13.3
Impact Factor
Theranostics 2024; 14(6):2396-2426. doi:10.7150/thno.95469 This issue Cite
Review
From molecules to medicine: thiol selective bioconjugation in synthesis of diagnostic and therapeutic radiopharmaceuticals
1. Affiliation Division of Applied RI, Korea Institute of Radiological & Medical Sciences (KIRAMS), 75 Nowon-ro, Nowon-gu, Seoul 01812, Republic of Korea.
2. University of Science and Technology (UST), 217, Gajeong-ro, Yuseong-gu, Daejeon 34113, Republic of Korea.
3. Department of Nuclear Engineering, Pakistan Institute of Engineering and Applied Sciences (PIEAS), P. O. Nilore, Islamabad 45650, Pakistan.
* These authors contributed equally to this work.
Abstract
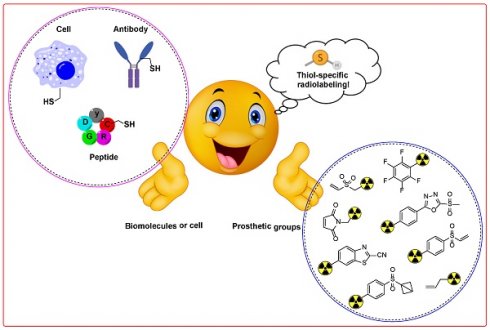
Radiolabeling of biomolecules and cells with radiolabeled prosthetic groups has significant implications for nuclear medicine, imaging, and radiotherapy. Achieving site-specific and controlled incorporation of radiolabeled prostheses under mild reaction conditions is crucial for minimizing the impact on the bioactivity of the radiolabeled compounds. The targeting of natural and abundant amino acids during radiolabeling of biomolecules often results in nonspecific and uncontrolled modifications. Cysteine is distinguished by its low natural abundance and unique nucleophilicity. It is therefore an optimal target for site-selective and site-specific radiolabeling of biomolecules under controlled parameters. This review extensively discusses thiol-specific radiolabeled prosthetic groups and provides a critical analysis and comprehensive study of the synthesis of these groups, their in vitro and in vivo stability profiles, reaction kinetics, stability of resulting adducts, and overall impact on the targeting ability of radiolabeled biomolecules. The insights presented here aim to facilitate the development of highly efficient radiopharmaceuticals, initially in preclinical settings and ultimately in clinical applications.
Keywords: Thiol-specific prosthetic groups, Radiopharmaceuticals, Imaging and radiotherapy, Maleimide
 Global reach, higher impact
Global reach, higher impact