13.3
Impact Factor
Theranostics 2024; 14(1):265-282. doi:10.7150/thno.90940 This issue Cite
Review
Lymphatic vessels: roles and potential therapeutic intervention in rheumatoid arthritis and osteoarthritis
1. War Trauma Medical Center, State Key Laboratory of Trauma and Chemical Poisoning, Army Medical Center, Daping Hospital, Army Medical University, Chongqing, 40038, People's Republic of China.
2. Department of Wound Repair and Rehabilitation Medicine, State Key Laboratory of Trauma and Chemical Poisoning, Army Medical Center, Daping Hospital, Army Medical University, Chongqing, 40038, People's Republic of China.
3. Department of Emergency Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People's Republic of China.
4. Rehabilitation Medicine Department, Army Medical Center, Daping Hospital, Army Medical University, Chongqing 400038, People's Republic of China.
5. The Department of Cardiology, General Hospital of Northern Theater Command, Shenyang 110015, People's Republic of China.
#These authors contributed equally to this work.
Received 2023-10-10; Accepted 2023-11-7; Published 2024-1-1
Abstract
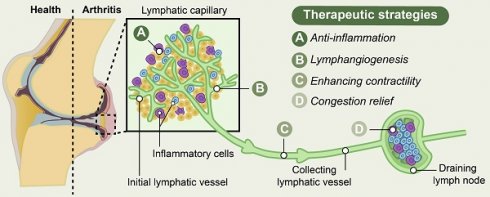
Lymphatic vessel networks are a main part of the vertebrate cardiovascular system, which participate in various physiological and pathological processes via regulation of fluid transport and immunosurveillance. Targeting lymphatic vessels has become a potent strategy for treating various human diseases. The presence of varying degrees of inflammation in joints of rheumatoid arthritis (RA) and osteoarthritis (OA), characterized by heightened infiltration of inflammatory cells, increased levels of inflammatory factors, and activation of inflammatory signaling pathways, significantly contributes to the disruption of cartilage and bone homeostasis in arthritic conditions. Increasing evidence has demonstrated the pivotal role of lymphatic vessels in maintaining joint homeostasis, with their pathological alterations closely associated with the initiation and progression of inflammatory joint diseases. In this review, we provide a comprehensive overview of the evolving knowledge regarding the structural and functional aspects of lymphatic vessels in the pathogenesis of RA and OA. In addition, we summarized the potential regulatory mechanisms underlying the modulation of lymphatic function in maintaining joint homeostasis during inflammatory conditions, and further discuss the distinctions between RA and OA. Moreover, we describe therapeutic strategies for inflammatory arthritis based on lymphatic vessels, including the promotion of lymphangiogenesis, restoration of proper lymphatic vessel function through anti-inflammatory approaches, enhancement of lymphatic contractility and drainage, and alleviation of congestion within the lymphatic system through the elimination of inflammatory cells. At last, we envisage potential research perspectives and strategies to target lymphatic vessels in treating these inflammatory joint diseases.
Keywords: Rheumatoid arthritis, Osteoarthritis, Inflammatory arthritis, Lymphatic vessels and Drainage function
1. Introduction
The lymphatic vascular network, found in virtually every organ of vertebrates, functions as a unidirectional flow system characterized by low pressure. Under physiological conditions, its primary roles include the elimination of interstitial capillary filtrates and tissue immunosurveillance [1]. The significance of lymphatic vessels in a wide range of human diseases is increasingly being acknowledged. Dysfunction of lymphatic vessels is observed in disorders such as pulmonary lymphatic anomalies [2], primary lymphoedema [3], adipose metabolism and obesity [4]. Targeting lymphatic vessels has become a potent strategy for treating for cancer [5], neurological disease [6] and facilitating post-myocardial infarction repair [7]. The precise regulation of lymphatic vessel structure and function has significant potential for informing the development of innovative therapeutics to a broad range of human diseases.
Inflammatory arthritis, such as rheumatoid arthritis (RA) and osteoarthritis (OA), are characterized by joint pain, swelling, and stiffness [8, 9], which have a high prevalence and is considered as the primary causes of global disability [10]. Although the regulation of RA- and OA-associated inflammation may involve distinct mechanisms, the excessive accumulation of catabolic factors, cytokines, and inflammatory cells in joints significantly contributes to the disruption of cartilage and bone homeostasis in these arthritic conditions. The impairment of lymphatic vessels leads to a decline in lymphatic clearance and hyperinflammation, which play crucial roles in the pathogenesis of inflammatory arthritis [11, 12]. In this review, we provide a comprehensive overview of the evolving knowledge regarding the structural and functional aspects of the lymphatic vessels in the pathogenesis of RA and OA. In addition, we summarized the potential regulatory mechanisms underlying the modulation of lymphatic function in maintaining joint homeostasis during inflammatory conditions, and further discuss the distinctions between RA and OA. Moreover, we describe therapeutic strategies for inflammatory arthritis based on lymphatic vessels, including the promotion of lymphangiogenesis, restoration of proper lymphatic vessel function through anti-inflammatory approaches, enhancement of lymphatic contractility and drainage, and alleviation of congestion within the lymphatic system through the elimination of inflammatory cells. At last, we envisage potential research perspectives and strategies to target lymphatic vessels in treating these inflammatory joint diseases.
1.1 The structure and function of lymphatic vessels
The lymphatic system is located in close proximity to the venous network, which plays crucial roles in conducting surveillance, and facilitating lipid absorption [13-15]. In brief, lymphatic vessels originate from preexisting blood vessels, which initiate from the lymphatic capillary, also known as initial lymphatic vessels, in the peripheral regions of the body. Subsequently, they converge through collecting lymphatics towards draining lymph nodes (DLNs), ultimately facilitating lymph drainage into the venous system via the right lymphatic trunk and thoracic duct [14, 16, 17] (Figure 1).
The hierarchical structure of synovial lymphatic system. (A) Synovial lymphatic system in joints start from the lymphatic capillary, also named as initial lymphatic vessels. Collecting lymphatics with anti-flowback valves (secondary valve) converge on draining lymph nodes, and then drain lymph to the venous system. (B) Initial lymphatic vessels are composed of a single layer of lymphatic endothelial cells and enveloped with discontinuous basal lamina. (C) Collecting lymphatic vessels are composed of a continuous basement membrane, a single layer of lymphatic endothelial cells and one or more layers of continuous lymphatic muscle cells.
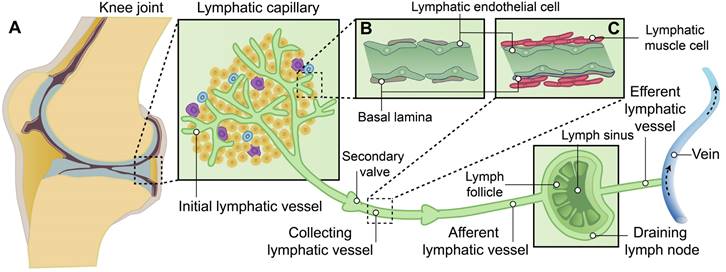
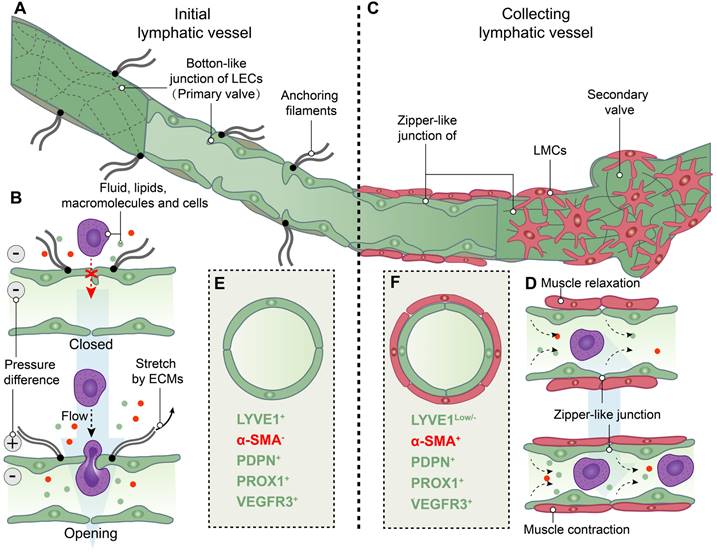
The formation of lymph takes place within the lymphatic capillaries upon the influx of interstitial fluid into these vessels. The initial lymphatic vessels in the human lymphatic capillary are slender-walled structures measuring approximately 35-70 μm in diameter and cul-de-sac in nature [18]. The initial lymphatic vessels consist of a monolayer of lymphatic endothelial cells (LECs) enveloped by an interrupted basal lamina [13, 19, 20]. LECs are firmly anchored to the elastic fibers in the surrounding tissue through anchoring filaments, facilitating the stretching and extension of lymphatic capillaries alongside extracellular matrix and elastic fibers under elevated interstitial fluid pressure [13, 19-23]. Moreover, LECs possess distinctive "button-like" junctions that facilitate the formation of highly permeable vessels, allowing for the transmigration of fluids, lipids, macromolecules and even cells [24]. Additionally, certain adjacent LECs within the lymphatic capillary exhibit overlapping regions and form the primary lymphatic valve, which serves as a crucial mechanism to prevent backflow of lymph. In the context of edema formation, augmented interstitial fluid pressure induces the opening of "button-like" junctions between LECs, thereby promoting enhanced generation and drainage of lymph within the lymphatic capillaries [13, 21-27] (Figure 2A-B).
Lymphatic outflow occurs through lymphatic capillaries, which converge into the collecting lymphatic vessel. The collecting lymphatic vessel is characterized by a continuous basement membrane, LECs connected via zipper-like junctions, and distinct lymphatic muscle cells (LMCs) that differentiate it from the initial lymphatic vessels in lymphatic capillary. Additionally, the collecting lymphatic vessel is equipped with secondary valves, each consisting of two layers of LECs. These secondary valves possess the ability to open or close in response to periodic fluctuations in fluid pressure, effectively preventing lymph backflow [13, 16, 19, 23, 24]. The flow of lymph in collecting lymphatic vessels is dependent on both intrinsic and extrinsic pumps. The intrinsic pump relies on the coordinated contraction or relaxation of LMCs, while the extrinsic pump is driven by the cyclical compression and expansion of lymphatics due to surrounding tissue forces [21, 24, 28]. The lymphatic pump and the lymphatic valves collaborate to counteract the gravitational force on lymph its smooth propulsion. Consequently, modulation of LMCs function can regulate lymph flow by modifying both pumping force and outflow resistance [13, 24] (Figure 2C-D).
Structure and function of initial and collecting lymphatic vessels. (A) Lymphatic endothelial cells (LECs) of initial lymphatic vessels directly connected to the surrounding extracellular matrixes (ECMs) by anchoring filaments, and LECs are connected by overlapped button-like junctions that act as primary valves. (B) When the internal and external pressure difference of vessels or/and the stretch force by ECMs on LECs are existed, primary valves not only permit initial lymphatic vessels to become highly permeable to fluid, lipids, macromolecules and even cells, but also prevent the backflow of these factors into tissues. (C) Collecting lymphatic vessels are composed of LECs tightly connected by zipper-like junctions, and lymphatic muscle cells (LMCs). In addition, secondary valves are specialized to prevent the flowback of lymph. (D) LMCs drive the contraction of collecting lymphatic vessels to move lymph forward. (E) Initial lymphatic vessels are composed of LECs with positive expression of lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), podoplanin (PDPN) and prospero homeobox 1 (PROX1) and vascular endothelial growth factor receptor 3 (VEGFR3), but not α-smooth muscle actin (α-SMA)-positive muscle cells. (F) Collecting lymphatic vessels have the low levels of LYVE1 expression compared to initial lymphatic vessels and the positive expression of a-SMA, PDPN, PROX1 and VEGFR3.
