13.3
Impact Factor
Theranostics 2024; 14(4):1464-1499. doi:10.7150/thno.92526 This issue Cite
Review
Degraders in epigenetic therapy: PROTACs and beyond
1. Department of Oncology, the Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou University, Zhengzhou, China.
2. Department of Oncology, the Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Henan Engineering Research Center of Precision Therapy of Gastrointestinal Cancer & Zhengzhou Key Laboratory for Precision Therapy of Gastrointestinal Cancer, Zhengzhou, China.
3. Key Laboratory of Advanced Drug Preparation Technologies, Ministry of Education, China; State Key Laboratory of Esophageal Cancer Prevention & Treatment; Key Laboratory of Henan Province for Drug Quality and Evaluation; Institute of Drug Discovery and Development; School of Pharmaceutical Sciences, Zhengzhou University, Zhengzhou, China.
4. XNA platform, School of Pharmaceutical Sciences, Zhengzhou University, Zhengzhou, China.
5. The School of Chinese Medicine, The University of Hong Kong, Pokfulam, Hong Kong, China.
6. Rega Institute for Medical Research, Medicinal Chemistry, KU Leuven, Herestraat 49-Box 1041, 3000 Leuven, Belgium.
Abstract
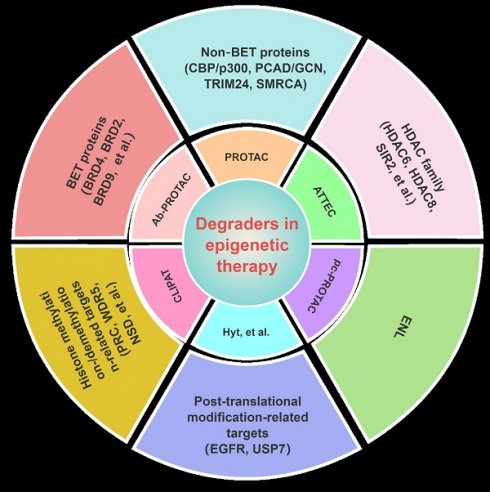
Epigenetics refers to the reversible process through which changes in gene expression occur without changing the nucleotide sequence of DNA. The process is currently gaining prominence as a pivotal objective in the treatment of cancers and other ailments. Numerous drugs that target epigenetic mechanisms have obtained approval from the Food and Drug Administration (FDA) for the therapeutic intervention of diverse diseases; many have drawbacks, such as limited applicability, toxicity, and resistance. Since the discovery of the first proteolysis-targeting chimeras (PROTACs) in 2001, studies on targeted protein degradation (TPD)—encompassing PROTACs, molecular glue (MG), hydrophobic tagging (HyT), degradation TAG (dTAG), Trim-Away, a specific and non-genetic inhibitor of apoptosis protein (IAP)-dependent protein eraser (SNIPER), antibody-PROTACs (Ab-PROTACs), and other lysosome-based strategies—have achieved remarkable progress. In this review, we comprehensively highlight the small-molecule degraders beyond PROTACs that could achieve the degradation of epigenetic proteins (including bromodomain-containing protein-related targets, histone acetylation/deacetylation-related targets, histone methylation/demethylation related targets, and other epigenetic targets) via proteasomal or lysosomal pathways. The present difficulties and forthcoming prospects in this domain are also deliberated upon, which may be valuable for medicinal chemists when developing more potent, selective, and drug-like epigenetic drugs for clinical applications.
Keywords: PROTACs, epigenetics, anti-cancer, drug design, protein degrader
 Global reach, higher impact
Global reach, higher impact