13.3
Impact Factor
Theranostics 2025; 15(13):6236-6252. doi:10.7150/thno.114676 This issue Cite
Review
Piezo-catalytic immunotherapy: mechanisms and feasibility in cancer treatment
Department of Ultrasound, The First Hospital of China Medical University, No. 155, Nanjing North Street, Heping District, Shenyang, 110001, Liaoning Province, China.
Received 2025-3-29; Accepted 2025-4-26; Published 2025-5-9
Abstract
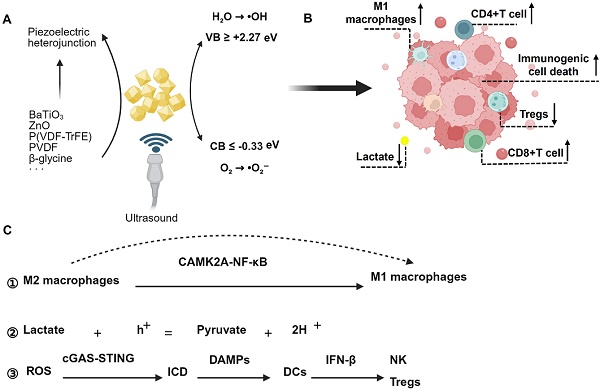
Over the past decade, immunotherapy has revolutionized the clinical management of numerous cancer types. However, only a subset of patients derives long-term durable tumor control from it. Intriguingly, several emerging therapeutic strategies harnessing reactive oxygen species (ROS)-mediated immunogenic cell death (ICD) hold promise for enabling precision immunotherapy in cancer, with sonodynamic therapy emerging as a notable example. The utilization of piezoelectric materials as sonosensitizers can effectively enhance ROS production, thereby augmenting the efficacy of ICD-induced immunotherapy. Additionally, electrical stimulation generated by the piezoelectric effect can further induce immune response activation and necroptosis, achieving a synergistic effect in piezo-catalytic immunotherapy. Herein, we will systematically review the pathways and potential mechanisms underlying piezo-catalytic immunotherapy, offering novel insights for the exploration of cancer immunotherapy strategies.
Keywords: Piezoelectric effect, Immunotherapy, Sonodynamic therapy, Sono-piezo dynamic therapy, Tumor immune microenvironment
Introduction
Cancer immunotherapy, which leverages the body's immune system to recognize and eliminate cancer cells, has emerged as a promising treatment modality for various cancer types [1]. This approach encompasses immune checkpoint blockade (ICB) [2], therapeutic vaccines [3], and adoptive cell therapy [4], among others. Over the past decade, immunotherapy has revolutionized the clinical management of numerous cancer types. However, due to significant interindividual variability in response, only a subset of patients achieves long-term durable tumor control [5]. Furthermore, immunotherapy can induce adverse events, potentially damaging multiple organ systems and even leading to death in some patients [6,7]. Therefore, achieving tumor-targeted immunotherapy is crucial for efficient and low-toxicity cancer treatment [8]. Studies have demonstrated that combining immunotherapy with other treatment modalities, such as chemotherapy, radiotherapy, and targeted therapy, can enhance tumor treatment efficacy [9-12].
Intriguingly, emerging therapeutic strategies leveraging reactive oxygen species (ROS)-mediated immunogenic cell death (ICD) have the potential to enable precision immunotherapy for cancer [13]. These strategies include sonodynamic therapy (SDT) [14], photodynamic therapy (PDT) [15], and radio-dynamic therapy (RDT) [16]. Compared to PDT and RDT, SDT-based immunotherapy offers advantages of low side effects and deep tissue penetration, thus holding greater promise for clinical translation [13]. The exact mechanism of SDT remains unclear, but it is believed that the ultrasonic cavitation effect plays a pivotal role. In the presence of sonosensitizers, stable cavitation during the ultrasonic process generates mechanical shearing, micro-jetting, and acoustic microstreaming, lowering the energy threshold required for cell membrane damage. Inertial cavitation triggers sonoluminescence, sonothermal, and sonomechanical effects, resulting in sufficient ROS production to kill cells [17,18]. Consequently, SDT can effectively induce ICD and activate antitumor immune responses through ROS-mediated apoptotic pathways in cancer cells [19]. However, traditional sonosensitizers face challenges due to low bioavailability, and SDT-induced antitumor immunity is limited by the hypoxic tumor microenvironment (TME) and insufficient ROS production [20]. Recent advances in ROS-based therapeutic strategies have been propelled by both endogenous response modulation and exogenous stimulation techniques. For endogenous potentiation, electron hybridization-mediated chemical dynamics (e.g., transition metal-catalyzed Fenton-like reactions) can amplify intratumoral oxidative stress through rational nanoplatform design [21,22]. Externally triggered approaches offer spatiotemporal control, including: (i) magneto-electrodynamic systems that convert alternating magnetic fields into localized ROS bursts via nano-transducers [23], (ii) energy-band-engineered photodynamic agents that overcome hypoxia limitations by modulating electron-hole separation [24], and (iii) multifield-coupled piezoelectric catalysis integrating mechanical, thermal, and electrical stimuli for deep-tissue therapy [25]. These paradigm-shifting strategies collectively expand the frontiers of nanocatalytic medicine by addressing tumor microenvironment constraints while enabling precision therapeutics.
Excitingly, piezoelectric materials have been utilized as sonosensitizers, generating more ROS upon ultrasonic excitation and demonstrating excellent therapeutic efficacy in cancer treatment. This method has been termed sono-piezo dynamic therapy (SPDT) [26-28]. In contrast to conventional SDT, which primarily depends on sonosensitizers (e.g., porphyrins or TiO2) to generate ROS via ultrasonic cavitation, SPDT introduces a paradigm shift by leveraging the intrinsic piezoelectric properties of materials to achieve dual mechanical-electrical stimulation. Under ultrasound excitation (1-3 MHz), piezoelectric materials undergo electron-hole pair separation, creating a built-in electric field with surface charges that directly drive redox reactions (e.g., H2O → •OH, O2 → •O2⁻) at potentials exceeding those of traditional SDT. Critically, engineered piezoelectric heterojunctions enhance charge separation efficiency, enabling sustained ROS production even under hypoxic condition—a limitation that severely restricts SDT efficacy [29]. Currently, piezoelectric materials are being explored in cancer immunotherapy, where ultrasonic excitation generates ROS and piezoelectric effects to induce immune response activation and necroptosis, thereby achieving a synergistic effect in tumor piezo-catalytic immunotherapy [30]. In this study, we aim to clarify the concept of piezo-catalytic immunotherapy, focusing on elucidating the pathways and potential mechanisms involved. Importantly, we will also discuss the feasibility, advantages, and disadvantages of using piezoelectric materials as sonosensitizers in cancer immunotherapy. Our findings provide new insights for exploring cancer immunotherapy strategies.
Tumor immune microenvironment (TIM)
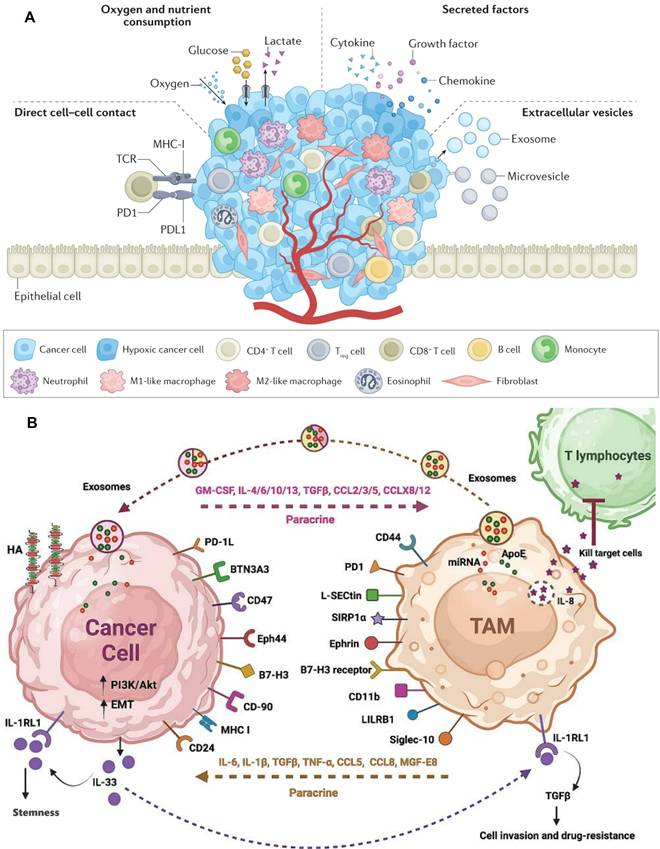
The TIM constitutes an intricate network comprising diverse immune cells, immune molecules, and non-immune cellular entities within the tumor tissue and its adjacent milieu. These constituents, through their intricate interactions and regulatory mechanisms, collectively modulate tumor growth, invasion, and metastasis [31,32]. Among these components, immune cells emerge as the most prevalent and complex participants within tumor tissue, exhibiting dual functionalities in cancer initiation and progression, exerting both antitumor and protumor effects [33]. This duality is primarily contingent on the dynamic crosstalk between cancer cells and immune cells, with cancer cells communicating and nurturing adjacent or distant immune cells via the secretion of extracellular vesicles, histones, cytokines, chemokines, growth factors, and proteases (Figure 1A).
Immune cells encompass T cells, B cells, macrophages, and dendritic cells (DCs). Antitumor immune cells primarily consist of effector T cells, specifically CD4+ T cells and CD8+ T cells, DCs, and M1-polarized macrophages [34]. The infiltration level of T cells is regarded as a pivotal determinant for the prognosis of cancer patients. In the natural TME, T cells serve as the primary effector cells of antitumor immune responses. CD4+ T cells can augment the proliferation of cytotoxic T lymphocytes and enhance antigen presentation by DCs, whereas DCs produce TNF-α, IL-6, IL-8, and IL-12 to participate in antitumor immunity [35,36]. Protumor immune cells predominantly include regulatory T cells (Tregs), M2-polarized macrophages, and N2-polarized neutrophils [31]. Notably, M2 macrophages dominate within tumor-associated macrophages (TAMs), inhibiting antitumor immune responses through the secretion of immunosuppressive cytokines such as TGF-β, IL-4, IL-10, among others [37]. Consequently, current TIM-targeted therapies primarily concentrate on inducing TAM depletion, inhibiting TAM recruitment, and polarizing M2 macrophages towards the M1 phenotype (Figure 1B) [38,39].