Following the collection by lymphatic vessels, lymph traverses the afferent lymphatics and reaches DLNs. Within the lymphatic sinuses of DLNs, an autoimmune response is initiated [15]. Subsequently, lymph exits these nodes via efferent lymphatic vessels to enter into the circulation of lymph.
1.2 Assessment and visualization of lymphatic vessel distribution within joints
Analogous to the circulatory system, lymphatic vessels are distributed across mammalian tissues. To assess and visualize lymphatic vessels, imaging and histological approaches are employed in the investigation. Imaging modalities, such as near-infrared indocyanine green (NIR-ICG) imaging, contrast-enhanced magnetic resonance imaging (CE-MRI), and power Doppler ultrasound (PD-US), enable the comprehensive assessment of lymphatic vessel and lymph node morphology and function in joints [29]. Further investigation of the distribution of the lymphatic system within the synovial joint necessitates histological observation, as distinguishing between lymphatic and vascular micro-vessels in histological sections at the light microscope level poses significant challenges. The ideal positive or negative marker should be exclusively localized within lymphatic vessels, rather than relying on relative differences in expression levels between blood and lymphatic vessels [30]. Furthermore, it is essential to distinguish between initial and collecting lymphatic vessels. Therefore, the visualization of lymphatic vessels relies on immunostaining markers for both LECs and LMCs (Figure 2E-F). Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), podoplanin (PDPN), prospero-related homeobox 1 (PROX1), and vascular endothelial growth factor receptor 3 (VEGFR3) are commonly employed for immunostaining of LECs, while α-smooth muscle actin (αSMA) is utilized for labeling of LMCs [31, 32]. LYVE1 is a widely used lymphatic marker, particularly of LECs with the absence of LMCs coverage in initial lymphatic vessels [33, 34]. The expression of PDPN and PROX1 is specific to LECs of lymphatic vessels rather than blood endothelial cells (BECs), and providing a crucial molecular distinction between LEC progenitors located within the veins and those emerging outside the cardinal veins [35]. Additionally, our group and other researchers have utilized the genetic model of LEC-specific inducible mouse, Prox1-CreERT2 mice [36], to investigate lymphatic vessels [37, 38]. During early development, VEGFR3, also named as Fms-like tyrosine kinase 4 (FLT4), is expressed in both BECs and LECs, but becomes restricted to LECs in adults [39]. The PROX1-VEGFR3 feedback loop plays a crucial role in the specification and maintenance of LECs [40, 41]. Additional markers, such as 5`-nucleotidase, cluster of differentiation (CD)31, CD34 and pathologische anatomie Leiden-endothelium (PAL-E), are selected based on specific research objectives [30]. The conjugation of fluorescent dyes with these markers is noteworthy, as they are highly expressed in initial lymphatic vessels or collecting lymphatic vessels [42-46]. To obtain more accurate highlights, multiple-immunostaining is employed. For instance, vessels that are PDPN+/α-SMA- are classified as initial lymphatic vessels, while those that are PDPN+/α-SMA+ are considered collecting lymphatic vessels. On the other hand, lacking PDPN but expressing α-SMA (PDPN-/α-SMA+) are identified as blood vessels [43, 46, 47].
In synovium of human joints, PDPN+ CD45- CD31- synovial fibroblasts were identified in RA and OA patients [48]. The presence of PDPNlow+/LYVE1low+/PROX1low+ BECs has been observed in chronic skin inflammation [49]. Furthermore, the expression of LYVE1 and VEGFR3 has been detected in BECs from patients with OA [50] and RA [51], respectively. LYVE1 is also expressed in some synovial tissue macrophage subsets [52, 53]. Therefore, the identification of lymphatic vessels in synovium necessitates the utilization of multiple markers, taking into account their tissue localization and morphology, as well as the specific tissue microenvironment and cell types involved. At the cellular tissue level, the synovial membrane in joints consists of two distinct layers: an inner layer and an outer layer positioned beneath it. Lymphatic vessels are predominantly found in the outer layer of the synovium, characterized by a loosely arranged collagenous extracellular matrix [54], while they are absent in the lining layer composed of macrophage-like and fibroblast-like synoviocytes [55]. In order to visualize the ensemble of the lymphatic vasculature, whole-slide digital imaging systems were employed for scanning images of stained joint tissue sections and quantifying the number and size of lymphatics, thereby enhancing credibility and comparability [43, 56]. Recently, a novel immunolabeling and imaging technique of intact bone tissues was employed on a light-sheet microscopy platform to investigate the lymphatic vessels involved in bone regeneration in mice [31].
Based on the aforementioned advancements in lymphatic vessel detection technology, the elucidation of lymphatic vessel distribution within joints has been consistently unveiled. Lymphatic vessels were identified within the stratified connective tissues surrounding the fetal cartilaginous knee joint tissues through immunostaining with PDPN and LYVE1 antibodies [57]. In adult mice, the presence of both lymphatic capillaries and collecting lymphatic vessels, as indicated by positive staining for PDPN and α-SMA, was observed in various soft tissues including capsule, ligaments, fat pads, muscles, and the patellar region; however, they were not detected in cartilage tissues [43, 46]. Moreover, lymphatic vessels have been identified within the periosteum of long bones [43]. Previous studies have demonstrated the limited efficacy of immunohistochemical staining in identifying vessels within the bone marrow [56, 58], suggesting that fluid clearance predominantly occurs through venous sinusoids rather than lymphatics. The present study provides strong evidence for the presence lymphatic vessels in mouse and human bones, which are involved in supporting bone regeneration following injury [31]. Therefore, lymphatic vessels are extensively distributed throughout the various tissues of the joint, excluding articular cartilage. The employment of additional high-resolution techniques is essential for subsequent research, which aims to evaluate the distribution of lymphatic vessels within the joint and discern diverse subpopulations.
2.1 Lymphatic vessels and RA
RA is a chronic autoimmune disorder that primarily affects the joints, leading to persistent inflammation and progressive damage. This results in the release of inflammatory mediators and the activation of immune cells, which further exacerbate the inflammation. Inflamed joints in RA patients are typically characterized by a marked increase in the number of activated and infiltrated immune cells, such as macrophages, lymphocytes, and plasma cells. These cells play a crucial role in the progression of joint inflammation, as they are responsible for producing and releasing various mediators, including cytokines, chemokines, and enzymes [59, 60]. Some of the most important cytokines involved in RA pathogenesis and progression are tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6. These inflammatory mediators can induce synovial inflammation and vasodilation, leading to joint pain, swelling, and functional impairment [61]. Both clinical studies and animal models suggest that lymphatic vessels likely play a crucial role in the clearance of these inflammatory cells and mediators from the inflamed synovium.
2.2 Animal models of RA in the investigation of lymphatic vessels
In classical animal models of RA, the utilization of K/BxN mice [45, 62], collagen-induced mice [63] and TNF-transgenic (TNF-Tg) [62, 64-69] mice was established to investigate the role of lymphatic vessels within joints. In K/BxN mice expressing both the T cell receptor (TCR) transgene KRN and the MHC class II molecule A, leading to recognition of glucose-6-phosphate-isomerase as an antigen by both T cells and B cells, a joint-specific autoimmune phenotype with numerous characteristics reminiscent of RA is observed [70]. Collagen, a critical autoantigen observed in human RA, is utilized to generate collagen-induced mice through active immunization via intradermal injection with heterologous type II collagen (CII) [71]. These two animal models exhibit a propensity to replicate the abrupt onset of RA. TNF-α plays a pivotal role in the pro-inflammatory cytokine cascade, and its activation elicits systemic inflammatory responses in RA [72]. The TNF-Tg mice, characterized by the overexpression of human TNF-α, exhibit a range of features reminiscent of those observed in patients with RA, including impaired mobility, joint inflammation, synovial hyperplasia, cartilage degradation, and osteoporosis; however, they do not manifest any discernible developmental abnormalities [73].
2.3 Vascular endothelial growth factor C (VEGF-C)/vascular endothelial growth factor receptor 3 (VEGFR3) signaling pathway in lymphangiogenesis of RA
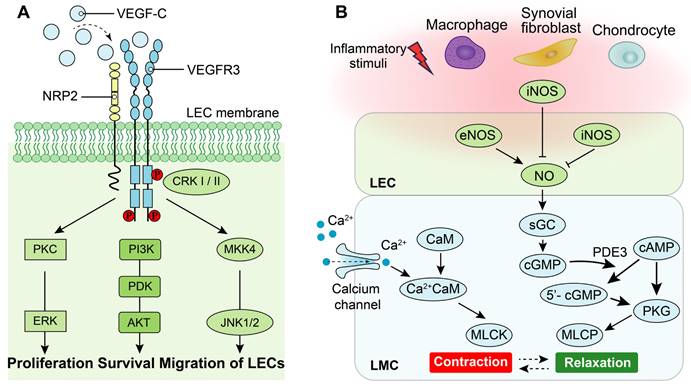
Lymphangiogenesis is a complex process regulated by cytokines and other factors, particularly the VEGF family [20, 74]. The VEGF family comprises essential regulators in the angiogenic process, including VEGF (also known as VEGF-A with multiple functional isoforms), VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placenta growth factor (PIGF) [75]. These ligands of the VEGF family activate signaling pathways by binding to tyrosine kinase receptors called vascular endothelial growth factor receptors (VEGFRs), which consist of three subtypes: VEGFR1, VEGFR2 and VEGFR3[76]. While both VEGFR1 and VEGFR2 are primarily associated with the regulation of angiogenesis, the signaling pathway of VEGFR3 plays a central role in the regulation of lymphangiogenesis [76]. Specifically, high-affinity binding between the ligands of VEGF-C/VEGF-D and their receptor VEGFR3 induces receptor dimerization and phosphorylation, which triggers the downstream signaling pathways that promote lymphangiogenesis [77]. Additionally, interaction between neuropilin (NRP)2 and VEGFR3 could improve lymphangiogenesis via mediating proper lymphatic vessel sprouting upon VEGF-C stimuli [77, 78]. The downstream signaling pathways activated by VEGF-C/VEGFR3 include mitogen-activated protein kinase/extracellular signal-related kinase (MAPK/ERK), phosphatidylinositol 3-kinase/protein kinase B (PI3k/AKT), as well as Jun N-terminal kinase1/2 (JNK1/2) pathways [79, 80]. Activation of these downstream signaling pathways leads to proliferation, survival, and migration of LECs along with remodeling of lymphatic vessels (Figure 3A).
The role of VEGF-C/VEGFR3 and nitric oxide synthase (NOS) signaling pathways on LECs and LMCs. (A) Neuropilin-2 (NRP2) promotes activation of VEGFR3 in LECs in response to VEGF-C. Phosphorylation of VEGFR3 activates the downstream PKC/ERK, PI3K/AKT, and JNK signaling pathways to regulate proliferation, survival and migration of LECs. (B) Under normal condition, calcium signaling induces LMC contraction, whereas LMC relaxation is regulated by NOS signaling. Endothelial NOS (eNOS) of adjacent LECs produces NO to inhibit LMC contraction which allows relaxation of lymphatic vessels. Under inflammatory condition, inducible NOS (iNOS) in adjacent LECs, activated-adherent macrophages and/or synovial fibroblasts and chondrocytes of joints suppresses NO function to disorder the cycle of lymphatic vessel contractions. PKC: Protein kinase C, ERK: Extracellular regulated kinases, PI3K: Phosphoinositide 3-kinase, PDK: Phosphoinositide 3-kinase, AKT: Protein kinase B, CRK-I/II: CT10 regulator of kinase adaptor proteins I/II, MKK4: Mitogen-activated protein kinase 4, JNK: c-Jun N-terminal kinase, sGC: Soluble guanylate cyclase, cGMP: Cyclic guanosine monophosphate, cAMP: Cyclic adenylic acid, PKG: Protein kinase G, MLCK: Myosin light chain kinase, MLCP: Myosin light chain phosphatase, CaM: Calmodulin.
