13.3
Impact Factor
Theranostics 2025; 15(13):6146-6183. doi:10.7150/thno.114836 This issue Cite
Review
Antioxidant nanozymes: current status and future perspectives in spinal cord injury treatments
Department of Spine Surgery, Honghui Hospital, Xi'an Jiaotong University, Xi'an, Youyidong Road, Shaanxi, 710054, China.
# Contribute equally
Received 2025-4-1; Accepted 2025-4-22; Published 2025-5-8
Abstract

Spinal cord injury (SCI) is a life - altering neurological condition that carries significant global morbidity and mortality. It results in the disruption of motor and sensory pathways below the site of injury, often leading to permanent functional impairments and severely diminished quality of life. Despite decades of clinical and research efforts, current treatment options remain largely supportive, with limited success in promoting meaningful functional recovery or neural regeneration. In recent years, nanozymes have emerged as a promising frontier in the therapeutic landscape for SCI. These nanomaterial - based artificial enzymes offer several compelling advantages over their natural counterparts, including superior stability under physiological conditions, adjustable catalytic activity, cost - effective production, and prolonged shelf life. Unlike traditional therapeutic agents, nanozymes can be engineered to closely mimic the activity of key endogenous antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase. By scavenging reactive oxygen species and attenuating oxidative damage, nanozymes help preserve neuronal integrity and support the intrinsic repair processes of the central nervous system. This review provides a comprehensive overview of the pathophysiological mechanisms underlying SCI and examines the classification and catalytic principles governing nanozyme activity. We delve into the molecular pathways through which nanozymes exert their neuroprotective effects, particularly their roles in modulating oxidative stress and suppressing inflammatory responses following injury. Additionally, we explore the current challenges associated with nanozyme development, such as biocompatibility, targeted delivery, and long - term safety, and discuss future directions for optimizing their therapeutic potential in clinical applications. By synthesizing emerging insights into antioxidant nanozyme - based strategies, this review aims to contribute to the evolving landscape of SCI treatment and to highlight the transformative potential of nanozymes in advancing neuroregenerative medicine. These innovative agents represent a new horizon in SCI management, offering renewed hope for improving neurological outcomes and quality of life in affected individuals.
Keywords: Spinal cord injury, Nanozymes, Reactive oxygen species, Oxidative stress, Inflammation
Introduction
Spinal cord injury (SCI) is a catastrophic neurological condition that results in profound and often irreversible impairments, posing a major global health burden. Characterized by the disruption of motor and sensory pathways below the level of injury, SCI significantly diminishes the quality of life of affected individuals [1]. According to global data from 2021, there were an estimated 574,502 new cases of SCI reported worldwide - of which 369,118 occurred in males and 205,385 in females. The global prevalence reached 15,400,682 cases, with 9,793,772 males and 5,606,910 females affected [2]. Beyond the physical consequences, SCI imposes long - term psychological, emotional, and socioeconomic burdens on patients, caregivers, and healthcare systems. Many individuals develop secondary mental health conditions, such as depression, as a result of prolonged disability and reduced independence [3]. The pathogenesis of SCI is multifaceted, involving both primary and secondary phases of injury. The initial mechanical insult - referred to as the primary injury - is typically irreversible and characterized by immediate disruption of neural tissue and vasculature. However, it is the subsequent cascade of secondary injury events that significantly amplifies tissue destruction and impairs neurological recovery, thereby presenting a key therapeutic window [4, 5]. Among the numerous mechanisms driving secondary damage, oxidative stress and neuroinflammation are particularly critical. The overaccumulation of reactive oxygen species (ROS) triggers lipid peroxidation, DNA fragmentation, and protein denaturation, ultimately leading to apoptosis and necrosis of neuronal and glial cells [6, 7]. Simultaneously, activated microglia and infiltrating immune cells secrete proinflammatory mediators such as tumor necrosis factor - alpha and interleukin - 1 beta, perpetuating a toxic microenvironment that further impedes axonal regeneration and functional recovery [8]. Consequently, the dual targeting of oxidative stress and inflammation has emerged as a promising therapeutic strategy to attenuate secondary injury and facilitate neural repair after SCI.
Current clinical approaches for SCI remain largely palliative and have shown limited effectiveness in reversing neurological deficits. Surgical interventions primarily focus on spinal stabilization and decompression of the injured cord. While essential for mitigating mechanical compression, these procedures offer minimal benefit in preventing the molecular and cellular sequelae of secondary injury [9]. Pharmacological strategies, most notably high - dose methylprednisolone, have been widely adopted to modulate inflammation in the acute phase of injury. However, the therapeutic efficacy of methylprednisolone remains controversial due to its associated risks, including heightened susceptibility to infection, gastrointestinal complications, electrolyte imbalances, and damage to peripheral organs. Moreover, the drug's low bioavailability, suboptimal tissue accumulation, and narrow therapeutic window further restrict its clinical utility [10]. In light of these limitations, the scientific community has increasingly turned toward advanced and multifaceted therapeutic modalities, including cell - based therapies, exosome - mediated interventions, and nanomaterial - driven approaches (Table 1) [11 - 15]. Cell transplantation offers a regenerative platform by providing cellular scaffolding, promoting neurotrophic support, and modulating immune responses. Nevertheless, translational challenges - such as poor cell survival, immunogenicity, tumorigenic potential, and difficulty achieving long - term engraftment - have slowed clinical progress [11]. Exosome - based therapies have emerged as a promising cell - free alternative, delivering biologically active molecules capable of reducing inflammation, countering oxidative stress, and enhancing neuroprotection. However, major obstacles remain, including the need for scalable manufacturing, precise targeting, and adherence to stringent regulatory standards [14]. Nanomaterial - based strategies have also gained traction due to their versatility in modulating key pathological processes in SCI. These platforms demonstrate potential in scavenging ROS, downregulating inflammatory responses, and supporting tissue regeneration. Despite encouraging preclinical data, hurdles such as limited biocompatibility, off - target effects, and challenges in large - scale synthesis and standardization must be addressed before clinical implementation can be realized [15]. As research progresses, overcoming these barriers will be critical to unlocking the full therapeutic potential of next - generation interventions for SCI.
As an innovative class of nanomaterials with intrinsic enzyme - mimicking catalytic activities, nanozymes have rapidly gained attention as a promising frontier in the therapeutic landscape of SCI [16, 17]. These artificial enzymes possess several distinct advantages over their natural counterparts, including enhanced stability under physiological and pathological conditions, adjustable catalytic efficiency, cost - effectiveness, prolonged shelf - life, and resilience in harsh biochemical environments. Such attributes make nanozymes particularly well - suited for addressing the multifaceted pathophysiology of SCI [18]. Recent advances have underscored the potential of nanozymes in facilitating neuroregeneration and restoring function in preclinical models of SCI. By emulating the enzymatic actions of critical antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), nanozymes are capable of scavenging excessive ROS, thereby mitigating oxidative stress and fostering cellular repair mechanisms [19]. An expanding body of evidence demonstrates that the therapeutic efficacy of nanozymes in SCI primarily derives from their capacity to suppress oxidative damage and modulate the neuroinflammatory milieu [17, 20, 21]. Our research group has made significant strides in the design and development of nanozyme - based systems for both therapeutic intervention and real - time diagnostic assessment of SCI. For instance, we successfully engineered a selenium (Se) - based nanodrug platform, Se@NADH, which exhibits dual neuroprotective and neuroregenerative properties by restoring mitochondrial function and eliminating intracellular ROS. This system effectively reduces oxidative injury and prevents neuronal apoptosis [22]. Furthermore, we developed a novel Se - based dual - signal - responsive antioxidant probe, Se@BDP - DOH, which not only inhibits ROS - mediated cellular damage but also enables the dynamic assessment of SCI severity through reversible photoacoustic imaging signals at 680 and 750 nm [23]. Furthermore, we developed a novel Se - based dual - signal - responsive antioxidant probe, Se@BDP - DOH, which not only inhibits ROS - mediated cellular damage but also enables the dynamic assessment of SCI severity through reversible photoacoustic imaging signals at 680 and 750 nm [24]. An additional strength of nanozyme platforms lies in their modular architecture, which allows for surface functionalization with targeting ligands. This feature enhances the precision of delivery to the injured spinal cord, thereby improving therapeutic outcomes and minimizing systemic off - target effects [25]. Moreover, certain nanozymes exhibit the unique capacity to traverse the blood - brain barrier, an otherwise formidable obstacle in central nervous system (CNS) therapeutics. This capability broadens the potential application of nanozymes as versatile agents for the treatment of SCI and other neurological disorders [26, 27].
In this comprehensive review, we first outline the pathological mechanisms underlying SCI, emphasizing the cellular and molecular processes that contribute to injury progression. We then provide a detailed overview of the classification and catalytic mechanisms of nanozymes, followed by an in - depth discussion of their emerging applications in SCI therapy. Finally, we address the current limitations and future directions in nanozyme research, highlighting the key challenges that must be overcome to fully realize their diagnostic and therapeutic potential. We believe this timely synthesis of current advancements will offer valuable insights for the scientific community, further demystifying the design principles of nanozymes and accelerating their development for clinical translation in SCI management.
Pathophysiology of Spinal Cord Injury
Primary injury
SCI most commonly arises from acute, high - impact trauma - such as motor vehicle accidents, falls, or sports injuries - that result in vertebral fractures or dislocations. These mechanical insults, including forces of hyperflexion, hyperextension, shearing, and compression, directly damage the spinal cord, constituting the primary injury phase [3, 4, 28] (Figure 1). This phase encompasses both the immediate mechanical insult and sustained compression due to displaced bone fragments or hemorrhage. The limited regenerative capacity of CNS neurons, compounded by the formation of a hostile microenvironment, significantly impairs functional recovery. Nevertheless, emerging evidence suggests that even in seemingly complete SCI, a subset of axonal pathways may remain anatomically intact at the lesion site, providing a substrate for potential functional restoration under appropriate therapeutic conditions [29].
The mechanism of action, pros and cons of different therapy strategies for SCI
| Therapy strategies | Mechanism | Pros | Cons | Reference |
|---|---|---|---|---|
| Methylprednisolone | Anti-inflammatory | Fast - acting Well - defined mechanism | Side effects, narrow window | [10] |
| Cell transplantation | Promoting endogenous regeneration, myelin regeneration, and axon regeneration Restoring interneuronal communication | Multi - mechanistic repair Source diversity Trophic support Potential for functional recovery. | Poor cell survival in cerebrospinal fluid insufficient migration to target sites Loss of plasticity attachment to SCI surfaces Immune rejection Tumor formation Ethical and regulatory concerns | [11] |
| Exosomes | Preserving the integrity of the BSCB Inhibiting apoptosis Regulating inflammation | Multi - mechanistic repair Source diversity Excellent biocompatibility cargo versatility Stable and small in size | Heterogeneity of exosomes Low yield and scalability issues Unclear pharmacokinetics Limited targeting specificity Regulatory and manufacturing hurdles | [13, 14] |
| Nanomaterials | Anti-oxidative stress Anti-inflammatory Stimuli - responsive delivery Promoting neural regeneration and remyelination Reconstructing the microenvironment promoting angiogenesis | Multifunctionality Targeted delivery potential Stimuli - responsiveness Scaffold - like structural supporting Versatile drug/gene loading platform | Biocompatibility and toxicity concerns Biodistribution and clearance Regulatory hurdles | [15] |
| Nanozymes | Anti-oxidative stress Anti-inflammatory Stimuli - responsive delivery | High stability to mimic natural enzymes Tunable catalytic activity Low cost and long - term storage Robustness in harsh environments | Unclear Catalytic Mechanisms Lack of Standardized Evaluation Metrics | [17 - 21] |
The pathophysiology process and therapy of SCI.
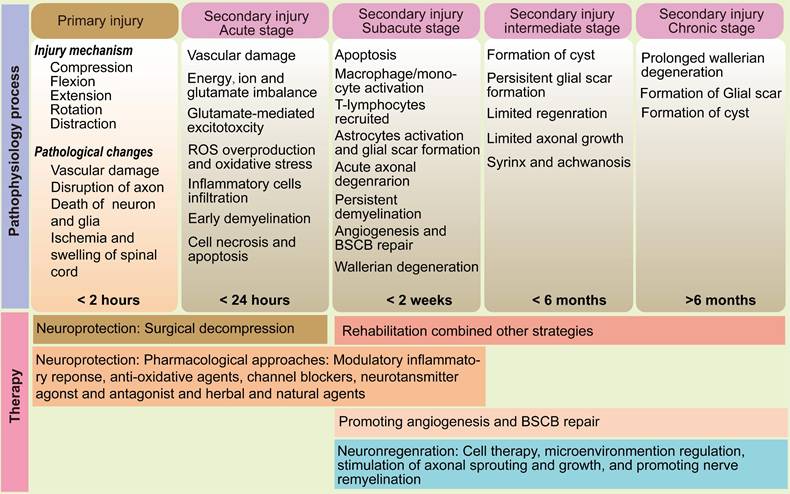
Secondary injury
Secondary injury unfolds as a complex and prolonged cascade of biochemical and cellular processes that exacerbate the initial damage and impede neurological recovery [30]. This pathological progression is classically divided into four overlapping phases: acute (< 2 days), subacute (2 days - 2 weeks), intermediate (2 weeks - 6 months), and chronic (>6 months) (Figure 1) [3]. In the acute phase, the disruption of vascular integrity leads to hemorrhage, ischemia, edema, and infiltration of immune cells. This is accompanied by the upregulation of proinflammatory cytokines and vasoactive mediators. The subacute phase is characterized by excitotoxicity - primarily driven by excess extracellular glutamate - which causes intracellular Ca2+ overload, mitochondrial dysfunction, and energy failure due to impaired adenosine triphosphate (ATP) synthesis [31, 32]. Neuroinflammation, a hallmark of secondary injury, involves the activation and recruitment of neutrophils, resident microglia, infiltrating macrophages, astrocytes, dendritic cells, and lymphocytes [33 - 35]. During this phase, acute axonal degeneration affects the proximal segments of injured axons, while Wallerian degeneration occurs distally. Both processes are mediated by shared downstream mechanisms such as cysteine protease activation, leading to progressive axonal fragmentation and demyelination [36, 37]. As the injury evolves into the intermediate and chronic stages, astrocyte proliferation and extracellular matrix deposition result in the formation of a dense glial scar. While this barrier limits the spread of inflammation and re - establishes the blood - spinal cord barrier (BSCB), it also presents a physical and biochemical obstacle to axonal regrowth [38]. Continued apoptosis of neurons and oligodendrocytes contributes to the development of fluid - filled cystic cavities surrounded by reactive astrocytes, further disrupting neural architecture and function.
Oxidative stress injury after SCI
Redox homeostasis is essential for maintaining cellular physiology and regulating numerous biological pathways, including metabolism, signal transduction, and gene expression [39]. Oxidative stress is a critical pathological mechanism in secondary SCI, resulting from a disrupted balance between oxidant generation and the capacity of endogenous antioxidant defense systems [40]. Among the most prominent reactive species implicated in this process are ROS and reactive nitrogen species (RNS), with ROS - including superoxide (O2·-), hydroxyl radical (·OH), and hydrogen peroxide (H2O2) - being particularly abundant. Antioxidant defenses are broadly categorized into enzymatic and non - enzymatic systems. Nonenzymatic antioxidants include glutathione (GSH), vitamin C, vitamin E, and β - carotene, while enzymatic systems comprise SOD, CAT, GPx, and glutathione reductase. Under normal conditions, neurons in the spinal cord generate relatively high levels of ROS due to their elevated metabolic activity [41], and reliance on oxidative phosphorylation for ATP production in mitochondria [42]. Physiologically, low concentrations of H2O2 play regulatory roles by reversibly oxidizing specific protein residues, thereby modulating protein function, intracellular signaling, and cellular processes such as migration, differentiation, angiogenesis, and cell cycle progression [43, 44]. Physiologically, low concentrations of H2O2 play regulatory roles by reversibly oxidizing specific protein residues, thereby modulating protein function, intracellular signaling, and cellular processes such as migration, differentiation, angiogenesis, and cell cycle progression [45].
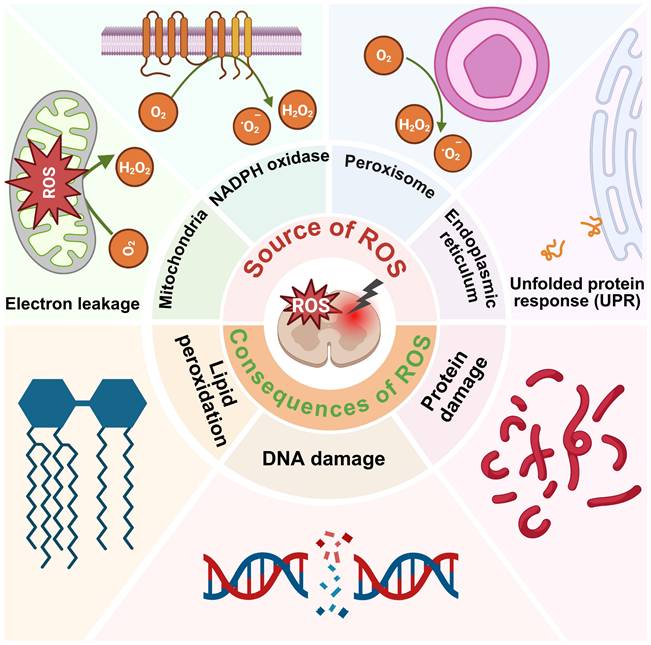
The Source of ROS in SCI
Under the pathophysiological conditions of SCI, vascular disruption leads to hypoxia, which contributes to electron leakage from the mitochondrial respiratory chain, excessive generation of ROS, and ATP synthesis [46]. Compromise of BSCB allows infiltration and activation of immune cells that, although initially protective, can exacerbate secondary damage. For example, activated macrophages generate O2·- via nicotinamide adenine dinucleotide phosphate oxidase activity, amplifying oxidative stress [47]. Iron, which plays a critical role in CNS functions such as oxidative phosphorylation and neurotransmitter synthesis, is typically sequestered by ferritin and transferrin. Following SCI, local acidosis disrupts iron homeostasis, releasing free iron that catalyzes the Fenton reaction, resulting in the formation of highly reactive ·OH [48]. Concurrently, inflammation and glutamate - induced excitotoxicity provoke endoplasmic reticulum (ER) stress, characterized by protein misfolding and intracellular Ca2+ dysregulation. During oxidative protein folding, enzymes such as protein disulfide isomerase and ERO1 transfer electrons to molecular oxygen (O2), generating additional ROS [49, 50]. Persistent ER stress and abnormal Ca2+ flux between the ER and mitochondria further augment ROS production and mitochondrial dysfunction, thereby promoting neural cell death (Figure 2) [50, 51].
Consequences of excessive ROS production after SCI
Due to its high O2 consumption and limited antioxidant defenses, the CNS is particularly vulnerable to oxidative stress. Overproduction of ROS damages proteins, lipids, and nucleic acids, leading to severe disruption of cellular homeostasis (Figure 2) [52, 53].
Lipid peroxidation is a key feature of secondary injury in SCI. ROS and RNS attack polyunsaturated fatty acids within cellular membranes, producing cytotoxic byproducts such as malondialdehyde and 4 - hydroxynonenal. These reactive aldehydes compromise membrane integrity, alter signaling pathways and initiate apoptotic or necrotic cell death [54 - 57]. ROS also induces protein damage through misfolding, chemical fragmentation, and irreversible cross - linking, resulting in the formation of insoluble aggregates that interfere with essential cellular processes [58]. In addition, ROS impairs the proteasome system, leading to the accumulation of damaged proteins and subsequent cell death [59]. Oxidative damage to nucleic acids is another major consequence of ROS overproduction. For instance, guanine residues in DNA are frequently oxidized to 8 - OHdG, which causes strand breaks, point mutations, and inhibition of DNA repair enzymes [60 - 62]. Persistent DNA damage ultimately leads to apoptosis or malignant transformation.
The catalytic mechanism and classification of nanozymes
As discussed, ROS are central mediators of oxidative damage in SCI, contributing to inflammation, blood - brain barrier disruption, and neuronal necrosis or apoptosis [63]. Therefore, therapeutic strategies aimed at enhancing antioxidant enzyme activity and scavenging ROS may offer significant potential for mitigating oxidative damage following SCI. The human body possesses several endogenous antioxidant enzymes - such as SOD, CAT, GPx, and glutathione reductase - which regulate redox homeostasis and protect against oxidative injury [64 - 66]. Although these enzymes exhibit high catalytic efficiency and substrate specificity, their application in clinical or industrial settings is hindered by limitations including costly purification, environmental sensitivity, low stability, and challenges in recycling and reuse [67].