However, during cancer progression, tumor cells incessantly absorb nutrients to sustain their rapid proliferation. Any alterations in the metabolic process can regulate the survival and function of immune cells, ultimately facilitating immune evasion and tumor progression [40]. Attributable to the active glycolysis of tumor cells, the interstitial glucose content within tumors diminishes, and such low glucose levels can impair T-cell functionality [41]. Concurrently, the extensive glucose metabolism in tumor cells results in lactate accumulation, with high lactate concentrations impeding CD8+ T-cell proliferation and cytokine secretion, thereby influencing the inactivation of tumor-infiltrating lymphocytes (TILs) [42]. Furthermore, the TME is abundant in lipids, encompassing fatty acids, lipoproteins, and cholesterol. Lipid accumulation in DCs induces endoplasmic reticulum (ER) stress, diminishing antigen presentation capabilities. ER stress induced by cholesterol accumulation leads to T-cell exhaustion. Enhanced lipid intake and peroxidation result in heightened oxidative stress, culminating in CD8+ T-cell dysfunction and ferroptosis [43,44]. Therefore, targeted interventions directed at any of these processes possess the potential to achieve immunotherapy for cancer.
Tumor immune microenvironment. A) The communication mechanism between cancer cells and immune cells. Adapted with permission from [33], copyright 2023 Springer Nature Limited; B) Interference between tumor cells and TAMs. Adapted with permission from [37], copyright 2024 The Author(s).
Sonodynamic therapy for TIM
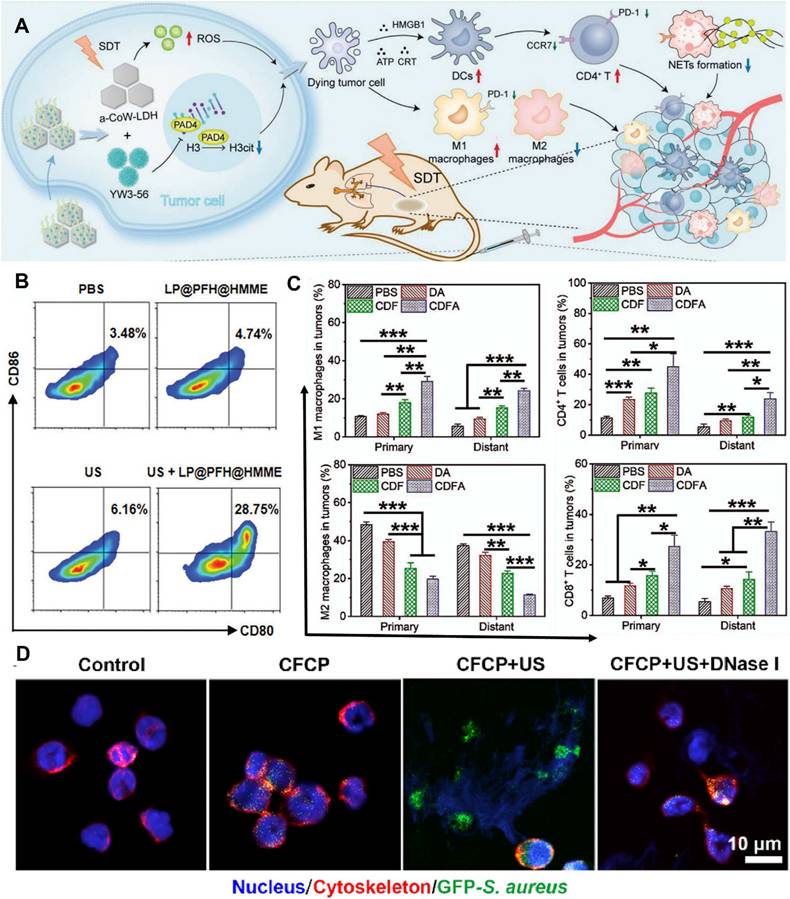
The three essential elements of SDT are ultrasound stimulation, sonosensitizers, and oxygen. The development of sonosensitizers has evolved from the earliest porphyrins and their derivatives to today's metal-organic framework sonosensitizers and novel piezoelectric sonosensitizers, undergoing a process from simplicity to complexity and from singularity to diversity [45]. As research deepens, scholars [46] have discovered that SDT can trigger systemic immune responses in the body and promote the treatment of metastatic tumors. For example, amorphous CoW-layered double hydroxide loaded with peptidylarginine deiminase 4 (PAD4) generates a large amount of ROS under ultrasound, inducing ICD while inhibiting the increase in citrullinated histone H3 (H3cit) and the release of neutrophil extracellular traps (NETs). This method upregulates the antitumor response while inhibiting immune escape (Figure 2A) and is defined as sono-immunotherapy [47].
Effective immunotherapy can be achieved by enhancing ICB through SDT. Nanoparticles loaded with anti-PD-L1 antibodies and containing sonosensitizers are constructed to generate a certain amount of ROS under ultrasound excitation, triggering ICD to sensitize the immune efficacy of ICB induced by anti-PD-L1 [48-50]. However, the mechanisms of action of SDT vary. Chen et al. [48] found that ICD induced by SDT may further trigger antitumor immune activation by promoting DC maturation (Figure 2B). In contrast, Ya et al. [49] found that amplified ICD combined with ICB increased the infiltration of cytotoxic T lymphocytes and natural killer (NK) cells, polarized M2 macrophages into M1 macrophages, and reduced regulatory T cells (Figure 2C).
SDT-mediated ICD activates the stimulator of interferon genes (STING) to amplify the immune stimulation of tumor-infiltrating myeloid cells. Based on STING agonists such as MSA-2 [51] and Mn2+ [52], nanomedicine delivery systems are constructed to generate ROS under ultrasound excitation, accurately damaging the cell nucleus while activating the cGAS-STING pathway to induce innate immune responses [53]. Alternatively, interference with iron ion metabolism increases the release of bacterial-associated double-stranded DNA (dsDNA), which promotes DC maturation by activating STING, thereby amplifying the immune stimulation of neutrophils (Figure 2D) [54].
By targeting the glycolysis process in the TME, SDT significantly inhibits cancer cell glucose uptake and lactate excretion while inducing cancer cell apoptosis and ICD, achieving ultrasound-triggered immune activation [55,56]. In addition, SDT synergizes with autophagy [57], oncolytic pyroptosis [58,59], and ferroptosis [56] to maximize tumor immunogenicity and reverse the immunosuppressive TME, ultimately triggering a powerful and durable systemic antitumor immune response.
However, traditional sensitizers have limited ability to generate ROS after ultrasound excitation, resulting in suboptimal efficacy of sono-immunotherapy. Therefore, it is crucial to explore potential sonosensitizers with higher ultrasound responsiveness. Promising candidate materials that have been explored include piezoelectric materials, defective semiconductors, hybrid assembly polymers with narrow band gaps, and novel sono-catalysts with heterojunctions [60].
The mechanism of SPDT
Traditional SDT relies on inertial cavitation to generate transient ROS bursts, which are often insufficient to induce robust ICD in hypoxic or immunosuppressive TMEs. In contrast, SPDT employs piezoelectric materials to achieve dual energy conversion. The dissociated electrons and holes migrate to opposite surfaces, leading to the production of a certain quantity of ROS through redox reactions. This process facilitates sonodynamic therapy for tumors. Furthermore, the charges induced on the surface of piezoelectric materials can directly impact cancer cells, altering the cell membrane potential, causing depolarization of both the mitochondrial membrane and the plasma membrane, and ultimately enhancing the effectiveness of sonodynamic therapy sensitized by the piezoelectric effect [28]. Consequently, piezoelectric materials are regarded as a promising class of sonosensitizers. Currently, inorganic piezoelectric materials are extensively studied due to their superior piezoelectric properties. However, their clinical application is hindered by factors such as high polarization temperatures, elevated preparation costs, poor stability, and potential toxicity. Recent advances in piezoelectric nanocomposites (e.g., 0.7BiFeO3-0.3BaTiO3 heterojunctions) demonstrate dual capabilities of ROS generation and PD-L1 downregulation, thereby enhancing checkpoint blockade efficacy [61]. Their team designed a tumor-targeted BTO-Pd-MnO2-HA piezoelectric nanocomposite. This system utilizes Schottky junctions to promote electron-hole separation, significantly boosting ROS production. Additionally, the TME triggers the degradation of MnO2, releasing Mn²⁺ ions that catalyze H2O2 decomposition via Fenton-like reactions to generate hydroxyl radicals (•OH). Concurrently, the nanocomposite depletes glutathione (GSH) and releases oxygen, synergistically enhancing SDT and chemodynamic therapy (CDT). This innovation expands the application of piezoelectric nanomaterials in biomedicine for precise regulation of the tumor microenvironment [62].
Sonodynamic therapy for TIM. A) Schematic illustration of the preparation of a-LDH@356-PEG nanosheets and their application in synergistic SDT/immunotherapy. Adapted with permission from [46], copyright 2024 The Author(s). B) A typical flow cytometry assay of mature BMDCs (CD80+CD86+). Adapted with permission from [48], copyright 2024 The Author(s). C) Quantitation analysis of M1 TAM(CD45+CD11b+CD80+F4/80+), M2 TAM(CD45+CD11b+CD206+F4/80+), CD4+ T cells (CD45+CD3e+CD4+), CD8+ T cells (CD45+CD3e+CD8+). Adapted with permission from [49], copyright 2023 Elsevier Ltd. D) CLSM images after co-culturing neutrophils from different DCM co-culture groups with fluorescent bacteria. Green for GFP-S. aureus, red for the neutrophil cytoskeleton, and blue for the nucleus. Adapted with permission from [54], copyright 2024 The Author(s).
