13.3
Impact Factor
Theranostics 2024; 14(8):3178-3192. doi:10.7150/thno.96743 This issue Cite
Review
Navigating new horizons: Prospects of NET-targeted radiopharmaceuticals in precision medicine
1. Department of Nuclear Medicine and Comprehensive Heart Failure Center, University Hospital of Würzburg, Würzburg, Germany.
2. Faculty of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan.
3. Nuclear Medicine, Faculty of Medicine, University of Augsburg, Augsburg, Germany.
4. DZHK (German Centre for Cardiovascular Research), Partner Site Frankfurt Rhine-Main, Frankfurt, Germany.
5. Goethe University Frankfurt, Department of Nuclear Medicine, Clinic for Radiology and Nuclear Medicine, Frankfurt, Germany.
6. German Cancer Consortium (DKTK), Partner Site Frankfurt/Mainz and German Cancer Research Center (DKFZ), Heidelberg, Germany.
7. The Russell H Morgan Department of Radiology and Radiological Sciences, Division of Nuclear Medicine and Molecular Imaging, Johns Hopkins School of Medicine, Baltimore, MD, United States.
Received 2024-3-28; Accepted 2024-4-23; Published 2024-5-19
Abstract
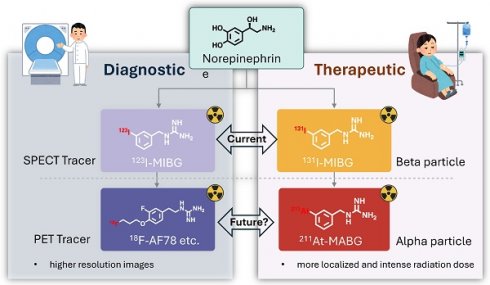
In the evolving landscape of precision medicine, NET-targeted radiopharmaceuticals are emerging as pivotal tools for the diagnosis and treatment of a range of conditions, from heart failure and neurodegenerative disorders to neuroendocrine cancers. This review evaluates the advancements offered by 18F-labeled PET tracers and 211At alpha-particle therapy, juxtaposed with current 123I-MIBG SPECT and 131I-MIBG therapies. The enhanced spatial resolution and capability for quantitative analysis render 18F-labeled PET tracers potential candidates for improved detection and management of diseases. Alpha-particle therapy with 211At may offer increased specificity and tumoricidal efficacy, pointing towards a shift in therapeutic protocols. While preliminary data is promising, these innovative approaches require thorough validation against current modalities. Ongoing clinical trials are pivotal to confirm the expected clinical benefits and to address safety concerns. This review underscores the need for rigorous research to verify the clinical utility of NET-targeted radiopharmaceuticals, which may redefine precision medicine paradigms and significantly impact patient care.
Keywords: Norepinephrine, PET, Astatine, MIBG, Alpha-particle, neuroendocrine tumor
Introduction
NET and its role in various diseases. The Norepinephrine Transporter (NET) is a critical protein that regulates the concentration of norepinephrine in both the central and peripheral nervous systems by facilitating the reuptake of this neurotransmitter from the synaptic cleft. The functional integrity of NET is essential for the maintenance of normal catecholaminergic neurotransmission, influencing a host of physiological processes ranging from mood regulation [1, 2] to the control of blood pressure [1]. Disruptions in NET function have been implicated in a diverse array of diseases. In cardiovascular medicine, NET abnormalities can lead to dysregulated sympathetic activity, contributing to the pathogenesis of conditions such as heart failure [3]. In the realm of psychiatric and neurodegenerative disorders, variations in NET expression and function are associated with conditions such as depression [1], attention-deficit/hyperactivity disorder [4], and the complex pathophysiology of Parkinson's syndrome [5], where altered norepinephrine signaling affects both motor and non-motor symptoms. In the case of Parkinson's disease (PD), NET's role extends beyond the central nervous system to implicate peripheral sympathetic denervation [6]. Accumulation of alpha-synuclein, a hallmark of Parkinson's pathology [7], in the peripheral autonomic nervous system may lead to impairment of NET function [8]. This phenomenon not only contributes to the diverse autonomic symptoms experienced by patients but also serves as a potential marker for early diagnosis [9]. Furthermore, NET is a key player in the growth and progression of certain tumors [10], including neuroblastoma [11], pheochromocytoma [12], and paraganglioma [13]. The expression of NET in these malignancies provides a unique target for both diagnostic imaging and radiotherapeutic interventions [14], allowing for the precise localization and treatment of these tumors. As we advance our understanding of NET's roles in these varied conditions, the development of targeted radiopharmaceuticals presents an opportunity to leverage this transporter for improved diagnostic accuracy and the delivery of targeted therapies, ultimately aiming to enhance patient outcomes in a multitude of clinical settings.
Introduction to radiopharmaceuticals and their diagnostic/therapeutic uses. Radiopharmaceuticals represent a unique intersection of pharmacy and nuclear medicine, serving as pivotal tools for both diagnosis and therapy in a myriad of diseases [15]. These specialized compounds are composed of a radioactive isotope, known as a radionuclide, which is bound to a pharmaceutical agent. The agent acts as a carrier, delivering the radionuclide to specific cells, tissues, or organs, while the emitted radiation allows for imaging or therapeutic action. In diagnostics, radiopharmaceuticals are utilized primarily for their ability to emit gamma rays, which can be detected by imaging techniques such as Single Photon Emission Computed Tomography (SPECT) [16]. These modalities offer a non-invasive means to visualize biological processes in vivo. For example, 123I-labeled meta-iodobenzylguanidine (MIBG) is specifically taken up by neuroendocrine cells via NET, enabling the imaging of diseases like pheochromocytoma and neuroblastoma [17], as well as providing cardiac sympathetic innervation insights in heart failure patients [18] (Figure 1). Therapeutically, radiopharmaceuticals exploit the cytotoxic effects of radiation, targeting and damaging diseased cells while sparing healthy ones to a certain extent. Beta-emitters like 131I, used in MIBG therapy, have been employed to deliver targeted radiotherapy to neuroendocrine tumor cells, which take up the MIBG compound, allowing the attached 131I to irradiate and kill the tumor from within [19, 20]. Over the past decade, the field of radiopharmaceuticals is progressively advancing towards 'theranostics' — a therapeutic strategy that combines specific targeted therapy based on specific targeted diagnostic tests, i.e. it combines therapeutic and diagnostic capabilities into a single agent or closely related agents. This approach ensures that the patients who are most likely to benefit from a particular treatment are identified using a corresponding diagnostic radiopharmaceutical [21].
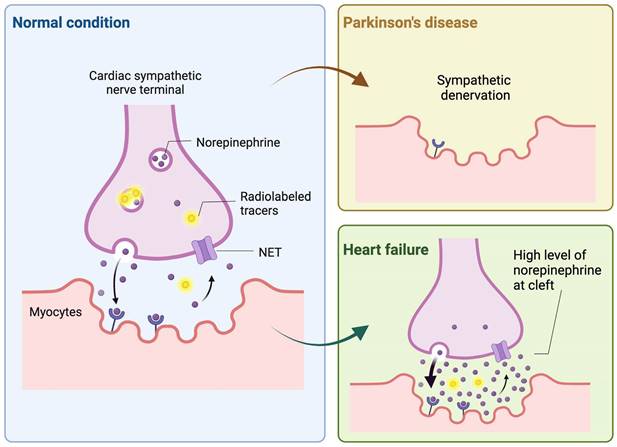
Illustration of the dynamics of norepinephrine and analogous radioactive tracers at cardiac sympathetic nerve endings. It depicts how Parkinson's disease and heart failure affect cardiac tracer uptake. In Parkinson's disease, the accumulation of alpha-synuclein at the terminals of cardiac sympathetic nerves leads to their denervation, resulting in reduced tracer uptake by the heart. Conversely, in heart failure, there is an upregulation of sympathetic nervous activity, which elevates norepinephrine levels in the synaptic cleft, thereby inhibiting tracer uptake due to competitive mechanisms. Created with BioRender.com.
Current challenges in NET targeting precision medicine. Precision medicine targeting NET faces several challenges that underscore the need for advancements in both diagnostic and therapeutic radiopharmaceuticals. One significant hurdle is the limited resolution of 123I-labeled MIBG imaging with SPECT. While SPECT is a mainstay of clinical nuclear medicine in the diagnosis of diseases like neuroblastoma [22], its dependency on lead collimators and the inherent physical limitations of gamma camera design result in less-than-optimal spatial resolution. This is particularly problematic in pediatric patients with neuroblastoma, where the small body size necessitates imaging that can discern fine anatomical details to accurately localize disease and guide treatment. Furthermore, the current gold-standard therapeutic radiopharmaceutical, 131I-labeled MIBG, demonstrates limited effectiveness in some clinical settings exemplified by a 39% objective response rate in neuroblastoma patients [23]. Despite being one of the most effective treatments for neuroblastoma, mortality rates remain concerning, with a significant proportion of patients not achieving long-term survival post-treatment [24]. This reality brings to the forefront an urgent need for the next generation of radiopharmaceuticals that can deliver more precise imaging and potent therapeutic effects. These challenges not only call for improved spatial resolution in diagnostic imaging to facilitate early and accurate detection, especially in smaller lesions, but also for the development of more effective therapeutic agents that can translate into improved survival rates. The next generation of precision medicine aims to address these gaps by allowing for tailored diagnosis and treatment based on individual patient characteristics, harnessing the power of advanced radiopharmaceuticals to enhance patient care and outcomes.