To address these challenges, substantial research efforts have focused on developing artificial enzyme mimetics. Studies have identified a range of synthetic compounds - including fullerenes, cyclodextrins, polymers, dendrimers, porphyrins, metal complexes, and various biomolecules - that exhibit enzyme - like activity and structural similarity to natural enzymes [68 - 71]. A landmark discovery in 2007 revealed that Fe3O4 nanoparticles (NPs) function as peroxidase (POD) mimics, marking the advent of a new class of catalytic nanomaterials known as nanozymes [72]. Since then, extensive investigations have explored the potential of nanozymes in biomedicine, catalysis, and environmental applications. The following section provides a detailed overview of the classification and catalytic mechanisms of nanozymes, highlighting their relevance and applicability in SCI treatment.
Mechanisms of antioxidant - like nanozymes catalytic in SCI
Nanozymes are a class of catalytically active nanomaterials that emulate the functional properties of natural enzymes. Based on their catalytic characteristics, CAT - like nanozymes can be classified into four major groups: oxidative enzymes, hydrolytic enzymes, synthetic enzymes, and isozymic enzymes. Among these, oxidative enzyme - type CAT - like nanozymes are the most prevalent, accounting for over 90% of all known CAT - like nanozymes [19]. This category includes catalytic nanozymes that mimic the activity of natural antioxidant enzymes such as SOD, CAT, and GPx [73]. Importantly, nanozymes that mimic antioxidant functions play a crucial role in modulating the production and elimination of ROS and RNS. By restoring redox homeostasis, these CAT - like nanozymes have demonstrated significant therapeutic potential in the treatment of SCI [74].
SOD - like nanozymes
O2•-, one of the primary ROS produced in mammalian cells, serves as a precursor to several other reactive species [41]. SOD enzymes tightly regulate intracellular O2·- levels by catalyzing its dismutation into H2O2 and O2 [75]. Disruptions in SOD function - due to mutations or dysregulation - can impair this catalytic activity, potentially leading to pathological consequences and contributing to the onset and progression of SCI.
The overproduction and consequence of ROS after SCI. Created with BioRender.com.
Mechanisms of the SOD - like activity of nanozymes. A - D. The surface structure of nanzymes contribute to the SOD - like activity of nanozyme. Schematic representation of the catalytic interaction mechanism of C3 and O2•-. Adapted with permission from [68], Copyright 2004 Elsevier. B. Illustration modifications of amino, carboxyl, and hydroxyl groups on the surface of C - dots. C. SOD - like activity of C - dots - SPI. D. SOD - like activities of C - dots - PS, and C - dots - PS - Hy. Adapted with permission from [77], Copyright 2023 Wiley. E. The reaction mechanism for the dismutation of superoxide of cerium oxide nanoparticle, which is the directly convertible valence state and oxygen vacancy of Ce3+ and Ce4+. Adapted with permission from [78], Copyright 2011, Royal Society of Chemistry.

To date, over 100 nanozymes have been reported to exhibit SOD - like activity. A breakthrough in this field was the discovery that fullerene and its derivatives could mimic SOD activity [76]. Mechanistic studies have shown that electron - deficient regions on the C60 molecule, in combination with malonyl functional groups, electrostatically stabilize O2·-, facilitating its dismutation [68]. Due to their robust ROS - scavenging capacity, fullerene derivatives have been widely explored in antioxidant applications, including neuroprotection and anti - aging therapies. Subsequently, a growing number of nanomaterials have been identified with SOD - like catalytic properties, capable of converting O2·- into H2O2 and O2, with the generated H2O2 further decomposed into H2O and O2 by CAT.
The SOD - like activity of many nanozymes is closely related to their nanostructure. For example, CD nanozymes have been constructed to display high SOD - like activity, comparable to that of the native SOD enzyme [68]. In the proposed model, electron - deficient sites on the C60 sphere act synergistically with malonyl groups attached to the C3 position (i.e., C3 - tris - malonyl - C60 derivatives) to guide and stabilize O2·- through electrostatic interactions, thereby facilitating its dismutation (Figure 3A). Additionally, CDs exhibit SOD - like activity through the binding of O2·- to functional groups such as ·OH, carboxyl, and amino moieties, while carbonyl groups oxidize the anions. This reaction produces O2 and reduces the CDs [77]. The reduced CDs are then reoxidized by another O2·-, generating H2O2 in the process (Figure 3B - D).
Charge transfer and electron relay also contribute to the SOD - like activity of certain nanozymes. CeO2 is a notable example, exhibiting SOD - like properties through redox - active transitions between Ce3+ and Ce4+ states. The catalytic performance of CeO2 primarily arises from its O2 vacancies and the ability of Ce3+ and Ce4+ to bind O2·- and transfer electrons during redox reactions involving H2O2 (Figure 3E) [78, 79]. Notably, decreasing the particle size of CeO2 enhances the density of O2 vacancies and promotes the formation of Ce3+, significantly improving its catalytic activity. Consequently, CeO2NPs smaller than 5 nm have been extensively studied as effective SOD mimics [80]. Unlike fullerene - based SOD nanozymes, the antioxidant properties of CeO2 stem from its combined SOD - and CAT - like activities [81]. This dual enzyme - mimetic capability positions CeO2 as a promising candidate for therapeutic applications in SCI [82].
CAT - like nanozymes
H2O2 is recognized as a major ROS involved in the redox regulation of biological processes. Under physiological conditions, intracellular H2O2 concentrations are tightly regulated and maintained at low nanomolar levels. At these concentrations, H2O2 functions as a signaling molecule, contributing to redox homeostasis. However, under pathological conditions, such as following SCI, the excessive accumulation of H2O2 induces oxidative stress and can ultimately lead to cellular damage and death [41]. CAT is an essential antioxidant enzyme that catalyzes the decomposition of H2O2 into water and O2, thereby protecting cells from oxidative damage. Its enzymatic activity plays a critical role in maintaining cellular redox balance and preventing damage associated with SCI.
The concept of CAT - mimetic nanozymes was first introduced in 2007, when CP - Au/Pt bimetallic NPs were found to exhibit both SOD - and CAT - like activities, offering promising therapeutic potential for oxidative stress - related diseases [84]. Since then, a wide variety of nanomaterials - including metals, metal oxides, MOFs, C - based nanomaterials, and single - atom catalysts - have been investigated for their CAT - mimetic properties, greatly expanding their potential applications in medicine [83]. These developments underscore the significant promise of nanozymes in combating oxidative stress and related disorders.
The catalytic mechanism of CAT - like nanozymes primarily involves redox reactions and substrate activation through adsorption and typically follows Michaelis-Menten kinetics. Their catalytic efficiency generally increases with higher concentrations of both substrates and nanozyme mimics [19]. These nanozymes promote electron transfer during redox reactions, effectively mimicking the function of natural CAT enzymes. The adsorption of substrate molecules onto the nanozyme's active surface is critical for enhancing catalytic performance, as it stabilizes reaction intermediates and optimizes the spatial arrangement for efficient electron transfer. For instance, the CAT - like activity of CeO2 is influenced by the valence state of Ce during redox cycling. H2O2 molecules interact with Ce4+ sites on the CeO2 surface, resulting in O - H bond cleavage and the release of water. Electrons from H2O2 are then transferred to Ce3+, reducing Ce4+ in the process. Thus, a higher proportion of Ce4+ on the surface enhances CAT - like catalytic activity [85]. The adsorption - activation mechanism is also closely associated with the catalytic behavior of noble metal nanozymes [86]. These NPs possess highly reactive surfaces capable of interacting strongly with H2O2. In acidic environments, the positive charge on the metal surface enhances H2O2 adsorption. Once adsorbed, the H2O2 molecules become more reactive and easier to decompose (Figure 4A) [87].
Density functional theory (DFT) calculations have also been used to explore the catalytic mechanisms of CAT - like nanozymes. H2O2 contains two types of chemical bonds: the H - O bond and the O - O bond. Its decomposition can proceed through two pathways: heterolytic or homolytic cleavage (Figure 4B). In the heterolytic cleavage pathway, the H - O bond is preferentially broken. DFT studies indicate that the CAT - like activity of CeO2 nanozymes follows this heterolytic pathway [88]. In contrast, the homolytic pathway involves breaking the O - O bond first. For example, Pd@TiO2 nanozymes have been synthesized, and both DFT analyses and experimental data show that the decomposition of H2O2 on Pd@SiO2 proceeds through a homolytic mechanism [83, 89].
GPx - like nanozymes
GPx is a key antioxidant enzyme responsible for preserving intracellular redox homeostasis. Enzymes in the GPx family catalyze the reduction of H2O2 to water, using GSH as a reducing agent. In addition to detoxifying H2O2, certain GPx isoforms also reduce lipid hydroperoxides to their corresponding alcohols, thereby safeguarding cells against oxidative damage [92, 93]. Several Se - containing small molecules with GPx - mimetic activity have been identified in mammalian systems [94]. The antioxidant efficacy of these compounds stems from their redox - active Se centers, which are integral to their catalytic behavior.
Mechanisms of the CAT - like activity of nanozymes. A. A model of the reaction mechanism for the complete dismutation of H2O2 by CeO2 nanopartcles (NPs). The reductive half involves binding of H2O2 to the two Ce3+ site (5), uptake of two protons and homolysis of the O - O bond with transfer of electrons to the two Ce3+ (6), and release of the H2O molecules to regenerate the initial Ce4+site (1). Adapted with permission from [78], Copyright 2011 Royal Society of Chemistry. B. Illustration of the mechanism of CAT - like nanozymes. The ring on the left represents the heterolysis catalytic reaction path, while the double ring on the right represents the homolytic catalytic reaction path. Adapted with permission from [83], Copyright 2022 Wiley.

These Se - based agents have shown promise in mitigating oxidative stress and may serve as potential therapeutic agents. For instance, Mugesh et al. reported that vanadium pentoxide (V2O5) nanowires exhibit GPx - like activity and provide robust protection against oxidative stress - induced cellular injury [95]. These nanowires are efficiently internalized by various cell types and confer significant cytoprotection by complementing the endogenous antioxidant machinery. They inhibit ROS - mediated damage to critical biomolecules, including proteins, lipids, and DNA. Although numerous nanomaterials have been developed to mimic GPx activity, their catalytic mechanisms remain relatively underexplored compared to those of natural GPx. Current studies suggest that GPx - like nanozymes operate via two principal catalytic pathways: the ping - pong mechanism and the ordered mechanism.
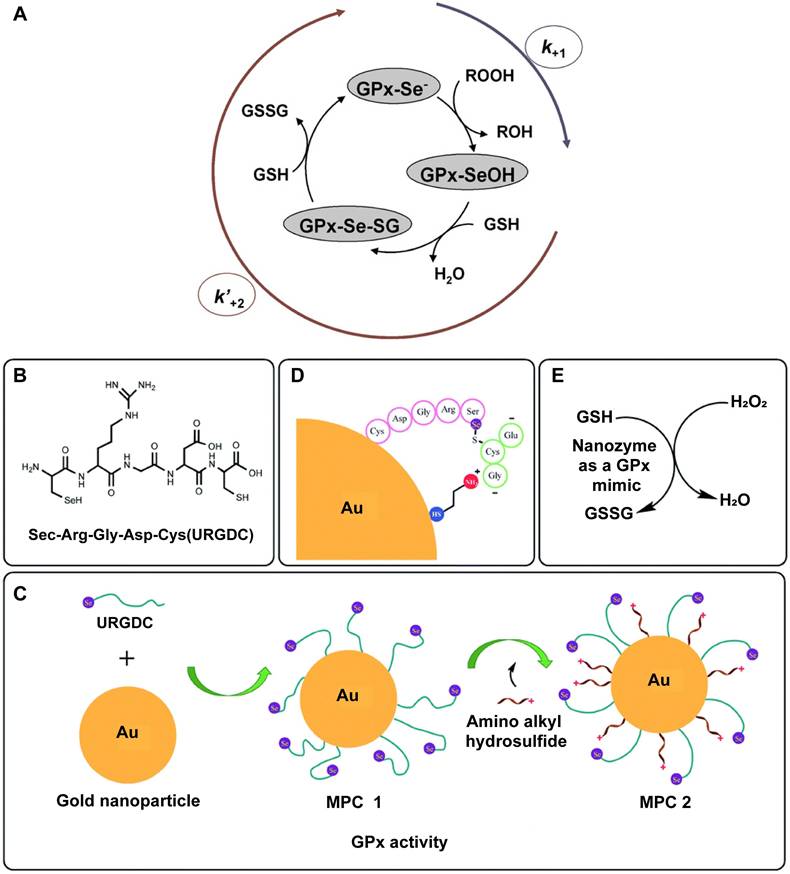
In the ping - pong mechanism, the enzyme alternately binds and releases substrates and products in a sequential manner. For natural GPx, hydroperoxides first oxidize the active selenol group ( - SeH) to selenenic acid ( - SeOH). This intermediate is then reduced by one GSH molecule to form a selenenyl - sulfide ( - SeSG), which is subsequently reduced by a second GSH molecule, regenerating the - SeH form and completing the catalytic cycle (Figure 5A) [90]. Analogously, several GPx - like nanozymes follow this ping - pong catalytic pathway. During this process, hydroperoxides oxidize the nanozyme's active sites to generate peroxide intermediates [95 - 97]. Initially, H2O2 interacts with the V2O5 surface, producing a vanadium - peroxide intermediate. Subsequently, GSH attacks the polarized peroxido linkage, forming a vanadium - dihydroxo species. This step concurrently yields glutathione sulfenic acid, which then reacts with an additional GSH molecule to form glutathione disulfide. The formation of the vanadium - peroxide intermediate via H2O2 binding to the V = O site is essential, as it initiates the entire catalytic sequence [95]. Beyond this classical catalytic route, an alternative mechanism involves redox changes in the valence state of the active centers. In this scenario, the active site interacts first with GSH, altering its oxidation state before engaging with H2O2. A notable example is the GPx - like catalytic cycle of copper vanadate (CuV2O6), in which GSH preconditions the vanadium center, resulting in a +IV oxidation state [98]. Upon subsequent exposure to excess H2O2, both +IV and +V vanadium species are detected, indicating active participation of H2O2 in redox cycling. Similar redox - mediated catalytic behavior has been observed in other metal - based nanozymes, including those incorporating Mn and Cu [99, 100].
In 1997, Santimone et al. proposed that GPx operates via a sequential ordered mechanism, rather than the previously suggested ping - pong mechanism [101]. TThis ordered mechanism involves the stepwise binding of substrates to the enzyme, forming an enzyme - multisubstrate complex. Product release only occurs after all substrates have successfully bound. Specifically, in one catalytic cycle, GPx interacts with one molecule of H2O2 followed by two molecules of GSH in a defined sequence, ultimately yielding glutathione disulfide and regenerating the enzyme. To date, only one nanozyme with GPx - like activity has been reported to follow this ordered mechanism. A representative example involves a structurally refined Se - containing pentapeptide, engineered with a cysteine residue at the C - terminus. The thiol group of this cysteine facilitates binding to AuNPs, which act as a scaffold to restrict the mobility of the peptide. This structural confinement maximizes the exposure of selenocysteine groups to the catalytic environment, promoting efficient interaction between GSH, H2O2, and the active site. Moreover, the AuNPs were further modified with cysteamine through Au - S covalent bonding, establishing an electrostatic framework that guides GSH - specifically its thiol group - toward the Se active site during each reduction step. This precise substrate orientation enhances the catalytic efficiency of the system (Figure 5B - E) [91]. In this mechanism, the enzyme - like activity of the nanozyme is contingent upon substrate binding in a strictly sequential order, mirroring the behavior of natural GPx.
Multiple enzyme - like nanozymes
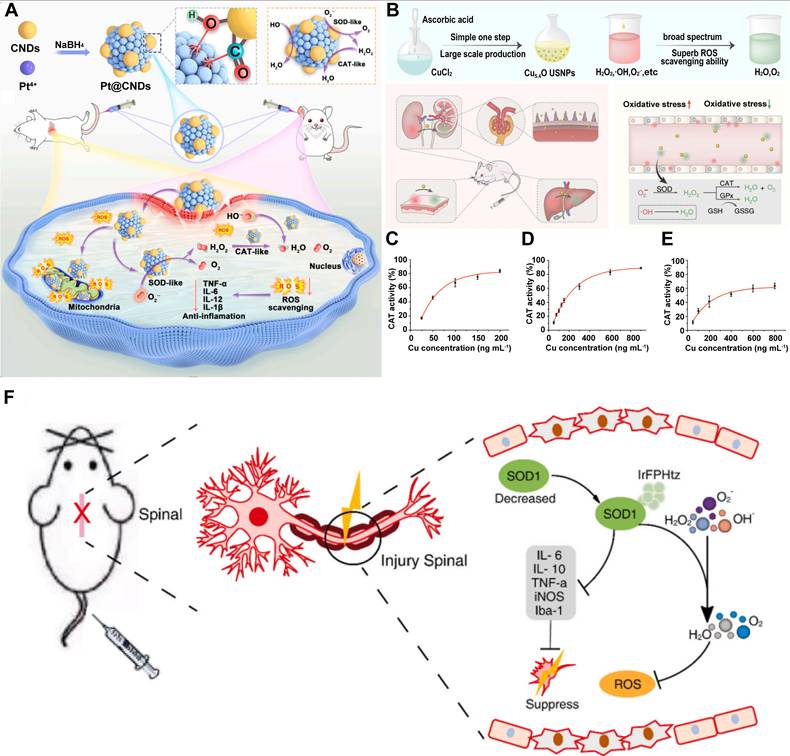
Natural enzymes typically exhibit a single catalytic function. However, in biological systems, multiple enzymes often operate in tandem to perform cascade reactions. For example, SOD and CAT act synergistically to convert O2·- first into H2O2 and then into water and molecular O2, thereby efficiently mitigating oxidative stress and preserving redox homeostasis [102]. To replicate these complex enzymatic networks, researchers have developed multienzyme - like nanozymes - nanomaterials that exhibit multiple enzyme - mimicking activities. These multifunctional nanozymes have attracted considerable interest for their potential applications in biomedicine, particularly in antioxidant therapy and disease treatment. One prominent example is the development of a Pt@CNDs nanocomposite, created by integrating CNDs with PtNPs. This nanozyme demonstrated exceptional SOD - and CAT - like activities, with specific activities of 12,605 U/mg and 3,172 U/mg, respectively.
The mechanisms of the GPx - like activity of nanozymes. A. Ping - pong mechanism of natural GPx. Adapted with permission from [90], Copyright 2013 Elsevier. B - E. Design of the nanozyme with GPx activity: B. The structural formula of the URGDC with -SeH at the N - terminus and -SH at the C - terminus. C. The construction of the nanozyme, with the GPx activity hypothesized to increase in order. This is a two - step process: first, URGDC was conjugated to gold nanoparticle (MPC 1); second, a mixed self - assembled monolayer was formed by further adding cationic amino alkyl hydrosulfide. D. The schematic representation of the active site of MPC 2. Structural insights into the recognition and binding of the substrate GSH with MPC 2. The positively charged amino group directs the donor substrate GSH towards the catalytic center in such a way that its sulfhydryl group must react with the selenium moiety. E. The mechanism for nanozyme - catalyzed reduction of H2O2 by GSH. Adapted with permission from [91], Copyright 2020 Royal Society of Chemistry.
Pt@CNDs effectively scavenged excessive ROS in both in vitro and in vivo models, thereby protecting biological systems from oxidative damage (Figure 6A) [103]. Similarly, Cu - based nanozymes have been engineered to possess multienzyme - mimicking capabilities for ROS elimination and inflammation mitigation. Liu et al.[104] for instance, synthesized ultrasmall Cu5.4ONPs that exhibited simultaneous SOD - , CAT - , and GPx - like activities. These nanozymes provided potent cytoprotection against ROS - induced cellular damage even at very low concentrations and significantly improved therapeutic efficacy (Figure 6B - E). Multienzyme - like nanozymes have also been designed for SCI treatment [105 - 107]. In our previous work, we reported a highly bioactive iridium metal complex (IrFPHtz) that displayed both SOD - and CAT - like enzymatic activities. This compound significantly enhanced resistance to oxidative stress and reduced inflammation, thereby improving therapeutic outcomes in SCI models (Figure 6F) [108]. Mechanistically, the functionality of multienzyme - like nanozymes arises from the combined catalytic activities of their individual enzyme - mimicking components. As the specific mechanisms underlying these individual activities have been discussed in previous sections, they are not repeated here.
