13.3
Impact Factor
Theranostics 2025; 15(13):6454-6475. doi:10.7150/thno.113665 This issue Cite
Review
Breaking the premetastatic niche barrier: the role of endothelial cells and therapeutic strategies
1. The First Clinical School of Medicine, Zhengzhou University, Zhengzhou 450001, China.
2. Department of Colorectal Surgery, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China.
#These authors contributed equally.
Received 2025-3-12; Accepted 2025-4-16; Published 2025-5-25
Abstract
The premetastatic niche (PMN) represents a metastasis-facilitative microenvironment established prior to tumor dissemination, initiated by vascular leakage and endothelial cell (EC) functional remodeling. ECs play pivotal roles as bridges in different stages of the metastatic cascade. As critical stromal components within the PMN, ECs not only drive angiogenesis but also actively orchestrate immune suppression, extracellular matrix (ECM) remodeling, and the inflammatory signaling characteristic of PMN formation, with multiple specific signaling pathways such as VEGF/Notch playing a crucial role. With the evolving understanding of the role of ECs in controlling tumor metastasis, therapeutic strategies targeting ECs within the PMN, such as antiangiogenic therapy (AAT), targeting of endothelial glycocalyx (GCX), inhibition of tumor-derived exosome (TDE) and angiocrine signaling, are becoming research hotspots. This review systematically delineates the cellular and molecular composition of PMNs, dynamically dissects their spatiotemporal evolution, and highlights organ-specific mechanisms of EC-driven PMN establishment. Furthermore, we summarize emerging EC-targeted therapeutic strategies, providing innovative insights for inhibiting tumor metastasis.
Keywords: Endothelial cells, Immunosuppression, Premetastatic niche, Targeted therapy, Angiogenesis
Introduction
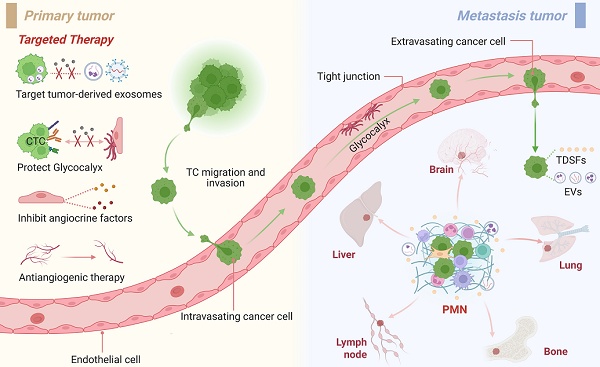
Tumor metastasis remains the leading cause of cancer-related mortality. Despite recent advancements in cancer therapeutics, metastatic cancers continue to pose significant clinical challenges [1]. A critical step in metastasis involves the shedding of circulating tumor cells (CTCs) from primary tumors, which disseminate through hematogenous or lymphatic systems to colonize distant organs [2]. The organotropism of metastasis can be attributed to specialized microenvironments that actively recruit tumor cells (TCs), as conceptualized by Paget's 1889 “seed and soil” hypothesis, which emphasizes bidirectional interactions between TCs (seeds) and host organs (soil) [3]. This metastasis-permissive microenvironment was later termed the PMN by Lyden et al. [4]. Emerging evidence reveals that tumors precondition distant organs by establishing PMNs prior to metastatic colonization [5]. Characterized by immune suppression, angiogenesis, vascular hyperpermeability, and organotropism, PMNs provide a hospitable ecosystem for TCs [5]. Consequently, PMN biology has garnered increasing recognition as a pivotal determinant of metastatic efficiency.
ECs, as active participants in the tumor microenvironment (TME), play a key role in angiogenesis and cancer progression. In addition to their canonical angiogenic function in supplying nutrients, ECs facilitate TC extravasation through vascular permeability modulation and immune evasion [6]. Moreover, TCs play a decisive role in promoting metastasis by promoting angiogenesis at distant sites [7]. ECs are uniquely positioned to orchestrate interactions among stromal, immune, and molecular components, distinguishing them from other cell types. Unlike other stromal cells, ECs directly shape the immune landscape through cytokine/chemokine secretion (e.g., IL-6, CCL2), which regulates immune cell trafficking and polarization. The unique ability of ECs to interact with immune and stromal components is further emphasized in ECM remodeling: post-translational modifications of ECM components dynamically regulate EC adhesion and paracrine signaling, thereby influencing cellular spatial organization within the TME [8]. Additionally, the cellular composition of the TME varies greatly among different tumor types, with ECs playing a central role. ECs induce the expression of tight junction proteins by expressing specific transcription factors (e.g., ETS1 and SOX7), enhancing the integrity of intercellular barriers, and thereby affecting the TME [9]. This review systematically examines the cellular and molecular composition of PMNs, with a particular emphasis on the multifaceted roles of ECs in PMN establishment. We further highlight the differential effects of ECs across distinct metastatic organs. Finally, we synthesized emerging therapeutic strategies targeting PMN-associated ECs, aiming to provide novel insights for preventing metastatic progression.
PMN: The Soil for Tumor Metastasis
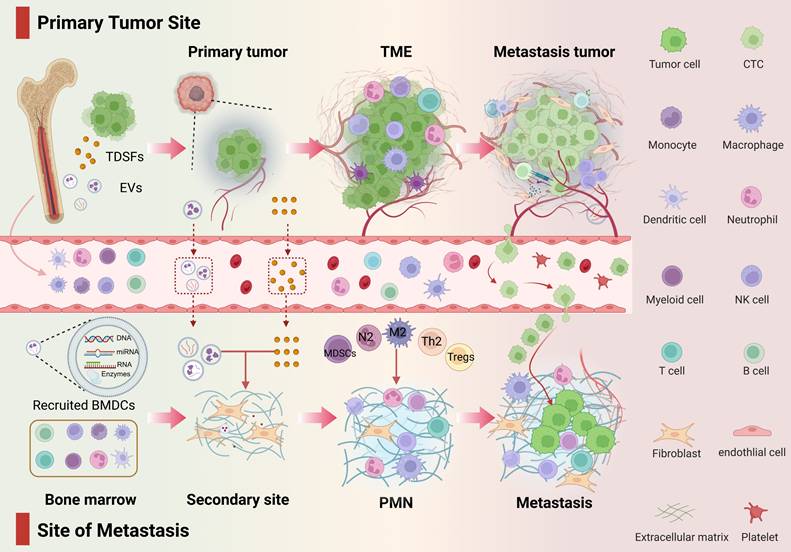
Over the past decade, our understanding of the PMN has deepened significantly, particularly with respect to the multifaceted roles of the immune system, stromal cells, tumor-derived secretory factor (TDSFs), and miRNA-enriched extracellular vesicles (EVs) in PMN dynamics. This progress has propelled PMN research from fundamental exploration toward clinical translation for preventing and treating metastatic progression. Here, we delineate the developmental stages and formation mechanisms underlying PMN establishment.
Cellular Constituents: Stromal-Immune Synergy
Stromal cells
The formation of the PMN involves intricate interactions among stromal cells, the vasculature, and the ECM, which are dynamically remodeled during crosstalk with primary tumors [10]. Stromal cells facilitate metastatic progression by secreting chemokines (e.g., CCL2) to recruit myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) [11]. Fibroblasts play pivotal roles in PMN establishment and therapeutic resistance [12]. For example, ovarian cancer cells extensively secrete exosomes enriched with miR-141, which activate the YAP1/GROα/CXCR signaling cascade to mediate tumor-fibroblast interactions, thereby fostering PMN development [13]. Moreover, pericytes within PMNs also exhibit protumorigenic properties. Murgai et al. demonstrated that tumor-derived factors induce KLF4 expression in pericytes, which results in the formation of fibronectin-rich PMNs, whereas conditional KLF4 knockout in pericytes suppresses their expansion and impedes lung metastasis [14]. Notably, organ-specific stromal responses occur—pulmonary ECs downregulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) under VEGF stimulation to promote PMN formation, whereas hepatic sinusoidal ECs increase fibronectin expression via TGF-β1 to drive tumor progression [15, 16]. Therefore, an in-depth exploration of stromal cell functions within PMNs has significant implications for the development of effective strategies to improve cancer therapeutics.
MDSCs
MDSCs, a heterogeneous population of immature myeloid cells with immunosuppressive functions, directly drive tumor metastasis by participating in PMN formation, promoting angiogenesis, and enhancing tumor invasion [17, 18]. Phenotypically, MDSCs are categorized into polymorphonuclear (PMN-MDSC) and monocytic (M-MDSC) subsets, which morphologically resemble neutrophils and monocytes [19]. Studies have demonstrated that the NLRP3-HSP70 axis in melanoma induces PMN-MDSC accumulation in lung tissues via TLR4 signaling-dependent mechanisms in pulmonary epithelial cells, fostering PMN development and conferring immunotherapy resistance [20]. Before TCs arrive, lung-infiltrated M-MDSCs enhance TC adhesion to ECs in the PMN by secreting IL-1β to upregulate ECs E-selectin expression [21]. Notably, specific TDSFs (e.g., TIMP-1 and macrophage migration inhibitory factor [MIF]) facilitate hepatic PMN formation through MDSC recruitment [22]. Chronic psychological stress activates the glucocorticoid-TAM-CXCL1 axis, driving splenic MDSC mobilization to construct breast cancer PMNs via CXCR2 signaling [23]. Mechanistically, galectin-1 recruits PMN-MDSCs through STING activation to mediate ECM remodeling, whereas PMN-MDSCs increase CTC metastatic potential via ROS/Notch/Nodal signaling crosstalk [24]. Thus, MDSC accumulation orchestrates PMN maturation and pulmonary metastasis progression.
