13.3
Impact Factor
Theranostics 2024; 14(1):17-32. doi:10.7150/thno.87345 This issue Cite
Review
Improving susceptibility of neuroendocrine tumors to radionuclide therapies: personalized approaches towards complementary treatments
1. Institute for Clinical Chemistry and Laboratory Medicine, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
2. Department of Internal Medicine III, University Clinic Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
3. Department of Endocrinology, Diabetology and Clinical Nutrition, University Hospital Zurich (USZ), and University of Zurich (UZH), Zurich, Switzerland.
4. Department of Radiopharmaceutical and Chemical Biology, Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden-Rossendorf (HZDR), Dresden, Germany.
5. University Hospital Würzburg, Division of Endocrinology and Diabetes, Würzburg, Germany.
6. Department of Medicine IV, University Hospital, Ludwig-Maximilians-University Munich, Munich, Germany.
7. Faculty of Chemistry and Food Chemistry, School of Science, Technische Universität Dresden, Dresden, Germany.
* Shared senior authorship.
Received 2023-6-20; Accepted 2023-9-30; Published 2024-1-1
Abstract
Radionuclide therapies are an important tool for the management of patients with neuroendocrine neoplasms (NENs). Especially [131I]MIBG and [177Lu]Lu-DOTA-TATE are routinely used for the treatment of a subset of NENs, including pheochromocytomas, paragangliomas and gastroenteropancreatic tumors. Some patients suffering from other forms of NENs, such as medullary thyroid carcinoma or neuroblastoma, were shown to respond to radionuclide therapy; however, no general recommendations exist. Although [131I]MIBG and [177Lu]Lu-DOTA-TATE can delay disease progression and improve quality of life, complete remissions are achieved rarely. Hence, better individually tailored combination regimes are required. This review summarizes currently applied radionuclide therapies in the context of NENs and informs about recent advances in the development of theranostic agents that might enable targeting subgroups of NENs that previously did not respond to [131I]MIBG or [177Lu]Lu-DOTA-TATE. Moreover, molecular pathways involved in NEN tumorigenesis and progression that mediate features of radioresistance and are particularly related to the stemness of cancer cells are discussed. Pharmacological inhibition of such pathways might result in radiosensitization or general complementary antitumor effects in patients with certain genetic, transcriptomic, or metabolic characteristics. Finally, we provide an overview of approved targeted agents that might be beneficial in combination with radionuclide therapies in the context of a personalized molecular profiling approach.
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of tumors affecting neuroendocrine cells and arise in many different organs. NENs can occur in the digestive tract from enterochromaffin cells, the pancreas from islet or ductal cells, the lungs from pulmonary neuroendocrine cells, and the thyroid from parafollicular C-cells. Furthermore, pheochromocytomas originate from chromaffin cells of the adrenal medulla and paragangliomas from extra-adrenal ganglia (together referred to as PPGL).
Gastroenteropancreatic (GEP) NENs are divided into three grades based on proliferation markers. Grade 3 denotes fast-progressing tumors and is further divided into the group of poorly differentiated neuroendocrine carcinomas and well-differentiated neuroendocrine tumors, which are genetically unrelated and show different responses to chemotherapy [1]. Some GEP-NENs are functionally active with various hormones, including serotonin, insulin, gastrin, glucagon, and vasoactive intestinal peptide, that can be produced and cause different clinical syndromes [2]. About 27-45% of patients present with distant metastasis at first diagnosis [3, 4]. Aggressiveness is generally dependent on the primary site, with NENs of the pancreas and small intestine having a high malignant potential but often indolent progression, and gastric and rectal NENs having lower rates of metastasis, but when metastases occur they progress rapidly. The liver is the most common site of metastasis in GEP-NENs. Currently used therapies are based on several successful phase III studies and include surgery (debulking or curative), local ablation of liver metastases, systemic therapy options with biotherapy using “cold” somatostatin analogs, PRRT, chemotherapy and molecularly targeted approaches [5].
Medullary thyroid carcinomas (MTCs) are a rare form of thyroid cancer with 5-10% of cases. MTCs often secrete calcitonin and can progress indolently after thyroidectomy due to their tendency of spreading to locoregional lymph nodes. Primary treatment is surgery of the thyroid gland and surrounding lymph nodes. Approaches for recurrent or metastatic MTCs include external beam radiotherapy, conventional chemotherapy, and tyrosine kinase inhibitors. Therapeutic strategies for metastatic MTC are rarely curative [6].
PPGL patients suffer from symptoms related to catecholamine excess, such as high blood pressure, sweating, headaches, and palpitations. The majority of PPGLs are curable by surgery, but up to 15% of pheochromocytoma and 40% of paraganglioma metastasize [7]. Therapeutic options for patients with metastatic disease are limited and not curative. Currently recommended approaches include chemotherapy according to the Averbuch scheme for rapidly growing tumors, radiopharmaceutical therapy with iodine-131 meta-iodobenzylguanidine ([131I]MIBG) for slowly to moderately progressing tumors, and somatostatin receptor 2 (SSTR2) targeted peptide receptor radionuclide therapy (PRRT) [8, 9].
The following sections summarize clinically approved radionuclide therapies for NENs, review molecular determinants of radioresistance in the light of what is known about cancer stem cells in NENs, and discuss strategies for improvement using adjuvant radiosensitizers with the potential to enhance treatment efficacy and reduce therapeutic escape.
Clinically Approved Radionuclide Theranostics in NENs
Somatostatin type 2 receptor targeting
Somatostatin receptors belong to the family of G-protein coupled receptors; they regulate hormone secretion and cell proliferation. Especially subtype 2 (SSTR2) is strongly expressed in NENs exceeding levels of healthy tissues [10, 11]. This tumor selectivity can be targeted using radiolabeled somatostatin analogs [12]. Depending on the radionuclide attached, these agents are valuable for diagnosis by positron emission tomography (PET) imaging or for radionuclide therapy. To express these dual benefits the term theranostics is used (Figure 1).
Preoperative PET imaging with the positron (β+)-emitting somatostatin analog [68Ga]Ga-DOTA-(Tyr3)octreotate (TATE) is recommended in the guidelines for NENs and was FDA (Food and Drug Administration)-approved in 2016 in the Unites States [13-16]. [68Ga]Ga-DOTA-TATE detects primary and metastatic lesions of NENs with high sensitivity and specificity of over 80%, as demonstrated in multiple studies [17-19]. Furthermore, [68Ga]Ga-DOTA-TOC and [64Cu]Cu-DOTA-TATE are available as FDA-approved diagnostic radiopharmaceuticals [20, 21]. Beta-minus (β-) particle-emitting SSTR2-binding somatostatin analogs are used for therapeutic intervention in GEP-NENs and PPGLs (Figure 1A/C) [12, 22]. In 2018, PRRT with [177Lu]Lu-DOTA-TATE was FDA approved as a promising treatment modality for patients with well-differentiated metastatic/inoperable GEP-NENs due to the NETTER-1 phase III trial that reported improved progression-free survival and quality-of-life in patients with progressing midgut NENs [23]. Disease was controlled in 82% of patients with progression-free survival of 29 months and overall survival of 63 months [24, 25]. Also poorly differentiated grade 3 GEP-NENs benefit from PRRT as long as increased uptake of [68Ga]Ga-DOTA-TATE is confirmed in all lesions [26].
MTCs express SSTR2, which positively correlates with improved overall survival [27, 28]. Imaging analysis with [68Ga]Ga-DOTA-TATE was reported to be especially positive in patients with high serum calcitonin, indicating that more differentiated MTCs have higher expression levels of SSTR2 [29, 30]. In accordance, [177Lu]Lu-DOTA-TATE therapy was recently shown to achieve favorable responses in patients with metastatic MTC [31].
In PPGLs, SSTR2 is expressed in about 50% of tumors depending on the underlying mutation [11, 32]. PPGLs that arise due to loss-of-function mutations in succinate dehydrogenase (SDH) subunit genes show excellent detection rates with [68Ga]Ga-DOTA-TATE, whereas PPGLs with gain-of-function mutations in hypoxia-inducible factor 2α (HIF2α or EPAS1) are less well detectable [33, 34]. Several clinical studies suggest that PRRT is one of the most effective therapies for metastatic PPGLs, especially for those with SDHx mutations [7, 35]. A recently published meta-analysis estimated the disease control rate in PPGLs treated with PRRT at 81% [36]. PRRT is well tolerated with limited acute and medium-term toxicity profiles and only low rates of nephrotoxicity and therapy-related myeloid neoplasms. Therefore, PRRT is a viable option for clinicians to delay tumor progression in patients with metastatic PPGL. Most patients show at least a partial response with progression-free survival in the range of 14 to 39 months and a median overall survival of 50 months [37, 38]. Unfortunately, many patients treated with PRRT will progress eventually and a recent report describes two patients with extensive metastatic spread after initial response to [177Lu]Lu-DOTA-TATE [39]. An ongoing phase II trial (NCT03206060) is evaluating [177Lu]Lu-DOTA-TATE in metastatic and inoperable PPGL.
Clinically approved targeted radionuclide theranostics in NENs mediated by somatostatin receptor type 2 (SSTR2, A) or norepinephrine transporter (NET, B). Panel C shows representative 68Ga-DOTATATE PET/CT images of an 83-year old male patient presenting with a well-differentiated neuroendocrine tumor of the pancreas with peritoneal, lymph node and hepatic metastases at baseline. After 4 cycles of PRRT, a good partial response with shrinkage of the pancreatic tumor, peritoneal metastases and the lymph node metastasis inguinal left was observed. Abbreviations: DOTA - tetraxetan, TATE - (Tyr3)octreotate, TOC - (Tyr3)octreotide, MIBG - meta-iodobenzylguanidine
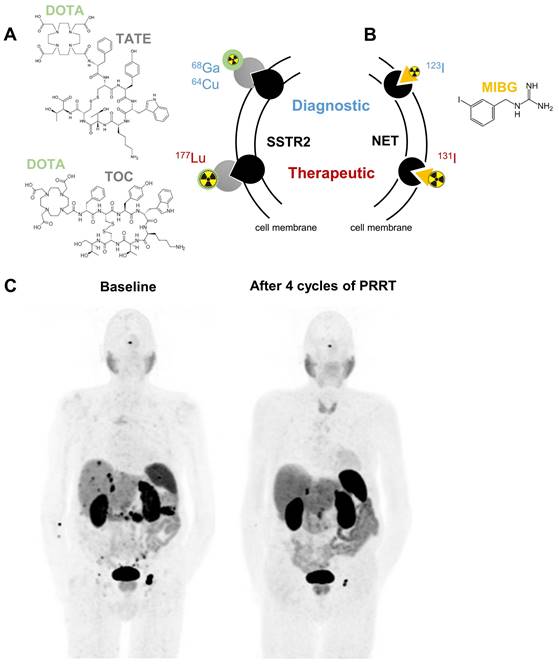
Norepinephrine transporter targeting
Another molecular target for theranostic radiopharmaceuticals is the norepinephrine transporter (encoded by SLC6A2). It is mainly expressed in noradrenergic neurons and chromaffin cells of the adrenal medulla and facilitates norepinephrine uptake. The norepinephrine transporter substrate meta-iodobenzylguanidine (MIBG) labeled with suitable radioisotopes of iodine is used for imaging (mainly [123I]MIBG) and therapy ([131I]MIBG) (Figure 1B). Planar scintigraphy or single-photon emission computed tomography (SPECT) are used as standard imaging techniques for these gamma-photon (γ)-emitting radiopharmaceuticals. Uptake of [123/131I]MIBG was demonstrated in all types of NENs, but predominantly in PPGLs and neuroblastomas of the adrenal [40].
