOPG-incorporated lanthanide-doped nanoparticle (LnNPs@PG) that enables dynamic monitoring of neutrophils in inflammation-associated disease [105]. In bloodstream, the hydroxyl groups of LnNPs@PG efficiently interact with complement C3 proteins, facilitating the hitchhiking of neutrophils through C3/C3R-mediated phagocytosis. As a result, LnNPs@PG achieves the dynamic monitoring of neutrophils during conditions such as cerebral ischemia/reperfusion and cutaneous wound healing. T cells play a crucial role in cancer immunotherapy, regulation and monitoring of T cell activity are essential for the prognostic assessment of cancer treatments that rely on immune activation. To this end, Zhou et al. presented a T-cell-targeting fusogenic liposomes (T-Fulips) to regulate and quantify T cell activity for cancer theranostics [39]. The surface of T-Fulips containing two types of functional ligands, 2,2,6,6-tetramethylpiperidine (TEMP) groups that neutralizing ROS and anti-CD3 antibodies (aCD3) that targeting CD3 on T cells. In the bloodstream, the aCD3 efficiently guides T-Fulips to T cells, followed by fusing with T cells to allow the anchoring of TEMP onto T cells. Subsequently, the TEMP molecules effectively neutralize ROS, thereby protecting T cells from oxidation-induced loss of activity. Moreover, TEMP undergoes a conversion into paramagnetic 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) radicals, enabling the quantification of T cell activity using magnetic resonance imaging. The ability to precisely visualize and quantify cellular behaviors in vivo offers great potential for personalized medicine and tailored treatment approaches. As these novel strategies continue to evolve, they have the potential to significantly impact disease management, improve treatment outcomes, and advance the field of non-invasive diagnostic imaging. Continued research and clinical validation are crucial to further unlock the full potential of hitchhiking-based imaging probes and their applications in precision medicine.
Summary and perspectives
Hitchhiking, which involves cruising on surfaces, has been adapted as a bio-inspired cargo delivery system for various applications. The rapid development of nanotechnology enables nanomaterials to interact with circulatory cells or proteins, either by phagocytosis or adsorption, thereby facilitating in vivo hitchhiking. In this system, circulatory cells (neutrophils, red blood cells, etc.) or proteins (serum albumin, ApoE, β2-GPI, Vtn, etc.) play a pivotal role as innate “moving gears” that facilitate the transport of hitchhiking nanoparticles to their desired target site. By combining the advantages of synthetic nanomaterials with circulatory cells/proteins, hitchhiking nanomaterials offer numerous benefits compared to conventional vectors. These advantages include enhanced circulatory stability, enhanced biosafety, and particularly improved targeting efficacy. The traditional ligand mediated active targeting is greatly influenced by the formation of protein corona in serum. However, by engineering the nanoparticle surface to preferentially adsorb certain types of proteins, hitchhiking strategies demonstrate an optimized protein corona that facilitates ligand-receptor interactions with specific target cells or tissues. This approach help overcome some of the limitations imposed by the protein corona and improve the nanoparticles' overall targeting efficacy. Consequently, hitchhiking nanomaterials have emerged as promising drug delivery vectors in various fields, such as anti-inflammation, cancer treatment, gene therapy, and diagnostic imaging.
Indeed, most hitchhiking systems involve the use of both cell-derived and synthetic materials, which present significant challenges in gaining regulatory approval for clinical applications. To ensure the safe and effective use of these systems, a series of standard and rigorous evaluations should be conducted prior to their implementation in clinical research. These evaluations should include the following: (1) Biocompatibility studies: thorough biocompatibility studies are essential to understand the interactions between hitchhiked cells and synthetic materials. These studies should assess potential adverse effects on cell function, viability, and immune response. Identifying any unexpected consequences of hitchhiking will enable researchers to optimize the system for safety and efficacy. (2) Immunogenicity assessment: The potential immunogenic response of the hitchhiking system should be carefully evaluated, especially when using foreign materials or nanoparticles. Understanding how the immune system responds to hitchhiked cells and materials is crucial for predicting possible immune reactions upon administration in vivo. (3) Long-term safety and toxicity: comprehensive investigations of the long-term safety and potential toxicity of the hitchhiking system are necessary. Researchers should assess how the attached materials degrade over time and whether they accumulate in the body, as such accumulation could lead to adverse effects. (4) In vivo studies: Conducting animal studies is crucial to evaluate the overall safety and efficacy of the hitchhiking system. These studies provide valuable insights into the distribution, biodistribution, and clearance of hitchhiked cells and materials in a living organism. By performing these evaluations meticulously, researchers may address regulatory concerns and pave the way for the safe and successful translation of hitchhiking systems from the laboratory to clinical applications. This comprehensive approach ensures that the potential benefits of hitchhiking in targeted drug delivery and imaging are harnessed while safeguarding patient safety and regulatory compliance.