Neutrophils
Neutrophils, the most abundant immune cells in peripheral blood, have garnered significant attention for their dual regulatory roles in tumor progression [25]. Lin28B, nicotine, and TLR3 drive the recruitment of N2-polarized neutrophils to establish an immunosuppressive PMN during breast cancer lung metastasis [26-28]. Neutrophil phenotypic polarization is dynamically regulated by microenvironmental cues: IFN-1 induces antitumor N1 polarization, whereas TGF-β suppresses N1 differentiation and promotes protumor N2 polarization, with the latter secreting arginase 1 to deplete arginine and impair T-cell cytotoxicity [29, 30]. The recruitment mechanism of neutrophils involves various signal molecules such as chemokines (e.g., CXCL1/8), exosomes, and bioactive factors. Notably, TANs dynamically interact with CTCs through adhesion molecule engagement, cytokine secretion, and neutrophil extracellular trap (NET) formation, thereby modulating the PMN and TME remodel. Experimental evidence confirms that SPP1-induced NETs facilitate hepatocellular carcinoma lung colonization by trapping CTCs [31], whereas strategies targeting CTC‒neutrophil interactions (e.g., biomimetic nanoparticle blockade) exhibit antimetastatic potential [32, 33]. Mechanistically, NETs degrade the ECM via protease release (e.g., MMP9) and reactivate dormant TCs, underscoring their critical role in metastatic cascades [34]. In summary, the interaction between neutrophils and ECs is critical for the migration of TCs and the formation of immunosuppressive PMN, as we will describe in further detail in the following sections [35-39].
Macrophages
TAMs dynamically shift their transcriptional programs along a continuous spectrum influenced by TME-derived stimuli, with M1 (antitumor) and M2 (protumor) phenotypes representing polar extremes [40]. The M2 subset dominates PMN establishment by orchestrating immune suppression and angiogenesis [41]. M1 TAMs are typically activated by LPS and IFN-γ, while M2 TAMs are activated by cytokines such as IL-4 and IL-13 [42]. Intermediate macrophage phenotypes (M2a, M2b, M2c, M2d) have been identified and described, and they significantly impact PMN formation and EC behavior [43]. For instance, M2a macrophages promote angiogenesis, whereas M2b macrophages are involved in immune cell recruitment and inflammatory responses [44]. TDEs deliver specific molecules (e.g., miR-934 and miR-4488) or regulate critical pathways (CAP2-mediated TGF-β1 secretion; Caveolin-1/PTEN-CCL2/VEGF-A axis) to drive M2 polarization, thereby enhancing hepatic/pulmonary metastasis and vascular remodeling [45-48]. Notably, distinct macrophage subsets predict divergent clinical outcomes, highlighting the importance of resolving TAM heterogeneity. Recent single-cell studies classify TAMs into resident tissue macrophages (RTMs) and monocyte-derived TAMs [42, 49]: RTMs activate fibroblasts and induce immunosuppression via phagocytosis of TDEs, whereas monocyte-derived TAMs remodel the PMN through proinflammatory cytokine secretion. This spatial-temporal regulatory mechanism underscores the therapeutic potential of targeting TAM subset differentiation.
T and B lymphocytes
As core components of adaptive immunity, T/B lymphocytes play complex regulatory roles within the tumor immune microenvironment. PMN establishment is closely associated with regulatory T-cell (Treg)-mediated immunosuppression [50]. Tregs shape CTC-disseminating microenvironments via the secretion of cytokines (e.g., TGF-β and IL-10), with hepatic infiltration of TNFR2+ Treg subsets correlating with poor prognosis in lung/colorectal cancer liver metastasis [51, 52]. Pharmacological inhibition of Tregs through NEDD8 pathway targeting effectively reduces postoperative pulmonary metastasis in patients with colorectal cancer [53]. Th2 polarization accelerates metastatic progression via STAT6-dependent complement C3 upregulation (driving neutrophil recruitment and NET formation) and macrophage phenotypic reprogramming [54, 55]. Notably, exosomal miR-135a-5p directly suppresses CD4+ T-cell activation, fostering liver metastasis immune tolerance [56].
B lymphocytes regulate the PMN through antibody-independent mechanisms, exhibiting unique roles in lymph node-breast cancer immune crosstalk [57]. In breast cancer, B cells drive lymph node metastasis via the HSPA4 glycosylation-IgG-CXCR4/SDF1α axis, while single-cell sequencing revealed that transcriptional reprogramming of marginal zone B cells in tumor-draining lymph nodes (angiogenic pathway activation) is negatively associated with prognosis [2, 58]. Intriguingly, T/B-cell synergism promotes breast cancer bone metastasis, although their spatiotemporal regulatory dynamics in the PMN remain incompletely characterized [59]. The current understanding of T/B lymphocyte interactions within the PMN remains limited; thus, conducting more comprehensive studies on these two types of cells is necessary.
Molecular Drivers: Dual Regulation by TDSFs and EVs
The formation of PMNs involves intricate interactions between diverse cellular and molecular components, which collectively orchestrate PMN development. These molecules originate not only from bone marrow-derived and stromal cells but also from TCs. While the previous sections focused on the roles of bone marrow-derived immune cells and stromal cells in PMN establishment, tumor-derived molecular components are pivotal in driving organ-specific PMN formation. Therefore, we focused on TDSFs and extracellular vesicles.
TDSFs
TDSFs include proteins, enzymes, cytokines, and other bioactive molecules secreted by TCs under hypoxic and inflammatory conditions, exerting critical regulatory effects on tumor progression and PMN establishment. The functional roles of TDSFs vary across tumor types, with accumulating evidence demonstrating that TDSFs promote PMN formation by mobilizing and recruiting bone marrow-derived cells. For example, in colorectal cancer, TDSFs recruit MDSCs via the S1PR1-STAT3 signaling axis to facilitate hepatic PMN formation [60]. Additionally, CCL2-mediated recruitment of Tregs and TAMs stimulates angiogenesis and immunosuppression, fostering pulmonary PMN development [61, 62]. Recent studies have revealed that gastric cancer-derived lipopolysaccharide-binding protein activates the TLR4/NF-κB pathway in hepatic macrophages and hepatic stellate cells, driving fibrotic PMN formation in the liver [63]. In summary, TDSFs interact with bone marrow-derived immune or host stromal cells through distinct signaling pathways, thereby inducing these cells to secrete specific molecular components to support PMN formation.
EVs
Tumor-derived EVs, pivotal mediators of TME crosstalk, exert pleiotropic regulatory effects on tumor growth and metastatic cascades by delivering bioactive cargoes, including nucleic acids, proteins, and metabolites [64, 65]. Classified by biogenesis pathways, EVs include exosomes, microvesicles, apoptotic bodies, oncosomes, and megasomes, with exosomes and microvesicles being extensively studied for their roles in PMN formation [66]. In recent years, mechanistic insights into EV-mediated metastasis and PMN establishment have expanded, notably identifying tumor exosomal integrins as predictive biomarkers for organotropic metastasis [67-70]. miRNAs are identified as early drivers of PMN driven by EVs, and are involved in regulating nearly all cancer-associated processes, including ECM remodeling, angiogenesis, and immune cell recruitment [71, 72]. For instance, miR-21 targets PTEN and the Akt signaling pathway, reducing the proliferation of Tregs and thereby modulating the function of immune cells [73]. miR-29a/c targets VEGF to inhibit angiogenesis in the gastric cancer microenvironment [74]. Additionally, in colorectal liver metastasis, circ-0034880-enriched EVs increase the activation of SPP1 high CD206+ protumorigenic macrophages, remodeling the host stromal microenvironment to foster overt metastasis [75]. Recent studies have focused on understanding the effects of specific factors on EV properties and function. For example, Snail overexpression in murine colon adenocarcinoma increases Glypican-1 levels in EVs, potentially augmenting PMN development and metastatic potential [76]. Notably, studies in xenograft models revealed that tumor EVs prime inflammatory responses in distant organs, accelerating PMN maturation [77].
Dynamic evolution of the PMN: Spatiotemporal orchestration by TCs
TCs establish permissive microenvironments in distant organs through spatiotemporally coordinated mechanisms to enable metastasis. As a critical metastatic checkpoint, PMN formation evolves through three dynamically interconnected phases.
Phase I: Molecular preprogramming. Primary tumors remotely precondition potential metastatic organs via the systemic secretion of exosomes, cytokines, and other signaling molecules. These tumor-derived components (e.g., miRNAs and integrins) establish organotropic molecular imprints through hematogenous/lymphatic circulation, systematically preconfiguring the PMN landscape. Recently, Wang et al. systematically organized the roles and mechanisms of known tumor-derived molecular components in PMN formation, which provided a novel understanding of PMN formation [78].
