13.3
Impact Factor
Theranostics 2024; 14(6):2544-2559. doi:10.7150/thno.93269 This issue Cite
Research Paper
Mechanosensitive protein polycystin-1 promotes periosteal stem/progenitor cells osteochondral differentiation in fracture healing
1. Department of Endocrinology, Endocrinology Research Center, Xiangya Hospital of Central South University, Changsha, Hunan, 410008, China.
2. Department of Medicine, University of Tennessee Health Science Center, Memphis, TN, 38163, USA.
3. Department of Orthopaedics, The Second Affiliated Hospital of Fuyang Normal University, Fuyang, Anhui, 236000, China.
4. Department of Orthopaedics, The First Affiliated Hospital of Shihezi University, Shihezi 832061, China.
5. Key Laboratory of Aging-related Bone and Joint Diseases Prevention and Treatment, Ministry of Education, Xiangya Hospital, Central South University, Changsha, Hunan, 410008, China.
6. National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, 410008, China.
7. Laboratory Animal Center, Xiangya Hospital, Central South University, Changsha, Hunan, 410008, China.
# All authors contributed equally
Abstract
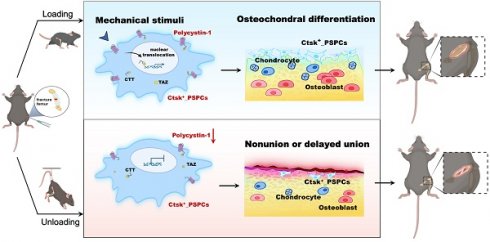
Background: Mechanical forces are indispensable for bone healing, disruption of which is recognized as a contributing cause to nonunion or delayed union. However, the underlying mechanism of mechanical regulation of fracture healing is elusive.
Methods: We used the lineage-tracing mouse model, conditional knockout depletion mouse model, hindlimb unloading model and single-cell RNA sequencing to analyze the crucial roles of mechanosensitive protein polycystin-1 (PC1, Pkd1) promotes periosteal stem/progenitor cells (PSPCs) osteochondral differentiation in fracture healing.
Results: Our results showed that cathepsin (Ctsk)-positive PSPCs are fracture-responsive and mechanosensitive and can differentiate into osteoblasts and chondrocytes during fracture repair. We found that polycystin-1 declines markedly in PSPCs with mechanical unloading while increasing in response to mechanical stimulus. Mice with conditional depletion of Pkd1 in Ctsk+ PSPCs show impaired osteochondrogenesis, reduced cortical bone formation, delayed fracture healing, and diminished responsiveness to mechanical unloading. Mechanistically, PC1 facilitates nuclear translocation of transcriptional coactivator TAZ via PC1 C-terminal tail cleavage, enhancing osteochondral differentiation potential of PSPCs. Pharmacological intervention of the PC1-TAZ axis and promotion of TAZ nuclear translocation using Zinc01442821 enhances fracture healing and alleviates delayed union or nonunion induced by mechanical unloading.
Conclusion: Our study reveals that Ctsk+ PSPCs within the callus can sense mechanical forces through the PC1-TAZ axis, targeting which represents great therapeutic potential for delayed fracture union or nonunion.
Keywords: Polycystin-1, Periosteal Stem/Progenitor Cells, Fracture healing, Mechanical stress
 Global reach, higher impact
Global reach, higher impact