Despite the significant success achieved by hitchhiking nanomaterials, there are still some challenges that limit their widespread application. To overcome these limitations, further efforts should be directed toward the following aspects: i) Targeting efficiency and specificity. Enhancing the targeting efficiency and specificity of nanomaterials to circulatory cells/proteins is crucial for their effective hitchhiking and delivery to specific tissues or cells. Further research is needed to optimize the selection and design of ligands or functional groups on the surface of nanomaterials to ensure efficient recognition and binding to the desired target. Ii) Precisely controlled drug release. The premature release of loaded drugs during hitchhiking may impair the migration, homing, and other functions of hitchhiked cells/proteins, resulting in unpredictable therapeutic efficacy. Further studies are needed to optimize the release behaviors of loaded drugs to ensure the precisely controlled release of the drug in the target. Iii) Scalability and manufacturing: To facilitate the practical application of hitchhiking nanomaterials, scalable and reproducible manufacturing methods need to be developed.
Acknowledgements
This work was supported by Tianjin Medical University Outstanding Young Scholars Startup Fund (Grant 116003-DW010037, 11502101/DW310415), The National Natural Science Foundation of China (NSFC; Grant Nos. 52203172 and 22077073), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-001A).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Liu Y, Shi L, Su L, van der Mei HC, Jutte PC, Ren Y. et al. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem Soc Rev. 2019;48:428-46
2. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101-24
3. Zhao Y, Zhang Z, Pan Z, Liu Y. Advanced bioactive nanomaterials for biomedical applications. Exploration (Beijing). 2021;1:20210089
4. Yan X, Wang F, Zheng B, Huang F. Stimuli-responsive supramolecular polymeric materials. Chem Soc Rev. 2012;41:6042-65
5. Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53:12320-64
6. Baig N, Kammakakam I, Falath W. Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv. 2021;2:1821-71
7. Zhang TX, Zhang ZZ, Yue YX, Hu XY, Huang F, Shi L. et al. A General Hypoxia-Responsive Molecular Container for Tumor-Targeted Therapy. Adv Mater. 2020;32:e1908435
8. Chen WH, Chen QW, Chen Q, Cui C, Duan S, Kang Y. et al. Biomedical polymers: synthesis, properties, and applications. Sci China Chem. 2022;65:1010-75
9. Yue Y-X, Zhang Z, Wang Z-H, Ma R, Chen M-M, Ding F. et al. Promoting Tumor Accumulation of Anticancer Drugs by Hierarchical Carrying of Exogenous and Endogenous Vehicles. Small Struct. 2022;3:2200067
10. James ND, Coker RJ, Tomlinson D, Harris JR, Gompels M, Pinching AJ. et al. Liposomal doxorubicin (Doxil): an effective new treatment for Kaposi's sarcoma in AIDS. Clin Oncol (R Coll Radiol). 1994;6:294-6
11. Ghosh S, Javia A, Shetty S, Bardoliwala D, Maiti K, Banerjee S. et al. Triple negative breast cancer and non-small cell lung cancer: Clinical challenges and nano-formulation approaches. J Control Release. 2021;337:27-58
12. Younis MA, Tawfeek HM, Abdellatif AAH, Abdel-Aleem JA, Harashima H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv Drug Deliv Rev. 2022;181:114083
13. Myhre BA. The first recorded blood transfusions: 1656 to 1668. Transfusion. 1990;30:358-62