Phase II: Microenvironmental remodeling. Resident organ cells respond to tumor-derived signals by initiating angiogenesis, immune evasion, and ECM remodeling to prime metastatic colonization. For example, KLF4-dependent perivascular cells mediate angiogenesis and ECM remodeling to promote PMN maturation [14]. Concurrently, cellular and molecular components within PMNs enhance TC invasiveness: tumor-associated neutrophils (TANs) facilitate CTC-endothelial adhesion during intravasation, whereas TANs directly interact with CTCs to increase their survival [79-82]. Hippo pathway inactivation further amplifies CTC aggressiveness via Wnt/β-catenin-mediated epithelial-mesenchymal transition (EMT), as evidenced by HCC EVs delivering miR-665/miR-1273f to suppress Hippo signaling and increase CTC stemness [83-85]. Through these spatiotemporal synergies, tumors engineer premetastatic soil long before physical CTC arrival [86].
Phase III: CTC Homing and Expansion. Mature PMNs guide CTC homing through chemotactic gradients. Bidirectional CTC‒PMN crosstalk critically regulates colonization: nicotine-induced N2 TANs activate CTC EMT via adiponectin 2, whereas CTC-derived IL-6 drives neutrophil N2 polarization through STAT3 signaling, forming a prometastatic feedforward loop [28, 87-89]. PMN-MDSCs further enhance CTC metastatic fitness through ROS/Notch crosstalk [24]. Postcolonization, CTCs either enter dormancy or exploit remodeled niches for proliferation, ultimately developing macroscopic metastases.
Progressive CTC colonization of mature PMNs drives pathological progression from micrometastases to macrometastases, with CTC-laden PMNs potentially promoting further dissemination. Collectively, PMN evolution reflects not stochastic events but a precision-engineered spatiotemporal program wherein tumors precondition distant microenvironments to license metastatic outgrowth (Figure 1).
EC contributions to PMN formation
The PMN represents an aberrant, tumor-permissive microenvironment devoid of cancer cells. Previous studies have indicated that PMN formation initiates with localized alterations (e.g., vascular leakage and stromal/ECM remodeling) before exerting systemic immunosuppressive effects. ECs, as pivotal PMN components, actively participate in niche establishment through immune suppression, inflammation, angiogenesis, and ECM remodeling. Here, we aimed to reveal the specific role of ECs in PMN formation by performing PMN studies related to ECs (Figure 2).
Developmental stages of the PMN. From the primary tumor to PMN, TCs promote metastasis in a spatiotemporal manner. This figure outlines the three-stage dynamic evolution of PMN from initiation to maturation. The entire process reflects the precise orchestration by TCs of the preparation of the “soil” in distant organs and their own colonization. Phase I: Molecular preprogramming—Primary tumors release exosomes, cytokines, and other factors to remotely regulate target organs, establishing chemotactic gradients and vascular leakage signatures. Phase II: Microenvironment remodeling—Resident cells in the target organ respond to tumor signals, constructing the PMN through angiogenesis, immune suppression, and ECM remodeling. Phase III: CTC Homing and Expansion—CTCs interact with the modified microenvironment, achieving metastatic focus formation through mechanisms such as immune evasion and stemness activation.
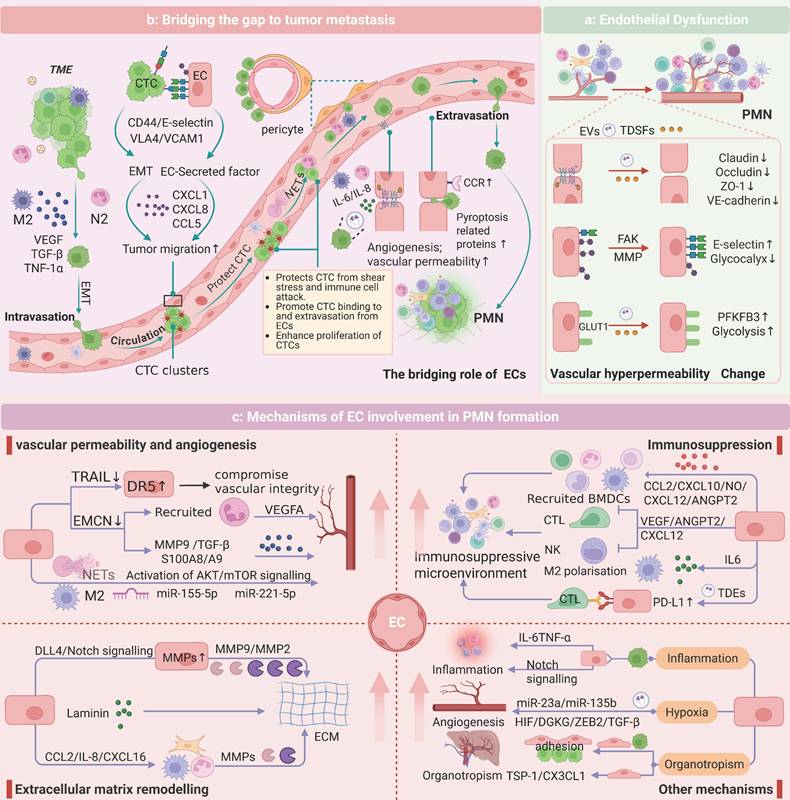
Endothelial Mechanisms in PMN Evolution. a: In the formation of the PMN, TDSFs and EVs induce endothelial dysfunction, compromising vascular barrier integrity. This manifests as increased vascular permeability and leakage. The underlying mechanisms involve multiple factors, including disruption of adherens junctions, activation of MMPs, degradation of the GCX, and metabolic reprogramming. b: ECs play a pivotal role in every stage of tumor metastasis. By modulating adhesion molecules, secreting chemokines, and maintaining the dormancy of CTCs, ECs facilitate the intravasation, survival in circulation, extravasation, and colonization of CTCs. ECs also provide a “bridge” for TCs and immune cells, creating a microenvironment conducive to metastasis. c: ECs contribute to the development of PMN characteristics, with recruited immune cells and tumor-derived molecular components interacting with ECs via the vasculature to prepare a mature environment for tumor metastasis within the PMN.
Stage-Specific Functions of ECs in the PMN
Endothelial Dysfunction: Vascular Barrier Disruption
Under physiological conditions, ECs maintain vascular integrity via VE-cadherin-mediated adherens junctions and occludin/claudin/ZO-1 tight junction complexes [90]. During PMN formation, TDSFs and EVs destabilize endothelial barriers, inducing pathological hyperpermeability [91]. For example, colorectal cancer-derived EVs (e.g., ADAM17 and miR-27b-3p) synergistically promote vascular leakage by interfering with the membrane localization of VE-cadherin and regulating the expression of tight junction proteins (ZO-1/occludin/Claudin5) via the KLF2/KLF4-VEGFR2 axis [92-94]. Breast cancer-derived angiopoietin 2 exacerbates junctional instability by activating MMPs [95]. Concurrently, GCX cleavage enzymes (e.g., heparanase, MMPs) in the PMN increase permeability via syndecan-3/4 ectodomain cleavage in a Rho-kinase-dependent manner [96-98]. Metabolic reprogramming also contributes: VEGF-enriched EVs drive endothelial hyperglycolysis via PFKFB3/GLUT1 upregulation, increasing leakage [99]. Increased vascular permeability facilitates bidirectional cellular trafficking and microenvironmental crosstalk, thereby accelerating PMN formation (Figure 2a).
Microenvironment remodeling: Endothelial-stromal-immune cross talk
ECs are driven by tumor-derived molecules and work together with stromal and immune cells to shape PMNs. On the one hand, stromal and immune cells within the PMN regulate EC functions via paracrine signaling. Fibroblasts activate the P38 MAPK pathway in ECs via the lncRNA SNHG5 to promote angiogenesis [100]. M2-polarized TAMs secrete exosomes enriched with miR-23a/155/221 to induce vascular leakage [101, 102], whereas interstitial macrophages increase vascular permeability through the IL-6/VEGF axis [103]. On the other hand, ECs autonomously contribute to the construction of an immunosuppressive microenvironment and ECM remodeling. For instance, tumor-derived autophagosomes upregulate PD-L1 expression on ECs via the TLR4-MyD88-p38/STAT3 cascade, suppressing T-cell activity and polarizing macrophages toward the M2 phenotype [104, 105]. Activated Notch1 receptors (N1ICDs) in ECs drive neutrophil infiltration and metastasis [39]. Additionally, CD36 upregulation in ECs exacerbates immunosuppression [106]. This reciprocal crosstalk between ECs and neighboring cells is highly important for the establishment of a mature PMN, which primes the microenvironment for subsequent TC dissemination and colonization.
