13.3
Impact Factor
Theranostics 2024; 14(1):33-55. doi:10.7150/thno.90093 This issue Cite
Review
Tumor-on-a-chip models combined with mini-tissues or organoids for engineering tumor tissues
Department of Precision Medicine, Sungkyunkwan University School of Medicine (SKKU-SOM) Suwon 16419, Republic of Korea
* The authors contributed equally.
Received 2023-9-12; Accepted 2023-10-15; Published 2024-1-1
Abstract
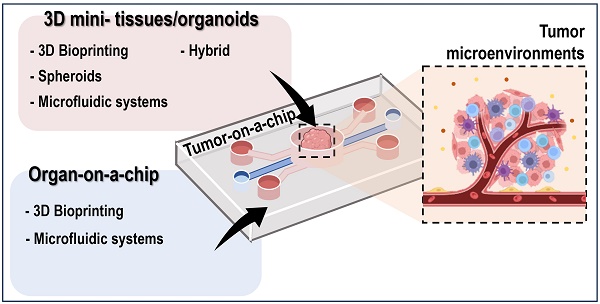
The integration of tumor-on-a-chip technology with mini-tissues or organoids has emerged as a powerful approach in cancer research and drug development. This review provides an extensive examination of the diverse biofabrication methods employed to create mini-tissues, including 3D bioprinting, spheroids, microfluidic systems, and self-assembly techniques using cell-laden hydrogels. Furthermore, it explores various approaches for fabricating organ-on-a-chip platforms. This paper highlights the synergistic potential of combining these technologies to create tumor-on-a-chip models that mimic the complex tumor microenvironment and offer unique insights into cancer biology and therapeutic responses.
Keywords: Tumor-on-a-chip, Tumor microenvironment, Organoids, Biofabrication, Cancer research
Introduction
Cancer remains one of the toughest challenges in modern medicine, affecting millions of people worldwide and presenting complex, multifaceted obstacles for researchers and clinicians alike [1]. Traditional two-dimensional (2D) cell cultures have been the backbone of cancer research for decades; however, they often fail to capture the intricacies and heterogeneity of tumors in vivo [2]. Consequently, there is a growing demand for more sophisticated and physiologically relevant models to study cancer biology, drug responses, and potential therapeutic interventions [2, 3].
In recent years, rapid advancements in biofabrication techniques have led to a new era in tissue engineering and regenerative medicine. These innovative methods have enabled the construction of three-dimensional (3D) mini-tissues and organoids that better mimic the structural and functional complexities of native tissues and organs [4-8]. By incorporating these cutting-edge biofabrication approaches, researchers have made significant steps in developing advanced in vitro models known as “organ-on-a-chip” systems [9].
Researchers have utilized various biofabrication methods such as 3D bioprinting [10, 11], spheroids [12], microfluidic systems [13], and self-assembly techniques [14] using cell-laden hydrogels to recapitulate tissue-like microenvironments. For instance, the introduction of cell-based components that imitate key features of human tumors and the surrounding microenvironment has allowed close representations of tumor microenvironments [9, 15, 16].
In the following sections, we delve into a range of biofabrication techniques used to fabricate mini-tissues. Here, we discuss both the strengths and limitations of each of the approaches. These methods encompass 3D Bioprinting, which offers precise control over the spatial arrangement of cells and biomaterials, enabling the creation of intricate structures [10, 11]. Furthermore, spheroid formation which leverages cell self-assembly to produce mini-tissues, providing a unique approach to tissue engineering [12, 14]. Additionally, we examine microfluidic systems, which have the capability to generate complex cellular structures. Lastly, self-assembly techniques rely on the inherent properties of cell-laden hydrogels, facilitating the formation of three-dimensional structures [13]. These techniques collectively offer a diverse toolkit for engineering mini-tissues, each with its own set of advantages and considerations.
Subsequently, we will explore the various techniques employed in fabricating organ-on-a-chip platforms. These platforms offer dynamic and controlled microenvironments that enable researchers to mimic the essential functions of specific organs, making them powerful tools for studying normal physiology and disease progression. Additionally, we will examine microfluidics-based organ-on-a-chip models, biomaterial-based approaches employing biologically relevant scaffolds, and hybrid systems that integrate both technologies [13, 17].
Building on this foundation, we will investigate the exciting area of tumor-on-a-chip models, where mini-tissues or organoids are merged with advanced microfluidic systems to create physiologically relevant tumor microenvironments (TMEs). These innovative platforms can provide new insights into cancer biology, including tumor heterogeneity, invasion, metastasis, and responses to therapeutic agents [18, 19]. By examining various tumor-on-a-chip models, we demonstrate the unique advantages of each biofabrication method and their potential applications in the context of cancer research.
This review aims to shed light on promising developments in the integration of tumor-on-a-chip technology with mini-tissues or organoids through diverse biofabrication methods. The synergy between these cutting-edge technologies can bridge the gap between traditional 2D cell culture and in vivo studies, providing researchers with more reliable and representative in vitro models to explore the complexities of cancer biology and therapeutic interventions. Through an improved understanding of tumor behavior and drug responses, these advanced models may pave the way for personalized medical approaches and propel us closer to more effective and targeted cancer treatments.
Biofabrication methods for mini-tissues/organoids
Mini-tissues, also known as organoids or tissue-like structures, have attracted considerable attention in recent years. They offer a more physiologically relevant and intricate representation of native tissues than traditional 2D cell cultures. Biofabrication methods employed to construct mini-tissues utilize various innovative techniques, each presenting unique advantages and challenges in generating tissue-like structures with cellular complexity and functionality. In this section, we examine several biofabrication methods in detail and discuss their principles, applications, and contributions to cancer research.
3D Bioprinting
3D bioprinting is a revolutionary biofabrication technique that allows the precise control of the spatial arrangement of cells, biomaterials, and biochemical cues, leading to the creation of complex multicellular structures [5, 20-22]. This method uses bioinks, which are cell-laden hydrogels or biomaterials, as building blocks that can be patterned layer-by-layer to form 3D tissue-like structures [23]. The ability to create tissues with intricate architectures and controlled cell distributions makes 3D bioprinting a valuable tool for mini-tissue construction.
Various biocompatible and biodegradable materials have been applied as bioinks in 3D bioprinting. In particular, hydrogels such as alginate, gelatin, collagen, and fibrin are commonly used because of their biochemical and biophysical abilities to mimic the extracellular matrix (ECM) and support cell growth and differentiation [24-26]. In addition, decellularized ECM-based bioinks derived from porcine, bovine, and fish tissues better simulate native microenvironments, facilitating the interaction and organization of cells [27, 28]. Various examples of 3D bioprinting processes used to obtain functional 3D mini-tissue constructs are listed in Table 1.
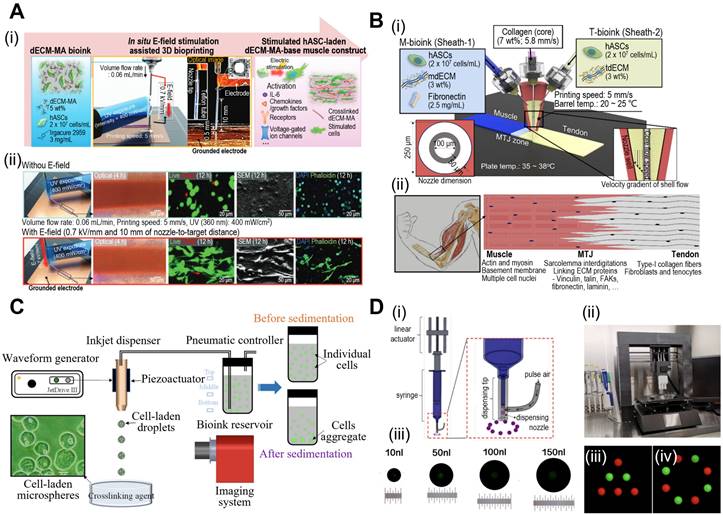
In 3D bioprinting, various printing techniques, including extrusion, inkjet, and laser-assisted methods are employed. Extrusion-based bioprinting utilizes a syringe or nozzle to deposit the bioink layer-by-layer, allowing precise control over cell and material placement. Furthermore, extrusion-based bioprinting can be enhanced through functionalization of the extrusion process. Kim et al. demonstrated simultaneous in situ E-field stimulation during bioink extrusion, as shown in Fig. 1A [29]. After 12 h of stimulation, cells developed branched actin filaments. E-field stimulation results in favorable cellular responses, such as increased cell proliferation, alignment, and myogenic activity in human adipose stem cells (hASCs). In addition, core-shell nozzles are commonly employed in tissue engineering applications to create hierarchical structures [30]. This nozzle application could be further expanded by incorporating double-sheath channels, as illustrated in Fig. 1B [31]. When this nozzle was used in extrusion-based bioprinting, researchers successfully developed a muscle-tendon unit with significant upregulation of genes associated with the muscle-tendon junction.
Fabrication methods of 3D mini-tissues/organoids.
| Fabrication type | Method | Material | Cell | Results | Ref. |
|---|---|---|---|---|---|
| 3D Bioprinting | Printing transwell dermis, vascular channel, hypodermis, epidermis | Hypodermis: adipose-derived dECM, fibrinogen Dermis: skin-derived dECM, fibrinogen Vascular channels: gelatin, glycerol, thrombin Transwell: PCL | HPAD HUVEC HDF HEK | Increased structural complexity Fully-matured perfusable vascularized 3D skin models were obtained Structural similarity compared with the native human skin using skin stemness markers | [120] |
| Printing bioink into the supportive bath followed by a crosslinking process | Bioink: alginate, carboxymethyl chitosan Printing bath: PEI | HAT-7 | Highly porous structure with sufficient structural integrity was obtained High degree of printability Mineral deposition and enamel-like tissue formation | [121] | |
| Bioink were crosslinked within the glass capillary nozzle and wall shear stress induced cell alignment | Gel-Ma | ASC | Significant reduction in the extrudate swelling phenomenon Higher cellular alignment and MHC expression Improved muscle regeneration | [122] | |
| Fibroblast and keratinocyte layers printed using fish derived bioink and UV crosslinking process | Tilapia skin derived dECM-Ma Cod skin derived dECM | HS27 HaCaT | Enhanced in vitro cellular activities Enhanced mechanical properties | [123] | |
| Spheroids | Positioning spheroids within the self-healing support hydrogel | Supportive hydrogel: HA modified with adamantane or β-cyclodextrin | iPSC-derived cardiomyocytes | 3D bioprinting of high cell density tissue models, with precise control over microtissue structure and local heterogeneity | [124] |
| Electrospinning spheroid-laden bioink on PCL strut | Bioink: alginate/PEO Supportive strut: PCL | C2C12 | Uniaxially elongated spheroids Spheroid-laden structure greatly promoted myotube alignment, fusion, and maturation. Upregulated myogenic-related gene (MyoD1, myog, and Myh2) expression | [50] | |
| Providing a rotational motion to the hanging droplets to facilitate the aggregation of suspended cells | N/A | HSF HepG2 | Overcome current challenges in the cell spheroid production process including long preparation time, low cell viability, and high costs | [125] | |
| Using magnetic field to form spheroids with magnetic nanoparticles containing cells | Nanoparticles isolated from magnetic bacteria | BMSC AMB-1 | Reduced the time required for spheroid generation Reinforced cell-cell contact for the activation of cellular interactions due to the magnetic forces Improve the various cellular functions such as proliferation and differentiation | [126] | |
| Using PCL-based buckyballs as micro-scaffolds for fabricating spheroids | PCL | ASC | Enhanced cell retention, the decreased compaction and the better control over the size Better chondrogenic and osteogenic differentiation | [127] | |
| Microfluidic systems | Cell laden bioink was extruded into pro-printed microchamber | Microchamber: PCL Bioink: Gel-Ma | MSC | Printing scalable arrays of spheroids within a reinforcing polymeric framework to orientate spheroid growth and fusion Supporting endochondral bone formation and maintaining the entire construct in bioreactor culture to enhance tissue development | [128] |
| 3D printing bioink containing cell-laden microgels which fabricated by microfluidic systems | Microgel: collagen/alginate Bioink: Sil-Ma, Gel-Ma | BMSC | Improved cell proliferation Better bone formation performance compared with 15%Sil-Ma/Gel-Ma construct | [62] | |
| Using multi-barrel capillary for fabricating complex microfibers | Alginate ECM protein | HepG2 NIH 3T3 HUVEC | Fabrication of bioactive microfibers with tunable morphological and structural features | [63] | |
| Epoxy resin-based T-junction microfluidic device for fabricating cell aggregated structures during printing process | Bioink core: Gel-Ma Bioink shell: collagen / PEGDA | NSC-34 C2C12 | Promotion of neurogenic activity Exhibited significantly higher myogenic and neurogenic differentiation Higher NMJ formation than conventional bioconstructs | [61] | |
| PDMS based microfluidic device for fabricating cell-imprinted substrate and dynamic cell culturing | Chip: PDMS | ASC Chondrocyte | Regular patterning of cell membranes, which increased the efficiency of culture compared to previous irregular methods Cell-imprinted substrate using a microfluidic chip derived from the cell membrane pattern can be used for various cell culture applications | [129] | |
| Hybrid fabrication methods | Using microfluidic device for formation cell-laden microgels | Thiolated gelatin Vinyl sulfonated hyaluronic acid | BMSC | Injected into defect site without sacrificing the cell viability and self-assemble into cartilage-like tissue via cell-cell interconnectivity Facilitate the cartilage repair | [130] |
| 4 × 105 cells were plated into one well of ultralow attachment 6-well plate for cell aggregation to self-assemble into vascular organoids | N/A | ESC-derived EVC | More accessible for complex vasculature engineering Engineered cardiac tissues with high-density microvasculature composed of hESCs-derived EVCs | [131] | |
| Using microfluidic device for generation of double emulsion droplets and formation cell-laden multicompartmental microgels by self-assembly | Alginate | MCF-7 MDA-MB-231 | Simple, affordable, and high-throughput approach for fabrication of complex microparticles Method offers co-encapsulation of viable cells with high resolution | [132] | |
| Loading MSC-laden micro-niches in meshed frames to induce self-assembly | Gelatin | Human umbilical cord MSC | Superior ability to maintain phenotypic characteristics and stemness in MSCs, while suppressing senescence and enhancing their paracrine functions Successfully repaired articular cartilage defects | [133] | |
| Cell-laden bioink extruded in cell-laden beads which fabricated by microfluidic | Collagen Gel-Ma Fibrin | BMSC iPSC-EC | Feasibility of 3D printing vasculature inside a bath of cell-laden microbeads to create centimeter-sized modular tissue engineered constructs | [134] |
Abbreviations: PCL (poly(ε-caprolactone)); PEO (poly(ethylene glycol)); Gel-Ma (methacrylated Gelatin); PEGDA (poly(ethylene glycol diacrylate)); dECM (decellularized extracellular matrix); HPAD (human preadipocyte); HUVEC (human umbilical vein endothelial cells); HDF (human dermal fibroblast); HAT-7 (dental epithelial cell line); ASC (adipose stem cells); HS27 (fibroblast cell line); HaCaT (keratinocyte); HEK (human embryonic kidney cell); HSF (human splenic fibroblast); HepG2 (human liver cancer cell line); MSC (mesenchymal stem cells); iPSC (induced pluripotent stem cells); hESCs (human embryonic stem cells); MyoD1 (myogenic differentiation 1); Myog (myogenin); Myh2 (myosin heavy chain 2).