Piezoelectric materials for immunotherapy
| Material Type | Example | Key Properties | Bandgap (eV) | ROS Generation Mechanism | ROS Efficiency | Immune Activation Potential | TIM Remodeling Effects | Biodegradability | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Inorganic Piezoelectrics | Barium Titanate (BaTiO₃) | Exhibits significant ferroelectric, piezoelectric, and pyroelectric properties; possesses photoluminescence and photocatalytic activity | 1.94 (pristine) | Piezoelectric potential drives redox reactions (e.g., H₂O → ·OH, O₂ → ·O₂⁻). | ROS levels increased by 2.5× vs. SDT; 80% tumor ablation in mice. | Induces ICD via ROS, promotes DC maturation and M1 macrophage polarization | Polarizes M1 macrophages (CD80⁺↑ 3×), enhances CD8⁺ T cell infiltration (51.7% in spleen). | Low (Non-degradable; potential hepatic toxicity at high doses) | [61, 86, 92] |
| ZnO | High melting point, simple preparation, low deposition temperature | 1.05 | Fenton-like reactions under TME (H₂O₂ → ·OH) + piezoelectric charge. | ·OH production increased by 4× in hypoxic TME. | Synergizes ROS and Ca²⁺ release for necroptosis, enhances CD8⁺ T cell infiltration | Triggers ferroptosis (lipid peroxidation ↑ 3.5×), reduces Tregs (↓40%). | Low (Similar to pristine BaTiO₃) | [62, 72] | |
| Organic Piezoelectrics | PVDF-TrFE | Flexibility, biocompatibility | 3.37 | Charge transfer via flexible polymer chains under ultrasound. | Moderate ROS (1.8× vs. control) but enhances Ca²⁺ influx. | Triggers ferroptosis, enhances antigen presentation via ZnO-PLLA composites | Promotes M1 polarization (CD86⁺↑ 2.3×) via Ca²⁺-CAMK2A-NF-κB axis. | Low (Non-degradable) | [77, 90] |
| PVDF | Exhibits excellent dielectric, ferroelectric, piezoelectric, and pyroelectric properties | - | - | Moderate (Charge transfer) | Modulates macrophage polarization via Ca²⁺-CAMK2A-NF-κB axis | - | Moderate (Biocompatible but non-degradable) | [26, 27, 74] | |
| PLLA | Biodegradable; exhibits good compatibility | - | Degradable piezoelectric scaffolds release charges gradually. | Low ROS (1.2× baseline) but sustains antigen presentation. | Enhances T cell responses via antigen delivery | Activates dendritic cells (CD80⁺↑ 2×), increases CD8⁺ T cells in tumors (↑3×). | High (Biodegradable; degrades within weeks) | [74, 94] | |
| Hybrid Materials | BiFeO₃ | Bandgap modulation (2.27 eV), glucose metabolism targeting | 2.8 | Schottky junction enhances charge separation; dual ROS and lactate depletion. | ROS ↑ 3×; lactate oxidation rate ↑ 68%. | Induces cellular senescence, amplifies ICD and checkpoint blockade | Repolarizes TAMs (M1/M2 ratio ↑ 2.1), inhibits PD-L1 glycosylation (↓60%). | Low (Non-degradable) | [82] |
| Defect-Engineered | CBNO-OV1 Nanosheets | Oxygen vacancies enhance charge separation | 3.16 | Oxygen vacancies inhibit electron-hole recombination, boosting ROS. | ROS ↑ 4× vs. pristine CBNO; necroptosis ↑ 2×. | Activates NF-κB pathway, induces necroptosis and systemic immune response | Increases CD8⁺ TILs (↑2.5×), reduces Tregs (↓50%), extends survival (28d → 49d in glioma). | Moderate (Partial degradation in vivo) | [69] |
| Natural Piezoelectric Biomaterials | β-glycine | Natural piezoelectricity, high biosafety | - | Supramolecular stacking generates high shear piezoelectricity (178 pm/V). | Moderate ROS (1.5× control) but biocompatible. | Promotes wound healing via electrical signals; potential for localized immune activation | Enhances epidermal regeneration (↑50% wound closure), minimal immune activation. | Very high (80% degradation within 4 weeks in chitosan composites) | [94, 95, 93] |
In previous research, a comprehensive review of the current status of piezoelectric materials in SDT applications was conducted. Additionally, the concept of SPDT was proposed, and the process of ROS generation by ultrasound-excited piezoelectric materials was elaborated. Specifically, ultrasound stimulates piezoelectric materials, causing the negative voltage at the conduction band (CB) edge to slightly exceed the O2/•O2⁻ redox potential (-0.33 V), thereby energetically favoring the O2/•O2⁻ redox reaction on the CB. Simultaneously, the positive voltage at the valence band (VB) edge is considerably higher than the H2O/•OH redox potential, facilitating the direct transfer of hole charges from the VB to water molecules to generate •OH. By constructing piezoelectric heterojunctions, semiconductor materials with lower energy levels are integrated with piezoelectric materials. Following ultrasound excitation, the electron-hole pairs within the piezoelectric materials transfer to the semiconductors with lower energy levels, preventing the rapid recombination of electron-hole pairs within the piezoelectric materials. This process increases ROS production and further promotes tumor cell death. For a detailed introduction, please refer to the recently published article [63]. Piezoelectric materials currently applied in immunotherapy are summarized in Table 1.
Piezo-catalyzed immunotherapy
As early as 2023, Shi and their team proposed the concept of Piezocatalytic Medicine (PCM), which refers to the application of piezocatalytic technology in the medical field. This encompasses the medical applications of piezocatalytic materials in tumor therapy, antibacterial treatments, organic degradation, tissue repair and regeneration, as well as biosensing [64]. In recent years, researchers have employed piezoelectric materials for cancer immunotherapy. Under ultrasound excitation, the generated ROS trigger ICD, thereby enhancing the efficacy of immunotherapy for cancer cells by remodeling the immune status of the tumor microenvironment. This approach is termed piezocatalytic immunotherapy [30]. While the underlying mechanisms remain incompletely understood, current research indicates that piezoelectric stimulation can modulate immune cells within the tumor immune microenvironment and regulate metabolic components, ultimately reshaping the tumor microenvironment to achieve immunotherapy for tumors.
Piezoelectric modulation of macrophage in TIM
The immune infiltration within the TME comprises macrophages, microglia, myeloid-derived suppressor cells, lymphocytes, NK cells, and neutrophils. The predominant populations are macrophages and microglia, collectively termed TAMs. The dynamic interplay between these components and glioma cells modulates tumor cell growth, proliferation, invasion, and survival [65,66]. Single-cell RNA sequencing has elucidated the evolution of the immune landscape throughout the progression of brain gliomas, revealing that early TME are primarily inhabited by microglia, whereas late-stage environments exhibit substantial infiltration of immunosuppressive macrophages and neutrophils [67]. Modulating the phenotype of TAMs can thus reshape the TIM, enabling immune sensitization therapy for tumors.
Montorsi et al. [68] treated microglia with piezoelectric polystyrene nanoparticles (PNPs) followed by ultrasound stimulation. They observed that microglia treated with CM-PNPs+US (conditioned medium-coated piezoelectric polystyrene nanoparticles combined with ultrasound stimulation) exhibited augmented inflammatory responses, evidenced by upregulated CD86 marker expression and increased ROS production. Glioblastoma cells cocultured with these stimulated microglia displayed reduced survival and proliferation rates, suggesting anticancer phenotype polarization. This study introduces a novel strategy of utilizing piezoelectric nanomaterials in conjunction with ultrasound stimulation to regulate microglia behavior for antitumor purposes, presenting a potential new approach for glioma immunotherapy. However, this study has limitations. Specifically, the underlying mechanisms through which piezoelectric stimulation enhances the inflammatory response of microglia require further exploration. Additionally, the long-term effects of this treatment on microglia function and cancer progression remain uncertain.
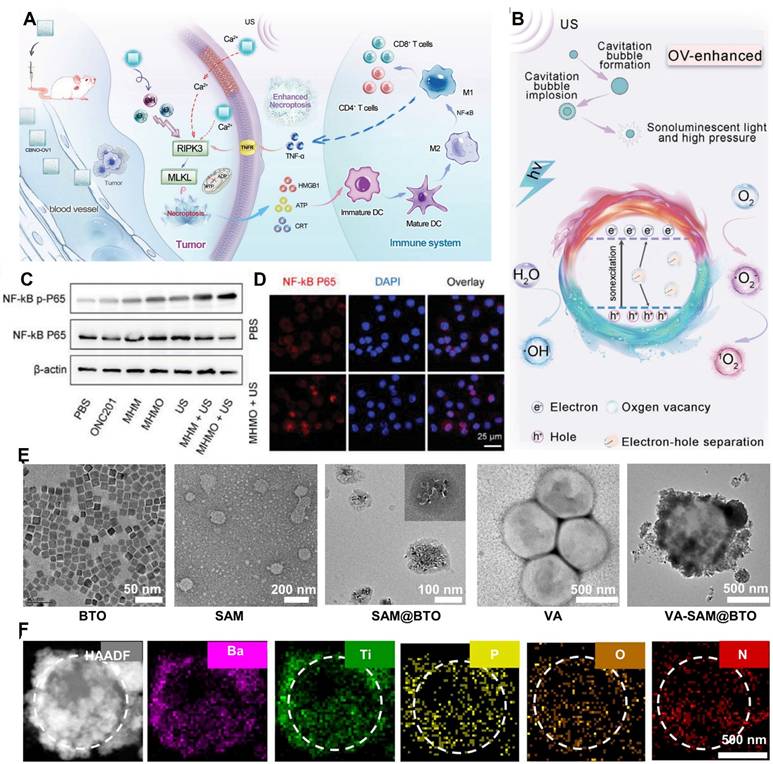
Du et al. [69] fabricated ultrathin CaBi2Nb2O9 nanosheets with tunable oxygen vacancies (CBNO-OV1). Oxygen vacancies enhance electron-hole separation efficiency by inhibiting electron-hole recombination, thereby substantially boosting ROS production. By adjusting the proportion of oxygen vacancies, they determined that the band gaps of CBNO, CBNO-OV0.5, CBNO-OV1, and CBNO-OV2 were 3.23 eV, 3.18 eV, 3.16 eV, and 3.13 eV, respectively. The CB of CBNO, CBNO-OV0.5, CBNO-OV1, and CBNO-OV2 relative to the normal hydrogen electrode was estimated at -0.348 eV, -0.388 eV, -0.438 eV, and -0.508 eV, respectively. Hence, a higher proportion of oxygen vacancies results in higher ROS generation efficiency. Furthermore, the efficient piezoelectric effect increases cell membrane permeability, leading to increased Ca2+ influx and necrotic apoptosis of cells. (Figure 3A and Figure 3B).
Yang et al. [70] designed piezoelectric Mg2+-doped hydroxyapatite (Mg-HAP) nanoparticles coated with a mesoporous silica layer and loaded with ONC201 as an agonist to specifically target the death receptor DR5 on tumor cells. They developed the Mg-HAP@MS/ONC201 nanoparticle (MHMO NP) system with a band gap of 5.3 eV, a VB of +3.55 eV, and a CB estimated at -1.75 eV, which is substantially higher than the redox potentials of H2O and O2. This study revealed for the first time that MHMO can promote significant ROS release under piezoelectric catalysis. Simultaneously, Ca2+ release synergizes with ROS to stimulate necrotic apoptosis of tumor cells, overcoming apoptosis resistance. (Figure 3C and Figure 3D).