Previous studies have demonstrated that VEGF-C/VEGFRs plays an important role in RA. Firstly, VEGF-C and its receptors VEGFR3 and 2 exhibit high expression levels in arthritic synovial tissue compared to healthy controls, serving as major regulators in lymphangiogenesis. Additionally, immunohistochemical staining reveals the presence of positively stained cells for VEGF-C protein primarily located within synovial lining cells such as synovial endothelial cells and fibroblasts [51, 81]. Macrophages exhibit abundant expression of VEGF-C and VEGFR3 in an inflammatory environment of RA[82]. Furthermore, a significant elevation of VEGF-C levels was observed in synovial fluid from patients with RA, exhibiting a strong positive correlation with TNF-α levels [83]. Potential molecular mechanisms were also researched in this field. In inflammatory microenvironment, PROX1 is activated by nuclear factor-κB (NF-κB) pathway and then regulate the VEGFR3 promoter activity, which strength the expression of receptors in LECs and the susceptibility of VEGF-C [84]. Moreover, VEGF-C/VEGFR3 signal pathway also suppress Toll-like receptors 4 (TLR4)/NF-κB pathway [85].
2.4 Nitric oxide synthase (NOS) signaling pathway in LMCs and the contraction of lymphatic vessels of RA
LMCs in lymphatic vessels exhibit a unique composition of both smooth and striated muscles, endowing them with the ability to generate robust rhythmic contractions [13] [24]. The contractile force of lymphatic vessels is primarily generated by the contraction of LMCs, which is tightly regulated by the interplay between cellular calcium dynamics and contractile proteins [24, 86] (Figure 3B). Specifically, stretch-induced contractions are mediated through the activation of L-type and T-type Ca2+ channels. Upon pacemaker-generated action potentials in lymphatic muscle cells, voltage-dependent Ca2+ channels open, allowing extracellular Ca2+ influx into LMCs. Subsequently, binding of Ca2+ to calmodulin triggers muscle contraction [86]. Moreover, the relaxation of LMCs is predominantly regulated by the nitric oxide (NO). In the lymphatic system of joints, the synthesis of NO is predominantly orchestrated by LECs and originates from endothelial NO synthase (eNOS) of LECs, while inducible NO synthase (iNOS) generated either directly or indirectly by LECs [65, 68], macrophages [65, 87], chondrocytes [88, 89] and fibroblasts [87]. NO activates cytoplasmic guanylate cyclase within LMCs to reduce vessel tone via cyclic guanosine monophosphate (cGMP)-dependent mechanisms [90]. Furthermore, NO inhibits intracellular Ca2+ entry from internal stores [91].
The expression of iNOS is primarily induced by inflammation, leading to the abundant production of NO, which plays a crucial role in the pathogenesis of inflammatory arthritis [92]. Previous study demonstrated that the iNOS mRNA level in LECs of efferent lymphatic vessels from TNF-Tg mice was significantly elevated by 8-fold compared to control mice [66]. The systolic function in ex vivo of popliteal lymphatic vessels from TNF-Tg mice is significantly impaired, which is NO synthase dependent [64]. Additionally, TNF directly induces LMC apoptosis through the production of NO by LECs [67]. The activation of NF-κB/iNOS signaling pathway in LECs by TNF leads to impaired lymphatic contraction function and subsequent production of NO mediated by iNOS [68]. In addition to LECs, macrophages stimulated by lipopolysaccharide significantly upregulate the expression of iNOS and induce lymphatic vessel dilation [93]. The expression of iNOS is also significantly upregulated in chondrocytes during inflammatory arthritis; Moreover, excessive production of NO can exacerbate inflammation by impeding matrix synthesis and facilitating its degradation [88]. Therefore, inflammation induces the upregulation of iNOS and leads to excessive production of NO, which impairs LMCs contraction and diminishes the function of lymphatic drainage.
2.5 Changes of lymphatic vessels during RA processes
Clinical studies have demonstrated a significant augmentation of lymphatic vessels within the arthritic synovial membrane in comparison to healthy controls [94, 95]. The present finding is further substantiated by the observation of significantly increased numbers and sizes of lymphatic vessels in synovial tissues from TNF-Tg mice model and K/BxN mice model [96]. Moreover, the whole-slide imaging system demonstrated an augmentation in capillary lymphatics and a reduction in collecting lymphatics were observed in TNF-Tg mice [46] as well as a mouse model of RA-associated periodontitis [97]. In a recent study, it was further observed that dilated capillary lymphatic vessels exhibited a decreased number of branch points compared to the wild control group. Additionally, collecting lymphatic vessels demonstrated decreased coverage of LMCs and impaired drainage function. Furthermore, elevated levels degenerative and apoptotic LMCs were observed [67].
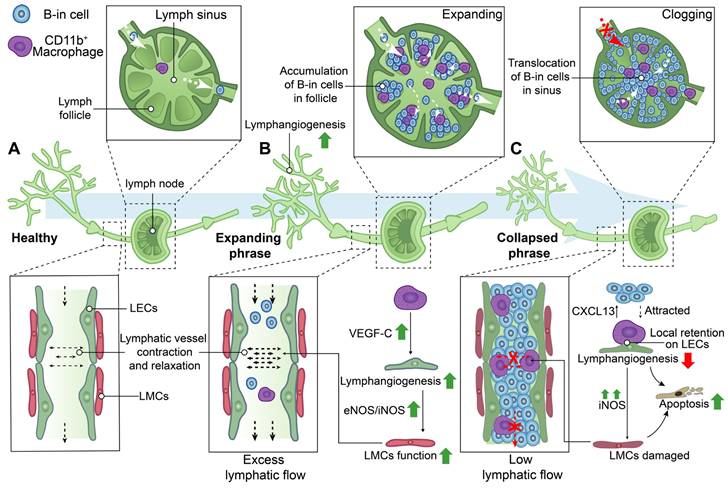
In addition to the modifications in the distribution and structure of lymphatic vessels, alterations in the clearance function of lymphatic vessels were also observed in the processes of RA (Figure 4). NIR-ICG lymphatic imaging revealed a significant increase in the frequency of lymphatic vessel contractions with the enlargement of DLNs in K/BxN mice during the acute phase of RA, subsequently normalizing the collapse of expanding DLNs during the chronic phase [45]. Therefore, the progression of RA is characterized by the presence of two distinct phenotypes of DLNs, namely expanding and collapsed DLNs.
During the expanding phase of DLNs, the augmented DLN volume is associated with an upregulation of lymphangiogenesis, infiltration of CD11b+ macrophages, and accumulation of a distinct subset of B cells characterized by high expression levels of CD23, CD21, IgM, IgD, and CD1d in inflamed nodes, also named B-in cells [45, 62, 96, 98-101]. CD11b+ macrophages actively contribute to lymphangiogenesis, play a crucial role in maintaining lymphatic function, and exhibit the potential to differentiate into osteoclasts as osteoclast precursors (OCPs) during inflammatory conditions [96, 102, 103] . CD11b+ macrophages alone possess the capability to generate lymphatic endothelial marker-expressing tube-like structures, including LYVE-1 and PDPN, in inflamed stromata of murine corneas [104]. Additionally, CD11b+/Gr-1+ macrophages, which exhibit a high expression of VEGF-C, were found to extensively infiltrate the inflamed skin and DLNs in a bacterial pathogen-induced acute inflammation model [105]. Furthermore, CD11b+ macrophages represent the primary source of VEGF-C in TNF-Tg mice, and neutralization of VEGFR3 resulted in a reduction in the number of CD11b+ cells expressing VEGF-C in DLNs [99]. These findings suggest that CD11b+ macrophages play a crucial role in promoting lymphangiogenesis, particularly during the expanding phase of DLNs.
During the stage of collapsed DLNs, a rapid decrease in volumes of DLNs was observed, which is associated with extensive bone loss in adjacent knee joints [45, 62, 64, 65, 99,97]. Lymphatic sinus serves as an essential conduit for lymph drainage [13]. B-in cells reside in the peripheral follicular regions during the expanding phase of DLNs, subsequently relocating to the lymphatic sinus during the collapsing phase. This translocation of B-in cells obstructs both lymph channels and the cavity of the lymphatic sinus, resulting in DLNs collapse and significantly diminished lymphatic drainage from inflamed joints. These findings elucidate the concurrent synovitis erosions, accumulation of B-in cells, and asymmetric onset of arthritis observed in TNF-Tg, K/BxN mice and RA patients [62, 98, 100, 101]. Noteworthy, the lymphatic vessels afferent to expanding DLNs exhibited a high-velocity flow of CD11b+ macrophages, whereas the lymphatic vessels afferent to collapsing DLNs showed stationary CD11b+ macrophages due to the loss of intrinsic lymphatic contractions and passive flow [106]. The local retention of CD11b+ macrophages lead to the damage of LECs and LMCs [82] [93]. Additionally, a gradient of chemokine C-X-C motif ligand 13 (CXCL13), released by static, aggregated, and activated macrophages in DLNs, attracts the migration of B-in cells [107]. CD11b+ macrophages also serve as a significant source of osteoclasts and are thus referred to as OCPs [96]. A study has reported a 4-7-fold increase in OCPs in the peripheral blood and spleen of TNF-Tg mice, which can be reversed by anti-TNF therapy [102]. Thus, on one hand, the increased and localized retention of CD11b+ macrophages could attract the migration of B-cells to lymphatic sinus, resulting in the obstruction of lymphatic vessels. On the other hand, these macrophages are directly involved in the extensive bone loss in adjacent knee joints during the collapsed phase of DLNs [108].
Schematic diagram demonstrating lymphatic phenotypes in the development of rheumatoid arthritis. Two different phenotypes of the lymph node, called expanding and collapsed phrase, were observed during rheumatoid arthritis development. (A) Lymphatic capillaries retrieve lymph and inflammatory factors which flow into lymphatic collecting vessels and be transported to lymph nodes by the flow force of lymphatic collecting vessels contraction. (B) During expanding phrase, lymphangiogenesis in the lymphatic system facilitates removal of inflammatory factors and lymph to the expanding lymph nodes with the accumulation of B-in cells in follicles. Macrophages are involved in regulating lymphangiogenesis and the contraction of lymphatic muscle cells (LMCs). (C) During collapsed phrase, lymph nodes have collapsed in volume owing to damaged LMC with low lymphatic flow, the disorders of lymphangiogenesis, and the cloggy of B-in cells in sinus. Local retention of CD11b+ macrophages on lymphatic endothelial cells (LECs) are involved in regulating the attraction of B-in cells, lymphangiogenesis and the function of LMCs. VEGF-C: Vascular endothelial growth factor C, eNOS: Endothelial nitric oxide synthase, iNOS: Inducible nitric oxide synthase, CXCL13: Chemokine C-X-C motif ligand 13.
3.1 Lymphatic vessels and OA
OA is a prevalent joint disorder characterized by the degeneration and damage of the articular cartilage, accompanied by inflammation of the surrounding tissues [109, 110]. While patients with OA and RA may experience similar symptoms of joint stiffness and pain, these two types of arthritis differ significantly in terms of their underlying causes. OA is primarily attributed to the gradual mechanical degradation of cartilage and the disorder of subchondral bone due to aging processes, whereas RA is characterized by a disease of autoimmune response. The pivotal role of synovial cells in OA is widely acknowledged, as they actively release a range of inflammatory mediators [109, 111]. These inflammatory mediators, acting as pivotal factors, stimulate the synthesis of inflammatory cytokines and matrix degrading enzymes such as matrix metalloproteinases (MMPs) [112] and the a disintegrin and metalloproteinase with thrombospondin motif (ADAMTS) family [113] of proteins in chondrocytes, thereby instigating cartilage destruction and degradation during OA progresses [114]. Therefore, the role of inflammation in the pathogenesis of OA has gained increasing consensus among researchers. Similar to RA, the peri-articular lymphatic system plays a significant role in the pathogenesis of OA [12]. Although extensive research has been conducted on the involvement of lymphatic vessels in human and murine RA, limited attention has been given to investigating their role in OA (Figure 5).