Inkjet-based bioprinting employs thermal or piezoelectric methods to eject bioink droplets from a specified location. In contrast, laser-assisted bioprinting uses lasers to propel bioink droplets to the target area. Consequently, this is considered a drop-on-demand (DOD) bioprinting technique, and recent advances suggest its great promise for fabricating functional tissues for implants and drug development. Xu et al. investigated the piezoelectric-actuated bioprinting of cell-bearing alginate bioink directly into a crosslinking agent to develop cell-laden microspheres (Fig. 1C) [32]. However, the current forms of DOD bioprinting are limited by inconsistent droplet volumes and negative effects on cell viability after dispensing at high pressures. To address these limitations, Grottkau et al. developed direct volumetric DOD bioprinting as described in Fig. 1D [33]. The described bioprinter utilizes linear actuator-driven syringes that can dispense <10 nL with high accuracy (±5%).
In recent years, digital light-processing (DLP)-based bioprinting methods have revolutionized the creation of mini-tissues with intricate architectures [34]. This technique employs concentrated light to solidify photo-crosslinkable solutions, resulting in the formation of 3D mini-tissues [35]. The method offers several advantages, including rapid production, flexibility, and high resolution, making DLP-based bioprinted mini-tissues highly desirable. Xie et al. developed a DLP-based bioprinting system to create osteo-callus organoids composed of microspheres loaded with BMSCs [36]. After 4 weeks of implantation, the organoid displayed significant new bone formation. Additionally, Carberry et al. crafted PEG-based sacrificial models with intricate architectures (37 ± 4 μm) [37]. These molds were cast into Matrigel to transfer arrays of features. The authors affirmed that utilizing DLP-printed sacrificial molds offers a robust and rapid method to obtain 3D mini-tissues.
From the perspective of cancer research, 3D bioprinted mini-tissues provide valuable insights into tumor growth, invasion, and drug responses. By incorporating different cell types, including several stem cells and ECM components, researchers can create tumorlike structures that closely mimic the TME, allowing the study of tumor-stromal interactions, angiogenesis, and immune cell infiltration [38, 39]. Among various types of tumor models, the 3D bioprinting of glioblastoma tumors has been well studied, as it is an aggressive form of cancer that affects the central nervous system [40]. For instance, Dai et al. fabricated glioma stem cell-laden bioconstructs composed of gelatin, alginate, and fibrinogen [41]. The cells proliferated well and showed high differentiation potential (glial fibrillary acidic protein and β-tubulin III). Moreover, the 3D tumor model showed higher drug resistance to temozolomide than the 2D model. Additionally, inkjet bioprinting was used to obtain copatterned hepatoma and glioma constructs to evaluate the efficacy of chemotherapeutics (in this case tegafur) against cells [42]. Researchers have concluded that the developed approach allows precise patterning (at the microscale) of various cells onto microchips for accurate analysis of drug efficacy. In addition, Wang et al. developed a 3D mini-tissue composed of human lung cancer cells (A549/95-D) using a 3D bioprinting process [43]. The cancer cells were combined with a gelatin-alginate-based bioink and extruded to form 3D constructs, which were subsequently crosslinked in a sodium alginate solution. To investigate cancer invasion, the authors assessed matrix metalloproteinases 2 (MMP2) and matrix metalloproteinases 9 (MMP9) using qPCR. The results revealed a significant upregulation of these genes in cells cultured within 3D constructs compared to those in 2D culture. Based on these examples, 3D bioprinting can enable the creation of patient-specific tumor models, paving the way for personalized medical approaches. However, the absence of adequate cell-cell interactions is a significant limitation in the realm of 3D bioprinted tissues. Research has demonstrated that robust cell-cell interactions, which affect intricate intracellular signaling, can substantially enhance the bioactivities of the cells within a tissue. To address this concern, researchers have explored cellular aggregates called cell spheroids, which can bolster the lacking cell-cell interactions in 3D bioprinted tissues. The subsequent sections delve into the fabrication and applications of cell-spheroids as mini-tissues.
Fabrication of mini-tissues/organoid using bioprinting system. (A) (i) Schematic diagram of in situ E-field stimulation and (ii) optical, live (green)/dead (red), SEM, and DAPI (blue)/phalloidin (green) images of hASCs fabricated W/ or W/O E-fields, where in situ E-field simulation evoked favourable cellular responses. Adapted with permission from [29], copyright Wiley 2021. (B) Schematic illustration of (i) the fabrication process of 3D bioprinting using a modified core/shell nozzle consisting of two sheath inlets and, and (ii) myotendinous junction (MTJ) unit. Adapted with permission from [31], copyright Wiley 2022. (C) Schematic illustration of inkjet bioprinting system containing several components including piezoactuator-attached inkjet nozzle, a waveform generator, a pneumatic controller, a bioink reservoir. Adapted with permission from [32], copyright MDPI 2022. (D) (i) Schematical diagram and (ii) optical images of drop-on-demand bioprinting method. (iii) Representative images of various patterns of dispensed solution volume by the drop-on-demand printing system. Adapted with permission from [33], copyright MDPI 2020.
Spheroids
Spheroids are self-assembled cellular aggregates that recapitulate certain aspects of tissue architecture and function [44]. These unique 3D spherical structures are formed through cell-cell interactions and exhibit oxygen, nutrient, and signaling molecule gradients, resulting in physiologically relevant tissue models [12]. Before the introduction of spheroids by Moscona and Moscona [45], 2D cell cultures were used for in vitro drug testing, disease modeling, and cellular response evaluations. Although 2D cell cultures are inexpensive and reproducible, their 2D characteristics do not reflect those of the 3D in vivo microenvironment. Thus, cellular spheroids can amend the limitations of 2D characteristics by providing extensive cell-to-cell interactions and mimicking the regulation of in vivo-like cellular functions including cell proliferation, migration, and differentiation [46, 47].
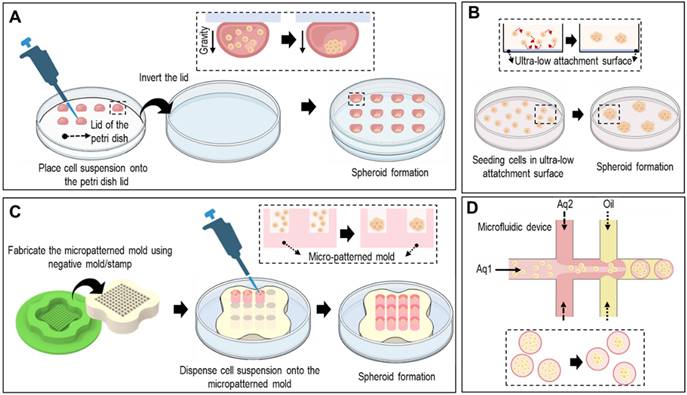
Spheroids can be formed using various methods, including hanging drop techniques, ultra-low-attachment plates, micropatterned molds, and microfluidic devices (Fig. 2). In the hanging-drop method, cell suspensions are pipetted onto the inverted lid of a culture dish, allowing the cells to self-assemble into spheroids through gravity-driven aggregation, as shown in Fig. 2A. Ultra-low attachment plates prevented cell attachment to the surface, promoting spheroid formation by facilitating cell-cell interactions (Fig. 2B). As shown in Fig. 2C, micropatterned molds with non-adhesive surfaces can be utilized to form spheroids. Briefly, seeded cells aggregate via the gravitational force. However, controlling spheroid size and handling is difficult. To overcome these limitations, microfluidic devices offer precise control over cell-seeding and culture conditions, enabling the generation of uniform spheroids of defined sizes (Fig. 2D).
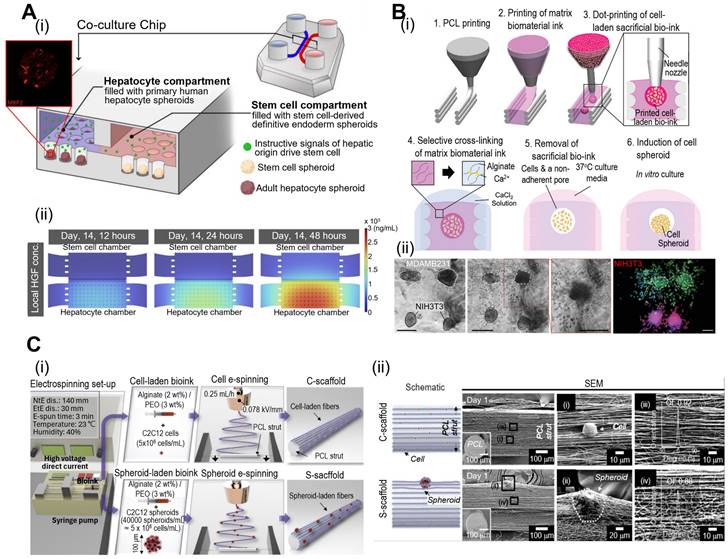
Spheroids offer several advantages such as ease of formation, low cost, and the ability to study multicellular interactions. Moreover, cell spheroids can exhibit a high expression of tissue-specific genes, thus regulating cell signaling and cytokine expression. For example, Fattahi et al. developed a microfluidic coculture device with two compartments containing human hepatocytes or human pluripotent stem cells (Fig. 3A) [48]. The compartments have interconnected grooves that allow for the exchange of paracrine signals. As a result, stem cells differentiate along the hepatic lineages owing to the release of hepatocyte growth factors in compartments containing human hepatocyte spheroids. However, cell spheroids also have limitations, such as limited size control and difficulties in vascularization, which can restrict their application in mimicking larger and more complex tissues.
To overcome these issues, cell spheroids have been incorporated into 3D bioconstructs to significantly enhance their bioactivity. Jeon et al. have developed a “3D Bio-Dot Printing” system that allows precise positioning of multi-type cell spheroids (Fig. 3B) [49]. As shown in Fig. 3B(i), this process involved sequential polycaprolactone (PCL) mold printing, biomaterial-based hydrogel printing, and bioink deposition. Consequently, the hydrostatic forces of the hydrogel induced cell aggregation and the formation of cell spheroids [Fig. 3B(ii)]. Fig. 3C shows the incorporation of myoblast (C2C12) spheroids into micro/nanofibrous structures via spheroid electrospinning [50]. Briefly, a C2C12 spheroid (~100 μm) bearing a bioink consisting of alginate (2 wt%) and PEO (3 wt%) was electrospun into a 3D bundle structure. Owing to the synergistic effects of appropriate topographical cues of micro/nanofibers and the strong cell-to-cell interactions of cell spheroids, myogenic activities were significantly upregulated compared to conventional structures. In addition to these spheroid applications, further advantages and disadvantages of various spheroid applications are explained in Table 1. As demonstrated by the examples provided, cell spheroids have shown the capacity to stimulate robust cell-cell interactions, ultimately enhancing cellular bioactivities. Nonetheless, there are limitations associated with the precise control of cell spheroid size and the extended duration required for their formation. To address these challenges, there have been developments in microfluidic systems for the construction of mini-tissues. These systems enable a rapid fabrication process with precise control over geometries. In the subsequent section, we will delve into the process and applications of microfluidic-based mini-tissue formations.
Various fabrication methods of cell-spheroids. Various schematical illustration of spheroid formations including (A) hanging drop that utilizes gravitational forces and surface tension to induce cell aggregation, (B) non-adherent surface which applies low attachment surfaces to induce self-assembly of cells into spheroids, (C) micro-patterned mold which utilizes seeding cells into micropatterned mold, and (D) microfluidic method which utilizes immiscible fluids and precise control of the flowrates to generate spheroids.
Fabrication of mini-tissues/organoid using cell-spheroids. (A) (i) Schematic diagrams of the co-culture microfluidic device and (ii) computational fluid dynamic model of the heatmaps of) demonstrating rapid accumulation HGF (hepatocyte growth factor) using COMSOL. Adapted with permission from [48], copyright MDPI 2023. (B) (i) “Bio-dot printing” fabrication procedure using polycaprolactone printing and cell spheroids, and (ii) Microscopy and fluorescence images of MDAMB231 and/or NIH3T3 cell spheroids demonstrating 3D spheroid invasion. Adapted with permission from [49] copyright Wiley 2020. (C) (i) Schematic diagram of cell/spheroid electrospinning process and (ii) scanning electron microscopy images demonstrating embedded cells (C-scaffold) and cell spheroid (S-scaffold) in electrospun PCL nanofibers. Adapted with permission from [50] copyright Ivyspring 2021.