Metastatic cascade regulation: endothelial-mediated CTC homing and extravasation
ECs serve as “bridges” for TCs and immune cells during the metastatic process. In the initial stage of metastasis, TAMs release EGF/TNF-α to promote the intravasation of TCs, and TANs guide TCs to the vascular endothelial interface through chemotaxis [80, 107]. CTCs in circulation face shear stress and immune clearance pressures, and their survival and metastatic efficiency are precisely regulated by adhesion molecules on the endothelial surface [82, 108]. E-selectin mediates transendothelial migration by binding to the CD44 receptor on the surface of CTCs, and its expression is positively regulated by IL-1β secreted by M-MDSCs [21, 109]. ECs in the leaky region form microenvironmental “hotspots” for metastatic cancer cell-specific homing through focal adhesion kinase (FAK)-dependent upregulation of E-selectin [110, 111]. For example, E-selectin is an important homing receptor for hematogenous dissemination in lung cancer, prostate cancer, and breast cancer [112-114]. In bone, E-selectin can also promote the EMT of CTCs, increasing bone metastasis. VCAM1 and ICAM1 are also key adhesion molecules that drive lung/breast-specific colonization of CTCs through interactions with VLA4 and integrins [115, 116]. Additionally, ECs reduce immune cell adhesion by suppressing VCAM-1/ICAM expression and form a physical barrier to protect CTCs from immune surveillance through platelet aggregation [117, 118]. Chemokines secreted by ECs (such as CXCL1/CXCL8/CCL5) guide the directional migration of CTCs through gradients, with CCL5 activating the androgen receptor to increase the invasiveness of prostate cancer [119, 120]. Molecules such as Biglycan and EphrinA1 also play roles in promoting TC migration [121]. Studies on pericytes have shown that CTCs replace pericytes by competing for L1CAM on ECs, thereby achieving vascular basement membrane infiltration [122]. During extravasation, the expression of CCR2 on ECs leads to endothelial retraction and TC extravasation [123]. Recently, high expression of pyroptosis-related proteins in ECs was shown to further accelerate this process [124]. Notably, ECs maintain the dormancy of CTCs through factors such as thrombospondin-1, providing a potential niche for metastatic relapse [91, 114] (Figure 2b).
In summary, ECs play a unique role in the formation of the PMN by altering their function, remodeling the microenvironment, and providing a “bridge,” thereby laying the foundation for CTC colonization. We also summarize the relevant molecular mechanisms by which TDEs target ECs to promote the formation of the PMN (Table 1).
Core mechanisms of EC involvement in PMN formation
Angiogenesis
As discussed, ECs critically regulate vascular permeability and TC migration. Angiogenesis, as one of the core mechanisms of PMN formation, is orchestrated by ECs through multiple pathways. For instance, EMCN-deficient ECs recruit Ly6G+ neutrophils and upregulate MMP9, S100A8/A9, and TGF-β to induce proangiogenic phenotypes and pulmonary PMN formation [35]. In breast cancer models, loss of TRAIL expression activates the death receptor DR5, triggering NF-κB/p38-dependent adhesion phenotype switching in ECs to promote myeloid cell infiltration and tumor colonization [15].
Mechanisms of TDEs targeting ECs to promote PMN formation.
| Exosome composition | Primary tumor | Function and mechanism | Target Organ | Refs |
|---|---|---|---|---|
| miR-BART2-5p | NPC | Induces EC pyroptosis and increases vascular permeability by regulating MRE11A | Bone/Liver/Lung | [124] |
| circ_0011496 | HCC | Circ_0011496 interacts with miR-486-5p to enhance angiogenesis and vascular permeability via the VEGF route | Lung | [125] |
| circ-ZNF609 | ESCC | Circ-ZNF609 disrupts EC tight junctions via miR-150-5p/VEGFA and HuR/ZO-1 pathways | Liver/Lung/Lymph node | [126] |
| miR-103a-3p | NPC | Promoting TC proliferation and vascular permeability by targeting ZO-1 and ACOX-1 | Lung/Lymph node | [127] |
| miR-1270 | BC | Disrupts EC tight junctions by down-regulating ZO-1 and occludin expression | Lung | [128] |
| miR-27b-3p | CRC | Post-transcriptional expression of VE-Cad and p120 was inhibited by targeting their 3'-UTR in ECs. | Liver/Lung | [92] |
| miR-374a-5p | NSCLC | Regulates the distribution of ZO1 and occludin in ECs by targeting γ-adducin, increasing vascular permeability | Brain | [129] |
| miR-605-3p | GC | By regulating the secretion of NOS3, it can increase the NO level of EC and promote angiogenesis. | Liver | [7] |
| MFI2-AS1 | NSCLC | Increased expression of NFAT5 through adsorption of miR-107, thereby activating the PI3K/AKT pathway and promoting angiogenesis | Lung | [130] |
| miR-455 | NPC | Disrupts EC tight junctions and increases vascular permeability by targeting ZO-1 | Lung | [131] |
| miR-519a-3p | GC | Targeting DUSP2 induces macrophage M2 polarisation and M2 macrophages promote angiogenesis | Liver | [132] |
| miR-3157-3p | NSCLC | By regulating the expression of VEGF/MMP2/MMP9 and occludin | Lung/Bone | [133] |
| miR-638 /miR-663a/ miR-3648 /miR-4258 | HCC | By down-regulating VE-cadherin and ZO-1 in ECs | Lung/Lymph node/Bone | [134] |
| miR-1260b | NSCLC | Inhibition of HIPK2 in ECs promotes angiogenesis and enhances tumour cell migration and drug resistance | Lung | [135] |
| miR-619-5p | NSCLC | Inhibition of RCAN1.4 promotes angiogenesis and tumour metastasis | Lung | [136] |
| miR-103 | HCC | Inhibition of VE-Cadherin, p120-catenin, and ZO-1 expression disrupts EC tight junctions and adhesion junctions | Liver/Lung | [137] |
| miR-25-3p | CRC | Regulation of VEGFR2, ZO-1, occludin and Claudin5 expression in ECs by targeting KLF2 and KLF4 | Liver/Lung | [93] |
| miR-23a | LC | Enhancement of angiogenesis and vascular permeability by targeting PHD1, PHD2, and ZO-1 | Lung | [138] |
| miR-105 | BC | Disrupts EC tight junctions by targeting ZO-1 | Bone/Lung/Brain | [139] |
| miR-135b | MM* | Enhancement of tube formation in ECs under hypoxic conditions via the HIF-FIH signalling pathway | Bone | [140] |
| miR-181b/ miR-27a/ miR-484/ miR-324-3p | RCC | Provision of pro-angiogenic mRNAs and miRNAs | Lung | [141] |
| miR-126 | BC | Targeting IGFBP2, PITPNC1, and MERTK to inhibit EC recruitment and angiogenesis | Lung/Bone | [142] |
| miR-9 | BC | Down-regulation of E-cadherin, activation of the β-catenin/VEGF pathway, and promotion of angiogenesis | Lung | [143] |
| CLTA | HCC | Stabilisation and up-regulation of BSG in ECs to remodel the pre-metastatic microvascular niche | Lung/Lymph node/Bone | [144] |
| vWF | HCC | Promoting angiogenesis and metastasis by facilitating the formation of a positive feedback loop between tumours and ECs by VEGF-A and FGF2 | Lung | [145] |
| Tspan8-α4/β1 Tspan8-α6/β4 | MT | Activation of EC membrane receptors induces activation of signalling pathways and up-regulation of transcription factors to promote angiogenesis. | Lung | [146] |
| ADAM17 | CRC | Regulates membrane localisation of VE-cadherin and enhances vascular permeability by targeting ECs | Liver/Lung/Peritoneum | [94] |
| NDPK-B | BC | Promotes EC migration and disrupts monolayer integrity, leading to vascular leakage in the lungs | Lung | [147] |
| NID1 | HCC | Enhancement of angiogenesis and pulmonary vascular endothelial permeability | Lung | [148] |
| uPAR | MM | Binds to ECs and activates VE-Cadherin, EGFR, and uPAR expression | Skin/Lymph node/Lung | [149] |
| ErbB2/ CRK | BC* | Promoting EC proliferation and invasion and increasing vascular permeability through FAK and PI3K/AKT signalling pathways. | Lung/Liver/Bone | [150] |
| TF | BC/PC | Activation of PAR-1 on EC causes upregulation of E-selectin expression and IL-8 secretion | Peritoneum/Liver/Bone | [151] |
*Abbreviation: NPC: Nasopharyngeal carcinoma, HCC: Hepatocellular carcinoma, ESCC: Esophageal squamous cell carcinoma, BC: Breast cancer, CRC: Colorectal cancer, NSCLC: Non-small cell lung cancer, GC: Gastric cancer, LC: Lung cancer, MM*: Multiple myeloma, RCC: Renal cell carcinoma, MT: Mouse Tumor, MM. Melanoma, BC*: Bladder cancer, PC: Pancreatic cancer.
On the other hand, M2 macrophage-derived exosomal miRNAs (e.g., miR-155-5p and miR-221-5p) regulate endothelial migration and angiogenesis by targeting molecules such as GJA1, whereas miR-30a-5p reprograms EC function via PDCD10-dependent mechanisms [152-154]. Neutrophils amplify angiogenesis via JAK/STAT3-mediated VEGFA activation in combination with G-CSF signaling, and NET-DNA enhances this effect by binding the ccdc25 receptor on HUVECs to activate the AKT/mTOR pathway [155, 156]. Notably, exosomal ANGPTL1 imposes vascular hyperpermeability and delays PMN maturation by reprogramming Kupffer cells and suppressing MMP9 expression [157]. In addition to TANs, the angiogenic mechanisms in PMNs involving diverse cellular and molecular components have been systematically elucidated.
Immunosuppression
Immune suppression is a well-recognized facilitator of PMN formation, in which ECs drive the immunosuppressive characteristics of the PMN through multiple mechanisms. EVs reprogram ECs to facilitate immunosuppressive cell infiltration and functional polarization [146]. Activated ECs mediate immune cell transendothelial migration by secreting chemokines (e.g., CCL2 and CXCL10), while IL-6 secretion drives macrophage polarization toward protumor phenotypes [158, 159]. Recently, the CXCL12+ EC subpopulation was shown to establish an HCC-specific immune escape microenvironment by inhibiting cytotoxic T lymphocyte activity and recruiting MDSCs [160].