The Evolution of Diagnostic Radiopharmaceuticals
History of SPECT and PET imaging. The evolution of SPECT and Positron Emission Tomography (PET) imaging has dramatically advanced nuclear medicine, each offering distinct capabilities based on their underlying technology. SPECT imaging, which matured as a clinical tool in the 1970s, relies on gamma cameras equipped with lead collimators to detect gamma rays emitted from radiopharmaceuticals [25]. These collimators, necessary to direct the photons onto the detector, are a fundamental component of SPECT technology. However, they inherently limit the system's spatial resolution due to the trade-off between sensitivity and resolution [26]. In practice, this results in SPECT practical resolutions often being larger than 10mm, which can be insufficient for detecting small lesions, a limitation especially critical in pediatric oncology.
PET technology, which became widely available in the 1980s [27], marked a significant improvement over SPECT in terms of both sensitivity and resolution [28]. In contrast, PET's ability to detect coincident photon pairs from positron annihilation events eliminates the need for lead collimators. This increases PET's sensitivity and allows for more precise spatial resolution, typically around 4-5mm [29]. The high sensitivity of PET also enables dynamic imaging [30], providing real-time tracking of radiotracer kinetics for comprehensive functional assessments [31]. The introduction of whole-body PET imaging has been revolutionary, especially in oncology, where it has become a cornerstone for accurate tumor staging and therapy monitoring [32]. PET's intrinsic technical advantages have made it a preferred modality in many clinical scenarios, leading to an increase in its adoption over SPECT. This shift is driven by PET's comprehensive diagnostic capabilities, which support a more personalized approach to patient care. The limitations of SPECT, including its reliance on lead collimators and lower spatial resolution, have prompted ongoing efforts to enhance its performance. Meanwhile, PET continues to advance with the development of new class of radiotracers and the integration of multimodal imaging systems such as PET/MRI [33]. The digitalization of detectors [34], improvement in scintillator materials [35], and refinements in image reconstruction algorithms [36] have all contributed to the superior performance of PET. Moreover, the introduction of time-of-flight (TOF) has opened new avenues for more detailed and informative imaging [37].
123I-MIBG in Neuroendocrine Imaging. 123I-MIBG, currently the primary clinically-used NET-targeting radiotracer, utilizes SPECT technology to diagnose cardiac diseases and provide independent risk stratification through adrenergic imaging [38]. As a pioneer in the benzylguanidine series, 123I-MIBG's polar structure allows for long-term storage in vesicles post-uptake, and its stability against monoamine oxidases breakdown exemplifies its well-designed nature [39]. The usefulness of 123I-MIBG has been substantiated by substantial clinical trial data, notably from such as ADMIRE-HF study [40]. Its application extends to risk stratification for the potential implantation of cardioverter defibrillators (ICD) in heart failure patients, with the ongoing ADMIRE-ICD trial set to further elucidate its impact on patient management [41]. For endocrine-related malignancies with high NET expression, 131I-MIBG serves as a therapeutic agent against in certain adult and pediatric patients with pheochromocytoma, paraganglioma and neuroblastoma [19, 42, 43], taking advantage of the cells' uptake and storage of monoamines. Beyond above applications, the utility of 123I-MIBG and related NET radiotracers is expanding into other pathophysiological assessments involving peripheral sympathetic activity, such as Parkinson Disease (PD) [44] (Figure 1). The presence of alpha-synuclein pathology in the peripheral nerve system is a characteristic feature of PD [45, 46], and utilizing these radiotracers aids in distinguishing PD from other neurodegenerative diseases. Furthermore, this diagnostic approach highlights the systemic nature of PD, extending its effects beyond the central nervous system [47]. Recent studies suggest that 123I-MIBG scintigraphy, often used alongside 123I-ioflupane SPECT, provides substantial diagnostic accuracy in differentiating PD from atypical parkinsonism and may contribute to the identification of early-stage PD [48]. Additionally, 123I-MIBG is proving valuable in distinguishing Alzheimer's disease from Lewy Body dementias [49]. Dynamic SPECT imaging using 123I-MIBG has also been applied to refine the quantification of cardiac nerve activity [50]. The process involved multiple scans and blood analyses for radiometabolites and a population-based correction method was then applied to the dataset, which significantly improved the measurement of the drug's distribution in heart tissue compared to previous methods. This advancement promises to enhance the precision of cardiac nerve function evaluations in medical practice.
NET targeting PET tracers. Despite its three-decade history, SPECT radiotracers like 123I-MIBG face limitations, such as inferior spatial resolution relative to PET, suboptimal sensitivity for small lesions or tumors, complex scanning protocols, and delayed imaging results. These drawbacks have spurred a shift in research focus towards developing PET tracers, which promise enhanced resolution and expedited diagnostic processes. 18F-labeled tracers, such as 18F-fluorodeoxyglucose (FDG), have revolutionized oncological imaging by enabling detailed whole-body scans that are critical for staging and assessing treatment response [51]. The development of 18F-labeled tracers extends beyond FDG to include specific molecules targeting various biological pathways [52]. These tracers are designed to bind to particular proteins or receptors, providing insights into a range of physiological and pathological processes. For instance, 18F-labeled compounds engineered to target NET offer enhanced precision in visualizing neurodegenerative diseases and sympathetically mediated cardiac conditions compared to the existing 123I-MIBG. The landscape of PET imaging has greatly evolved through the ongoing quest to enhance tracer specificity and NET binding affinity, key factors for precise diagnostic imaging [39]. The narrative began with the advent of 11C-labeled tracers such as 11C-hydroxyephedrine (HED), an innovative tracer for delineating cardiac sympathetic nerve function [53-56]. Although 11C-HED demonstrates high affinity for norepinephrine transport system and has been instrumental in cardiac [57] and oncological imaging [58], its clinical utility is curtailed by the short half-life of 11C (20.4 min), which requires the proximity of a cyclotron and constrains the imaging time window. Despite the achievements with 11C-labeled tracers, the shift toward 18F-labeling over the past two decades is driven by several advantages: cost-effectiveness due to centralized distribution, the feasibility of delayed and prolonged scans owing to a longer half-life (110 min), enhanced avaliablity of PET imaging, and the design flexibility that accommodates improved metabolic stability. These benefits are steering the development of new NET tracers towards 18F to leverage the full potential of PET imaging technology. With the unveiling of 18F-6F-dopamine [59, 60], 18F-LMI1195 [61-66] and 18F-meta-fluorobenzylguanidine (MFBG) [67-69], the field witnessed enhanced tracers offering superior cardiac imaging contrast, thereby improving the delineation of sympathetic nervous activity within the heart [69, 70]. This was a significant step forward in the cardiac applications of PET imaging, building on the foundations laid by its predecessors. The introduction of 18F-AF78 [71-73] and 18F-PHPG/MHPG [74, 75] is a testament to the rapid progress in this domain, delivering tracers with pronounced NET affinity [72], potentially reshaping the diagnostic landscape for a variety of NET-related disorders. The high binding affinity of 18F-AF78 (Figure 2) [73] could lead to superior sensitivity and specificity in disease detection, especially in conditions where NET dysfunction is implicated, including certain neurodegenerative pathologies and neuroendocrine tumors. The evolution of these PET tracers showcases a decisive march toward imaging modalities that are not only more efficient but also molecularly astute, aligning with the overarching objectives of precision medicine. As we anticipate the future, the progression of NET-targeted PET tracers carries the potential to not only refine diagnostic accuracy (Figure 3) [67, 68] but also guide therapeutic interventions tailored to the unique molecular profiles of individual patients, truly embodying the essence of personalized care. An overview of the radiophamaceuticals targeting NET is shown in Table 1 and Figure 4.
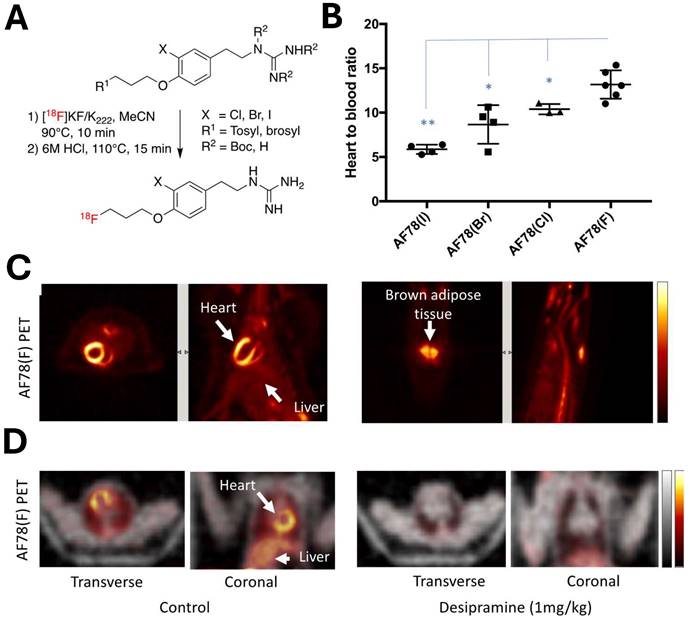
18F-AF78 demonstrated high NET affinity and advantageous in vivo radiotracer kinetics across various species. (A) A protocol for the one-pot, two-step radiolabeling of NET-targeting tracer 18F-AF78. (B) In vivo studies of radiotracer distribution in healthy rats confirm that 18F-AF78(F) showing highest contrast cardiac uptake among meta-substituents on the benzene ring. (C) PET scans reveal clear imaging of cardiac tissue (left) and brown adipose tissue uptake (right) in healthy rats using 18F-AF78(F). (D) Comparative PET scans of non-human primates (NHPs), with and without the NET-selective inhibitor desipramine, demonstrate the specificity of 18F-AF78 in cardiac imaging. Adapted with permission from [73], Copyright 2023, MDPI.