Microfluidic systems
Microfluidic systems have emerged as powerful tools for creating mini-tissues owing to their ability to control fluid flow, cell seeding, and culture conditions in microscale environments. Microfluidic devices are typically manufactured from biocompatible materials, such as poly(dimethyl siloxane) (PDMS), poly(methyl methacrylate) (PMMA), and cyclic olefin polymer (COP). In this context, silicone-based elastomer (PDMS) is the most commonly used material owing to its diverse characteristics, including optical transparency, low cost, easy fabrication of intricate structures, bioinertness, and gas permeability [51, 52]. Similarly, PMMA has been extensively studied for use in microfluidic devices using various methods including milling, hot embossing, micromachining, laser ablation, and microinjection molding [53]. Furthermore, PMMA is a notably more rigid polymer compared to PDMS, rendering it more suitable for mass production [54, 55]. However, owing to its rigidity, PMMA is not suitable for valve applications in microfluidic devices. In addition, COPs have demonstrated high resistance to both chemical and biological factors while maintaining optical transparency [56-58]. This makes them an excellent choice of polymer for microfluidic applications. Various fabrication methods, such as laser ablation, micromilling, injection molding, hot embossing, and nanoimprint lithography, can be employed in the production of microfluidic devices. The chips were designed to accommodate multiple channels, reservoirs, and chambers to regulate fluid flow and cell culture. These platforms enable the generation of precise tissue architectures and dynamic microenvironments [13, 59].
Microfluidic systems are commonly used to obtain spheroid-like structures. As shown in Fig. 4A(i), the microfluidic device can be used to aggregate cells into a bead structure [60]. Based on careful consideration of the flow rates in each channel, cell beads with sizes that enhance cell-to-cell interactions were obtained, as shown in Fig. 4A(ii). This approach can be further enhanced by introducing two side channels that provide a pitching flow [Fig. 4B(i)] [61]. By controlling the side channel flow rate, various core structures, including beads, rosaries, and fibers, were obtained, as demonstrated in the live (green)/dead (red) and DAPI (blue)/phalloidin (red) images shown in Fig. 4B(ii).
Microfluidic systems enable the controlled seeding of cells and the establishment of tissue-like structures within defined microenvironments. By adjusting the flow rates and culture conditions, researchers can create gradients of nutrients, oxygen, and other factors to mimic the in vivo microenvironment. Chai et al. fabricated a microgel structure consisting of a core (collagen type I) and a shell (alginate) using a multichannel microfluidic device [62]. Cell-laden microgels were incorporated into methacrylated silk fibroin (Sil-Ma) and methacrylated gelatin (Gel-Ma) hydrogels for bioprinting. As a result of microgel incorporation, cellular proliferation significantly improved. Cheng et al. fabricated microfibers using a multibarrel capillary microfluidic device [Fig. 4C(ii)] [63]. The described system can be further elaborated via the incorporation of several microchannels, thereby obtaining fibers with a variety of microarchitectures. Microfluidic systems offer excellent reproducibility and scalability, making them ideal for high-throughput studies and drug-screening applications. The ability to generate mini-tissues in a controlled and standardized manner enhances the reliability of experimental results. Microfluidic-based fabrication methods, while advantageous, come with certain drawbacks. These include the complex design and fabrication process, restricted selection of biomaterials, and careful management of cell viability due to potential shear stress in microfluidic environments. Consequently, researchers have sought to address these challenges by integrating various fabrication methods into hybrid approaches. These hybrid methods offer potential solutions to the limitations posed by individual techniques. In the subsequent section, we will explore the applications of hybrid fabrication methods in the creation of mini-tissues.
Hybrid fabrication methods using cell-laden hydrogels
Hybrid fabrication techniques in tissue engineering capitalize on the unique properties of cell-laden hydrogels. These hydrogels, which contain living cells, provide an ideal environment for cellular growth and tissue formation. By combining various methods, these techniques enable the creation of intricate three-dimensional structures that closely resemble natural tissues. This versatility allows for the generation of mini-tissues with crucial cell-cell interactions, along with tissue-specific functionalities and microarchitectures. This level of precision holds significant promise for advancing the field of tissue engineering as well as tumor development and therapeutics assessments. For instance, microfluidic-assisted bioprinting of intricate structures has been reported [64]. Costantini et al. utilized a three-channeled microfluidic bioprinting system consisting of the cell-bearing bioink in the core channel, and the crosslinker-bearing hydrogel in the side channel which formed a pitching flow to the core [65].
Consequently, the diameter of the core fiber can be reduced and crosslinked simultaneously. Similarly, Dickman et al. utilized a microfluidic chip with a calcium chloride solution in the side channels to crosslink the alginate hydrogel in the core simultaneously during the extrusion process. Consequently, the printability of the proposed system was significantly increased [66].
Challenges such as inadequate vascularization and limited size can impede the mimicking of larger and more complex tissues in cell spheroids. To address this issue, cell spheroids are incorporated into 3D bioprinted structures. Kim et al. developed a new bioprinting system that utilizes bioink droplets to obtain interlayered human umbilical vein endothelial cell (HUVEC) spheroids within 3D bioconstructs containing hASCs [67]. DAPI (blue)/phalloidin (red) and DAPI (blue)/OPN (green) images indicate that F-actin was significantly more developed with the incorporation of cell spheroids, and the osteogenic activities of hASCs were elevated compared to conventionally obtained bioconstructs. Similarly, osteogenesis-related genes (BMP-2, ALP, OCN, and OPN) and angiogenesis-related genes (VEGF, PECAM1, and VWF) were significantly upregulated in the described constructs.
Mini-tissue assembled using hybrid fabrication processes holds great promise for tissue engineering and cancer research. By modulating the hydrogel properties and incorporating different cell types, researchers can create tissue-specific models. In tissue engineering, upon the implantation of tissue-specific bioconstructs, tissue integration with native tissue can be accelerated, resulting in functional recovery. Furthermore, tissue-specific models can be developed to recreate the TME for studying tumor development, invasion, and therapeutic responses.
Fabrication of mini-tissues/organoid using microfluidic system. (A) (i) Schematic diagrams, and (ii) live (green)/dead (red) and DAPI (blue)/phalloidin (green) images demonstrating cell-bead laden structures fabricated with the microfluidic device. Adapted with permission from [60], copyright Wiley 2022. (B) (i) Schematic diagram of the T-junction microfluidic system and the influence of the Rayleigh instability of cell-laden methacrylated gelatin (Gel-Ma) and collagen/polyethylene glycol diacrylate (PEGDA). (ii) Live (green)/dead (red) and DAPI (blue)/phalloidin (red) images of cells in fiber, rosary, and bead core structures. Adapted with permission from [61], copyright Elsevier 2023. (C) Schematic diagram of various geometries of microchannels (i-ii) and (iii) optical images of the bioink flowing process. Adapted with permission from [62], copyright Elsevier 2021.
Diverse biofabrication methods for mini-tissues offer unique capabilities to generate tissue-like structures with cellular complexity and physiological relevance. By connecting the potential of 3D bioprinting, spheroids, microfluidic systems, and hybrid fabrication approaches, researchers have paved the way for more advanced and sophisticated tumor-on-a-chip models [40, 68-70]. In the subsequent sections, we explore the integration of these mini-tissues with organ-on-a-chip (OOC) platforms to create comprehensive tumor-on-a-chip models, offering unprecedented opportunities to unravel the complexities of cancer biology and advance personalized medicine.
Fabrication techniques for OOCs
OOC technology has revolutionized biomedical research by creating advanced in vitro models that replicate human organ structure and function [9, 16]. These platforms incorporate microfluidics, biomaterials, and cell culture techniques to mimic dynamic organ microenvironments. Researchers can use them to explore organ physiology, disease mechanisms, and drug responses. In this section, we delve into various fabrication techniques for OOC systems, including microfluidics-based approaches, biomaterial-based strategies, and hybrid systems combining both methods. OOC systems aim to emulate key features of specific organs, employing controlled microfluidic environments to culture relevant cell types. They typically include microchannels, chambers, and porous membranes for cell culture, nutrient exchange, and simulating physiological fluid flow. Next, we discuss the integration of mini-tissues, produced using methods such as bioprinting, cell spheroids, microfluidics, and hybrid approaches, into OOC systems.
Bioprinting-based OOCs
Recent advances in 3D bioprinting processes have enabled the recapitulation of in vivo-like conditions and the multiplex microarchitectures of human tissues. Therefore, bioprinting strategies are promising tools for designing and manufacturing OOC devices. In this regard, bioprinted OOCs can provide sophisticated structures using ECMs and cells with a fast-manufacturing process and easy modification of device design.
The integration of additional complexity through the incorporation of various cell types such as endothelial or nervous cells into the bioink of 3D bioprinting systems offers a notable advantage in generating biomimetic tissue constructs to create OOC models. This approach enables drug screening and disease modeling to represent native human tissues and disease responses more accurately.
For instance, Zhang et al. fabricated an endothelialized myocardium using 3D bioprinting of HUVECs, followed by seeding cardiomyocytes. The resulting organoid was placed within a perfusable microfluidic device, creating an “endothelialized-myocardium-on-a-chip” system for evaluating cardiovascular toxicity [71]. Similarly, Lind et al. introduced a fully 3D-printed device capable of continuously detecting the contractile forces of cardiac tissues [72]. This device incorporates multiple components, including a cantilever, strain sensor, and electrical interconnects. These results suggest that this device provides a novel platform for evaluating the morphogenesis and pathogenesis of drug-induced tissues through structural and functional assessments. In addition to the described applications, various examples of 3D bioprinting-based OOCs are presented in Table 2.
Microfluidics-based OOCs
Microfluidics-based OOC platforms leverage microfabrication techniques to create precise and controllable fluid flow patterns, thereby enabling the emulation of organ-specific microenvironments. The design of microfluidic OOC devices involves integrating channels and chambers to mimic the vasculature and tissue architecture of the target organ [73]. Microfluidic flow systems facilitate the transport of nutrients, oxygen, and other factors, thereby promoting cell viability and physiological functions. Additionally, microfluidic OOCs with multiple compartments can support crosstalk between various cells via the interchange of secreted factors. Shim et al. examined this phenomenon by designing microfluidic OOCs with two compartments containing tumor-draining lymph nodes and a tumor [74]. The authors observed that the lymph nodes were significantly immunosuppressed when cultured with tumor cells compared with healthy tissues, suggesting that the designed microfluidic OOCs can recapitulate tumor-induced immune suppression in healthy lymph nodes.
Microfluidic systems allow the seeding of multiple cell types within a device, mimicking the cellular composition of the organ of interest. Additionally, these platforms enable the creation of barrier models, such as blood-brain or intestinal barriers, which are crucial for drug transport and disease modeling [75]. Endothelial and epithelial cells create biological barriers between tissues. Griep et al. incorporated the blood-brain barrier by using a human brain endothelial cell line (hCMEC/D3) [76].
Biomaterials for organ-on-a-chip (OOC).
| Type | Material | Advantages | Disadvantages | OOC applications | Ref. |
|---|---|---|---|---|---|
| Natural | Collagen | Biocompatible Biodegradation High cell adhesion Appropriate permeability Main component of native tissue | Insufficient mechanical stability High cost of production | Lung Liver Intestine Brain | [135-138] |
| Chitosan | Biocompatible Biodegradation | Unstable mechanical properties | Barrier model Gut | [139, 140] | |
| Alginate | Low cost of production Biocompatible Biodegradation Controllable mechanical properties Appropriate permeability | Inadequate cell adhesion Poor bioactivities Lack mammalian representation | Liver Vessel | [141, 142] | |
| Cellulose | High mechanical strength Insoluble in water Biocompatible | Lack mammalian representation Microbial and fungal degradation | Barrier model Liver | [143, 144] | |
| Gelatin | Low cost of production Biocompatible Biodegradation Appropriate permeability | Insufficient mechanical stability Temperature sensitive | Cardiac Liver Neuromuscular | [145-147] | |
| Matrigel | High cell adhesion Similar biochemical cues to the native tissue Appropriate permeability | High cost of production High variability from batch to batch Insufficient mechanical stability | Pancreas Brain Vascular | [148-150] | |
| dECM | Accurate representation of the native tissue High cellular activities Biocompatible Biodegradable Appropriate permeability | High cost of production High variability from batch to batch Insufficient mechanical stability | Multi-OOC | [151] | |
| Synthetic | PCL | Low cost of production Biocompatible Sufficient mechanical properties | Low cell adhesion and activities Slow degradation | Supportive structure to bioconstructs | [78] |
| PDMS | Optical transparent Highly elastic Biocompatible Can withstand long cyclic loading durations Low cost of production | Non-biodegradable Hydrophobic Low permeability | External OOC structure | [51, 152] | |
| PMMA | High mechanical properties Optical transparent Biocompatible Low cost of production | Non-biodegradable Hydrophobic | External OOC structure | [153, 154] | |
| PLA | Biodegradable Tunable mechanical properties Biocompatible | High stiffness not suitable for OOC Hydrophobic | External OOC structure Supportive structure to bioconstructs Membrane structure | [155, 156] | |
| PGA | Biodegradable Biocompatible | High stiffness not suitable for OOC Hydrophobic | Controlled drug release in OOC Membrane structure | [78, 157] |
Abbreviations: PDMS (poly(dimethylsiloxane)); PMMA (poly(methyl methacrylate)); PLA (poly(lactic acid)); PGA (poly(glycolic acid))
Barrier function was evaluated by analyzing barrier tightness using transendothelial electrical resistance measurements. As a result, the barrier functionality was enhanced when fluid shear stress was applied, whereas exposure to tumor necrosis factor inhibited this functionality. Moreover, Kim et al. incorporated the intestinal barrier using intestinal epithelial cells (Caco-2) cultured in microfluidic OOCs and exposed them to a cyclic mechanical strain similar to that in the native environment [77]. As a result, mechanical stress can evoke the differentiation of Caco-2 cells, assist in villous structure formation, and perform intestinal barrier functions.