Moreover, proangiogenic molecules such as VEGF induce immune exhaustion by increasing PD-1/CTLA-4 expression on Tregs and CD8+ T cells [161]. VEGF also suppresses dendritic cell activation, thereby impairing T-cell priming [162]. Under inflammatory stimuli, angiopoietin-2 (ANGPT2) synergizes with TNF-α to recruit Tregs/MDSCs via adhesion molecule modulation, amplifying immunosuppression [163, 164]. In breast cancer, tumor-derived autophagosomes activate the TLR4‒MyD88 signaling pathway in ECs to upregulate PD-L1 expression, directly inhibiting T-cell function [105]. These findings collectively highlight ECs as pivotal regulators of immune dynamics, coordinating spatiotemporally resolved molecular networks to establish immunosuppressive niches within PMNs.
ECM Remodeling
The ECM, a complex network of proteins and glycosaminoglycans, plays a pivotal role in TC motility and invasion. TDSFs remodel PMN matrix stiffness and topology by regulating the expression of ECM structural proteins (e.g., laminin), degradative enzymes (MMP family), and processing proteins [165]. Mechanistically, ECs directly cleave ECM components via MMP-2/MMP-9 and activate stromal cell MMP secretion through paracrine cytokines such as CCL2/IL-8 [166]. Additionally, the activated DLL4/Notch signaling axis upregulates endothelial MMP9 expression, whereas neutrophil-derived MMPs disrupt vascular integrity by degrading VE-cadherin, synergistically facilitating CTC extravasation [167, 168].
Notably, tumor-specific ECM remodeling features significantly impact clinical outcomes. Melanoma ECs secrete laminin to drive invasive phenotypes, while elevated laminin expression in renal cell carcinoma is correlated with poor prognosis [169]. Prior studies using glioblastoma 3D bioprinted cultures containing TCs, ECs, and hyaluronic acid derivatives demonstrated how ECM stiffness modulates transcriptional programs and tumor‒endothelial crosstalk [170]. In summary, endothelium-mediated ECM remodeling provides both physical scaffolding and chemotactic gradients to support tumor metastasis.
Other mechanisms: inflammation, hypoxia, and organotropism
Inflammation: Chronic inflammation drives PMN formation via endothelial dysfunction [171]. ECs mediate tumor-endothelial interactions through ICAM-1, triggering IL-6/TNF-α inflammatory cascades to establish liver-metastatic microenvironments [172]. Notch signaling activation induces endothelial senescence, amplifying neutrophil infiltration and proinflammatory cytokine secretion to accelerate tumor adhesion and metastasis [38]. EC-derived CCL5 recruits monocytes to promote breast cancer dissemination [173], whereas macrophage‒endothelial crosstalk exacerbates inflammatory responses through hypoxia-dependent mechanisms [174]. Additionally, IGFBP7hi endothelial subpopulations disrupt GCX integrity, exposing adhesion molecules to facilitate T-cell extravasation and amplify inflammatory microenvironments [175]. These findings highlight ECs as pivotal orchestrators of inflammatory niche establishment.
Hypoxia: Hypoxia, a central driver of angiogenesis, coordinates PMN formation through differential endothelial regulation of hypoxia inducible factors (HIFs) [176]. Dynamic HIF-1α/HIF-2α expression under acute vs. chronic hypoxia reshapes lung metastatic niches, enhancing TC dissemination [176, 177]. HIF signaling induces endothelial-specific DGKG expression, activating the ZEB2/TGF-β1 axis to promote proangiogenic phenotypes and Treg differentiation [178]. Hypoxia-derived exosomal miR-23a/135b regulates PMN maturation by targeting vascular permeability and angiogenesis pathways [138, 140]. Notably, sarcoma-derived hypoxia-modified collagen VI disrupts pulmonary endothelial barrier integrity, providing structural support for metastasis [179].
Organotropism: ECs critically regulate organ-specific metastasis. Transfer of tumor-derived microRNAs to ECs modulates their migratory properties and organ selectivity [180]. The causative role of blood flow is a key factor in the direct regulation of organ-specificity by the vasculature. For example, the liver's unique blood supply pattern can cause primary tumors to metastasize to the liver through the portal vein. Hemodynamics and fluid flow patterns not only facilitate the transport of CTCs but also influence their ability to colonize distant sites, as evidenced by the patterns observed in liver and lung interactions [181]. Moreover, the regulation of organotropism is multifaceted, involving various biological factors, including the intermittent nature of blood flow in specific vascular structures, such as liver sinusoids [182]. This intermittent flow can affect the survival and colonization potential of metastatic cells, as they must adapt to the changing hemodynamic conditions within the circulatory system [183]. CTC-EC adhesion determines organotropism, with TSP-1 and CX3CL1 secreted by ECs influencing TC self-renewal and immune cell recruitment, respectively, to shape “congenial soil” for metastasis [182, 184, 185]. Interestingly, mitochondrial transfer from ECs to TCs via tunneling nanotubes enhances invasiveness through metabolic reprogramming [186]. Understanding these EC-tumor interactions is vital for deciphering organ-specific metastatic mechanisms [187] (Figure 2c).
Role of ECs in organ-specific PMNs
Clinical evidence highlights that most cancers metastasize to specific organs, a phenomenon termed organotropism. Investigating the shared and divergent mechanisms of PMNs across metastatic organs is critical for deciphering organotropic metastasis and developing targeted therapies. This section discusses EC-driven mechanisms in organ-specific PMN landscapes.
Lymph node
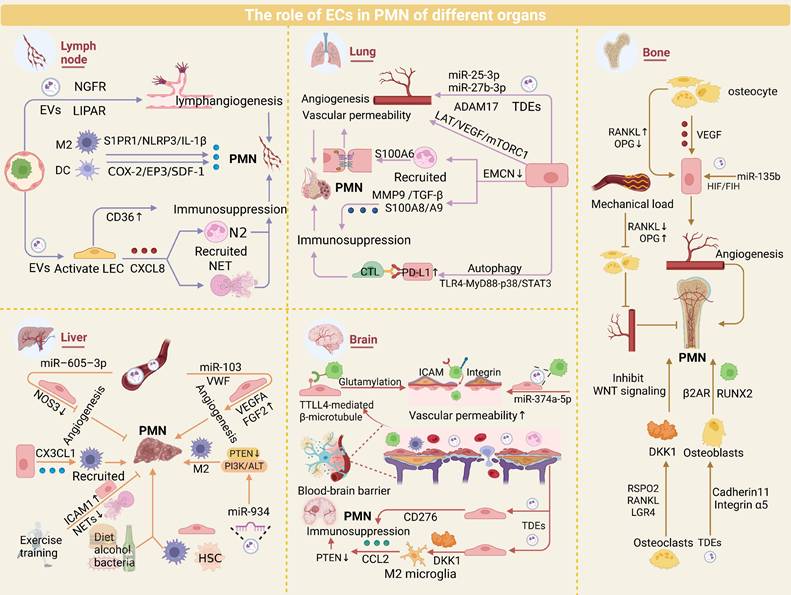
Lymph nodes (LNs), as critical hubs of the lymphatic system, favor PMN formation by providing tumor-permissive microenvironments that enhance TC immune evasion and invasiveness. LN-associated PMNs are characterized by lymphangiogenesis and high endothelial venule remodeling [188]. TDEs promote characteristic changes in the PMN by remodeling lymphatic endothelial function. For example, melanoma-derived NGFR-enriched EVs are internalized by LECs, activating ERK/NF-κB signaling and upregulating ICAM-1 to increase lymphangiogenesis and TC adhesion [189]. ITGA6+ EVs deliver circRNA-LIPAR into LECs, triggering E-selectin-mediated lymphatic remodeling [190]. Tumor EVs also coordinate immunosuppression during LN remodeling via interactions with LECs [191]. LEC-derived CXCL8 recruits TANs to form NETs, whereas CD36-dependent immune checkpoint signaling reinforces immunosuppression [106, 192]. POSTN deposition amplifies lymphangiogenesis via VEGF-C upregulation, facilitating tumor colonization [193]. Macrophage S1PR1/NLRP3/IL-1β signaling synergizes with the dendritic cell COX-2/EP3/SDF-1 pathway to increase lymphangiogenesis and PMN maturation [194, 195]. Collectively, these findings underscore the intricate crosstalk among ECs, LNs, and PMN formation in metastasis.
Lung
The high vascular perfusion and oxygen-rich microenvironment of the lungs foster a unique PMN. Tumor-derived EVs (e.g., miR-25-3p, ADAM17, and miR-27b-3p) promote lung PMN vascularization by targeting ECs [92-94]. Breast cancer-derived autophagosomes activate the TLR4‒MyD88‒p38/STAT3 cascade in ECs to upregulate PD-L1, suppressing T-cell immune function and facilitating pulmonary metastasis [104, 105]. Interestingly, chemotherapy-induced ANXA6+ EVs remodel ECs into prometastatic phenotypes via NF-κB activation [196]. Zhang et al. demonstrated that EMCN-deficient ECs (genetically engineered in murine models of breast and lung adenocarcinoma) recruit Ly6G+ neutrophils and increase MMP9, S100A8/A9, and TGF-β expression to drive lung PMN formation [35].