For diagnostic use, low-dose [131I]MIBG was already approved in 1994 by the FDA as an imaging agent for the localization of PPGL, later augmented by [123I]MIBG in 2008. Since 2018 the formulation of high-specific-activity (HSA) [131I]MIBG, which contains only a negligible amount of unlabeled MIBG, is currently the only FDA-approved therapy for metastatic PPGLs in the United States. Therapeutic benefits include sustained blood pressure control with partial response or stable disease over 12 months in over 90% of patients; median overall survival was 36.7 months [41]. Generally, a more differentiated tumor phenotype with a mature storage and secretion apparatus for catecholamines or other hormones, such as serotonin, are favorable for MIBG uptake [42]. For this reason, a currently running phase II study in Brazil is recruiting patients with well-differentiated NENs for [131I]I-MIBG therapy (NCT04831567). Further details on [131I]MIBG therapy in different NENs have been summarized elsewhere [43]. Especially for PPGLs, there might be cases with positive imaging results for [131I]MIBG and [68Ga]Ga-DOTA-TATE, making the patient eligible to both kinds of targeted radionuclide therapy. Practical recommendations for decision-making in such instances were recently published [44].
Besides FDA-approved radiopharmaceuticals for theranostic targeting of SSTR2 and norepinephrine transporters in NENs, a broad range of theranostic agents using other radionuclides or directed towards other tumor-specific molecular targets are currently under preclinical and clinical evaluation. This topic was extensively reviewed elsewhere [45-47].
Biophysical and radiobiological effects of beta-minus particle-emitting radiopharmaceuticals
Most of our current knowledge on radiobiological effects in cancer emerged from studies using external beam radiation therapy (EBRT). However, the biophysical and radiobiological effects of EBRT differ significantly from those of radionuclide therapy. Unlike EBRT, the effects of radionuclide therapy depend not only on the type of radiation applied at a specific dose, but are also considerably influenced by the distribution and retention of the radiopharmaceutical within the target tissue, the half-life of the radionuclide, and the varying ionization density of the emitted particles.
To date, targeted radionuclide-based therapies of solid tumors, including NENs, are carried out mainly with β- particle emitters. Despite their rather low in vitro cytotoxicity compared to α particle or Auger electron emitters, these radionuclides continue to be pursued for targeted therapies, mainly due to their availability and favorable physical characteristics, such as particle energy and range (leading to crossfire irradiation of tumor cells with no radiopharmaceutical bound) and physical half-lives compatible with the biological half-lives of the carrier molecules [48]. Nevertheless, especially non-uniformities in the activity distribution as well as declining activity concentration over time will usually result in sub-lethal low-dose irradiation at least in some regions of the target tissue, where the tumor cells will escape from treatment or eventually develop a radioresistant phenotype. Crucial factors contributing to a non-uniform radiopharmaceutical distribution in solid tumors are (i) differences in perfusion, e.g. depending on the amount of connective tissue and/or vascularization density, (ii) region-specific differences in interstitial pressure, and (iii) differences in binding-site densities among tumor cells.
There are two main strategies for enhancing the efficacy of conventional radionuclide therapies: increasing the absorbed radiation dose (dose maximization), or enhancing the tumor's susceptibility to the biophysical and radiobiological effects of ionizing radiation (radiosensitization). The absorbed dose of a target-specific radiopharmaceutical can be improved by (i) optimizing treatment schemes and sequences, e.g., via personalized fractionating and dosing [49]; (ii) minimizing off-target retention, in particular in kidneys, thereby allowing for increased total applied doses of the radiopharmaceutical [16, 50, 51]; (iii) upregulating molecular targets for radiopharmaceuticals in tumors, e.g., through epigenetic modifiers [52-55]; (iv) increasing the bound fraction of radiopharmaceuticals in tumors, e.g., using albumin binder conjugates providing increased blood circulation times [56] or receptor antagonists [57, 58]; and (v) attaching alternative radionuclides with suitable chemical properties for radiolabeling as well as favorable decay properties providing radiation with high ionization densities such as α particles, Auger electrons and/or high-energy β‒ particles, and half-lives compatible with the pharmacologic properties of the targeting vectors, e.g. 225Ac, 213Bi, 211At, 212Pb, 161Tb, and 67Cu [59-61]. Such dose maximization approaches were reported elsewhere and will only be discussed in respect to modulation of SSTR2 expression. The following sections outline pathways causing radioresistance in NENs and highlight concepts for combining clinically approved radionuclide therapy with targeted small molecules to achieve radiosensitizing effects (Figure 2).
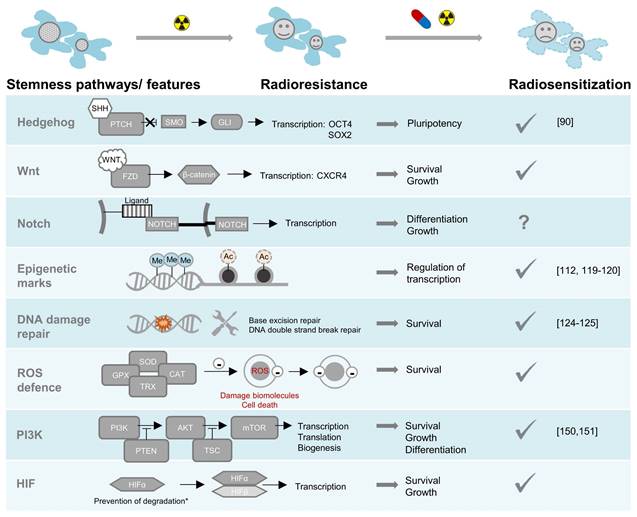
Radioresistance pathways in NENs: pharmacological targeting sensitizes to radiotherapy. A tick under radiosensitization indicates that preclinical evidence for radiosensitization exists, the citations specify that evidence was given specifically in NEN models. *HIFα degradation can be perturbed by pathogenic variants in several genes or through metabolic effects of oncometabolites. Abbreviations: SHH-sonic hedgehog, PTCH-patched, SMO-smoothened, GLI-glioma-associated oncogene, FZD-frizzled, Me-methylation, Ac-acetylation, ROS-reactive oxygen species, SOD-superoxide dismutase, CAT-catalase, GPX-glutathione peroxidase, TRX-thioredoxin, PI3K-phosphatidylinositol 3-kinases, AKT-serine/threonine-protein kinase, PTEN-phosphatase and tensin homolog, TSC-tuberous sclerosis complex, mTOR-mammalian target of rapamycin, HIF-hypoxia inducible factor.
Strategies for Enhancing Susceptibility to Conventional Radionuclide Therapies: Molecular Targets in NENs
Cancer stem cell-like traits in NENs
Radioresistance is closely linked to stem cell traits, i.e. the ability of self-renewal. Reports from different tumor entities have shown that so called cancer stem cells (CSCs) are inherently resistant to radiation treatment through different mechanisms, including enhanced DNA repair capabilities, ROS detoxification strategies, and activation of cell survival pathways, such as PI3K/Akt, Wnt/β-catenin, and Notch signaling [62]. Here we review what is known about CSCs in NENs.
CSCs have been identified in gastrointestinal NENs based on aldehyde dehydrogenase (ALDH) activity, a marker known to promote self-renewal [63]. ALDH-positive cells showed upregulated signaling of tyrosine-protein kinase Src, which was important for tumor growth in a xenograft model. In pancreatic NENs, a population of CD90 and ALDHA1 expressing cells was found to be highly tumorigenic and characterized by C-Met signaling, another tyrosine-protein kinase [64]. As an additional marker for these stem-like cells, CD47 was identified. Targeting CD47 increased tumor cell destruction by macrophages and resulted in reduced tumor growth and prolonged survival in mice. Additional anti-EGFR therapy increased survival even further. In MTC, a CD133-positive cell population was found to be enriched in tumor spheres [65]. Additionally, the authors showed sphere formation to be dependent on the receptor tyrosine kinase Ret. CD133-positive cells were also involved in chemoresistance to 5-fluorouracil [66]. A study identified co-expression of the stem cell markers CD133 and CD44 to be correlated with invasion and metastases and decreased patient survival [67]. CD133-positive cells and other stem cell markers were also identified in small cell lung cancer (SCLC) in multiple studies [68-70]. A side population of cells with high proliferation rates, efficient tumor-inducing ability, higher angiogenic potential, and decreased neuronal markers of CD56 and CD90, was identified in different SCLC cell lines [71]. These cells expressed high levels of genes associated with cancer stem cells, Notch and Hedgehog pathways. Both pathways are involved in the regulation of stem cell features.
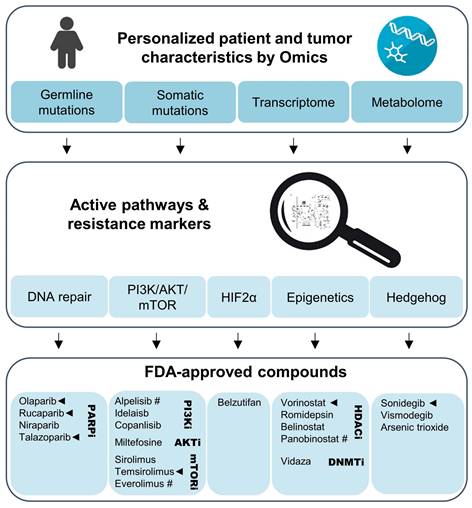
Personalized evaluation of promising radiosensitizers for combination therapy with PRRT in NENs. Omics studies on gene mutations, transcriptional and metabolic pathways in the primary tumor or biopsy material should lead the selection of adjuvant therapies for NENs eligible for PRRT. Clinical trials using such a personalized approach could evaluate agents that have been FDA-approved for use in other cancers. Especially compounds with preclinical evidence for radiosensitization effects together [177Lu]Lu-DOTA-TATE (marked with ◄) and those with evidence for anti-tumor effects in NENs (marked with #) should be prioritized.
