13.3
Impact Factor
Theranostics 2024; 14(4):1450-1463. doi:10.7150/thno.87201 This issue Cite
Research Paper
Re-analysis of single-cell transcriptomics reveals a critical role of macrophage-like smooth muscle cells in advanced atherosclerotic plaque
1. Department of Cardiology, Daping Hospital, The Third Military Medical University (Army Medical University), P.R. China.
2. Department of Cardiology, the Sixth Medical Centre, Chinese PLA General Hospital, Beijing, P.R. China.
3. Key Laboratory of Geriatric Cardiovascular and Cerebrovascular Disease Research, Ministry of Education of China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, P. R. China.
4. College of Pulmonary and Critical Care Medicine, The 8th Medical Centre, Chinese PLA General Hospital, Beijing, P.R. China.
5. Department of Cardiac Surgery, Daping Hospital, The Third Military Medical University (Army Medical University), P.R. China.
6. Department of Cardiology, No. 926 Hospital, Joint Logistics Support Force of PLA, P.R. China.
* Co-first authors.
Abstract
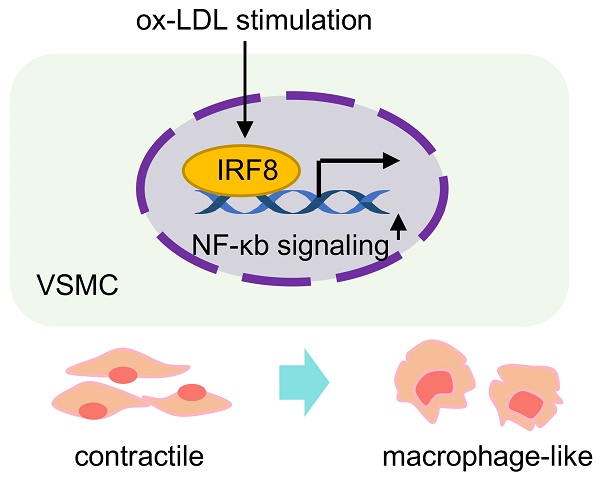
Aims: Smooth muscle cell (SMC) remodeling poses a critical feature in the development and progression of atherosclerosis. Although fate mapping and in silicon approaches have expanded SMC phenotypes in atherosclerosis, it still remains elusive about the contributions of individual SMC phenotypes and molecular dynamics to advanced atherosclerotic plaque.
Methods: Using single-cell transcriptome, we investigated cellular compositions of human carotid plaque laden with atherosclerotic core, followed by in vivo experiments utilizing SMC-lineage tracing technology, bulk RNA sequencing (RNA-seq) and both in vivo and in vitro validation of the underlying molecular mechanism.
Results: 5 functionally distinct SMC subtypes were uncovered based on transcriptional features (described as contractile, fibroblast-like, osteogenic, synthetic and macrophage-like) within the niche. A proinflammatory, macrophage-like SMC subtype displaying an intermediary phenotype between SMC and macrophage, exhibits prominent potential in destabilizing plaque. At the molecular level, we explored cluster-specific master regulons by algorithm, and identified interferon regulatory factor-8 (IRF8) as a potential stimulator of SMC-to-macrophage transdifferentiation via activating nuclear factor-κB (NF-κB) signaling.
Conclusions: Our study illustrates a comprehensive cell atlas and molecular landscape of advanced atherosclerotic lesion, which might renovate current understanding of SMC biology in atherosclerosis.
Keywords: atherosclerosis, single-cell transcriptome, smooth muscle cell, macrophage, phenotypic transition
 Global reach, higher impact
Global reach, higher impact