Recent insights highlight LAT1 as a regulator of EC proliferation and VEGF-A/mTORC1-driven angiogenesis, while LAT1 inhibitors suppress lung metastasis by inducing vascular normalization [197, 198]. Notably, EC‒EC-neutrophil crosstalk enhances neutrophil transendothelial migration through S100A6-mediated tight junction disruption and TRPM2-dependent VE-cadherin phosphorylation [36, 37]. In addition, alveolar epithelial cells modulate endothelial barrier integrity via Wnt/β-catenin signaling, whereas M2 macrophages promote vascular leakage through TGF-β1-induced EMT [199-201]. In short, the unique microenvironment of the lungs helps to generate PMNs.
Liver
ECs are pivotal components of the hepatic microenvironment and critically regulate liver homeostasis and disease pathogenesis [202]. EV delivery of von Willebrand factor (VWF) enhances angiogenesis in HCC via a VEGF-A/FGF2-FGFR4/ERK1 positive feedback circuit [145]. Recent studies revealed that miR-605-3p suppresses vascularization in hepatic PMNs by reducing exosomal NOS3 levels [7]. Experimental evidence has demonstrated that exercise training mitigates liver metastasis susceptibility by inhibiting NET formation and modulating EC adhesion molecule expression [203].
Macrophages are key contributors to hepatic PMN establishment. ECs recruit CX3CR1+ macrophages through CX3CL1 secretion, driving MMP9 upregulation and liver metastasis progression, potentially through TNF-α signaling [204]. TDEs (e.g., miR-934/203a-3p) induce macrophage M2 polarization via PTEN/PI3K/AKT pathway activation, synergizing with CXCL12/CXCR4 signaling to promote colorectal cancer liver metastasis [45, 205, 206]. Additionally, the high permeability and lack of tight junctions in liver sinusoidal endothelial cells (LSECs) enable the liver to more effectively filter TCs from the bloodstream. For instance, the fenestrated structure of LSECs allows them to inhibit the activation of hepatic stellate cells under normal conditions, thereby maintaining the homeostasis of the liver environment. However, TCs tend to induce the defenestration of LSECs, leading to enhanced TC adhesion. These structural changes provide a more favorable environment for the growth of TCs [207]. Notably, hepatic PMN formation is modulated by external factors, including gut bacteria, diet, and alcohol, which collectively shape immunosuppressive microenvironments [208-210].
Brain
The formation of brain PMNs involves unique mechanisms due to the presence of the blood-brain barrier (BBB). TDEs regulate BBB permeability to orchestrate the spatiotemporal evolution of the brain PMN. Recent studies revealed that small-cell carcinoma-derived miR-374a-5p enhances BBB permeability by targeting γ-adducin and disrupting the distribution of ZO1 and occludin [129]. Cytoskeletal remodeling drives tumor transendothelial migration, where TTLL4-mediated glutamylation of β-tubulin promotes the transport of multiple vesicular bodies, enhancing breast cancer cell adhesion to the BBB endothelium [211]. TCs upregulate adhesion molecules such as ICAM1 and β3-integrin to strengthen their anchorage to the BBB endothelium and induce endothelial apoptosis, facilitating brain PMN formation [212]. Notably, single-cell sequencing revealed CD276 upregulation in metastatic ECs, highlighting the unique immune checkpoint regulatory properties of the BBB [213]. The abundance of microglia in the brain is important for the establishment of the PMN. Microglia-derived exosomal miR-19a recruits myeloid cells via CCL2 activation by suppressing PTEN, whereas endothelial-derived Dkk-1 induces M1-to-M2 microglial polarization, synergistically fostering an immunosuppressive microenvironment [214, 215]. Targeted interventions (e.g., ESTA blocking the E-selectin/CD44 interaction) significantly inhibit breast cancer brain metastasis, underscoring the therapeutic potential of endothelial-specific targets [216, 217]. Exogenous stimuli, such as nicotine exposure, accelerate brain PMN maturation by inducing TC metabolic reprogramming and enhancing stemness [218]. These findings reveal that the BBB finely regulates the cascade of brain metastases through the dual mechanisms of structural remodeling and immunomodulation.
Bone
Owing to their abundant blood supply and marrow microenvironment, bone tissue allows cancer cells to infiltrate and proliferate via hematogenous or lymphatic dissemination. Bone remodeling and homeostasis involve crosstalk between osteocytes and ECs in the marrow. Osteocytes promote angiogenesis by secreting VEGF to activate ECs [219], while the RANKL/OPG balance regulates endothelial permeability to influence metastatic efficiency: elevated RANKL/OPG ratios increase vascular permeability, facilitating bone infiltration [220-222]. Mechanical loading suppresses vascular permeability and PMN formation by reducing the RANKL/OPG ratio via fluid shear stress-induced inhibition of MMP9 secretion and weakened tumor-endothelial adhesion [223]. Tumor-derived miR-135b enhances angiogenesis under hypoxia through the HIF-FIH pathway, synergizing with hypoxic microenvironments to accelerate bone metastasis [140], whereas siRNA nanodelivery systems targeting the bone marrow endothelium offer novel strategies for PMN intervention [224].
Osteoblasts and osteoclasts critically contribute to PMN formation. Cadherin 11 and integrin α5 mediate specific recognition of tumor-derived EVs by osteoblasts, creating PMNs that promote RUNX2-high breast cancer cell colonization [225]. Concurrently, β2-adrenergic receptor activation in osteoblasts triggers VEGF-dependent angiogenesis, accelerating tumor colonization [226]. RSPO2 and RANKL signaling through LGR4 recruits osteoclasts to remodel the bone matrix microenvironment, facilitating PMN development [227].
In summary, ECs exhibit common regulatory mechanisms and organ-specific functions in the process of tumor metastasis. In major metastatic organs such as lymph nodes, lungs, liver, brain, and bones, ECs generally drive the formation of PMNs by promoting angiogenesis and secreting exosomes to modulate the immune microenvironment. Meanwhile, ECs in each organ have unique roles. In lymph nodes, ECs enhance the stealthiness and invasiveness of TCs through lymphangiogenesis and high endothelial venous remodeling; in the lungs, the dense branching of the capillary network and the slow blood flow characteristics make CTCs more likely to be retained at the endothelial interface; in the liver, TCs induce the fenestration loss of LSECs, thereby enhancing the adhesion capacity of TCs; in the brain, ECs promote the infiltration of TCs by dismantling the “protective net” of the BBB (using certain small molecules to disrupt the barrier structure) and upregulating immune checkpoints; in bones, ECs regulate vascular permeability through the RANKL/OPG dynamic balance, providing a favorable growth environment for TCs. In conclusion, the commonalities of ECs in different metastatic organs provide the foundation for tumor metastasis, while the organ-specificity of ECs endows TCs with “niche selectivity” (Figure 3).
Strategies and recent advances in targeting EC
Targeting TDEs: Molecular Interventions in EC-Mediated PMNs
In recent years, interest in the role of TDEs in promoting the formation of the PMN through their interaction with ECs has increased (Table 1), highlighting the potential of targeting exosomes in the regulation of the PMN [228]. Several drugs that inhibit the release of TDEs to impede cancer progression have been identified. For example, cannabidiol suppresses TDE release, potentially through alterations in mitochondrial function [229]. TDE inhibitors such as chloramidine and bisindolylmaleimide-I have also been shown to increase the efficacy of chemotherapeutic agents [230].
Role of ECs in organ-specific PMN. ECs play pivotal roles in PMN formation across major metastatic organs, including lymph nodes, lungs, liver, brain, and bone, by orchestrating angiogenesis and modulating immunosuppressive microenvironments. While these conserved EC-driven mechanisms underpin PMN development, organ-specific EC adaptations—such as BBB remodeling in the brain or sinusoidal fenestration regulation in the liver—further fine-tune metastatic tropism. Elucidating the shared and distinct mechanisms of EC-mediated PMN regulation provides critical insights into organotropism.
Additionally, Y27632, an inhibitor of ROCK1 and ROCK2, can block proteins involved in cell motility, thereby reducing TDE release [231]. On the other hand, traditional Chinese medicine is also emerging as a potential method to inhibit TDE release [232]. Recently, Jia et al. elucidated the mechanisms by which the Jiedu recipe and oleanolic acid inhibit PMN formation, which is associated with TDE release [233, 234]. Another approach to targeting TDEs involves the inhibition of exosome-related components. For example, TDEs containing BART2-5p promote metastasis by inducing pyroptosis in ECs, and BART2-5p inhibitors can attenuate this effect [93]. In addition to pharmacological inhibition, genetic manipulation is another extensively studied strategy for targeting and inhibiting TDEs. Disruption of genes regulating TDE biogenesis and secretion using RNAi and CRISPR-Cas9 systems has achieved TDE inhibition. For instance, RNA interference screening of 23 components of the endosomal sorting complex required for transport in MHC II-expressing HeLa-CIITA cells revealed that silencing HRS, STAM1, and TSG101 reduced the secretion of CD63 and MHC II associated with exosomes [235]. However, this strategy still faces some adverse effects and challenges. Genetic manipulation may lead to off-target effects, such as unintended gene insertions, deletions, or mutations, thereby causing safety issues [236]. Moreover, the current inhibition strategies have limited specificity, as they block both TDEs and non-TDEs, potentially inducing adverse reactions in tumor treatment. On the other hand, since TDE biogenesis involves multiple signaling pathways, single-target blockade can be easily weakened by compensatory mechanisms [237]. Additionally, the high heterogeneity of exosomes in the bloodstream makes specific identification difficult [228]. Therefore, it is necessary to develop multitarget pharmacological inhibitors. Meanwhile, to minimize side effects caused by non-TDEs, precise release of TDE inhibitors is also required (Figure 4a).