3.2 Dynamic alterations of lymphatic vessels involves in various stages of OA progression
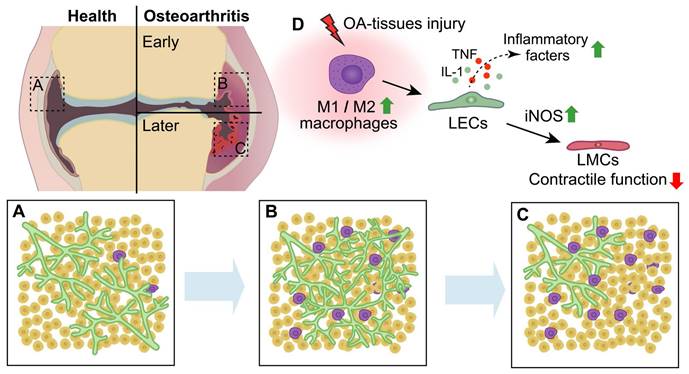
Based on clinical reports, immunohistochemical analysis of synovial specimens from patients with OA revealed an increased presence of lymphatic vessels infiltrated by numerous inflammatory cells across all zones of the synovial membrane [94]. These findings imply the potential involvement of synovial lymphatic vessels in the inflammatory cascade underlying OA pathogenesis. On the other hand, dysfunction of microcirculation and lymphatic drainage was observed in samples of OA patients by transmission electron microscopy [115]. Furthermore, a study has reported that lymphatic vessel density and the fractional area of LECs in knee synovium sections from patients with OA are lower compared to normal control knees in post-mortem analysis. Additionally, there is a negative correlation between lymphatic vessel density and synovial effusion, which is a characteristic feature of advanced stages of OA development [50]. The reduced lymphatic vessel density may contribute to the retention of synovial fluid in OA patients, potentially exacerbating joint inflammation by impairing lymphatic pumping function [116]. In addition to the number of lymphatic vessels, the relationship between the severity of OA and the number, size, and central fatty changes of DLNs observed in MR images of patients does not exhibit a linear trend [117]. Therefore, these findings suggest that dynamic alterations in the structure and functions of the lymphatic system may play a significant role in various stages of OA progression. Further investigation is warranted to elucidate the long-term mechanisms by which the lymphatic system maintains joint homeostasis during OA progresses.
Schematic diagram demonstrating lymphatic phenotypes in the development of osteoarthritis. (A-C) In the process of osteoarthritis (OA) development, lymphangiogenesis of lymphatic capillaries are observed in the early stage of OA, whereas decreased lymphatic capillaries and impaired lymphatic pumping function are observed in the late stage of OA. (D) After OA-tissues injury, M1 macrophages is significantly increased compared to M2 macrophages, which induces the production of lymphatic endothelial cells (LECs) inflammatory factors and damages the function of lymphatic muscle cells (LMCs) via increasing inducible nitric oxide synthase (iNOS) of LECs.
3.3 Synovial lymphatic drainage is impaired during OA progression
In the mice model of meniscal-ligamentous injury (MLI)-induced OA, increased capillary lymphatics and decreased collecting lymphatic vessels in OA joints were detected at 3 months post-MLI by the whole-slide imaging system [46]. Further study found significantly increased lymphatic vessels, especially lymphatic capillaries, in the thicker synovial membrane of mice at 12 weeks post-MLI and cartilage-specific knockout of transforming growth factor beta (TGF beta) type II receptor mice at 6 weeks after tamoxifen induction [43]. Potentially, an increase in lymphatic capillaries could enhance lymphatic drainage; however, a decline in lymphatic drainage function was also detected in these mice [43, 118]. Recent findings suggest that the increased lymphangiogenesis of lymphatic capillaries does not exhibit a positive correlation with lymphatic vessel contractility and transport properties in visceral adipose tissues of rodents and humans with obesity and/or diabetes [119]. One potential explanation for this phenomenon is that the emergence of extensively branched lymphatic capillaries in their juvenile stage could elicit a leaky phenotype, thereby hindering their drainage function. The lymphatic capillaries and collecting lymphatic vessels were significantly reduced, leading to impaired lymphatic pumping function; Consequently, lymph clearance was significantly diminished and pro-inflammatory factors accumulated in the knees of both 20-week MLI-induced OA [43] and aging-related OA [120] mice, which is further supported by OA samples from patients [43]. Therefore, the above results show that synovial lymphatic drainage is impaired during OA progression.
3.4 The collaboration of macrophages and lymphatic vessels regulates OA
Although the predominance of T-lymphocytes and monocyte infiltration, as well as synovitis and concentrations of inflammation mediators in joints, appears to be less pronounced in OA compared to RA, it is widely acknowledged that macrophages play a significant role in the process of joint inflammation and bone destruction in OA [121-124], potentially influencing this process through collaboration with lymphatic vessels. Macrophages are highly adaptable cells that can be categorized into two distinct phenotypes, namely classically activated M1 (pro-inflammatory) macrophages and alternatively activated M2 (anti-inflammatory) macrophages, which exhibit differential responses to microenvironmental stimuli [125]. The M1 macrophages secrete substantial quantities of proinflammatory cytokines and mediators, such as TNF-α, IL-1, and IL-6[126]. The M2 macrophages, also referred to as healing macrophages, exhibit an anti-inflammatory phenotype and contribute significantly to tissue repair, remodeling and pro-tumorigenic functions [127, 128]. Additionally, the production of VEGF-C by M2 macrophages is associated with lymphangiogenesis in human tumors [129]. Macrophages accumulate and undergo polarization (M1 or M2) within the synovium and articular cavity during the progression of OA [130, 131]. In the early stage of OA (5-6 weeks post-MLI), synovitis and accumulation of M1 macrophages adjacent to lymphatic vessels were observed in the synovium of mice joints. Furthermore, M1 macrophages play a crucial role in promoting destructive processes by regulating the expression of inflammatory mediators, such as TNF, IL-1 and iNOS, in LECs and synovial lymphatic drainage in inflamed synovial tissue [118]. Itch serves as a crucial negative regulator of NF-κB, a pivotal inflammatory signaling pathway, and has been observed to suppress the pro-inflammatory polarization of macrophages and the release of IL-1α, an inflammatory cytokine [132, 133]. Global knockout of itch in mice results in severe phenotypes associated with OA and impairs synovial lymphatic drainage through M1 macrophage-induced inflammatory response in LECs [134]. In our group, we observed decreased fibroblast growth factor receptor 3 (FGFR3) expression in monocytes derived from patients with OA. Conditional knockout of FGFR3 in macrophages using lysozyme-Cre mice exacerbated joint destruction by promoting synovitis and enhancing macrophage accumulation through CXCL12/CXCR7-dependent chemotaxis in both aging and MLI-induced OA models [135]. Given that the secretion of CXCL12 from LECs is crucial for tissue regeneration after injury [31], it is imperative to further investigate the potential interplay between synovial tissue-resident macrophages and lymphatic vessels in OA.
4. Potential impact of inflammatory environment on lymphatic vessels in RA and OA
The inflammatory response observed in RA exhibits distinct characteristics compared to that seen in OA [136]. RA exhibit a chronic and high-grade systemic inflammation [137], whereas the pathogenesis of OA is characterized by mild inflammation within the synovium [138]. Specially, the expression of TNF-α and IL-1β is higher in both the number of cells and levels in the synovium of RA compared to OA, while the OA synovium shows an increased presence of cells expressing the anti-inflammatory marker IL-10 [139], indicating a more inflammatory environment in RA compared to OA. Therefore, variations in the inflammatory environment within the synovium of RA compared to OA may exert an influence on the structure and functionality of lymphatic vessels, thereby contributing to disparities in disease pathology and progression.
In the synovium, RA exhibits a higher presence of infiltrating inflammatory cells compared to OA [140]. Recently, the application of high-resolution techniques has provided a comprehensive understanding of the cellular composition within the synovium in both RA and OA [48]. In this study, OA serves as a reference for controlling the highly inflammatory RA synovium, establishing the maximum levels of OA inflammation as the threshold for defining leukocyte-rich or leukocyte-poor RA. Leukocyte-poor RA synovium exhibits a closer resemblance to OA synovium in terms of the proportion of leukocytes and PDPN+ cells, including lymphatic vessels and fibroblasts within the tissue, suggests leukocyte-poor RA and OA have similar inflammatory states to some extent. In contrast, leukocyte-rich RA synovial tissue exhibits a profusion of monocyte, B and T cell infiltrates alongside comparatively fewer PDPN+ cells, suggesting a pronounced impairment in lymphatic vessel drainage function during RA progression that requires further investigation.
Various subgroups of macrophages significantly contribute to the inflammatory responses in both RA and OA. Studies have shown that macrophages are directly involved in the regulation of lymphangiogenesis and lymphatic vessel function in a variety of diseases [141, 142]. In the synovium, the co-localization of M1 macrophages and PDPN+ LECs in OA, along with elevated gene expression levels of pro-inflammatory factors in LECs [118], suggests the influence of macrophages on LEC function. Furthermore, transcriptional changes of single-cell RNA-sequencing on the joints of mice with RA progression exhibited an elevation in inflammatory monocytes, accompanied by a decrease in LMCs of lymphatic vessels and M2 macrophage populations [143]. Of note, the synovium of leukocyte-rich RA exhibits an increased population of monocytes polarized towards IL-1β+ and interferon (IFN)-γ-activated secreted phosphoprotein (SPP1)+ pro-inflammatory M1-like macrophages compared to OA synovium, where the majority of macrophages are nuclear protein 1 (NUPR1)+ and Mer proto-oncogene tyrosine kinase (MerKT)+ M2-like polarized in response to the unique homeostatic requirements of the synovium [48, 52]. Thus, further investigation into the interactions between different macrophage subgroups in RA and OA and lymphatic vessels is necessary to elucidate their involvement in the distinct states of the inflammatory process.
5. Targeting the lymphatic vessels as a potential therapeutic strategy
The statement highlights the potential for distinct regulatory mechanisms and varying treatment approaches in addressing disorders of lymphatic vessels associated with RA and OA. In accordance with the pathogenesis of RA, potential lymphatic-modulating treatments for RA development include strategies to anti-inflammation and restore lymphatic vessel contraction, as well as remove inflammatory cells to unclog lymphatic vessels. Additionally, it is evident that reduced lymphatic vessel numbers and impaired lymphatic contraction contribute to compromised lymphatic drainage, which correlates positively with the severity of arthritic inflammation in both RA and OA. Therefore, enhancing lymphangiogenesis and improving lymphatic draining function represent potential viable treatment strategies (Table 1).
Anti-TNF treatment. As previously mentioned, TNF-α plays a significant role in the pathogenesis of RA [69]. TNF-Tg mice, which overexpress human TNF-α, exhibit numerous features observed in RA patients. Consequently, therapeutic interventions targeting TNF-α blockade have been extensively investigated and implemented in clinical practice [144-147]. The systemic administration of anti-TNF therapy leads to a reduction in macrophage numbers through the promotion of lymphatic vessel recovery and enhanced lymphatic contraction, ultimately resulting in improved lymphatic drainage of a RA mouse model and provides the most significant pain relief for symptomatic knee joints in RA patients [82, 98, 101, 148]. Furthermore, the notable reduction of inflammatory responses in the synovium through anti-TNF treatment is not attributed to an increase in macrophage apoptosis or impaired monocyte influxion [149-151]. Although systemic anti-TNF therapy is generally considered efficacious in the treatment of RA, a significant proportion of patients (about 40%) exhibit inadequate response [152, 153]. Considering the crucial role of lymphatic drainage in RA, localized delivery of anti-TNF drug targeting the immune system through reversing lymphatic dysfunction and reducing RA-associated swelling may exhibit more favorable effects compared to systemic administration in a collagen-induced RA [154]. For OA treatment, the efficacy of TNF inhibition strategy was evaluated in two clinical trials, wherein Adalimumab and Etanercept (TNF inhibitors) were administered for 12 weeks and 24 weeks, respectively. However, no significant differences in pain control were observed in patients with hand OA [155, 156].