Summary of NET targeting radiophamacuticals
| Name | Development stage | Major characteristics | References |
|---|---|---|---|
| Diagnostic | |||
| 123I-MIBG | FDA approved for pheochromocytoma and neuroblastoma, Phase 3 completed (NCT02043522: heart failure) | SPECT tracer | [38, 41, 44] |
| 11C-HED | Used in clinical trials, NCT00756366: heart failure, NCT 01400334: arrhythmic events) | PET tracer with short half-life | [55, 56] |
| 18F-6F-dopamine | Investigational New Drug application Nr.138638: neuroblastoma | First 18F PET tracer for NET | [59, 60] |
| 18F-MFBG | Phase 3 ongoing (NCT04724369: neuroblastoma) Clinical trial (NCT06149195: heart failure) | Structure comparable to MIBG | [67, 69] |
| 18F-LMI1195 | Phase 1 completed (NCT00891241, heart failure) | High radiochemical yield | [70] |
| 18F-4F-PHPG | Early Phase 1 completed (NCT02385877: neuroendocrine tumors) | Slow NET kinetics and fast liver washout | [74] |
| 18F-AF78 | Preclinical stage (non-human primates) | High NET affinity in cellular assay | [71-73] |
| 124I-MIBG | Phase 1 / 2 completed (NCT02043899: neuroblastoma) | PET tracer identical chemical property with 123I-MIBG | [77, 78] |
| Therapeutic | |||
| 131I-MIBG | FDA approved: pheochromocytoma and paraganglioma Phase 2 ongoing (NCT03561259: neuroblastoma) | Beta radionuclide therapy | [19] |
| 211At-MABG | Phase 1 ongoing (JRCT2021220012: pheochromocytoma, paraganglioma) | Alpha radionuclide therapy | [94, 101, 102] |
124I-labeled MIBG offers a promising PET/CT imaging alternative to the current SPECT/CT radiotracer 123I-MIBG, despite some inherent challenges. Research by Seo et al. has shown the potential of 124I-MIBG PET/CT in streamlining pretherapy dosimetry for 131I-MIBG therapy using just two time-point scans, providing an accurate estimation of tumor and organ doses [76]. Aboian et al. further demonstrated 124I-MIBG PET/CT's superior lesion detection in children with neuroblastoma, identifying significantly more lesions than 123I-MIBG SPECT/CT [77]. Furthermore, Maric et al. have successfully utilized dosimetry derived from 124I-MIBG PET scans to guide dosing in high-activity 131I-MIBG therapy, resulting in durable disease control with manageable side effects [78]. These findings underline the diagnostic and therapeutic advantages of 124I-MIBG PET/CT, suggesting its role in enhancing the precision of metastatic neuroblastoma treatment. However, the broader clinical use of 124I is limited due to its high production costs and technical constraints [79]. The isotope is produced via the 124Te(p,n)124I nuclear reaction in a cyclotron and has a half-life of 4.2 days, favorable for long-term biological studies. Yet, the high positron energy of 124I, at 2.14 MeV, leads to a wide positron range, potentially reducing PET imaging resolution due to the long travel distance of positrons before annihilation [79, 80]. Nevertheless, the benefits of 124I, such as its longer half-life and robust chemistry that allows for versatile compound labeling, continue to drive interest in its use for medical imaging and therapeutic applications.
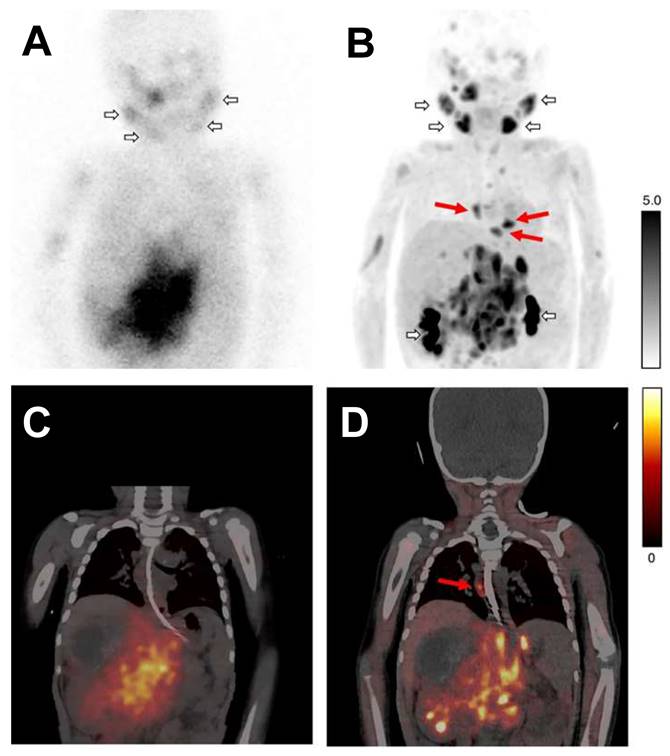
Planar scintigraphy with 123I-MIBG (A) and the corresponding fused SPECT/CT (B), along with 18F-MFBG PET maximum intensity projection (MIP) (C) and fused PET/CT (D), were conducted on a patient with neuroblastoma. Short arrows in images (A and C) illustrate normal physiological uptake in the salivary glands and the renal pelvicalyceal system. Areas of additional uptake represent tumor lesions. Long red arrows identify extra mediastinal lymph node metastases that are captured by 18F-MFBG PET, showcasing its superior sensitivity, but these were not on 123I-MIBG scans. Adapted with permission from [67] Copyright 2022, Springer Nature.
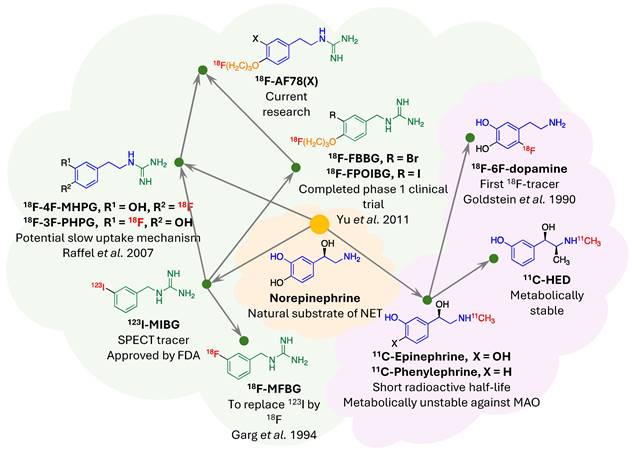
Evolutionary phylogeny of Norepinephrine Transporter (NET) radiotracers. This phylogenetic tree traces the development of selected NET-targeting radiotracers, classified into two main categories: monoamines (indicated by the violet cloud) and guanidines (denoted by the light green cloud). Shared molecular frameworks within the radiotracers are color-coded to illustrate structural similarities—core structures in blue and green, and the ubiquitous "tail" motif in orange. The placement of radionuclides within the molecular structures is highlighted in red. Adapted with permission from [73], Copyright 2023, MDPI.
In a recent comparative study, 18F-MFBG PET/CT was shown to outperform 123I-MIBG SPECT/CT in detecting neuroblastoma lesions in children [68]. The research included 40 patients, averaging 6 years old. It revealed that while both imaging modalities had negative results in six patients, 18F-MFBG PET/CT detected lesions in 11.8% of patients who were negative on 18F-MFBG. Overall, 18F-MFBG PET/CT identified 252 more lesions than 123I-MIBG SPECT/CT, marking a significant increase in detection. Additionally, Curie scores, which quantify tumor load, were considerably higher with 18F-MFBG PET/CT PET/CT, with 88.2% of patients showing active disease having higher scores compared to those from 123I-MIBG imaging. This suggests that 18F-MFBG PET/CT could offer improved diagnostic accuracy in pediatric neuroblastoma.