A. Synthesis of Pt@CNDs with cascade superoxide dismutase - catalase activities and applications of Pt@CNDs in eliminating intracellular ROS. Adapted with permission from [103], Copyright 2023 Elsevier. B. Schematic illustration of Cu5.4O ultrasmall nanoparticles in the treatment of ROS - related diseases. Cu5.4O ultrasmall nanoparticles with multenzyme - mimickin g and broad - spectrum ROS scavenging ability are synthesized by a simple and green method. Due to the robust ROS scavenging ability in vivo, Cu5.4O ultrasmall nanoparticles exhibit therapeutic effect against broad ROS - related diseases. C. CAT - like, D. SOD - like, and E. GPx - like activity of Cu5.4O USNPs. Adapted with permission from [104], Copyright 2020 Springer Nature. F. Schematicof the synthetic approach of the complex IrFPHtz and its neuroprotective application mechanisms against SCI. Adapted with permission from [108], Copyright 2022 Elsevier.
Classification of nanozymes
The field of nanozymes is advancing at an extraordinary rate, as evidenced by the exponential rise in scientific publications and patent filings each year. This growth underscores their scientific importance and broad application potential. Despite the absence of a universally accepted classification system - owing to their structural and functional diversity - this review categorizes nanozymes into five main types: metal - based nanozymes, metal - organic framework (MOF) - based nanozymes, single - atom nanozyme (SAzymes), carbon (C) - based nanozymes, and other nanozymes. This classification primarily reflects their core material composition and catalytic architecture, in line with prevailing trends in recent nanozyme research.
Metal - based nanozymes
Metal - based nanozymes, the first to be discovered, have attracted significant interest as therapeutic candidates due to their outstanding catalytic performance [109]. They can be further subdivided into monometallic, metal oxide, and metal - hybrid nanozymes. Owing to the presence of unsaturated metal atom sites and valence variability - particularly among transition metals - these nanozymes often exhibit multi - enzyme mimetic activities. Their potent antioxidant and anti - inflammatory properties position them as promising agents for mitigating oxidative stress and inflammation, particularly in the context of oxidative stress - induced inflammatory disorders [110]. Typically, metal - based antioxidant nanozymes are composed of metallic or metal - doped nanomaterials that possess at least two or more enzyme - like activities, such as SOD - like, CAT - like, and GPx - like functions. Those with both SOD - like and CAT - like activity are particularly effective in scavenging ROS while simultaneously generating molecular O2. The SOD - like function catalyzes the dismutation of O2·- into H2O2 and O2). Subsequently, CAT - like activity decomposes H2O2 into water (H2O) and O2, thereby preventing the harmful accumulation of H2O2 and maintaining redox homeostasis [114].
Mechanistically, the enzyme - mimetic activities of metal - based nanozymes stem from the intrinsic characteristics of monometallic structures, valence fluctuations in metal oxides, and atomically dispersed metal sites in metal coordination frameworks [115 - 117].
Size and Morphology Optimization
The catalytic activity of nanozymes is significantly influenced by their size and morphology. For instance, manganese(II,III) oxide (Mn3O4) nanomaterials synthesized in various shapes and sizes were evaluated for their enzyme - mimicking properties. Among them, Mn3O4 NPs with a flower - like morphology exhibited superior SOD - like activity, attributed to their increased surface area and substantially larger pore size (Figure 7A - D) [111]. This underscores the importance of surface architecture in enhancing nanozyme efficacy.
Enhancing Oxygen Vacancies
Another key strategy to boost nanozyme catalytic performance is the enhancement of surface O2 vacancies. The introduction of electron - rich catalytic sites can significantly improve electron redistribution and promote electron accumulation at defect sites, which facilitates multielectron redox reactions [118]. In one example, electron - dense Ru clusters were integrated into nonstoichiometric copper hydroxide (Ru/def - Cu(OH)2), a material rich in O2 vacancy defects. This nanozyme demonstrated effective ROS - scavenging capabilities, efficiently attenuating inflammatory cascade responses and modulating the endogenous microenvironment in SCI models (Figure 7E - G) [112].
Valence State Tuning and Composition Optimization
Adjusting the composition ratio represents another effective strategy to optimize the enzyme - mimetic activity of nanozymes. Metal - based nanozymes, owing to their flexible valence states, can mimic a broad range of cascade oxidase activities. By fine - tuning the ratio between oxidized and reduced states, nanozymes exhibiting both oxidizing and reducing functions can be progressively optimized, thereby enhancing their potential for clinical application. A representative example is copper (Cu) - based nanozymes, where different Cu valence states impart distinct catalytic functions. Cu NPs demonstrate excellent catalytic efficiency in scavenging H2O2 and O2·-, although they are less effective against ·OH. In contrast, cuprous oxide (Cu2O) NPs exhibit strong catalytic properties that facilitate electron transfer reactions, enabling the neutralization of H2O2 and ·OH, thus partially replicating POD activity. Notably, Cu2O NPs coated with a Cu2O shell, forming a Cu/Cu2O core - shell structure, show optimized ratios of Cu⁰ and Cu⁺, and demonstrate enhanced scavenging of H2O2, ·OH, and O2·- [104]. Another example is the cerium oxide (CeO2)/Mn3O4 nanozyme system, in which enzyme - like activity is significantly enhanced through compositional optimization. These nanozymes are composed of CeO2, enriched in Ce3+, and finely dispersed across root - like Mn3O4 nanostructures that contain O2 vacancies and reactive Mn3+ sites. This unique configuration provides superior catalytic activity compared to pure CeO2 or Mn3O4, especially in terms of CAT - and SOD - like functionalities (Figure 7I - J) [113].
In summary, the catalytic activity of metal - based nanozymes can be modulated through various strategies, including size and morphology modification to increase active surface area, introduction of O2 vacancies to enhance redox activity, and compositional tuning to optimize valence states [117, 119, 120].
Strategies of promoting enzyme mimetic activities of mental - based nanozymes. A - D. Modifying the size and morphology for enhancing the number of active sites: A. Structural parameters of different morphology of Mn3O4 nanoparticles including M1 (cubes), M2 (polyhedron), M3 (hexagonal plates), M4 (flakes) with Mnf (flower) determined by SEM, BET and BJH analyses. B - D. Comparison of CAT (B), GPx (C) and SOD - like (D) activity of different morphology of Mn3O4 nanoparticles. Adapted with permission from [111], Copyright 2017 Wiley. E - H. Increasing surface oxygen vacancies to influence enzymatic activity: E. The unique oxygen vacancies in Cu(OH)2 promote the formation of electron - rich Ru clusters, thus increasing the biocatalytic ROS scavenging capabilities of Ru/def - Cu(OH)2. F. Optimized pentahedral crystal structure model for Ru/def - Cu(OH)2. G. The left shows the optimized structure models for Ru/def - Cu(OH)2. The right shows the corresponding 2D plane average charge. H. The left side exhibits charge density differences of Ru/def - Cu(OH)2 before H2O2 adsorption, after one H2O2 adsorption, and after two H2O2 adsorption (cyan and yellow represent charge depletion and accumulation, respectively, the cutoff of the density difference isosurface is 0.01 e/Bohr3) and the right side exhibits the corresponding PDOS analysis. Adapted with permission from [112], Copyright 2024 Wiley. I - J. Adjusting the composition ratio to optimize the valence states ratio: I. Three steps of CeOx/Mn3O4 synthesis. J. Diagram of electron transfer between Mn and Ce atoms. Adapted with permission from [113], Copyright 2025 Wiley.

MOF - based nanozymes
MOFs, formed through the coordination of metal ions or clusters with organic ligands, have emerged as a highly promising class of materials due to their ordered porous structures. These frameworks are structurally tunable, easily modifiable, and possess excellent biocompatibility, making them particularly attractive for therapeutic applications [105]. The abundant channels and cavities within MOF structures facilitate the transport and diffusion of small molecules. Additionally, the ordered arrangement of secondary building units (SBUs) maximizes the utilization of active sites, offering a significant advantage over conventional granular materials [121]. Certain MOFs inherently exhibit enzyme - like activity and can directly participate in biochemical reactions within biological systems, thus functioning as nanozymes. Their stable mesoporous architecture and interconnected channels promote efficient substrate interaction and product transport. These structural features, coupled with synergistic interactions among components, result in enhanced catalytic performance [122]. Furthermore, nanozymes derived from a post - synthetic modification of MOFs can exhibit intrinsic defects or atomic unsaturation at coordination sites, thereby increasing the availability of active catalytic sites. Thus, the development of MOF - based nanozymes represents a viable and effective strategy for significantly improving catalytic activity. Given the considerable advantages of MOF materials in enhancing nanozyme functionality, the following sections describe various strategies and synthetic approaches for the fabrication of MOF - derived nanozymes.
Constructing MOF Directly
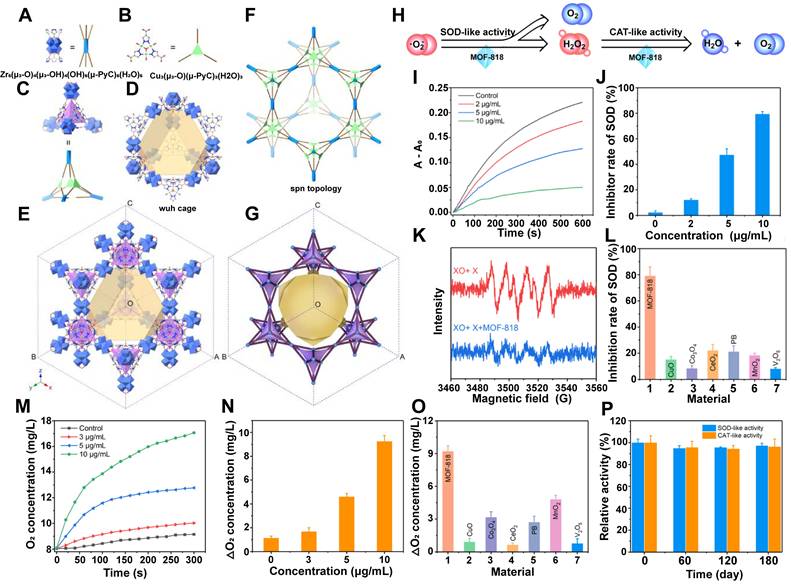
As previously described, MOFs are porous crystalline materials composed of metal ions or clusters coordinated with organic ligands. Some MOFs demonstrate inherent enzyme - like activity by facilitating catalytic reactions through their metal centers or organic ligands. This implies that MOF - based nanozymes can be obtained by directly constructing MOF nanomaterials with catalytic capabilities. A representative example is MOF - 818, a mesoporous cage - like nanostructure composed of dual metal centers (Cu and Zr) and H2PyC organic ligands [123]. As shown in figure 8A - G, the architecture of MOF - 818 primarily comprises Zr - SBU, formed by the coordination of carboxylate groups in H2PyC with Zr6O8 clusters. Additionally, Cu - SBU is formed by coordinating the pyrazole groups in H2PyC with Cu ions, collectively yielding a unique wuh - cage structure. MOF - 818 exhibits exceptional SOD - and CAT - like enzyme activities, effectively scavenging ROS (Figure 8H - P). When combined with hydrogels, it has demonstrated therapeutic efficacy in the treatment of diabetic chronic wounds [122]. Moreover, MOF - 818 has been successfully employed in the prevention of calcium oxalate kidney stones by mitigating oxidative stress and inflammation [124].
Co - precipitation
Co - precipitation represents another effective strategy for synthesizing MOF - based nanozymes. This method enables the simultaneous formation of MOF and the incorporation of catalytic species. Mechanistically, it utilizes MOFs as protective scaffolds that encapsulate and uniformly distribute catalytic NPs throughout the structure. For example, Ptzyme@ZIFs were synthesized by dispersing and confining platinum (Pt) NPs within the zeolitic imidazolate framework - 8 (ZIF - 8) framework. These materials exhibit cascade SOD - and CAT - like activities (Figure 9A) [125]. One of the major advantages of this approach is the mild reaction conditions, which are especially suitable for encapsulating sensitive biological molecules, such as natural enzymes, into MOFs. ZIF - type MOFs are frequently employed in this context because they can be synthesized in aqueous media at room temperature, facilitating integration with biomolecules [129].
3.1.2.3 Surface Modification
In addition to the aforementioned strategies, MOF - based nanozymes can also be synthesized by functionalizing the MOF surface with catalytic species via physical adsorption or covalent bonding. For instance, Xu et al. covalently attached Se molecules (PhSeBr) to a Zr(IV) - based UiO - 66 - NH2 framework. In this system, the PhSeBr molecules acted as functional catalytic sites with GPx - like activity. The high surface area and uniform porosity of the MOF facilitated the formation of numerous catalytically active centers (Figure 9B) [126]. Another example is the synthesis of Ce - based UiO - 66 - CH3, which demonstrated the ability to rapidly neutralize excessive ROS, restore mitochondrial energy balance, and modulate the immune microenvironment. These combined effects supported continuous regeneration in SCI models (Figure 9C) [127]. A multifunctional nanozyme, Cu - TCPP - Mn, was developed by integrating Cu and manganese (Mn) atoms into a tetrachloroporphyrin (TCPP) framework. Initially, TCPP was combined with Cu(NO3)2 using a bottom - up synthesis approach assisted by benzoic acid. Subsequently, Mn2+ ions were introduced into the Cu - TCPP structure, yielding bimetallic Cu - TCPP - Mn nanosheets (Figure 9D). This bimetallic MOF nanozyme exhibited enhanced SOD - and CAT - like activities and proved to be an effective ROS scavenger and inflammation suppressor [128].
Directly Constructing of MOF - based Nanozymes:A - G. Structures of MOF - 818, including the structure of Zr - SBU (A), Cu - SBU (B) used to construct mesoporous cages, supertetrahedron (I) (C), structure of mesoporous wuh cage (D), structure of MOF - 818 (E), spn topology (F), and corresponding tiling (G). Adapted with permission from [123], Copyright 2019 American Chemical Society. H. Schematic illustration of the SOD - and CAT - like activities of MOF - 818. I. Time - dependent absorbance changes of A - A0 (560 nm) with different concentrations of MOF - 818. J. Elimination efffciency of O₂·- with different concentrations of MOF - 818. K. EPR spectra analysis of O₂·- - scavenging by MOF - 818 (10 μg/mL). L. Comparison of the SOD - like activity of MOF - 818 with other typical antioxidative materials. M. Kinetic curves of O2 generation from the decomposition of H2O2 (20 mM) in the presence of different concentrations of MOF - 818. N. Net oxygen generation from H2O2 catalyzed by different concentrations of MOF - 818. O. Comparison of CAT - like activity of MOF - 818 with other typical antioxidative materials. P. Time - dependent activities of MOF - 818 dispersed in aqueous solution. Adapted with permission from [122], Copyright 2022, American Chemical Society.
Pyrolysis
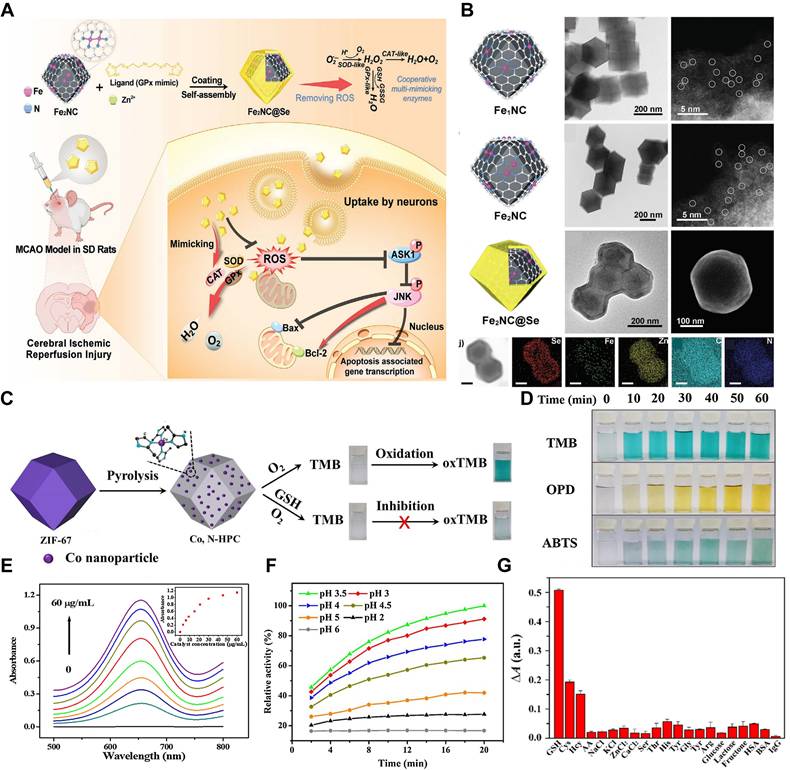
Pyrolysis represents another key strategy for constructing MOF - based nanozymes. In this approach, MOFs act as sacrificial templates, undergoing direct carbonization or oxidation to yield functional nanomaterials. The resulting products - such as metal - nitrogen (N) - doped carbon (M - NC), metal/C nanocomposites, and metal oxide/C hybrids - exhibit intrinsic enzyme - mimicking activities [130]. For example, a binuclear Fe2NC nanozyme was synthesized using a “precursor - preselected“ wet - chemical carbonization method. Specifically, Fe2(CO)9 was encapsulated within a ZIF - 8 framework to form Fe2@ZIF - 8 composites, which were then pyrolyzed at 800°C for 3 h under an argon atmosphere. The resulting Fe2NC nanozyme displayed robust SOD - , CAT - , and oxidase - like activities. To further enhance its functionality, Se - containing diimidazole was used as an organic ligand to form a MOF shell around the Fe2NC nanozyme, with Se serving as the GPx - active site and Zn2+ coordinating with the ligand. The final product, Fe2NC@Se, provided strong protection against oxidative stress by scavenging ROS, reducing cellular damage, and inhibiting apoptosis (Figure 10A - B) [131]. Pyrolysis has also been instrumental in developing novel MOF - derived nanozymes. For instance, a cobalt - and N - co - doped high surface area porous carbon (Co, N - HPC) was synthesized by pyrolyzing ZIF - 67. This nanozyme exhibited strong oxidase - like activity and catalyzed the transformation of colorless substrates - such as TMB, OPD, and ABTS - into chromogenic products without requiring H2O2. The high catalytic efficiency of Co, N - HPC was attributed to its large surface area, high N content, and reduced particle agglomeration (Figure 10C - G) [132]. Furthermore, these pyrolysis - derived nanozymes maintained the desirable characteristics of the original MOFs, including high structural stability, uniform pore size distribution, and resistance to physiological degradation.
In summary, MOFs have emerged as a highly attractive class of materials for biomedical and catalytic applications, owing to their tunable structures, excellent biocompatibility, and porous architecture that supports efficient molecular transport. Among these, MOF - based nanozymes stand out for their ability to mimic natural enzymes, making them particularly effective in ROS scavenging and inflammation control. Several fabrication approaches - including direct synthesis, co - precipitation, surface functionalization, and pyrolysis - have been successfully employed to enhance the catalytic performance of these nanozymes. Compared to conventional granular materials, MOF - derived nanozymes offer distinct advantages such as higher surface area, controlled porosity, structural integrity, and adaptability, positioning them as highly promising candidates for therapeutic and catalytic use.
Synthesis strategy of MOF - based nanozymes:Co - precipitation and Surface Modification:A. Schematic illustration of Ptzymes integrated L - and D - chiral ZIFs and their therapeutic mechanism on PD based on remission of both apoptosis and ferroptosis on neurons injured by excessively produced ROS and disordered inflammation. Adapted with permission from [125], Copyright 2023 Springer Nature. B. Schematic illustration of the UiO - 66 - Se catalysts obtained by PSM performed on UiO - 66 - NH2. Adapted with permission from [126], Copyright 2018 Springer Nature. C - E. Preparation and the potential mechanisms of Gel - Cr/Ce@PDA in treating SCI. C. Preparation and comparison of Ce - Uio - 66 - X. D. Preparation of Gel - Cr/Ce@PDA. E. Schematic diagram showing the mechanism Gel - Cr/Ce@PDA promoting macrophages M2 polarization and neuronal cells regeneration. Adapted with permission from [127], Copyright 2024 Wiley. F - G. Schematic illustration of the design and synthesis of Cu - TCPP - Mn nanozyme for myocardial injury treatment. F. The bimetallic Cu - TCPP - Mn nanozyme was fabricated by embedding manganese and copper into the porphyrin via solvothermal method, followed by sonication into small MOF nanodots. G. Cu - TCPP - Mn nanozyme retained cascade activity that has been shown to scavenge ROS, inhibit inflammation, reduce myocardium fibrosis and promote constructive remodeling and vascularization in MI and I/R injury animal models. Adapted with permission from [128], Copyright 2023 Ivyspring international publisher.