Sun et al. [71] prepared one-dimensional oxygen-deficient BaTiO3 nanorod arrays (BaTiO3-x) on Ti to enhance sonocatalytic efficiency. L-Arginine (LA) was covalently immobilized on the surface of BaTiO3-x (BaTiO3-x/LA). Under US irradiation (1.5 W/cm2, 1 MHz, 50% duty cycle), the ROS generated by BaTiO3-x decomposed LA to release NO, initiating a chain reaction of ROS-NO-ONOO-. The weakly antibacterial ·O2- reacts with NO to produce the more antibacterial ONOO-. Concurrently, the rapid consumption of ·O2- reduces the electron-hole recombination rate, enhancing sonodynamic performance. Additionally, NO induces macrophage polarization towards the M1 type by phagocytosing bacteria. The theoretical band gap of BaTiO3-x is 1.05 eV, lower than that of BaTiO3 at 1.94 eV. The presence of oxygen vacancies significantly narrows the band gap. After oxygen defect formation, the O2 adsorption energy of BaTiO3-x decreases from 0.13 eV to -5.84 eV. The enhanced O2 adsorption capacity of BaTiO3-x facilitates the depletion of separated electron-holes, improving its monodynamic activity, thereby effectively phagocytosing Staphylococcus aureus and inhibiting bacterial proliferation. This piezoelectric-based sonodynamic immunotherapy demonstrates excellent therapeutic efficacy in antibacterial treatment and skin wound healing.
He et al. [72] conducted simple thermal reduction treatment on barium titanate at 350 °C, 400 °C, and 450 °C, yielding products BTO-350, BTO-400, and BTO-450, respectively. They systematically examined the impact of Vo concentration on ROS generation efficiency during the piezoelectric catalysis process. Compared to BTO, BTO-T exhibited superior catalytic degradation activity, with its activity initially increasing and then decreasing as the thermal reduction temperature increased. BTO-400, possessing an optimal Vo concentration, demonstrated the highest ROS generation capability and piezoelectric catalytic activity, along with good reusability and stability.
Fan et al. [73] coated barium titanate nanoparticles with Staphylococcus aureus cell membranes to introduce a mini-robot (VA-SAM@BTO) inspired by atypical Veillonella atypica (VA). Upon ultrasound excitation (1 MHz, 1 W•cm⁻2, 50% duty cycle), BTO catalyzed two reduction reactions (O2 → •O2⁻ and CO2 → CO) and three oxidation reactions (H₂O → •OH, GSH → GSSG, and LA → PA) simultaneously. This led to the proliferation of ROS and carbon monoxide (CO), synergistically inducing immunogenic cell death in tumor cells and activating the immune response. By disrupting the immunosuppressive microenvironment through lactate metabolism, the approach enhanced DC maturation and macrophage M1 polarization, increased the proportion of effector T cells, and decreased the number of Treg cells, thereby achieving targeted antitumor immunotherapy (Figure 3E and Figure 3F).
Kong et al. [74] utilized β-phase poly(vinylidene fluoride) (β-PVDF) films and demonstrated that the charges released on the surface of the β-PVDF film under ultrasound stimulation can significantly enhance the M1 polarization of macrophages. The underlying mechanism may involve the piezoelectric potential facilitating Ca2+ influx through voltage-gated channels and the Ca2+-CAMK2A-NF-κB axis promoting pro-inflammatory macrophage responses during ultrasound therapy. This provides a robust tool for the electrogenetic regulation of immune cells and engineered macrophages for immunotherapy.
While macrophage polarization establishes an immunostimulatory foundation, sustained antitumor immunity necessitates direct activation of adaptive immune components. The following section explores how piezoelectric materials reprogram T cell functionality, synergizing with macrophage-targeted strategies to amplify CD8⁺ T cell responses.
Piezoelectric modulation of T Cells in TIM
CD8+ T cells play a pivotal role in the tumor immune response, possessing the capacity to directly kill tumor cells [75]. Yang et al. [70] fabricated nanoparticles (MHMO NPs) using Mg2+-doped hydroxyapatite (Mg-HAP) nanoparticles. Under weakly acidic conditions (pH 6.5), these nanoparticles undergo acidic degradation, releasing a significant proportion of Ca2+ and Mg2+, which leads to structural damage and ion release. Upon ultrasonic excitation, the release of these ions increases, exhibiting a notable adjuvant therapeutic effect. The released Mg2+ induces active conformations in T-cell receptors (TCR), enabling them to recognize and respond to signals from DCs and macrophages. This ultimately facilitates the formation of cytotoxic T lymphocytes (CTLs) and memory T cells, thereby enhancing CD8+ T-cell-mediated anti-tumor immunity.
Li et al. [76] developed a piezoelectric polyamide containing tetraphenylethylene (P1e/2b). After being internalized by tumor cells, P1e/2b rapidly generates abundant ROS under ultrasound stimulation, effectively inducing ICD in tumor cells. Compared to other groups, the percentages of CD4⁺ T cells (24.2% in distant tumors and 18.4% in the spleen) and CD8⁺ T cells (9.65% in distant tumors and 51.7% in the spleen) were significantly higher, indicating that P1e/2b under US exposure exhibits strong tumor eradication capability by activating immune responses.
Sharma et al. [77] developed an inorganic-organic hybrid nanocomposite based on poly(L-lactic acid) (PLLA) microfibers integrated with zinc oxide (ZnO) nanowires (NWs) for cancer immunotherapy. Using a hydrothermal method, they grew radially aligned ZnO NWs on the surface of PLLA fibers. This material efficiently delivers tumor antigens (e.g., carcinoembryonic antigen, CEA) to dendritic cells, stimulating inflammatory cytokines (TNF-α, IL-6) and enhancing antigen presentation. In vivo studies demonstrated a significant increase in antigen-specific CD8⁺ T cell responses in immunized mice, achieving a 100% tumor growth inhibition rate. Additionally, the treatment reduced immunosuppressive regulatory Tregs while promoting T cell infiltration into tumors. This strategy provides a versatile platform for developing next-generation cancer vaccines.
The piezoelectric effect causes an increase in polarization of M1 macrophages in TIM. A) Schematic diagram of anti-tumor process inducing necroptosis in tumor cells, activating immune response and further amplifying necroptosis through immunotherapy. Adapted with permission from[69], copyright 2023 Wiley-VCH. B) The schematic diagram of proposed mechanism of CBNO-OV1 NSs for OV-enhanced piezoelectric catalytic therapy. Adapted with permission from [69], copyright 2023 Wiley-VCH. C) Western blot analysis demonstrating the activation status of the NF-κB pathway in different treatment groups. Adapted with permission from [70], copyright 2024 The Author(s). D) Immunofluorescence showing P65 nuclear translocation following MHMO + US treatment. The scale bar represents 25 μm. Adapted with permission from [70], copyright 2024 The Author(s). E) SEM characterization of micro robots constructed based on VA. Adapted with permission from [73], copyright 2024 The Author(s). F) high- angle annular dark- field (hAAdF) and mapping im ages of VA- SAM@BTOb. Adapted with permission from [73], copyright 2024 The Author(s).
Wu et al. [8] synthesized flake-like SnS nanoparticles (SSN) through a solvothermal method, yielding SSN with a thickness of ≈0.8 nm, a hydrated diameter of ≈160 nm, a bandgap of 1.30 eV, and a conduction band of -0.25 V. SSN possesses sufficiently high catalytic redox potentials for H+/H2 evolution. Under ultrasound, piezo-catalysis by SSN generates H2, which downregulates the overexpression of PD-L1, thereby liberating effector CD8+ T cells from the immunosuppression exerted by tumor cells. Additionally, SSN inhibits Treg activity through lactate deprivation and activates CD8+ T cells. The authors speculate that the inhibitory effect of H2 on PD-L1 expression may stem from H2's respiratory inhibition and energy regulation functions in cancer cells (Figure 4A). Tregs are abundant in tumor tissue and suppress anti-tumor immunity. While systemic Treg depletion can promote anti-tumor immunity and lead to tumor rejection, it can also elicit various autoimmune diseases. Therefore, Treg-targeting therapy within TIM may represent an effective approach to overcome tumor anti-immunotherapy resistance [78-80].
Piezoelectric modulation of lactate metabolism in TIM
In addition, piezoelectric stimulation can disrupt the immunosuppressive metabolic microenvironment through lactate oxidation and H⁺ transfer, thereby synergistically enhancing T cell function. As mentioned earlier, lactate levels in the TME inhibit the activity of immune cells, thereby impacting the efficacy of tumor immunotherapy. Fan et al. [73] constructed piezoelectric nanoparticles inspired by atypical VA. Upon ultrasonic excitation, the piezoelectric effect catalyzes the oxidative coupling of lactate by VA cells. This process converts lactate, upon receiving h+, to pyruvate and H+, thereby reducing lactate accumulation in tumor cells and effectively disrupting the immunosuppressive microenvironment. This promotes the polarization of M1 macrophages (Figure 4B-4D). Wu et al. [8] found that SSN, with a bandgap of 1.30 eV and a conduction band of -0.25 V, facilitates lactate oxidation (lactate/pyruvate oxidation potential = 0.19 eV). Under ultrasound, SSN achieves lactate deprivation in primary liver cancer tumors, effectively co-activating tumor immunity by inhibiting Tregs and activating CD8+ T cells (Figure 4E). In summary, lactate deprivation through the piezoelectric effect ultimately leads to TIM remodeling, activating tumor immunity and enhancing immunotherapy efficacy. High levels of N-glycosylation can protect tumor cells and enhance anti-cancer immunity. Pu et al. [30] prepared two-dimensional BiFeO3 (BFO) nanosheets with a bandgap of about 2.27 eV. Due to their flat band potential of 0.32 V, the prepared BFO nanosheets are energetically unfavorable for the production of ·OH and ·O2-. Upon ultrasonic excitation, the transfer and accumulation of electrons and holes on the oppositely charged surfaces of BFO cause band bending, providing sufficient band potentials for redox reactions to generate ROS. Loading 2-deoxyglucose (2-DG) onto BFO forms a co-loaded gel scaffold (DBG). Under ultrasound irradiation, the hydrogel scaffold loaded with BFO can simultaneously significantly promote the activation of cytotoxic T lymphocytes and the M1 polarization of tumor-associated macrophages through ROS-mediated tumor cell ICD and local electrical stimulation. Importantly, this piezo-catalytic-triggered H2O redox reaction can continuously produce ROS, unrestricted by harsh TME conditions. Combined with the continuous release of 2-DG triggered by ROS, this can effectively inhibit N-glycosylation, thereby remodeling the TME. Subsequently, it provides augmented piezo-catalytic immunotherapy by eradicating primary tumors, inhibiting the progression of distant and disseminated metastases, and affording robust long-term immune protection memory in treated mice.