As the field of NET-targeting PET tracers continues to expand, we recognize that there are significant knowledge gaps and clinical challenges that must be addressed [39]. A primary recommendation is to create a comprehensive database to systematically track and analyze the performance of 18F-labeled tracers across various clinical scenarios. This would not only help in recognizing patterns of efficacy and patient outcomes but also contribute to refining clinical guidelines and protocols. Further investigation comparing the biodistribution, such as liver and kidney uptake, and clearance rates across diverse patient cohorts would be beneficial, particularly when different tracers are used. This research is critical for optimizing both the performance of imaging and the dosing regimens, which in turn, can enhance the safety profile of these diagnostic agents. A lack of longitudinal data linking PET imaging results with long-term patient outcomes presents another gap. Consequently, there is a need for extended follow-up in multi-institutional studies to elucidate the prognostic value of these tracers in cardiology as well as in oncology, particularly in relation to therapeutic decisions and patient survival. Clinically, the underutilization of advanced NET-targeting tracers due to logistical barriers is a pressing issue. By forging partnerships between academia, industry, and healthcare systems, we can enhance the availability and application of these tracers, ensuring equitable access to this crucial diagnostic technology. Moreover, yet in the early development stage, with tracers such as 18F-AF78 [72, 73] and 18F-PHPG/MHPG [75] demonstrating high NET affinity, refining imaging protocols becomes possible, which may significantly improve disease detection and characterization. By integrating these imaging results with molecular and genetic data, we could potentially further tailor the diagnosis and treatment of NET-related disorders, moving closer to the personalized medicine paradigm.
NET-Targeted Therapy with Beta and Alpha Emitters
131I-MIBG therapy's mechanisms and applications. 131I-MIBG therapy, which harnesses the therapeutic properties of a radioactive isotope for the targeted treatment of certain neuroendocrine tumors, has seen a reaffirmation of its clinical value through the FDA approval in July 2018. 131I-MIBG functions on the principle of molecular mimicry. Structurally analogous to norepinephrine, it is actively transported into neuroendocrine cells via the NET. Once internalized, the radioactive decay of 131I releases high energy beta-particles, leading to localized cell damage and apoptosis, primarily within neuroendocrine tumors that overexpress NET. It is now an FDA-approved therapy for adult and pediatric patients (12 years and older) with locally advanced or metastatic pheochromocytoma or paraganglioma who require systemic anticancer therapy. This regulatory milestone has cemented 131I-MIBG's role in the treatment paradigm of these conditions. The implementation of 131I-MIBG therapy post-FDA approval requires multidisciplinary coordination to ensure patient safety and treatment efficacy [24]. The procedure necessitates isolation due to the radioactive nature of the compound and involves specialized nuclear medicine facilities. Looking forward, optimizing dosing, mitigating side effects, and integrating 131I-MIBG with other therapies [81] remain areas of active research. 131I-MIBG therapy would provide a much-needed option for children with high-risk neuroblastoma, who may have limited treatment choices, and has been associated with improved survival outcomes in this patient population [81]. As clinical experience with 131I-MIBG grows and further studies refine its application, it is poised to improve outcomes for patients facing limited treatment options, symbolizing progress in personalized cancer therapy.
Dosimetry in radionuclide therapy is the process of measuring and assessing the radiation dose that a patient receives during treatment with radioactive pharmaceuticals such as 131I-MIBG [82]. The process begins with an initial tracer dose of the radiopharmaceutical to understand its distribution and clearance within the body, often involving several imaging sessions over time. From this, specialists calculate the radiation absorbed by different tissues, striving to maximize the dose to the tumor while limiting exposure to healthy organs to reduce toxicity. For 131I-MIBG therapy specifically, these dosimetry calculations are critical in shaping a personalized treatment plan. The therapeutic activity is carefully determined based on the patient's unique physiological and disease characteristics to deliver a defined whole-body dose aimed at achieving the best therapeutic effect. Advancements in dosimetry techniques have evolved to include SPECT-based tumor dosimetry, with studies showing the ability to deliver whole-body doses, fine-tuning the activity administered based on whole-body retention from the initial therapy [83]. In 2016, a more individualized approach to 131I-MIBG therapy considered both dosimetry data and clinical judgment to deliver high activities of 131I-MIBG that patients could tolerate, especially with stem cell support [84]. This personalized method demonstrated high response rates and manageable toxicity. Comparatively, low-dose diagnostic scans with 123I-MIBG were found to be less effective than post-treatment scans with 131I-MIBG in detecting malignant pheochromocytoma and paraganglioma, underscoring the importance of scan timing and dose in diagnostic accuracy [85].
Edmondson et al. explored gene expression analysis as an alternative biodosimetric approach to calculate internal radiation doses for neuroblastoma patients treated with 131I-MIBG [86]. Blood samples from 40 patients, with a median age of 9, were collected before and 72 to 96 hours post-infusion. Absorbed doses were calculated using patient-specific data and compared with gene expression changes. Six genes were significantly modulated by the 131I-MIBG treatment. A predictive model using three gene transcripts could account for 98% of the variance in gene expression changes post-treatment, suggesting that these biomarkers are indicative of internal radiation exposure. This novel approach holds promise for biodosimetry in radiopharmaceutical therapy.
The treatment with 131I-MIBG can lead to certain side effects such as bone marrow suppression, thyroid gland damage, nausea, hypertension, and inflammation of the salivary glands [82, 87]. Protective measures like thyroid-blocking agents, anti-emetics, blood pressure control, and hydration are standard to alleviate these effects. The integration of 131I-MIBG with 90Y-labeled DOTA-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) has been proposed as a synergistic approach to enhance tumor dose delivery in neuroendocrine tumors while potentially reducing side effects [14]. The theoretical model developed by Madsen et al. suggests that this combined therapy could significantly increase tumor dosage without surpassing critical organ dose limits, as these agents impact different dose-limiting tissues [88]. Further research by Bushnell et al. through a phase 1 clinical trial has affirmed the practicality of this approach for patients with nonoperable progressive neuroendocrine tumors [89]. In this trial, the combination of 90Y-DOTATOC with 131I-MIBG was adjusted based on subject-specific dosimetry with respect to renal and bone marrow dose constraints. The preliminary results showed that the combined therapy could achieve a tumor dose increment between 34-83% compared to 90Y-DOTATOC PRRT alone, without encountering dose-limiting toxicities and adhering to a dose limit of 1,900 cGy for kidneys and 150 cGy for bone marrow. These studies pave the way for more personalized and potentially more effective radionuclide therapy regimens for treating neuroendocrine tumors, underlining the necessity of comprehensive dosimetric analysis to fully harness the benefits of combined-agent therapy.
Introduction of alpha-particle therapy. Among various alpha-emitting radionuclides that are gaining interest [90, 91], astatine-211 (211At) [92] stands out for its potential to advance targeted cancer therapies, following the path laid by the halogen 131I [93]. Both 211At and 131I are adept at creating stable, target-specific radiopharmaceuticals, especially for targeting the NET. 211At is particularly advantageous due to its emission of High-Linear Energy Transfer (LET) irradiation alpha particles [94], which confer a concentrated therapeutic effect. While the beta-particle emission of 131I-MIBG has established its role in treating neuroendocrine tumors, 211At-astatobenzylguanidine (211At-MABG), a structural analogue of MIBG, promises enhanced precision and cytotoxicity at the tumor site due to the localized impact of alpha particles. In addition, actinium-225 (225Ac) is also highly valued, particularly for its use in peptide-based tracers or antibody radioconjugates, due to its affinity for larger, metal-affinitive biological molecules and the possibility to be chelated by several commonly used macrocyclic chelators such as DOTA, making it a versatile choice for targeted therapy [95]. In contrast, 211At is more compatible with small organic molecules to form stable covalent bond exemplified by MABG, broadening its applicability for various radiopharmaceuticals [96].
Both 211At and 225Ac show promise as alpha-emitting agents for medical purposes, yet they are markedly different in their production and decay characteristics. The generation of 211At generally requires a cyclotron [97], whereas 225Ac is typically sourced from nuclear reactors, with its longer half-life of 10 days enhancing its potential for wider distribution [98]. Establishing in-house production of 211At using a medium-energy cyclotron signifies a significant capital investment for medical or research institutions. This outlay encompasses not only the purchase price but also the subsequent costs related to maintenance, operations, and the skilled personnel necessary to ensure safe and efficient facility operation. Given the short half-life of 211At — a mere 7.2 hours in contrast to the 10-day half-life of 225Ac — there are critical differences impacting both the biodistribution for effective dose delivery and logistical considerations [99]. The relatively short half-life of 211At necessitates a production site close to the clinical application venue or an expedient transport system to deliver the isotope promptly and with minimal loss of activity. Such logistics require a synchronized supply chain to guarantee consistent availability.
A significant advantage of 211At come from its non-serial decay property. It transitions directly to stable Bismuth-207 (207Bi), in contrast to 225Ac, which initiates a decay chain yielding multiple radioactive daughters [95]. The immediate transition to a stable state for 211At means there is no additional radiation emitted following its decay, streamlining many facets of its therapeutic application and related safety measures. In clinical practice, managing 211At focuses on the direct administration and adherence to safety protocols until it decays to its stable form. This is unlike the approach required for serially decaying nuclides like 225Ac, which might need consideration for the management of radioactive decay products over a more extended period. These production nuances, along with subsequent purification, logistical arrangements, and regulatory considerations, highlight the unique challenges and potential of each radionuclide within precision medicine's evolving landscape.