14. Berséus O, Boman K, Nessen SC, Westerberg LA. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion. 2013;53(Suppl 1):114s-23s
15. Cap AP, Beckett A, Benov A, Borgman M, Chen J, Corley JB. et al. Whole Blood Transfusion. Mil Med. 2018;183:44-51
16. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341-7
17. Szydlak R. Mesenchymal stem cells in ischemic tissue regeneration. World J Stem Cells. 2023;15:16-30
18. Miatmoko A, Hariawan BS, Cahyani DM, Dewangga SS, Handoko KK, Purwati. et al. Prospective use of amniotic mesenchymal stem cell metabolite products for tissue regeneration. J Biol Eng. 2023;17:11
19. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909-15
20. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273-90
21. Hovhannisyan L, Riether C, Aebersold DM, Medová M, Zimmer Y. CAR T cell-based immunotherapy and radiation therapy: potential, promises and risks. Mol Cancer. 2023;22:82
22. Anselmo AC, Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. J Control Release. 2014;190:531-41
23. Haschek-Hock WM, Rousseaux CG, Wallig MA, Bolon B. NEW: Haschek and Rousseaux's Handbook of Toxicologic Pathology, Volume 1: Principles and Practice of Toxicologic Pathology, 4th Edition - 2021. Int J Toxicol. 2022;41:253-4
24. Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17:349-62
25. Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799-820
26. Mogre SS, Christensen JR, Niman CS, Reck-Peterson SL, Koslover EF. Hitching a Ride: Mechanics of Transport Initiation through Linker-Mediated Hitchhiking. Biophys J. 2020;118:1357-69
27. Jennings HS. Behavior of the lower organisms: Columbia University Press; 1931
28. Binford LR. Organization and formation processes: looking at curated technologies. Journal of anthropological research. 1979;35:255-73
29. Padmakumar A, Koyande NP, Rengan AK. The Role of Hitchhiking in Cancer Therapeutics-A Review. Adv Ther (Weinh). 2022;5:2200042
30. Stephan MT, Irvine DJ. Enhancing Cell therapies from the Outside In: Cell Surface Engineering Using Synthetic Nanomaterials. Nano Today. 2011;6:309-25
31. Brenner JS, Pan DC, Myerson JW, Marcos-Contreras OA, Villa CH, Patel P. et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun. 2018;9:2684
32. Sofias AM, Bjørkøy G, Ochando J, Sønstevold L, Hegvik M, Davies CdL. et al. Cyclic Arginine-Glycine-Aspartate-Decorated Lipid Nanoparticle Targeting toward Inflammatory Lesions Involves Hitchhiking with Phagocytes. Adv Sci (Weinh). 2021;8:2100370
33. Wu JR, Hernandez Y, Miyasaki KF, Kwon EJ. Engineered nanomaterials that exploit blood-brain barrier dysfunction for delivery to the brain. Adv Drug Deliv Rev. 2023;197:114820
34. Wu LP, Ficker M, Christensen JB, Simberg D, Trohopoulos PN, Moghimi SM. Dendrimer end-terminal motif-dependent evasion of human complement and complement activation through IgM hitchhiking. Nat Commun. 2021;12:4858
35. Gao C, Cheng Q, Li J, Chen J, Wang Q, Wei J. et al. Supramolecular Macrophage-Liposome Marriage for Cell-Hitchhiking Delivery and Immunotherapy of Acute Pneumonia and Melanoma. Adv Funct Mater. 2021;31:2102440
36. Chen KH, Nguyen N, YuHuang T, Lin YJ, Yu YT, Song HL. et al. Macrophage-Hitchhiked Orally Administered Β-Glucans-Functionalized Nanoparticles as "Precision-Guided Stealth Missiles" for Targeted Pancreatic Cancer Therapy. Adv Mater. 2023: e2304735.