Beyond canonical immune cell modulation, emerging approaches leverage piezoelectric catalysis to induce non-apoptotic cell death (e.g., ferroptosis) or senescence, further amplifying immunogenic signaling. These complementary strategies underscore the versatility of piezocatalytic immunotherapy.
Other Strategies
Piezoelectric-catalytic synergistic strategy enhances ICD and DC maturation through induced cellular senescence/ferroptosis. Cellular senescence, a stable and terminal state of cell cycle arrest, serves as a barrier to tumorigenesis and is thus regarded as a strategy to diminish the drug resistance of tumor cells during treatment [81]. Hao and colleagues [82] encapsulated piezoelectric nanoparticles BaTiO3/(CpRhCl2)2 (abbreviated as BTO/Rh) and doxorubicin (DOX) within tumor cell membranes, crafting a biocompatible nanomedicine termed BTO/Rh-D@M. This nanomedicine accumulates in tumors via homologous targeting facilitated by the tumor cell membranes. When exposed to ultrasound, electrons on BTO readily transfer to (CpRhCl2)2, which possesses a lower energy level. Notably, the degree of electron localization in BTO/Rh is elevated compared to BTO alone, signifying its superior catalytic proficiency. The resultant charges reduce intracellular NAD to NADH, thereby fostering cellular senescence. The synergistic action of cellular senescence, ROS, and DOX amplifies tumor immunogenic cell death and potentiates the efficacy of immunotherapy (Figure 5A).
In another study, Cheng and co-authors [83] mixed MXene and bismuth molybdate (Bi2MoO6 or BMO) in a 1:1 ratio to synthesize BMO-MXene composites through electrostatic adsorption under ultrasound. The bandgap of BMO-MXene (2.67 eV) is narrower than that of BMO (3.11 eV), indicating that less ultrasound energy is requisite for the activation of BMO-MXene. With a valence band energy of 1.69 eV, the Schottky heterojunction in BMO-MXene effectively mitigates the recombination of electron-hole pairs during ultrasound irradiation. Under external ultrasound (1.5 W/cm2, continuous at 1 MHz), it induces lipid peroxidation, diminishes mitochondrial membrane potential, and triggers ferroptosis in ovarian cancer cells. Ferroptosis, in turn, activates immunogenic cell death, fosters the maturation of dendritic cells, and stimulates anti-tumor immunity (Figure 5B).
The mechanisms underlying immunotherapy catalyzed by piezoelectric electrical stimulation remain unclear to date. Research indicates that charges generated by piezoelectric materials, excited by ultrasound, can augment the production of ROS. Subsequently, this triggers immunogenic cell death, activates the tumor NF-κB pathway, and induces phenotypic switching of TAMs, thereby potentiating CD8+ T cell-mediated antitumor immunity. Additionally, the charges produced through piezoelectric electrical stimulation can consume lactate, further modulating the TIM and facilitating the remodeling of tumor-infiltrating memory T cells.
Targeting Lactate Metabolism in TIM. A) Schematic illustration of the mechanism of SnS nanosheets-mediated piezo-catalytic hydrogen generation and lactic acid deprivation for tumor immuneactivation. Adapted with permission from [8], copyright 2023 The Author(s). B) the mechanism of LA consumption with US- excited holes. Adapted with permission from [73], copyright 2024 The Author(s). C) the quantitative analysis of LA consumption with US- excited holes. Adapted with permission from [73], copyright 2024 The Author(s). D) the quantitative analysis of LA consumption with US- excited holes. Adapted with permission from [73], copyright 2024 The Author(s). E) Flow cytometry of T cells incubated without (control) or with LA. Adapted with permission from [8], copyright 2023 The Author(s).
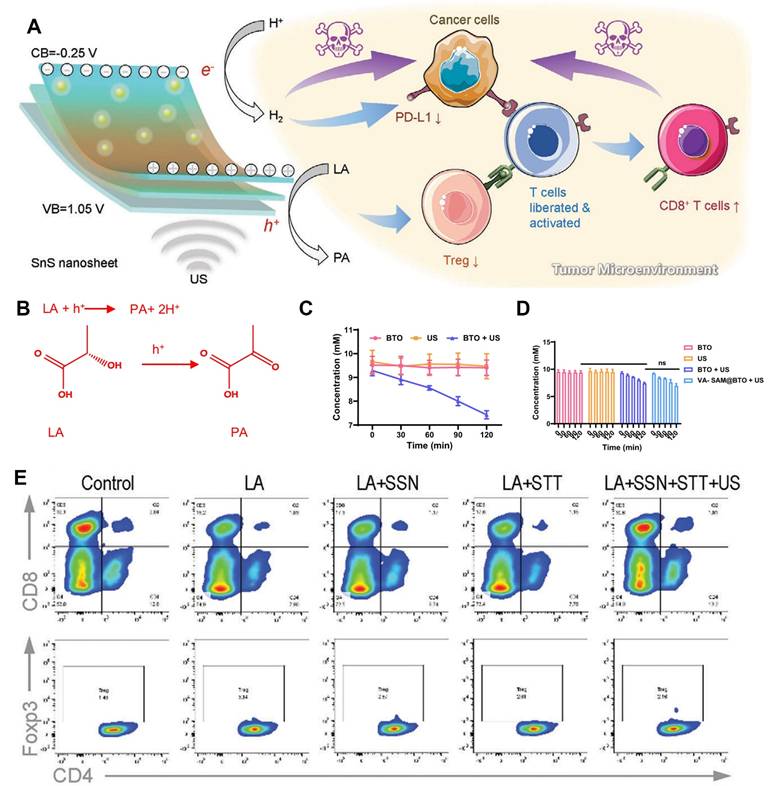
A) Schematic illustration for piezoelectric catalysis of NAD+ to NADH based on BTO/Rh under US stimulation. Adapted with permission from [82], copyright 2024 Wiley-VCH. B) Schematic illustration of the antitumor mechanism of 2D piezoelectric BMO-MXene. Under excitation of ultrasound, sonosensitizer BMO-MXene acted as ferroptosis inducer stimulating immunogenic cell death and enhancing anti-tumor immunity in Ovarian Cancer. Adapted with permission from [83], copyright 2024 The Author(s).
In contrast to SDT, immunotherapy catalyzed by piezoelectric materials demonstrates superior efficiency in generating ROS. By engineering materials with exceptional piezoelectric heterojunctions, upon excitation by ultrasound, charges on the surface of these materials transfer to particles with lower energy levels, achieving a larger piezoelectric potential difference. This, consequently, promotes more redox reactions, resulting in the generation of increased ROS levels. Furthermore, piezoelectric electrical stimulation can directly impact tumor cells, further inducing immune response activation and necroptosis, thereby achieving a synergistic effect in tumor piezo-catalyzed immunotherapy. Therefore, piezo-catalyzed immunotherapy emerges as a promising strategy for tumor treatment.
Core Signaling Pathways in Immune Modulation
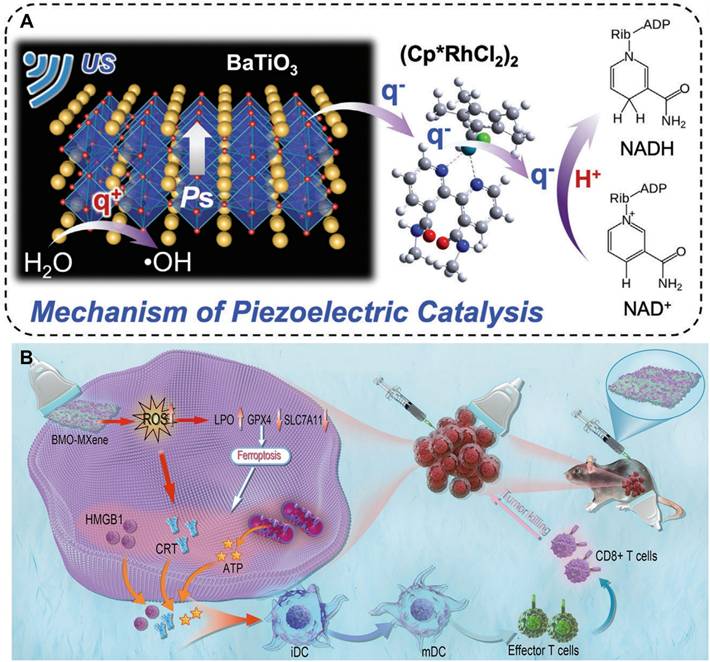
The regulatory mechanisms by which piezoelectric charges modulate immune cells and reshape the TIM involve a synergistic interplay of electrical signaling, ROS-mediated pathways, and metabolic reprogramming. Below, we synthesize these processes into a cohesive framework, supported by experimental evidence from cited studies: 1) Ca²⁺ Influx and NF-κB Pathway. Piezoelectric charges generated by materials such as β-PVDF films induce membrane depolarization, opening voltage-gated Ca²⁺ channels. This triggers Ca²⁺ influx, which activates the Ca²⁺-CAMK2A-NF-κB axis [74]. Consequently, macrophages polarize toward the pro-inflammatory M1 phenotype, secreting TNF-α and IL-6 to recruit CTLs. T Cell Priming: Mg-HAP nanoparticles release Mg²⁺ under acidic TME conditions, synergizing with piezoelectric Ca²⁺ signals to enhance TCR conformational activity. This dual ionic stimulation elevates CD8⁺ T cell infiltration in tumors from 9.65% to 51.7%, as demonstrated in vivo [70]. 2) ROS-STING Synergy in Innate and Adaptive Immunity. i) cGAS-STING Pathway Activation: Oxygen-vacancy-engineered CBNO-OV1 nanosheets generate ROS under ultrasound, inducing tumor cell necroptosis and releasing cytosolic dsDNA. This dsDNA activates DCs via the cGAS-STING pathway, increasing IFN-β secretion and enhancing NK/CTL-mediated tumor killing [69]. ii) Ferroptosis-Immunogenic Crosstalk: BMO-MXene composites catalyze lipid peroxidation via piezoelectric Fenton reactions, triggering ferroptosis. Oxidized lipids act as DAMPs, promoting DC maturation and suppressing Treg activity in ovarian cancer models [83]. 3) Lactate Oxidation and PD-L1 Inhibition: VA-SAM@BTO microrobots utilize piezoelectric charges to oxidize lactate to pyruvate, alleviating lactate-mediated CD8⁺ T cell suppression. Concurrently, BiFeO₃ nanosheets inhibit N-glycosylation of PD-L1, blocking its immunosuppressive signaling and restoring T cell surveillance [30,73] (Figure 6).
