13.3
Impact Factor
Theranostics 2024; 14(1):249-264. doi:10.7150/thno.87306 This issue Cite
Research Paper
Reprogramming of endothelial gene expression by tamoxifen inhibits angiogenesis and ERα-negative tumor growth
1. INSERM U1297, Institut des Maladies Métaboliques et Cardiovasculaires, Université de Toulouse, BP 84225, 31 432 Toulouse cedex 04, France.
2. Laboratoire de Biologie des Tumeurs et du Développement, GIGA-Cancer, Université de Liège, B23, Liège, Belgium.
3. Departments of Molecular and Integrative Physiology and Chemistry, University of Illinois, Urbana, Illinois, USA.
4. INSERM U1037, CRCT, Oncopole- 31 037 Toulouse cedex, France.
5. Institut de Génétique De Rennes (IGDR). UMR 6290 CNRS-Université de Rennes, ERL INSERM U1305. CS 74205- 35042 Rennes Cedex, France.
Abstract
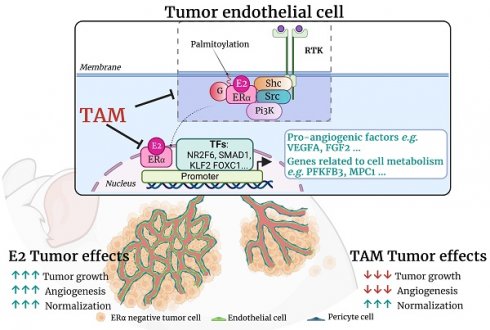
Rationale: 17β-estradiol (E2) can directly promote the growth of ERα-negative cancer cells through activation of endothelial ERα in the tumor microenvironment, thereby increasing a normalized tumor angiogenesis. ERα acts as a transcription factor through its nuclear transcriptional AF-1 and AF-2 transactivation functions, but membrane ERα plays also an important role in endothelium. The present study aims to decipher the respective roles of these two pathways in ERα-negative tumor growth. Moreover, we delineate the actions of tamoxifen, a Selective Estrogen Receptor Modulator (SERM) in ERα-negative tumors growth and angiogenesis, since we recently demonstrated that tamoxifen impacts vasculature functions through complex modulation of ERα activity.
Methods: ERα-negative B16K1 cancer cells were grafted into immunocompetent mice mutated for ERα-subfunctions and tumor growths were analyzed in these different models in response to E2 and/or tamoxifen treatment. Furthermore, RNA sequencings were analyzed in endothelial cells in response to these different treatments and validated by RT-qPCR and western blot.
Results: We demonstrate that both nuclear and membrane ERα actions are required for the pro-tumoral effects of E2, while tamoxifen totally abrogates the E2-induced in vivo tumor growth, through inhibition of angiogenesis but promotion of vessel normalization. RNA sequencing indicates that tamoxifen inhibits the E2-induced genes, but also initiates a specific transcriptional program that especially regulates angiogenic genes and differentially regulates glycolysis, oxidative phosphorylation and inflammatory responses in endothelial cells.
Conclusion: These findings provide evidence that tamoxifen specifically inhibits angiogenesis through a reprogramming of endothelial gene expression via regulation of some transcription factors, that could open new promising strategies to manage cancer therapies affecting the tumor microenvironment of ERα-negative tumors.
Keywords: Tamoxifen, Angiogenesis, Estrogen Receptor ERα, Endothelial cells, Tumor growth
 Global reach, higher impact
Global reach, higher impact