13.3
Impact Factor
Theranostics 2024; 14(2):593-607. doi:10.7150/thno.85437 This issue Cite
Research Paper
Combined BET and MEK Inhibition synergistically suppresses melanoma by targeting YAP1
1. Department of Dermatology, Hunan Engineering Research Center of Skin Health and Disease, Hunan Key Laboratory of Skin Cancer and Psoriasis, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China.
2. Department of Dermatology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
3. Department of Pathology, Yale School of Medicine, New Haven, CT 06520, USA.
4. Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China.
5. The first clinical college of Chongqing Medical University, Chongqing, China.
6. Health Management of Xiangya Hospital, Central South University, Changsha, Hunan 410008, China.
7. Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, Hunan 410013, China.
8. Clinical Research Center (CRC), Clinical Pathology Center (CPC), Cancer Early Detection and Treatment Center (CEDTC), Chongqing University Three Gorges Hospital, Chongqing University, Wanzhou, Chongqing, China.
9. Translational Medicine Research Center (TMRC), School of Medicine Chongqing University, Shapingba, Chongqing, China.
*Rui Hu, Huihui Hou and Yao Li contributed equally.
Abstract
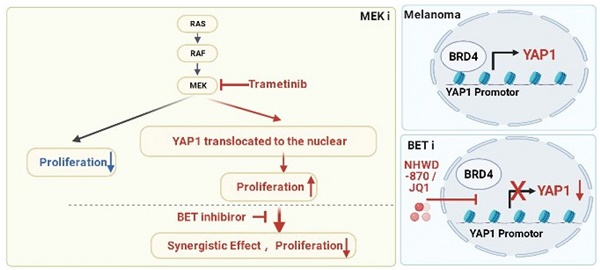
Rationale: The response rate to the MEK inhibitor trametinib in BRAF-mutated melanoma patients is less than 30%, and drug resistance develops rapidly, but the mechanism is still unclear. Yes1-associated transcriptional regulator (YAP1) is highly expressed in melanoma and may be related to MEK inhibitor resistance. The purpose of this study was to investigate the mechanism of YAP1 in MEK inhibitor resistance in melanoma and to screen YAP1 inhibitors to further determine whether YAP1 inhibition reverses MEK inhibitor resistance.
Methods: On the one hand, we analyzed paired melanoma and adjacent tissue samples using RNA-seq and found that the Hippo-YAP1 signaling pathway was the top upregulated pathway. On the other hand, we evaluated the transcriptomes of melanoma samples from patients before and after trametinib treatment and investigated the correlation between YAP1 expression and trametinib resistance. Then, we screened for inhibitors that repress YAP1 expression and investigated the mechanisms. Finally, we investigated the antitumor effect of YAP1 inhibition combined with MEK inhibition both in vitro and in vivo.
Results: We found that YAP1 expression levels upon trametinib treatment in melanoma patients were correlated with resistance to trametinib. YAP1 was translocated into the nucleus after trametinib treatment in melanoma cells, which could render resistance to MEK inhibition. Thus, we screened for inhibitors that repress YAP1 expression and identified multiple bromodomain and extra-terminal (BET) inhibitors, including NHWD-870, as hits. BET inhibition repressed YAP1 expression by decreasing BRD4 binding to the YAP1 promoter. Consistently, YAP1 overexpression was sufficient to reverse the proliferation defect caused by BRD4 depletion. In addition, the BET inhibitor NHWD-870 acted synergistically with trametinib to suppress melanoma growth in vitro and in vivo.
Conclusions: We identified a new vulnerability for MEK inhibitor-resistant melanomas, which activated Hippo pathway due to elevated YAP1 activity. Inhibition of BRD4 using BET inhibitors suppressed YAP1 expression and led to blunted melanoma growth when combined with treatment with the MEK inhibitor trametinib.
Keywords: Melanoma, MEK inhibitor, BET inhibitor, YAP1, BRD4
 Global reach, higher impact
Global reach, higher impact