Constructing of MOF - based Nanozymes by Pyrolysis. A. Schematic illustration of the synthetic route of Fe2NC@Se with multi - enzyme mimicking activities and its therapeutic use for reperfusion injury in ischemic stroke. B. Characterization of Fe1NC, Fe2NC, and Fe2NC@Se. Adapted with permission from [131], Copyright 2022 Wiley. C. Schematic illustration of the design and synthesis of Co, N co - doped hierarchically porous carbon hybrid. D. Photographs of the colored product obtained from the reaction of Co, N - HPC with different chromogenic substrates. E. The UV - vis absorption spectra of TMB with different concentration of Co, N - HPC (0, 3, 6, 9, 12, 21, 30, 45 and 60 μg/mL). Inset: Effect of Co, N - HPC concentration on absorbance. F. Time - evolution of the catalytic activity of Co, N - HPC to TMB in different pH buffers (2.0-6.0). G. Selectivity of Co, N - HPC - TMB system for GSH over other potential interferences. Adapted with permission from [132], Copyright 2018 Elsevier.
SAzymes
SAzymes represent a novel class of nanomaterials that replicate the catalytic properties of natural enzymes at the atomic level. By integrating advanced single - atom technology with intrinsic enzyme - like active sites, these materials maximize both atomic efficiency and catalytic site density. Their well - defined geometric and electronic structures, combined with complete atom utilization, result in superior catalytic activity and substrate specificity. Furthermore, SAzymes serve as a bridge between homogeneous and heterogeneous catalysis, offering a promising solution to the design limitations of conventional catalytic materials [133]. Consequently, they exhibit high selectivity and catalytic performance, effectively addressing the drawbacks associated with traditional nanozymes and natural enzymes [134 - 136]. The typical architectures of SAzymes include metal - metal (M - M), metal - metal oxide, metal - metal sulfide, and metal - N - C (M - N - C) configurations [137 - 140]. These nanozymes possess distinct active sites that enable enzyme - like activities similar to those of GPx, CAT, and SOD. Therefore, selecting an appropriate synthetic strategy is crucial to ensure that the resulting nanozymes exhibit the desired enzymatic functionalities. However, reducing the material size to the atomic scale leads to the formation of unsaturated coordination sites, which increases the metal surface free energy. This, in turn, poses challenges in isolating and stabilizing individual atomic sites [141]. To overcome these challenges, various synthetic strategies have been developed for the fabrication of SAzymes. These methods can be broadly categorized into “bottom - up” and “top - down” approaches, depending on the choice of metal precursor. The bottom - up strategy involves the use of mononuclear metal complexes as starting materials, whereas the top - down approach utilizes bulk metals or NPs as precursors [133].
“Bottom-up” synthetic strategy
The bottom - up synthetic approach involves directly generating monoatomic metal species from metal complex precursors, which are then introduced into support materials via techniques such as impregnation, electrostatic adsorption, and coprecipitation. A critical step in this strategy is the effective anchoring of isolated metal atoms onto the support, which ensures the stability and high enzymatic activity of the resulting nanobiocatalysts [142, 143]. Spatial confinement is considered an ideal method for preventing the migration and aggregation of single atoms. For instance, using a biomineralization strategy, ferric ions were encapsulated within ZIF - 8 to generate a series of iron - centered single - atom nanozymes. These nanozymes varied depending on the ferric precursor employed. During synthesis, the N coordination number of the isolated iron atoms was finely tuned by adjusting both the type of precursor and the pyrolysis temperature. Specifically, pyrolyzing a ferritin - containing precursor at 800 °C resulted in a Fe - N4 configuration. In contrast, using FeCl3 under the same conditions produced a Fe - N3 structure. When the pyrolysis temperature was raised to 950°C, Fe-N2 configuration was obtained from ferritin. These findings highlight the synergistic influence of the metal precursor and pyrolysis conditions in modulating the metal coordination environment (Figure 11A) [144]. Additionally, a PEGylated Mn - based SAzyme was successfully synthesized, in which Mn atoms were effectively captured by N - rich porous C (Figure 11B) [145]. Another strategy to enhance metal - support interactions involve coordination stabilization. This is achieved by forming metal - nonmetal bonds between unsaturated coordination atoms on the support and the vacant orbitals of metal atoms. A notable example is the synthesis of a series of precious metal - based single - atom catalysts with densely distributed PM - Nx sites, which displayed exceptional turnover frequencies in O2 reduction reactions (Figure 11C) [146]. NC materials are commonly used as efficient support matrices because they contain abundant unsaturated N coordination sites and can be readily prepared through the pyrolysis of N - rich precursors [147]. Furthermore, defect anchoring has proven to be a reliable method for immobilizing metal atoms. This approach has been widely applied to various supports, including NC, metal oxides, and metal hydroxides. For example, Fe - based SAzymes with tunable coordination numbers were synthesized by introducing Fe atoms into defect sites stabilized by N atoms within an NC matrix. This was achieved by incorporating melamine into the system during synthesis (Figure 11D) [148].
“Top - down” synthetic strategy
In contrast to bottom - up methods, the “top - down” synthetic strategy has emerged as a powerful approach for developing efficient, stable, and tunable single - atom catalysts. Unlike bottom - up strategies, which build catalysts from molecular or atomic precursors, top - down approaches begin with bulk metals or NPs and break them down into isolated single atoms. This is typically achieved using controlled physical and chemical treatments that allow for precise tuning of structural and catalytic properties. The mechanisms used to capture dissociated metal atoms through metal - support interactions are similar to those described in the bottom - up approach. Therefore, this section primarily focuses on the key techniques used to disrupt strong M - M bonds within metal NPs or bulk materials [149]. For instance, a notable example involves reversing the sintering process to disassemble PtNPs into individual Pt atoms. This transformation yields a thermally stable Pt - based SAzyme with significantly enhanced POD - like catalytic activity and kinetics compared to nanozymes composed of PtNPs (Figure 12 A - G) [150]. The conversion is carried out via pyrolysis at 1050°C for 5 h under a flowing N2 atmosphere. Similar nanoparticle - to - single - atom (NP - to - SA) transitions have also been observed in other noble metals. PdNPs and AuNPs can be converted into thermally stable single atoms (Pd - SAs and Au - SAs) when exposed to temperatures above 900°C in an inert environment [151].
Bottom - up synthetic route of Single - Atom nanozymes. A. Schematic illustration depicting of regulation of the coordination environment of mesopore - confined single atoms from metalloprotein - MOFs. Adapted with permission from [144], Copyright 2022 Wiley. B. Synthesis procedure of Mn - based and PEGylated SAE (Mn/PSAE). Adapted with permission from [145], Copyright 2021 Wiley. C. Schematic illustration of a series of PM - based (PM = Ru, Pt, and Pd) SACs were prepared by molten salt - assisted pyrolysis strategy and compared with traditional encapsulation pyrolysis strategy. Adapted with permission from [146], Copyright 2024 Wiley. D. Schematic illustration of synthesis process for FeN5 SAzyme. Adapted with permission from [148], Copyright 2022 Wiley.

SAzymes emulate natural enzymes at the atomic level, offering improved catalytic efficiency and substrate specificity. These nanozymes serve as a unique interface between homogeneous and heterogeneous catalysis, combining the benefits of both systems. Common structural frameworks include M - M, metal - oxide, and M - N - C configurations. Their synthesis relies on two primary approaches: bottom - up techniques, which use metal complexes and stabilize them on supports such as N - doped C, and top - down strategies, which deconstruct bulk materials or NPs into isolated metal atoms. These methods address challenges related to the instability and coordination environment of single atoms, resulting in highly tunable and high - performance nanozymes with enzyme - like behavior.
Carbon - based nanozymes
C - based nanomaterials have drawn significant interest due to their remarkable enzyme - mimicking properties. A wide range of C allotropes - including C nanotubes, graphene oxide, C nitride, carbon nanodots (CNDs), and fullerenes - exhibit catalytic activities similar to those of key antioxidant enzymes such as SOD, CAT, and GPx [152]. Their structural diversity, chemical tunability, and inherent biocompatibility make them ideal candidates for nanozyme development. Since the identification of fullerene as a radical scavenger, both pristine fullerenes and their derivatives have been widely used to eliminate free radicals and protect neurons from oxidative stress. A particularly notable compound is C60[C(COOH)2]3, known as C60 - C3, which features C3 symmetry. This molecule exhibits enhanced antioxidant activity and provides superior neuroprotection compared to unmodified C60. Its catalytic efficiency in eliminating O2·- underlies this activity. Mechanistic analyses have shown that during this reaction, C60 - C3 remains chemically unaltered, while O2·- is converted into O2 and H2O2, mimicking the mechanism of SOD [153]. In addition to fullerenes, hydrophilic C clusters have also been developed as efficient SOD mimics [154]. Similarly, graphene - based nanozymes have demonstrated promising applications in scavenging ROS and RONS, which are implicated in a wide range of diseases [155]. These materials possess large surface areas and distinctive surface properties, which contribute to their catalytic performance and biological compatibility. Carbon dots (CDs), a class of C - based nanomaterials with diameters typically less than 10 nm, offer an abundance of surface functional groups and high surface area - to - volume ratios. CDs feature C - rich, N - rich, and O2 - rich surface moieties that are crucial for their enzyme - like activities [156, 157]. Based on their structural characteristics and synthesis methods, CDs are typically classified into four types: graphene CDs, C quantum dots, CNDs, and carbonized polymer dots. Each type exhibits unique catalytic properties and potential biomedical applications [158]. The CAT - like activity of graphene oxide quantum dots demonstrates their potential as antioxidant nanozymes [159]. Similarly, graphene quantum dots (GQDs) have been explored for their POD - like activity. Theoretical evaluations suggest that ketone groups on the GQD surface function as catalytically active sites, while carboxyl groups serve to bind substrates. In contrast, ·OH groups may suppress enzymatic activity by interfering with catalytic processes. However, definitive evidence from both in vitro and in vivo experiments confirming the POD - like activity of GQDs is still lacking. Addressing this gap in future research is essential for validating the full potential of GQDs as nanozymes [160].
Heteroatom doping in nanocarbon materials is a powerful strategy for developing high - performance C - based nanozymes. Pristine graphene and C nanotubes generally exhibit limited enzyme - like activity and a narrow range of catalytic functions [25, 161]. However, when N atoms are incorporated into the C framework, the resulting doped nanozymes demonstrate significantly enhanced catalytic performance, displaying four types of microenvironment - responsive enzyme - like activities under physiological conditions - specifically, POD, CAT, and SOD - like activities [162]. In addition to N, various other heteroatoms have been successfully introduced into C nanostructures to mimic the catalytic behavior of natural enzymes [163 - 165]. These findings support the feasibility of engineering a diverse library of C - based nanozymes with tunable, multi - enzyme functionalities, which could potentially serve as alternatives to natural enzymes in biological systems.
Other nanozymes
Beyond metal - , MOF - , single - atom - , and C - based nanozymes, several novel nanozyme systems have demonstrated promising enzyme - mimetic properties but do not fall within the conventional classifications. These include peptide - based nanozymes (PepNzymes) and Prussian blue nanozymes (PBNzs), both of which are gaining increasing attention for their unique structures and tunable functionalities.
PepNzymes represent a growing class of artificial enzymes that integrate the catalytic versatility of peptides with the structural advantages of nanoscale materials. Owing to their inherent biocompatibility, molecular recognition capability, and self - assembly behavior, PepNzymes exhibit diverse enzyme - like activities, including SOD - , CAT - , POD - , and hydrolase - like functions [166]. These nanozymes are typically constructed through the self - assembly of functional peptides, peptide - cofactor complexes, or peptide-nanoparticle hybrids [167, 168]. Key structural parameters - such as amino acid sequence, secondary structure, and the coordination environment - critically influence their catalytic activity and substrate specificity. Supramolecular assemblies can enhance catalytic efficiency by facilitating electron transfer and improving substrate accessibility. Beyond their catalytic potential, PepNzymes also hold promise for targeted delivery, biosensing, and regenerative medicine applications [166]. Given their programmability, structural tunability, and functional diversity, PepNzymes are particularly appealing for modulating oxidative stress and providing neuroprotection in SCI. However, only a limited number of PepNzymes have been explored in the context of SCI repair. Future research should prioritize the design of antioxidant and anti - inflammatory peptide - based systems specifically tailored to the complex microenvironment of SCI. Approaches such as metal coordination, hierarchical self - assembly, and targeted delivery could be strategically employed to enhance catalytic efficiency and therapeutic outcomes. With continued innovation, PepNzymes may offer a cell - free, precisely controllable strategy for promoting neuroregeneration and functional recovery following SCI.
Top - Down synthetic route of Single - Atom nanozymes. A - G: Synthesis and structural characterizations of Pt TS - SAzyme. A. Illustration of the preparation process of PtTS - SAzyme. B. TEM image of ZIF - 8/Pt NPs@PZS. TEM and enlarged TEM image (C), HAADF - STEM (D), and the corresponding EDS mapping images (E) of Pt TS - SAzyme. F. HAADF - STEM image and G. an enlarged image of PtTS - SAzyme. Adapted with permission from [150], Copyright 2021 American Chemical Society.

PBNzs have also emerged as a promising therapeutic platform for SCI due to their inherent redox activity, biocompatibility, and multifunctional capabilities. Functioning as biomimetic enzymes, PBNzs exhibit both CAT - and SOD - like activities, enabling efficient scavenging of ROS and alleviation of oxidative stress [169]. Furthermore, PBNzs contribute to inflammation modulation by promoting M2 macrophage polarization, thereby creating a neuroprotective microenvironment conducive to tissue repair and regeneration [170]. In a recent development, researchers have designed a rapamycin - loaded, hollow mesoporous Prussian blue - based nanozyme - termed RHPAzyme. This multifunctional system not only scavenges ROS via Fe2+/Fe3+ valence transitions and interactions with the cyanide groups of hollow mesoporous Prussian blue but also exhibits multienzyme - like activity. As a result, RHPAzyme effectively reduces oxidative stress and inflammation while inhibiting the MAPK/AKT signaling pathway [106]. Despite these promising findings, several challenges remain - particularly in terms of achieving targeted delivery, ensuring long - term biosafety, and translating these systems into clinically scalable therapies. Addressing these issues through systematic investigation will be essential to unlocking the full therapeutic potential of PBNzs in SCI and other oxidative stress - related disorders.
In summary, these emerging nanozyme systems represent innovative directions for designing multifunctional, adaptive, and biocompatible catalytic platforms. However, their catalytic mechanisms, in vivo stability, and long - term biosafety remain incompletely understood. Further research is needed to standardize experimental protocols and therapeutic assessments to facilitate their clinical translation.
Nanozymes application in SCI
The pathophysiology of SCI is well known to be complex, involving both primary mechanical damage and secondary injury processes. The primary injury is irreversible, while the secondary injury exacerbates tissue damage and impedes neurological recovery, making it a critical target for therapeutic intervention. Owing to their inherent enzyme - mimetic properties, nanozymes have emerged as promising therapeutic agents for mitigating secondary damage following SCI. By combining the structural advantages of nanomaterials with the catalytic functions of natural enzymes, nanozymes offer a novel approach to SCI treatment.
The mechanism of nanozymes for SCI treatment
Alleviation oxidative stress
It is widely accepted that excessive production of ROS following SCI leads to oxidative stress, which damages cellular structures - including lipids, proteins, and DNA - ultimately exacerbating neuronal injury and cell death [45, 63]. Therefore, eliminating ROS and reducing oxidative damage are key strategies for neuroprotection. Nanozymes with SOD - like and CAT - like enzymatic activities have shown significant efficacy in neutralizing ROS. Those with CAT - like activity catalyze the decomposition of H2O2 into H2O and O2, while SOD - like nanozymes convert O2·- into H2O2 and O2 [102, 171]. Moreover, certain nanozymes exhibit both SOD and CAT - like activities, which synergistically inhibit ·OH production and enhance overall ROS scavenging. Some nanozymes have also demonstrated GPx - like activity, enabling additional pathways for ROS detoxification [172].
To date, various nanozymes have been designed and applied to alleviate oxidative stress in SCI treatment. For instance, a Ce - based MOF was synthesized through ligand screening, resulting in a structure capable of rapidly neutralizing excess ROS while supplying mitochondrial energy. This dual action helped modulate the immune microenvironment and supported continuous nerve regeneration. These nanozymes exhibited both SOD - and CAT - like activities, significantly reducing mitochondrial ROS levels in macrophages and promoting their polarization from the proinflammatory M1 to the anti - inflammatory M2 phenotype. Ultimately, these effects led to improved locomotor and urinary function recovery in SCI rat models [127]. Among MOFs, ZIF - 8 has emerged as a promising candidate due to its excellent biocompatibility, high drug - loading capacity, and intrinsic ROS - scavenging properties [173]. ZIF - 8 is commonly employed as a delivery platform for drugs and genes, releasing Zn2+ ions in a sustained manner under acidic conditions. This zinc release activates the JNK1/p38/MAPK signaling pathway, promoting neural differentiation and angiogenesis in dental pulp stem cells (DPSCs) [174]. Se, an essential component of many natural enzyme active sites, has also drawn considerable attention. SeNPs are particularly notable for their strong ROS - scavenging capabilities and anti - inflammatory effects [175]. These NPs have been used in animal models of SCI to reduce ROS accumulation and mitigate associated damage [175, 176]. CeO2 nanozymes, a prototypical example of metal oxide - based nanozymes, have been extensively investigated for their high ROS - scavenging efficiency via combined SOD - and CAT - like activity [177, 178]. For example, L - arginine - loaded hollow CeO2 nanospheres were developed to simultaneously eliminate ROS, induce M2 microglial polarization, and release nitric oxide (NO), thereby promoting neural stem cell (NSC) differentiation and aiding SCI recovery [177]. Prussian blue (PB), an FDA - approved antidote for thallium and cesium poisoning, also has strong ROS - scavenging properties due to its CAT - , SOD - , and POD - like activities [179, 180]. Building on this, multifunctional RHPAzymes - based on PB - were synthesized to exhibit in vitro SOD - , POD - , and CAT - mimicking activities. These nanozymes alleviated mitochondrial dysfunction, suppressed ROS overproduction, and prevented apoptosis by modulating the MAPK/AKT signaling pathway [106]. Recently, SAzymes have been applied in the treatment of CNS injuries. A single - atom Pt/CeO2 catalyst was developed for the topical treatment of brain trauma. This nanozyme provided robust metal - support interactions and exhibited strong ROS - scavenging capabilities, with CAT - , SOD - , and GPx - like activities. Both in vitro and in vivo experiments demonstrated that this nanozyme - based therapeutic dressing effectively reduced oxidative stress markers and inflammation in neuronal cells, ultimately improving impaired neurocognitive function [138].
Inhibiting Inflammation
Neuroinflammation is another major extrinsic factor that inhibits neural regeneration after SCI [181]. SCI triggers a robust immune response, marked by the infiltration of peripheral leukocytes (particularly M1 macrophages) and the release of various cytokines and chemokines. Persistent infiltration of inflammatory cells contributes to further cell death and the formation of cystic microcavities. As the primary effectors of the immune response, macrophages serve as key targets for regulating neuroinflammation following SCI [182].
Nanozymes have shown considerable promise in modulating the proinflammatory response during SCI by reducing the expression of proinflammatory cytokines, facilitating the transition of macrophages from a proinflammatory (M1) to an anti - inflammatory (M2) phenotype, and eliminating ROS [183]. These properties position nanozymes as potential therapeutic agents for SCI, capable of mitigating oxidative damage, preventing neuronal death, and suppressing inflammation. A range of nanozymes has been developed to inhibit macrophage activation, a major driver of proinflammatory responses. For example, engineered multifunctional zinc - organic framework - based AIE - active Zn@MOF - TPD nanozymes have demonstrated ROS - neutralizing properties that protect neurons from oxidative stress while exerting anti - inflammatory and neuroprotective effects. These nanozymes target the NF - κB-MMP - 9 signaling pathway, inhibiting NF - κB activation and MMP - 9 expression. Consequently, they reduce glial scar formation, promote axonal preservation, and maintain myelin integrity, ultimately supporting motor function recovery in SCI models [105]. Similarly, bioactive ZnMn - layered double hydroxides embedded in hydrogels have released Zn2+ and Mn2+, which suppress M1 macrophage activation and promote their polarization to the M2 phenotype. This material enhances spinal cord tissue regeneration, exhibits potent anti - inflammatory effects, and significantly improves functional recovery in mice with SCI [184]. Another innovative material - a conductive molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel - has demonstrated the ability to reduce interleukin - 6 and tumor necrosis factor - alpha levels, inhibit M1 macrophage differentiation, and promote M2 macrophage activation, resulting in a pronounced anti - inflammatory effect [185]. Selenium - doped carbon quantum dots (Se - CQDs) have also been synthesized with notable anti - inflammatory properties. These NPs actively reduce inflammation in injured tissues, thereby protecting cells from inflammatory damage, particularly in SCI - induced neuroinflammation. By inhibiting proinflammatory cytokine production and modulating the immune response, Se - CQDs effectively suppress inflammatory cascades [20].