In addition to residing CSCs being the cause of tumor development and malignant transformation, there are indications that populations of dedifferentiated cancer cells contribute to tumor progression and invasiveness, in which mutations cause the acquisition of more stem-like cell features. This hypothesis in the context of NENs was outlined in a recent review [72]. Specific experimental evidence for either theory is, however, scarce for these tumor entities. Intra-tumor heterogeneity caused through genome instability and the occurrence of cells with different mutational changes can drive progressive dedifferentiation of tumors and varies considerably between different types of NENs. High numbers of subclonal cell populations were identified in pancreatic NENs, e.g. with MEN1 mutations as subclonal drivers [73]. Additionally, different methylation signatures corresponding to genomic subgroups have been described in pancreatic NENs, in which the group with the highest methylation pattern is enriched for MEN1 mutations [74]. Pancreas-specific knockout of Men1 in mice was shown to result in insulinomas with progressive loss of insulin production, indicating dedifferentiation processes [75]. In contrast, PPGLs have low numbers of subclonal populations and a low mutational burden compared to other tumors [76, 77]. A model has been proposed for PPGLs, in which tumors with more immature features, e.g. those due to SDHx mutations, arise from earlier progenitors, like Schwann cell precursors and carry more stem-like features, while others originate from mutations in later developmental stages [78].
Radiation and chemotherapy can also enrich and transform more differentiated cells into stem-like cells resulting in relapse or therapy resistance. These cell populations are characterized by a transition to a mesenchymal state; they have increased survival, DNA damage repair capacity, and activated Hedgehog, Wnt, Notch, HIF, and phosphoinositide 3-kinase (PI3K) signaling [79].
Hedgehog signaling
Several mechanisms can lead to activation of Hedgehog signaling, mutations of pathway components, such as loss-of-function of the Smoothened (SMO) repressor PTCH1, or constitutive activation of SMO, excessive expression of Hedgehog ligands either by the tumor cell itself or by stromal components [80]. One of the transcriptional targets of Hedgehog signaling is NANOG, known as a master transcription factor regulating the recruitment of other transcription factors, including those involved in pluripotency and self-renewal, e.g. OCT4 and SOX2 [81, 82]. Several studies have shown that the growth of SCLC cells is strongly dependent on Hedgehog signaling [83, 84]. The majority of ileum NENs express the ligand SHH, other components of the Hedgehog pathway are also expressed in gastrointestinal NENs [85, 86]. Additionally, a subgroup of PPGLs with MAML3 fusion or CSDE1 somatic variants is characterized by activation of Hedgehog and Wnt signaling [87]. Although specific information for NENs is limited, currently available studies indicate that Hedgehog plays an important role in these tumors. Whether the activation of Hedgehog signaling only occurs in CSCs remains to be elucidated, but at least it suggests a poorly differentiated phenotype, which in turn might lead to increased invasiveness and therapy resistance, e.g., against radiation treatment [88, 89]. In fact, the Hedgehog inhibitor sonidegib was shown to potentiate peptide receptor radiotherapy in a NEN cell line [90]. Sonidegib is FDA-approved for advanced basal cell carcinoma. Severe side effects can occur with high doses, but lower doses achieved better benefit-to-risk profiles [91].
Canonical Wnt signaling
Wnt proteins activate transmembrane receptors of the Frizzled family, and downstream actions prevent phosphorylation of β-catenin, which in turn accumulates and forms transcription-initiating complexes. Inhibition of this pathway demonstrated reduced viability, growth and colony-forming ability in NEN cell line models [92, 93]. Furthermore, the promoter of the Wnt signaling inhibitor secreted frizzled-related protein 1 (SFRP-1) was found to be methylated in many NEN tissues and cell line models [93]. Treatment of cell lines with DNA methylation inhibitor 5-aza-2′-deoxycytidine increased expression of SFRP-1 and other negative regulators of Wnt. Pathway inhibitors were shown to have radiosensitizing effects in other cancer entities [94]. Wnt signaling was also identified to regulate CXCR4 expression, indicating that NENs with Wnt signaling activation might benefit from [68Ga]Ga-pentixafor and [177Lu]Lu-/[90Y]Y-pentixather theranostics rather than SSTR2 targeting [95]. Especially pancreatic NENs with MEN1 mutations show activated Wnt signaling and could be susceptible to this form of theranostics [96].
Notch signaling
Notch is another evolutionarily conserved pathway that regulates proliferation, stem cell features and differentiation. Notch ligands bind to their receptors and activate canonical signaling through the transcription factor CSL (CBF-1/Suppressor of Hairless/LAG1); alternatively, non-canonical signaling independently of CSL can occur, e.g., through antagonistic interaction with Wnt/β-catenin [97]. Notch signaling was shown to be a regulator of neuroendocrine differentiation in pancreatic, lung, thyroid, and gastrointestinal tissues and NENs [98-102]. Moreover, head and neck paragangliomas were shown to have amplifications in Notch pathway components [103]. NOTCH1 and its receptor were also expressed in paragangliomas without copy number variations through a possible mechanism involving miRNA. Nevertheless, activation of NOTCH1 through epigenetic modulation in NENs was associated with treatment response in cell line models and patients [104, 105], emphasizing the importance of careful target evaluation in each disease setting or even patient.
Epigenetic reprogramming
Epigenetic gene signatures, including DNA methylation and histone methylation, and acetylation, play an important role in maintaining stemness. Alterations in epigenetic marks during carcinogenesis affect cellular plasticity and reprogramming. Methylation signatures corresponding to different subtypes were found in pancreatic NENs [74]. Additionally, mutations in genes involved in epigenetic processes, including histone modification, were identified [106]. Several histone deacetylases (HDACs) were elevated in high-grade (G3) pancreatic NENs compared to lower grades. Especially upregulation of nuclear HDAC5 was associated with reduced disease-free survival and overall survival [107]. The non-selective HDAC inhibitor panobinostat showed promising results in vitro and stimulated redifferentation evaluated by insulin production and SSTR2 expression [108]. The latter effect is of particular interest, since increased presence of the target molecule SSTR2 is likely to improve efficacy. Several preclinical studies evaluated the effects of DNA methyltransferase and HDAC inhibitors in GEP-NEN cell lines for its ability to increase SSTR2 [109-111]. Combination of 5-fluorouracil with either decitabine (DNA methyltransferase inhibitor) or tacedinaline (HDAC inhibitor) increased cell apoptosis compared to either drug alone, had radiosensitization effects when given before gamma irradiation, and increased SSTR2 in several human NEN cell lines [112]. As a result, cellular uptake of [68Ga]Ga -DOTA-TOC was higher with the drug combination. Although SSTR2 upregulating effects have been shown in vitro and in vivo, the underlying mechanism may be unrelated to epigenetic reprogramming [111]. To this end, our group showed that epigenetic modification with valproic acid and 5-aza-2'-deoxycytidine modulates SSTR2 levels and sensitivity to [177Lu]Lu-DOTA-TATE in mouse models for PPGL [53]. Similar to experiences in GEP-NEN models, SSTR2 promoter methylation was also not the cause of SSTR2 induction in PPGL.
In PPGLs, mutations in genes encoding Krebs cycle enzymes, such as SDH or fumarate hydratase (FH), lead to a CpG island methylator phenotype (CIMP) associated with transition to a mesenchymal state and increased malignancy due to inhibition of ten-eleven translocation (TET) methylcytosine dioxygenases by oncometabolites [113]. Importantly, it was demonstrated that the CIMP only leads to metastatic features in conjunction with HIF2α activation in PPGL [114, 115]. This suggests that epigenetic targeting alone may not be successful, but might be worth investigating in combination and in patients with rare germline pathogenic variants in DNA methyltransferases [116].
Heat shock protein 90 (HSP90) is a molecular chaperone that binds to chromatin regulators, is highly expressed in NEN primary tumors and metastases, and targeting was shown to have antiproliferative effects [117, 118]. Combination treatment of [177Lu]Lu-DOTA-TATE with HSP90 inhibitor onalespib in a NEN xenograft model increased tumor doubling time, the percentage of cases with complete remissions, and overall survival compared to control or monotherapy [119, 120]. Another study investigated CC-90011, an oral inhibitor of lysine-specific demethylase 1 (LSD1/KDM1A) in a phase I trial [121]. Monotherapy was well-tolerated in patients with refractory NENs, and four patients achieved prolonged stable disease (longer than 6 months). In addition, proteins reading histone modifications have been targeted in NENs. Inhibitors for bromodomain-containing proteins from the bromo and extra-terminal (BET) domain family inhibited proliferation and increased apoptosis in preclinical NEN models [122]. Currently, one trial tests [177Lu]Lu-DOTA-TATE together with DNA hypomethylating agent ASTX727 (NCT05178693).
DNA damage repair
Enhanced DNA repair capacity protects genome integrity and is required for maintaining cell stemness. Hence, it is not surprising that also CSCs are characterized by enhanced DNA damage response and/or elongated cycling times through activation of cyclin-dependent kinases, which in turn leads to more time for DNA repair processes [123]. Inhibiting the DNA damage capacity in tumors is a valid strategy. Preclinical studies in SSTR2-expressing neuroendocrine cell lines or tumor slices demonstrated that poly(ADP-ribose) polymerase-1 (PARP) inhibitors talazoparib, olaparib, and 1,5-dihydroxyisochinolin in combination with [177Lu]Lu-DOTA-TATE increased the number of double-strand breaks and reduced cell/tumor growth further than either treatment alone [124, 125]. In a PPGL cell line model with SDHB knockdown, the combination of olaparib with temozolomide resulted in reduced metastatic lesions and prolonged overall survival in mice [126]. Several clinical studies are underway to further investigate safety and efficacy of combining PARP inhibitors with [177Lu]Lu-DOTA-TATE (NCT04375267, NCT04086485, NCT05053854, NCT05870423). At least in combination with EBRT, olaparib demonstrated an excellent toxicity profile [127]. Further radiosensitizing agents that are currently tested in clinical trials include Triapine, an inhibitor of ribonucleotide reductase, which is the rate-limiting enzyme of DNA synthesis and repair (NCT05724108), and the DNA-dependent protein kinase inhibitor Peposertib (NCT04750954).