Potential therapy by targeting the function of lymphatic vessels on RA and OA
| Drug | Administration | Model | Outcome | References | |
|---|---|---|---|---|---|
| Anti-TNF treatment | Anti-TNF IgG1 antibody | Systemic administration 10 mg/kg/week | TNF-Tg mice | Decreased synovial and lymph node volumes without a reduction of lymphatic vessels; Restored lymphatic contractions; Potential enhancement of inflammatory cell egress; Not improved LMCs defects. | [82, 98, 148, 182] |
| Certolizumab pegol | Systemic administration 400 mg monthly | RA patients with active flare of a single wrist or knee | Linear inverse correlation between lymph node volume and joint pain | [101] | |
| Ginsenoside Rg1 | Systemic administration 20 mg/kg daily | TNF-Tg mice | Improved lymphatic drainage; Increased LMCs coverage; Reduced inflammation in LECs and bone erosion. | [47, 183] | |
| Etanercept | Local lymphatic delivery by nanotopography (SOFUSA™) | A rat model of collagen-induced arthritis | More favorable pharmacodynamics than subcutaneous or intravenous administration; Increased lymphatic pumping and reduced swelling of joints. | [154] | |
| Etanercept | Systemic administration 25-50 mg/week | Inflammatory hand OA patients | Not relieve pain; Radiographic remodeling of subchondral bone. | [155] | |
| Adalimumab | Systemic administration 40 mg/week | Inflammatory hand OA patients | Not show any effect on pain, synovitis or bone marrow lesions. | [156] | |
| Proteasome inhibitors | Bortezomib | Systemic administration 0.25 mg/ml in a 5-μl | MLI-induced OA | Decreased cartilage loss; Reduced the expression of inflammatory genes by LECs; Improved lymphatic drainage. | [118] |
| B cell depletion therapy | Anti CD20 mAbs (18B12 IgG2a) | Systemic administration 10 mg/kg every 2 weeks | TNF-Tg mice | Decreased synovial volumes; Converted collapsed DLNs to expanding DLNs; Increased lymphatic clearance Without recovery of the lymphatic pulse Decreased cartilage loss. | [62, 106] |
| Rituximab | Systemic administration 1000 mg twice | RA patients on methotrexate with resistance to TNF inhibitors | Inhibit the progression of structural joint damage; Mild to moderate side effects. | [147, 161, 162] | |
| Anti-CD20 antibody (Cy5-αCD20) Rituximab | IA compared to SC or IV administration | Collagen-induced arthritis mice Sprague-Dawley rat | IA with a greater B cell depletion in DLNs of joints. | [163, 164] | |
| VEGF-C/VEGFR3 treatment | AAV-VEGF-C | IA administration | TNF-Tg mice | Increased lymphangiogenesis; Improved lymphatic drainage; Attenuated joint tissue damage. | [56, 169] |
| VEGF-C156S | IA administration | Age-Related OA | Improved lymphatic drainage; Attenuated joint tissue damage. | [120] | |
| iNOS inhibitors | Ferulic acid, L-NIL L-NAME | Local administration L-NIL (4 mg/kg) Systemic administration Ferulic acid (20 mg/kg/day) L-NIL or L-NAME (100 ng/ml in drinking water) | TNF-Tg mice | Improved lymphatic drainage; Restored lymphatic contractions; Attenuated joint tissue damage. | [66] |
| Fang-Ji-Huang-Qi-Tang decoction | Systemic administration | Collagen-induced arthritis mice | Increased lymphangiogenesis; Improved lymphatic drainage; Attenuated joint tissue damage. | [177] | |
| Du-Huo-Ji-Sheng-Tang decoction | Systemic administration | TNF-Tg mice Zebrafish | Increased lymphangiogenesis Improved lymphatic drainage; Attenuated joint tissue damage. | [178] | |
| Total saponins of Panax notoginseng | Systemic administration | TNF-Tg mice | Prevented LMCs apoptosis; Improved lymphatic drainage; Attenuated joint tissue damage. | [67] | |
| Cindunistat (SD-6010) | Systemic administration 50 or 200 mg/day | Symptomatic knee OA patients (KLG 2 or 3) | Less joint space narrowing KLG2 patients during early stage of treatment; Not slow OA progression in KLG3 patients. | [180] | |
| GW274150 | Systemic administration 60 mg/day | RA patients with DAS28 scores ≥4.0 | A trend towards reduction in synovial thickness and vascularity without statistically significant. | [181] | |
Abbreviations: AAV: Adeno-associated virus; IA: Intra-articular; iNOS: Inducible Nitric oxide synthase; IV: intravenous; KLG: Kellgren and Lawrence Grade; L-NAME: Nω-nitro-L-arginine methyl ester; L-NIL: L-N6-(1-iminoethyl) lysine 5-tetrazole-amide; MLI: Meniscal ligamentous injury; OA: Osteoarthritis; RA: Rheumatoid arthritis; SC: Subcutaneous; TNF: Tumor necrosis factor, VEGF-C: Vascular endothelial growth factor
Bortezomib treatment. The proteasome inhibitor Bortezomib has been approved by the US Food and Drug Administration for the first-line treatment of patients diagnosed with multiple myeloma [157]. Studies conducted on murine models of experimentally induced arthritis have demonstrated the efficacy of proteasome inhibitors, such as MG132 [158] and Bortezomib [159, 160], in ameliorating inflammatory joint manifestations through modulation of the NF-κB signaling pathway. In MLI-induced OA mouse model, intra-articular administration of Bortezomib significantly improves synovial lymphatic drainage, decreases numbers of M1 macrophages and the inflammatory gene expression by LECs [118]. Thus, Proteasome inhibitor, Bortezomib, may potentially serve as a novel therapeutic approach for restoring synovial lymphatic function in arthritis. However, further investigation through human clinical trials is necessary to validate its therapeutic potential.
B cell depletion therapy. The translocated B-in cells from the DLN follicles into the sinuses during the stage of collapsed DLNs are believed to exert a mechanical hindrance on lymphatic flow, thereby exacerbating RA. Thus, the depletion of local B-in cells in the joint and DLNs is therefore anticipated to confer therapeutic benefits in RA by attenuating both the inflammatory response and lymphatic dysfunction. The depletion of B-in cells using anti-CD20 mAbs in TNF-Tg mice in the onset of RA effectively prevented knee flare, while therapeutic intervention targeting collapsed DLNs ameliorated inflammatory-erosive arthritis [62, 106]. Furthermore, the B cell depleting antibody Rituximab is employed as an alternative therapy for RA in clinical practice [147, 161, 162]. However, progression of RA was observed in a subset of patients, while mild to moderate side effects associated with systemic Rituximab treatment were reported about 85% of patients [162]. Recently, investigation has been conducted on the localized delivery of B cell depletion therapy in RA joints with the aim of mitigating off-target adverse effects. The results revealed that intra-articular injection of a B cell depletion antibody effectively enhances local exposure to DLNs of joints, thereby facilitating dose reduction and minimizing systemic toxicities in RA mice models [163, 164]. Despite OA not being classified as an immune-mediated inflammatory disease within adaptive immunity, there have been documented instances of autoantibodies in synovial tissue and alterations in the characteristics of circulating and local B-cells with immunological functions [165-167]. These findings provide evidence suggesting that B-cells may contribute to the pathogenesis of OA. The potential for therapeutic intervention in OA by targeting B cells, as well as the possibility of excessive B-cell activation serving as a biomarker for predicting disease progression or clinical severity, remains to be elucidated [168].
VEGF-C/VEGFR3 treatment. The VEGF-C/VEGFR3 signaling pathway represents a prominent target for increasing lymphangiogenesis and enhancing lymphatic drainage capacity, thereby suggesting that elevating the content of VEGF-C or selectively targeting LECs with VEGFR3 within joints may hold promising therapeutic potential in arthritis. For RA, inhibition of lymphangiogenesis and lymphatic drainage through systemic blockade of VEGFR3 exacerbates the severity of inflammation [99], whereas intra-articular administration of VEGF-C adeno-associated virus (AAV) mitigates joint damage in RA by promoting local lymphatic drainage in mice [169]. For OA, synovial lymphatic drainage is impaired during OA progression. A recent study found that a decrease in the expression of VEGF-C and genes associated with the VEGFR3 signaling pathway was observed in the OA synovium of aged mice; furthermore, intra-articular injection of VEGF-C156S, a mutant form of VEGF-C that specifically binds to VEGFR3[170], enhances synovial lymphatic drainage and attenuates tissue damage in aged mice [120]. Based on these evidences, targeting VEGF-C/ VEGFR3 signaling is a promising approach for regulating lymphatic vessels in arthritis treatment; however, further investigation is required to address existing unclears. Firstly, AAV-mediated delivery of VEGF-C overcomes the short half-life of recombinant VEGF-C, and studies were reported to assess the short-term safety of this approach for potential clinical use [171, 172]. Given that arthritis is a chronic disease, it is imperative to investigate the long-term effects and safety profile of AAV-based delivery of VEGF-C for joints. Additionally, the VEGF-C/VEGFR3 signaling pathway potentially modulates chondrocytes [120], macrophages [173], and bone homeostasis [31, 58, 174] in joints, necessitating further investigation.
iNOS inhibitors. Inflammation triggers the upregulation of iNOS, resulting in excessive production of NO, which impairs the contraction of LMCs and diminishes lymphatic drainage. Thus, inhibitors targeting iNOS may serve as potential treatments to preserve lymphatic function in joints with inflammation. For RA, preliminary findings of iNOS inhibition in animal models of acute arthritis exhibited promising outcomes [175, 176]. Furthermore, local administration of L-N6-(1-iminoethyl) lysine 5-tetrazole-amide, an iNOS inhibitor, into inflamed paws of TNF-Tg mice led to restoration of lymphatic vessel contractions and improved drainage [66]. Additionally, numerous plant extracts and small molecules derived from plants have demonstrated promising potential in regulating lymphatic function through the inhibition of iNOS, as evidenced by animal models of arthritis [66, 67, 177-179]. For OA, the inhibition of iNOS in previous OA studies is considered to have chondrocyte-protective effects, leading to a decrease in general MMPs activity, as well as a reduction in the incidence and size of osteophytes and cartilage lesions in OA models [179]. However, the impact of iNOS inhibition on lymphatic vessels remains unclear during OA progresses. Although animal studies provide support for the investigation of iNOS inhibitors as potential disease-modifying interventions for arthritis, including OA and early RA, no successful clinical trials evaluating the efficacy of these agents have been reported [180, 181]. The possible explanation is that an early iNOS-independent phase followed by a subsequent iNOS-dependent phase have identified in the development of RA, suggesting that targeted inhibition of selective iNOS would yield more pronounced effects on severe RA [64, 65]. Moreover, considering the irreversible nature of LMCs damage and its limited ability to fully recover after severe injury [32, 182], it is crucial to further investigate the specific timepoints at which iNOS-dependent arthritic attenuation may occur.