Application of nanozymes for SCI treatment
Metal - based naozymes for SCI treatment
Metal - based nanozymes have recently emerged as a promising strategy for treating SCI. These nanozymes combine the intrinsic properties of metal elements with enzyme - like activities, offering unique advantages for neural repair and regeneration [105, 186].
Se is an essential nonmetallic micronutrient for both humans and animals, playing a vital role in maintaining physiological homeostasis. It is crucial to the body's antioxidant systems, particularly through its involvement in the biosynthesis of selenoproteins such as GPx and thioredoxin reductase, both of which exhibit strong antioxidant capabilities and help regulate immune responses to mitigate inflammation [187, 188]. Se NPs possess notable antioxidant, anti - aging, and antitumor properties, and are further characterized by low toxicity, high drug - loading capacity, good biocompatibility, and biodegradability [189 - 191]. The therapeutic effects of SeNPs in SCI treatment have been well established [175, 192 - 194]. One notable example is the development of ultra - small - diameter lentinan Se nanoparticles (LNT - UsSeNPs) by Liu et al., which exhibit high ROS - scavenging capacity, suppress inflammatory responses and cell apoptosis, and promote motor function recovery in SCI mice (Figure 13A) [195]. ABTS free radical scavenging assays and electron paramagnetic resonance spectroscopy confirmed the excellent ROS - scavenging ability of LNT - UsSeNPs (Figure 13B - C). Flow cytometry and immunofluorescence - based JC - 1 staining demonstrated a reduction in mitochondrial depolarization (Figure 13D - E). ehavioral studies in mice revealed that LNT - UsSeNPs enhanced motor function and neuronal survival (Figure 13F - I). Mechanistically, these nanozymes exert antioxidant and anti - inflammatory effects by inhibiting neuronal apoptosis through the PI3K - AKT - mTOR - eIF4EBP1 and Ras - Raf - MEK - WIPI2 signaling pathways (Figure 13J - K).
CeO2, a representative metal oxide, is widely recognized for its diverse enzyme - mimetic properties, particularly in managing inflammatory diseases through activities resembling those of SOD and CAT. These properties are attributed to the material's ability to modulate its electronic structure via reversible redox cycling between Ce3+ (reduced state) and Ce4+ (oxidized state) [85, 196, 197]. In the context of SCI, CeO2 has demonstrated promising therapeutic potential by mitigating oxidative stress and exerting anti - inflammatory effects [17, 198]. Wang et al. developed Ce@UCNP - BCH that not only efficiently scavenges ROS at the site of injury but also enables real - time monitoring of oxidative stress levels during the SCI repair process through ratiometric luminescence signaling (Figure 14A) [74]. Electron paramagnetic resonance spectroscopy confirmed that Ce@UCNP - BCH mimics both SOD - and CAT - like enzymatic functions (Fiugre 14B - C). Furthermore, this nanozyme served as an effective indicator of the redox microenvironment and the severity of injury at the lesion site (Figure 14D - F). Behavioral assessments and immunofluorescence analyses demonstrated that Ce@UCNP - BCH significantly promotes spinal cord regeneration, including remyelination, and facilitates functional recovery in mice with SCI (Figure 14G - M).
Metal - based nanozymes represent one of the most promising strategies for the treatment of SCI, owing to their high catalytic efficiency, ROS - scavenging capacity, and structural stability. Among various nanozyme platforms, metal - based systems are particularly notable for their potential to achieve clinical translation. However, despite their therapeutic advantages, several critical gaps remain unaddressed. To date, there is a lack of systematic investigation into the in vivo metabolism, long - term toxicity, and the impact of exogenous metal ions on normal cellular metabolic processes. These issues are especially relevant given the sensitivity of neural tissue to metabolic perturbations. Therefore, for metal - based nanozymes to advance toward clinical application in SCI therapy, comprehensive studies are urgently needed to evaluate their biocompatibility, biodistribution, clearance mechanisms, and potential metabolic interference in both injured and healthy tissues.
MOF - based nanozymes for SCI treatment
MOF - based nanozymes have emerged as highly promising tools in regenerative medicine due to their intrinsic enzyme - like activities [199]. These nanomaterials offer distinct advantages, including high catalytic efficiency, site - specific targeting, and large surface areas suitable for drug loading [200, 201].
Such properties make MOF - based nanozymes particularly attractive for the treatment of SCI [105, 127, 202]. A notable subfamily of MOFs is ZIFs, which exhibit a crystalline, zeolite - like structure consisting of interconnected porous cavities [203]. Among them, ZIF - 8 stands out due to its superior biocompatibility, high cargo - loading capacity, and ROS - scavenging capability [173, 204]. Lin et al. constructed a multifunctional ZIF - 8 - based nanoplatform incorporating Prussian blue and Schisandrin B for targeted metabolic intervention in SCI (Figure 15A) [204]. This platform effectively suppressed M1 macrophage accumulation, decreased ROS levels (Figure 15B - C), and promoted the phenotypic switch of macrophages from a proinflammatory (M1) to an anti - inflammatory (M2) state. This polarization enhanced tissue infiltration and repair by reprogramming macrophage metabolism (Figure 15D - J). Treatment with the MOF nanoplatform significantly improved motor function in SCI rats (Figure 15K - O).
Additionally, several studies have explored integrating ZIF - 8 into hydrogels to optimize the microenvironment at the injury site, reduce localized cell death, and accelerate tissue regeneration. For instance, Zhou et al. incorporated ZIF - 8 into a gelatin methacryloyl hydrogel, facilitating the controlled release of Zn2+ under acidic conditions. This strategy protected transplanted DPSCs from apoptosis and enhanced their neural differentiation and angiogenic capacity via activation of the MAPK signaling pathway. Consequently, there was an observed increase in both axon length and density in DPSC - derived neuro - like cells, ultimately contributing to improved motor function in SCI rats [174].
MOF - based nanozymes offer unique advantages in the treatment of SCI due to their high porosity, tunable structure, and exceptional drug - loading capacity. These characteristics enable MOF - based nanozymes to serve as multifunctional therapeutic platforms, capable of targeted drug delivery, ROS scavenging, and controlled release of anti - inflammatory or neuroprotective agents. Moreover, the modularity of MOF - based nanozymes allows for precise engineering of their chemical composition and surface properties to improve biocompatibility and targeting efficiency. However, despite these promising features, several challenges hinder their clinical translation. Key concerns include their structural stability under physiological conditions, potential toxicity of metal ions and organic linkers, and limited understanding of their in vivo degradation, biodistribution, and long - term safety. Additionally, the interactions between MOF - based nanozymes and neural tissue microenvironments remain insufficiently explored. Addressing these challenges through advanced material design, systematic toxicological evaluation, and mechanism - based studies is essential to fully realize the therapeutic potential of MOF - based nanozymes in SCI.
SAzymes for SCI treatment
SAzymes, characterized by atomically dispersed active metal centers and maximal atomic utilization, represent an innovative class of biomimetic catalysts. These nanozymes emulate the active sites of metalloenzymes and exhibit catalytic activities similar to SOD, CAT, and GPx [205, 206].
Metal - based naozymes for SCI treatment. A. Schematic of LNT - UsSeNPs scavenging ROS suppressing inflammatory response and cell apoptosis, and promote the recovery of motor function in SCI mice. B. The in vitro antioxidant activity of LNT - SeNPs and LNT - UsSeNPs was determined using the ABTS radical scavenging assay. C. EPR spectral analysis of LNT - SeNPs and LNT - UsSeNPs for scavenging O2•-. D. The ΔΨm of PC - 12 cells was detected via JC - 1 staining. E. PC - 12 cells in JC - 1 fluorescence image. F - I. Improvement effect of LNT - UsSeNPs on motor recovery and neuronal survival in SCI mice: F. BMS scores and slope test. G. The results of mice at different times. H. Footprint analysis was conducted for each group of mice to assess gait and locomotor function. I. A snapshot from the recorded video shows the sequence of hind limb movements for each group of mice during walking. Points and lines are used to represent the iliac crest, knee joint, and ankle joint. The direction of hind limb movement is indicated by an arrow. J. Western blot analysis of the differentially expressed proteins identified using TMT proteomics (1: Sham, 2: SCI, 3: SCI+MPSS, 4: SCI+LNT - UsSeNPs). K. The molecular mechanism LNT - UsSeNPs inhibit neuronal apoptosis. Adapted with permission from [195], Copyright 2025 Spring Nature.
Metal oxide nanozymes for SCI treatment. A. Schematic of ameliorating SCI via Ce@UCNP - BCH promotes real - time luminescence and single - cell multi - omics evaluation. B - C. EPR spectra of the H2O2 (B) and O2•-(C) under different concentrations of Ce@UCNP - BCH. D. Redox state detection using Ce@UCNP - BCH. E. UCL imaging of Ce@UCNP - BCH in vivo. F. Quantified ratios of I650/I690 intensity of n (n = 5). G. BMS scores of SCI mice. H. Mouse footprints from the control, sham, injured, injured + Ce@UCNP, and injured + Ce@UCNP - BCH groups. Histograms of the stride length (I) and stride width (J) of mouse in the control, sham, injury, injury + Ce@UCNP, and injury+ Ce@UCNP - BCH groups (n = 9). K. The images showing the location of the damaged spinal cord tissue in each experimental group after being stained with H&E (left) and LFB (right), scale bar=1 mm. L. The fluorescent images showing the spinal cord for immunofluorescence analysis co - stained with anti - NeuN antibodies (red) and anti - BrdU (green), scale bar = 200 μm. M. The fluorescent images showing the spinal cord for immunofluorescence analysis co - stained with anti - GFAP antibodies (red) and anti - NFH (green), scale bar = 200 μm. Adapted with permission from [74], Copyright 2025 Wiley.

MOF - based nanozymes for SCI treatment. A. SEM image of ZIF - 8, ZIF - 8@PB and ZIF - 8@PB - SB. B - C. In vitro anti - ROS of ZIF - 8@PB - SB: B. DCFH - DA images of modiffed ZIF - 8 NPs; C. CLSM images of modiffed ZIF - 8 NPs tested by the [Ru(dpp)3]2+ Cl2 probe. D - J. In vivo macrophages polarization of ZIF - 8@PB - SB in SCI mice: D. Representative images of colocalization in vivo. E - G. Quantitative analysis of iNOS (E), IL - 1β (F), and CD86 (G) in SCI mice; H - J. Quantification of colocalization in vivo. K - O. In vivo therapeutic effects of ZIF - 8@PB - SB in SCI mice: K. Motor function scores of SCI mice with different treatments. L - O. Quantitative analysis of the mobility frequency (L), the total distance to point area (M), the total distance moved (N), the distance to point area mean (O),in SCI mice. Adapted with permission from [204], Copyright 2024 American Chemical Society.

Owing to these properties, SAzymes are considered highly promising for neural repair; however, studies specifically targeting SCI remain limited [207]. A pioneering study by Jiang et al. introduced a cobalt - based single - atom nanozyme (Co - SAzyme) with a porous hollow structure designed to attenuate oxidative stress and inflammation associated with secondary injury in SCI [208]. The Co - SAzyme demonstrated SOD - like activity by catalyzing the dismutation of O2·- into H2O2 and O2, as well as CAT - and GPx - like activities for the decomposition of H2O2. Density functional theory (DFT) calculations further supported these ROS - scavenging mechanisms. Western blot analyses confirmed the strong anti - inflammatory properties of the nanozyme. Moreover, the nanozyme's hollow architecture enabled the encapsulation and sustained release of the neuroprotective drug minocycline. Behavioral testing revealed significant functional improvements in motor recovery in the treated group, while hematoxylin-eosin and Nissl staining at 28 days post - injury demonstrated a marked reduction in neuronal apoptosis in rats receiving minocycline - loaded Co - SAzyme.
SAzymes hold great promise for treating SCI due to their high catalytic efficiency and ROS - scavenging capability. However, several critical challenges hinder their clinical translation. These include poor targeting efficiency and limited penetration across the BSCB, unclear long - term biocompatibility and metabolic pathways, and insufficient catalytic stability under the complex in vivo microenvironment. Technical barriers in synthesis, including batch variability and structural instability, further limit their scalability. Addressing these issues through smart delivery systems, integrated therapeutic platforms, and standardized fabrication protocols is essential for advancing Sazyme - based therapies in SCI.
C - based nanozymes for SCI treatment
As a novel class of nanozymes for biochemical applications, C - based nanozymes are more operationally stable and cost - effective than natural enzymes. They are also easy to prepare and exhibit high resilience under harsh conditions [209 - 211]. Owing to their unique surface functional groups and electronic structure, C - based nanozymes possess excellent electrical conductivity and antioxidant properties, making them promising candidates for enhancing functional recovery after SCI [212].
Li et al. developed and characterized novel Ejiao carbon dots (EJCDs), synthesized specifically to enhance the self - renewal of hematopoietic stem cells (HSCs) and promote their differentiation into erythroid progenitors (Figure 16A) [213]. These EJCDs were designed to modulate the dysregulated hematopoietic system following SCI, influencing HSC lineage commitment and the subsequent maturation of immune cells, thereby offering therapeutic potential. The results demonstrated that EJCDs not only promoted HSC proliferation and regulated lineage differentiation after SCI (Figure 16B - F), but also restored the homeostasis of the bone marrow microenvironment (Figure 16G - H). Ultimately, the CDs reduced local inflammatory cell infiltration, mitigated secondary injury, protected residual neurons, and facilitated neurological function recovery (Figure 16I - K). Luo et al. reported Se - CQDs, which exhibited favorable biocompatibility and a significant protective effect against H2O2 - induced oxidative damage in vitro [20]. These Se - CQDs showed potent anti - inflammatory and anti - apoptotic properties, effectively limiting glial scar formation and enhancing the survival of neurons with intact myelin sheaths in vivo. Consequently, Se - CQDs markedly improved locomotor function in rats with TSCI.
In parallel, some researchers have explored loading C - based nanozymes into hydrogels to create a favorable microenvironment for transplanted stem cells, supporting their survival, encouraging their differentiation into neurons, and thus promoting SCI repair. Qi et al. designed an injectable hydrogel loaded with CDs and FTY720, a sphingosine - 1 - phosphate receptor modulator, for SCI therapy (Figure 17A) [214]. The hydrogel displayed potent ROS scavenging ability, as confirmed by DCFH - DA and DHE assays (Figure 17B - C). In vitro, the hydrogel facilitated NSC proliferation and promoted their differentiation into neurons, while suppressing differentiation into astrocytes (Figure 17B - C). In vitro, the hydrogel facilitated NSC proliferation and promoted their differentiation into neurons, while suppressing differentiation into astrocytes (Figure 17D - E). In vivo experiments revealed that combining the hydrogel with NSCs significantly reduced injury cavity size and demyelination (Figure 17F - J). Additionally, the treatment enhanced the generation of new neurons and synapses in the injured area and attenuated glial scar formation (Figure 17K - N).
C - based nanozymes hold promise for SCI treatment due to their excellent biocompatibility, high surface area, and tunable catalytic activities, exhibiting antioxidant, anti - inflammatory, and neuroprotective properties. However, concerns remain regarding long - term toxicity, biodegradability, and batch - to - batch consistency. To achieve clinical translation, future efforts should focus on improving lesion - site targeting and conducting comprehensive biosafety evaluations.
Delivery strategies for nanozymes in SCI
Although nanozymes hold great potential in promoting SCI repair by alleviating oxidative stress and inflammation, their clinical application is limited by BSCB, which impedes the accumulation of therapeutic agents at the lesion site. To overcome this limitation, exosomes, cell membrane encapsulation, and hydrogel - based delivery systems are being explored to enhance the permeability and targeting of nanozymes across the BSCB.
Carbon - based nanozymes for SCI treatment. A. Schematic representation of EJCDs modulating the immune system to repair SCI in the bone marrow and spleen. B - F: EJCDs promote the proliferation of HSCs and regulate their lineage differentiation After SCI. B - C: Impact of EJCDs treatment at various time points post - SCI on hematopoietic progenitor cell numbers. B: Frequency and absolute numbers LSK cells of within the Lin- cells determined; C: Frequency and absolute numbers of LT - HSC within Lin- cells measured. D - F: Effects of EJCDs administration at different time points post - SCI on HSC lineage changes. D: MEP, GMP, and CMP at different time points; E: Proportions of MEP, GMP, and CMP on 3 DPI. F: Absolute number and frequency of MEP at different times. G - H. Effects of EJCDs administration at different time points post - SCI on myeloid cell numbers in the BM. G: Frequency and absolute numbers of monocytes determined H: Frequency and absolute numbers of macrophages determined. I. Frequency of monocytes and macrophages as a percentage of all live cells in the spinal cord 3 DPI (n = 6). J - K: Assessment of motor function recovery 6 weeks post - SCI, J: Evaluation of hindlimb functional recovery in mice using Catwalk Gait Analysis (n = 6); K: Electrophysiological assessment measuring motor - evoked potential conduction amplitude and latency in the spinal cord (n = 6). 1 DPI and 3 DPI: n = 8; 7 DPI and 14 DPI: n = 6. ****p < 0.0001. Adapted with permission from [213], Copyright 2025 Elsevier.
C - based nanozymes for SCI treatment. A. FTY720 - CDs@GelMA hydrogel was prepared, filled with NSCs, and implanted into a rat spinal cord total transection damage model. B - C: Scavenging of reactive oxygen species by different groups of hydrogels. B. DCFH images of different groups of hydrogels. Scale bar: 200 μm. C. DHE images of different groups of hydrogels. Scale bar: 200 μm. D - E: Proliferation of NSCs in different hydrogels. D. Identification of isolated cells, which were spherical in shape and positively expressed Nestin (green). Cell nuclei were stained with DAPI (blue). Scale bar: 100 μm; E. Normal growth of neural stem cells in hydrogels. Cell nuclei were stained with DAPI (blue) and neurons were stained with Tuj - 1 (red), scale bar: 100 μm F - j: Evaluation of motor function recovery and tissue repair in rats 8 weeks after spinal cord injury. F. HE staining of rat spinal cord in each group. Left: scale bar: 500 μm; right: scale bar: 100 μm. G. LFB staining of the spinal cord of each group. The image on the right is a magnified image of the area shown in the black square on the left. Left: scale bar: 500 μm; right: scale bar: 100 μm. H. BBB scores of different groups at 8 weeks post - injury. I. Percentage of cavity area in each group. J. Percentage of LFB - positive area in each group. *Indicates P<0.05. K. Regeneration of neurons at the lesion site in different groups. Scale bar: 100 μm. L. nerve fiber regeneration and axonal myelin re - formation in different groups. Scale bar: 100μm. M. Percentage of Tuj - 1 positive area in different groups. N. Percentage of GFAP positive area in different groups. *, **P<0.05 and P<0.01, respectively. Adapted with permission from [214], Copyright 2024 Taylor & Franci
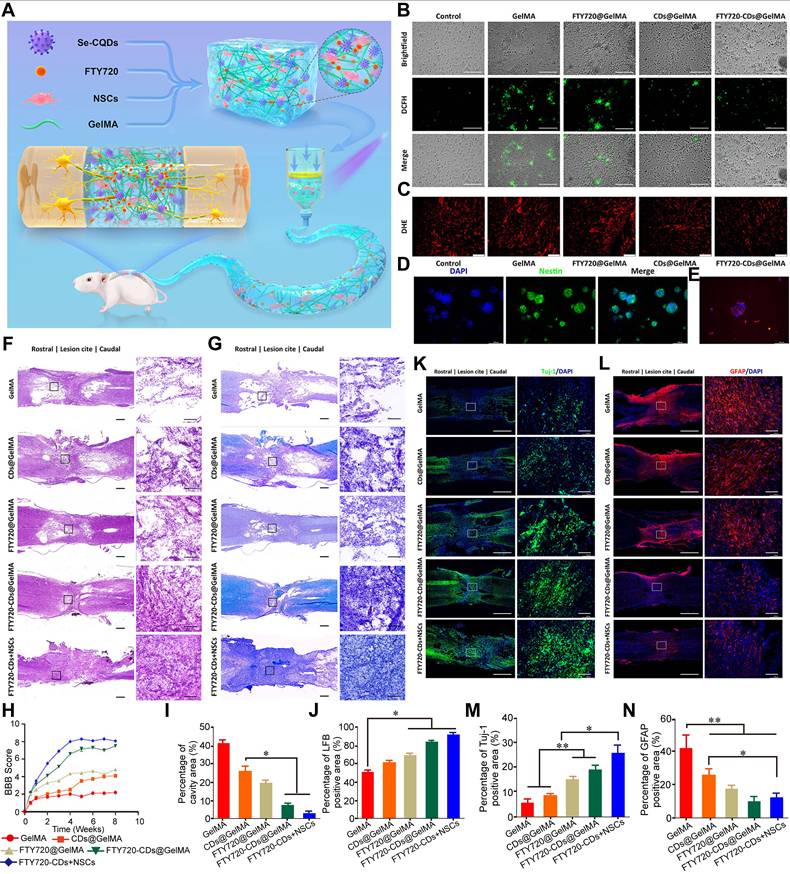
Exosomes
Exosomes are nanosized extracellular vesicles secreted by various cell types and serve as critical mediators of intercellular communication [215, 216]. Typically ranging from 30 to 150 nanometers in diameter, exosomes originate within multivesicular endosomes and are released into the extracellular space [217]. Encased in a lipid bilayer, exosomes protect their cargo - comprising proteins, lipids, and nucleic acids - from enzymatic degradation, enabling them to influence recipient cells and modulate biological activities effectively [218]. Due to their inherent biocompatibility, stability, and ability to traverse biological barriers, exosomes are gaining recognition as promising therapeutic tools for SCI [219, 220]. Exosomes can serve as carriers for nanozymes, facilitating their targeted delivery across the BSCB to the injury site. For example, biocompatible macrophage - derived exosome - enclosed Mn - iron Prussian blue analogs have been employed for immunomodulatory therapy in SCI. These exosome - coated Mn - iron Prussian blue analogs significantly reduced ROS levels, improved the H2O2 - rich microenvironment, and inhibited apoptosis and inflammation in vitro [221]. Such exosome - encapsulated nanozymes combine the unique catalytic properties of nanozymes with the targeting capabilities and biological safety of exosomes, presenting a compelling strategy for SCI repair. However, research in this area remains in its infancy, and more extensive investigations are needed to fully realize their therapeutic potential.