Biomaterials in OOCs
Biomaterial-based OOC platforms employ biologically relevant scaffolds to support cell culture and tissue organization, thereby providing a more native microenvironment. A variety of natural biomaterials including collagen, alginate, gelatin, and decellularized extracellular matrix (dECM), and synthetic biomaterials including PDMS, PMMA, and PCL have been employed to serve as scaffolds in biomaterial-based OOC systems (Table 2). These materials offer structural support, cell adhesion sites, and signaling cues to guide cell behavior and tissue formation. In general, biomaterials with pH, oxygen, and carbon dioxide characteristics that are compatible with cell types should be considered. Previous studies have suggested that a balanced pH of approximately 7.4, and a carbon dioxide concentration of 4-10% are appropriate for supporting cellular functions [78, 79]. In the context of tissue-specific biomaterials in OOC, the dECM, which consists of collagen, elastin, glycoproteins, and various tissue-specific growth factors, has been regularly employed to recapitulate the native environment of cells.
The mechanical properties of the biomaterial matrix are important criteria for the fabrication of OOCs [80]. The integrins located on the membranes of cells reportedly can detect the mechanical properties of the substrates and relay these signals in multiplex intercellular pathways to control the biological functions [73, 81, 82]. Generally, mechanical properties that resemble native tissues can evoke favorable cellular bioactivities. For example, the elastic modulus of an arterial wall is approximately 1 MPa, similar to that of PDMS (approximately 700 kPa) [83]. Therefore, researchers used PDMS in OOCs to recapitulate their native mechanical properties. In addition, the stiffness of the lung tissue was measured as approximately 3.4 kPa [84]. To obtain similar mechanical properties, Huang et al. examined Gel-Ma bioconstructs with a stiffness of 6.23 kPa [85].
The cells were seeded within the biomaterial scaffold, which allowed them to self-organize and differentiate into tissue-like structures. The 3D architecture of the scaffold facilitates cell-cell interactions and recapitulates tissue-specific functions [86]. The framework of biomaterial-based scaffolds requires an interconnected pore network that allows the continuous flow of the medium [87]. The exchange of nutrients and gases, as well as the removal of metabolic waste and byproducts from scaffold degradation, can assist in supporting cellular functions. However, the porosity of the 3D scaffold must be finely balanced so that the mechanical integrity and stability are not compromised. Based on previous literature, the selection of an appropriate biomaterial with a fine balance between mechanical properties, porosity, and bioactivity is a crucial factor to consider in the successful design of OOC systems.
Hybrid OOC systems
The previously outlined fabrication techniques for Organ-on-Chip (OOC) platforms offer valuable tools for in vitro modeling of human organs. However, it's important to recognize that these methods come with both advantages and disadvantages. For instance, 3D bioprinting systems have notable strengths for OOC applications. They are compatible with a wide range of hydrogels, making them suitable for multi-material bioprinting. Additionally, they excel in achieving high manufacturing consistency, enabling the printing of solutions with high cell density. Nevertheless, it's crucial to consider their limitations. Hydrogels in this context can degrade rapidly, and the shear forces and pressures involved in extrusion may affect cell viability. Moreover, 3D bioprinting systems for OOCs may face restrictions in terms of printing speed and resolution. To address the challenges associated with bioprinting, precise fluid control in microfluidic-based approaches allows for the fabrication of mini-tissues with high resolution and cell viability. However, it's worth noting that the complex setup and potential absence of tissue-specific biochemical cues could potentially hinder the bioactivities of the residing cells. In light of this, biomaterial-based strategies may offer a more native-like microenvironment.
Currently, hybrid OOC systems that combine bioprinting, microfluidics, and biomaterials are being introduced to create more comprehensive and functional organ models. In hybrid systems, microfluidic channels and chambers are integrated into biomaterial-based scaffolds, enabling the precise control of fluid flow and cell culture conditions. This integration enhances the physiological relevance of the OOC model. For instance, cell spheroids have been intensively studied as they are relatively easy and inexpensive to form, while providing a valid evaluation to study multicellular interactions with external stimuli. Although spheroids have regularly been studied, their static culture environment can cause the accumulation of biochemical wastes and affect cell viability. To overcome these issues, cell spheroids have frequently been integrated into OOCs to evaluate drug efficacy and the influence of stimuli on cellular behavior. For instance, Alexander et al. fabricated 3 × 3 microwells using a commercially available 3D printer to place the HepG2 spheroids into each well [88]. Subsequently, various parameters, including extracellular acidification, cellular respiration, and morphology, were monitored to assess the metabolic activity of the spheroids. However, they have several drawbacks, including fragility, limited vascularization, and limited dimensions and lifetimes [89].
Hybrid OOC systems represent a powerful tool in biomedical research, combining the adaptability of microfluidics with the biomimetic qualities of scaffold materials. These systems enable researchers to delve into the intricacies of cellular interactions and tissue behaviors in a controlled laboratory environment. However, it's worth noting that the integration of diverse components can present technical challenges, particularly in terms of both fabrication and precise fluidic control. Overcoming these hurdles is pivotal in realizing the full potential of these systems for advancing our understanding of biological processes.
Microfluidics-based approaches offer precise fluid control and cellular integration, whereas biomaterial-based strategies provide a more native-like microenvironment. Hybrid systems combine these technologies to create comprehensive and functional organ models. For instance, Urbaczek et al. investigated OOC integrated with microfluidic channels containing endothelial cells. To enhance the bioactivities of the endothelial cells, the channel was treated with oxygen plasma followed by fibronectin coating [90]. As a result of these treatments, a notable increase in vascular endothelial growth factor (VEGF) secreted from the cells was observed. These OOC platforms have tremendous potential for advancing disease modeling, drug screening, and personalized medicine, contributing significantly to the fields of tissue engineering and regenerative medicine. In the following section, we explore the integration of mini-tissues or organoids with these OOC platforms to create tumor-on-a-chip models, presenting a powerful approach to cancer research and therapeutic development.
Applications of tumor-on-a-chip (TOC) combined with mini-tissues/organoids
The integration of mini-tissues or organoids with advanced microfluidic platforms has provided exciting opportunities in cancer research, culminating in the development of TOC models. These innovative systems combine the physiological relevance of 3D mini-tissues with the dynamic microenvironments provided by microfluidics, enabling the recreation of the complex tumor microenvironments (TME) in vitro [40, 91, 92]. In this section, we explore different approaches for creating TOC models using various biofabrication methods, highlighting their contributions to understanding tumor biology, drug responses, and personalized medicine.
TOC with 3D bioprinted tissue constructs
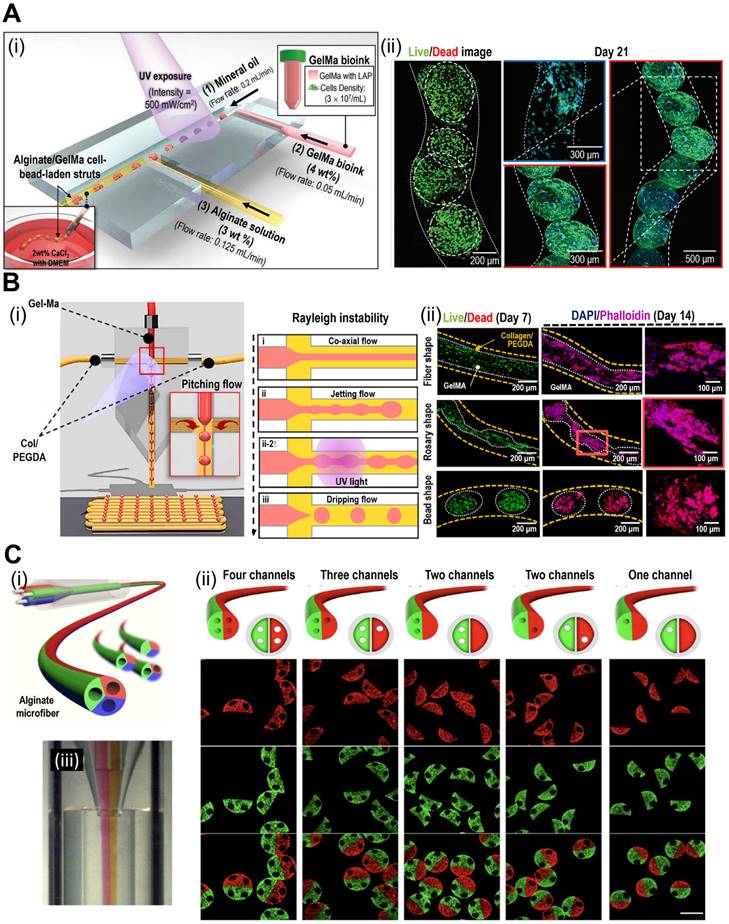
The precise control of cell positioning and tissue architecture afforded by 3D bioprinting makes it an excellent choice for creating TOC models with mini-tissues or organoids. By bioprinting different cell types within mini-tissues, researchers can recreate the heterogeneity observed in tumors in vivo, including cancer, stromal, and immune cells. The spatial arrangement of these cells can mimic the tumor architecture and cellular interactions, enabling the study of tumor growth and progression. For instance, Kim et al. have demonstrated in situ bioprinting of metastatic cancer spheroids (500-1000 μm) to a perusable vascular system (Fig. 5A) [93]. The authors used two cancer types [malignant melanoma (SK-MEL-28) and gastric carcinoma (HTB-103)]. The cancer spheroid with a diameter of approximately 600 μm showed optimal hypoxia, invasion, and angiogenic activities. Based on these results, the authors concluded that the developed platform can recapitulate patient-specific cancer progression. Similarly, Cao et al. incorporated perusable blood and lymphatic vessels around human breast cancer cells (HTB-22) to recreate the in vivo TME, as shown in Fig. 5B(i)-(iii) [94]. They have investigated various combinations of blood and lymphatic vessels using polyethylene glycol diacrylate as the vessel wall to observe the diffusion profiles [Fig. 5B(iv)-(vi)]. As shown in Fig. 5C(i), the vascular network can also be incorporated into TOC via submerged bioprinting [95]. Submerged bioprinting methods have regularly been utilized to support bioprinting and enhance resolution [23, 96]. The research conducted by Neufeld et al. demonstrated the deposition of a thermoreversible synthetic polymer (Pluronic F127) as a sacrificial material to provide a vascular network for 3D bioprinted glioblastoma (the most aggressive form of brain cancer). Compared with the 2D cancer model, the dynamic microenvironment provided by the 3D bioprinted model can provide a more accurate and reliable evaluation of cancer progression and drug efficacy.
TOC models with 3D bioprinted mini-tissues offer a valuable platform for drug screening and personalized medical approaches. These models can be derived from patient-specific cells, allowing researchers to test the efficacy of various therapies and identify personalized treatment strategies. Moreover, a multi-barrel bioprinting strategy can provide an appropriate TME for the accurate evaluation of cancer therapeutics [97, 98]. Yi et al. utilized a multi-barrel 3D bioprinting method for human glioblastoma cells in the core and endothelial cells in the peripheral layer to provide an oxygen gradient for the bioconstruct [99]. To provide biochemical cues such as the TME, the research group utilized brain dECM. The authors evaluated patient-specific reactions to various cancer treatments, including chemoradiation and temozolomide. Because of patient-specific compartments, including the core (glioblastoma) and peripheral (vascular) compartments, the reaction to treatment can provide personalized treatment. Bioprinted TOC, while a promising tool in cancer research, does have a significant drawback of relatively weak cell-cell interactions [100, 101]. This may result in difficulties in accurately replicating the TME, potentially leading to simplified representations. Additionally, bioprinted TOC faces challenges in emulating the dynamic nature of tumors and their responses to treatment over time. To address these issues, cell-spheroid based TOC address the formal challenges via providing robust cell-cell interactions, while microfluidic-based TOC can overcome the latter challenge via providing dynamic culture environment to better mimic the native TME. In addition to the applications of bioprinted TOC, various examples are listed in Table 3.
Applications of 3D bioprinted tissue-constructs incorporated in TOC. (A) (i) Schematic illustrations of the bioprinting process for the organ-on-a-chip (OOC), and (ii) optical images of the perfusion of OOC and flurescent images of DAPI (blue)/CD31 (green) demonstrating vascularization of perfuable OOC. Adapted with permission from [93], copyright Wiley 2021. (B) Schematic showing (i) native and (ii) designed lymphatic-blood system of tumor microenvironment. (iii-vi) Schematic diagram of the fabrication process of the OOC demontrating perfusable blood (red) and flymphatic (yellow) hollow tubes. Adapted with permission from [94], copyright Wiley 2019. (C) (i) schematical diagram illustrating 3D bioprinting of multistage microfluidic OOC and (ii) confocal imaging demonstrating HUVEC (red) and pericyte cells (blue) within the OOC. Adapted with permission from [95], copyright AAAS 2021.
Spheroid-based TOC models
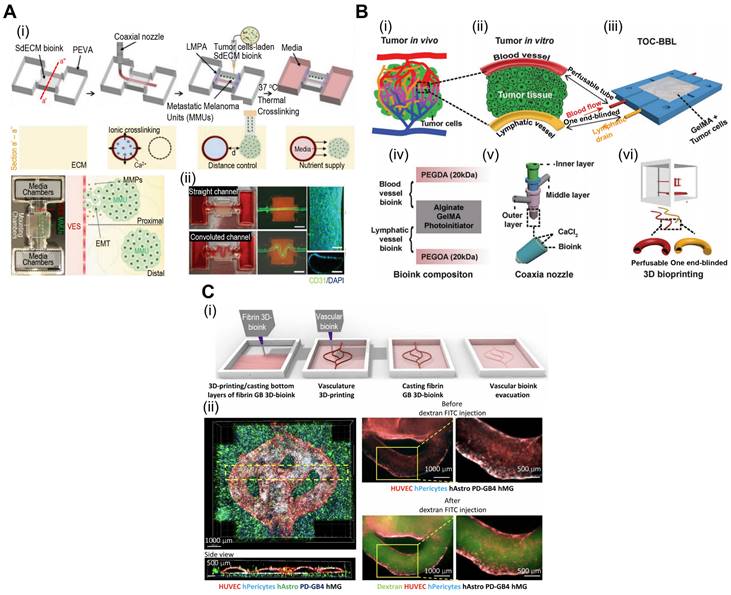
Spheroids serve as building blocks for TOC models, enabling the formation of mini-tissues that recapitulate aspects of TME gradients and cell-cell interactions. Spheroid-based TOC models enable the creation of nutrient and oxygen gradients, simulating the heterogeneous microenvironments observed in solid tumors. These gradients can influence tumor growth, metabolism, and drug responses, providing valuable insights into tumor behavior. As shown in illustrations of Fig. 6A(i), neutrophils can be extravasated through a phenomenon known as chemotaxis in response to tumor-derived stimuli [102]. However, interactions between cancer cells and neutrophils are not fully understood. To evaluate these dynamics, Surendran et al. used spheroids composed of ovarian cancer cells and placed them in microwell plates coated with collagen [Fig. 6A(ii)] [103]. Neutrophil migration and tumor invasion can be stimulated by incorporating microfluidic channels into microwell plates, which act as a vascular network. In the described work, the researchers noted elevated migration of neutrophils towards the cancer spheroids and the formation of neutrophil extracellular traps (NETs). Subsequently, the buildup of NETs can induce aggregated cancer cells in the spheroids to migrate and invade the surrounding collagen matrix.