37. Torrieri G, Iqbal I, Fontana F, Talman V, Liljenback H, Putri A. et al. Macrophage Hitchhiking Nanoparticles for the Treatment of Myocardial Infarction: An In Vitro and In Vivo Study. Adv Funct Mater. 2023
38. Liu Y, Hu D, Gao D, Gong P, Zheng H, Sun M. et al. Engineered apoptotic bodies hitchhiking across the blood-brain barrier achieved a combined photothermal-chemotherapeutic effect against glioma. Theranostics. 2023;13:2966-78
39. Shi C, Zhang Q, Yao Y, Zeng F, Du C, Nijiati S. et al. Targeting the activity of T cells by membrane surface redox regulation for cancer theranostics. Nat Nanotechnol. 2023;18:86-97
40. Sofias AM, Toner YC, Meerwaldt AE, van Leent MMT, Soultanidis G, Elschot M. et al. Tumor Targeting by α(v)β(3)-Integrin-Specific Lipid Nanoparticles Occurs via Phagocyte Hitchhiking. ACS Nano. 2020;14:7832-46
41. Wang J, Li Y, Nie G. Multifunctional biomolecule nanostructures for cancer therapy. Nat Rev Mater. 2021;6:766-83
42. Varki A. Biological roles of glycans. Glycobiology. 2017;27:3-49
43. Shao Q, Jiang S. Molecular understanding and design of zwitterionic materials. Adv Mater. 2015;27:15-26
44. Erfani A, Seaberg J, Aichele CP, Ramsey JD. Interactions between biomolecules and zwitterionic moieties: a review. Biomacromolecules. 2020;21:2557-73
45. Deirram N, Zhang C, Kermaniyan SS, Johnston AP, Such GK. pH-responsive polymer nanoparticles for drug delivery. Macromol Rapid Commun. 2019;40:1800917
46. Wang Y, Guo J, Li B, Li D, Meng Z, Sun SK. Biocompatible therapeutic albumin/genipin bioglue for postoperative wound adhesion and residual tumor ablation. Biomaterials. 2021;279:121179
47. Kell DB. Hitchhiking into the cell. Nat Chem Biol. 2020;16:367-8
48. Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D. et al. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759-65
49. Choi MR, Bardhan R, Stanton-Maxey KJ, Badve S, Nakshatri H, Stantz KM. et al. Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer Nanotechnol. 2012;3:47-54
50. Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV. et al. A macrophage-nanozyme delivery system for Parkinson's disease. Bioconjug Chem. 2007;18:1498-506
51. Brynskikh AM, Zhao Y, Mosley RL, Li S, Boska MD, Klyachko NL. et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson's disease. Nanomedicine (Lond). 2010;5:379-96
52. Mu Q, Yao K, Syeda MZ, Zhang M, Cheng Q, Zhang Y. et al. Ligustrazine Nanoparticle Hitchhiking on Neutrophils for Enhanced Therapy of Cerebral Ischemia-Reperfusion Injury. Adv Sci (Weinh). 2023;10:e2301348
53. Xue X, Huang Y, Bo R, Jia B, Wu H, Yuan Y. et al. Trojan Horse nanotheranostics with dual transformability and multifunctionality for highly effective cancer treatment. Nat Commun. 2018;9:3653
54. Zhan Q, Yi K, Cui X, Li X, Yang S, Wang Q. et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro Oncol. 2022;24:1871-83
55. Nguyen PHD, Jayasinghe MK, Le AH, Peng B, Le MTN. Advances in Drug Delivery Systems Based on Red Blood Cells and Their Membrane-Derived Nanoparticles. ACS Nano. 2023;17:5187-210
56. Wang Z, Li J, Cho J, Malik AB. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat Nanotechnol. 2014;9:204-10
57. Li S, Li M, Huo S, Wang Q, Chen J, Ding S. et al. Voluntary-Opsonization-Enabled Precision Nanomedicines for Inflammation Treatment. Adv Mater. 2021;33:e2006160
58. Wang Y, Wang Z, Li G. Engineered retroviruses map ligand-receptor interactions. Nat Methods. 2022;19:408-10
59. Zheng J, Zheng Z, Fu C, Weng Y, He A, Ye X. et al. Deciphering intercellular signaling complexes by interaction-guided chemical proteomics. Nat Commun. 2023;14:4138
60. Ferri-Borgogno S, Zhu Y, Sheng J, Burks JK, Gomez JA, Wong KK. et al. Spatial Transcriptomics Depict Ligand-Receptor Cross-talk Heterogeneity at the Tumor-Stroma Interface in Long-Term Ovarian Cancer Survivors. Cancer Res. 2023;83:1503-16
61. Choi JH, Lee BS, Jang JY, Lee YS, Kim HJ, Roh J. et al. Single-cell transcriptome profiling of the stepwise progression of head and neck cancer. Nat Commun. 2023;14:1055
62. Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D. et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813-20
63. Cruz MA, Bohinc D, Andraska EA, Alvikas J, Raghunathan S, Masters NA. et al. Nanomedicine platform for targeting activated neutrophils and neutrophil-platelet complexes using an α(1)-antitrypsin-derived peptide motif. Nat Nanotechnol. 2022;17:1004-14
64. Xie S, Tao Y, Pan Y, Qu W, Cheng G, Huang L. et al. Biodegradable nanoparticles for intracellular delivery of antimicrobial agents. J Control Release. 2014;187:101-17
65. Patil V, Patel A. Biodegradable Nanoparticles: A Recent Approach and Applications. Curr Drug Targets. 2020;21:1722-32
66. Yu L, Chen Y, Wu M, Cai X, Yao H, Zhang L. et al. "Manganese Extraction" Strategy Enables Tumor-Sensitive Biodegradability and Theranostics of Nanoparticles. J Am Chem Soc. 2016;138:9881-94
67. Khodabakhshaghdam S, Khoshfetrat AB, Rahbarghazi R. Alginate-chitosan core-shell microcapsule cultures of hepatic cells in a small scale stirred bioreactor: impact of shear forces and microcapsule core composition. J Biol Eng. 2021;15:1-12
68. Osmani N, Follain G, León MJG, Lefebvre O, Busnelli I, Larnicol A. et al. Metastatic tumor cells exploit their adhesion repertoire to counteract shear forces during intravascular arrest. Cell reports. 2019;28:2491-500 e5
69. Davis KA, Wu PJ, Cahall CF, Li C, Gottipati A, Berron BJ. Coatings on mammalian cells: interfacing cells with their environment. J Biol Eng. 2019;13:5
70. Lenders V, Escudero R, Koutsoumpou X, Armengol Álvarez L, Rozenski J, Soenen SJ. et al. Modularity of RBC hitchhiking with polymeric nanoparticles: testing the limits of non-covalent adsorption. J Nanobiotechnology. 2022;20:333
71. Zelepukin IV, Yaremenko AV, Shipunova VO, Babenyshev AV, Balalaeva IV, Nikitin PI. et al. Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale. 2019;11:1636-46
72. Kang Z, Liu Q, Zhang Z, Zheng Y, Wang C, Pan Z. et al. Arginine-Rich Polymers with Pore-Forming Capability Enable Efficient Intracellular Delivery via Direct Translocation Across Cell Membrane. Adv Healthc Mater. 2022;11:e2200371
73. Wang Y, Zhang Z, Zheng C, Zhao X, Zheng Y, Liu Q. et al. Multistage Adaptive Nanoparticle Overcomes Biological Barriers for Effective Chemotherapy. Small. 2021;17:e2100578
74. Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662-8
75. Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322-37
76. Zhang Z, Yue YX, Li Q, Wang Y, Wu X, Li X. et al. Design of Calixarene-Based ICD Inducer for Efficient Cancer Immunotherapy. Adv Funct Mater. 2023;33:2213967
77. Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1-16
78. Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME. et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613-8
79. Xu DD, Ma RR, Fu AP, Guan Z, Zhong N, Peng H. et al. Ion adsorption-induced reversible polarization switching of a van der Waals layered ferroelectric. Nat Commun. 2021;12:655
80. Li J, Luo W, Zhang S, Ma C, Xiao X, Duan W. et al. The effect of multiple pairs of meta-dicarboxyl groups on molecular self-assembly and the selective adsorption of coronene by hydrogen bonding and van der Waals forces. Nano Research. 2022;15:1691-7
81. Chen S, Zhong Y, Fan W, Xiang J, Wang G, Zhou Q. et al. Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion-drug conjugates with cell-membrane affinity. Nat Biomed Eng. 2021;5:1019-37
82. Zhao Z, Feng Y, Xiang J, Liu J, Piao Y, Shao S. et al. Screening of Zwitterionic Liposomes with Red Blood Cell-Hitchhiking and Tumor Cell-Active Transporting Capability for Efficient Tumor Entrance. Adv Funct Mater. 2023;33:2214369
83. Ehlerding EB, Lan X, Cai W. "Albumin Hitchhiking" with an Evans Blue Analog for Cancer Theranostics. Theranostics. 2018;8:812-4
84. Pan J, Wang Z, Huang X, Xue J, Zhang S, Guo X. et al. Bacteria-derived Outer-membrane Vesicles Hitchhike Neutrophils to Enhance Ischemic Stroke Therapy. Adv Mater. 2023: 2301779.
