13.3
Impact Factor
Theranostics 2024; 14(9):3693-3707. doi:10.7150/thno.96944 This issue Cite
Research Paper
Towards effective CAIX-targeted radionuclide and checkpoint inhibition combination therapy for advanced clear cell renal cell carcinoma
1. Department of Medical Imaging, Nuclear Medicine, Radboud University Medical Center, Nijmegen, the Netherlands.
2. Department of Urology, Radboud University Medical Center, Nijmegen, the Netherlands.
3. Department of Radiation Oncology, Radboud University Medical Center, Nijmegen, the Netherlands.
4. Department of Radiology and Nuclear Medicine, Erasmus MC Cancer Institute, Erasmus University Medical Center, Rotterdam, The Netherlands.
5. Telix Pharmaceuticals Limited, Melbourne, VIC 3051, Australia.
Abstract
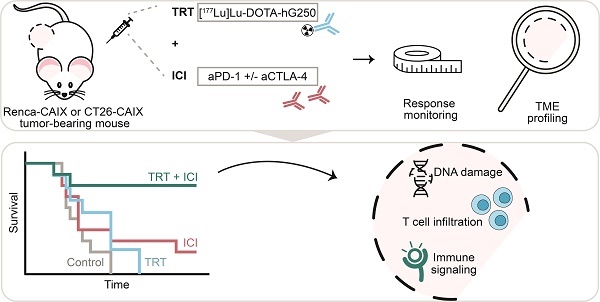
Background: Immune checkpoint inhibitors (ICI) are routinely used in advanced clear cell renal cell carcinoma (ccRCC). However, a substantial group of patients does not respond to ICI therapy. Radiation is a promising approach to increase ICI response rates since it can generate anti-tumor immunity. Targeted radionuclide therapy (TRT) is a systemic radiation treatment, ideally suited for precision irradiation of metastasized cancer. Therefore, the aim of this study is to explore the potential of combined TRT, targeting carbonic anhydrase IX (CAIX) which is overexpressed in ccRCC, using [177Lu]Lu-DOTA-hG250, and ICI for the treatment of ccRCC.
Methods: In this study, we evaluated the therapeutic and immunological action of [177Lu]Lu-DOTA-hG250 combined with aPD-1/a-CTLA-4 ICI. First, the biodistribution of [177Lu]Lu-DOTA-hG250 was investigated in BALB/cAnNRj mice bearing Renca-CAIX or CT26-CAIX tumors. Renca-CAIX and CT26-CAIX tumors are characterized by poor versus extensive T-cell infiltration and homogeneous versus heterogeneous PD-L1 expression, respectively. Tumor-absorbed radiation doses were estimated through dosimetry. Subsequently, [177Lu]Lu-DOTA-hG250 TRT efficacy with and without ICI was evaluated by monitoring tumor growth and survival. Therapy-induced changes in the tumor microenvironment were studied by collection of tumor tissue before and 5 or 8 days after treatment and analyzed by immunohistochemistry, flow cytometry, and RNA profiling.
Results: Biodistribution studies showed high tumor uptake of [177Lu]Lu-DOTA-hG250 in both tumor models. Dose escalation therapy studies in Renca-CAIX tumor-bearing mice demonstrated dose-dependent anti-tumor efficacy of [177Lu]Lu-DOTA-hG250 and remarkable therapeutic synergy including complete remissions when a presumed subtherapeutic TRT dose (4 MBq, which had no significant efficacy as monotherapy) was combined with aPD-1+aCTLA-4. Similar results were obtained in the CT26-CAIX model for 4 MBq [177Lu]Lu-DOTA-hG250 + a-PD1. Ex vivo analyses of treated tumors revealed DNA damage, T-cell infiltration, and modulated immune signaling pathways in the TME after combination treatment.
Conclusions: Subtherapeutic [177Lu]Lu-DOTA-hG250 combined with ICI showed superior therapeutic outcome and significantly altered the TME. Our results underline the importance of investigating this combination treatment for patients with advanced ccRCC in a clinical setting. Further investigations should focus on how the combination therapy should be optimally applied in the future.
Keywords: Targeted Radionuclide Therapy, Immune Checkpoint Inhibitors, Combined Modality Therapy, Tumor Microenvironment, Cancer Treatment
 Global reach, higher impact
Global reach, higher impact