6. Perspective
In general, lymphatic vessels plays a pivotal role in maintaining joint homeostasis, dysfunction is intricately linked to inflammatory joint diseases. Further investigation is warranted to explore the dynamic changes and functional significance of lymphatic vessels at different stages of RA and OA, facilitating the identification of an optimal intervention window for clinical practice. Moreover, it is imperative to conduct a comprehensive investigation utilizing techniques such as single-cell sequencing and lineage tracing in order to thoroughly explore the key cellular subsets and molecular characteristics that underlie pathological alterations in lymphatic vessels of these diseases. The exploration of more specific molecular targets, in conjunction with tailored drug delivery modes, is crucial for optimizing therapeutic strategies and enhancing treatment efficacy efficiently. We anticipate that future advancements will yield more efficacious strategies for modulating joint lymphatic vessels, thereby enhancing the clinical outcomes of inflammatory joint diseases, such as RA and OA.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81802205, 81830075, 82372495, 82202770, 82122044), Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX1267, CSTB2022NSCQ-MSX1267), 2020 Chongqing Municipal Healthcare Technology Promotion Project (2020jstg028), Chongqing Emergency Critical Care Clinical Medical Research Center and Innovation Developing Program of Army Medical Center (2019CXJX018).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Petrova TV, Koh GY. Biological functions of lymphatic vessels. Science. 2020 369
2. Itkin M, Rabinowitz DA, Nadolski G, Stafler P, Mascarenhas L, Adams D. Abnormal Pulmonary Lymphatic Flow in Patients With Lymphatic Anomalies and Respiratory Compromise. Chest. 2020;158:681-91
3. Brouillard P, Witte MH, Erickson RP, Damstra RJ, Becker C, Quere I. et al. Primary lymphoedema. Nat Rev Dis Primers. 2021;7:77
4. Escobedo N, Oliver G. The Lymphatic Vasculature: Its Role in Adipose Metabolism and Obesity. Cell Metab. 2017;26:598-609
5. Vaahtomeri K, Alitalo K. Lymphatic Vessels in Tumor Dissemination versus Immunotherapy. Cancer Res. 2020;80:3463-5
6. Li X, Qi L, Yang D, Hao S, Zhang F, Zhu X. et al. Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat Neurosci. 2022;25:577-87
7. Wang W, Li X, Ding X, Xiong S, Hu Z, Lu X. et al. Lymphatic endothelial transcription factor Tbx1 promotes an immunosuppressive microenvironment to facilitate post-myocardial infarction repair. Immunity. 2023
8. Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS. et al. Rheumatoid arthritis. Nature Reviews Disease Primers. 2018 4
9. Scanzello CR. Role of low-grade inflammation in osteoarthritis. Current Opinion in Rheumatology. 2017;29:79-85
10. Roberts NLS, Mountjoy-Venning WC, Anjomshoa M, Banoub JAM, Yasin YJ. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study (vol 392, pg 1789, 2018). Lancet. 2019;393:E44-E
11. Bouta EM, Bell RD, Rahimi H, Xing L, Wood RW, Bingham CO 3rd. et al. Targeting lymphatic function as a novel therapeutic intervention for rheumatoid arthritis. Nat Rev Rheumatol. 2018;14:94-106
12. Cao M, Ong MTY, Yung PSH, Tuan RS, Jiang Y. Role of synovial lymphatic function in osteoarthritis. Osteoarthritis Cartilage. 2022;30:1186-97
13. Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic Vessel Network Structure and Physiology. Comprehensive Physiology. 2019;9:207-99
14. Skandalakis JE, Skandalakis LJ, Skandalakis PN. Anatomy of the lymphatics. Surgical Oncology Clinics of North America. 2007;16:1 -+
15. Padera TP, Meijer EFJ, Munn LL. The Lymphatic System in Disease Processes and Cancer Progression. In: Yarmush ML, editor. Annual Review of Biomedical Engineering, Vol 18. 2016 p. 125-58
16. Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. Journal of Clinical Investigation. 2014;124:888-97
17. Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ. et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes & Development. 2007;21:2422-32
18. Fischer M, Franzeck UK, Herrig I, Costanzo U, Wen S, Schiesser M. et al. Flow velocity of single lymphatic capillaries in human skin. Am J Physiol. 1996;270:H358-63
19. Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. Journal of Cell Biology. 2011;193:607-18
20. Tammela T, Alitalo K. Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell. 2010;140:460-76
21. Zawieja D. Lymphatic biology and the microcirculation: Past, present, and future. Microcirculation. 2005;12:141-50
22. Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. Journal of Experimental Medicine. 2007;204:2349-62
23. Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiological reviews. 1990;70:987-1028
24. Zawieja DC. Contractile Physiology of Lymphatics. Lymphatic Research and Biology. 2009;7:87-96
25. Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural Flow Modulates Cell and Fluid Transport Functions of Lymphatic Endothelium. Circulation Research. 2010;106:920-U181
26. Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. Plasticity of Button-Like Junctions in the Endothelium of Airway Lymphatics in Development and Inflammation. American Journal of Pathology. 2012;180:2561-75
27. Trzewik J, Mallipattu SK, Artmann GM, Delano FA, Schmid-Schonbein GW. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2001;15:1711-7
28. von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. International Journal of Biochemistry & Cell Biology. 2004;36:1147-53
29. Polomska AK, Proulx ST. Imaging technology of the lymphatic system. Adv Drug Deliv Rev. 2021;170:294-311
30. Sleeman JP, Krishnan J, Kirkin V, Baumann P. Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech. 2001;55:61-9
31. Biswas L, Chen J, De Angelis J, Singh A, Owen-Woods C, Ding Z. et al. Lymphatic vessels in bone support regeneration after injury. Cell. 2023;186:382-97 e24
32. Kenney HM, Peng Y, de Mesy Bentley KL, Xing L, Ritchlin CT, Schwarz EM. The Enigmas of Lymphatic Muscle Cells: Where Do They Come From, How Are They Maintained, and Can They Regenerate? Current rheumatology reviews. 2023;19:246-59
33. Lutter S, Xie S, Tatin F, Makinen T. Smooth muscle-endothelial cell communication activates Reelin signaling and regulates lymphatic vessel formation. The Journal of cell biology. 2012;197:837-49
34. Tammela T, Saaristo A, Holopainen T, Lyytikkä J, Kotronen A, Pitkonen M. et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nature medicine. 2007;13:1458-66
35. Yang Y, García-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK. et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120:2340-8
36. Bazigou E, Lyons OTA, Smith A, Venn GE, Cope C, Brown NA. et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. Journal of Clinical Investigation. 2011;121:2984-92
37. Marchand T, Akinnola KE, Takeishi S, Maryanovich M, Pinho S, Saint-Vanne J. et al. Periosteal skeletal stem cells can migrate into the bone marrow and support hematopoiesis after injury. bioRxiv. 2023
38. Zhang D, Huang J, Sun X, Chen H, Huang S, Yang J. et al. Targeting local lymphatics to ameliorate heterotopic ossification via FGFR3-BMPR1a pathway. Nat Commun. 2021;12:4391
39. Kaipainen A, Korhonen J, Mustonen T, Van Hinsbergh VWM, Fang G-H, Dumont D. et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3566-70
40. Ma WS, Gil HJ, Liu XL, Diebold LP, Morgan MA, Oxendine-Burns MJ. et al. Mitochondrial respiration controls the Prox1-Vegfr3 feedback loop during lymphatic endothelial cell fate specification and maintenance. Science Advances. 2021 7
41. Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D. et al. The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes & Development. 2014;28:2175-87
42. Del Rey MJ, Fare R, Izquierdo E, Usategui A, Rodriguez-Fernandez JL, Suarez-Fueyo A. et al. Clinicopathological Correlations of Podoplanin (gp38) Expression in Rheumatoid Synovium and Its Potential Contribution to Fibroblast Platelet Crosstalk. Plos One. 2014 9
43. Shi JX, Liang QQ, Zuscik M, Shen J, Chen D, Xu H. et al. Distribution and Alteration of Lymphatic Vessels in Knee Joints of Normal and Osteoarthritic Mice. Arthritis & Rheumatology. 2014;66:657-66
44. Suzuki T, Takakubo Y, Oki H, Liu X, Honma R, Naganuma Y. et al. Immunohistochemical Analysis of Inflammatory Rheumatoid Synovial Tissues Using Anti-Human Podoplanin Monoclonal Antibody Panel. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy. 2018 37
45. Zhou QA, Wood R, Schwarz EM, Wang YJ, Xing LP. Near-Infrared Lymphatic Imaging Demonstrates the Dynamics of Lymph Flow and Lymphangiogenesis During the Acute Versus Chronic Phases of Arthritis in Mice. Arthritis and Rheumatism. 2010;62:1881-9
46. Shi JX, Liang QQ, Wang YJ, Mooney RA, Boyce BF, Xing L. Use of a whole-slide imaging system to assess the presence and alteration of lymphatic vessels in joint sections of arthritic mice. Biotechnic & Histochemistry. 2013;88:428-39
47. Hou T, Liu Y, Wang XY, Jiao DL, Xu H, Shi Q. et al. Ginsenoside Rg1 promotes lymphatic drainage and improves chronic inflammatory arthritis. Journal of Musculoskeletal & Neuronal Interactions. 2020;20:526-34
48. Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nature Immunology. 2019;20:928 -+
49. Gröger M, Niederleithner H, Kerjaschki D, Petzelbauer P. A previously unknown dermal blood vessel phenotype in skin inflammation. Journal of Investigative Dermatology. 2007;127:2893-900
50. Walsh DA, Verghese P, Cook GJ, McWilliams DP, Mapp PI, Ashraf S. et al. Lymphatic vessels in osteoarthritic human knees. Osteoarthritis and Cartilage. 2012;20:405-12
51. Paavonen K, Mandelin J, Partanen T, Jussila L, Li T-F, Ristimaki A. et al. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. Journal of Rheumatology. 2002;29:39-45
52. Alivernini S, MacDonald L, Elmesmari A, Finlay S, Tolusso B, Gigante MR. et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nature medicine. 2020;26:1295 -+
53. Lim HY, Lim SY, Tan CK, Thiam CH, Goh CC, Carbajo D. et al. Hyaluronan ReceptorLYVE-1-Expressing Macrophages Maintain Arterial Tone through Hyaluronan-Mediated Regulation of Smooth Muscle Cell Collagen. Immunity. 2018;49:326 -+
54. Simkin PA. Synovial perfusion and synovial fluid solutes. Annals of the rheumatic diseases. 1995;54:424-8
55. Buckley CD, Ospelt C, Gay S, Midwood KS. Location, location, location: how the tissue microenvironment affects inflammation in RA. Nature Reviews Rheumatology. 2021;17:195-212
56. Kakei Y, Akashi M, Shigeta T, Hasegawa T, Komori T. Alteration of Cell-Cell Junctions in Cultured Human Lymphatic Endothelial Cells with Inflammatory Cytokine Stimulation. Lymphatic Research and Biology. 2014;12:136-43
57. Melrose J, Little CB. Immunolocalization of lymphatic vessels in human fetal knee joint tissues. Connective Tissue Research. 2010;51:289-305
58. Zhang Q, Guo R, Lu Y, Zhao L, Zhou Q, Schwarz EM. et al. VEGF-C, a lymphatic growth factor, is a RANKL target gene in osteoclasts that enhances osteoclastic bone resorption through an autocrine mechanism. J Biol Chem. 2008;283:13491-9
59. Smolen JS. Rheumatoid arthritis Primer - behind the scenes. Nat Rev Dis Primers. 2020;6:32
60. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England journal of medicine. 2011;365:2205-19
61. Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunological reviews. 2010;233:233-55