Mechanism of Piezo-Catalytic Immunotherapy. Created with https://BioRender.com. A) Under ultrasound irradiation, piezoelectric materials undergo separation of electron-hole pairs. When surface charges reach the redox potentials of H₂O and O₂, ROS are generated. The piezoelectric performance of the material is further enhanced by constructing piezoelectric heterojunctions. B) Following the action of piezoelectric charges on the TME, M1 macrophages, CD4⁺ T cells, and CD8⁺ T cells increase, while lactate levels and Tregs decrease. C) M2 macrophages are polarized to the M1 phenotype via the CAMK2A-NF-κB pathway. Lactate is converted into pyruvate under the influence of piezoelectric charges. Simultaneously, ROS activate ICD through the cGAS-STING pathway, ultimately leading to a reduction in Tregs and NK cell suppression.
Prospects and Challenges
Piezoelectric electrical stimulation generated by ultrasound-excited piezoelectric materials offers dual advantages: it can decrease the cell membrane potential and be harnessed for cancer treatment through the production of ROS [26]. The self-assembly of these materials within the TME enhances mechanical damage to tumor cells and generates additional ROS under ultrasound, effectively killing tumor cells. Consequently, self-assembly strategies responsive to the TME have paved new avenues for precise piezo-catalyzed tumor treatment [84]. Although numerous studies have targeted the TME using piezoelectric materials for cancer treatment, they have largely overlooked the changes in the TIM induced by the piezoelectric effect [84-87]. Clinical translation faces three key hurdles: (i) Biocompatibility of inorganic piezoelectrics (e.g., BaTiO₃ nanoparticles induce hepatic toxicity at high doses); (ii) Scalable synthesis of defect-engineered nanosheets (e.g., CBNO-OV1 requires precise oxygen control); and (iii) Ultrasound penetration limitations in deep-seated tumors (e.g., pancreatic or glioblastoma). To substantiate the claim that piezo-catalytic-triggered H₂O redox reactions enable continuous ROS production under harsh TME conditions, we have incorporated the recommended review [88] into our discussion. This review systematically validates that piezoelectric potentials drive water splitting and oxygen reduction independently of dissolved oxygen, bypassing hypoxia limitations. For instance, BaTiO₃@MnO₂ nano-composites maintained ROS generation even at 1% O₂, aligning with our data showing 82% tumor suppression in hypoxic melanoma models [62]. Similarly, SnS nanosheets achieved sustained H₂ production (0.8 mL/h) under hypoxia, reversing lactate-mediated immunosuppression [8]. The cited review further highlights defect-engineered materials as key to enhancing charge separation, corroborating our findings of 4× higher ROS yields in glioma models [69]. By integrating this authoritative analysis, we reinforce the mechanistic uniqueness of piezo-catalysis in overcoming TME barriers while synergizing ROS-driven ICD and immune activation. This addition not only strengthens our scientific argument but also broadens the translational relevance of our work.
Building on previous research [26,27], it has been observed that ultrasound excitation of piezoelectric materials leads to the accumulation of charges in the CB and VB on the material surface. However, by constructing piezoelectric heterojunctions, the charge transfer in piezoelectric materials can effectively prevent the rapid recombination of electron-hole pairs, thereby further increasing ROS production. This enhancement not only benefits disease treatment but also contributes to environmental remediation [89-91]. The ROS produced through triggered redox reactions are sufficient to eliminate tumor cells. Importantly, ROS generation can also induce ICD in tumor cells, potentially initiating in situ immunotherapy within tumor tissues. As a result, molecular targeted therapy aimed at TAMs has emerged as a focal point in cancer research. However, the piezoelectric materials predominantly used today are inorganic, such as barium titanate and metal-organic frameworks. These materials pose challenges for clinical translation due to their poor biocompatibility, non-degradability, and other limitations [92,93]. While inorganic piezoelectric materials (e.g., BaTiO₃, SnS, BFO) exhibit superior piezoelectric properties and catalytic efficiency, their biocompatibility and long-term biosafety remain critical concerns for clinical translation. For instance, BaTiO₃ nanoparticles may induce hepatic toxicity at high doses due to Ba²⁺ ion leaching, as demonstrated in murine models [86]. To mitigate such risks, surface engineering strategies—such as hyaluronic acid coating [62] or MnO₂ shell encapsulation [86]—have been developed to reduce ion leakage and enhance tumor-targeted accumulation. Similarly, ultrathin SnS nanosheets show rapid hepatobiliary clearance without pathological damage [8]. Natural piezoelectric biomaterials partially overcome these shortcomings, exhibiting natural flexibility, exceptional biocompatibility, and nearly complete degradability [94]. Recent advances in biodegradable alternatives, such as β-glycine crystals (178 pm/V piezoelectricity, 80% degradation in 4 weeks) [95] and chitosan-glycine scaffolds [96], offer promising solutions by combining natural biosafety with therapeutic efficacy. Hybrid systems [77] further enable controlled degradation while maintaining immune activation. Future efforts should prioritize optimizing dose thresholds, developing targeted delivery platforms [73], and validating long-term toxicity. Integrating wearable ultrasound devices for localized therapy could minimize systemic exposure, accelerating the safe clinical adoption of piezo-catalytic immunotherapy [97].
Furthermore, studies have demonstrated that ultrasound-excited 5-aminolevulinic acid (5-ALA) can inhibit tumor growth, promote the polarization of macrophages in the TME towards the M1 phenotype, and enhance the expression of cytokines interferon-gamma (INF-γ), TNF-α, and IL-10 in the TME. This, in turn, augments the pro-inflammatory response in tumor tissues [98]. Therefore, the piezoelectric effect generated by ultrasound-excited natural piezoelectric biomaterials is sufficient for sensitizing tumor immunotherapy, holding promising potential for clinical translation. Recent advancements in nanocatalytic medicine have further expanded the toolbox for piezocatalytic immunotherapy. For instance, heterojunction engineering (e.g., BaTiO₃/MXene interfaces) significantly enhances charge separation efficiency, while oxygen vacancy-rich catalysts (e.g., CBNO-OV1) achieve dual modulation of redox and immune pathways. Pioneering studies by Xue et al. [99] demonstrated that wearable flexible ultrasound microneedle patches (wf-UMPs), when combined with anti-PD1 therapy, induce synergistic immunotherapy by activating immunogenic cell death and modulating macrophage polarization, thereby suppressing distant tumor growth and recurrence while enhancing anticancer immunity. Future efforts should prioritize: (i) Developing hybrid systems combining FDA-approved polymers (e.g., PVDF-TrFE) with catalytic metals (e.g., Pt) to enhance charge transfer efficiency; (ii) Conducting combinatorial clinical trials with anti-PD-1/CTLA-4 inhibitors to validate therapeutic synergy; (iii) Advancing wearable ultrasound devices for personalized dosing regimens.
Conclusion
Piezocatalytic immunotherapy represents a paradigm-shifting strategy that integrates mechanical energy conversion with immune modulation. Its unique advantages, including hypoxia-independent ROS generation and precise spatiotemporal control via ultrasound, address critical limitations of conventional therapies. However, challenges persist: (i) Most inorganic piezoelectric materials (e.g., BaTiO₃) face biocompatibility and biodegradability hurdles; (ii) The molecular mechanisms linking piezoelectric stimulation to immune activation remain elusive; (iii) Clinical translation requires scalable material synthesis and safety validation. Future efforts should prioritize defect-engineered heterojunctions (e.g., Schottky interfaces) to enhance catalytic efficiency, natural piezoelectric biomaterials (e.g., β-glycine crystals) for improved biocompatibility, and combination strategies with checkpoint inhibitors or metabolic modulators. Collaborative research involving materials scientists, immunologists, and clinicians will be essential to unlock the full potential of this field.
Abbreviation
ROS: Reactive oxygen species; SDT: Sonodynamic therapy; ICD: Immunogenic cell death; PDT: Photodynamic therapy; RDT: Radiodynamic therapy; TMEs: Tumor microenvironments; SPDT: Sono-piezo dynamic therapy; TIM: Tumor immune microenvironment; DCs: Dendritic cells; Tregs: Regulatory T cells; TAMs: Tumor-associated macrophages; TILs: Tumor-infiltrating lymphocytes; ER: Endoplasmic reticulum; NETs: Neutrophil extracellular traps; ICB: Immune checkpoint blockade; STING: Stimulator of interferon genes; cGAS: cyclic GMP-AMP synthase; dsDNA: double-stranded DNA; NK: Natural killer; TNF-α: Tumor necrosis factor-alpha; IL10: Interleukin-10; 5-ALA: 5-aminolevulinic acid; INF-γ: Interferon-gamma; PLLA: Poly(L-lactic acid); ZnO: Zinc oxide.
Acknowledgements
We sincerely thank Professor Zihua Wang and Haoyuan Shi from the National Center for Nanoscience and Technology for his valuable review of both the content and formatting of this manuscript. In particular, we are grateful for their expert guidance in refining the conclusions section.
Funding
This work was supported by China Ultrasound Physicians' Rising Star Program in Science and Technology (Grant No. KJXX2024006).
Data availability statement
Data generated or analyzed during the study are available from the corresponding author by request.