Antiangiogenic therapies: Reducing pathways for tumor metastasis
PMN formation relies on endothelial “bridging” functions; inhibiting angiogenesis disrupts tumor intravasation, circulation, extravasation, and distant colonization while impairing immune cell recruitment for the establishment of an immunosuppressive niche [238]. Anti-angiogenic therapies primarily target the VEGF signaling pathway to suppress tumor angiogenesis. Multiple kinase inhibitors (e.g., sorafenib and sunitinib) block VEGF/PDGF signaling and are widely used to curtail tumor progression by preventing bypass pathway activation [239]. Paradoxically, sunitinib promotes PMN formation in metastatic breast cancer by inducing EC senescence, chemokine secretion, and cell junction loosening [240], necessitating cautious clinical application. Nanoparticle-based strategies enhance antiangiogenic efficacy [241]. Apatinib-loaded nanoparticles inhibit tumor dissemination via VEGF/VEGFR2 blockade [242]. In addition, antiangiogenic immunotherapy, which can increase the sensitivity of tumors to angiogenic therapy, has been a popular research direction in recent years [243]. Preclinical evidence, such as the use of the bispecific antibody to jointly block ANGPT2 and VEGFA, can significantly improve antitumor immunity. Clinically, the combination of PD-1 and VEGF2 inhibitors for the treatment of HCC outperforms monotherapy in clinical trials [244]. Currently, drug resistance remains the main challenge faced by AAT, with complex and diverse resistance mechanisms [245]. For instance, bevacizumab, an anti-VEGFA antibody, has been approved for the treatment of various advanced metastatic cancers, including lung, colorectal, renal, breast, and recurrent glioblastoma. However, many patients treated with VEGF inhibitors, especially when combined with chemotherapy, may initially survive longer but eventually succumb to their disease due to the development of resistance. After inhibition of VEGF, ECs maintain survival through alternative signaling pathways such as Angiopoietin/Tie2, FGF, and Notch [246-251]. In addition, multiple studies have shown that tumors escape therapeutic pressure by activating alternative angiogenic patterns, including intussusceptive angiogenesis, vessel co-option, and vasculogenic mimicry [252-254]. In the future, exploring more effective AAT combination therapy regimens, developing specific biomarkers, and accurately grasping the “timing window” for treatment can significantly improve the clinical application of AAT [255]. For example, the CXCR4 inhibitor AMD3100 can only effectively inhibit angiogenesis by blocking SDF-1-mediated precursor recruitment early after radiotherapy [256, 257]. Alternative approaches, such as vascular promotion and vascular disruption, remain exploratory [258-260] (Figure 4b).
Targeting endothelial GCX: structurally targeted intervention for ECs in the PMN
GCX and adhesion molecules regulate endothelial-tumor/immune cell interactions, modulating adhesion and permeability to inhibit metastasis. Previously, hemodynamic shear stress triggered GCX degradation, facilitating TC homing to ECs [261]. Further studies have indicated that TCs can alter the endothelial GCX to form adhesion sites, thereby enhancing their ability to extravasate into surrounding tissues. This manipulation of the GCX is a crucial step in the metastatic process, suggesting that therapeutic strategies targeting these interactions may be feasible [262]. Okorafor et al. focused on the impact of the physical environment on the extravasation of triple-negative breast cancer, emphasizing that the endothelial GCX acts as a barrier regulating this process. They proposed that understanding the physical mechanisms underlying these interactions could help identify new therapeutic targets to prevent metastasis [263]. VEGF differentially reorganizes heparan sulfate and hyaluronic acid in ECs vs. TCs, creating proadhesive niches [264, 265]. Fu et al. conducted an in-depth analysis of the effects of VEGF on the endothelial GCX in the context of the BBB. Their research indicates that while VEGF reduces GCX coverage on ECs, it increases GCX coverage on malignant breast cancer cells. This differential effect may facilitate the adhesion and migration of TCs across the BBB [264]. Recently, Shi et al. provided a detailed map of the composition and structure of the GCX layer in the aged brain endothelium and revealed the significant impact of its dysregulation on BBB integrity and brain health [266]. GCX degradation by macrophage-derived factors was shown to promote PMN maturation, whereas macrophage depletion preserves GCX integrity [97].
Therapeutic strategies for targeting ECs in PMN. Many therapeutic strategies for PMN have been proposed, with most targeting various cells within the PMN still in the developmental stage. Among these, we summarize four common therapeutic strategies targeting ECs. Parts a and c are preclinical therapies, and parts b and d are clinical therapies.
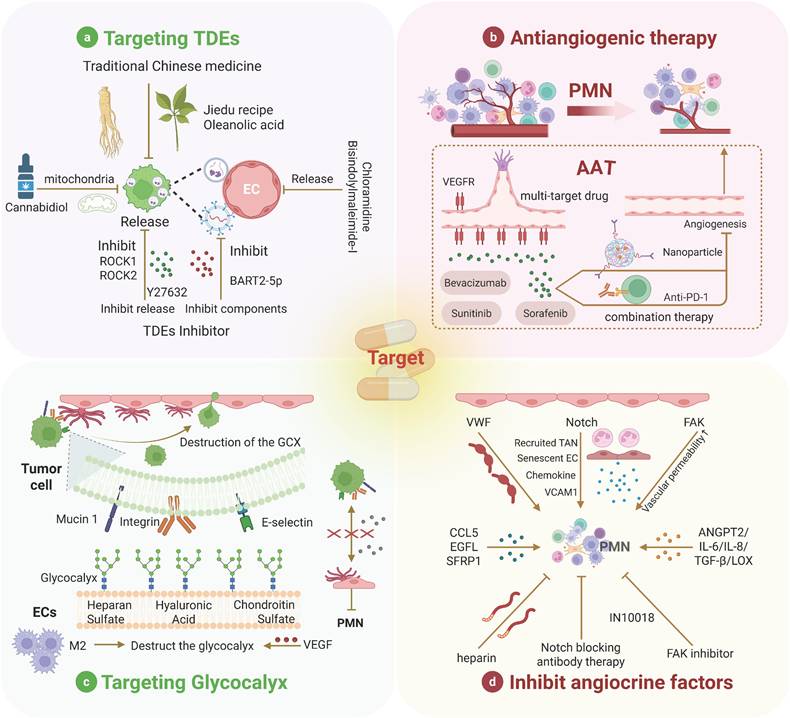
Disrupting hyaluronic acid‒CD44 interactions (key for TC‒EC adhesion) significantly reduces TC extravasation [262, 267, 268]. GCX also modulates TDE uptake/release to drive angiogenesis and metastasis [269]. However, research on endothelial GCX is mostly based on animal models or cultured cells, with more studies remaining in the preclinical stage, which cannot accurately reflect the human condition. In summary, understanding the mechanisms that regulate the interactions between ECs and TCs, as well as their responses to the physical environment and factors such as VEGF, is crucial for developing targeted therapies to inhibit metastatic progression [263] (Figure 4c).
Inhibiting angiocrine signaling: blocking the tumor-promoting effects of EC
The concept of angiocrine signaling has evolved from the traditional understanding of ECs as mere participants in angiogenesis to their more complex role in controlling tumor metastasis [270]. ECs express membrane-bound and secreted factors that influence tumor progression [108]. For example, VWF, an angiocrine factor, has been demonstrated to enhance TC adhesion and transendothelial migration. The use of low-molecular-weight heparin to negatively regulate VWF secretion can inhibit tumor metastasis [271, 272]. The Notch signaling pathway plays a central role in angiocrine regulation during tumor development, with sustained Notch1 activity inducing EC senescence and the expression of chemokines and adhesion molecules such as VCAM1, thereby promoting metastasis [39, 273]. Treatment with Notch1- or VCAM1-blocking antibodies can prevent Notch-driven metastasis. Moreover, FAK in ECs has been identified as a major regulator of chemosensitivity in cancer therapy, with FAK inhibition reducing metastasis following gemcitabine treatment [274, 275]. On the other hand, the role of TGF-β signaling has also been emphasized in advanced tumors. It has been observed that endogenous TGF-β signaling can promote TCs to evade inhibitory effects, which suggests that blocking this pathway may enhance the efficacy of anti-tumor therapies [276]. This is in line with the finding that some signaling pathways have dual roles, and inhibiting these pathways can reduce tumor growth and alter immune responses. For instance, in colorectal cancer, inhibiting specific molecules such as ADAM17 and soluble JAGGED-1 is associated with the disruption of angiocrine signaling, further supporting the view that targeting these pathways can mitigate tumor-promoting effects [277, 278]. EGFL7, which is associated with the ECM, is linked to primary tumor growth, angiogenesis, tumor metastasis, and drug resistance, highlighting the multifaceted role of angiocrine factors in cancer progression [279]. Recently, Sfrp1 derived from TECs was shown to support cancer stem cell maintenance through WNT signaling, further emphasizing the complex interplay between TECs and TCs [280]. While combinatorial targeting of angiocrine factors with established therapies has demonstrated clinical promise, the precise mechanisms underlying these agents' vascular remodeling effects necessitate further in vivo validation. Additionally, the spatiotemporal heterogeneity of angiocrine signaling within solid tumors and tumor-type specificity of individual angiocrine factors remain formidable challenges requiring multi-omics characterization [270]. Overall, angiocrine factors are involved in various aspects of cancer progression, including proliferation, stemness, EMT, invasion, and immune suppression, making them promising platforms for developing effective therapeutic strategies (Figure 4d).