Reactive oxygen species (ROS) and detoxification mechanisms
Ionizing radiation triggers ROS in cells through radiolysis of water and the induction of ROS formation in mitochondria [128]. Excessive ROS in turn damages biomolecules and activates different cell death pathways [129]. Consequently, the efficacy of radiation treatment is linked to a cell's ability to control ROS levels through various detoxification mechanisms. These include enzymes like manganese or copper-zinc superoxide dismutase, catalases, and glutathione peroxidase (GPX)/reductase that convert oxygen radicals to hydrogen peroxide and subsequently water, and nonenzymatic antioxidants, such as urate, glutathione, polyamines, vitamins E, A, and C. Tight regulation of ROS levels is also important for maintaining stemness; hence all pathways described above influence redox homeostasis [130]. GPX4 is one such regulator that protects cells from lipid oxidation and thereby protects from ferroptotic cell death [131]. SCLC cell lines with a non-neuroendocrine phenotype were more sensitive to ferroptosis induction by GPX4 inhibition than SCLC cell lines with a more neuroendocrine subtype, which relied more on the thioredoxin antioxidant pathway [132]. Both pathways have been implicated in radiosensitization of cancer cells [133, 134]. Another important regulator of redox homeostasis is the nuclear factor erythroid 2-related factor 2 (NRF2). It induces the expression of a multitude of antioxidant enzymes and metabolic components participating in the pentose phosphate pathway, which generates NADPH necessary for the regeneration of glutathione. In a model for PPGL with low SDHB expression the NRF2 inhibitor brusatol showed cytotoxic effects [135]. It was also demonstrated to enhance radiosensitivity in other cancer cells [136].
PI3K signaling
The PI3K pathway with its downstream regulators, the serine/threonine kinase AKT and mammalian target of rapamycin (mTOR), has been implicated in the development and progression of many cancers due to its role in mediating cell survival and proliferation. Furthermore, this pathway can be activated in response to radiation treatment, thereby facilitating radioresistance. Current knowledge was gathered mainly through studies in more common cancers, such as glioblastoma, non-small cell lung cancer, head and neck cancer, colorectal cancer, and prostate cancer, and is summarized in a recent review [137]. Interestingly, the use of single inhibitors of pathway components generated variable results, while dual PI3K/mTOR inhibitors demonstrated more consistent radiosensitization effects that were associated with inhibition of double strand break repair and cell proliferation, and elevation of apoptosis and autophagy. Additionally, effects on the tumor microenvironment contributed to radiosensitization by normalizing the tumor vasculature and thereby reducing areas of low oxygen.
Different mechanisms were shown to result in PI3K/AKT/mTOR activation in GEP-NENs, including gene mutations in pathway components, copy number gain of AKT1 or AKT2, decreased expression of mTOR regulators PTEN and TSC2 or increased expression of tumorigenic pathway components [106, 138-140]. PPGLs are classified based on their expression profile in clusters. Especially tumors grouped into cluster 2 are characterized by PI3K/AKT/mTOR activation due to mutations in the RET proto-oncogene (RET), neurofibromin 1 (NF1), transmembrane protein 127 (TMEM127), MYC-associated factor X (MAX), and other related genes [141, 142]. Mutations in RET, both germline and somatic, also cause the development of MTC, where activated PI3K/AKT/mTOR signaling was demonstrated in primary tumors and metastasis [143, 144].
Inhibition of the PI3K/AKT/mTOR pathway demonstrated anti-tumor effects in several models of GEP-NENs, MTC, and PPGL [145-149]. In one study a link between anti-tumor activity and the induction of differentiation was demonstrated in GEP-NEN cell lines [149]. Additionally, radiosensitizing effects of PI3K/AKT/mTOR inhibitors have been reported in in vitro models of GEP-NENs [150, 151]. In PPGLs with known metastatic progression, a microRNA signature was identified that functions as a potential marker for tumors with higher sensitivity to mTOR pathway inhibition [152]. Three trials are registered that investigate the combination of tyrosine kinase or mTOR inhibitors with [177Lu]Lu-DOTA-TATE (NCT05687123, NCT05249114, NCT03629847). A previous study found that everolimus caused manageable toxicity as a second agent to [177Lu]Lu-DOTA-TATE [153].
HIF signaling
Another important pathway in NENs that is associated with PI3K/AKT/mTOR signaling is mediated through the transcription factors HIF1α and HIF2α. The latter appears to be more relevant for NEN development and progression, since HIF2α is a regulator of trunk neural crest stemness and migration towards sympathoadrenal sites [154].
PPGLs of the expression cluster 1 arise due to mutations in genes activating hypoxia-inducible factors (HIFs), but especially HIF2α, and are more prone to metastasize [115]. Genes conferring susceptibility for cluster 1 PPGLs also include SDH subunit genes and FH that result in production of the oncometabolites succinate or fumarate, which inhibit α-ketoglutarate-dependent enzymes and thereby induce HIF signaling and global epigenetic modifications [113, 114]. These metabolic features can be used to identify such tumors, also those where succinate increases are not caused by gene mutations but rather by epigenetic mechanisms [155, 156]. Cluster 1 PPGLs most likely arise from less differentiated chromaffin cells than cluster 2 PPGLs, indicated by the more immature nature of the catecholamine production and secretion machinery and the earlier age of disease onset [78].
In PPGL cell models, HIF2α activation is associated with increased migratory and invasive features, a less differentiated cellular state, and a more radioresistant phenotype [114, 115, 157]. Radiosensitizing properties have been described for SN-38 through the inhibition of radiation-induced HIF1α in colorectal cancer [158]; however, whether HIF2α inhibition can lead to similar effects in NENs has to be explored. The specific HIF2α inhibitor belzutifan is currently tested as a monotherapy in a phase II clinical trial in patients with pancreatic NENs and PPGL (NCT04924075). Preclinical evidence whether belzutifan can act as a radiosensitizer in combination with 177Lu]Lu-DOTA-TATE is, unfortunately, still outstanding.
HIF-regulated processes open up further possibilities for targeted radiosensitizing approaches. Exemplarily, an attractive molecular target in the specific context is cyclooxygenase-2 (COX-2) and subsequent prostaglandin-mediated signaling pathways [159]. Prostaglandins produced due to increased COX-2 activity, such as PGE2, are pro-inflammatory cytokine-like factors that substantially modulate the immune response in cancer. They are also involved in the establishment of the tumor-associated microenvironment by promoting angiogenesis. In this regard, prostaglandins are directly involved in tumor progression and the acquisition of radioresistance, which leads to unsatisfactory results of radiotherapy [160]. Additionally, the role of COX-2 in CSC survival and recolonization after therapy has been considered in detail elsewhere, making it clear that inhibiting COX-2 is an effective way to prevent treatment failure due to tumor repopulation [161]. Addressing COX-2 by selective inhibitors or dual drugs as an adjuvant approach is also feasible in NENs [162-165].
Metabolic interventions
The acquisition of proliferative, malignant, and stem cell features during tumor progression is accompanied by changes in cell metabolism, raising the possibility of targeting deregulated metabolic features. Highly glycolytic tumors, evident from strong uptake of [¹⁸F] fluorodeoxyglucose (FDG) by PET, could benefit from radiosensitization with glycolytic inhibitors. NENs double positive for [68Ga]Ga-DOTA-TATE and [¹⁸F]-FDG have been observed previously [166]. On the other hand, targeting mitochondrial metabolism reduces oxygen usage and can result in elevated generation of reactive oxygen species. Both approaches are still experimental. Radiosensitizing effects of glycolytic inhibitor 2-deoxy-D-glucose and respiratory complex I inhibitor metformin have been demonstrated in neuroblastoma, glioma and other cell lines in combination with ionizing radiation [167]. Additionally, retrospective analysis of patients taking metformin as an anti-diabetic drug during radiotherapy suggests potentiating effects [168]. Interestingly, CSCs either exhibit a glycolytic or mitochondrial phenotype depending on the tissue of origin [169]. Further research is required to understand these mechanisms in NENs. Targeting of other metabolic pathways may also be beneficial in certain subgroups of NENs. PPGLs with defects in SDHx genes show increased dependency on glutamine metabolism for replenishing Krebs cycle intermediates [170]. The feasibility of targeting glutamine metabolism in combination with radiation to enhance radioresponse was recently demonstrated in prostate cancer cell lines [171]. Furthermore, preclinical evidence suggests that pharmacological inhibition of nicotineamide phosphoribosyltransferase (NAMPT), an enzyme involved in NAD+ metabolism, sensitizes the NEN cell line GOT1 to [177Lu]Lu-DOTA-TATE [172]. NAMPT is responsible for regeneration of NAD+, which is required amongst others for activation of PARP-1, thereby indirectly influencing DNA repair.
Personalized radiosensitization strategies for SSTR2-based PRRT
Although many promising approaches towards improving SSTR2-based PRRT in NENs have been demonstrated in preclinical models, for patients it will be important to select the potentially most effective radiosensitization agent on an individual basis. Patients should be characterized based on germline mutational status, as well as the mutational, transcriptomic, and metabolomic profile of the primary tumor. Additionally, biopsies of metastases or inoperable tumors could be analyzed where possible.
Genetic or metabolic markers can guide selection of an appropriate combination therapy with agents that have been approved for other cancers (Figure 3). Tumors with transcriptional profiles, somatic or germline mutations indicating upregulation of the PI3K/AKT/mTOR might benefit from combinations with temsirolimus or one of the approved PI3K inhibitors. Such an approach might be effective in RET-mutated MTCs [173]. On the other hand, PGLs with SDHx mutations or metabolic profiles indicating impairment of SDH or other Krebs cycle enzymes that are characterized by a CIMP might respond to combinations including HDAC or DNA methyltransferase inhibitors. PARP inhibitors might improve radiotherapeutic effects across a wider range of tumors, but should certainly be considered for tumors with mutations in DNA damage repair pathways, including BRCA1/2, ATM, and ATRX. Transcriptomics might further aid in predicting PARP resistance, including loss of TP53BP1 or Shieldin complex expression [174]. Bioinformatic approaches might also assist in the selection of potential complementary therapies. The PanDrugs.org platform is one such example and provides suggestions for chemotherapeutics based on the tumor's mutational landscape [175].
Due to massive reductions in the costs of next generation sequencing, multi-gene panel genetic diagnostics are performed on a routine basis in the Western world. Especially for patients with PPGLs, genetic screening for mutations in known susceptibility genes is recommended to identify hereditary causes and to better manage the risk of metastatic disease [176]. Additionally, several countries are running precision oncology-based cancer trials, including the National Institutes of Health in the USA and the Molecularly Aided Stratification for Tumor Eradication (MASTER) program in Germany. Personalized PRRT approaches could be incorporated in such existing infrastructures. The proposed combinational treatments might become important, especially, in the context of rare diseases, where dedicated clinical trials for new agents are difficult to perform.