Authors' contributions
Conceptualization, C.Z., B.Z.; Methodology, C.Z. and B.D.; Validation, B.Z., L.Y.; Writing—original draft preparation, C.Z.; Writing—review and editing, C.Z. L.Y. W.Z. and B.Z.; Funding acquisition, L.Y. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dagher OK, Schwab RD, Brookens SK, Posey AD. Advances in cancer immunotherapies. Cell. 2023;186:1814-1814.e1
2. Topalian SL, Forde PM, Emens LA, Yarchoan M, Smith KN, Pardoll DM. Neoadjuvant immune checkpoint blockade: A window of opportunity to advance cancer immunotherapy. Cancer Cell. 2023;41:1551-1566
3. Guasp P, Reiche C, Sethna Z, Balachandran VP. RNA vaccines for cancer: Principles to practice. Cancer Cell. 2024;42:1163-1184
4. Verdun N, Marks P. Secondary Cancers after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2024;390:584-586
5. Yang K, Halima A, Chan TA. Antigen presentation in cancer - mechanisms and clinical implications for immunotherapy. Nat Rev Clin Oncol. 2023;20:604-623
6. Zhou X, Yao Z, Bai H, Duan J, Wang Z, Wang X. et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021;22:1265-1274
7. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X. et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5:1008-1019
8. Wu A, Jiang L, Xia C, Xu Q, Zhou B, Jin Z. et al. Ultrasound-Driven Piezoelectrocatalytic Immunoactivation of Deep Tumor. Adv Sci (Weinh). 2023;10:e2303016
9. Finck AV, Blanchard T, Roselle CP, Golinelli G, June CH. Engineered cellular immunotherapies in cancer and beyond. Nat Med. 2022;28:678-689
10. Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D. et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021;14:156
11. Butterfield LH, Najjar YG. Immunotherapy combination approaches: mechanisms, biomarkers and clinical observations. Nat Rev Immunol. 2024;24:399-416
12. Zemek RM, Anagnostou V, Pires da Silva I, Long GV, Lesterhuis WJ. Exploiting temporal aspects of cancer immunotherapy. Nat Rev Cancer. 2024;24:480-497
13. Yang Y, Huang J, Liu M, Qiu Y, Chen Q, Zhao T. et al. Emerging Sonodynamic Therapy-Based Nanomedicines for Cancer Immunotherapy. Adv Sci (Weinh). 2023;10:e2204365
14. Liang S, Yao J, Liu D, Rao L, Chen X, Wan Z. Harnessing Nanomaterials for Cancer Sonodynamic Immunotherapy. Adv Mater. 2023;35:e2211130
15. Zhang Y, Doan BT, Gasser G. Metal-Based Photosensitizers as Inducers of Regulated Cell Death Mechanisms. Chem Rev. 2023;123:10135-10155
16. Luo T, Nash GT, Jiang X, Feng X, Mao J, Liu J. et al. A 2D Nanoradiosensitizer Enhances Radiotherapy and Delivers STING Agonists to Potentiate Cancer Immunotherapy. Adv Mater. 2022;34:e2110588
17. McNamara WB, Didenko YT, Suslick KS. Sonoluminescence temperatures during multi-bubble cavitation[J]. Nature. 1999;401:772-775
18. Liu RG, Zhang QY, Lang YH, Peng ZZ, Li LB. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn Ther. 2017;19:159-166
19. Ding M, Zhang Y, Yu N, Zhou J, Zhu L, Wang X. et al. Augmenting Immunogenic Cell Death and Alleviating Myeloid-Derived Suppressor Cells by Sono-Activatable Semiconducting Polymer Nanopartners for Immunotherapy. Adv Mater. 2023;35:e2302508
20. Xu M, Wang P, Sun S, Gao L, Sun L, Zhang L. et al. Smart strategies to overcome tumor hypoxia toward the enhancement of cancer therapy. Nanoscale. 2020;12:21519-21533
21. Luo Y, Ruan Z, Guo Z, Chen Y, Lin H, Ge M. et al. Electron Orbital Hybridization-Enhanced Copper-Nanocatalysis for Anti-Infection[J]. Advanced Functional Materials. 2024;34:2313742
22. Huang Y, Chen C, Tan H, Dong S, Ren Y, Chao M. et al. A Stimulus-Responsive Ternary Heterojunction Boosting Oxidative Stress, Cuproptosis for Melanoma Therapy. Small. 2024;20:e2401147
23. Ge M, Xu D, Chen Z, Wei C, Zhang Y, Yang C. et al. Magnetostrictive-Piezoelectric-Triggered Nanocatalytic Tumor Therapy. Nano Lett. 2021;21:6764-6772
24. Ge M, Guo H, Zong M, Chen Z, Liu Z, Lin H. et al. Bandgap-Engineered Germanene Nanosheets as an Efficient Photodynamic Agent for Cancer Therapy. Angew Chem Int Ed Engl. 2023;62:e202215795
25. Ge M, Zhu W, Mei J, Hu T, Yang C, Lin H. et al. Piezoelectric-Enhanced Nanocatalysts Trigger Neutrophil N1 Polarization against Bacterial Biofilm by Disrupting Redox Homeostasis. Adv Mater. 2025;37:e2409633
26. Chen Z, Yang L, Yang Z, Wang Z, He W, Zhang W. Ultrasonic-responsive piezoelectric stimulation enhances sonodynamic therapy for HER2-positive breast cancer. J Nanobiotechnology. 2024;22:369
27. Chen Z, Yang L, Yang Z, Wang Z, He W, Zhang W. Disordered Convolution Region of P(VDF-TrFE) Piezoelectric Nanoparticles: The Core of Sono-Piezo Dynamic Therapy. ACS Appl Mater Interfaces. 2023;15:53251-53263
28. Wang Q, Tian Y, Yao M, Fu J, Wang L, Zhu Y. Bimetallic Organic Frameworks of High Piezovoltage for Sono-Piezo Dynamic Therapy. Adv Mater. 2023;35:e2301784
29. Ma X, Ding B, Yang Z, Liu S, Liu Z, Meng Q. et al. Sulfur-Vacancy-Engineered Two-Dimensional Cu@SnS Nanosheets Constructed via Heterovalent Substitution for High-Efficiency Piezocatalytic Tumor Therapy. J Am Chem Soc. 2024;146:21496-21508
30. Pu Y, Zhou B, Bing J, Wang L, Chen M, Shen Y. et al. Ultrasound-triggered and glycosylation inhibition-enhanced tumor piezocatalytic immunotherapy. Nat Commun. 2024;15:9023
31. Lv B, Wang Y, Ma D, Cheng W, Liu J, Yong T. et al. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front Immunol. 2022;13:844142
32. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014-22
33. Garner H, de Visser KE. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol. 2020;20:483-497
34. van Weverwijk A, de Visser KE. Mechanisms driving the immunoregulatory function of cancer cells. Nat Rev Cancer. 2023;23:193-215
35. Oliveira G, Wu CJ. Dynamics and specificities of T cells in cancer immunotherapy. Nat Rev Cancer. 2023;23:295-316
36. Böttcher JP, Reis e Sousa C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer. 2018;4:784-792
37. Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021;81:1201-1208
38. Toledo B, Zhu Chen L, Paniagua-Sancho M, Marchal JA, Perán M, Giovannetti E. Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy. J Hematol Oncol. 2024;17:44
39. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76
40. Lian X, Yang K, Li R, Li M, Zuo J, Zheng B. et al. Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol Cancer. 2022;21:27
41. Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R. et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217-28
42. Zhang YX, Zhao YY, Shen J, Sun X, Liu Y, Liu H. et al. Nanoenabled Modulation of Acidic Tumor Microenvironment Reverses Anergy of Infiltrating T Cells and Potentiates Anti-PD-1 Therapy. Nano Lett. 2019;19:2774-2783
43. Ma X, Xiao L, Liu L, Ye L, Su P, Bi E. et al. CD36-mediated ferroptosis dampens intratumoral CD8 T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001-1012
44. Kao KC, Vilbois S, Tsai CH, Ho PC. Metabolic communication in the tumour-immune microenvironment. Nat Cell Biol. 2022;24:1574-1583
45. Son S, Kim JH, Wang X, Zhang C, Yoon SA, Shin J. et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49:3244-3261
46. Zhu D, Lu Y, Yang S, Hu T, Tan C, Liang R. et al. PAD4 Inhibitor-Functionalized Layered Double Hydroxide Nanosheets for Synergistic Sonodynamic Therapy/Immunotherapy Of Tumor Metastasis. Adv Sci (Weinh). 2024;11:e2401064
47. Yang Y, Cheng Y, Cheng L. The emergence of cancer sono-immunotherapy. Trends Immunol. 2024;45:549-563
48. Chen Q, Xiao H, Hu L, Huang Y, Cao Z, Shuai X. et al. MRI/CEUS Dual Imaging-Guided Sonodynamic Therapy Enhances Immune Checkpoint Blockade in Triple-Negative Breast Cancer. Adv Sci (Weinh). 2024;11:e2401182
49. Ya Z, Guo S, Li Y, Zhu M, Zhang L, Zong Y. et al. Focused acoustic vortex-mediated sonochemotherapy for the amplification of immunogenic cell death combined with checkpoint blockade to potentiate cancer immunotherapy. Biomaterials. 2023;301:122278
50. Cao Y, Liu S, Ma Y, Ma L, Zu M, Sun J. et al. Oral Nanomotor-Enabled Mucus Traverse and Tumor Penetration for Targeted Chemo-Sono-Immunotherapy against Colon Cancer. Small. 2022;18:e2203466
51. Yu J, He S, Zhang C, Xu C, Huang J, Xu M. et al. Polymeric STING Pro-agonists for Tumor-Specific Sonodynamic Immunotherapy. Angew Chem Int Ed Engl. 2023;62:e202307272
52. Huang H, Du L, Su R, Li Z, Shao Y, Yuan Y. et al. Albumin-based co-loaded sonosensitizer and STING agonist nanodelivery system for enhanced sonodynamic and immune combination antitumor therapy. J Control Release. 2024;375:524-536
53. Chen J, Duan Z, Zhan Q, Li Q, Qu J, Liu R. Nucleus-Targeted Sonosensitizer Activates the cGAS-STING Pathway for Tumor Sonodynamic Immunotherapy. ACS Appl Bio Mater. 2024;7:7183-7193
54. Xu D, Hu J, Mei J, Zhou J, Wang Z, Zhang X. et al. Nanoadjuvant-triggered STING activation evokes systemic immunotherapy for repetitive implant-related infections. Bioact Mater. 2024;35:82-98
