13.3
Impact Factor
Theranostics 2024; 14(8):3300-3316. doi:10.7150/thno.96027 This issue Cite
Review
Building consensus on the application of organoid-based drug sensitivity testing in cancer precision medicine and drug development
1. State Key Laboratory of Systems Medicine for Cancer, Shanghai Cancer Institute, Shanghai Jiaotong University School of Medicine, Shanghai 200232, PRC
2. Department of Biliary-Pancreatic Surgery, Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai 200127, PRC
3. Department of Oncology, Shanghai Jiaotong University Affiliated Sixth People's Hospital, Shanghai 200233 PRC
4. Department of Oral and Maxillofacial-Head and Neck Oncology, Ninth People's Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200125, PRC
5. National Center of Stomatology, National Clinical Research Center for Oral Disease, Shanghai 200011, PRC
6. Department of Oncology, Ren Ji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200127, PRC
7. Department of Oncology, Tianjin Medical University General Hospital, Tianjin 300052, PRC
8. Department of Neurology and Institute of Neurology, Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai 200025, PRC
9. Department of Neurology, Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine (Boao Research Hospital), Hainan 571434, PRC
10. Department of Surgical Oncology, Second Affiliated Hospital, Zhejiang University School of Medicine, No. 88, Jiefang Road, Hangzhou, Zhejiang 310009, PRC
11. PDO-based DST Consortium
12. Department of Hepatobiliary Surgery, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Anhui 230001, PRC
13. Anhui Province Key Laboratory of Hepatopancreatobiliary Surgery, Anhui Provincial Clinical Research Center for Hepatobiliary Diseases, Hefei, Anhui 230001, PRC
14. The State Key Laboratory of Membrane Biology, Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing 100190, PRC
15. The MOE Basic Research and Innovation Center for the Targeted Therapeutics of Solid Tumors, School of Basic Medicine, Jiangxi Medical College, Nanchang University, Nanchang 330047, China
16. Translational Medical Center for Stem Cell Therapy & Institute for Regenerative Medicine, Shanghai East Hospital, School of Life Sciences and Technology, Tongji University, Shanghai 200120, PRC
17. Frontier Science Center for Stem Cell Research, Tongji University, 1239 Siping Road, Shanghai 200092, PRC
18. Shanghai Key Laboratory of Maternal-Fetal Medicine, Clinical and Translational Research Center of Shanghai First Maternity and Infant Hospital, School of Life Sciences and Technology, Tongji University, Shanghai 200092, PRC
#These authors contribute equally to the work.
Abstract
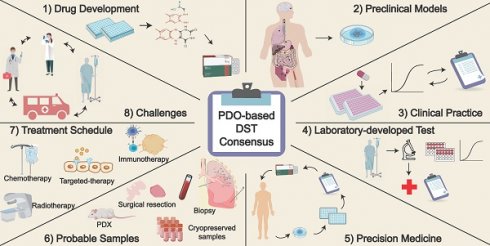
Patient-derived organoids (PDOs) have emerged as a promising platform for clinical and translational studies. A strong correlation exists between clinical outcomes and the use of PDOs to predict the efficacy of chemotherapy and/or radiotherapy. To standardize interpretation and enhance scientific communication in the field of cancer precision medicine, we revisit the concept of PDO-based drug sensitivity testing (DST). We present an expert consensus-driven approach for medication selection aimed at predicting patient responses. To further standardize PDO-based DST, we propose guidelines for clarification and characterization. Additionally, we identify several major challenges in clinical prediction when utilizing PDOs.
Keywords: Organoid, Patient-derived organoids (PDOs), Precision medicine, Drug sensitivity testing (DST), Expert consensus
 Global reach, higher impact
Global reach, higher impact