Vascularized tumors are essential criteria for providing an appropriate TME and oxygen gradient for evaluating cancer therapies. Hu et al. developed tumor spheroids composed of human esophageal carcinoma (ECA 109) cells using microfluidic methods [104]. Subsequently, these spheroids were placed in a vascular bed composed of HUVEC to induce vascularization [Fig. 6B(i)]. As shown in Fig. 6B(ii), vascular networks formed around the cancer spheroids. Previous studies have shown, that prolyl hydroxyl inhibitors can normalize tumor blood vessels, thereby improving cancer treatment efficacy [105, 106]. Using the described TOC, researchers have evaluated the effects of dimethylallyl glycine (DMOG, a prolyl hydroxyl inhibitor). DMOG significantly improved the vascularization of cancer spheroids and the efficacy of cancer treatments (paclitaxel and cisplatin).
With the ability to create complex 3D structures, spheroid-based TOC platforms offer an opportunity to study tumor invasion and metastasis. The integration of microfluidics enables the observation of tumor cell migration and dissemination in real time, contributing to a better understanding of cancer metastatic processes. Moreover, microfluidics-incorporated TOC with tumor spheroids can be utilized for appropriate drug delivery. For instance, as shown in Fig. 6C, Zhuang et al. evaluated the effects of mesoporous silica nanoparticles (MSNs) on an array of breast cancer spheroids [107]. Because of their biodegradability and biocompatibility, MSNs have been regularly used as nano-delivery systems for cancer therapy [108, 109]. As such, the research group evaluated various conditions under which MSNs were administered to cancer spheroids. The group noted that continuous MSNs delivery resulted in better infiltration, and the size of the MSNs had a greater effect than the static 2D model. Additional examples of microfluidic TOCs are listed in Table 4.
The utilization of cell spheroids in Tumor-on-Chip (TOC) technology presents several notable limitations [110]. Firstly, cell spheroids have inherent size restrictions, which may hinder the accurate representation of complex tumor structures and the heterogeneity typically observed in real tumors. Additionally, replicating the intricate vascular networks present in tumors proves challenging with cell spheroids alone, impacting the representation of oxygen gradients within the tumor. Further, the static culture conditions commonly used for spheroids do not adequately capture the dynamic nature of tumor growth and responses to treatment over time. To overcome these challenges, integration of complex vascular networks can inherently increase size of tumor spheroids while maintaining the oxygen gradient. Moreover, introduction of microfluidics can provide dynamic culturing conditions that recapitulate the native TME.
Fabrication techniques for organ-on-a-chip (OOC).
| Type | Method | Material | Cell | Results | Ref. |
|---|---|---|---|---|---|
| 3D printing based OOC | By printing OOC and embedded sensor | Ink for OOC: dextran, TPU, CB, PDMS, Ag:PA | hiPS-CMs NRVMs | 3D printed cardiac microphysiology device fabricated by sequentially printing the OOC base, tissue template, and well | [72] |
| By printing tissues and chip at a once | Ink for OOC: PCL Ink for tissue: type I collagen, gelatin | HUVEC HepG2 | Liver-on-a-chip fabricated via simultaneously printing cavity, the cell laden hydrogel and cover Heterotypic cell types and biomaterials were successfully positioned at the desired position | [158] | |
| By printing microchannel within the PDMS bath | Ink for channel: carbopol Ink for OOC: PDMS | BMEC HUVEC | OOC with microchannel was developed by printing sacrificial carbopol within the PDMS bath | [159] | |
| By printing silicone bioink | Ink for OOC: silicone Ink for the tissue: brain dECM | U-87 MG HUVECs | Chamber of the vascularized GBM-on-a-chip was fabricated by printing permeable silicone-based ink Bioprinted glioblastoma-on-a-chip reproduces the results of patient-specific resistances to treatment | [99] | |
| Microfluidic based OOC | Molding | Chip: PDMS, PTFE tube, PMMA Tissue: patient derived tissue slice | TDLN NDLN | Chip is composed of three-layer which containing reservoir, tissue culture well, PCL membrane Co-culture of pairs of tissue slices under continuous recirculating flow resulting in modeling of complex inter-organ communication ex vivo | [74] |
| Laser cutting technique | Chip: PMMA Hydrogel for fluid: matrigel | hiPSCs-F | Custom and reconfigurable assembled chip composed of microfluidic chip and a fluidic routing unit is developed Various chip assemblies demonstrate the versatile utility of the fluidic routing system which enables custom design of the chip-to-chip communication and fitting of variety of multicell biological models | [149] | |
| Molding | PDMS | HepG2 hiPSC-HC | Integrated network of liver-lobule-like hexagonal tissue-culture chambers constructed in a hybrid layout with a separate seed-feed network integrated to chip Provides a microphysiological niche for hepatocytes | [160] |
Abbreviations: TPU (thermoplastic polyurethane); CB (carbon black nanoparticles); Ag:PA (silver particle-filled, polyamide); GBM (glioblastoma); PTFE (Poly(tetrafluoroethylene)); hiPSC-CMs (Human-induced pluripotent stem cell-derived cardiomyocytes); NRVMs (Neonatal rat ventricular myocytes); HAT-7 (dental epithelial cell line); HepG2 (human hepatocellular carcinoma); HUVEC (human umbilical vein endothelial cells); BMEC (human bone marrow microvascular endothelial cell line); U-87 MG (GBM-like cell line); TDLN (tumor-draining lymph nodes); NDLN (non-draining lymph nodes); hiPSCs-F (human induced pluripotent stem cells-derived skin fibroblasts); hiPSC-HC (human induced pluripotent stem cells- derived hepatocytes).
Applications of tumor-on-a-chip (TOC) combined with mini-tissues/organoids.
| Type | Method | Material | Cells | Results | Ref. |
|---|---|---|---|---|---|
| Bioprinted tissue constructs-based TOC | 3D bioprinted vessel, tumor within the dECM bath | Bioink for tumor: skin dECM Bioink for vessel: vascular tissue dECM, alginate Chip: PDMS | HTB-103 SK-MEL-28 A548 U-87 HUVEC | Metastatic cancer unit (MCU) shows hypoxia, invasion, and angiogenetic signaling Observed the proximity of MCU to vascular endothelium system (VES) augments the epithelial-mesenchymal transition (EMT) in MCU and vascular Dysfunction/inflammation in VES | [93] |
| 3D bioprinted cell laden perfusable hollow vessel and single oulet lymphatic vessel | Bioink for vessel: Gel-Ma, alginate, PEGDA Bioink for lymphatic vessel: alginate, Gel-Ma, PEGOA Chip: PDMS, PMMA | MCF-7 HUVEC | Systems with the bioprinted blood/lymphatic vessels exhibit varying levels of diffusion profiles for biomolecules and anticancer drugs | [94] | |
| 3D bioprinted GBM and vessel | Bioink for GBM: fibrin/gelatin Bioink for vessel: PF-127 Chip: PDMS | U-87 MG T98G U373 293T cells Saos-2 MDAMB-231 GL261 HUVEC Vascular brain pericytes PD-GB4 cell | Bioprinted human and murine GB model was able to recapitulate fundamental aspects of in vivo GB models, including cell proliferation, invasion, response to therapies, and gene profiling | [95] | |
| 3D bioprinted neuroblastoma and vessel, endothelial cells seeded vascular channel | Bioink: Gel-Ma, fibrinogen Chip: PMMA | HDMEC STA-NB15 ASC iPSc HUVEC/hTERT-EYFP HFF/hTERT-ECFP | Micro-vascularized neuroblastoma tumor-environment model directly printed into fluidic chips and observed the interaction between vascular system and neuroblastoma tumor | [161] | |
| Spheroid-based TOC models | Spheroid was placed within the chip | Chip: type-I bovine collagen, PAAm ring magnet | NIH OVCAR-3 | Distinct neutrophil responses and functional modalities respond to the growing tumor spheroids | [103] |
| Spheroid generated within the chip | Chip: PDMS (coated with PVA) | HCT11 T47D HepG2 | Tumor spheroids were generated within the chip and used to evaluate drug efficiency (anticancer drug efficacy, apoptosis) Results indicate negative correlation between the dimension of tumor spheroids and drug sensitivity | [162] | |
| Spheroids was placed within the vascularized chip | Chip: PDMS | HUVECs hLFs Eca-109 | Perfusable, vascularized tumor spheroid-on-a-chip was used to observe the vasoprotective and angiogenic effects of the drugs | [104] | |
| Microfluidic-based TOC models | Cell suspension was flowed into the microfluidic chamber | Cell suspension: fibrinogen, thrombin Chip: PDMS | ECFC-EC NHLF SW480 HCT116 | Colorectal cancer (CRC) confirmed using gene expression, and tumor heterogeneity Treatment responses in the bioconstructs closely recreate the CRC tumor clinicopathology | [163] |
| Cell suspension was injected into the microfluidic chip | Cell suspension: fibrin, thrombin Chip: PDMS | HUVEC GS5 | Microvasculature-on-a-chip system model can recapitulate in vivo tumor cell dynamics and heterogeneity, representing a new route to study patient-specific tumor cell functions | [114] |
Abbreviations: PVA (Poly(vinyl alcohol)), HTB-103 (gastric carcinoma cells); SK-MEL-28 (Malignant melanoma cell); A548 (lung carcinoma cell); U-87 (glioblastoma); MCF-7 (breast cancer cell line); A-172 (Human Glioblastoma cells); hCMEC/D3 cerebral micro-vessel endothelial cells (hCMEC/D3); GBM (Glioblastoma multiform); T98G, U373, U-87 MG (human glioblastoma cell lines); Saos-2 (human osteosarcoma cells); MDAMB-231 (human breast cancer cell); GL261 (murine glioma cell line); STA-NB15 (neuroblastoma cell line); ASC (adipose derived stem cell); HFF/hTERT-ECFP (human foreskin fibroblasts); NK-92 (cellosaurus cell line); ECFC-EC (Human endothelial colony-forming cell-derived endothelial cells); NHLF (Normal human lung fibroblasts); SW480 (colorectal cancer cells); HCT116 (colorectal cancer cell); NIH:OVCAR-3 (Chemoresistant ovarian cancer cell line); PAAm (poly(acrylamide)); T47D (human breast cancer cell line); hLFs (normal human lung fibroblasts); Eca-109 (human esophageal carcinoma cell line); GS5 (patient-derived glioma stem-like cells).
Microfluidic TOC models
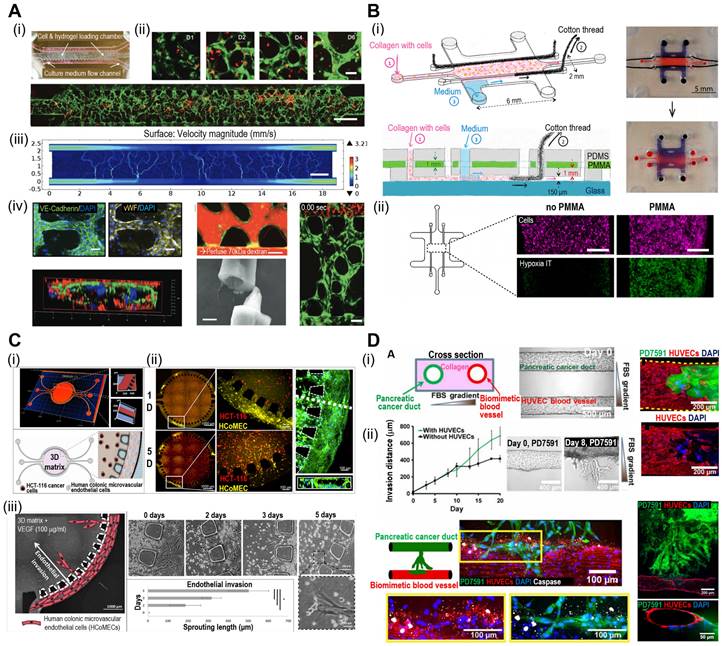
Microfluidic systems provide dynamic and controlled microenvironments for TOC models, allowing researchers to study tumor behavior under conditions that mimic the in vivo environment. Microfluidic TOC models can mimic fluid flow and shear stress in the TME. These dynamic conditions influence tumor cell behavior, including proliferation, invasion, and response to therapies, thereby providing critical insights into cancer biology. For instance, the perivascular niche (PVN) plays an important role in the intercellular activities of cancer cells, including cell fate, tumor invasion, and drug resistance [111, 112]. In the context of brain cancer, the PVN refers to the region around the micro-vessels in which brain tumor stem-like cells (BTSC) reside, thereby providing a path for the migration of cancerous cells [113]. Xiao et al. utilized TOC incorporated into the microvascular track to evaluate the role of the PVN in brain tumor stem-like cells (BTSC), as shown in Fig. 7A(i) [114]. As shown in Fig. 7A(ii), the endothelial cells formed extensive vascular networks after 4 d of culture. To further elucidate the velocity profile, computational fluid dynamics (CFD) was conducted to determine the local flow rates, revealing heterogeneity within the microvessels [Fig. 7A(iii)]. Immunofluorescence images of VE-cadherin and vWF revealed the formation of cell-adherent junctions [Fig. 7A(iv)].