It should be emphasized that evaluating the effectiveness of radionuclide therapies might require expanding the conventional approaches for measuring response. This expansion is necessary to adequately encompass the specific objectives and impacts of this therapeutic approach. Given the distinct mechanism and goals of radionuclide therapy, the objective response rates, such as tumor shrinkage, alone might not fully capture the actual clinical benefit for the patient. Hence, it's crucial to consider other factors, such as improvements in quality of life, reducing tumor burden, alleviating symptoms, slowing disease progression, or increased survival time, to gain a more comprehensive picture of treatment effectiveness. In this regard, the utilization of [18F]FDG-PET/CT in assessing therapy response can be a valuable complement to gain a more comprehensive understanding of treatment efficacy [177].
Currently running clinical trials that investigate combinational therapies with [177Lu]Lu-DOTA-TATE (Lutathera) target some of the pathways mentioned above, including DNA repair, epigenetics, and kinase/mTOR signaling (Table 1). The important point, however, is that although patients are selected based on SSTR2 positivity by [68Ga]Ga-DOTA-TATE PET/CT, they are not specifically chosen to receive inhibitors based on their tumor profile. In future clinical trials should incorporate personalized selection of complementary drugs also in the setting of PRRT.
Clinical trials directed towards [177Lu]Lu-DOTA-TATE (Lutathera) in combination with other agents
| Identifier | Phase | Conditions | Drugs | Category 2nd drug | Status |
|---|---|---|---|---|---|
| NCT05053854 | I | metastatic GEP-NEN | Lutathera + Talazoparib | PARP inhibitor | recruiting |
| NCT04375267 | I | NEN, ThymomaMesothelioma (SSTR2+) | Lutathera + Olaparib | PARP inhibitor | recruiting |
| NCT05870423 | I | NEN | Lutathera + Olaparib | PARP inhibitor | recruiting |
| NCT04086485 | I, II | GEP-NEN | Lutathera + Olaparib | PARP inhibitor | recruiting |
| NCT05724108 | II | metastatic NENs | Lutathera + Triapine | ribonucleotide reductase inhibitor (DNA synthesis/repair) | recruiting |
| NCT04750954 | I | NEN | Lutathera + Peposertib | DNA-dependent protein kinase inhibitor (DNA repair) | recruiting |
| NCT05178693 | I | NEN | Lutathera + ASTX727 | DNA hypomethylating agent | recruiting |
| NCT05687123 | I | pancreatic NEN | Lutathera + Sunitinib | tyrosine kinase inhibitor | recruiting |
| NCT05249114 | I | NEN | Lutathera + Carbozantinib | tyrosine kinase inhibitor | recruiting |
| NCT03629847 | I, II | GEP- and lung NEN | Lutathera + Everolimus | mTOR inhibitor | unknown |
| NCT05142696 | I | SCLC | Lutathera + Carboplatin+ Etoposide + Tislelizumab | alkylating agent, topoisomerase inhibitor, immunotherapy | recruiting |
| NCT02358356 | II | GEP-NEN | Lutathera + Capecitabine + Temozolomide | antimetabolite, alkylating agent | completed |
| NCT03325816 | I, II | SCLC | Lutathera + Nivolumab | immunotherapy | completed |
| NCT04525638 | II | grade 3 neuroendocrine tumours,neuroendocrine carcinomas | Lutathera + Nivolumab | immunotherapy | recruiting |
| NCT03457948 | II | NEN | Lutathera + Pembrolizumab | immunotherapy | recruiting |
| 2014-003067-38* | II | GEP-NEN | Lutathera + Capecitabine | antimetabolite | ongoing |
Sourced from ClinicalTrials.gov or *clinicaltrialsregister.eu
Since [177Lu]Lu-DOTA-TATE itself was shown to have a mild toxicity profile [178], adverse effects are expected to originate from the second agent. Drug combinations with [177Lu]Lu-DOTA-TATE have so far resulted in little increase in toxicities, although the combination with everolimus had to be managed under close observation and dose reductions were necessary in some patients [153, 179]. As the proposed strategy would address targets with high abundance in tumor tissue, lower doses of the second agent might be required, thereby limiting normal tissue toxicities.
Conclusions and Outlook
Present systemic treatments offered to patients with NENs will halt disease progression in some individuals but rarely lead to a cure. Hence, further improvements and an increased level of personalization are urgently required. As radionuclide therapy shows promising responses in some patients and inhibitors of tumorigenic pathways were shown to be effective in certain patient groups, the next logical step is the application of combination therapies based on the knowledge of active tumorigenic pathways. The currently most promising strategies are targeting DNA repair pathways, which is applicable across a wide range of NENs, and inhibitors of kinase signaling that could be effective in subgroups of GEP-NENs, MTCs, and PPGLs. In this regard, clinical trials should incorporate adequate patient selection into their trial design. Genetic mutations, transcriptional or even microRNA signatures are feasible biomarkers. A third pathway with the potential for yielding improved treatments for some patients with SCLC, GEP-NEN, and PPGL is Hedgehog, a regulator of stemness features. In combination with other strategies, e.g. those directed towards dose maximization, personalized radiosensitization should become a mainstay of NEN therapy.
Acknowledgements
This work was supported by the German Research Foundation [Deutsche Forschungsgemeinschaft (DFG)] within the CRC/Transregio 205/2, Project number: 314061271 - TRR 205 “The Adrenal: Central Relay in Health and Disease” (to all authors).
Author contributions
All authors have jointly conceived and written this review article. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Assarzadegan N, Montgomery E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch Pathol Lab Med. 2021;145:664-77
2. Hofland J, Falconi M, Christ E, Castano JP, Faggiano A, Lamarca A. et al. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J Neuroendocrinol. 2023: e13318.
3. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y. et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-42
4. Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R. et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8-21
5. Auernhammer CJ, Spitzweg C, Angele MK, Boeck S, Grossman A, Nölting S. et al. Advanced neuroendocrine tumours of the small intestine and pancreas: clinical developments, controversies, and future strategies. Lancet Diabetes Endocrinol. 2018;6:404-15
6. Ceolin L, Duval M, Benini AF, Ferreira CV, Maia AL. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr Relat Cancer. 2019;26:R499-R518
7. Nölting S, Bechmann N, Taieb D, Beuschlein F, Fassnacht M, Kroiss M. et al. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr Rev. 2022;43:199-239
8. Nölting S, Grossman A, Pacak K. Metastatic Phaeochromocytoma: Spinning Towards More Promising Treatment Options. Exp Clin Endocrinol Diabetes. 2019;127:117-28
9. Nölting S, Ullrich M, Pietzsch J, Ziegler CG, Eisenhofer G, Grossman A. et al. Current Management of Pheochromocytoma/Paraganglioma: A Guide for the Practicing Clinician in the Era of Precision Medicine. Cancers (Basel). 2019 11
10. Sun LC, Coy DH. Somatostatin receptor-targeted anti-cancer therapy. Curr Drug Deliv. 2011;8:2-10
11. Leijon H, Remes S, Hagstrom J, Louhimo J, Maenpaa H, Schalin-Jantti C. et al. Variable somatostatin receptor subtype expression in 151 primary pheochromocytomas and paragangliomas. Hum Pathol. 2019;86:66-75
12. Park S, Parihar AS, Bodei L, Hope TA, Mallak N, Millo C. et al. Somatostatin Receptor Imaging and Theranostics: Current Practice and Future Prospects. J Nucl Med. 2021;62:1323-9
13. Pavel M, Oberg K, Falconi M, Krenning EP, Sundin A, Perren A. et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-60
14. Sundin A, Arnold R, Baudin E, Cwikla JB, Eriksson B, Fanti S. et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology. 2017;105:212-44
15. Knigge U, Capdevila J, Bartsch DK, Baudin E, Falkerby J, Kianmanesh R. et al. ENETS Consensus Recommendations for the Standards of Care in Neuroendocrine Neoplasms: Follow-Up and Documentation. Neuroendocrinology. 2017;105:310-9
16. Iravani A, Parihar AS, Akhurst T, Hicks RJ. Molecular imaging phenotyping for selecting and monitoring radioligand therapy of neuroendocrine neoplasms. Cancer Imaging. 2022;22:25
17. Mojtahedi A, Thamake S, Tworowska I, Ranganathan D, Delpassand ES. The value of (68)Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging. 2014;4:426-34
18. Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40-7
19. Maurice JB, Troke R, Win Z, Ramachandran R, Al-Nahhas A, Naji M. et al. A comparison of the performance of (68)Ga-DOTATATE PET/CT and (123)I-MIBG SPECT in the diagnosis and follow-up of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39:1266-70
20. Hennrich U, Benesova M. [(68)Ga]Ga-DOTA-TOC: The First FDA-Approved (68)Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals (Basel). 2020 13
21. Jha A, Patel M, Carrasquillo JA, Chen CC, Millo C, Maass-Moreno R. et al. Choice Is Good at Times: The Emergence of [(64)Cu]Cu-DOTATATE-Based Somatostatin Receptor Imaging in the Era of [(68)Ga]Ga-DOTATATE. J Nucl Med. 2022;63:1300-1
22. Kunikowska J, Zemczak A, Kolodziej M, Gut P, Lon I, Pawlak D. et al. Tandem peptide receptor radionuclide therapy using (90)Y/(177)Lu-DOTATATE for neuroendocrine tumors efficacy and side-effects - polish multicenter experience. Eur J Nucl Med Mol Imaging. 2020;47:922-33
23. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B. et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-35
24. Kim SJ, Pak K, Koo PJ, Kwak JJ, Chang S. The efficacy of (177)Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42:1964-70
25. Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW. et al. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res. 2017;23:4617-24