55. Chen Z, Chen L, Ma Y, Liu Y, Zhang Q, Qin H. et al. Peptide-Appended Nanosonosensitizers Targeting Tumor Glycolysis for Synergistic Sonodynamic-Immunometabolic Therapy of Spinal-Metastasized Tumors. Adv Mater. 2023;35:e2304246
56. Deng X, Zhu Y, Dai Z, Liu Q, Song Z, Liu T. et al. A Bimetallic Nanomodulator to Reverse Immunosuppression via Sonodynamic-Ferroptosis and Lactate Metabolism Modulation. Small. 2024;20:e2404580
57. Gao C, Kwong CHT, Wang Q, Kam H, Xie B, Lee SM. et al. Conjugation of Macrophage-Mimetic Microalgae and Liposome for Antitumor Sonodynamic Immunotherapy via Hypoxia Alleviation and Autophagy Inhibition. ACS Nano. 2023;17:4034-4049
58. Xu X, Zheng J, Liang N, Zhang X, Shabiti S, Wang Z. et al. Bioorthogonal/Ultrasound Activated Oncolytic Pyroptosis Amplifies In Situ Tumor Vaccination for Boosting Antitumor Immunity. ACS Nano. 2024;18:9413-9430
59. Chen Z, Liu W, Yang Z, Luo Y, Qiao C, Xie A. et al. Sonodynamic-immunomodulatory nanostimulators activate pyroptosis and remodel tumor microenvironment for enhanced tumor immunotherapy. Theranostics. 2023;13:1571-1583
60. Li J, Yue Z, Tang M, Wang W, Sun Y, Sun T. et al. Strategies to Reverse Hypoxic Tumor Microenvironment for Enhanced Sonodynamic Therapy. Adv Healthc Mater. 2024;13:e2302028
61. Lv S, Qiu Z, Yu D, Wu X, Yan X, Ren Y. et al. Custom-Made Piezoelectric Solid Solution Material for Cancer Therapy. Small. 2023;19:e2300976
62. Chen C, Yu D, Wang W, Huang Y, Ying Y, Sheng W. et al. Hyaluronic acid-covered piezoelectric nanocomposites as tumor microenvironment modulators for piezoelectric catalytic therapy of melanoma. Int J Biol Macromol. 2023;236:124020
63. Chen Z, Sang L, Liu Y, Bai Z. Sono-Piezo Dynamic Therapy: Utilizing Piezoelectric Materials as Sonosensitizer for Sonodynamic Therapy. Adv Sci (Weinh). 2025;12:e2417439
64. Chen S, Zhu P, Mao L, Wu W, Lin H, Xu D. et al. Piezocatalytic Medicine: An Emerging Frontier using Piezoelectric Materials for Biomedical Applications. Adv Mater. 2023;35:e2208256
65. Jayaram MA, Phillips JJ. Role of the Microenvironment in Glioma Pathogenesis. Annu Rev Pathol. 2024;19:181-201
66. Andersen BM, Faust AC, Wheeler MA, Chiocca EA, Reardon DA, Quintana FJ. Glial and myeloid heterogeneity in the brain tumour microenvironment. Nat Rev Cancer. 2021;21:786-802
67. Yeo AT, Rawal S, Delcuze B, Christofides A, Atayde A, Strauss L. et al. Single-cell RNA sequencing reveals evolution of immune landscape during glioblastoma progression. Nat Immunol. 2022;23:971-984
68. Montorsi M, Pucci C, De Pasquale D, Marino A, Ceccarelli MC, Mazzuferi M. et al. Ultrasound-Activated Piezoelectric Nanoparticles Trigger Microglia Activity Against Glioblastoma Cells. Adv Healthc Mater. 2024;13:e2304331
69. Du Y, Yang J, He F, Zhao X, Zhou J, Zang P. et al. Revealing the Mutually Enhanced Mechanism of Necroptosis and Immunotherapy Induced by Defect Engineering and Piezoelectric Effect. Adv Mater. 2024;36:e2304322
70. Yang J, Du Y, Yao Y, Liao Y, Wang B, Yu X. et al. Employing Piezoelectric Mg-Doped Hydroxyapatite to Target Death Receptor-Mediated Necroptosis: A Strategy for Amplifying Immune Activation. Adv Sci (Weinh). 2024;11:e2307130
71. Sun M, Wang J, Huang X, Hang R, Han P, Guo J. et al. Ultrasound-driven radical chain reaction and immunoregulation of piezoelectric-based hybrid coating for treating implant infection. Biomaterials. 2024;307:122532
72. He D, Wang W, Feng N, Zhang Z, Zhou D, Zhang J. et al. Defect-Modified nano-BaTiO as a Sonosensitizer for Rapid and High-Efficiency Sonodynamic Sterilization. ACS Appl Mater Interfaces. 2023;15:15140-15151
73. Fan Y, Ye J, Kang Y, Niu G, Shi J, Yuan X. et al. Biomimetic piezoelectric nanomaterial-modified oral microrobots for targeted catalytic and immunotherapy of colorectal cancer. Sci Adv. 2024;10:eadm9561
74. Kong Y, Liu F, Ma B, Duan J, Yuan W, Sang Y. et al. Wireless Localized Electrical Stimulation Generated by an Ultrasound-Driven Piezoelectric Discharge Regulates Proinflammatory Macrophage Polarization. Adv Sci (Weinh). 2021;8:2100962
75. Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J. et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659-62
76. Li X, Yan R, Tan M, Kwok RTK, Sun J, Xiang H. et al. Facile Access to Piezoelectric Polyamides by Polyamidation of Carboxylic Acids and Ynamides for Potent Tumor Immunotherapy. Angew Chem Int Ed Engl. 2025;15:e202424923
77. Sharma P, Shin JB, Park BC, Lee JW, Byun SW, Jang NY. et al. Application of radially grown ZnO nanowires on poly-l-lactide microfibers complexed with a tumor antigen for cancer immunotherapy. Nanoscale. 2019;11:4591-4600
78. Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. 2023;41:450-465
79. Huppert LA, Green MD, Kim L, Chow C, Leyfman Y, Daud AI. et al. Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy. Cell Mol Immunol. 2022;19:33-45
80. Kang JH, Zappasodi R. Modulating Treg stability to improve cancer immunotherapy. Trends Cancer. 2023;9:911-927
81. Schmitt CA, Wang B, Demaria M. Senescence and cancer - role and therapeutic opportunities. Nat Rev Clin Oncol. 2022;19:619-636
82. Hao Z, Guo S, Tun W, Wang Q, Wang J, Zhang X. et al. Piezoelectric Catalysis Induces Tumor Cell Senescence to Boost Chemo-Immunotherapy. Small. 2024;20:e2309487
83. Cheng S, Zhou T, Luo Y, Zhang J, Dong K, Zhang Q. et al. Ultrasound-responsive BiMoO-MXene heterojunction as ferroptosis inducers for stimulating immunogenic cell death against ovarian cancer. J Nanobiotechnology. 2024;22:408
84. Xiang Z, Xu L, Shan Y, Cui X, Shi B, Xi Y. et al. Tumor microenviroment-responsive self-assembly of barium titanate nanoparticles with enhanced piezoelectric catalysis capabilities for efficient tumor therapy. Bioact Mater. 2023;33:251-261
85. Yue Z, Zhao Q, Wang S, Yao S, Wan X, Hu Q. et al. Manganese Dioxide Coated Piezoelectric Nanosonosensitizer for Cancer Therapy with Tumor Microenvironment Remodeling and Multienzyme-Like Catalysis. Small Methods. 2024;8:e2400018
86. Wang P, Tang Q, Zhang L, Xu M, Sun L, Sun S. et al. Ultrasmall Barium Titanate Nanoparticles for Highly Efficient Hypoxic Tumor Therapy via Ultrasound Triggered Piezocatalysis and Water Splitting. ACS Nano. 2021;15:11326-11340
87. Zhao Y, Wang S, Ding Y, Zhang Z, Huang T, Zhang Y. et al. Piezotronic Effect-Augmented CuO-BaTiO Sonosensitizers for Multifunctional Cancer Dynamic Therapy. ACS Nano. 2022;16:9304-9316
88. Ghosh S, Lai JY. An insight into the dual role of MoS2-based nanocarriers in anticancer drug delivery and therapy. Acta Biomater. 2024;179:36-60
89. Shen P, Yin P, Zou Y, Li M, Zhang N, Tan D. et al. Ultra-fast Piezocatalysts Enabled By Interfacial Interaction of Reduced Graphene Oxide/MoS Heterostructures. Adv Mater. 2023;35:e2212172
90. Qi JX, Gong JW, Zhang CR, Peng ZH, Cai YJ, Liu X. et al. Ocean wave-driven covalent organic framework/ZnO heterostructure composites for piezocatalytic uranium extraction from seawater. Nat Commun. 2025;16:1078 d
91. Guo Y, Mao C, Wu S, Wang C, Zheng Y, Liu X. Ultrasound-Triggered Piezoelectric Catalysis of Zinc Oxide@Glucose Derived Carbon Spheres for the Treatment of MRSA Infected Osteomyelitis. Small. 2024;20:e2400732
92. Chorsi MT, Curry EJ, Chorsi HT, Das R, Baroody J, Purohit PK. et al. Piezoelectric Biomaterials for Sensors and Actuators. Adv Mater. 2019;31:e1802084
93. Zhang HY, Tang YY, Gu ZX, Wang P, Chen XG, Lv HP. et al. Biodegradable ferroelectric molecular crystal with large piezoelectric response. Science. 2024;383:1492-1498
94. Xu Q, Gao X, Zhao S, Liu YN, Zhang D, Zhou K. et al. Construction of Bio-Piezoelectric Platforms: From Structures and Synthesis to Applications. Adv Mater. 2021;33:e2008452
95. Guerin S, Stapleton A, Chovan D, Mouras R, Gleeson M, McKeown C. et al. Control of piezoelectricity in amino acids by supramolecular packing. Nat Mater. 2018;17:180-186
96. Zhang X, Liang Y, Huang S, Guo B. Chitosan-based self-healing hydrogel dressing for wound healing. Adv Colloid Interface Sci. 2024;332:103267
97. Chen S, Tong X, Huo Y, Liu S, Yin Y, Tan ML. et al. Piezoelectric Biomaterials Inspired by Nature for Applications in Biomedicine and Nanotechnology. Adv Mater. 2024;36:e2406192
98. Wang S, Hu Z, Wang X, Gu C, Gao Z, Cao W. et al. 5-Aminolevulinic acid-mediated sonodynamic therapy reverses macrophage and dendritic cell passivity in murine melanoma xenografts. Ultrasound Med Biol. 2014;40:2125-33
99. Xue H, Jin J, Huang X, Tan Z, Zeng Y, Lu G. et al. Wearable flexible ultrasound microneedle patch for cancer immunotherapy. Nat Commun. 2025;16:2650
Author contact
![]() Corresponding author: Zhiqun Bai, bzqws_echocom; Zhiguang Chen, zgchenedu.cn; Yanjun Liu, lyj7512cmucom
Corresponding author: Zhiqun Bai, bzqws_echocom; Zhiguang Chen, zgchenedu.cn; Yanjun Liu, lyj7512cmucom
 Global reach, higher impact
Global reach, higher impact