211At-MABG's superior localized effect is a consequence of the alpha-particles' short path length, resulting in intense cellular damage confined to a narrow range, thereby mitigating the risk to adjacent healthy tissue (Figure 5). This enhanced localized effect positions 211At as a formidable candidate for more focused and potent treatment modalities. Building upon iodine-based therapies, 211At-MABG has already demonstrated significant promise in preclinical experiments with neuroendocrine tumor models (Figure 6) [100], indicating potential for high efficacy and a favorable safety margin.
In Japan, a Phase 1 clinical trial (jRCT2021220012) is currently evaluating the pharmacokinetics, safety, and efficacy of 211At-MABG in adult patients with pheochromocytoma or paraganglioma who are not candidates for surgery or curative external radiation. This study utilizes a 3+3 dose-escalation design to determine the maximum tolerated dose, starting with three cohorts receiving increasing doses of the radiopharmaceutical (Cohort 1 = 0.65 MBq/kg, Cohort 2 = 1.3 MBq/kg and Cohort 3 = 2.1 MBq/kg). Key endpoints include dose-limiting toxicity, radioactive pharmacokinetics, urinary discharge of radioactivity, catecholamine response, objective response rate, progression-free survival, the impact of 131I-MIBG scintigraphy on tumor accumulation, and patient quality of life. As 211At-MABG is on early phase human trials, backed by encouraging animal study results, it stands on the cusp of transitioning from an experimental therapy to a tangible clinical reality. Research is now concentrated on refining 211At delivery and dosimetry accounting for its shorter physiological half-life compared to 131I [94, 101-104].
The therapeutic effects of 211At-MABG on malignant pheochromocytoma using RNA-sequencing to understand its molecular mechanisms and identify potential biomarkers for therapeutic response have also been explored [104, 105]. In this regard, in vitro experiments on a rat pheochromocytoma cell line (PC12) were conducted, revealing significant anti-tumor effects. Transcriptomic analyses were performed at 3, 6, and 12 hours post-treatment with 211At-MABG, using iso-survival doses of 0.8 and 0.1 kBq/mL. Comparison with 60Co gamma-ray irradiation highlighted 211At-MABG-specific gene expression changes. Notably, four genes (Mien1, Otub1, Vdac1, and Vegfa) showed promise as potential therapy biomarkers due to their expression patterns correlating with survival decrease. This study's findings underscore the potential of 211At-MABG in treating malignant pheochromocytoma and pave the way for using molecular imaging to target identified biomarkers for enhanced therapeutic efficacy.
Enhanced imaging and computational modeling are needed to ensure accurate tumor targeting and minimized radiation exposure to non-targeted tissues, optimizing both therapeutic impact and patient safety [103]. Clinical challenges also include establishing clear protocols for managing potential side effects unique to alpha-emitting therapies and ensuring access to these treatments across diverse healthcare settings. A roadmap for the field should involve the development of multicenter collaborations to share knowledge, standardize treatment protocols, and pool resources for large-scale trials that can provide the level of evidence needed for regulatory approval and clinical acceptance.
PET diagnostics using 18F-tracers and 211At therapy can complement each other. The synergy of NET-targeting PET diagnostics using 18F-labeled radiotracers together with 211At-MABG therapy embodies a promising approach to enhance the management of neuroendocrine tumors. The 18F-labeled NET-targeting PET tracers offer superior spatial resolution and the potential for precise quantification, which could significantly improve the identification and characterization of tumors with high NET expression [68, 106]. This advanced diagnostic capability is expected to refine patient selection, incorporating potential biomarkers, specific patient characteristics, and disease stages, ensuring that the potent 211At-MABG therapy is administered to individuals most likely to benefit. 211At-MABG, targeting the NET for therapeutic delivery, leverages the cell-killing properties of alpha-particles [94, 99]. This therapeutic strategy aims to maximize tumor eradication while limiting collateral damage to surrounding tissues due to the short range of alpha emission. When integrated with 18F-tracer PET imaging, clinicians could monitor therapeutic response, allowing for dynamic treatment adjustments.
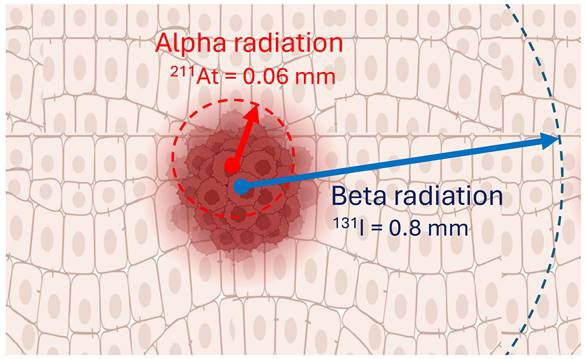
Alpha-particles, like 211At, offer the potential to combine cell-specific molecular targets with radiation that has a range in tissue of only a few cells (0.06 mm), which is different to beta-particles, such as 131I, that have a maximum range of around 0.8 mm. The distinct advantages of alpha particles, such as their potent cytotoxicity and selective targeting, position targeted alpha therapy as a promising avenue in the field of cancer treatment. Created with BioRender.com.
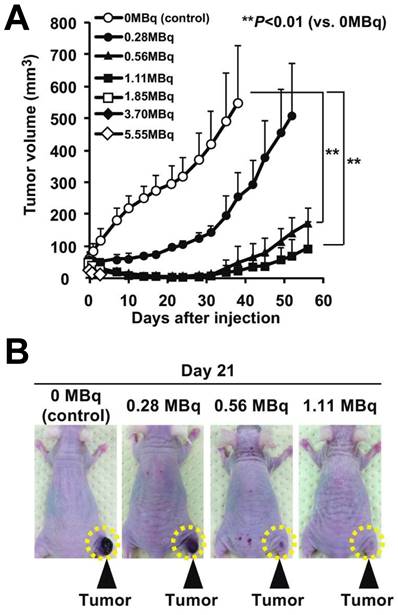
The 211At-MABG-treated mice showed significantly lower relative tumor growth in mice with PC12 pheochromocytoma cells: a) Tumor growth trajectories following 211At-MABG administration demonstrate significant reduction. b) Day 21 post-treatment images of mice, comparing 211At-MABG treatment, with tumors highlighted by dashed circles. Adapted with permission from [100]. Copyright 2018, Springer Nature.
While this integrative model of 18F NET-targeting PET diagnostics and 211At-MABG therapy is intuitively appealing, it remains a hypothesis that demands empirical validation. It outlines the design of future studies that should aim to establish a correlation between 18F-labeled PET tracer uptake and NET expression with patient outcomes post 211At-MABG therapy to confirm the predictive value of PET imaging and to evaluate long-term outcomes and quality of life in patients undergoing this combined treatment approach, to ensure that the clinical benefits justify its use over conventional methods. In essence, there is a need for prospective clinical trials with clearly defined endpoints, encompassing both response criteria and patient-centric outcomes. Such trials would not only substantiate the therapeutic merits but also refine the selection criteria for patients most likely to benefit from this approach, thereby advancing the field towards more effective and individualized cancer therapies. If proven successful, this approach could significantly impact the trajectory of personalized cancer therapy, aligning treatment strategies closely with the unique biological signatures of each patient's tumor (Figure 7).
Conclusion
The advent of 18F-labeled NET-targeting PET tracers and 211At therapy marks a transformative era in precision medicine. These novel approaches have opened up unprecedented avenues for managing neuroendocrine-related conditions. The sensitivity and specificity of 18F-labeled PET tracers for the NET allow for earlier and more accurate detection of diseases, thus streamlining the diagnosis and staging process for conditions such as heart failure, Parkinson's syndrome, and various neuroendocrine tumors. 211At therapy, leveraging targeted alpha-particle radiation, stands on the cusp of redefining the treatment landscape for NET-expressing tumors with its potential to deliver highly potent cytotoxic effects precisely where needed. The promise of these radiopharmaceuticals is predicated on the continuation of rigorous research to substantiate their therapeutic value and confirm their safety profile. As clinical trials progress, it is imperative that the medical community nurtures cross-disciplinary collaboration to enhance treatment methodologies, fully elucidate the long-term ramifications of these therapies, and innovate further in the development of even more targeted radiopharmaceuticals. Looking forward, NET-targeted radiopharmaceuticals are anticipated to significantly influence patient care. The integration of PET diagnostics using 18F-tracers, along with the therapeutic potential of 211At exemplified by MABG, could herald an era of highly individualized care, optimizing treatment plans to match the unique biological landscape of each patient. The potential of these modalities extends beyond immediate clinical outcomes, offering the possibility of not just improved management but also potentially increasing survival rates and the quality of life for those battling complex neuroendocrine conditions. As we stand at the threshold of this new chapter in medical science, NET-targeted radiopharmaceuticals offer a beacon of hope, illuminating the path toward a future where precision medicine is not just an aspiration but a reality.