62. Li J, Kuzin I, Moshkani S, Proulx ST, Xing LP, Skrombolas D. et al. Expanded CD23(+)/CD21(hi) B Cells in Inflamed Lymph Nodes Are Associated with the Onset of Inflammatory-Erosive Arthritis in TNF-Transgenic Mice and Are Targets of Anti-CD20 Therapy. Journal of Immunology. 2010;184:6142-50
63. Han YP, Li X, Zhou QY, Jie HY, Lao XB, Han JC. et al. FTY720 Abrogates Collagen-Induced Arthritis by Hindering Dendritic Cell Migration to Local Lymph Nodes. Journal of Immunology. 2015;195:4126-35
64. Scallan JP, Bouta EM, Rahimi H, Kenney HM, Ritchlin CT, Davis MJ. et al. Ex vivo Demonstration of Functional Deficiencies in Popliteal Lymphatic Vessels From TNF-Transgenic Mice With Inflammatory Arthritis. Frontiers in Physiology. 2021 12
65. Bell RD, Slattery PN, Wu EK, Xing LP, Ritchlin CT, Schwarz EM. iNOS dependent and independent phases of lymph node expansion in mice with TNF-induced inflammatory-erosive arthritis. Arthritis Research & Therapy. 2019 21
66. Liang QQ, Ju YW, Chen Y, Wang WS, Li JL, Zhang L. et al. Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice. Arthritis Research & Therapy. 2016 18
67. Liang Q, Zhang L, Xu H, Li J, Chen Y, Schwarz EM. et al. Lymphatic muscle cells contribute to dysfunction of the synovial lymphatic system in inflammatory arthritis in mice. Arthritis Res Ther. 2021;23:58
68. Chen YX, Rehal S, Roizes S, Zhu HL, Cole WC, von der Weid PY. The pro-inflammatory cytokine TNF-alpha inhibits lymphatic pumping via activation of the NF-kappa B-iNOS signaling pathway. Microcirculation. 2017 24
69. Li P, Schwarz EM. The TNF-alpha transgenic mouse model of inflammatory arthritis. Springer Seminars in Immunopathology. 2003;25:19-33
70. Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732-5
71. Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nature protocols. 2007;2:1269-75
72. Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA. et al. Adalimumab, a fully human anti-tumor necrosis factor a monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate - The ARMADA trial. Arthritis and Rheumatism. 2003;48:35-45
73. Li G, Wu Y, Jia H, Tang L, Huang R, Peng Y. et al. Establishment and evaluation of a transgenic mouse model of arthritis induced by overexpressing human tumor necrosis factor alpha. Biology open. 2016;5:418-23
74. Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Experimental Cell Research. 2006;312:549-60
75. Melincovici CS, Bosca AB, Susman S, Marginean M, Mihu C, Istrate M. et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Romanian Journal of Morphology and Embryology. 2018;59:455-67
76. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298-307
77. Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. Journal of Biochemistry. 2013;153:13-9
78. Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I. et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. The Journal of cell biology. 2010;188:115-30
79. Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood. 2005;106:3423-31
80. Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. Journal of Clinical Investigation. 2014;124:878-87
81. Wauke K, Nagashima M, Ishiwata T, Asano G, Yoshino S. Expression and localization of vascular endothelial growth factor-C in rheumatoid arthritis synovial tissue. Journal of Rheumatology. 2002;29:34-8
82. Bouta EM, Kuzin I, Bentley KD, Wood RW, Rahimi H, Ji RC. et al. Treatment of Tumor Necrosis Factor-Transgenic Mice With Anti-Tumor Necrosis Factor Restores Lymphatic Contractions, Repairs Lymphatic Vessels, and May Increase Monocyte/Macrophage Egress. Arthritis & Rheumatology. 2017;69:1187-93
83. Cha HS, Bae EK, Koh JH, Chai JY, Jeon CH, Ahn KS. et al. Tumor necrosis factor-alpha induces vascular endothelial growth factor-C expression in rheumatoid synoviocytes. Journal of Rheumatology. 2007;34:16-9
84. Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE. et al. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappa B and Prox1. Blood. 2010;115:418-29
85. Zhang YB, Lu Y, Ma L, Cao XD, Xiao J, Chen JX. et al. Activation of Vascular Endothelial Growth Factor Receptor-3 in Macrophages Restrains TLR4-NF-kappa B Signaling and Protects against Endotoxin Shock. Immunity. 2014;40:501-14
86. Lee S, Roizes S, von der Weid PY. Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. Journal of Physiology-London. 2014;592:5409-27
87. Grabowski PS, Wright PK, Van 't Hof RJ, Helfrich MH, Ohshima H, Ralston SH. Immunolocalization of inducible nitric oxide synthase in synovium and cartilage in rheumatoid arthritis and osteoarthritis. British journal of rheumatology. 1997;36:651-5
88. Scher JU, Pillinger MH, Abramson SB. Nitric oxide synthases and osteoarthritis. Current rheumatology reports. 2007;9:9-15
89. Yonekura Y, Koshiishi I, Yamada K, Mori A, Uchida S, Nakamura T. et al. Association between the expression of inducible nitric oxide synthase by chondrocytes and its nitric oxide-generating activity in adjuvant arthritis in rats. Nitric Oxide-Biology and Chemistry. 2003;8:164-9
90. Klinger JR, Kadowitz PJ. The Nitric Oxide Pathway in Pulmonary Vascular Disease. Am J Cardiol. 2017;120:S71-S9
91. Munn LL. Mechanobiology of lymphatic contractions. Seminars in Cell & Developmental Biology. 2015;38:67-74
92. Stichtenoth DO, Frolich JC. Nitric oxide and inflammatory joint diseases. British journal of rheumatology. 1998;37:246-57
93. Liao S, Cheng G, Conner DA, Huang YH, Kucherlapati RS, Munn LL. et al. Impaired lymphatic contraction associated with immunosuppression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18784-9
94. Xu H, Edwards J, Banerji S, Prevo R, Jackson DG, Athanasou NA. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Annals of the Rheumatic Diseases. 2003;62:1227-9
95. Polzer K, Baeten D, Soleiman A, Distler J, Gerlag DM, Tak PP. et al. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Annals of the Rheumatic Diseases. 2008;67:1610-6
96. Zhang Q, Lu Y, Proulx ST, Guo R, Yao Z, Schwarz EM. et al. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res Ther. 2007;9:R118
97. Wang H, Chen YY, Li WL, Sun L, Chen HY, Yang QD. et al. Effect of VEGFC on lymph flow and inflammation-induced alveolar bone loss. Journal of Pathology. 2020;251:323-35
98. Proulx ST, Kwok E, You ZG, Papuga MO, Beck CA, Shealy DJ. et al. Longitudinal assessment of synovial, lymph node, and bone volumes in inflammatory arthritis in mice by in vivo magnetic resonance Imaging and microfocal computed tomography. Arthritis and Rheumatism. 2007;56:4024-37
99. Guo RL, Zhou Q, Proulx ST, Wood R, Ji RC, Ritchlin CT. et al. Inhibition of Lymphangiogenesis and Lymphatic Drainage via Vascular Endothelial Growth Factor Receptor 3 Blockade Increases the Severity of Inflammation in a Mouse Model of Chronic Inflammatory Arthritis. Arthritis and Rheumatism. 2009;60:2666-76
100. Li J, Zhou Q, Wood RW, Kuzin I, Bottaro A, Ritchlin CT. et al. CD23(+)/CD21(hi) B-cell translocation and ipsilateral lymph node collapse is associated with asymmetric arthritic flare in TNF-Tg mice. Arthritis Research & Therapy. 2011 13
101. Kuzin II, Kates SL Ju YW, Zhang LZ Rahimi H, Wojciechowski W et al. Increased numbers of CD23(+)CD21(hi) Bin-like B cells in human reactive and rheumatoid arthritis lymph nodes. European Journal of Immunology. 2016;46:1752-7
102. Li P, Schwarz EM, O'Keefe RJ, Ma L, Looney RJ, Ritchlin CT. et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11b(high) osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis and Rheumatism. 2004;50:265-76
103. Watari K, Nakao S, Fotovati A, Basaki Y, Hosoi F, Bereczky B. et al. Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-kappa B activation. Biochemical and Biophysical Research Communications. 2008;377:826-31
104. Maruyama K, Li M, Cursiefen C, Jackson DG, Keino H, Tomita M. et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11 b-positive macrophages. Journal of Clinical Investigation. 2005;115:2363-72
105. Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE. et al. Critical role of CD11b(+) macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650-9
106. Li J, Ju YW, Bouta EM, Xing LP, Wood RW, Kuzin I. et al. Efficacy of B cell depletion therapy for murine joint arthritis flare is associated with increased lymphatic flow. Arthritis and Rheumatism. 2013;65:130-8
107. Bouta EM, Li J, Ju YW, Brown EB, Ritchlin CT, Xing LP. et al. The role of the lymphatic system in inflammatory-erosive arthritis. Seminars in Cell & Developmental Biology. 2015;38:90-7
108. Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunological reviews. 2010;233:301-12
109. Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258-75
110. Dainese P, Wyngaert KV, De Mits S, Wittoek R, Van Ginckel A, Calders P. Association between knee inflammation and knee pain in patients with knee osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2022;30:516-34
111. Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Current rheumatology reports. 2013;15:323
112. Grillet B, Pereira RVS, Van Damme J, Abu El-Asrar A, Proost P, Opdenakker G. Matrix metalloproteinases in arthritis: towards precision medicine. Nat Rev Rheumatol. 2023;19:363-77
113. Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the 'usual suspects'. Osteoarthritis Cartilage. 2017;25:1000-9
114. Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302-11
115. Borodin YI, Liubarskiy MS, Bgatova NP, Mustafayev NR, Dryomov YY. Morphological criteria of the state of the microcirculation and of the lymphatic drainage in the synovial membrane of the knee joint under normal and pathological conditions. Morfologiya. 2008;133:51-5
116. Michalaki E, Nepiyushchikh Z, Rudd JM, Bernard FC, Mukherjee A, McKinney JM. et al. Effect of Human Synovial Fluid From Osteoarthritis Patients and Healthy Individuals on Lymphatic Contractile Activity. Journal of biomechanical engineering. 2022 144
117. Huh YM, Kim S, Suh JS, Song HT, Song K, Shin KH. The role of popliteal lymph nodes in differentiating rheumatoid arthritis from osteoarthritis by using CE 3D-FSPGR MR imaging: Relationship of the inflamed synovial volume. Korean Journal of Radiology. 2005;6:117-24
118. Wang W, Lin X, Xu H, Sun W, Bouta EM, Zuscik MJ. et al. Attenuated Joint Tissue Damage Associated With Improved Synovial Lymphatic Function Following Treatment With Bortezomib in a Mouse Model of Experimental Posttraumatic Osteoarthritis. Arthritis Rheumatol. 2019;71:244-57
119. Cao E, Watt MJ, Nowell CJ, Quach T, Simpson JS, Ferreira VD. et al. Mesenteric lymphatic dysfunction promotes insulin resistance and represents a potential treatment target in obesity. Nature Metabolism. 2021;3:1175 -+
120. Lin X, Bell RD, Catheline SE, Takano T, McDavid A, Jonason JH. et al. Targeting Synovial Lymphatic Function as a Novel Therapeutic Intervention for Age-Related Osteoarthritis in Mice. Arthritis & Rheumatology. 2023
121. Liu BL, Zhang MQ, Zhao JM, Zheng M, Yang H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Experimental and Therapeutic Medicine. 2018;16:5009-14
122. Zhang H, Cai D, Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28:555-61
123. Thomson A, Hilkens CMU. Synovial Macrophages in Osteoarthritis: The Key to Understanding Pathogenesis? Front Immunol. 2021;12:678757
124. Wu CL, Harasymowicz NS, Klimak MA, Collins KH, Guilak F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthritis Cartilage. 2020;28:544-54
125. Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C. et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192-7
126. Lopa S, Leijs MJ, Moretti M, Lubberts E, van Osch GJ, Bastiaansen-Jenniskens YM. Arthritic and non-arthritic synovial fluids modulate IL10 and IL1RA gene expression in differentially activated primary human monocytes. Osteoarthritis Cartilage. 2015;23:1853-7
127. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F. et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425-40
128. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-37
129. Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C. et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947-56
130. Manferdini C, Paolella F, Gabusi E, Gambari L, Piacentini A, Filardo G. et al. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthritis Cartilage. 2017;25:1161-71
131. Fahy N, de Vries-van Melle ML, Lehmann J, Wei W, Grotenhuis N, Farrell E. et al. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014;22:1167-75
132. Lin X, Wang W, McDavid A, Xu H, Boyce BF, Xing L. The E3 ubiquitin ligase Itch limits the progression of post-traumatic osteoarthritis in mice by inhibiting macrophage polarization. Osteoarthritis and Cartilage. 2021;29:1225-36
133. Lin X, Zhang HW, Boyce BF, Xing LP. Ubiquitination of interleukin-1 alpha is associated with increased pro-inflammatory polarization of murine macrophages deficient in the E3 ligase ITCH. Journal of Biological Chemistry. 2020;295:11764-75
134. Wang W, Xu H, Sun W, Wang H, Zuscik M, Xing L. Mice deficient in the NF-κB negative regulator, itch, develop severe osteoarthritis and reduced synovial lymphatic drainage due to m1 macrophages-induced lymphatic endothelial cell inflammation. Osteoarthritis and Cartilage. 2016;24:S31-S2
135. Kuang L, Wu JY, Su N, Qi HB, Chen HG, Zhou SR. et al. FGFR3 deficiency enhances CXCL12-dependent chemotaxis of macrophages via upregulating CXCR7 and aggravates joint destruction in mice. Annals of the Rheumatic Diseases. 2020;79:112-22
136. Schuster R, Rockel JS, Kapoor M, Hinz B. The inflammatory speech of fibroblasts. Immunological Reviews. 2021;302:126-46
137. Lazzerini PE, Capecchi PL, Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. European Heart Journal. 2017;38:1717-U42
138. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nature Reviews Rheumatology. 2016;12:580-92
139. Al-Madol MA, Shaqura M, John T, Likar R, Ebied RS, Schäfer M. et al. Comparative Expression Analyses of Pro- versus Anti-Inflammatory Mediators within Synovium of Patients with Joint Trauma, Osteoarthritis, and Rheumatoid Arthritis. Mediators of Inflammation. 2017. 2017
140. Haraoui B, Pelletier JP, Cloutier JM, Faure MP, Martel-Pelletier J. Synovial membrane histology and immunopathology in rheumatoid arthritis and osteoarthritis. In vivo effects of antirheumatic drugs. Arthritis and rheumatism. 1991;34:153-63
141. Cahill TJ, Sun X, Ravaud C, Villa Del Campo C, Klaourakis K, Lupu IE. et al. Tissue-resident macrophages regulate lymphatic vessel growth and patterning in the developing heart. Development. 2021 148
142. Zhang Y, Zhang C, Li L, Liang X, Cheng P, Li Q. et al. Lymphangiogenesis in renal fibrosis arises from macrophages via VEGF-C/VEGFR3-dependent autophagy and polarization. Cell Death Dis. 2021;12:109
143. Kenney HM, Wu CL, Loiselle AE, Xing LP, Ritchlin CT, Schwarz EM. Single-cell transcriptomics of popliteal lymphatic vessels and peripheral veins reveals altered lymphatic muscle and immune cell populations in the TNF-Tg arthritis model. Arthritis Research & Therapy. 2022 24
144. Smolen JS, Landewe RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3-18
145. Benucci M, Saviola G, Baiardi P, Manfredi M, Sarzi-Puttini P, Atzeni F. EFFICACY AND SAFETY OF LEFLUNOMIDE OR METHOTREXATE PLUS SUBCUTANEOUS TUMOUR NECROSIS FACTOR-ALPHA BLOCKING AGENTS IN RHEUMATOID ARTHRITIS. International Journal of Immunopathology and Pharmacology. 2011;24:269-74
146. Kameda H, Ueki Y, Saito K, Nagaoka S, Hidaka T, Atsumi T. et al. Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: a randomized trial. Mod Rheumatol. 2010;20:531-8
147. Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-806
148. Proulx ST, Kwok E, You Z, Beck CA, Shealy DJ, Ritchlin CT. et al. MRI and quantification of draining lymph node function in inflammatory arthritis. In: Zaidi M, editor. Skeletal Biology and Medicine, Pt B: Disease Mechanisms and Therapeutic Challenges. 2007 p. 106-23
149. Smeets TJM, Kraan MC, van Loon ME, Tak PP. Tumor necrosis factor a blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis and Rheumatism. 2003;48:2155-62
150. Wijbrandts CA, Remans PH, Klarenbeek PL, Wouters D, Weerman MAV, Smeets TJ. et al. Analysis of Apoptosis in Peripheral Blood and Synovial Tissue Very Early After Initiation of Infliximab Treatment in Rheumatoid Arthritis Patients. Arthritis and Rheumatism. 2008;58:3330-9
151. Herenius MM, Thurlings RM, Wijbrandts CA, Bennink RJ, Dohmen SE, Voermans C. et al. Monocyte migration to the synovium in rheumatoid arthritis patients treated with adalimumab. Ann Rheum Dis. 2011;70:1160-2
152. Maini RN, Feldmann M. How does infliximab work in rheumatoid arthritis? Arthritis Res. 2002;4(Suppl 2):S22-8
153. Oliver J, Plant D, Webster AP, Barton A. Genetic and genomic markers of anti-TNF treatment response in rheumatoid arthritis. Biomark Med. 2015;9:499-512
154. Aldrich MB, Velasquez FC, Kwon S, Azhdarinia A, Pinkston K, Harvey BR. et al. Lymphatic delivery of etanercept via nanotopography improves response to collagen-induced arthritis. Arthritis Res Ther. 2017;19:116
155. Kloppenburg M, Ramonda R, Bobacz K, Kwok WY, Elewaut D, Huizinga TWJ. et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77:1757-64
156. Aitken D, Laslett LL, Pan F, Haugen IK, Otahal P, Bellamy N. et al. A randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis - the HUMOR trial. Osteoarthritis Cartilage. 2018;26:880-7
157. Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12:2955-60
158. Ahmed AS, Li J, Ahmed M, Hua L, Yakovleva T, Ossipov MH. et al. Attenuation of pain and inflammation in adjuvant-induced arthritis by the proteasome inhibitor MG132. Arthritis Rheum. 2010;62:2160-9
159. Lee SW, Kim JH, Park YB, Lee SK. Bortezomib attenuates murine collagen-induced arthritis. Ann Rheum Dis. 2009;68:1761-7
160. Yannaki E, Papadopoulou A, Athanasiou E, Kaloyannidis P, Paraskeva A, Bougiouklis D. et al. The proteasome inhibitor bortezomib drastically affects inflammation and bone disease in adjuvant-induced arthritis in rats. Arthritis Rheum. 2010;62:3277-88
161. Keystone E, Emery P, Peterfy CG, Tak PP, Cohen S, Genovese MC. et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Annals of the Rheumatic Diseases. 2009;68:216-21
162. Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. The New England journal of medicine. 2004;350:2572-81
163. Lam AD, Cao E, Leong N, Gracia G, Porter CJH, Feeney OM. et al. Intra-articular injection of biologic anti-rheumatic drugs enhances local exposure to the joint-draining lymphatics. Eur J Pharm Biopharm. 2022;173:34-44
164. Lam AD, Styles IK, Senyschyn D, Cao EY, Anshabo A, Abdallah M. et al. Intra-articular Injection of a B Cell Depletion Antibody Enhances Local Exposure to the Joint-Draining Lymph Node in Mice with Collagen-Induced Arthritis. Molecular Pharmaceutics. 2023;20:2053-66
165. Xie X, van Delft MAM, Shuweihdi F, Kingsbury SR, Trouw LA, Doody GM. et al. Auto-antibodies to post-translationally modified proteins in osteoarthritis. Osteoarthritis Cartilage. 2021;29:924-33
166. Da RR, Qin Y, Baeten D, Zhang Y. B cell clonal expansion and somatic hypermutation of Ig variable heavy chain genes in the synovial membrane of patients with osteoarthritis. J Immunol. 2007;178:557-65
167. Xie X, Doody GM, Shuweihdi F, Conaghan PG, Ponchel F. B-cell capacity for expansion and differentiation into plasma cells are altered in osteoarthritis. Osteoarthritis Cartilage. 2023;31:1176-88
168. Burt KG, Scanzello CR. B cells in osteoarthritis: simply a sign or a target for therapy? Osteoarthritis Cartilage. 2023;31:1148-51
169. Zhou Q, Guo RL, Wood R, Boyce BF, Liang QQ, Wang YJ. et al. Vascular Endothelial Growth Factor C Attenuates Joint Damage in Chronic Inflammatory Arthritis by Accelerating Local Lymphatic Drainage in Mice. Arthritis and Rheumatism. 2011;63:2318-28
170. Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O. et al. A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J Biol Chem. 1998;273:6599-602
171. Hartiala P, Lahdenperä O, Vuolanto A, Saarikko A. Lymfactin, an investigational adenoviral gene therapy expressing VEGF-C, is currently studied in a double-blind, randomized, placebo-controlled, multicenter, phase 2 clinical study in patients suffering from breast cancer associated secondary lymphedema (BCAL). Cancer Research. 2020 80
172. Hartiala P, Suominen S, Suominen E, Kaartinen I, Kiiski J, Viitanen T. et al. Phase 1 Lymfactin (R) Study: Short-term Safety of Combined Adenoviral VEGF-C and Lymph Node Transfer Treatment for Upper Extremity Lymphedema. Journal of Plastic Reconstructive and Aesthetic Surgery. 2020;73:1612-21
173. Yamashita M, Niisato M, Kawasaki Y, Karaman S, Robciuc MR, Shibata Y. et al. VEGF-C/VEGFR-3 signalling in macrophages ameliorates acute lung injury. Eur Respir J. 2022 59
174. Orlandini M, Spreafico A, Bardelli M, Rocchigiani M, Salameh A, Nucciotti S. et al. Vascular endothelial growth factor-D activates VEGFR-3 expressed in osteoblasts inducing their differentiation. J Biol Chem. 2006;281:17961-7
175. McCartney-Francis N, Allen JB, Mizel DE, Albina JE, Xie QW, Nathan CF. et al. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993;178:749-54
176. Santos LL, Morand EF, Yang Y, Hutchinson P, Holdsworth SR. Suppression of adjuvant arthritis and synovial macrophage inducible nitric oxide by N-iminoethyl-L-ornithine, a nitric oxide synthase inhibitor. Inflammation. 1997;21:299-311
177. Han H, Ma Y, Wang X, Yun R, Lu S, Xia M. et al. Fang-Ji-Huang-Qi-Tang attenuates degeneration of early-stage KOA mice related to promoting joint lymphatic drainage function. Evid Based Complement Alternat Med. 2020;2020:3471681
178. Chen Y, Li J, Li Q, Wang T, Xing L, Xu H. et al. Du-Huo-Ji-Sheng-Tang Attenuates Inflammation of TNF-Tg Mice Related to Promoting Lymphatic Drainage Function. Evid Based Complement Alternat Med. 2016;2016:7067691
179. Ahmad N, Ansari MY, Haqqi TM. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J Cell Physiol. 2020;235:6366-76
180. Hellio le Graverand MP, Clemmer RS, Redifer P, Brunell RM, Hayes CW, Brandt KD. et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2013;72:187-95
181. Seymour M, Petavy F, Chiesa F, Perry H, Lukey PT, Binks M. et al. Ultrasonographic measures of synovitis in an early phase clinical trial: a double-blind, randomised, placebo and comparator controlled phase IIa trial of GW274150 (a selective inducible nitric oxide synthase inhibitor) in rheumatoid arthritis. Clin Exp Rheumatol. 2012;30:254-61
182. Kenney HM, Dieudonne G, Yee S, Maki JH, Wood RW, Schwarz EM. et al. Near-Infrared Imaging of Indocyanine Green Identifies Novel Routes of Lymphatic Drainage from Metacarpophalangeal Joints in Healthy Human Hands. Lymphatic Research and Biology. 2023
183. Jiao D, Liu Y, Hou T, Xu H, Wang X, Shi Q. et al. Notoginsenoside R1 (NG-R1) Promoted Lymphatic Drainage Function to Ameliorating Rheumatoid Arthritis in TNF-Tg Mice by Suppressing NF-kappaB Signaling Pathway. Front Pharmacol. 2021;12:730579
Author contact
![]() Corresponding authors: xieyangli841015com; linchen70com; nizhenhong1986com.
Corresponding authors: xieyangli841015com; linchen70com; nizhenhong1986com.
 Global reach, higher impact
Global reach, higher impact