26. Sorbye H, Kong G, Grozinsky-Glasberg S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr Relat Cancer. 2020;27:R67-R77
27. Mato E, Matias-Guiu X, Chico A, Webb SM, Cabezas R, Berna L. et al. Somatostatin and somatostatin receptor subtype gene expression in medullary thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:2417-20
28. de Vries LH, Lodewijk L, Willems SM, Dreijerink KMA, de Keizer B, van Diest PJ. et al. SSTR2A expression in medullary thyroid carcinoma is correlated with longer survival. Endocrine. 2018;62:639-47
29. Tran K, Khan S, Taghizadehasl M, Palazzo F, Frilling A, Todd JF. et al. Gallium-68 Dotatate PET/CT is superior to other imaging modalities in the detection of medullary carcinoma of the thyroid in the presence of high serum calcitonin. Hell J Nucl Med. 2015;18:19-24
30. Tuncel M, Kilickap S, Suslu N. Clinical impact of (68)Ga-DOTATATE PET-CT imaging in patients with medullary thyroid cancer. Ann Nucl Med. 2020;34:663-74
31. Parghane RV, Naik C, Talole S, Desmukh A, Chaukar D, Banerjee S. et al. Clinical utility of (177) Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck. 2020;42:401-16
32. Fischer A, Kloos S, Maccio U, Friemel J, Remde H, Fassnacht M. et al. Metastatic pheochromocytoma and paraganglioma: Somatostatin receptor 2 expression, genetics and therapeutic responses. J Clin Endocrinol Metab. 2023
33. Jha A, Ling A, Millo C, Gupta G, Viana B, Lin FI. et al. Superiority of (68)Ga-DOTATATE over (18)F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx )-related pheochromocytoma and paraganglioma in the pediatric population. Eur J Nucl Med Mol Imaging. 2018;45:787-97
34. Darr R, Nambuba J, Del Rivero J, Janssen I, Merino M, Todorovic M. et al. Novel insights into the polycythemia-paraganglioma-somatostatinoma syndrome. Endocr Relat Cancer. 2016;23:899-908
35. Taieb D, Wanna GB, Ahmad M, Lussey-Lepoutre C, Perrier ND, Nölting S. et al. Clinical consensus guideline on the management of phaeochromocytoma and paraganglioma in patients harbouring germline SDHD pathogenic variants. Lancet Diabetes Endocrinol. 2023;11:345-61
36. Marretta AL, Ottaiano A, Iervolino D, Bracigliano A, Clemente O, Di Gennaro F. et al. Response to Peptide Receptor Radionuclide Therapy in Pheocromocytomas and Paragangliomas: A Systematic Review and Meta-Analysis. J Clin Med. 2023 12
37. Vyakaranam AR, Crona J, Norlen O, Granberg D, Garske-Roman U, Sandstrom M. et al. Favorable Outcome in Patients with Pheochromocytoma and Paraganglioma Treated with (177)Lu-DOTATATE. Cancers (Basel). 2019 11
38. Nastos K, Cheung VTF, Toumpanakis C, Navalkissoor S, Quigley AM, Caplin M. et al. Peptide Receptor Radionuclide Treatment and (131)I-MIBG in the management of patients with metastatic/progressive phaeochromocytomas and paragangliomas. J Surg Oncol. 2017;115:425-34
39. Wolf KI, Jha A, van Berkel A, Wild D, Janssen I, Millo CM. et al. Eruption of Metastatic Paraganglioma After Successful Therapy with (177)Lu/(90)Y-DOTATOC and (177)Lu-DOTATATE. Nucl Med Mol Imaging. 2019;53:223-30
40. Desai H, Borges-Neto S, Wong TZ. Molecular Imaging and Therapy for Neuroendocrine Tumors. Curr Treat Options Oncol. 2019;20:78
41. Pryma DA, Chin BB, Noto RB, Dillon JS, Perkins S, Solnes L. et al. Efficacy and Safety of High-Specific-Activity (131)I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J Nucl Med. 2019;60:623-30
42. Carrasquillo JA, Chen CC, Jha A, Ling A, Lin FI, Pryma DA. et al. Imaging of Pheochromocytoma and Paraganglioma. J Nucl Med. 2021;62:1033-42
43. Kayano D, Kinuya S. Current Consensus on I-131 MIBG Therapy. Nucl Med Mol Imaging. 2018;52:254-65
44. Jha A, Taieb D, Carrasquillo JA, Pryma DA, Patel M, Millo C. et al. High-Specific-Activity-(131)I-MIBG versus (177)Lu-DOTATATE Targeted Radionuclide Therapy for Metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res. 2021;27:2989-95
45. Refardt J, Hofland J, Kwadwo A, Nicolas GP, Rottenburger C, Fani M. et al. Theranostics in neuroendocrine tumors: an overview of current approaches and future challenges. Rev Endocr Metab Disord. 2021;22:581-94
46. Jang A, Kendi AT, Johnson GB, Halfdanarson TR, Sartor O. Targeted Alpha-Particle Therapy: A Review of Current Trials. Int J Mol Sci. 2023 24
47. Harris PE, Zhernosekov K. The evolution of PRRT for the treatment of neuroendocrine tumors; What comes next? Front Endocrinol (Lausanne). 2022;13:941832
48. Kassis AI. Therapeutic radionuclides: biophysical and radiobiologic principles. Semin Nucl Med. 2008;38:358-66
49. Del Prete M, Buteau FA, Arsenault F, Saighi N, Bouchard LO, Beaulieu A. et al. Personalized (177)Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging. 2019;46:728-42
50. Park EA, Graves SA, Menda Y. The Impact of Radiopharmaceutical Therapy on Renal Function. Semin Nucl Med. 2022;52:467-74
51. Brandt F, Ullrich M, Wodtke J, Kopka K, Bachmann M, Loser R. et al. Enzymological Characterization of (64)Cu-Labeled Neprilysin Substrates and Their Application for Modulating the Renal Clearance of Targeted Radiopharmaceuticals. J Med Chem. 2023
52. Klomp MJ, Dalm SU, de Jong M, Feelders RA, Hofland J, Hofland LJ. Epigenetic regulation of somatostatin and somatostatin receptors in neuroendocrine tumors and other types of cancer. Rev Endocr Metab Disord. 2021;22:495-510
53. Ullrich M, Richter S, Liers J, Drukewitz S, Friedemann M, Kotzerke J. et al. Epigenetic drugs in somatostatin type 2 receptor radionuclide theranostics and radiation transcriptomics in mouse pheochromocytoma models. Theranostics. 2023;13:278-94
54. Pollard JH, Menda Y, Zamba KD, Madsen M, O'Dorisio MS, O'Dorisio T. et al. Potential for Increasing Uptake of Radiolabeled (68)Ga-DOTATOC and (123)I-MIBG in Patients with Midgut Neuroendocrine Tumors Using a Histone Deacetylase Inhibitor Vorinostat. Cancer Biother Radiopharm. 2021;36:632-41
55. Refardt J, Klomp MJ, van Koetsveld PM, Dogan F, Konijnenberg M, Brabander T. et al. Effect of epigenetic treatment on SST(2) expression in neuroendocrine tumour patients. Clin Transl Med. 2022;12:e957
56. Brandt F, Ullrich M, Laube M, Kopka K, Bachmann M, Loser R. et al. "Clickable" albumin binders for modulating the tumor uptake of targeted radiopharmaceuticals. J Med Chem. 2022;65:710-33
57. Albrecht J, Exner S, Grotzinger C, Prasad S, Konietschke F, Beindorff N. et al. Multimodal Imaging of 2-Cycle PRRT with (177)Lu-DOTA-JR11 and (177)Lu-DOTATOC in an Orthotopic Neuroendocrine Xenograft Tumor Mouse Model. J Nucl Med. 2021;62:393-8
58. Baum RP, Zhang J, Schuchardt C, Muller D, Macke H. First-in-Humans Study of the SSTR Antagonist (177)Lu-DOTA-LM3 for Peptide Receptor Radionuclide Therapy in Patients with Metastatic Neuroendocrine Neoplasms: Dosimetry, Safety, and Efficacy. J Nucl Med. 2021;62:1571-81
59. Kim YS, Brechbiel MW. An overview of targeted alpha therapy. Tumour Biol. 2012;33:573-90
60. Borgna F, Haller S, Rodriguez JMM, Ginj M, Grundler PV, Zeevaart JR. et al. Combination of terbium-161 with somatostatin receptor antagonists-a potential paradigm shift for the treatment of neuroendocrine neoplasms. Eur J Nucl Med Mol Imaging. 2021
61. Cullinane C, Jeffery CM, Roselt PD, van Dam EM, Jackson S, Kuan K. et al. Peptide Receptor Radionuclide Therapy with (67)Cu-CuSarTATE Is Highly Efficacious Against a Somatostatin-Positive Neuroendocrine Tumor Model. J Nucl Med. 2020;61:1800-5
62. Krause M, Dubrovska A, Linge A, Baumann M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev. 2017;109:63-73
63. Gaur P, Sceusi EL, Samuel S, Xia L, Fan F, Zhou Y. et al. Identification of cancer stem cells in human gastrointestinal carcinoid and neuroendocrine tumors. Gastroenterology. 2011;141:1728-37
64. Krampitz GW, George BM, Willingham SB, Volkmer JP, Weiskopf K, Jahchan N. et al. Identification of tumorigenic cells and therapeutic targets in pancreatic neuroendocrine tumors. Proc Natl Acad Sci U S A. 2016;113:4464-9
65. Zhu W, Hai T, Ye L, Cote GJ. Medullary thyroid carcinoma cell lines contain a self-renewing CD133+ population that is dependent on ret proto-oncogene activity. J Clin Endocrinol Metab. 2010;95:439-44
66. Kucerova L, Feketeova L, Kozovska Z, Poturnajova M, Matuskova M, Nencka R. et al. In vivo 5FU-exposed human medullary thyroid carcinoma cells contain a chemoresistant CD133+ tumor-initiating cell subset. Thyroid. 2014;24:520-32
67. Bi Y, Meng Y, Wu H, Cui Q, Luo Y, Xue X. Expression of the potential cancer stem cell markers CD133 and CD44 in medullary thyroid carcinoma: A ten-year follow-up and prognostic analysis. J Surg Oncol. 2016;113:144-51
68. Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A. et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504-14
69. Qiu X, Wang Z, Li Y, Miao Y, Ren Y, Luan Y. Characterization of sphere-forming cells with stem-like properties from the small cell lung cancer cell line H446. Cancer Lett. 2012;323:161-70
70. Sarvi S, Mackinnon AC, Avlonitis N, Bradley M, Rintoul RC, Rassl DM. et al. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist. Cancer Res. 2014;74:1554-65
71. Salcido CD, Larochelle A, Taylor BJ, Dunbar CE, Varticovski L. Molecular characterisation of side population cells with cancer stem cell-like characteristics in small-cell lung cancer. Br J Cancer. 2010;102:1636-44
72. Waldum HL, Oberg K, Sordal OF, Sandvik AK, Gustafsson BI, Mjones P. et al. Not only stem cells, but also mature cells, particularly neuroendocrine cells, may develop into tumours: time for a paradigm shift. Therap Adv Gastroenterol. 2018;11:1756284818775054
73. Dentro SC, Leshchiner I, Haase K, Tarabichi M, Wintersinger J, Deshwar AG. et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell. 2021;184:2239-54 e39
74. Lakis V, Lawlor RT, Newell F, Patch AM, Mafficini A, Sadanandam A. et al. DNA methylation patterns identify subgroups of pancreatic neuroendocrine tumors with clinical association. Commun Biol. 2021;4:155