Cell-spheroids incorporated in TOC applications. (A) (i) Tumor microenvironment (TME) schematic illustration between interactional dynamics of pre-metastatic tumor and neutrophils and (ii) optical images demonstrating tumor spheroids incased within microwells. Adapted with permission from [103], copyright IOP publishing 2021. (B) (i) Schematic illustration of vascularized tumor spheroid organ-on-a-chip (OOC) comprised of five microchannels and (ii) fluorescence images of Eca-109 and HUVEC cells demonstrating vascularized tumor spheroids. Adapted with permission [104], copyright ACS publishing 2022. (C) Schematics and optical images of microfluidic OOCs containing MCF-7 cells demonstrating evaluation of nanoparticle penetration, and (ii) bright-field and fluorescent images of spheroid arrays containing 160 MCF-7 tumor spheroids. Adapted with permission [107], copyright Wiley 2019.
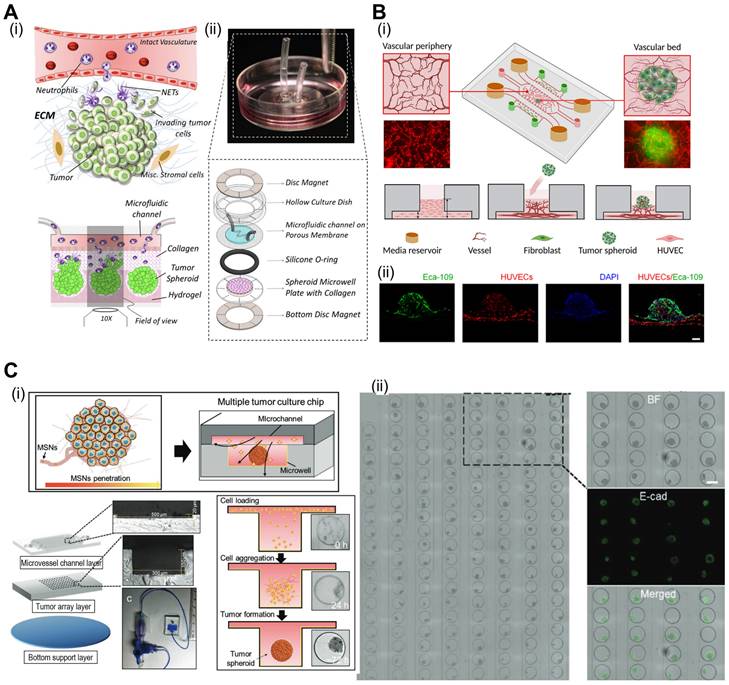
The oxygen gradient is an essential part of the TME and promotes tumor heterogeneity (hypoxic core and normoxic surroundings). The microfluidic device was integrated into the TOC to maintain an oxygen gradient that mimicked the native TME. Palacio-Castañeda et al. fabricated TOC-containing U-251 MG glioblastoma cells (Fig. 7B) [115]. As a result, the research group indicated that under hypoxic conditions, the metabolic activities of cancerous cells increased, as indicated by higher glucose uptake and hydrogel acidity.
Microfluidic systems enable the real-time monitoring of cellular behavior and responses to various stimuli, such as drug treatments or changes in nutrient availability. This capability allows researchers to study the dynamic and temporal aspects of tumor responses, further enhancing our understanding of cancer progression and therapeutic outcomes. For instance, Carvalho et al. developed a 3D TOC integrated with microfluidic channels that simulated the TME of human colorectal tumors to evaluate anticancer drugs [116]. Fig. 7C(i) and (ii) show the 3D surface mapping of the TOC and cell tracker of a human colorectal carcinoma cell line (HCT-116 CRC; red) and human colonic microvascular endothelial cells (yellow), respectively. Inside the circular core compartment, a 3D matrix composed of Matrigel and VEGF facilitated endothelial cell invasion [Fig. 7C(iii)]. The developed TOC allowed for the evaluation of real-time drug delivery and its cellular effects. Similarly, Nguyen et al. developed a TOC consisting of two ducts (pancreatic cancer and vascular ducts) separated by a collagen matrix, as shown in Fig. 7D(i) [117]. Cancerous cells (pancreatic ductal adenocarcinoma) invaded the vascular network, resulting in endothelial ablation [Fig 7D(ii)]. Additional examples of microfluidic TOCs are listed in Table 4.
Microfluidic-based TOCs. (A) (i) Normal optical and (ii) fluorescent images showing live (green)/dead (red) microvasculature-based organ-on-a-chip OOC containing brain tumor stem-like cells. (iii) Finite element analysis (FEA) results of flow velocity profile measured using COMSOL software and (iv) immunofluorescent images (VE-cadherin and vWF), cross-sectional images of the microvessels. Adapted with permission [114], copyright Wiley 2019. (B) (i) Schematic diagram and optical images of OOC consisting of a central culture compartment and perfusion mechanism (blue: trypan blue). (ii) Evaluation of hypoxia of a PMMA-incorporated OOC. Adapted with permission [115], copyright MDPI 2019. (C) (i) A 3D heatmap and design of an OOC, (ii) fluorescent images demonstrating prevascularization of OOC, and (iii) schematic and optical images of endothelial cell sprout formations. Adapted with permission [116] copyright AAAS 2019. (D) (i) Schematic illustration and optical images of OOC consisting of blood vessels and pancreatic cancer ducts, and distance of pancreatic ductal adenocarcinoma cell invasion distance. (ii) Fluorescent images of YFP PD7591 cells invading the blood vessel. Adapted with permission [117] copyright AAAS 2019.
Microfluidic-based TOC platforms, while immensely powerful for emulating the intricacies of the tumor microenvironment, face a notable challenge in scalability [118]. The intricate networks of microchannels and chambers that define these systems can be complex and time-consuming to fabricate, limiting the ease with which they can be scaled up to accommodate larger-scale experiments or high-throughput screening. To address the scalability challenge inherent in microfluidic-based Tumor-on-Chip (TOC) systems, a multifaceted approach can be employed. Recent advanced fabrication techniques, such as 3D printing and soft lithography, offer a scalable means to create intricate microfluidic structures efficiently. Additionally, Modular design principles facilitate scalability by allowing the assembly of smaller, standardized components into larger, more complex systems. By integrating these strategies, the scalability issue can be effectively addressed, unlocking the full potential of microfluidic-based TOC systems for advancing cancer research and drug development.
TOC technology, while a promising tool in cancer research, does come with inherent limitations. These models are simplified representations of real tumors, often lacking the full complexity and heterogeneity found in the human body. They may focus on specific aspects of tumor biology, potentially missing critical interactions. Furthermore, the absence of immune system representation and challenges in modeling metastasis are notable drawbacks. Size restrictions, maintaining long-term viability, and ethical considerations also pose challenges. Additionally, the resource-intensive nature and potential variations between models can impede widespread adoption. Integrating systemic effects and validating against clinical data remain ongoing concerns. Despite these limitations, TOC models have significantly advanced our understanding of cancer, though ongoing research seeks to address and overcome these constraints for even greater efficacy.
Future perspectives
TOC technology, which combines mini-tissues or organoids with advanced microfluidic systems, has been accompanied by a new era of cancer research and therapeutic development. The integration of various biofabrication methods into TOC models offers several applications and holds significant promise for personalized medicine, high-throughput drug screening, and advancements in cancer biology. In this section, we explore the various applications of TOC technology and discuss future perspectives that could shape its impact on cancer research and healthcare.
TOC models provide a physiologically relevant representation of the tumor microenvironment, allowing researchers to study tumor growth, invasion, metastasis, and therapeutic responses in a controlled and reproducible manner [119]. These models can bridge the gap between traditional 2D cell culture and in vivo studies, enabling more accurate and predictive preclinical testing of drugs and therapeutic strategies. The ability to incorporate different cell types, such as cancer, stromal, immune, and vascular cells, into mini-tissues or organoids adds to the complexity of the model and better reflects tumor heterogeneity in vivo. This facilitates the investigation of tumor-stromal interactions, immune responses, and the effects of the TME on therapeutic efficacy.
Although the TOC technology shows great promise, there are still challenges to be addressed in terms of scaling and standardization. The complexity of these models and the need for specialized equipment may limit their accessibility. To fully utilize the potential of TOC technology, efforts should be made to optimize and standardize fabrication processes, making them more accessible and reproducible.
The integration of TOC technology with other cutting-edge biotechnologies such as artificial intelligence (AI) and high-throughput screening has opened new avenues for cancer research and drug development. AI algorithms can analyze the complex data generated by these models and provide valuable insights into tumor behavior, drug responses, and potential therapeutic targets. High-throughput screening platforms can rapidly test many drugs or combinations, thereby accelerating the identification of novel anticancer agents and personalized treatment regimens.
TOC technology can revolutionize personalized medical approaches. Using patient-derived cells to create mini-tissues or organoids on chips, researchers can tailor treatments for individual patients, leading to more effective and targeted therapies. These personalized models can be used to predict drug responses and optimize treatment strategies to achieve better clinical outcomes. Furthermore, TOC platforms can be instrumental in testing novel drug candidates, evaluating drug combinations, and screening for potential toxicity, thereby expediting drug development and reducing reliance on animal testing.
The future of TOC technology is expected to be positive, with many exciting directions and emerging technologies. Advances in biofabrication techniques such as improved 3D bioprinting strategies, novel biomaterials, and more sophisticated microfluidic platforms will enhance the capabilities and versatility of TOC models. The incorporation of immune cells and patient-specific immune responses adds another layer of complexity to these models, facilitating the study of cancer immunotherapy and immunomodulatory drugs. Furthermore, the integration of OOC systems representing different organs and tissues into a single “body-on-a-chip” platform may enable comprehensive studies of systemic drug effects and organ crosstalk.
Conclusion
TOC technology represents a promising frontier in cancer research, offering sophisticated and physiologically relevant models with the potential to reshape the landscapes of cancer treatment and care. Integrating biofabrication methods, mini-tissues, and microfluidics offers a powerful toolkit for understanding the complexities of tumor biology and personalized medicine, bringing us closer to more effective and targeted cancer therapies. With continued research, innovation, and collaboration, TOC technology holds tremendous promise for shaping the fight against cancer and advancing the boundaries of biomedical science.
Acknowledgements
This research was supported by the “Korea National Institute of Health” research project (2022ER130500) and the grant from the Ministry of Trade, Industry & Energy (MOTIE, Korea) under Industrial Technology Innovation Program (20009652: Technology on commercialization and materials of Bioabsorbable Hydroxyapatite that is less than micrometer in size). This research was also supported by the SungKyunKwan University and the BK21 FOUR (Graduate School Innovation) funded by the Ministry of Education (MOE, Korea) and National Research Foundation of Korea (NRF).
