13.3
Impact Factor
Theranostics 2023; 13(14):4974-4992. doi:10.7150/thno.86466 This issue Cite
Research Paper
Application of Lung-Targeted Lipid Nanoparticle-delivered mRNA of soluble PD-L1 via SORT Technology in Acute Respiratory Distress Syndrome
1. Department of Biochemistry and Molecular Cell Biology, Shanghai Key Laboratory for Tumor Microenvironment and Inflammation, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
2. Department of Gastrointestinal Surgery, Renji Hospital Affiliated, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China.
3. State Key Laboratory of Genetic Engineering, School of Life Sciences, Fudan University, Shanghai, 200433, China.
4. Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
5. Shanghai RNACure Biopharma Co., Ltd. Shanghai, 200438, China.
6. Shanghai Frontiers Science Center of Cellular Homeostasis and Human Diseases. Shanghai, 200025, China.
Abstract
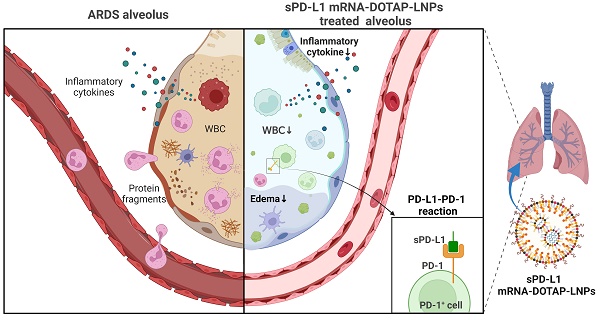
Rationale: Acute respiratory distress syndrome (ARDS) is a life-threatening condition characterized by excessive immune response usually due to lung inflammation. Local immunosuppression is crucial for effective ARDS treatment. However, current methods are limited in their ability to target the lungs specifically.
Methods: This study utilized lung-targeted lipid nanoparticles (LNPs) with 1,2-dioleoyl-3-trimethylammonium-propane (termed DOTAP-LNPs) to encapsulate chemically modified soluble programmed death ligand-1 (sPD-L1) mRNA and examined its physiological characteristics and therapeutic efficacy. A comparative analysis was performed between sPD-L1 mRNA delivered by DOTAP-LNPs, sPD-L1 mRNA delivered by regular LNPs (MC3-LNPs), and PD-L1-Fc recombinant protein administered systemically. Additionally, the survival rate of ARDS mice treated with different drugs was assessed.
Results: Administration of sPD-L1 mRNA-LNPs to ARDS model mice significantly reduced leukocyte chemotaxis and protein accumulation in lung tissue, along with a decrease in pulmonary edema. Notably, in situ intervention using sPD-L1 mRNA-DOTAP-LNPs exhibited superior therapeutic effects compared to PD-L1-Fc recombinant protein and sPD-L1 mRNA encapsulated in MC3-LNPs. Importantly, treatment with sPD-L1 mRNA-DOTAP-LNPs improved the survival rate of ARDS model mice.
Conclusion: This study demonstrates the feasibility of utilizing stable and reliable mRNA to express the immunosuppressive molecule sPD-L1 specifically in the lungs. The findings provide proof of concept for localized nanoparticle delivery and offer a novel therapeutic strategy for treating acute inflammation in ARDS.
Keywords: immunosuppression, PD-L1, mRNA therapy, LNPs, ARDS
 Global reach, higher impact
Global reach, higher impact