13.3
Impact Factor
Theranostics 2024; 14(3):1241-1259. doi:10.7150/thno.91180 This issue Cite
Research Paper
Single-cell RNA sequencing reveals S100a9hi macrophages promote the transition from acute inflammation to fibrotic remodeling after myocardial ischemia‒reperfusion
1. Department of Cardiology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China.
2. Department of Pediatrics, The First Affiliated Hospital of Anhui Medical University, Hefei, China.
3. Department of Cardiology, The Second Affiliated Hospital of Anhui Medical University, Hefei, China.
4. School of Cardiovascular and Metabolic Health, University of Glasgow, Glasgow, United Kingdom.
5. The First Clinical Medical school of Anhui Medical university, Hefei, China.
6. Department of Maternal, Child and Adolescent Health, School of Public Health, Anhui Medical University, No 81 Meishan Road, Hefei 230032, Anhui, China.
7. MOE Key Laboratory of Population Health Across Life Cycle, Hefei, China.
Abstract
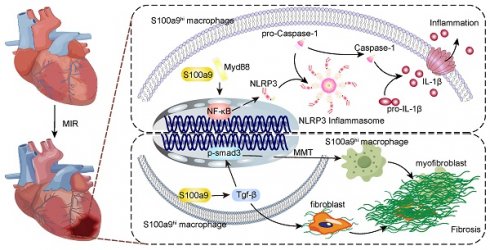
Rationale: The transition from acute inflammation to fibrosis following myocardial ischemia‒reperfusion (MIR) significantly affects prognosis. Macrophages play a pivotal role in inflammatory damage and repair after MIR. However, the heterogeneity and transformation mechanisms of macrophages during this transition are not well understood.
Methods: In this study, we used single-cell RNA sequencing (scRNA-seq) and mass cytometry to examine murine monocyte-derived macrophages after MIR to investigate macrophage subtypes and their roles in the MIR process. S100a9-/- mice were used to establish MIR model to clarify the mechanism of alleviating inflammation and fibrosis after MIR. Reinfusion of bone marrow-derived macrophages (BMDMs) after macrophage depletion (MD) in mice subjected to MIR were performed to further examine the role of S100a9hi macrophages in MIR.
Results: We identified a unique subtype of S100a9hi macrophages that originate from monocytes and are involved in acute inflammation and fibrosis. These S100a9hi macrophages infiltrate the heart as early as 2 h post-reperfusion and activate the Myd88/NFκB/NLRP3 signaling pathway, amplifying inflammatory responses. As the tissue environment shifts from proinflammatory to reparative, S100a9 activates transforming growth factor-β (Tgf-β)/p-smad3 signaling. This activation not only induces the transformation of myocardial fibroblasts to myofibroblasts but also promotes fibrosis via the macrophage-to-myofibroblast transition (MMT). Targeting S100a9 with a specific inhibitor could effectively mitigate acute inflammatory damage and halt the progression of fibrosis, including MMT.
Conclusion: S100a9hi macrophages are a promising therapeutic target for managing the transition from inflammation to fibrosis after MIR.
Keywords: myocardial ischemia-reperfusion injury, inflammation, cardiac fibrosis, S100a9hi macrophages, macrophage-to-myofibroblast transition
 Global reach, higher impact
Global reach, higher impact