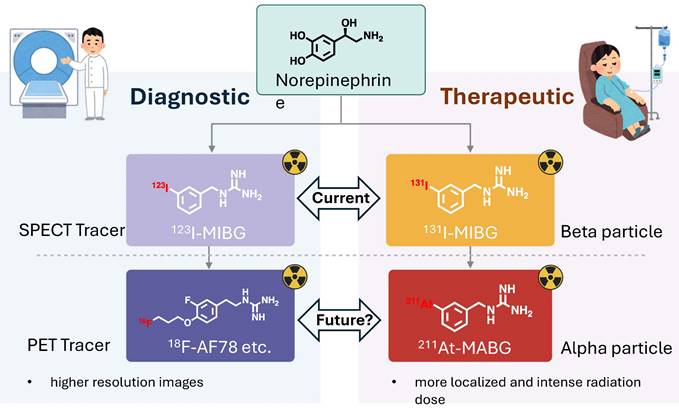
Advancements in Radiopharmaceuticals for NET Targeting: This illustration summarizes the progress in diagnostic and therapeutic agents, highlighting the integration of PET for high-resolution, sensitive imaging, and the employment of alpha-emitting nuclides for focused radiation therapy. These innovations herald a new era of precision medicine with enhanced diagnostic and treatment capabilities. Created with BioRender.com.
Acknowledgements
Funding
This project is partially supported by the Okayama University “RECTOR” Program, KAKENHI grant (22H03027) from the Japan Society for the Promotion of Science (TH) and the German Research Foundation (453989101, RAW, TH; 507803309, RAW). All other authors declare no conflict of interest.
Author contributions
Conceptualization, T.H., R.A.W., X.C.; resources, T.H.; writing—original draft preparation, T.H., X.C.; writing — review and editing, R.A.W., T.H.; visualization, T.H.; funding acquisition, R.A.W., T.H. All authors have read and agreed to the published version of the manuscript.
Other
ChatGPT has been employed for editing tasks.
Competing Interests
RAW: Speaker honoraria from Novartis/AAA and PentixaPharm, advisory board work for Novartis/AAA and Bayer. All other authors declare no conflict of interest.
References
1. Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7:9-13
2. Werner RA, Kobayashi R, Javadi MS, Kock Z, Wakabayashi H, Unterecker S. et al. Impact of Novel Antidepressants on Cardiac (123)I-Metaiodobenzylguanidine Uptake: Experimental Studies on SK-N-SH Cells and Healthy Rabbits. J Nucl Med. 2018;59:1099-103
3. Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739-53
4. Vanicek T, Spies M, Rami-Mark C, Savli M, Hoflich A, Kranz GS. et al. The norepinephrine transporter in attention-deficit/hyperactivity disorder investigated with positron emission tomography. JAMA Psychiatry. 2014;71:1340-9
5. Delaville C, Deurwaerdere PD, Benazzouz A. Noradrenaline and Parkinson's disease. Front Syst Neurosci. 2011;5:31
6. Chen Z, Li G, Liu J. Autonomic dysfunction in Parkinson's disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis. 2020;134:104700
7. Peng H, Chen S, Wu S, Shi X, Ma J, Yang H. et al. Alpha-synuclein in skin as a high-quality biomarker for Parkinson's disease. J Neurol Sci. 2023;451:120730
8. Pfeiffer RF. Autonomic Dysfunction in Parkinson's Disease. Neurotherapeutics. 2020;17:1464-79
9. Malek N, Lawton MA, Grosset KA, Bajaj N, Barker RA, Burn DJ. et al. Autonomic Dysfunction in Early Parkinson's Disease: Results from the United Kingdom Tracking Parkinson's Study. Mov Disord Clin Pract. 2017;4:509-16
10. Calvani M, Pelon F, Comito G, Taddei ML, Moretti S, Innocenti S. et al. Norepinephrine promotes tumor microenvironment reactivity through beta3-adrenoreceptors during melanoma progression. Oncotarget. 2015;6:4615-32
11. Godugu K, Karakus OO, Fujioka K, Glinsky GV, Mousa SA. Anticancer Efficacy and Mechanisms of a Dual Targeting of Norepinephrine Transporter and Thyrointegrin alphavbeta3 Antagonist in Neuroblastoma. J Cancer. 2022;13:2594-606
12. Lee M, Minaskan N, Wiedemann T, Irmler M, Beckers J, Yousefi BH. et al. Targeting PI3K/mTOR signaling exerts potent antitumor activity in pheochromocytoma in vivo. Endocr Relat Cancer. 2017;24:1-15
13. Kantorovich V, Pacak K. Pheochromocytoma and paraganglioma. Prog Brain Res. 2010;182:343-73
14. Pandit-Taskar N, Modak S. Norepinephrine Transporter as a Target for Imaging and Therapy. J Nucl Med. 2017;58:39S-53S
15. Dhoundiyal S, Srivastava S, Kumar S, Singh G, Ashique S, Pal R. et al. Radiopharmaceuticals: navigating the frontier of precision medicine and therapeutic innovation. Eur J Med Res. 2024;29:26
16. Holman BL, Tumeh SS. Single-photon emission computed tomography (SPECT). Applications and potential. JAMA. 1990;263:561-4
17. Harisankar CN, Mittal BR, Bhattacharya A, Kashyap R, Bhansali A. Iodine-131 meta-iodobezylguanidine single photon emission computed tomography/computerized tomography in diagnosis of neuro-endocrine tumors. Indian J Nucl Med. 2012;27:55-8
18. Verschure DO, Nakajima K, Verberne HJ. Cardiac (123)I-mIBG Imaging in Heart Failure. Pharmaceuticals (Basel). 2022 15
19. Thorpe MP, Kane A, Zhu J, Morse MA, Wong T, Borges-Neto S. Long-Term Outcomes of 125 Patients With Metastatic Pheochromocytoma or Paraganglioma Treated With 131-I MIBG. J Clin Endocrinol Metab. 2020;105:e494-501
20. Hoefnagel CA, De Kraker J, Valdes Olmos RA, Voute PA. 131I-MIBG as a first-line treatment in high-risk neuroblastoma patients. Nucl Med Commun. 1994;15:712-7
21. Herrmann K, Schwaiger M, Lewis JS, Solomon SB, McNeil BJ, Baumann M. et al. Radiotheranostics: a roadmap for future development. Lancet Oncol. 2020;21:e146-e56
22. Theerakulpisut D, Raruenrom Y, Wongsurawat N, Somboonporn C. Value of SPECT/CT in Diagnostic I-131 MIBG Scintigraphy in Patients with Neuroblastoma. Nucl Med Mol Imaging. 2018;52:350-8
23. He H, Xu Q, Yu C. The efficacy and safety of Iodine-131-metaiodobenzylguanidine therapy in patients with neuroblastoma: a meta-analysis. BMC Cancer. 2022;22:216
24. Pryma DA, Chin BB, Noto RB, Dillon JS, Perkins S, Solnes L. et al. Efficacy and Safety of High-Specific-Activity (131)I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J Nucl Med. 2019;60:623-30
25. Groch MW, Erwin WD. SPECT in the year 2000: basic principles. J Nucl Med Technol. 2000;28:233-44
26. Van Audenhaege K, Van Holen R, Vandenberghe S, Vanhove C, Metzler SD, Moore SC. Review of SPECT collimator selection, optimization, and fabrication for clinical and preclinical imaging. Med Phys. 2015;42:4796-813
27. Jones T, Townsend D. History and future technical innovation in positron emission tomography. J Med Imaging (Bellingham). 2017;4:011013
28. Lee I, Paeng JC, Lee SJ, Shin CS, Jang JY, Cheon GJ. et al. Comparison of Diagnostic Sensitivity and Quantitative Indices Between (68)Ga-DOTATOC PET/CT and (111)In-Pentetreotide SPECT/CT in Neuroendocrine Tumors: a Preliminary Report. Nucl Med Mol Imaging. 2015;49:284-90
29. Watakabe T, Toya R, Saito T, Matsuyama T, Shiraishi S, Kai Y. et al. High Spatial Resolution Digital Positron Emission Tomography Images With Dedicated Source-to-background Algorithm for Radiotherapy Planning. Anticancer Res. 2020;40:2567-72
30. Slomka P, Berman DS, Alexanderson E, Germano G. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging. 2014;2:343-58
31. Bengel FM, Higuchi T, Javadi MS, Lautamaki R. Cardiac positron emission tomography. J Am Coll Cardiol. 2009;54:1-15
32. Surti S, Pantel AR, Karp JS. Total Body PET: Why, How, What for? IEEE Trans Radiat Plasma Med Sci. 2020;4:283-92
33. Ehman EC, Johnson GB, Villanueva-Meyer JE, Cha S, Leynes AP, Larson PEZ. et al. PET/MRI: Where might it replace PET/CT? J Magn Reson Imaging. 2017;46:1247-62
34. Surti S, Viswanath V, Daube-Witherspoon ME, Conti M, Casey ME, Karp JS. Benefit of Improved Performance with State-of-the Art Digital PET/CT for Lesion Detection in Oncology. J Nucl Med. 2020;61:1684-90
35. Gonzalez-Montoro A, Ullah MN, Levin CS. Advances in Detector Instrumentation for PET. J Nucl Med. 2022;63:1138-44
36. Hellwig D, Hellwig NC, Boehner S, Fuchs T, Fischer R, Schmidt D. Artificial Intelligence and Deep Learning for Advancing PET Image Reconstruction: State-of-the-Art and Future Directions. Nuklearmedizin. 2023;62:334-42