85. Senbanjo LT, Chellaiah MA. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front Cell Dev Biol. 2017;5:18
86. Xu H, Niu M, Yuan X, Wu K, Liu A. CD44 as a tumor biomarker and therapeutic target. Exp Hematol Oncol. 2020;9:36
87. Ludwig N, Szczepanski MJ, Gluszko A, Szafarowski T, Azambuja JH, Dolg L. et al. CD44(+) tumor cells promote early angiogenesis in head and neck squamous cell carcinoma. Cancer Lett. 2019;467:85-95
88. Swiston AJ, Cheng C, Um SH, Irvine DJ, Cohen RE, Rubner MF. Surface functionalization of living cells with multilayer patches. Nano Lett. 2008;8:4446-53
89. Ferguson LT, Hood ED, Shuvaeva T, Shuvaev VV, Basil MC, Wang Z. et al. Dual Affinity to RBCs and Target Cells (DART) Enhances Both Organ- and Cell Type-Targeting of Intravascular Nanocarriers. ACS Nano. 2022;16:4666-83
90. Topçu AA, Kılıç S, Özgür E, Türkmen D, Denizli A. Inspirations of Biomimetic Affinity Ligands: A Review. ACS Omega. 2022;7:32897-907
91. Gao C, Cheng Q, Wei J, Sun C, Lu S, Kwong CHT. et al. Bioorthogonal supramolecular cell-conjugation for targeted hitchhiking drug delivery. Mater Today. 2020;40:9-17
92. Glassman PM, Hood ED, Ferguson LT, Zhao Z, Siegel DL, Mitragotri S. et al. Red blood cells: The metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv Drug Deliv Rev. 2021;178:113992
93. Malech HL, DeLeo FR, Quinn MT. The Role of Neutrophils in the Immune System: An Overview. Methods Mol Biol. 2020;2087:3-10
94. Liew PX, Kubes P. The Neutrophil's Role During Health and Disease. Physiol Rev. 2019;99:1223-48
95. Akbar N, Braithwaite AT, Corr EM, Koelwyn GJ, van Solingen C, Cochain C. et al. Rapid neutrophil mobilization by VCAM-1+ endothelial cell-derived extracellular vesicles. Cardiovasc Res. 2023;119:236-51
96. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519-31
97. Villanueva V, Li X, Jimenez V, Faridi HM, Gupta V. CD11b agonists offer a novel approach for treating lupus nephritis. Transl Res. 2022;245:41-54
98. Sas AR, Carbajal KS, Jerome AD, Menon R, Yoon C, Kalinski AL. et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat Immunol. 2020;21:1496-505
99. Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19:177-91
100. Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y. et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692-700
101. Li T, Zhang Z, Li X, Dong G, Zhang M, Xu Z. et al. Neutrophil Extracellular Traps: Signaling Properties and Disease Relevance. Mediators Inflamm. 2020;2020:9254087
102. Chu D, Gao J, Wang Z. Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection. ACS Nano. 2015;9:11800-11
103. Zhang CY, Dong X, Gao J, Lin W, Liu Z, Wang Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv. 2019;5:eaax7964
104. Bachmaier K, Stuart A, Singh A, Mukhopadhyay A, Chakraborty S, Hong Z. et al. Albumin Nanoparticle Endocytosing Subset of Neutrophils for Precision Therapeutic Targeting of Inflammatory Tissue Injury. ACS Nano. 2022;16:4084-101
105. Zhang M, Wang Z, Shao Y, Zhao Y, Liu Z. Complement-Opsonized NIR-IIb Emissive Immunotracers for Dynamically Monitoring Neutrophils in Inflammation-Related Diseases. Adv Mater. 2022;34:e2203477
106. Farshbaf M, Valizadeh H, Panahi Y, Fatahi Y, Chen M, Zarebkohan A. et al. The impact of protein corona on the biological behavior of targeting nanomedicines. Int J Pharm. 2022;614:121458
107. Xiao W, Gao H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system. Int J Pharm. 2018;552:328-39
108. Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc Natl Acad Sci U S A. 1973;70:2663-6
109. Villa CH, Anselmo AC, Mitragotri S, Muzykantov V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv Drug Deliv Rev. 2016;106:88-103
110. Ford J. Red blood cell morphology. Int J Lab Hematol. 2013;35:351-7
111. Hoffman JF. Biconcave shape of human red-blood-cell ghosts relies on density differences between the rim and dimple of the ghost's plasma membrane. Proc Natl Acad Sci U S A. 2016;113:14847-51