Cell membranes encapsulation
Cell membrane - based biomimetic systems offer enhanced targeting capabilities, immune evasion, and improved biocompatibility. By coating NPs or nanozymes with natural cell membranes, synthetic nanomaterials can acquire biological identity, enabling them to cross biological barriers, evade immune surveillance, and accumulate at sites of injury [222, 223]. A representative example is the work by Yu et al., who developed GSH - modified macrophage - derived cell membranes encapsulating metformin nanogels. These nanogels effectively alleviated oxidative stress and suppressed inflammation. Pharmacokinetic and drug release studies showed that GSH - modified macrophage - derived cell membranes encapsulating metformin nanogels displayed a sustained release profile, while in vivo imaging confirmed their targeted accumulation at the injury site, demonstrating efficient delivery [222]. Similarly, Liu et al. designed macrophage - derived nanovesicles encapsulating SeNPs - Met - MVs for SCI therapy. These nanovesicles successfully traversed the BSCB and delivered their cargo - SeNPs and metformin - directly to the lesion site, where they exerted potent anti - inflammatory and antioxidant effects [223].
Cell membrane - coated nanozymes represent an effective delivery platform due to their ability to evade immune rejection, cross the BSCB, and specifically target injured spinal cord tissue. Future research should focus on refining membrane - coating techniques to improve the stability, targeting specificity, and cargo delivery efficiency of these nanozymes. Moreover, challenges related to large - scale manufacturing, long - term biosafety, and the interactions of these systems with the SCI microenvironment must be addressed to advance clinical translation.
Hydrogel
Hydrogels are highly hydrated materials composed of water and hydrophilic polymer networks. Their exceptional characteristics, including biocompatibility, permeability, biodegradability, and capacity to support cell interactions, make them ideal candidates for replicating the natural molecular microenvironment [224]. Various types of hydrogels have been employed in SCI treatment to improve the local tissue environment. These include phase - change hydrogels, self - healing hydrogels, oriented fiber hydrogels, self - assembled microsphere hydrogels, and multifunctional hydrogels such as conductive, stimuli - responsive, adhesive, antioxidant, and sustained - release variants [225].
Based on these properties, hydrogels have been explored as delivery platforms for nanozymes, facilitating their slow and sustained release across BSCB. This enables them to exert antioxidant, anti - inflammatory, and neuroregenerative effects, ultimately contributing to the restoration of neurological function [226]. For example, Gong et al. developed a nanozyme hydrogel loaded with cerium - manganese nanoparticles and nerve growth factor (NGF) (CeMnNPs - PEG) [16]. Locally injected at the injury site, the hydrogel enabled the gradual release of CeMnNPs and NGF, effectively scavenging ROS, reducing inflammation, and promoting M2 macrophage polarization by inhibiting the cyclic GMP - AMP synthase - stimulator of interferon genes signaling pathway. Additionally, microenvironment - responsive hydrogels have been used as carriers for nanozymes to enhance therapeutic outcomes in SCI. Xu et al. reported the development of a ceria nanozyme - integrated thermoresponsive in situ - forming hydrogel, which reduced oxidative stress, improved mesenchymal stem cell viability, accelerated angiogenesis, facilitated nerve regeneration, and promoted motor function recovery after SCI [227].
In summary, hydrogels, due to their unique physicochemical properties and compatibility with nanozymes, hold great promise in advancing SCI treatment strategies. Their ability to modulate the injury microenvironment and support the controlled release of therapeutic agents makes them highly suitable for addressing the complex challenges of spinal cord repair. The integration of nanozymes within these hydrogel systems enables precise targeting, sustained activity, and multifaceted therapeutic benefits. Nevertheless, further studies are required to optimize their performance, scale up production, and evaluate long - term biosafety to ensure successful clinical translation.
Current challenge
Over the past decade, nanozymes have attracted increasing attention as stable, cost - effective alternatives to natural enzymes. In the context of spinal cord injury (SCI), antioxidant nanozymes offer promising therapeutic potential, particularly for mitigating oxidative stress and neuroinflammation. However, several critical challenges must be addressed to facilitate their clinical translation.
Firstly, the injured spinal cord presents a highly complex and dynamic microenvironment characterized by excessive ROS, proinflammatory cytokines, pH fluctuations, and a disrupted BSCB. These factors can significantly compromise the structural stability, catalytic efficiency, and functional durability of nanozymes. Moreover, while some nanozymes have demonstrated the ability to cross the BSCB, the mechanisms underlying their trans - barrier transport, accumulation at the lesion site, and cellular uptake remain poorly understood, which limits the development of effective targeted delivery systems.
Secondly, the biocompatibility and long - term biosafety of nanozymes remain important concerns. Although many nanozymes exhibit low short - term toxicity in vitro or in small animal models, their long - term fate, potential accumulation in the central nervous system, and chronic effects on cellular metabolism and immune responses have not been adequately investigated. In particular, nanozymes containing transition metals may release ions that interfere with normal physiological functions or elicit oxidative damage if not tightly controlled.
Thirdly, the substrate specificity and selectivity of nanozymes in SCI models are often suboptimal. Their interactions with non - target substrates may result in off - target effects, impairing normal biochemical pathways and reducing therapeutic precision. Surface modifications, such as ligand conjugation or cell membrane coating, may enhance targeting capabilities and minimize adverse interactions, but these strategies require further optimization and validation.
Fourthly, most current nanozyme designs focus primarily on ROS scavenging and anti - inflammatory effects, whereas SCI pathophysiology also involves excitotoxicity, glial scar formation, axonal degeneration, and impaired neuroregeneration. Therefore, the development of multifunctional nanozymes capable of responding to multiple pathological signals could enable more effective and holistic therapeutic strategies.
Fifthly, the mechanistic understanding of intracellular signaling, immune modulation, and long - term interactions between nanozymes and host tissue remains limited. Advanced methodologies such as proteomics, transcriptomics, and real - time in vivo imaging are needed to elucidate these processes and guide rational design.
Lastly, the field lacks standardized and comparable evaluation systems for nanozyme catalytic activity and kinetics. Variations in experimental protocols, including pH, buffer systems, temperature, and substrate concentrations, complicate comparisons across studies. Moreover, inconsistent reporting units (e.g., U/mg, nmol/min/mg, absorbance change) and variable kinetic analyses (e.g., Km, Vmax) hinder reproducibility and scalability.
In summary, future efforts should focus on improving the biocompatibility, long - term safety, and targeting efficiency of nanozymes in SCI, optimizing catalytic performance under physiological conditions, developing multifunctional therapeutic platforms, and establishing standardized evaluation criteria. Addressing these challenges will be essential to unlock the full translational potential of nanozymes for SCI treatment.
Future prospects and innovation directions
Although antioxidant nanozymes hold significant promise for SCI therapy, several obstacles must be overcome to facilitate their clinical application. The pathological SCI microenvironment can compromise nanozyme stability and catalytic performance. Moreover, issues such as poor substrate specificity, limited targeting to lesion sites, and poorly understood long - term biological interactions reduce therapeutic accuracy and safety. Comprehensive toxicological evaluations, including assessments of chronic toxicity, biodistribution, metabolic pathways, and physiological responses, are urgently needed to support clinical translation.
Future research must go beyond incremental optimization and embrace interdisciplinary approaches to develop more effective, clinically viable nanozymes. One promising avenue is the design of nanozymes with multiple enzyme - mimetic activities, such as SOD - , CAT - , and GPx - like functions. These multifunctional nanozymes can better replicate endogenous antioxidant systems and manage the complex ROS dynamics present in SCI. Additionally, the incorporation of stimuli - responsive elements, activated by local changes in pH, ROS levels, or GSH concentrations, can allow for spatiotemporal control of nanozyme activity and reduce off - target effects. Innovations such as gene - editing nanozymes and photoresponsive nanozymes also represent emerging directions for SCI therapy.
Targeted delivery remains a significant challenge. Strategies such as ligand - based surface engineering and the use of biomimetic carriers (e.g., exosomes or cell membrane - coated NPs) can facilitate nanozyme transport across the BSCB and promote accumulation at the injury site. In parallel, next - generation nanozymes should be engineered not only for antioxidative and anti - inflammatory purposes but also to promote neuroregeneration. This could involve integrating neurotrophic factors such as NGF or brain - derived neurotrophic factor, or coupling nanozymes with extracellular matrix - mimetic molecules to encourage axonal growth and synaptic plasticity.
Emerging approaches also include embedding nanozymes in three - dimensional - printed scaffolds or injectable hydrogels, which enable both structural support and biochemical modulation of the injury site. For enhanced therapeutic precision, technologies such as single - cell RNA sequencing and spatial proteomics can inform the rational design of nanozymes tailored to specific cell types or lesion regions. Meanwhile, advanced imaging and molecular profiling techniques will be vital for deciphering nanozyme - induced intracellular pathways and long - term biological effects.
Finally, the development of standardized evaluation systems specific to SCI, covering criteria such as catalytic activity, neurocompatibility, biodegradation, and functional outcomes, will be crucial for harmonizing research and accelerating translation. These standards, along with scalable manufacturing protocols, early - phase clinical modeling, and regulatory alignment, will pave the way for nanozymes to evolve from experimental tools into clinically effective therapies for SCI.
Acknowledgements
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Author contributions
Y.M., J.P: Writing - original draft, Methodology, Investigation. C.J: Methodology, Investigation. X.Y., Y, W: Writing - review & editing, Supervision. R.L., Writing - review & editing, Supervision. H.H., X.W., D.H.: Writing - review & editing, Supervision, Conceptualization, Visualization. All authors contributed to the article and approved the final manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fouad K, Popovich PG, Kopp MA, Schwab JM. The neuroanatomical - functional paradox in spinal cord injury. Nat Rev Neurol. 2021;17:53-62
2. Lu Y, Shang Z, Zhang W, Hu X, Shen R, Zhang K. et al. Global, regional, and national burden of spinal cord injury from 1990 to 2021 and projections for 2050: A systematic analysis for the Global Burden of Disease 2021 study. Ageing Res Rev. 2025;103:102598
3. Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A. et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018
4. Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A. et al. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020 21
5. Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant - based intervention. Spinal Cord. 2012;50:264-74
6. Gao X, Cheng W, Zhang X, Zhou Z, Ding Z, Zhou X. et al. Nerve Growth Factor - Laden Anisotropic Silk Nanofiber Hydrogels to Regulate Neuronal/Astroglial Differentiation for Scarless Spinal Cord Repair. ACS Appl Mater Interfaces. 2022;14:3701-15
7. Xiao CL, Lai HT, Zhou JJ, Liu WY, Zhao M, Zhao K. Nrf2 Signaling Pathway: Focus on Oxidative Stress in Spinal Cord Injury. Mol Neurobiol. 2025;62:2230-49
8. Shahrezaei A, Sohani M, Sohouli M, Taherkhani S, Nasirinezhad F. The involvement and significance of M2 macrophages in neuropathic pain following spinal cord injury: a systematic review. J Physiol Sci. 2024;74:45
9. Tetreault LA, Kwon BK, Evaniew N, Alvi MA, Skelly AC, Fehlings MG. A Clinical Practice Guideline on the Timing of Surgical Decompression and Hemodynamic Management of Acute Spinal Cord Injury and the Prevention, Diagnosis, and Management of Intraoperative Spinal Cord Injury: Introduction, Rationale, and Scope. Global Spine J. 2024;14:10s-24s
10. Yang LY, Tsai MY, Juan SH, Chang SF, Yu CR, Lin JC. et al. Exerting the Appropriate Application of Methylprednisolone in Acute Spinal Cord Injury Based on Time Course Transcriptomics Analysis. Int J Mol Sci. 2021 22
11. Zhang N, Hu J, Liu W, Cai W, Xu Y, Wang X. et al. Advances in Novel Biomaterial - Based Strategies for Spinal Cord Injury Treatment. Mol Pharm. 2024;21:4764-85
12. Ma W, Li X. Spinal cord injury repair based on drug and cell delivery: From remodeling microenvironment to relay connection formation. Mater Today Bio. 2025;31:101556
13. Liao Z, Zeng J, Lin A, Zou Y, Zhou Z. Pre - treated mesenchymal stem cell - derived exosomes: A new perspective for accelerating spinal cord injury repair. Eur J Pharmacol. 2025;992:177349
14. Li Y, Luo W, Meng C, Shi K, Gu R, Cui S. Exosomes as promising bioactive materials in the treatment of spinal cord injury. Stem Cell Res Ther. 2024;15:335
15. Gong W, Zhang T, Che M, Wang Y, He C, Liu L. et al. Recent advances in nanomaterials for the treatment of spinal cord injury. Mater Today Bio. 2023;18:100524
16. Gong Z, Chen Z, Li D, Lu X, Wu J, Sun H. et al. Hydrogel loaded with cerium - manganese nanoparticles and nerve growth factor enhances spinal cord injury repair by modulating immune microenvironment and promoting neuronal regeneration. J Nanobiotechnology. 2025;23:29
17. Liu D, Niu R, Wang S, Shao L, Yang X, Liu X. et al. Nitric Oxide - Releasing Mesoporous Hollow Cerium Oxide Nanozyme - Based Hydrogel Synergizes with Neural Stem Cell for Spinal Cord Injury Repair. ACS Nano. 2025;19:2591-614
18. Liang M, Yan X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc Chem Res. 2019;52:2190-200
19. Zhang Y, Zhang L, Wang M, Li P. The applications of nanozymes in neurological diseases: From mechanism to design. Theranostics. 2023;13:2492-514
20. Luo W, Wang Y, Lin F, Liu Y, Gu R, Liu W. et al. Selenium - Doped Carbon Quantum Dots Efficiently Ameliorate Secondary Spinal Cord Injury via Scavenging Reactive Oxygen Species. Int J Nanomedicine. 2020;15:10113-25
21. Rahimi B, Behroozi Z, Motamednezhad A, Jafarpour M, Hamblin MR, Moshiri A. et al. Study of nerve cell regeneration on nanofibers containing cerium oxide nanoparticles in a spinal cord injury model in rats. J Mater Sci Mater Med. 2023;34:9
22. Gao G, Li J, Ma Y, Xie M, Luo J, Wang K. et al. Dual - Responsive Multi - Functional Silica Nanoparticles with Repaired Mitochondrial Functions for Efficient Alleviation of Spinal Cord Injury. Exploration. 270012.
23. Ji Z, Zheng J, Ma Y, Lei H, Lin W, Huang J. et al. Emergency Treatment and Photoacoustic Assessment of Spinal Cord Injury Using Reversible Dual - Signal Transform - Based Selenium Antioxidant. Small. 2023;19:e2207888
24. Wang K, Zheng J, Li R, Chen T, Ma Y, Wu P. et al. Single - Cell Multi - omics Assessment of Spinal Cord Injury Blocking via Cerium - doped Upconversion Antioxidant Nanoenzymes. Adv Sci (Weinh). 2025;12:e2412526
25. Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y. et al. Nanomaterials with enzyme - like characteristics (nanozymes): next - generation artificial enzymes (II). Chem Soc Rev. 2019;48:1004-76
26. Chen Q, Wang J, Xiong X, Chen J, Wang B, Yang H. et al. Blood - Brain Barrier - Penetrating Metal - Organic Framework Antioxidant Nanozymes for Targeted Ischemic Stroke Therapy. Adv Healthc Mater. 2024: e2402376.