37. Vandenberghe S, Mikhaylova E, D'Hoe E, Mollet P, Karp JS. Recent developments in time-of-flight PET. EJNMMI Phys. 2016;3:3
38. Nakajima K, Nakata T. Cardiac 123I-MIBG Imaging for Clinical Decision Making: 22-Year Experience in Japan. J Nucl Med. 2015;56(Suppl 4):11S-9S
39. Chen X, Kudo T, Lapa C, Buck A, Higuchi T. Recent advances in radiotracers targeting norepinephrine transporter: structural development and radiolabeling improvements. J Neural Transm (Vienna). 2020;127:851-73
40. Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA. et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212-21
41. Al Badarin FJ, Wimmer AP, Kennedy KF, Jacobson AF, Bateman TM. The utility of ADMIRE-HF risk score in predicting serious arrhythmic events in heart failure patients: incremental prognostic benefit of cardiac 123I-mIBG scintigraphy. J Nucl Cardiol. 2014;21:756-62 quiz 3-55, 63-5
42. Sheikhbahaei S, Sadaghiani MS, Rowe SP, Solnes LB. Neuroendocrine Tumor Theranostics: An Update and Emerging Applications in Clinical Practice. AJR Am J Roentgenol. 2021;217:495-506
43. Weiss BD, Yanik G, Naranjo A, Zhang FF, Fitzgerald W, Shulkin BL. et al. A safety and feasibility trial of (131) I-MIBG in newly diagnosed high-risk neuroblastoma: A Children's Oncology Group study. Pediatr Blood Cancer. 2021;68:e29117
44. Skowronek C, Zange L, Lipp A. Cardiac 123I-MIBG Scintigraphy in Neurodegenerative Parkinson Syndromes: Performance and Pitfalls in Clinical Practice. Front Neurol. 2019;10:152
45. Park DG, Kang J, An YS, Chang J, Yoon JH. Association of plasma alpha-synuclein with cardiac (123)I-MIBG scintigraphy in early Parkinson's disease. Neurosci Lett. 2022;770:136399
46. Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH. et al. Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2013;72:119-29
47. Pitton Rissardo J, Fornari Caprara AL. Cardiac 123I-Metaiodobenzylguanidine (MIBG) Scintigraphy in Parkinson's Disease: A Comprehensive Review. Brain Sci. 2023 13
48. Asahi T, Kashiwazaki D, Yoneyama T, Noguchi K, Kuroda S. Importance of (123)I-ioflupane SPECT and Myocardial MIBG Scintigraphy to Determine the Candidate of Deep Brain Stimulation for Parkinson's Disease. Neurol Med Chir (Tokyo). 2016;56:125-31
49. Slaets S, Van Acker F, Versijpt J, Hauth L, Goeman J, Martin JJ. et al. Diagnostic value of MIBG cardiac scintigraphy for differential dementia diagnosis. Int J Geriatr Psychiatry. 2015;30:864-9
50. Wu J, Lin SF, Gallezot JD, Chan C, Prasad R, Thorn SL. et al. Quantitative Analysis of Dynamic 123I-mIBG SPECT Imaging Data in Healthy Humans with a Population-Based Metabolite Correction Method. J Nucl Med. 2016;57:1226-32
51. Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med. 2011;31:3-13
52. Jiang L, Tu Y, Shi H, Cheng Z. PET probes beyond (18)F-FDG. J Biomed Res. 2014;28:435-46
53. Werner RA, Chen X, Maya Y, Eissler C, Hirano M, Nose N. et al. The Impact of Ageing on 11C-Hydroxyephedrine Uptake in the Rat Heart. Sci Rep. 2018;8:11120
54. Rischpler C, Fukushima K, Isoda T, Javadi MS, Dannals RF, Abraham R. et al. Discrepant uptake of the radiolabeled norepinephrine analogues hydroxyephedrine (HED) and metaiodobenzylguanidine (MIBG) in rat hearts. Eur J Nucl Med Mol Imaging. 2013;40:1077-83
55. Hall AB, Ziadi MC, Leech JA, Chen SY, Burwash IG, Renaud J. et al. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation. 2014;130:892-901
56. Fallavollita JA, Heavey BM, Luisi AJ Jr, Michalek SM, Baldwa S, Mashtare TL Jr. et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141-9
57. Munch G, Nguyen NT, Nekolla S, Ziegler S, Muzik O, Chakraborty P. et al. Evaluation of sympathetic nerve terminals with [(11)C]epinephrine and [(11)C]hydroxyephedrine and positron emission tomography. Circulation. 2000;101:516-23
58. Shulkin BL, Wieland DM, Baro ME, Ungar DR, Mitchell DS, Dole MG. et al. PET hydroxyephedrine imaging of neuroblastoma. J Nucl Med. 1996;37:16-21
59. Vavere AL, Neumann KD, Butch ER, Hu B, DiMagno SG, Snyder SE. Improved, one-pot synthesis of 6-[(18) F]fluorodopamine and quality control testing for use in patients with neuroblastoma. J Labelled Comp Radiopharm. 2018;61:1069-80
60. Pacak K, Eisenhofer G, Carrasquillo JA, Chen CC, Li ST, Goldstein DS. 6-[18F]fluorodopamine positron emission tomographic (PET) scanning for diagnostic localization of pheochromocytoma. Hypertension. 2001;38:6-8
61. Yu M, Bozek J, Lamoy M, Guaraldi M, Silva P, Kagan M. et al. Evaluation of LMI1195, a novel 18F-labeled cardiac neuronal PET imaging agent, in cells and animal models. Circ Cardiovasc Imaging. 2011;4:435-43
62. Chen X, Werner RA, Lapa C, Nose N, Hirano M, Javadi MS. et al. Subcellular storage and release mode of the novel (18)F-labeled sympathetic nerve PET tracer LMI1195. EJNMMI Res. 2018;8:12
63. Werner RA, Rischpler C, Onthank D, Lapa C, Robinson S, Samnick S. et al. Retention Kinetics of the 18F-Labeled Sympathetic Nerve PET Tracer LMI1195: Comparison with 11C-Hydroxyephedrine and 123I-MIBG. J Nucl Med. 2015;56:1429-33
64. Higuchi T, Yousefi BH, Reder S, Beschorner M, Laitinen I, Yu M. et al. Myocardial Kinetics of a Novel [(18)F]-Labeled Sympathetic Nerve PET Tracer LMI1195 in the Isolated Perfused Rabbit Heart. JACC Cardiovasc Imaging. 2015;8:1229-31
65. Gaertner FC, Wiedemann T, Yousefi BH, Lee M, Repokis I, Higuchi T. et al. Preclinical evaluation of 18F-LMI1195 for in vivo imaging of pheochromocytoma in the MENX tumor model. J Nucl Med. 2013;54:2111-7
66. Higuchi T, Yousefi BH, Kaiser F, Gartner F, Rischpler C, Reder S. et al. Assessment of the 18F-labeled PET tracer LMI1195 for imaging norepinephrine handling in rat hearts. J Nucl Med. 2013;54:1142-6
67. Samim A, Blom T, Poot AJ, Windhorst AD, Fiocco M, Tolboom N. et al. [(18)F]mFBG PET-CT for detection and localisation of neuroblastoma: a prospective pilot study. Eur J Nucl Med Mol Imaging. 2023;50:1146-57
68. Wang P, Li T, Liu Z, Jin M, Su Y, Zhang J. et al. [(18)F]MFBG PET/CT outperforming [(123)I]MIBG SPECT/CT in the evaluation of neuroblastoma. Eur J Nucl Med Mol Imaging. 2023;50:3097-106
69. Grkovski M, Zanzonico PB, Modak S, Humm JL, Narula J, Pandit-Taskar N. F-18 meta-fluorobenzylguanidine PET imaging of myocardial sympathetic innervation. J Nucl Cardiol. 2022;29:3179-88
70. Sinusas AJ, Lazewatsky J, Brunetti J, Heller G, Srivastava A, Liu YH. et al. Biodistribution and radiation dosimetry of LMI1195: first-in-human study of a novel 18F-labeled tracer for imaging myocardial innervation. J Nucl Med. 2014;55:1445-51
71. Chen X, Fritz A, Werner RA, Nose N, Yagi Y, Kimura H. et al. Initial Evaluation of AF78: a Rationally Designed Fluorine-18-Labelled PET Radiotracer Targeting Norepinephrine Transporter. Mol Imaging Biol. 2020;22:602-11
72. Chen X, Werner RA, Koshino K, Nose N, Muhlig S, Rowe SP. et al. Molecular Imaging-Derived Biomarker of Cardiac Nerve Integrity - Introducing High NET Affinity PET Probe (18)F-AF78. Theranostics. 2022;12:4446-58
73. Tutov A, Chen X, Werner RA, Muhlig S, Zimmermann T, Nose N. et al. Rationalizing the Binding Modes of PET Radiotracers Targeting the Norepinephrine Transporter. Pharmaceutics. 2023 15
74. Raffel DM, Jung YW, Koeppe RA, Jang KS, Gu G, Scott PJH. et al. First-in-Human Studies of [(18)F] Fluorohydroxyphenethylguanidines. Circ Cardiovasc Imaging. 2018;11:e007965