75. Bertolino P, Tong WM, Herrera PL, Casse H, Zhang CX, Wang ZQ. Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res. 2003;63:4836-41
76. Crona J, Backman S, Maharjan R, Mayrhofer M, Stalberg P, Isaksson A. et al. Spatiotemporal Heterogeneity Characterizes the Genetic Landscape of Pheochromocytoma and Defines Early Events in Tumorigenesis. Clin Cancer Res. 2015;21:4451-60
77. Raynaud F, Mina M, Tavernari D, Ciriello G. Pan-cancer inference of intra-tumor heterogeneity reveals associations with different forms of genomic instability. PLoS Genet. 2018;14:e1007669
78. Scriba LD, Bornstein SR, Santambrogio A, Mueller G, Huebner A, Hauer J. et al. Cancer Stem Cells in Pheochromocytoma and Paraganglioma. Front Endocrinol (Lausanne). 2020;11:79
79. Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH. et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16:10
80. Cochrane CR, Szczepny A, Watkins DN, Cain JE. Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers (Basel). 2015;7:1554-85
81. Heurtier V, Owens N, Gonzalez I, Mueller F, Proux C, Mornico D. et al. The molecular logic of Nanog-induced self-renewal in mouse embryonic stem cells. Nat Commun. 2019;10:1109
82. Po A, Ferretti E, Miele E, De Smaele E, Paganelli A, Canettieri G. et al. Hedgehog controls neural stem cells through p53-independent regulation of Nanog. EMBO J. 2010;29:2646-58
83. Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313-7
84. Park KS, Martelotto LG, Peifer M, Sos ML, Karnezis AN, Mahjoub MR. et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med. 2011;17:1504-8
85. Fendrich V, Waldmann J, Esni F, Ramaswamy A, Mullendore M, Buchholz M. et al. Snail and Sonic Hedgehog activation in neuroendocrine tumors of the ileum. Endocr Relat Cancer. 2007;14:865-74
86. Shida T, Furuya M, Nikaido T, Hasegawa M, Koda K, Oda K. et al. Sonic Hedgehog-Gli1 signaling pathway might become an effective therapeutic target in gastrointestinal neuroendocrine carcinomas. Cancer Biol Ther. 2006;5:1530-8
87. Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR. et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell. 2017;31:181-93
88. Gan GN, Eagles J, Keysar SB, Wang G, Glogowska MJ, Altunbas C. et al. Hedgehog signaling drives radioresistance and stroma-driven tumor repopulation in head and neck squamous cancers. Cancer Res. 2014;74:7024-36
89. Chen YJ, Lin CP, Hsu ML, Shieh HR, Chao NK, Chao KS. Sonic hedgehog signaling protects human hepatocellular carcinoma cells against ionizing radiation in an autocrine manner. Int J Radiat Oncol Biol Phys. 2011;80:851-9
90. Spetz J, Langen B, Rudqvist N, Parris TZ, Helou K, Nilsson O. et al. Hedgehog inhibitor sonidegib potentiates (177)Lu-octreotate therapy of GOT1 human small intestine neuroendocrine tumors in nude mice. BMC Cancer. 2017;17:528
91. Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P. et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716-28
92. Jin XF, Spoettl G, Maurer J, Nölting S, Auernhammer CJ. Inhibition of Wnt/beta-Catenin Signaling in Neuroendocrine Tumors in vitro: Antitumoral Effects. Cancers (Basel). 2020 12
93. Kim JT, Li J, Jang ER, Gulhati P, Rychahou PG, Napier DL. et al. Deregulation of Wnt/beta-catenin signaling through genetic or epigenetic alterations in human neuroendocrine tumors. Carcinogenesis. 2013;34:953-61
94. Huang Y, Sheng H, Xiao Y, Hu W, Zhang Z, Chen Y. et al. Wnt/beta-catenin inhibitor ICG-001 enhances the antitumor efficacy of radiotherapy by increasing radiation-induced DNA damage and improving tumor immune microenvironment in hepatocellular carcinoma. Radiother Oncol. 2021;162:34-44
95. Weich A, Rogoll D, Gawlas S, Mayer L, Weich W, Pongracz J. et al. Wnt/beta-Catenin Signaling Regulates CXCR4 Expression and [(68)Ga] Pentixafor Internalization in Neuroendocrine Tumor Cells. Diagnostics (Basel). 2021 11
96. Jiang X, Cao Y, Li F, Su Y, Li Y, Peng Y. et al. Targeting beta-catenin signaling for therapeutic intervention in MEN1-deficient pancreatic neuroendocrine tumours. Nat Commun. 2014;5:5809
97. Crabtree JS, Singleton CS, Miele L. Notch Signaling in Neuroendocrine Tumors. Front Oncol. 2016;6:94
98. Oser MG, Sabet AH, Gao W, Chakraborty AA, Schinzel AC, Jennings RB. et al. The KDM5A/RBP2 histone demethylase represses NOTCH signaling to sustain neuroendocrine differentiation and promote small cell lung cancer tumorigenesis. Genes Dev. 2019;33:1718-38
99. Shan L, Aster JC, Sklar J, Sunday ME. Notch-1 regulates pulmonary neuroendocrine cell differentiation in cell lines and in transgenic mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L500-9
100. Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T. et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877-81
101. Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, Hsiao EC, Schuebel KE, Borges MW. et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90:4350-6
102. Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819-30
103. Cama A, Verginelli F, Lotti LV, Napolitano F, Morgano A, D'Orazio A. et al. Integrative genetic, epigenetic and pathological analysis of paraganglioma reveals complex dysregulation of NOTCH signaling. Acta Neuropathol. 2013;126:575-94
104. Aspuria PJ, Lunt SY, Varemo L, Vergnes L, Gozo M, Beach JA. et al. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab. 2014;2:21
105. Mohammed TA, Holen KD, Jaskula-Sztul R, Mulkerin D, Lubner SJ, Schelman WR. et al. A pilot phase II study of valproic acid for treatment of low-grade neuroendocrine carcinoma. Oncologist. 2011;16:835-43
106. Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P. et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65-71
107. Klieser E, Urbas R, Stattner S, Primavesi F, Jager T, Dinnewitzer A. et al. Comprehensive immunohistochemical analysis of histone deacetylases in pancreatic neuroendocrine tumors: HDAC5 as a predictor of poor clinical outcome. Hum Pathol. 2017;65:41-52
108. Schmitz RL, Weissbach J, Kleilein J, Bell J, Huttelmaier S, Viol F. et al. Targeting HDACs in Pancreatic Neuroendocrine Tumor Models. Cells. 2021 10
109. Veenstra MJ, van Koetsveld PM, Dogan F, Farrell WE, Feelders RA, Lamberts SWJ. et al. Epidrug-induced upregulation of functional somatostatin type 2 receptors in human pancreatic neuroendocrine tumor cells. Oncotarget. 2018;9:14791-802
110. Taelman VF, Radojewski P, Marincek N, Ben-Shlomo A, Grotzky A, Olariu CI. et al. Upregulation of Key Molecules for Targeted Imaging and Therapy. J Nucl Med. 2016;57:1805-10
111. Klomp MJ, Hofland LJ, van den Brink L, van Koetsveld PM, Dogan F, de Ridder CMA. et al. The Effect of VPA Treatment on Radiolabeled DOTATATE Uptake: Differences Observed In Vitro and In Vivo. Pharmaceutics. 2022 14
112. Jin XF, Auernhammer CJ, Ilhan H, Lindner S, Nölting S, Maurer J. et al. Combination of 5-Fluorouracil with Epigenetic Modifiers Induces Radiosensitization, Somatostatin Receptor 2 Expression, and Radioligand Binding in Neuroendocrine Tumor Cells In Vitro. J Nucl Med. 2019;60:1240-6
113. Letouze E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C. et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739-52
114. Morin A, Goncalves J, Moog S, Castro-Vega LJ, Job S, Buffet A. et al. TET-Mediated Hypermethylation Primes SDH-Deficient Cells for HIF2alpha-Driven Mesenchymal Transition. Cell Rep. 2020;30:4551-66 e7
115. Bechmann N, Moskopp ML, Ullrich M, Calsina B, Wallace PW, Richter S. et al. HIF2alpha supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr Relat Cancer. 2020;27:625-40
116. Remacha L, Curras-Freixes M, Torres-Ruiz R, Schiavi F, Torres-Perez R, Calsina B. et al. Gain-of-function mutations in DNMT3A in patients with paraganglioma. Genet Med. 2018;20:1644-51
117. Gilbert JA, Adhikari LJ, Lloyd RV, Halfdanarson TR, Muders MH, Ames MM. Molecular markers for novel therapeutic strategies in pancreatic endocrine tumors. Pancreas. 2013;42:411-21
118. Zitzmann K, Ailer G, Vlotides G, Spoettl G, Maurer J, Goke B. et al. Potent antitumor activity of the novel HSP90 inhibitors AUY922 and HSP990 in neuroendocrine carcinoid cells. Int J Oncol. 2013;43:1824-32
119. Lundsten S, Spiegelberg D, Raval NR, Nestor M. The radiosensitizer Onalespib increases complete remission in (177)Lu-DOTATATE-treated mice bearing neuroendocrine tumor xenografts. Eur J Nucl Med Mol Imaging. 2020;47:980-90
120. Lundsten S, Spiegelberg D, Stenerlow B, Nestor M. The HSP90 inhibitor onalespib potentiates 177LuDOTATATE therapy in neuroendocrine tumor cells. Int J Oncol. 2019;55:1287-95
121. Hollebecque A, Salvagni S, Plummer R, Isambert N, Niccoli P, Capdevila J. et al. Phase I Study of Lysine-Specific Demethylase 1 Inhibitor, CC-90011, in Patients with Advanced Solid Tumors and Relapsed/Refractory Non-Hodgkin Lymphoma. Clin Cancer Res. 2021;27:438-46
122. Lines KE, Stevenson M, Filippakopoulos P, Muller S, Lockstone HE, Wright B. et al. Epigenetic pathway inhibitors represent potential drugs for treating pancreatic and bronchial neuroendocrine tumors. Oncogenesis. 2017;6:e332
123. Abad E, Graifer D, Lyakhovich A. DNA damage response and resistance of cancer stem cells. Cancer Lett. 2020;474:106-17
124. Cullinane C, Waldeck K, Kirby L, Rogers BE, Eu P, Tothill RW. et al. Enhancing the anti-tumour activity of (177)Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci Rep. 2020;10:10196
125. Nonnekens J, van Kranenburg M, Beerens CE, Suker M, Doukas M, van Eijck CH. et al. Potentiation of Peptide Receptor Radionuclide Therapy by the PARP Inhibitor Olaparib. Theranostics. 2016;6:1821-32
126. Pang Y, Lu Y, Caisova V, Liu Y, Bullova P, Huynh TT. et al. Targeting NAD(+)/PARP DNA Repair Pathway as a Novel Therapeutic Approach to SDHB-Mutated Cluster I Pheochromocytoma and Paraganglioma. Clin Cancer Res. 2018;24:3423-32