27. Yang Y, Li Z, Fan X, Jiang C, Wang J, Rastegar - Kashkooli Y. et al. Nanozymes: Potential Therapies for Reactive Oxygen Species Overproduction and Inflammation in Ischemic Stroke and Traumatic Brain Injury. ACS Nano. 2024;18:16450-67
28. Choo AM, Liu J, Dvorak M, Tetzlaff W, Oxland TR. Secondary pathology following contusion, dislocation, and distraction spinal cord injuries. Exp Neurol. 2008;212:490-506
29. Liu Z, Xiang C, Zhao X, Aizawa T, Niu R, Zhao J. et al. Regulation of dynamic spatiotemporal inflammation by nanomaterials in spinal cord injury. J Nanobiotechnology. 2024;22:767
30. Al Mamun A, Quan Z, Geng P, Wang S, Shao C, Xiao J. Targeting Remyelination in Spinal Cord Injury: Insights and Emerging Therapeutic Strategies. CNS Neurosci Ther. 2024;30:1-15
31. Liu X, Jiang X, Yu Q, Shen W, Tian H, Mei X. et al. Sodium alginate and naloxone loaded macrophage - derived nanovesicles for the treatment of spinal cord injury. Asian J Pharm Sci. 2022;17:87-101
32. Scholpa NE, Schnellmann RG. Mitochondrial - Based Therapeutics for the Treatment of Spinal Cord Injury: Mitochondrial Biogenesis as a Potential Pharmacological Target. J Pharmacol Exp Ther. 2017;363:303-13
33. Wang P, Chen Z, Li P, Al Mamun A, Ning S, Zhang J. et al. Multi - targeted nanogel drug delivery system alleviates neuroinflammation and promotes spinal cord injury repair. Mater Today Bio. 2025;31:101518
34. Tian H, Zheng J, Wang F, Zhang W, Chen Y, Wang X. et al. NLRP3 inflammasome promotes functional repair after spinal cord injury in mice by regulating autophagy and its mechanism. Int Immunopharmacol. 2025;149:114230
35. Liu H, Zhang J, Xu X, Lu S, Yang D, Xie C. et al. SARM1 promotes neuroinflammation and inhibits neural regeneration after spinal cord injury through NF - κB signaling. Theranostics. 2021;11:4187-206
36. Wu W, He Y, Chen Y, Fu Y, He S, Liu K. et al. In vivo imaging in mouse spinal cord reveals that microglia prevent degeneration of injured axons. Nat Commun. 2024;15:8837
37. Noristani HN. Intrinsic regulation of axon regeneration after spinal cord injury: Recent advances and remaining challenges. Exp Neurol. 2022;357:114198
38. Bhatt M, Sharma M, Das B. The Role of Inflammatory Cascade and Reactive Astrogliosis in Glial Scar Formation Post - spinal Cord Injury. Cell Mol Neurobiol. 2024;44:78
39. Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE. et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23:499-515
40. Shen YJ, Huang YC, Cheng YC. Advancements in Antioxidant - Based Therapeutics for Spinal Cord Injury: A Critical Review of Strategies and Combination Approaches. Antioxidants (Basel). 2024 14
41. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363-83
42. Wang Q, Wang X, Shang Z, Zhao L. Mechanism and prospects of mitochondrial transplantation for spinal cord injury treatment. Stem Cell Res Ther. 2024;15:457
43. Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C. et al. Hormesis and Oxidative Distress: Pathophysiology of Reactive Oxygen Species and the Open Question of Antioxidant Modulation and Supplementation. Antioxidants (Basel). 2022 11
44. Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017;11:613-9
45. Yin Z, Wan B, Gong G, Yin J. ROS: Executioner of regulating cell death in spinal cord injury. Front Immunol. 2024;15:1330678
46. Schmidt J, Quintá HR. Mitochondrial dysfunction as a target in spinal cord injury: intimate correlation between pathological processes and therapeutic approaches. Neural Regen Res. 2023;18:2161-6
47. Han Y, Li X, Yang L, Zhang D, Li L, Dong X. et al. Ginsenoside Rg1 attenuates cerebral ischemia - reperfusion injury due to inhibition of NOX2 - mediated calcium homeostasis dysregulation in mice. J Ginseng Res. 2022;46:515-25
48. Liu W, Zhu Y, Ye W, Xiong J, Wang H, Gao Y. et al. Redox regulation of TRIM28 facilitates neuronal ferroptosis by promoting SUMOylation and inhibiting OPTN - selective autophagic degradation of ACSL4. Cell Death Differ. 2025
49. Shiiba I, Ito N, Oshio H, Ishikawa Y, Nagao T, Shimura H. et al. ER - mitochondria contacts mediate lipid radical transfer via RMDN3/PTPIP51 phosphorylation to reduce mitochondrial oxidative stress. Nat Commun. 2025;16:1508
50. Jangra A, Gola P, Singh J, Gond P, Ghosh S, Rachamalla M. et al. Emergence of taurine as a therapeutic agent for neurological disorders. Neural Regen Res. 2024;19:62-8
51. Zhou K, Zheng Z, Li Y, Han W, Zhang J, Mao Y. et al. TFE3, a potential therapeutic target for Spinal Cord Injury via augmenting autophagy flux and alleviating ER stress. Theranostics. 2020;10:9280-302
52. Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883-901
53. Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490-503
54. Catalá A, Díaz M. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Front Physiol. 2016;7:423
55. Li QS, Jia YJ. Ferroptosis: a critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen Res. 2023;18:506-12
56. Yu Z, Cheng X, Pan W, Yu C, Duan Y. The ferroptosis activity is associated with neurological recovery following chronic compressive spinal cord injury. Neural Regen Res. 2023;18:2482-8
57. Liu C, Liu Z, Dong Z, Liu S, Kan H, Zhang S. Multifaceted interplays between the essential players and lipid peroxidation in ferroptosis. J Genet Genomics. 2025
58. Tiek D, Song X, Yu X, Wu R, Iglesia R, Catezone A. et al. Oxidative stress induced protein aggregation via GGCT produced pyroglutamic acid in drug resistant glioblastoma. iScience. 2025;28:111769
59. Liu X, Wang W, Nie Q, Liu X, Sun L, Ma Q. et al. The Role and Mechanisms of Ubiquitin - Proteasome System - Mediated Ferroptosis in Neurological Disorders. Neurosci Bull. 2025
60. Scheijen EE, Veeningen N, Duwé S, Ivanova A, Van Broeckhoven J, Hendrix S. et al. Temporal and spatial pattern of DNA damage in neurons following spinal cord Injury in mice. J Biomed Sci. 2025;32:12
61. Scheijen EEM, Hendrix S, Wilson DM 3rd. Oxidative DNA Damage in the Pathophysiology of Spinal Cord Injury: Seems Obvious, but Where Is the Evidence? Antioxidants (Basel). 2022 11
62. Zewail - Foote M, Del Mundo IMA, Klattenhoff AW, Vasquez KM. Oxidative damage within alternative DNA structures results in aberrant mutagenic processing. Nucleic Acids Res. 2025 53
63. Song Q, Cui Q, Sun S, Wang Y, Yuan Y, Zhang L. Crosstalk Between Cell Death and Spinal Cord Injury: Neurology and Therapy. Mol Neurobiol. 2024;61:10271-87
64. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. 2015;97:55-74
65. Lin H, Wang L, Jiang X, Wang J. Glutathione dynamics in subcellular compartments and implications for drug development. Curr Opin Chem Biol. 2024;81:102505
66. Lee JH. Oxidative stress and the multifaceted roles of ATM in maintaining cellular redox homeostasis. Redox Biol. 2024;75:103269
67. Chen Y, Li B, Li K, Lin Y. Superoxide dismutase nanozymes: current status and future perspectives on brain disease treatment and diagnosis. Chem Commun (Camb). 2024;60:4140-7
68. Ali SS, Hardt JI, Quick KL, Kim - Han JS, Erlanger BF, Huang TT. et al. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic Biol Med. 2004;37:1191-202
69. Kataky R, Morgan E. Potential of enzyme mimics in biomimetic sensors: a modified - cyclodextrin as a dehydrogenase enzyme mimic. Biosens Bioelectron. 2003;18:1407-17
70. Kirkorian K, Ellis A, Twyman LJ. Catalytic hyperbranched polymers as enzyme mimics; exploiting the principles of encapsulation and supramolecular chemistry. Chem Soc Rev. 2012;41:6138-59
71. Gong L, Zhao Z, Lv YF, Huan SY, Fu T, Zhang XB. et al. DNAzyme - based biosensors and nanodevices. Chem Commun (Camb). 2015;51:979-95
72. Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N. et al. Intrinsic peroxidase - like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577-83
73. Chen Z, Yu Y, Gao Y, Zhu Z. Rational Design Strategies for Nanozymes. ACS Nano. 2023;17:13062-80
74. Wang K, Zheng J, Li R, Chen T, Ma Y, Wu P. et al. Single - Cell Multi - omics Assessment of Spinal Cord Injury Blocking via Cerium - doped Upconversion Antioxidant Nanoenzymes. Adv Sci (Weinh). 2025: e2412526.
75. Mei L, Zhu S, Liu Y, Yin W, Gu Z, Zhao Y. An overview of the use of nanozymes in antibacterial applications. Chemical Engineering Journal. 2021;418:129431
76. Markovic Z, Trajkovic V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials. 2008;29:3561-73
77. Liu C, Fan W, Cheng W - X, Gu Y, Chen Y, Zhou W. et al. Red Emissive Carbon Dot Superoxide Dismutase Nanozyme for Bioimaging and Ameliorating Acute Lung Injury. Advanced Functional Materials. 2023;33:2213856
78. Celardo I, Pedersen JZ, Traversa E, Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3:1411-20
79. Tarnuzzer RW, Colon J, Patil S, Seal S. Vacancy engineered ceria nanostructures for protection from radiation - induced cellular damage. Nano Lett. 2005;5:2573-7
80. Soh M, Kang DW, Jeong HG, Kim D, Kim DY, Yang W. et al. Ceria - Zirconia Nanoparticles as an Enhanced Multi - Antioxidant for Sepsis Treatment. Angew Chem Int Ed Engl. 2017;56:11399-403
81. Sardesai NP, Andreescu D, Andreescu S. Electroanalytical evaluation of antioxidant activity of cerium oxide nanoparticles by nanoparticle collisions at microelectrodes. J Am Chem Soc. 2013;135:16770-3
82. Zheng G, Yu W, Xu Z, Yang C, Wang Y, Yue Z. et al. Neuroimmune modulating and energy supporting nanozyme - mimic scaffold synergistically promotes axon regeneration after spinal cord injury. J Nanobiotechnology. 2024;22:399
83. Xu D, Wu L, Yao H, Zhao L. Catalase - Like Nanozymes: Classification, Catalytic Mechanisms, and Their Applications. Small. 2022;18:e2203400
84. Kajita M, Hikosaka K, Iitsuka M, Kanayama A, Toshima N, Miyamoto Y. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic Res. 2007;41:615-26
85. Othman A, Gowda A, Andreescu D, Hassan MH, Babu SV, Seo J. et al. Two decades of ceria nanoparticle research: structure, properties and emerging applications. Mater Horiz. 2024;11:3213-66
86. Mou X, Wu Q, Zhang Z, Liu Y, Zhang J, Zhang C. et al. Nanozymes for Regenerative Medicine. Small Methods. 2022;6:e2200997
87. Li J, Liu W, Wu X, Gao X. Mechanism of pH - switchable peroxidase and catalase - like activities of gold, silver, platinum and palladium. Biomaterials. 2015;48:37-44
88. Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS. et al. Nanoceria exhibit redox state - dependent catalase mimetic activity. Chem Commun (Camb). 2010;46:2736-8
89. Plauck A, Stangland EE, Dumesic JA, Mavrikakis M. Active sites and mechanisms for H₂O₂ decomposition over Pd catalysts. Proc Natl Acad Sci U S A. 2016;113:E1973-82
90. Brigelius - Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289-303
91. Zhang D, Shen N, Zhang J, Zhu J, Guo Y, Xu L. A novel nanozyme based on selenopeptide - modified gold nanoparticles with a tunable glutathione peroxidase activity. RSC Adv. 2020;10:8685-91
92. Margis R, Dunand C, Teixeira FK, Margis - Pinheiro M. Glutathione peroxidase family - an evolutionary overview. Febs j. 2008;275:3959-70
93. Terman A, Brunk UT. Oxidative stress, accumulation of biological 'garbage', and aging. Antioxid Redox Signal. 2006;8:197-204
94. Huang X, Liu X, Luo Q, Liu J, Shen J. Artificial selenoenzymes: designed and redesigned. Chem Soc Rev. 2011;40:1171-84
95. Vernekar AA, Sinha D, Srivastava S, Paramasivam PU, D'Silva P, Mugesh G. An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat Commun. 2014;5:5301
96. Ghosh S, Roy P, Karmodak N, Jemmis ED, Mugesh G. Nanoisozymes: Crystal - Facet - Dependent Enzyme - Mimetic Activity of V(2) O(5) Nanomaterials. Angew Chem Int Ed Engl. 2018;57:4510-5
97. Huang Y, Liu C, Pu F, Liu Z, Ren J, Qu X. A GO - Se nanocomposite as an antioxidant nanozyme for cytoprotection. Chem Commun (Camb). 2017;53:3082-5
98. Ghosh S, Roy P, Prasad S, Mugesh G. A GPx - mimetic copper vanadate nanozyme mediates the release of nitric oxide from S - nitrosothiols. Faraday Discuss. 2022;234:284-303
99. Zeng W, Yu M, Chen T, Liu Y, Yi Y, Huang C. et al. Polypyrrole Nanoenzymes as Tumor Microenvironment Modulators to Reprogram Macrophage and Potentiate Immunotherapy. Adv Sci (Weinh). 2022;9:e2201703
100. Singh N, Geethika M, Eswarappa SM, Mugesh G. Manganese - Based Nanozymes: Multienzyme Redox Activity and Effect on the Nitric Oxide Produced by Endothelial Nitric Oxide Synthase. Chemistry. 2018;24:8393-403
101. Carsol MA, Pouliquen - Sonaglia I, Lesgards G, Marchis - Mouren G, Puigserver A, Santimone M. A new kinetic model for the mode of action of soluble and membrane - immobilized glutathione peroxidase from bovine erythrocytes - - effects of selenium. Eur J Biochem. 1997;247:248-55
102. Yang B, Chen Y, Shi J. Reactive Oxygen Species (ROS) - Based Nanomedicine. Chem Rev. 2019;119:4881-985
103. Zhang Y, Gao W, Ma Y, Cheng L, Zhang L, Liu Q. et al. Integrating Pt nanoparticles with carbon nanodots to achieve robust cascade superoxide dismutase - catalase nanozyme for antioxidant therapy. Nano Today. 2023;49:101768
104. Liu T, Xiao B, Xiang F, Tan J, Chen Z, Zhang X. et al. Ultrasmall copper - based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11:2788
105. Zheng J, Chen T, Wang K, Peng C, Zhao M, Xie Q. et al. Engineered Multifunctional Zinc - Organic Framework - Based Aggregation - Induced Emission Nanozyme for Accelerating Spinal Cord Injury Recovery. ACS Nano. 2024;18:2355-69
106. Shen K, Li X, Huang G, Yuan Z, Xie B, Chen T. et al. High rapamycin - loaded hollow mesoporous Prussian blue nanozyme targets lesion area of spinal cord injury to recover locomotor function. Biomaterials. 2023;303:122358
107. Xiong T, Yang K, Zhao T, Zhao H, Gao X, You Z. et al. Multifunctional Integrated Nanozymes Facilitate Spinal Cord Regeneration by Remodeling the Extrinsic Neural Environment. Adv Sci (Weinh). 2023;10:e2205997
108. Ji ZS, Gao GB, Ma YM, Luo JX, Zhang GW, Yang H. et al. Highly bioactive iridium metal - complex alleviates spinal cord injury via ROS scavenging and inflammation reduction. Biomaterials. 2022;284:121481
109. Zandieh M, Liu J. Nanozymes: Definition, Activity, and Mechanisms. Adv Mater. 2024;36:e2211041
110. Fan R, Chuan D, Liu Z, Chen H, Chen C, Guo G. et al. Antioxidant MnO2 nanozymes - encapsulated hydrogel synergistically regulate the spinal ROS microenvironment and promote spinal cord repair. Chemical Engineering Journal. 2023;478:147148
111. Singh N, Savanur MA, Srivastava S, D'Silva P, Mugesh G. A Redox Modulatory Mn(3) O(4) Nanozyme with Multi - Enzyme Activity Provides Efficient Cytoprotection to Human Cells in a Parkinson's Disease Model. Angew Chem Int Ed Engl. 2017;56:14267-71
112. Liu J, Wang T, Liao C, Geng W, Yang J, Ma S. et al. Constructing Electron - Rich Ru Clusters on Non - Stoichiometric Copper Hydroxide for Superior Biocatalytic ROS Scavenging to Treat Inflammatory Spinal Cord Injury. Adv Mater. 2024;36:e2411618
113. Zhang J, Wang Z, Lin X, Gao X, Wang Q, Huang R. et al. Mn - Ce Symbiosis: Nanozymes with Multiple Active Sites Facilitate Scavenging of Reactive Oxygen Species (ROS) Based on Electron Transfer and Confinement Anchoring. Angew Chem Int Ed Engl. 2025;64:e202416686
114. Li D, Dai D, Xiong G, Lan S, Zhang C. Metal - Based Nanozymes with Multienzyme - Like Activities as Therapeutic Candidates: Applications, Mechanisms, and Optimization Strategy. Small. 2023;19:e2205870
115. Jiang Y, Kang Y, Liu J, Yin S, Huang Z, Shao L. Nanomaterials alleviating redox stress in neurological diseases: mechanisms and applications. J Nanobiotechnology. 2022;20:265
116. Shi Y, Liu C, Gui Y, Guo Y, Zhang Y, Pan J. et al. A Nattokinase - Loaded Nanozyme for Alleviating Acute Myocardial Infarction via Thrombolysis and Antioxidation. Adv Healthc Mater. 2024: e2402763.
117. Che H, Xu J, Wu D, Chen S, Liu C, Zhao C. et al. Reactive oxygen species - responsive polydopamine - PtCuTe nanoparticle - loaded microneedle system for promoting the healing of infected skin wounds. J Control Release. 2024;376:999-1013
118. Zhao S, Hung SF, Deng L, Zeng WJ, Xiao T, Li S. et al. Constructing regulable supports via non - stoichiometric engineering to stabilize ruthenium nanoparticles for enhanced pH - universal water splitting. Nat Commun. 2024;15:2728
119. Wang Q, Cheng C, Zhao S, Liu Q, Zhang Y, Liu W. et al. A Valence - Engineered Self - Cascading Antioxidant Nanozyme for the Therapy of Inflammatory Bowel Disease. Angew Chem Int Ed Engl. 2022;61:e202201101
120. Wang Y, Tan Z, Zhang Z, Zhu P, Tam SW, Zhang Z. et al. Facet - Dependent Activity of CeO(2) Nanozymes Regulate the Fate of Human Neural Progenitor Cell via Redox Homeostasis. ACS Appl Mater Interfaces. 2022;14:35423-33
121. Yao W, Hu A, Ding J, Wang N, Qin Z, Yang X. et al. Hierarchically Ordered Macro - Mesoporous Electrocatalyst with Hydrophilic Surface for Efficient Oxygen Reduction Reaction. Adv Mater. 2023;35:e2301894
122. Chao D, Dong Q, Yu Z, Qi D, Li M, Xu L. et al. Specific Nanodrug for Diabetic Chronic Wounds Based on Antioxidase - Mimicking MOF - 818 Nanozymes. J Am Chem Soc. 2022;144:23438-47
123. Liu Q, Song Y, Ma Y, Zhou Y, Cong H, Wang C. et al. Mesoporous Cages in Chemically Robust MOFs Created by a Large Number of Vertices with Reduced Connectivity. J Am Chem Soc. 2019;141:488-96
124. Tian Y, Ye Z, Wang X, Guan H, Liu W, Duan X. et al. MOF - 818 Nanozyme Suppresses Calcium Oxalate Kidney Stones by Alleviating Oxidative Stress and Inflammatory Injury. Adv Healthc Mater. 2024: e2401574.
125. Jiang W, Li Q, Zhang R, Li J, Lin Q, Li J. et al. Chiral metal - organic frameworks incorporating nanozymes as neuroinflammation inhibitors for managing Parkinson's disease. Nat Commun. 2023;14:8137
126. Zhou W, Li H, Xia B, Ji W, Ji S, Zhang W. et al. Selenium - functionalized metal - organic frameworks as enzyme mimics. Nano Research. 2018;11:5761-8
127. Jiang X, Wang W, Tang J, Han M, Xu Y, Zhang L. et al. Ligand - Screened Cerium - Based MOF Microcapsules Promote Nerve Regeneration via Mitochondrial Energy Supply. Adv Sci (Weinh). 2024;11:e2306780
128. Xiang K, Wu H, Liu Y, Wang S, Li X, Yang B. et al. MOF - derived bimetallic nanozyme to catalyze ROS scavenging for protection of myocardial injury. Theranostics. 2023;13:2721-33
129. Man T, Xu C, Liu XY, Li D, Tsung CK, Pei H. et al. Hierarchically encapsulating enzymes with multi - shelled metal - organic frameworks for tandem biocatalytic reactions. Nat Commun. 2022;13:305
130. Wang D, Jana D, Zhao Y. Metal - Organic Framework Derived Nanozymes in Biomedicine. Acc Chem Res. 2020;53:1389-400
131. Tian R, Ma H, Ye W, Li Y, Wang S, Zhang Z. et al. Se - Containing MOF Coated Dual - Fe - Atom Nanozymes With Multi - Enzyme Cascade Activities Protect Against Cerebral Ischemic Reperfusion Injury. Advanced Functional Materials. 2022;32:2204025
132. Li S, Wang L, Zhang X, Chai H, Huang Y. A Co,N co - doped hierarchically porous carbon hybrid as a highly efficient oxidase mimetic for glutathione detection. Sensors and Actuators B: Chemical. 2018;264:312-9
133. Peng C, Pang R, Li J, Wang E. Current Advances on the Single - Atom Nanozyme and Its Bioapplications. Adv Mater. 2024;36:e2211724
134. Zhao D, Zhuang Z, Cao X, Zhang C, Peng Q, Chen C. et al. Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation. Chem Soc Rev. 2020;49:2215-64
135. Yang J, Wang D, Li Y. Identifying the Types and Characterization of the Active Sites on M - X - C Single - Atom Catalysts. Chemphyschem. 2020;21:2486-96
136. Wang D, Zhao Y. Single - atom engineering of metal - organic frameworks toward healthcare. Chem. 2021;7:2635-71
137. Liu H, Li Y, Sun S, Xin Q, Liu S, Mu X. et al. Catalytically potent and selective clusterzymes for modulation of neuroinflammation through single - atom substitutions. Nat Commun. 2021;12:114
138. Yan R, Sun S, Yang J, Long W, Wang J, Mu X. et al. Nanozyme - Based Bandage with Single - Atom Catalysis for Brain Trauma. ACS Nano. 2019;13:11552-60
139. Wang Y, Qi K, Yu S, Jia G, Cheng Z, Zheng L. et al. Revealing the Intrinsic Peroxidase - Like Catalytic Mechanism of Heterogeneous Single - Atom Co - MoS(2). Nanomicro Lett. 2019;11:102
140. Zhang R, Xue B, Tao Y, Zhao H, Zhang Z, Wang X. et al. Edge - Site Engineering of Defective Fe - N(4) Nanozymes with Boosted Catalase - Like Performance for Retinal Vasculopathies. Adv Mater. 2022;34:e2205324
141. Fu Z, Fan K, He X, Wang Q, Yuan J, Lim KS. et al. Single - Atom - Based Nanoenzyme in Tissue Repair. ACS Nano. 2024;18:12639-71
142. Lu Y, Liu T, Dong CL, Huang YC, Li Y, Chen J. et al. Tuning the Selective Adsorption Site of Biomass on Co(3) O(4) by Ir Single Atoms for Electrosynthesis. Adv Mater. 2021;33:e2007056
143. Hai X, Xi S, Mitchell S, Harrath K, Xu H, Akl DF. et al. Scalable two - step annealing method for preparing ultra - high - density single - atom catalyst libraries. Nat Nanotechnol. 2022;17:174-81
144. Liang J, Johannessen B, Wu Z, Webster RF, Yong J, Zulkifli MYB. et al. Regulating the Coordination Environment of Mesopore - Confined Single Atoms from Metalloprotein - MOFs for Highly Efficient Biocatalysis. Adv Mater. 2022;34:e2205674
145. Zhu Y, Wang W, Cheng J, Qu Y, Dai Y, Liu M. et al. Stimuli - Responsive Manganese Single - Atom Nanozyme for Tumor Therapy via Integrated Cascade Reactions. Angew Chem Int Ed Engl. 2021;60:9480-8
146. Fan C, Gao X, Tang P, Wang Q, Li B. Molten Salt - Assisted Synthesis of Porous Precious Metal - Based Single - Atom Catalysts for Oxygen Reduction Reaction. Adv Sci (Weinh). 2024: e2410784.