112. Singh MP, Flynn NH, Sethuraman SN, Manouchehri S, Ritchey J, Liu J. et al. Reprogramming the rapid clearance of thrombolytics by nanoparticle encapsulation and anchoring to circulating red blood cells. J Control Release. 2021;329:148-61
113. Liu Z, Janzen J, Brooks DE. Adsorption of amphiphilic hyperbranched polyglycerol derivatives onto human red blood cells. Biomaterials. 2010;31:3364-73
114. Li J, Ding Y, Cheng Q, Gao C, Wei J, Wang Z. et al. Supramolecular erythrocytes-hitchhiking drug delivery system for specific therapy of acute pneumonia. J Control Release. 2022;350:777-86
115. Jin M, Zhu S, Hou Y. Insight on Serum Albumin: From Structure and Biological Properties to Functional Biomaterials for Bone Repair. ACS Biomater Sci Eng. 2023;9:2235-50
116. Takakura Y, Takahashi Y. Strategies for persistent retention of macromolecules and nanoparticles in the blood circulation. J Control Release. 2022;350:486-93
117. Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73-89
118. Lin T, Zhao P, Jiang Y, Tang Y, Jin H, Pan Z. et al. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano. 2016;10:9999-10012
119. Kratz F, Warnecke A, Scheuermann K, Stockmar C, Schwab J, Lazar P. et al. Probing the cysteine-34 position of endogenous serum albumin with thiol-binding doxorubicin derivatives. Improved efficacy of an acid-sensitive doxorubicin derivative with specific albumin-binding properties compared to that of the parent compound. J Med Chem. 2002;45:5523-33
120. Lou X, Zhang D, Ling H, He Z, Sun J, Sun M. et al. Pure redox-sensitive paclitaxel-maleimide prodrug nanoparticles: Endogenous albumin-induced size switching and improved antitumor efficiency. Acta Pharm Sin B. 2021;11:2048-58
121. Xu Y, Yang C, Wu Y, Jiang W, Cheng Q, Yan L. et al. In Situ Albumin-Hitchhiking NIR-II Probes for Accurate Detection of Micrometastases. Nano Lett. 2023;23:5731-7
122. Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519-22
123. Jin C, Zhang H, Zou J, Liu Y, Zhang L, Li F. et al. Floxuridine Homomeric Oligonucleotides "Hitchhike" with Albumin In Situ for Cancer Chemotherapy. Angew Chem Int Ed Engl. 2018;57:8994-7
124. Wen X, Xu P, Shi M, Liu J, Zeng X, Zhang Y. et al. Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics. Theranostics. 2022;12:422-33
125. Jacobson O, Kiesewetter DO, Chen X. Albumin-Binding Evans Blue Derivatives for Diagnostic Imaging and Production of Long-Acting Therapeutics. Bioconjug Chem. 2016;27:2239-47
126. Yamamoto T, Ikuta K, Oi K, Abe K, Uwatoku T, Hyodo F. et al. In vivo MR detection of vascular endothelial injury using a new class of MRI contrast agent. Bioorg Med Chem Lett. 2004;14:2787-90
127. Zhang J, Lang L, Zhu Z, Li F, Niu G, Chen X. Clinical Translation of an Albumin-Binding PET Radiotracer 68Ga-NEB. J Nucl Med. 2015;56:1609-14
128. Liu Y, Wang G, Zhang H, Ma Y, Lang L, Jacobson O. et al. Stable Evans Blue Derived Exendin-4 Peptide for Type 2 Diabetes Treatment. Bioconjug Chem. 2016;27:54-8
129. Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264-74
130. Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914-8
131. Guo R, Deng M, He X, Li M, Li J, He P. et al. Fucoidan-functionalized activated platelet-hitchhiking micelles simultaneously track tumor cells and remodel the immunosuppressive microenvironment for efficient metastatic cancer treatment. Acta Pharm Sin B. 2022;12:467-82
132. Rouzet F, Bachelet-Violette L, Alsac JM, Suzuki M, Meulemans A, Louedec L. et al. Radiolabeled fucoidan as a p-selectin targeting agent for in vivo imaging of platelet-rich thrombus and endothelial activation. J Nucl Med. 2011;52:1433-40
133. Juenet M, Aid-Launais R, Li B, Berger A, Aerts J, Ollivier V. et al. Thrombolytic therapy based on fucoidan-functionalized polymer nanoparticles targeting P-selectin. Biomaterials. 2018;156:204-16
134. Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216:176-94
135. Wang W, Zhang Z, Liu Y, Kong L, Li W, Hu W. et al. Nano-integrated cascade antioxidases opsonized by albumin bypass the blood-brain barrier for treatment of ischemia-reperfusion injury. Biomater Sci. 2022;10:7103-16
136. Butt Y, Kurdowska A, Allen TC. Acute Lung Injury: A Clinical and Molecular Review. Arch Pathol Lab Med. 2016;140:345-50
137. Mokrá D. Acute lung injury - from pathophysiology to treatment. Physiol Res. 2020;69:S353-s66
138. Manicone AM. Role of the pulmonary epithelium and inflammatory signals in acute lung injury. Expert Rev Clin Immunol. 2009;5:63-75
139. Jimi E, Fei H, Nakatomi C. NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis. Int J Mol Sci. 2019 20
140. Luo L, Luo Z, Zhang J, Liu X, Huang J, Wang S. et al. Preventing acute lung injury from progressing to pulmonary fibrosis by maintaining ERS homeostasis through a multistage targeting nanomicelle. Nano Today. 2023;48:101719
141. Zhang Z, Wang Q, Liu Q, Zheng Y, Zheng C, Yi K. et al. Dual-Locking Nanoparticles Disrupt the PD-1/PD-L1 Pathway for Efficient Cancer Immunotherapy. Adv Mater. 2019;31:e1905751
142. Wellenstein MD, de Visser KE. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity. 2018;48:399-416
143. Zhang Y, Guo Z, Cao Z, Zhou W, Zhang Y, Chen Q. et al. Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials. 2018;183:243-57
144. Wang X, Chen D, Huang K, Li M, Zhan C, Dong Z. et al. Albumin-Hitchhiking Drug Delivery to Tumor-Draining Lymph Nodes Precisely Boosts Tumor-Specific Immunity through Autophagy Modulation of Immune Cells. Adv Mater. 2023: e2211055.
145. Tang L, Wang Z, Mu Q, Yu Z, Jacobson O, Li L. et al. Targeting Neutrophils for Enhanced Cancer Theranostics. Adv Mater. 2020;32:e2002739
146. Yu X, Xing G, Sheng S, Jin L, Zhang Y, Zhu D. et al. Neutrophil Camouflaged Stealth Nanovehicle for Photothermal-Induced Tumor Immunotherapy by Triggering Pyroptosis. Adv Sci (Weinh). 2023;10:e2207456
147. Pai JA, Satpathy AT. High-throughput and single-cell T cell receptor sequencing technologies. Nat Methods. 2021;18:881-92
148. Chen C, Jing W, Chen Y, Wang G, Abdalla M, Gao L. et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci Transl Med. 2022;14:eabn1128
149. Liu Q, Zhao K, Wang C, Zhang Z, Zheng C, Zhao Y. et al. Multistage Delivery Nanoparticle Facilitates Efficient CRISPR/dCas9 Activation and Tumor Growth Suppression In Vivo. Adv Sci (Weinh). 2019;6:1801423
150. Zhang S, Shen J, Li D, Cheng Y. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics. 2021;11:614-48
151. Liu Q, Cai J, Zheng Y, Tan Y, Wang Y, Zhang Z. et al. NanoRNP Overcomes Tumor Heterogeneity in Cancer Treatment. Nano Lett. 2019;19:7662-72
152. Dilliard SA, Cheng Q, Siegwart DJ. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc Natl Acad Sci U S A. 2021 118
153. Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15:313-20
154. Qiu M, Glass Z, Chen J, Haas M, Jin X, Zhao X. et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc Natl Acad Sci U S A. 2021 118
155. Qiu M, Tang Y, Chen J, Muriph R, Ye Z, Huang C. et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2022 119
156. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353-6
157. Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer's disease: Past, present and future. Neuropharmacology. 2014;76:27-50
158. Huang Q, Jiang C, Xia X, Wang Y, Yan C, Wang X. et al. Pathological BBB Crossing Melanin-like Nanoparticles as Metal-ion Chelator and Neuroinflammation Regulator against Alzheimer's Disease Research (Wash D C). 2023; 6: 0180.
Author contact
![]() Corresponding authors: Yang Liu (yliuedu.cn) and Zhanzhan Zhang (zzzedu.cn).
Corresponding authors: Yang Liu (yliuedu.cn) and Zhanzhan Zhang (zzzedu.cn).
 Global reach, higher impact
Global reach, higher impact