75. Jang KS, Jung YW, Gu G, Koeppe RA, Sherman PS, Quesada CA. et al. 4-[18F]Fluoro-m-hydroxyphenethylguanidine: a radiopharmaceutical for quantifying regional cardiac sympathetic nerve density with positron emission tomography. J Med Chem. 2013;56:7312-23
76. Seo Y, Huh Y, Huang SY, Hernandez-Pampaloni JM, Hawkins RA, Gustafson WC. et al. Technical Note: Simplified and practical pretherapy tumor dosimetry - A feasibility study for (131) I-MIBG therapy of neuroblastoma using (124) I-MIBG PET/CT. Med Phys. 2019;46:2477-86
77. Aboian MS, Huang SY, Hernandez-Pampaloni M, Hawkins RA, VanBrocklin HF, Huh Y. et al. (124)I-MIBG PET/CT to Monitor Metastatic Disease in Children with Relapsed Neuroblastoma. J Nucl Med. 2021;62:43-7
78. Maric I, Weber M, Prochnow A, Schmitz J, Unger N, Schaarschmidt BM. et al. Efficacy and Safety of (124)I-MIBG Dosimetry-Guided High-Activity (131)I-MIBG Therapy of Advanced Pheochromocytoma or Neuroblastoma. J Nucl Med. 2023;64:885-91
79. Bzowski P, Borys D, Gorczewski K, Chmura A, Daszewska K, Gorczewska I. et al. Efficiency of (124)I radioisotope production from natural and enriched tellurium dioxide using (124)Te(p,xn)(124)I reaction. EJNMMI Phys. 2022;9:41
80. Huang SY, Savic D, Yang J, Shrestha U, Seo Y. The Effect of Magnetic Field on Positron Range and Spatial Resolution in an Integrated Whole-Body Time-Of-Flight PET/MRI System. IEEE Nucl Sci Symp Conf Rec (1997). 2014. 2014
81. Yanik GA, Villablanca JG, Maris JM, Weiss B, Groshen S, Marachelian A. et al. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A new approaches to neuroblastoma therapy (NANT) phase II study. Biol Blood Marrow Transplant. 2015;21:673-81
82. Fielding SL, Flower MA, Ackery D, Kemshead JT, Lashford LS, Lewis I. Dosimetry of iodine 131 metaiodobenzylguanidine for treatment of resistant neuroblastoma: results of a UK study. Eur J Nucl Med. 1991;18:308-16
83. Buckley SE, Saran FH, Gaze MN, Chittenden S, Partridge M, Lancaster D. et al. Dosimetry for fractionated (131)I-mIBG therapies in patients with primary resistant high-risk neuroblastoma: preliminary results. Cancer Biother Radiopharm. 2007;22:105-12
84. George SL, Falzone N, Chittenden S, Kirk SJ, Lancaster D, Vaidya SJ. et al. Individualized 131I-mIBG therapy in the management of refractory and relapsed neuroblastoma. Nucl Med Commun. 2016;37:466-72
85. Kayano D, Taki J, Fukuoka M, Wakabayashi H, Inaki A, Nakamura A. et al. Low-dose (123)I-metaiodobenzylguanidine diagnostic scan is inferior to (131)I-metaiodobenzylguanidine posttreatment scan in detection of malignant pheochromocytoma and paraganglioma. Nucl Med Commun. 2011;32:941-6
86. Edmondson DA, Karski EE, Kohlgruber A, Koneru H, Matthay KK, Allen S. et al. Transcript Analysis for Internal Biodosimetry Using Peripheral Blood from Neuroblastoma Patients Treated with (131)I-mIBG, a Targeted Radionuclide. Radiat Res. 2016;186:235-44
87. Polishchuk AL, Dubois SG, Haas-Kogan D, Hawkins R, Matthay KK. Response, survival, and toxicity after iodine-131-metaiodobenzylguanidine therapy for neuroblastoma in preadolescents, adolescents, and adults. Cancer. 2011;117:4286-93
88. Madsen MT, Bushnell DL, Juweid ME, Menda Y, O'Dorisio MS, O'Dorisio T. et al. Potential increased tumor-dose delivery with combined 131I-MIBG and 90Y-DOTATOC treatment in neuroendocrine tumors: a theoretic model. J Nucl Med. 2006;47:660-7
89. Bushnell DL, Bodeker KL, O'Dorisio TM, Madsen MT, Menda Y, Graves S. et al. Addition of (131)I-MIBG to PRRT ((90)Y-DOTATOC) for Personalized Treatment of Selected Patients with Neuroendocrine Tumors. J Nucl Med. 2021;62:1274-7
90. Dadachova E. Cancer therapy with alpha-emitters labeled peptides. Semin Nucl Med. 2010;40:204-8
91. Jang A, Kendi AT, Johnson GB, Halfdanarson TR, Sartor O. Targeted Alpha-Particle Therapy: A Review of Current Trials. Int J Mol Sci. 2023 24
92. Wunderlich G, Franke WG, Doberenz I, Fischer S. Two ways to establish potential At-211 radiopharmaceuticals. Anticancer Res. 1997;17:1809-13
93. Allen BJ. Clinical trials of targeted alpha therapy for cancer. Rev Recent Clin Trials. 2008;3:185-91
94. Batra V, Samanta M, Makvandi M, Groff D, Martorano P, Elias J. et al. Preclinical Development of [211At]meta- astatobenzylguanidine ([211At]MABG) as an Alpha Particle Radiopharmaceutical Therapy for Neuroblastoma. Clin Cancer Res. 2022;28:4146-57
95. Hooijman EL, Radchenko V, Ling SW, Konijnenberg M, Brabander T, Koolen SLW. et al. Implementing Ac-225 labelled radiopharmaceuticals: practical considerations and (pre-)clinical perspectives. EJNMMI Radiopharm Chem. 2024;9:9
96. Lindegren S, Albertsson P, Back T, Jensen H, Palm S, Aneheim E. Realizing Clinical Trials with Astatine-211: The Chemistry Infrastructure. Cancer Biother Radiopharm. 2020;35:425-36
97. Zalutsky MR, Pruszynski M. Astatine-211: production and availability. Curr Radiopharm. 2011;4:177-85
98. Engle JW. The Production of Ac-225. Curr Radiopharm. 2018;11:173-9
99. Rabiei M, Asadi M, Yousefnia H. Astatine-211 Radiopharmaceuticals; Status, Trends, and the Future. Curr Radiopharm. 2023
100. Ohshima Y, Sudo H, Watanabe S, Nagatsu K, Tsuji AB, Sakashita T. et al. Antitumor effects of radionuclide treatment using alpha-emitting meta-(211)At-astato-benzylguanidine in a PC12 pheochromocytoma model. Eur J Nucl Med Mol Imaging. 2018;45:999-1010
101. Ukon N, Higashi T, Hosono M, Kinuya S, Yamada T, Yanagida S. et al. Manual on the proper use of meta-[(211)At] astato-benzylguanidine ([(211)At] MABG) injections in clinical trials for targeted alpha therapy (1st edition). Ann Nucl Med. 2022;36:695-709
102. Sakashita T, Matsumoto S, Watanabe S, Hanaoka H, Ohshima Y, Ikoma Y. et al. Nonclinical study and applicability of the absorbed dose conversion method with a single biodistribution measurement for targeted alpha-nuclide therapy. EJNMMI Phys. 2021;8:80
103. Alcocer-Avila ME, Larouze A, Groetz JE, Hindie E, Champion C. Physics and small-scale dosimetry of alpha -emitters for targeted radionuclide therapy: The case of 211At. Med Phys. 2024
104. Doudard A, Corroyer-Dulmont A, Jaudet C, Bernaudin M, Valable S, Ledoux X. et al. Application of a new spectral deconvolution method for in vitro dosimetry in assessment of targeted alpha therapy. Med Phys. 2023;50:3762-72
105. Ohshima Y, Kono N, Yokota Y, Watanabe S, Sasaki I, Ishioka NS. et al. Anti-tumor effects and potential therapeutic response biomarkers in alpha-emitting meta-(211)At-astato-benzylguanidine therapy for malignant pheochromocytoma explored by RNA-sequencing. Theranostics. 2019;9:1538-49
106. Piccardo A, Treglia G, Fiz F, Bar-Sever Z, Bottoni G, Biassoni L. et al. The evidence-based role of catecholaminergic PET tracers in Neuroblastoma. A systematic review and a head-to-head comparison with mIBG scintigraphy. Eur J Nucl Med Mol Imaging. 2024;51:756-67
Author contact
![]() Corresponding author: Takahiro Higuchi — Department of Nuclear Medicine and Comprehensive Heart Failure Center, University Hospital Würzburg, 97080 Würzburg, Germany; orcid.org/0000-0002-1989-736X; Email: thiguchicom.
Corresponding author: Takahiro Higuchi — Department of Nuclear Medicine and Comprehensive Heart Failure Center, University Hospital Würzburg, 97080 Würzburg, Germany; orcid.org/0000-0002-1989-736X; Email: thiguchicom.
 Global reach, higher impact
Global reach, higher impact