147. Chen J, Ma Q, Zheng X, Fang Y, Wang J, Dong S. Kinetically restrained oxygen reduction to hydrogen peroxide with nearly 100% selectivity. Nat Commun. 2022;13:2808
148. Xu B, Li S, Zheng L, Liu Y, Han A, Zhang J. et al. A Bioinspired Five - Coordinated Single - Atom Iron Nanozyme for Tumor Catalytic Therapy. Adv Mater. 2022;34:e2107088
149. Giulimondi V, Kaiser SK, Agrachev M, Krumeich F, Clark AH, Mitchell S. et al. Redispersion strategy for high - loading carbon - supported metal catalysts with controlled nuclearity. J Mater Chem A Mater. 2022;10:5953-61
150. Chen Y, Wang P, Hao H, Hong J, Li H, Ji S. et al. Thermal Atomization of Platinum Nanoparticles into Single Atoms: An Effective Strategy for Engineering High - Performance Nanozymes. J Am Chem Soc. 2021;143:18643-51
151. Wei S, Li A, Liu JC, Li Z, Chen W, Gong Y. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat Nanotechnol. 2018;13:856-61
152. Zhuang Z, Yu Y, Dong S, Sun X, Mao L. Carbon - based nanozymes: design, catalytic mechanisms, and environmental applications. Anal Bioanal Chem. 2024;416:5949-64
153. Dugan LL, Turetsky DM, Du C, Lobner D, Wheeler M, Almli CR. et al. Carboxyfullerenes as neuroprotective agents. Proc Natl Acad Sci U S A. 1997;94:9434-9
154. Samuel EL, Marcano DC, Berka V, Bitner BR, Wu G, Potter A. et al. Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. Proc Natl Acad Sci U S A. 2015;112:2343-8
155. Wen Y, Yan L, Ling Y - C. The designing strategies of graphene - based peroxidase mimetic materials. Science China Chemistry. 2018;61:266-75
156. Farshidfar N, Fooladi S, Nematollahi MH, Iravani S. Carbon dots with tissue engineering and regenerative medicine applications. RSC Adv. 2023;13:14517-29
157. Alafeef M, Srivastava I, Aditya T, Pan D. Carbon Dots: From Synthesis to Unraveling the Fluorescence Mechanism. Small. 2024;20:e2303937
158. Szczepankowska J, Khachatryan G, Khachatryan K, Krystyjan M. Carbon Dots - Types, Obtaining and Application in Biotechnology and Food Technology. Int J Mol Sci. 2023 24
159. Ren C, Hu X, Zhou Q. Graphene Oxide Quantum Dots Reduce Oxidative Stress and Inhibit Neurotoxicity In vitro and In vivo through Catalase - Like Activity and Metabolic Regulation. Adv Sci (Weinh). 2018;5:1700595
160. Zheng AX, Cong ZX, Wang JR, Li J, Yang HH, Chen GN. Highly - efficient peroxidase - like catalytic activity of graphene dots for biosensing. Biosens Bioelectron. 2013;49:519-24
161. Kotov NA. Chemistry. Inorganic nanoparticles as protein mimics. Science. 2010;330:188-9
162. Fan K, Xi J, Fan L, Wang P, Zhu C, Tang Y. et al. In vivo guiding nitrogen - doped carbon nanozyme for tumor catalytic therapy. Nat Commun. 2018;9:1440
163. Bai B, Qi S, Yang K, Yu X, Jian R, Zhang T. et al. Self - Assembly of Selenium - Doped Carbon Quantum Dots as Antioxidants for Hepatic Ischemia - Reperfusion Injury Management. Small. 2023;19:e2300217
164. Wang H, Chu D, Zhang M, Huang X, Shi Y, Zhao Y. et al. Manganese - doped carbon dots with catalase - like activity enable MRI - guided enhanced photodynamic therapy. Colloids Surf B Biointerfaces. 2025;246:114398
165. Sang Y, Huang Y, Li W, Ren J, Qu X. Bioinspired Design of Fe(3+) - Doped Mesoporous Carbon Nanospheres for Enhanced Nanozyme Activity. Chemistry. 2018;24:7259-63
166. He S, Ma L, Zheng Q, Wang Z, Chen W, Yu Z. et al. Peptide nanozymes: An emerging direction for functional enzyme mimics. Bioact Mater. 2024;42:284-98
167. Sinha NJ, Langenstein MG, Pochan DJ, Kloxin CJ, Saven JG. Peptide Design and Self - assembly into Targeted Nanostructure and Functional Materials. Chemical Reviews. 2021;121:13915-35
168. Liu Q, Wan K, Shang Y, Wang ZG, Zhang Y, Dai L. et al. Cofactor - free oxidase - mimetic nanomaterials from self - assembled histidine - rich peptides. Nat Mater. 2021;20:395-402
169. Chen C, Wu H, Li Q, Liu M, Yin F, Wu M. et al. Manganese Prussian blue nanozymes with antioxidant capacity prevent acetaminophen - induced acute liver injury. Biomater Sci. 2023;11:2348-58
170. Gao J, Chen S, Lin S, Mei X. Prussian blue analogues improves the microenvironment after spinal cord injury by regulating Zn. Int Immunopharmacol. 2024;131:111868
171. Zhang S, Wang L, Kang Y, Wu J, Zhang Z. Nanomaterial - based reactive oxygen species scavengers for osteoarthritis therapy. Acta Biomater. 2023;162:1-19
172. Lai Y, Wang J, Yue N, Zhang Q, Wu J, Qi W. et al. Glutathione peroxidase - like nanozymes: mechanism, classification, and bioapplication. Biomater Sci. 2023;11:2292-316
173. Abdelhamid HN. Zeolitic Imidazolate Frameworks (ZIF - 8) for Biomedical Applications: A Review. Curr Med Chem. 2021;28:7023-75
174. Zhou H, Jing S, Xiong W, Zhu Y, Duan X, Li R. et al. Metal - organic framework materials promote neural differentiation of dental pulp stem cells in spinal cord injury. J Nanobiotechnology. 2023;21:316
175. Wang S, Liu Y, Sun Q, Zeng B, Liu C, Gong L. et al. Triple Cross - linked Dynamic Responsive Hydrogel Loaded with Selenium Nanoparticles for Modulating the Inflammatory Microenvironment via PI3K/Akt/NF - κB and MAPK Signaling Pathways. Adv Sci (Weinh). 2023;10:e2303167
176. Zhou H, Li Z, Jing S, Wang B, Ye Z, Xiong W. et al. Repair spinal cord injury with a versatile anti - oxidant and neural regenerative nanoplatform. J Nanobiotechnology. 2024;22:351
177. Liu D, Niu R, Wang S, Shao L, Yang X, Liu X. et al. Nitric Oxide - Releasing Mesoporous Hollow Cerium Oxide Nanozyme - Based Hydrogel Synergizes with Neural Stem Cell for Spinal Cord Injury Repair. ACS Nano. 2024
178. Das M, Patil S, Bhargava N, Kang JF, Riedel LM, Seal S. et al. Auto - catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28:1918-25
179. Zhang W, Hu S, Yin JJ, He W, Lu W, Ma M. et al. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J Am Chem Soc. 2016;138:5860-5
180. Busquets MA, Estelrich J. Prussian blue nanoparticles: synthesis, surface modification, and biomedical applications. Drug Discovery Today. 2020;25:1431-43
181. Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma. 2000;17:203-18
182. Milich LM, Ryan CB, Lee JK. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 2019;137:785-97
183. Kim JW, Mahapatra C, Hong JY, Kim MS, Leong KW, Kim HW. et al. Functional Recovery of Contused Spinal Cord in Rat with the Injection of Optimal - Dosed Cerium Oxide Nanoparticles. Adv Sci (Weinh). 2017;4:1700034
184. Cui X, Huang C, Huang Y, Zhang Y, Wu J, Wang G. et al. Amplification of Metalloregulatory Proteins in Macrophages by Bioactive ZnMn@SF Hydrogels for Spinal Cord Injury Repair. ACS Nano. 2024;18:33614-28
185. Chen L, Wang W, Lin Z, Lu Y, Chen H, Li B. et al. Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. J Nanobiotechnology. 2022;20:210
186. Peng C, Luo J, Wang K, Li J, Ma Y, Li J. et al. Iridium metal complex targeting oxidation resistance 1 protein attenuates spinal cord injury by inhibiting oxidative stress - associated reactive oxygen species. Redox Biol. 2023;67:102913
187. Xu Y, Lai H, Pan S, Pan L, Liu T, Yang Z. et al. Selenium promotes immunogenic radiotherapy against cervical cancer metastasis through evoking P53 activation. Biomaterials. 2024;305:122452
188. Zou B, Xiong Z, Yu Y, Shi S, Li X, Chen T. Rapid Selenoprotein Activation by Selenium Nanoparticles to Suppresses Osteoclastogenesis and Pathological Bone Loss. Adv Mater. 2024;36:e2401620
189. Hao M, Zhang D, Wang W, Wei Z, Duan J, He J. et al. HAp Thermosensitive Nanohydrogel Cavities Act as Brood Pouches to Incubate and Control - Release NSCs for Rapid Spinal Cord Injury Therapy. Advanced Functional Materials. 2022;32:2203492
190. Yu Y, Wang Y, Zhang J, Bu Q, Jiang D, Jiang Y. et al. Anaerobic probiotics - in situ Se nanoradiosensitizers selectively anchor to tumor with immuno - regulations for robust cancer radio - immunotherapy. Biomaterials. 2025;318:123117
191. Umapathy S, Pan I, Issac PK, Kumar MSK, Giri J, Guru A. et al. Selenium Nanoparticles as Neuroprotective Agents: Insights into Molecular Mechanisms for Parkinson's Disease Treatment. Mol Neurobiol. 2024
192. Luo W, Li Y, Zhao J, Niu R, Xiang C, Zhang M. et al. CD44 - targeting hyaluronic acid - selenium nanoparticles boost functional recovery following spinal cord injury. J Nanobiotechnology. 2024;22:37
193. Liu X, Mao Y, Huang S, Li W, Zhang W, An J. et al. Selenium nanoparticles derived from Proteus mirabilis YC801 alleviate oxidative stress and inflammatory response to promote nerve repair in rats with spinal cord injury. Regen Biomater. 2022;9:rbac042
194. Rao S, Lin Y, Lin R, Liu J, Wang H, Hu W. et al. Traditional Chinese medicine active ingredients - based selenium nanoparticles regulate antioxidant selenoproteins for spinal cord injury treatment. J Nanobiotechnology. 2022;20:278
195. Liu P, Liu X, Wu Z, Shen K, Li Z, Li X. et al. Size effect - based improved antioxidant activity of selenium nanoparticles regulating Anti - PI3K - mTOR and Ras - MEK pathways for treating spinal cord injury to avoid hormone shock - induced immunosuppression. J Nanobiotechnology. 2025;23:17
196. Saifi MA, Seal S, Godugu C. Nanoceria, the versatile nanoparticles: Promising biomedical applications. J Control Release. 2021;338:164-89
197. Gao Y, Zou J, Chen B, Cao Y, Hu D, Zhang Y. et al. Hyaluronic acid/serotonin - decorated cerium dioxide nanomedicine for targeted treatment of ulcerative colitis. Biomater Sci. 2023;11:618-29
198. Behroozi Z, Rahimi B, Hamblin MR, Nasirinezhad F, Janzadeh A, Ramezani F. Injection of Cerium Oxide Nanoparticles to Treat Spinal Cord Injury in Rats. J Neuropathol Exp Neurol. 2022;81:635-42
199. Li D, Dai D, Xiong G, Lan S, Zhang C. Metal - Based Nanozymes with Multienzyme - Like Activities as Therapeutic Candidates: Applications, Mechanisms, and Optimization Strategy. Small. 2023;19:2205870
200. Sha F, Wang X, Kirlikovali KO, Farha OK. Enhancing Biocatalysis: Metal - Organic Frameworks as Multifunctional Enzyme Hosts. Acc Chem Res. 2024;57:3500-11
201. Haider J, Shahzadi A, Akbar MU, Hafeez I, Shahzadi I, Khalid A. et al. A review of synthesis, fabrication, and emerging biomedical applications of metal - organic frameworks. Biomater Adv. 2022;140:213049
202. Zhai X, Chen K, Wei X, Zhang H, Yang H, Jiao K. et al. Microneedle/CD - MOF - mediated transdural controlled release of methylprednisolone sodium succinate after spinal cord injury. J Control Release. 2023;360:236-48
203. Troyano J, Carné - Sánchez A, Avci C, Imaz I, Maspoch D. Colloidal metal - organic framework particles: the pioneering case of ZIF - 8. Chem Soc Rev. 2019;48:5534-46
204. Lin S, Cui J, Li X, Chen S, Gao K, Mei X. Modified ZIF - 8 Nanoparticles for Targeted Metabolic Treatment of Acute Spinal Cord Injury. ACS Appl Mater Interfaces. 2024;16:14503-9
205. Cao F, Zhang L, You Y, Zheng L, Ren J, Qu X. An Enzyme - Mimicking Single - Atom Catalyst as an Efficient Multiple Reactive Oxygen and Nitrogen Species Scavenger for Sepsis Management. Angew Chem Int Ed Engl. 2020;59:5108-15
206. Li B, Bai Y, Yion C, Wang H, Su X, Feng G. et al. Single - Atom Nanocatalytic Therapy for Suppression of Neuroinflammation by Inducing Autophagy of Abnormal Mitochondria. ACS Nano. 2023;17:7511-29
207. Zhang S, Li Y, Sun S, Liu L, Mu X, Liu S. et al. Single - atom nanozymes catalytically surpassing naturally occurring enzymes as sustained stitching for brain trauma. Nat Commun. 2022;13:4744
208. Jiang Y, Rong H, Wang Y, Liu S, Xu P, Luo Z. et al. Single - atom cobalt nanozymes promote spinal cord injury recovery by anti - oxidation and neuroprotection. Nano Research. 2023;16:9752-9
209. McHugh EA, Liopo AV, Mendoza K, Robertson CS, Wu G, Wang Z. et al. Oxidized Activated Charcoal Nanozymes: Synthesis, and Optimization for In vitro and In vivo Bioactivity for Traumatic Brain Injury. Adv Mater. 2024;36:e2211239
210. Sun H, Zhou Y, Ren J, Qu X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew Chem Int Ed Engl. 2018;57:9224-37
211. Johnson AP, Gangadharappa HV, Pramod K. Graphene nanoribbons: A promising nanomaterial for biomedical applications. J Control Release. 2020;325:141-62
212. Rahbar A, Shakyba S, Ghaderi M, Kazemi K, Fagheh AF, Farsinejad P. et al. Ivermectin - functionalized multiwall carbon nanotube enhanced the locomotor activity and neuropathic pain by modulating M1/M2 macrophage and decrease oxidative stress in rat model of spinal cord injury. Heliyon. 2021;7:e07311
213. Li J, Wang H, Li Y, Wang C, Feng H, Pang Y. et al. Novel carbon dots with dual Modulatory effects on the bone marrow and spleen as a potential therapeutic candidate for treating spinal cord injury. Bioact Mater. 2025;45:534-50
214. Qi Z, Pan S, Yang X, Zhang R, Qin C, Yan H. et al. Injectable Hydrogel Loaded with CDs and FTY720 Combined with Neural Stem Cells for the Treatment of Spinal Cord Injury. Int J Nanomedicine. 2024;19:4081-101
215. Iorio R, Petricca S, Di Emidio G, Falone S, Tatone C. Mitochondrial Extracellular Vesicles (mitoEVs): emerging mediators of cell - to - cell communication in health, aging and age - related diseases. Ageing Res Rev. 2024: 102522.
216. Garcia - Martin R, Wang G, Brandão BB, Zanotto TM, Shah S, Kumar Patel S. et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446-51
217. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 367
218. Zhao J, Zhu W, Mao Y, Li X, Ling G, Luo C. et al. Unignored intracellular journey and biomedical applications of extracellular vesicles. Adv Drug Deliv Rev. 2024;212:115388
219. Nie X, Liu Y, Yuan T, Yu T, Yun Z, Xue W. et al. Platelet - rich plasma - derived exosomes promote blood - spinal cord barrier repair and attenuate neuroinflammation after spinal cord injury. J Nanobiotechnology. 2024;22:456
220. Miron RJ, Estrin NE, Sculean A, Zhang Y. Understanding exosomes: Part 2 - Emerging leaders in regenerative medicine. Periodontol 2000. 2024;94:257-414
221. Bai L, Gao J, Zhang P, Lin S, Zhang C. Immunotherapy of M2 macrophage derived from exosome - based nanoparticles for spinal cord injury. Int Immunopharmacol. 2024;132:111983
222. Yu Q, Jiang X, Liu X, Shen W, Mei X, Tian H. et al. Glutathione - modified macrophage - derived cell membranes encapsulated metformin nanogels for the treatment of spinal cord injury. Biomater Adv. 2022;133:112668
223. Liu X, Sun J, Du J, An J, Li Y, Hu Y. et al. Encapsulation of Selenium Nanoparticles and Metformin in Macrophage - Derived Cell Membranes for the Treatment of Spinal Cord Injury. ACS Biomater Sci Eng. 2023;9:5709-23
224. Yin P, Liang W, Han B, Yang Y, Sun D, Qu X. et al. Hydrogel and Nanomedicine - Based Multimodal Therapeutic Strategies for Spinal Cord Injury. Small Methods. 2024;8:e2301173
225. Li N, He J. Hydrogel - based therapeutic strategies for spinal cord injury repair: Recent advances and future prospects. Int J Biol Macromol. 2024;277:134591
226. Li L, Xiao B, Mu J, Zhang Y, Zhang C, Cao H. et al. A MnO(2) Nanoparticle - Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano. 2019;13:14283-93
227. Xu L, Mu J, Ma Z, Lin P, Xia F, Hu X. et al. Nanozyme - Integrated Thermoresponsive In situ Forming Hydrogel Enhances Mesenchymal Stem Cell Viability and Paracrine Effect for Efficient Spinal Cord Repair. ACS Appl Mater Interfaces. 2023;15:37193-204
Author contact
![]() Corresponding authors: Ruoyu Li: 349508872com; Huimin Hu: dochuhuimincom; Xiaodong Wang: ZSSL_70com; Dingjun Hao: haodingjunxjtu.edu.cn
Corresponding authors: Ruoyu Li: 349508872com; Huimin Hu: dochuhuimincom; Xiaodong Wang: ZSSL_70com; Dingjun Hao: haodingjunxjtu.edu.cn
 Global reach, higher impact
Global reach, higher impact