Data availability
Additional data are available from the corresponding author upon reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clinical therapeutics. 2016;38:1551-66
2. Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M. et al. Comparison of 2D-and 3D-culture models as drug-testing platforms in breast cancer. Oncology reports. 2015;33:1837-43
3. Chen G, Xu Y, Kwok PCL, Kang L. Pharmaceutical applications of 3D printing. Additive Manufacturing. 2020;34:101209
4. Ali MA, Hu C, Yttri EA, Panat R. Recent advances in 3D printing of biomedical sensing devices. Advanced functional materials. 2022;32:2107671
5. Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioactive materials. 2018;3:144-56
6. Hwangbo H, Lee H, Roh EJ, Kim W, Joshi HP, Kwon SY. et al. Bone tissue engineering via application of a collagen/hydroxyapatite 4D-printed biomimetic scaffold for spinal fusion. Applied Physics Reviews. 2021 8
7. Hwangbo H, Lee H, Jin EJ, Jo Y, Son J, Woo HM. et al. Photosynthetic Cyanobacteria can Clearly Induce Efficient Muscle Tissue Regeneration of Bioprinted Cell-Constructs. Advanced Functional Materials. 2023;33:2209157
8. Pei M, Hwangbo H, Kim G. Hierarchical fibrous collagen/poly (ε-caprolactone) structure fabricated with a 3D-printing process for tissue engineering applications. Composites Part B: Engineering. 2023;259:110730
9. Zhang B, Korolj A, Lai BFL, Radisic M. Advances in organ-on-a-chip engineering. Nature Reviews Materials. 2018;3:257-78
10. Heath CA. Cells for tissue engineering. Trends in biotechnology. 2000;18:17-9
11. Hubbell JA. Biomaterials in tissue engineering. Bio/technology. 1995;13:565-76
12. Chae S, Hong J, Hwangbo H, Kim G. The utility of biomedical scaffolds laden with spheroids in various tissue engineering applications. Theranostics. 2021;11:6818
13. Richard C, Neild A, Cadarso VJ. The emerging role of microfluidics in multi-material 3D bioprinting. Lab on a Chip. 2020;20:2044-56
14. Peck M, Dusserre N, McAllister TN, L'Heureux N. Tissue engineering by self-assembly. Materials Today. 2011;14:218-24
15. Wu Q, Liu J, Wang X, Feng L, Wu J, Zhu X. et al. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomedical engineering online. 2020;19:1-19
16. Zheng F, Fu F, Cheng Y, Wang C, Zhao Y, Gu Z. Organ-on-a-Chip Systems: microengineering to biomimic living systems. Small. 2016;12:2253-82
17. Ma J, Wang Y, Liu J. Bioprinting of 3D tissues/organs combined with microfluidics. RSC advances. 2018;8:21712-27
18. Shukla P, Yeleswarapu S, Heinrich MA, Prakash J, Pati F. Mimicking tumor microenvironment by 3D bioprinting: 3D cancer modeling. Biofabrication. 2022;14:032002
19. Portillo-Lara R, Annabi N. Microengineered cancer-on-a-chip platforms to study the metastatic microenvironment. Lab on a chip. 2016;16:4063-81
20. Wang Z, Wang L, Li T, Liu S, Guo B, Huang W. et al. 3D bioprinting in cardiac tissue engineering. Theranostics. 2021;11:7948
21. Choi Y-J, Yi H-G, Kim S-W, Cho D-W. 3D cell printed tissue analogues: a new platform for theranostics. Theranostics. 2017;7:3118
22. Kim W, Kim G. Bioprinting 3D muscle tissue supplemented with endothelial-spheroids for neuromuscular junction model. Applied Physics Reviews. 2023 10
23. Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR. Bioinks for 3D bioprinting: an overview. Biomaterials science. 2018;6:915-46
24. Tang M, Rich JN, Chen S. Biomaterials and 3D bioprinting strategies to model glioblastoma and the blood-brain barrier. Advanced Materials. 2021;33:2004776
25. Chae S, Cho D-W. Biomaterial-based 3D bioprinting strategy for orthopedic tissue engineering. Acta biomaterialia. 2023;156:4-20
26. Kim W, Kim GH. An intestinal model with a finger-like villus structure fabricated using a bioprinting process and collagen/SIS-based cell-laden bioink. Theranostics. 2020;10:2495
27. Kim BS, Kim H, Gao G, Jang J, Cho D-W. Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication. 2017;9:034104
28. Kim BS, Das S, Jang J, Cho D-W. Decellularized extracellular matrix-based bioinks for engineering tissue-and organ-specific microenvironments. Chemical reviews. 2020;120:10608-61
29. Kim W, Lee H, Lee CK, Kyung JW, An SB, Han IB. et al. A bioprinting process supplemented with in situ electrical stimulation directly induces significant myotube formation and myogenesis. Advanced Functional Materials. 2021;31:2105170
30. Perez RA, Kim H-W. Core-shell designed scaffolds for drug delivery and tissue engineering. Acta biomaterialia. 2015;21:2-19
31. Kim WJ, Kim GH. A bioprinted complex tissue model for myotendinous junction with biochemical and biophysical cues. Bioengineering & Translational Medicine. 2022;7:e10321
32. Xu H, Martinez Salazar DM, Xu C. Investigation of cell concentration change and cell aggregation due to cell sedimentation during inkjet-based bioprinting of cell-laden bioink. Machines. 2022;10:315
33. Grottkau BE, Hui Z, Pang Y. A novel 3D bioprinter using direct-volumetric drop-on-demand technology for fabricating micro-tissues and drug-delivery. International Journal of Molecular Sciences. 2020;21:3482
34. Zhang Z, Jin Y, Yin J, Xu C, Xiong R, Christensen K. et al. Evaluation of bioink printability for bioprinting applications. Applied Physics Reviews. 2018 5
35. Goodarzi Hosseinabadi H, Dogan E, Miri AK, Ionov L. Digital light processing bioprinting advances for microtissue models. ACS Biomaterials Science & Engineering. 2022;8:1381-95
36. Xie C, Liang R, Ye J, Peng Z, Sun H, Zhu Q. et al. High-efficient engineering of osteo-callus organoids for rapid bone regeneration within one month. Biomaterials. 2022;288:121741
37. Carberry BJ, Hergert JE, Yavitt FM, Hernandez JJ, Speckl KF, Bowman CN. et al. 3D printing of sacrificial thioester elastomers using digital light processing for templating 3D organoid structures in soft biomatrices. Biofabrication. 2021;13:044104
38. Arneth B. Tumor microenvironment. Medicina. 2019;56:15
39. Kim HN, Habbit NL, Su CY, Choi N, Ahn EH, Lipke EA. et al. Microphysiological systems as enabling tools for modeling complexity in the tumor microenvironment and accelerating cancer drug development. Advanced Functional Materials. 2019;29:1807553
40. Datta P, Dey M, Ataie Z, Unutmaz D, Ozbolat IT. 3D bioprinting for reconstituting the cancer microenvironment. NPJ precision oncology. 2020;4:18
41. Dai X, Ma C, Lan Q, Xu T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication. 2016;8:045005
42. Zhang J, Chen F, He Z, Ma Y, Uchiyama K, Lin J-M. A novel approach for precisely controlled multiple cell patterning in microfluidic chips by inkjet printing and the detection of drug metabolism and diffusion. Analyst. 2016;141:2940-7
43. Wang X, Zhang X, Dai X. Tumor-like Lung Cancer Model Based on 3D Bioprinting. 3 Biotech, 8: 501. 2018
44. Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, De Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends in biotechnology. 2013;31:108-15
45. Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. Journal of anatomy. 1952;86:287
46. Siltanen C, Yaghoobi M, Haque A, You J, Lowen J, Soleimani M. et al. Microfluidic fabrication of bioactive microgels for rapid formation and enhanced differentiation of stem cell spheroids. Acta biomaterialia. 2016;34:125-32
47. Kim W, Gwon Y, Park S, Kim H, Kim J. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioactive Materials. 2023;19:50-74
48. Fattahi P, de Hoyos-Vega JM, Choi JH, Duffy CD, Gonzalez-Suarez AM, Ishida Y. et al. Guiding Hepatic Differentiation of Pluripotent Stem Cells Using 3D Microfluidic Co-Cultures with Human Hepatocytes. Cells. 2023;12:1982
49. Jeon S, Heo JH, Kim MK, Jeong W, Kang HW. High-precision 3D bio-dot printing to improve paracrine interaction between multiple types of cell spheroids. Advanced Functional Materials. 2020;30:2005324
50. Yeo M, Chae S, Kim G. An in vitro model using spheroids-laden nanofibrous structures for attaining high degree of myoblast alignment and differentiation. Theranostics. 2021;11:3331
51. Raj M K, Chakraborty S. PDMS microfluidics: A mini review. Journal of Applied Polymer Science. 2020;137:48958
52. Chen C, Mehl BT, Munshi AS, Townsend AD, Spence DM, Martin RS. 3D-printed microfluidic devices: fabrication, advantages and limitations—a mini review. Analytical Methods. 2016;8:6005-12
53. Hong T-F, Ju W-J, Wu M-C, Tai C-H, Tsai C-H, Fu L-M. Rapid prototyping of PMMA microfluidic chips utilizing a CO 2 laser. Microfluidics and nanofluidics. 2010;9:1125-33
54. Ma X, Li R, Jin Z, Fan Y, Zhou X, Zhang Y. Injection molding and characterization of PMMA-based microfluidic devices. Microsystem Technologies. 2020;26:1317-24
55. Zhang W, Lin S, Wang C, Hu J, Li C, Zhuang Z. et al. PMMA/PDMS valves and pumps for disposable microfluidics. Lab on a Chip. 2009;9:3088-94
56. Wan Z, Zhong AX, Zhang S, Pavlou G, Coughlin MF, Shelton SE. et al. A robust method for perfusable microvascular network formation in vitro. Small Methods. 2022;6:2200143
57. Nunes PS, Ohlsson PD, Ordeig O, Kutter JP. Cyclic olefin polymers: emerging materials for lab-on-a-chip applications. Microfluidics and nanofluidics. 2010;9:145-61
58. Alsharhan AT, Acevedo R, Warren R, Sochol RD. 3D microfluidics via cyclic olefin polymer-based in situ direct laser writing. Lab on a Chip. 2019;19:2799-810
59. Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A. et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Advanced materials. 2016;28:677-84
60. Kim J, Lee H, Jin EJ, Jo Y, Kang BE, Ryu D. et al. A microfluidic device to fabricate one-step cell bead-laden hydrogel struts for tissue engineering. Small. 2022;18:2106487
61. Kim J, Kim G. Formation of various cell-aggregated structures in the core of hydrogel filament using a microfluidic device and its application as an in vitro neuromuscular junction model. Chemical Engineering Journal. 2023: 144979.
62. Chai N, Zhang J, Zhang Q, Du H, He X, Yang J. et al. Construction of 3D printed constructs based on microfluidic microgel for bone regeneration. Composites Part B: Engineering. 2021;223:109100
63. Cheng Y, Yu Y, Fu F, Wang J, Shang L, Gu Z. et al. Controlled fabrication of bioactive microfibers for creating tissue constructs using microfluidic techniques. ACS applied materials & interfaces. 2016;8:1080-6
64. Davoodi E, Sarikhani E, Montazerian H, Ahadian S, Costantini M, Swieszkowski W. et al. Extrusion and microfluidic-based bioprinting to fabricate biomimetic tissues and organs. Advanced materials technologies. 2020;5:1901044
65. Costantini M, Testa S, Mozetic P, Barbetta A, Fuoco C, Fornetti E. et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials. 2017;131:98-110
66. Dickman CT, Russo V, Thain K, Pan S, Beyer ST, Walus K. et al. Functional characterization of 3D contractile smooth muscle tissues generated using a unique microfluidic 3D bioprinting technology. The FASEB Journal. 2020;34:1652-64
67. Kim W, Jang CH, Kim G. Bone tissue engineering supported by bioprinted cell constructs with endothelial cell spheroids. Theranostics. 2022;12:5404
68. Kang Y, Datta P, Shanmughapriya S, Ozbolat IT. 3D bioprinting of tumor models for cancer research. ACS Applied Bio Materials. 2020;3:5552-73
69. Tabatabaei Rezaei N, Kumar H, Liu H, Lee SS, Park SS, Kim K. Recent Advances in Organ-on-Chips Integrated with Bioprinting Technologies for Drug Screening. Advanced healthcare materials. 2023: 2203172.
70. Monteiro MV, Ferreira LP, Rocha M, Gaspar VM, Mano JF. Advances in bioengineering pancreatic tumor-stroma physiomimetic Biomodels. Biomaterials. 2022;287:121653
71. Zhang YS, Arneri A, Bersini S, Shin S-R, Zhu K, Goli-Malekabadi Z. et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45-59
72. Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M. et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nature materials. 2017;16:303-8
73. Wang M, Cui C, Ibrahim MM, Han B, Li Q, Pacifici M. et al. Regulating mechanotransduction in three dimensions using sub-cellular scale, crosslinkable fibers of controlled diameter, stiffness, and alignment. Advanced Functional Materials. 2019;29:1808967
74. Shim S, Belanger MC, Harris AR, Munson JM, Pompano RR. Two-way communication between ex vivo tissues on a microfluidic chip: application to tumor-lymph node interaction. Lab on a Chip. 2019;19:1013-26
75. Li Z, Hui J, Yang P, Mao H. Microfluidic organ-on-a-chip system for disease modeling and drug development. Biosensors. 2022;12:370
76. Griep LM, Wolbers F, de Wagenaar B, ter Braak PM, Weksler BB, Romero IA. et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomedical microdevices. 2013;15:145-50
77. Kim HJ, Ingber DE. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integrative Biology. 2013;5:1130-40
78. Osório LA, Silva E, Mackay RE. A review of biomaterials and scaffold fabrication for organ-on-a-chip (OOAC) systems. Bioengineering. 2021;8:113
79. Cao UM, Zhang Y, Chen J, Sayson D, Pillai S, Tran SD. Microfluidic organ-on-A-chip: A guide to biomaterial choice and fabrication. International Journal of Molecular Sciences. 2023;24:3232
80. Tran T, Hamid Z, Cheong K. A review of mechanical properties of scaffold in tissue engineering: Aloe vera composites. Journal of Physics: Conference Series: IOP Publishing. 2018 p. 012080
81. Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature reviews Molecular cell biology. 2009;10:63-73
82. Sun Q, Hou Y, Chu Z, Wei Q. Soft overcomes the hard: Flexible materials adapt to cell adhesion to promote cell mechanotransduction. Bioactive Materials. 2022;10:397-404
83. Abulaiti M, Yalikun Y, Murata K, Sato A, Sami MM, Sasaki Y. et al. Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function. Scientific reports. 2020;10:19201
84. Sicard D, Haak AJ, Choi KM, Craig AR, Fredenburgh LE, Tschumperlin DJ. Aging and anatomical variations in lung tissue stiffness. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2018;314:L946-L55
85. Huang D, Liu T, Liao J, Maharjan S, Xie X, Pérez M. et al. Reversed-engineered human alveolar lung-on-a-chip model. Proceedings of the National Academy of Sciences. 2021;118:e2016146118
86. O'brien FJ. Biomaterials & scaffolds for tissue engineering. Materials today. 2011;14:88-95
87. Hollister SJ. Porous scaffold design for tissue engineering. Nature materials. 2005;4:518-24
88. Alexander Jr F, Eggert S, Wiest J. A novel lab-on-a-chip platform for spheroid metabolism monitoring. Cytotechnology. 2018;70:375-86
89. Gunti S, Hoke AT, Vu KP, London Jr NR. Organoid and spheroid tumor models: Techniques and applications. Cancers. 2021;13:874
90. Urbaczek AC, Leão PAGC, Souza FZRd, Afonso A, Vieira Alberice J, Cappelini LTD. et al. Endothelial cell culture under perfusion on a polyester-toner microfluidic device. Scientific reports. 2017;7:10466
91. Anderson NM, Simon MC. The tumor microenvironment. Current Biology. 2020;30:R921-R5
92. Wang X, Wang X, Zhong X, Li G, Yang Z, Gong Y. et al. V-TiO2 nanospindles with regulating tumor microenvironment performance for enhanced sonodynamic cancer therapy. Applied Physics Reviews. 2020 7
93. Kim BS, Cho WW, Gao G, Ahn M, Kim J, Cho DW. Construction of Tissue-Level Cancer-Vascular Model with High-Precision Position Control via In Situ 3D Cell Printing. Small Methods. 2021;5:2100072
94. Cao X, Ashfaq R, Cheng F, Maharjan S, Li J, Ying G. et al. A tumor-on-a-chip system with bioprinted blood and lymphatic vessel pair. Advanced functional materials. 2019;29:1807173
95. Neufeld L, Yeini E, Reisman N, Shtilerman Y, Ben-Shushan D, Pozzi S. et al. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Science advances. 2021;7:eabi9119
96. Kim D, Hwangbo H, Kim G. Engineered Myoblast-Laden Collagen Filaments Fabricated Using a Submerged Bioprinting Process to Obtain Efficient Myogenic Activities. Biomacromolecules. 2021;22:5042-51
97. Lee G, Kim SJ, Chun H, Park J-K. Multilayered and heterogeneous hydrogel construct printing system with crosslinking aerosol. Biofabrication. 2021;13:045027
98. Lee JM, Ng WL, Yeong WY. Resolution and shape in bioprinting: Strategizing towards complex tissue and organ printing. Applied Physics Reviews. 2019 6
99. Yi H-G, Jeong YH, Kim Y, Choi Y-J, Moon HE, Park SH. et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nature biomedical engineering. 2019;3:509-19
100. Velayudhan S, Pr AK. Biocompatibility evaluation of antioxidant cocktail loaded gelatin methacrylamide as bioink for extrusion-based 3D bioprinting. Biomedical Materials. 2023;18:044101
101. Belgodere JA, King CT, Bursavich JB, Burow ME, Martin EC, Jung JP. Engineering breast cancer microenvironments and 3D bioprinting. Frontiers in bioengineering and biotechnology. 2018;6:66
102. Tazzyman S, Barry ST, Ashton S, Wood P, Blakey D, Lewis CE. et al. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. International journal of cancer. 2011;129:847-58
103. Surendran V, Rutledge D, Colmon R, Chandrasekaran A. A novel tumor-immune microenvironment (TIME)-on-Chip mimics three dimensional neutrophil-tumor dynamics and neutrophil extracellular traps (NETs)-mediated collective tumor invasion. Biofabrication. 2021;13:035029
104. Hu Z, Cao Y, Galan EA, Hao L, Zhao H, Tang J. et al. Vascularized tumor spheroid-on-a-Chip model verifies synergistic vasoprotective and chemotherapeutic effects. ACS Biomaterials Science & Engineering. 2022;8:1215-25
105. Hsu Y, Hung J, Chang W, Lin Y, Pan Y, Tsai P. et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929-42
106. Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer cell. 2005;7:77-85
107. Zhuang J, Zhang J, Wu M, Zhang Y. A dynamic 3D tumor spheroid chip enables more accurate nanomedicine uptake evaluation. Advanced Science. 2019;6:1901462
108. Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J. et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11:313-27
109. Watermann A, Brieger J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials. 2017;7:189
110. Li W, Zhou Z, Zhou X, Khoo BL, Gunawan R, Chin YR. et al. 3D Biomimetic Models to Reconstitute Tumor Microenvironment In Vitro: Spheroids, Organoids, and Tumor-on-a-Chip. Advanced Healthcare Materials. 2023: 2202609.