127. Loap P, Loirat D, Berger F, Cao K, Ricci F, Jochem A. et al. Combination of Olaparib with radiotherapy for triple-negative breast cancers: One-year toxicity report of the RADIOPARP Phase I trial. Int J Cancer. 2021;149:1828-32
128. Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894-901
129. Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G. et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192-203
130. Lendeckel U, Wolke C. Redox-Regulation in Cancer Stem Cells. Biomedicines. 2022 10
131. Liu H, Schreiber SL, Stockwell BR. Targeting Dependency on the GPX4 Lipid Peroxide Repair Pathway for Cancer Therapy. Biochemistry. 2018;57:2059-60
132. Bebber CM, Thomas ES, Stroh J, Chen Z, Androulidaki A, Schmitt A. et al. Ferroptosis response segregates small cell lung cancer (SCLC) neuroendocrine subtypes. Nat Commun. 2021;12:2048
133. Patwardhan RS, Sharma D, Sandur SK. Thioredoxin reductase: An emerging pharmacologic target for radiosensitization of cancer. Transl Oncol. 2022;17:101341
134. Shibata Y, Yasui H, Higashikawa K, Miyamoto N, Kuge Y. Erastin, a ferroptosis-inducing agent, sensitized cancer cells to X-ray irradiation via glutathione starvation in vitro and in vivo. PLoS One. 2019;14:e0225931
135. Liu Y, Pang Y, Caisova V, Ding J, Yu D, Zhou Y. et al. Targeting NRF2-Governed Glutathione Synthesis for SDHB-Mutated Pheochromocytoma and Paraganglioma. Cancers (Basel). 2020 12
136. Sun X, Wang Q, Wang Y, Du L, Xu C, Liu Q. Brusatol Enhances the Radiosensitivity of A549 Cells by Promoting ROS Production and Enhancing DNA Damage. Int J Mol Sci. 2016 17
137. Wanigasooriya K, Tyler R, Barros-Silva JD, Sinha Y, Ismail T, Beggs AD. Radiosensitising Cancer Using Phosphatidylinositol-3-Kinase (PI3K), Protein Kinase B (AKT) or Mammalian Target of Rapamycin (mTOR) Inhibitors. Cancers (Basel). 2020 12
138. Di Domenico A, Wiedmer T, Marinoni I, Perren A. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr Relat Cancer. 2017;24:R315-R34
139. Banck MS, Kanwar R, Kulkarni AA, Boora GK, Metge F, Kipp BR. et al. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest. 2013;123:2502-8
140. Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M. et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28:245-55
141. Jochmanova I, Pacak K. Genomic Landscape of Pheochromocytoma and Paraganglioma. Trends Cancer. 2018;4:6-9
142. Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14:108-19
143. Rapa I, Saggiorato E, Giachino D, Palestini N, Orlandi F, Papotti M. et al. Mammalian target of rapamycin pathway activation is associated to RET mutation status in medullary thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:2146-53
144. Tamburrino A, Molinolo AA, Salerno P, Chernock RD, Raffeld M, Xi L. et al. Activation of the mTOR pathway in primary medullary thyroid carcinoma and lymph node metastases. Clin Cancer Res. 2012;18:3532-40
145. Lee M, Minaskan N, Wiedemann T, Irmler M, Beckers J, Yousefi BH. et al. Targeting PI3K/mTOR signaling exerts potent antitumor activity in pheochromocytoma in vivo. Endocr Relat Cancer. 2017;24:1-15
146. Fankhauser M, Bechmann N, Lauseker M, Goncalves J, Favier J, Klink B. et al. Synergistic Highly Potent Targeted Drug Combinations in Different Pheochromocytoma Models Including Human Tumor Cultures. Endocrinology. 2019;160:2600-17
147. Somnay Y, Simon K, Harrison AD, Kunnimalaiyaan S, Chen H, Kunnimalaiyaan M. Neuroendocrine phenotype alteration and growth suppression through apoptosis by MK-2206, an allosteric inhibitor of AKT, in carcinoid cell lines in vitro. Anticancer Drugs. 2013;24:66-72
148. Gild ML, Landa I, Ryder M, Ghossein RA, Knauf JA, Fagin JA. Targeting mTOR in RET mutant medullary and differentiated thyroid cancer cells. Endocr Relat Cancer. 2013;20:659-67
149. Nölting S, Rentsch J, Freitag H, Detjen K, Briest F, Mobs M. et al. The selective PI3Kalpha inhibitor BYL719 as a novel therapeutic option for neuroendocrine tumors: Results from multiple cell line models. PLoS One. 2017;12:e0182852
150. Chow Z, Johnson J, Chauhan A, Izumi T, Cavnar M, Weiss H. et al. PI3K/mTOR Dual Inhibitor PF-04691502 Is a Schedule-Dependent Radiosensitizer for Gastroenteropancreatic Neuroendocrine Tumors. Cells. 2021 10
151. Exner S, Arrey G, Prasad V, Grotzinger C. mTOR Inhibitors as Radiosensitizers in Neuroendocrine Neoplasms. Front Oncol. 2020;10:578380
152. Calsina B, Castro-Vega LJ, Torres-Perez R, Inglada-Perez L, Curras-Freixes M, Roldan-Romero JM. et al. Integrative multi-omics analysis identifies a prognostic miRNA signature and a targetable miR-21-3p/TSC2/mTOR axis in metastatic pheochromocytoma/paraganglioma. Theranostics. 2019;9:4946-58
153. Claringbold PG, Turner JH. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): A Phase I Study. Cancer Biother Radiopharm. 2015;30:261-9
154. Niklasson CU, Fredlund E, Monni E, Lindvall JM, Kokaia Z, Hammarlund EU. et al. Hypoxia inducible factor-2alpha importance for migration, proliferation, and self-renewal of trunk neural crest cells. Dev Dyn. 2021;250:191-236
155. Richter S, Gieldon L, Pang Y, Peitzsch M, Huynh T, Leton R. et al. Metabolome-guided genomics to identify pathogenic variants in isocitrate dehydrogenase, fumarate hydratase, and succinate dehydrogenase genes in pheochromocytoma and paraganglioma. Genet Med. 2019;21:705-17
156. Richter S, Klink B, Nacke B, de Cubas AA, Mangelis A, Rapizzi E. et al. Epigenetic Mutation of the Succinate Dehydrogenase C Promoter in a Patient With Two Paragangliomas. J Clin Endocrinol Metab. 2016;101:359-63
157. Seifert V, Richter S, Bechmann N, Bachmann M, Ziegler CG, Pietzsch J. et al. HIF2alpha-Associated Pseudohypoxia Promotes Radioresistance in Pheochromocytoma: Insights from 3D Models. Cancers (Basel). 2021 13
158. Okuno T, Kawai K, Hata K, Murono K, Emoto S, Kaneko M. et al. SN-38 Acts as a Radiosensitizer for Colorectal Cancer by Inhibiting the Radiation-induced Up-regulation of HIF-1alpha. Anticancer Res. 2018;38:3323-31
159. Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C. et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377-86
160. Kim W, Son B, Lee S, Do H, Youn B. Targeting the enzymes involved in arachidonic acid metabolism to improve radiotherapy. Cancer Metastasis Rev. 2018;37:213-25
161. Pang LY, Hurst EA, Argyle DJ. Cyclooxygenase-2: A Role in Cancer Stem Cell Survival and Repopulation of Cancer Cells during Therapy. Stem Cells Int. 2016;2016:2048731
162. Ullrich M, Richter S, Seifert V, Hauser S, Calsina B, Martinez-Montes AM. et al. Targeting Cyclooxygenase-2 in Pheochromocytoma and Paraganglioma: Focus on Genetic Background. Cancers (Basel). 2019 11
163. Brandt F, Ullrich M, Seifert V, Haase-Kohn C, Richter S, Kniess T. et al. Exploring Nitric Oxide (NO)-Releasing Celecoxib Derivatives as Modulators of Radioresponse in Pheochromocytoma Cells. Molecules. 2022 27
164. Gao F, Zafar MI, Juttner S, Hocker M, Wiedenmann B. Expression and Molecular Regulation of the Cox2 Gene in Gastroenteropancreatic Neuroendocrine Tumors and Antiproliferation of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). Med Sci Monit. 2018;24:8125-40
165. Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A. et al. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64:7210-5
166. Panagiotidis E, Alshammari A, Michopoulou S, Skoura E, Naik K, Maragkoudakis E. et al. Comparison of the Impact of 68Ga-DOTATATE and 18F-FDG PET/CT on Clinical Management in Patients with Neuroendocrine Tumors. J Nucl Med. 2017;58:91-6
167. Nile DL, Rae C, Walker DJ, Waddington JC, Vincent I, Burgess K. et al. Inhibition of glycolysis and mitochondrial respiration promotes radiosensitisation of neuroblastoma and glioma cells. Cancer Metab. 2021;9:24
168. Koritzinsky M. Metformin: A Novel Biological Modifier of Tumor Response to Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;93:454-64
169. Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016;114:1305-12
170. Kluckova K, Thakker A, Vettore L, Escribano-Gonzalez C, Hindshaw RL, Tearle JLE. et al. Succinate dehydrogenase deficiency in a chromaffin cell model retains metabolic fitness through the maintenance of mitochondrial NADH oxidoreductase function. FASEB J. 2020;34:303-15
171. Mukha A, Kahya U, Linge A, Chen O, Lock S, Lukiyanchuk V. et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics. 2021;11:7844-68
172. Elf AK, Bernhardt P, Hofving T, Arvidsson Y, Forssell-Aronsson E, Wangberg B. et al. NAMPT Inhibitor GMX1778 Enhances the Efficacy of 177Lu-DOTATATE Treatment of Neuroendocrine Tumors. J Nucl Med. 2017;58:288-92
173. Subbiah V, Cote GJ. Advances in Targeting RET-Dependent Cancers. Cancer Discov. 2020;10:498-505
174. Noordermeer SM, van Attikum H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends Cell Biol. 2019;29:820-34
175. Pineiro-Yanez E, Reboiro-Jato M, Gomez-Lopez G, Perales-Paton J, Troule K, Rodriguez JM. et al. PanDrugs: a novel method to prioritize anticancer drug treatments according to individual genomic data. Genome Med. 2018;10:41
176. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH. et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915-42
177. Parihar AS, Dehdashti F, Wahl RL. FDG PET/CT-based Response Assessment in Malignancies. Radiographics. 2023;43:e220122
178. NETTER-1 Phase III in Patients With Midgut Neuroendocrine Tumors Treated With 177Lu-DOTATATE. Efficacy and Safety Results. Clin Adv Hematol Oncol. 2016;14:8-9
179. Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27:561-9
Author contact
![]() Corresponding authors: Dr. Martin Ullrich: m.ullrichde; Dr. Susan Richter: Susan.Richterde.
Corresponding authors: Dr. Martin Ullrich: m.ullrichde; Dr. Susan Richter: Susan.Richterde.
 Global reach, higher impact
Global reach, higher impact