111. Oh M, Nör JE. The perivascular niche and self-renewal of stem cells. Frontiers in physiology. 2015;6:367
112. Marturano-Kruik A, Nava MM, Yeager K, Chramiec A, Hao L, Robinson S. et al. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proceedings of the National Academy of Sciences. 2018;115:1256-61
113. Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL. et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell stem cell. 2014;14:357-69
114. Xiao Y, Kim D, Dura B, Zhang K, Yan R, Li H. et al. Ex vivo dynamics of human glioblastoma cells in a microvasculature-on-a-chip system correlates with tumor heterogeneity and subtypes. Advanced Science. 2019;6:1801531
115. Palacio-Castañeda V, Kooijman L, Venzac B, Verdurmen WP, Le Gac S. Metabolic switching of tumor cells under hypoxic conditions in a tumor-on-a-chip model. Micromachines. 2020;11:382
116. Carvalho M, Barata D, Teixeira L, Giselbrecht S, Reis R, Oliveira J. et al. Colorectal tumor-on-a-chip system: A 3D tool for precision onco-nanomedicine. Science advances. 2019;5:eaaw1317
117. Nguyen D-HT, Lee E, Alimperti S, Norgard RJ, Wong A, Lee JJ-K. et al. A biomimetic pancreatic cancer on-chip reveals endothelial ablation via ALK7 signaling. Science advances. 2019;5:eaav6789
118. Choi G, Murphy E, Guan W. Microfluidic time-division multiplexing accessing resistive pulse sensor for particle analysis. ACS sensors. 2019;4:1957-63
119. Lin Z, Luo G, Du W, Kong T, Liu C, Liu Z. Recent advances in microfluidic platforms applied in cancer metastasis: Circulating tumor cells'(CTCs) isolation and tumor-on-a-chip. Small. 2020;16:1903899
120. Kim BS, Gao G, Kim JY, Cho DW. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Advanced healthcare materials. 2019;8:1801019
121. Mohabatpour F, Duan X, Yazdanpanah Z, Tabil XL, Lobanova L, Zhu N. et al. Bioprinting of alginate-carboxymethyl chitosan scaffolds for enamel tissue engineering in vitro. Biofabrication. 2022;15:015022
122. Hwangbo H, Lee H, Jin E-J, Lee J, Jo Y, Ryu D. et al. Bio-printing of aligned GelMa-based cell-laden structure for muscle tissue regeneration. Bioactive materials. 2022;8:57-70
123. Lee H, Chun W, Kim G. Three-Dimensional Artificial Skin Construct Bioprinted with a Marine-Based Biocomposite. Biomacromolecules. 2023
124. Daly AC, Davidson MD, Burdick JA. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nature communications. 2021;12:753
125. Tang T, Zhang P, Wei Y, Jia H, Feng L, Xu Y. High-efficiency 3D cell spheroid formation via the inertial focusing effect in rotating droplets. Bio-Design and Manufacturing. 2023;6:90-7
126. Kim JA, Choi J-H, Kim M, Rhee WJ, Son B, Jung H-K. et al. High-throughput generation of spheroids using magnetic nanoparticles for three-dimensional cell culture. Biomaterials. 2013;34:8555-63
127. Guillaume O, Kopinski-Grünwald O, Weisgrab G, Baumgartner T, Arslan A, Whitmore K. et al. Hybrid spheroid microscaffolds as modular tissue units to build macro-tissue assemblies for tissue engineering. Acta Biomaterialia. 2023;165:72-85
128. Daly AC, Kelly DJ. Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials. 2019;197:194-206
129. Kashani SY, Moraveji MK, Taghipoor M, Kowsari-Esfahan R, Hosseini AA, Montazeri L. et al. An integrated microfluidic device for stem cell differentiation based on cell-imprinted substrate designed for cartilage regeneration in a rabbit model. Materials Science and Engineering: C. 2021;121:111794
130. Feng Q, Li Q, Wen H, Chen J, Liang M, Huang H. et al. Injection and self-assembly of bioinspired stem cell-laden gelatin/hyaluronic acid hybrid microgels promote cartilage repair in vivo. Advanced Functional Materials. 2019;29:1906690
131. Liu Y, Zhang Y, Mei T, Cao H, Hu Y, Jia W. et al. hESCs-derived early vascular cell spheroids for cardiac tissue vascular engineering and myocardial infarction treatment. Advanced Science. 2022;9:2104299
132. Samandari M, Alipanah F, Tamayol A, Javanmard SH, Sanati-Nezhad A. Controlled self-assembly of microgels in microdroplets. Sensors and Actuators B: Chemical. 2021;348:130693
133. Xing D, Liu W, Li JJ, Liu L, Guo A, Wang B. et al. Engineering 3D functional tissue constructs using self-assembling cell-laden microniches. Acta biomaterialia. 2020;114:170-82
134. Yang G, Mahadik B, Choi JY, Justine RY, Mollot T, Jiang B. et al. Fabrication of centimeter-sized 3D constructs with patterned endothelial cells through assembly of cell-laden microbeads as a potential bone graft. Acta biomaterialia. 2021;121:204-13
135. Khalid MAU, Kim YS, Ali M, Lee BG, Cho Y-J, Choi KH. A lung cancer-on-chip platform with integrated biosensors for physiological monitoring and toxicity assessment. Biochemical Engineering Journal. 2020;155:107469
136. Zamprogno P, Wüthrich S, Achenbach S, Thoma G, Stucki JD, Hobi N. et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Communications biology. 2021;4:168
137. Lee K-H, Lee J, Lee S-H. 3D liver models on a microplatform: well-defined culture, engineering of liver tissue and liver-on-a-chip. Lab on a Chip. 2015;15:3822-37
138. Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab on a Chip. 2012;12:2165-74
139. Tibbe MP, Leferink AM, van den Berg A, Eijkel JC, Segerink LI. Microfluidic Gel Patterning Method by Use of a Temporary Membrane for Organ-On-Chip Applications. Advanced Materials Technologies. 2018;3:1700200
140. Jing B, Xia K, Zhang C, Jiao S, Zhu L, Wei J. et al. Chitosan oligosaccharides regulate the occurrence and development of enteritis in a human gut-on-a-chip. Frontiers in Cell and Developmental Biology. 2022;10:877892
141. Christoffersson J, Aronsson C, Jury M, Selegård R, Aili D, Mandenius C-F. Fabrication of modular hyaluronan-PEG hydrogels to support 3D cultures of hepatocytes in a perfused liver-on-a-chip device. Biofabrication. 2018;11:015013
142. Jia L, Han F, Yang H, Turnbull G, Wang J, Clarke J. et al. Microfluidic fabrication of biomimetic helical hydrogel microfibers for blood-vessel-on-a-chip applications. Advanced Healthcare Materials. 2019;8:1900435
143. Sathe T, Bodas D. Development and characterization of a polydimethylsiloxane-cellulose acetate hybrid membrane for application in organ-on-a-chip. Materials Science and Engineering: B. 2023;291:116366
144. Agarwal T, Borrelli MR, Makvandi P, Ashrafizadeh M, Maiti TK. Based Cell Culture: Paving the Pathway for Liver Tissue Model Development on a Cellulose Paper Chip. ACS Applied Bio Materials. 2020;3:3956-74
145. McCain ML, Agarwal A, Nesmith HW, Nesmith AP, Parker KK. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials. 2014;35:5462-71
146. Lee H, Kim J, Choi Y, Cho D-W. Application of gelatin bioinks and cell-printing technology to enhance cell delivery capability for 3D liver fibrosis-on-a-chip development. ACS Biomaterials Science & Engineering. 2020;6:2469-77
147. Besser RR, Bowles AC, Alassaf A, Carbonero D, Claure I, Jones E. et al. Enzymatically crosslinked gelatin-laminin hydrogels for applications in neuromuscular tissue engineering. Biomaterials science. 2020;8:591-606
148. Lai BFL, Lu RXZ, Hu Y, Davenport Huyer L, Dou W, Wang EY. et al. Recapitulating Pancreatic Tumor Microenvironment through Synergistic Use of Patient Organoids and Organ-on-a-Chip Vasculature. Advanced functional materials. 2020;30:2000545
149. Wang Y, Wang L, Guo Y, Zhu Y, Qin J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC advances. 2018;8:1677-85
150. Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A. et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nature materials. 2016;15:669-78
151. Kim JJ, Park JY, Nguyen VVT, Bae M, Kim M, Jang J. et al. Pathophysiological Reconstruction of a Tissue-Specific Multiple-Organ On-A-Chip for Type 2 Diabetes Emulation using 3D Cell Printing. Advanced Functional Materials. 2023;33:2213649
152. Campbell SB, Wu Q, Yazbeck J, Liu C, Okhovatian S, Radisic M. Beyond polydimethylsiloxane: alternative materials for fabrication of organ-on-a-chip devices and microphysiological systems. ACS biomaterials science & engineering. 2020;7:2880-99
153. Ali U, Karim KJBA, Buang NA. A review of the properties and applications of poly (methyl methacrylate)(PMMA). Polymer Reviews. 2015;55:678-705
154. Busek M, Nøvik S, Aizenshtadt A, Amirola-Martinez M, Combriat T, Grünzner S. et al. Thermoplastic elastomer (TPE)-poly (methyl methacrylate)(PMMA) hybrid devices for active pumping pdms-free organ-on-a-chip systems. Biosensors. 2021;11:162
155. Ongaro AE, Di Giuseppe D, Kermanizadeh A, Miguelez Crespo A, Mencattini A, Ghibelli L. et al. Polylactic is a sustainable, low absorption, low autofluorescence alternative to other plastics for microfluidic and organ-on-chip applications. Analytical chemistry. 2020;92:6693-701
156. Chuchuy J, Rogal J, Ngo T, Stadelmann K, Antkowiak L, Achberger K. et al. Integration of electrospun membranes into low-absorption thermoplastic organ-on-chip. ACS biomaterials science & engineering. 2021;7:3006-17
157. Li X, Jiang X. Microfluidics for producing poly (lactic-co-glycolic acid)-based pharmaceutical nanoparticles. Advanced drug delivery reviews. 2018;128:101-14
158. Lee H, Cho D-W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab on a Chip. 2016;16:2618-25
159. Ozbolat V, Dey M, Ayan B, Ozbolat IT. Extrusion-based printing of sacrificial Carbopol ink for fabrication of microfluidic devices. Biofabrication. 2019;11:034101
160. Banaeiyan AA, Theobald J, Paukštyte J, Wölfl S, Adiels CB, Goksör M. Design and fabrication of a scalable liver-lobule-on-a-chip microphysiological platform. Biofabrication. 2017;9:015014
161. Nothdurfter D, Ploner C, Coraça-Huber DC, Wilflingseder D, Müller T, Hermann M. et al. 3D bioprinted, vascularized neuroblastoma tumor environment in fluidic chip devices for precision medicine drug testing. Biofabrication. 2022;14:035002
162. Chen Y, Gao D, Liu H, Lin S, Jiang Y. Drug cytotoxicity and signaling pathway analysis with three-dimensional tumor spheroids in a microwell-based microfluidic chip for drug screening. Analytica chimica acta. 2015;898:85-92
163. Hachey SJ, Movsesyan S, Nguyen QH, Burton-Sojo G, Tankazyan A, Wu J. et al. An in vitro vascularized micro-tumor model of human colorectal cancer recapitulates in vivo responses to standard-of-care therapy. Lab on a Chip. 2021;21:1333-51
Author contact
![]() Corresponding author: Prof. GeunHyung Kim. Department of Precision Medicine, Sungkyunkwan University School of Medicine (SKKU-SOM) Suwon 16419, Republic of Korea. E-mail: gkimbmeedu; Tel.: +82-31-290-7828.
Corresponding author: Prof. GeunHyung Kim. Department of Precision Medicine, Sungkyunkwan University School of Medicine (SKKU-SOM) Suwon 16419, Republic of Korea. E-mail: gkimbmeedu; Tel.: +82-31-290-7828.
 Global reach, higher impact
Global reach, higher impact