Conclusion and Future Perspective
The PMN hypothesis is an emerging concept concerning tumor metastasis, primarily involving changes in vascular permeability, activation of stromal cells, remodeling of the ECM, and recruitment of immune cells. Its importance in cancer metastasis is increasingly recognized. In this study, we thoroughly discuss the complex relationship between the PMN and ECs, highlighting the crucial role of ECs in tumor metastasis and PMN formation. Additionally, we further synthesized current strategies for targeting ECs within the PMN, ranging from exosome inhibition to GCX modulation and angiocrine signaling blockade.
However, research gaps persist. While myeloid cells (e.g., macrophages and neutrophils) dominate PMN studies, EC-centric investigations remain underrepresented. Nevertheless, the role of ECs in tumor metastasis should not be overlooked, as their interactions with tumor and immune cells are crucial for understanding the mechanisms underlying tumor metastasis. Additionally, many therapeutic approaches, although promising in preclinical models, face translational challenges, particularly in clinical validation.
Future efforts should prioritize combinatorial therapies integrating EC-targeted interventions with immune modulation or chemotherapy to enhance efficacy. For example, combining AAT with immune checkpoint inhibitors can induce tumor vessel normalization, improve immune cell infiltration and function, and thus achieve a synergistic antitumor effect. Evaluating whether the combination therapy is synergistic or additive, as well as shifting the focus of antiangiogenic drugs from VEGF/R to other candidates (e.g., FGF/R), can help further optimize antiangiogenic immunotherapy. Moreover, using cutting-edge technologies such as single-cell transcriptomics and spatial transcriptomics can provide in-depth insights into the interactions between endothelial and immune cells, revealing their dynamic changes and functional differences in the PMN. These technologies can also help us revolutionize our understanding of PMN cellular heterogeneity. For example, macrophages and neutrophils exhibit diverse functional subsets within the PMN, the complexity of which is now resolvable at single-cell resolution. Leveraging these tools will clarify EC communication networks with PMN components (e.g., immune cells and the ECM) and unveil novel drivers of metastasis.
Abbreviations
PMN: Premetastatic niche; EC: Endothelial cell; TC: Tumor cell; CTCs: Circulating tumor cells; EV: Extracellular vesicle; TDE: Tumor-derived exosome; ECM: Extracellular matrix; MDSC: Myeloid-derived suppressor cell; TDSF: Tumor-derived secretory factor; TME: Tumor microenvironment; NET: Neutrophil extracellular trap; TAN: Tumor-associated neutrophil; EMT: Epithelial-mesenchymal transition; FAK: Focal adhesion kinase; TAM: Tumor-associated macrophage; Treg: Regulatory T cell; HIF: Hypoxia inducible factor; TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand; BBB: Blood-brain barrier; GCX: Glycocalyx; LSEC: Sinusoidal endothelial cell; AAT: antiangiogenic therapy.
Acknowledgements
Funding
This work was supported by grants from Henan Medical Science and Technology Research Plan (No. LHG120230294, to S.Y.), the China Postdoctoral Science Foundation (No. 2023M743201, to S.Y.), the National Fund Postdoctoral Researcher Program (No. GZB20230671, to S.Y.). We thank Biorender (https://www.biorender.com/) for its ability to improve our figures.
Author Contributions
Y.F., W.C., and Y.Y. conceived and designed the study, with conceptual oversight from corresponding authors S.Y., Z.L., and W.Y. X.Z., M.T., Z.X., and Y.G. performed data collection, analysis, and drafted the initial manuscript. All authors critically revised the manuscript for intellectual content, with final critical evaluation and approval led by S.Y., Z.L., and W.Y. All authors reviewed and endorsed the final submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298-306
2. Gu Y, Liu Y, Fu L, Zhai L, Zhu J, Han Y. et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nature medicine. 2019;25:312-22
3. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer metastasis reviews. 1989;8:98-101
4. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820-7
5. Liu Y, Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer cell. 2016;30:668-81
6. Oria VO, Erler JT. Tumor Angiocrine Signaling: Novel Targeting Opportunity in Cancer. Cells. 2023 12
7. Hu Y, Zang W, Feng Y, Mao Q, Chen J, Zhu Y. et al. mir-605-3p prevents liver premetastatic niche formation by inhibiting angiogenesis via decreasing exosomal nos3 release in gastric cancer. Cancer cell international. 2024;24:184
8. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nature communications. 2020;11:5120
9. Roudnicky F, Kim BK, Lan Y, Schmucki R, Küppers V, Christensen K. et al. Identification of a combination of transcription factors that synergistically increases endothelial cell barrier resistance. Scientific reports. 2020;10:3886
10. Korneva YS, Ukrainets RV. Principles of premetastatic niche formation. Journal of Modern Oncology. 2020;21:6-9
11. Masuda T, Noda M, Kogawa T, Kitagawa D, Hayashi N, Jomori T. et al. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer science. 2020;111:924-31
12. Wang Z, Liu J, Huang H, Ye M, Li X, Wu R. et al. Metastasis-associated fibroblasts: an emerging target for metastatic cancer. Biomarker research. 2021;9:47
13. Mo Y, Leung LL, Mak CSL, Wang X, Chan WS, Hui LMN. et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Molecular cancer. 2023;22:4
14. Murgai M, Ju W, Eason M, Kline J, Beury DW, Kaczanowska S. et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nature medicine. 2017;23:1176-90
15. Riera-Domingo C, Leite-Gomes E, Charatsidou I, Zhao P, Carrá G, Cappellesso F. et al. Breast tumors interfere with endothelial TRAIL at the premetastatic niche to promote cancer cell seeding. Science advances. 2023;9:eadd5028
16. Kim J, Lee C, Kim I, Ro J, Kim J, Min Y. et al. Three-Dimensional Human Liver-Chip Emulating Premetastatic Niche Formation by Breast Cancer-Derived Extracellular Vesicles. ACS nano. 2020;14:14971-88
17. Tesi RJ. MDSC; the Most Important Cell You Have Never Heard Of. Trends in pharmacological sciences. 2019;40:4-7
18. Wang Y, Ding Y, Guo N, Wang S. MDSCs: Key Criminals of Tumor Pre-metastatic Niche Formation. Frontiers in immunology. 2019;10:172
19. Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer immunology research. 2017;5:3-8
20. Theivanthiran B, Yarla N, Haykal T, Nguyen YV, Cao L, Ferreira M. et al. Tumor-intrinsic NLRP3-HSP70-TLR4 axis drives premetastatic niche development and hyperprogression during anti-PD-1 immunotherapy. Science translational medicine. 2022;14:eabq7019
21. Shi H, Zhang J, Han X, Li H, Xie M, Sun Y. et al. Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1β-mediated increase in E-selectin expression. International journal of cancer. 2017;140:1370-83
22. Krüger A. Premetastatic niche formation in the liver: emerging mechanisms and mouse models. Journal of molecular medicine (Berlin, Germany). 2015;93:1193-201
23. Zheng Y, Wang N, Wang S, Zhang J, Yang B, Wang Z. Chronic psychological stress promotes breast cancer pre-metastatic niche formation by mobilizing splenic MDSCs via TAM/CXCL1 signaling. Journal of experimental & clinical cancer research: CR. 2023;42:129
24. Sprouse ML, Welte T, Boral D, Liu HN, Yin W, Vishnoi M. et al. PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling. International journal of molecular sciences. 2019 20
25. Chen E, Yu J. The role and metabolic adaptations of neutrophils in premetastatic niches. Biomarker research. 2023;11:50
26. Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X. et al. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer cell. 2016;30:243-56
27. Qi M, Xia Y, Wu Y, Zhang Z, Wang X, Lu L. et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nature communications. 2022;13:897
28. Tyagi A, Sharma S, Wu K, Wu SY, Xing F, Liu Y. et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nature communications. 2021;12:474
29. Xing X, Bai Y, Song J. The Heterogeneity of Neutrophil Recruitment in the Tumor Microenvironment and the Formation of Premetastatic Niches. Journal of immunology research. 2021;2021:6687474
30. Jia J, Wang Y, Li M, Wang F, Peng Y, Hu J. et al. Neutrophils in the premetastatic niche: key functions and therapeutic directions. Molecular cancer. 2024;23:200
31. Xie SZ, Yang LY, Wei R, Shen XT, Pan JJ, Yu SZ. et al. Targeting SPP1-orchestrated neutrophil extracellular traps-dominant pre-metastatic niche reduced HCC lung metastasis. Experimental hematology & oncology. 2024;13:111
32. Hu C, Long L, Lou J, Leng M, Yang Q, Xu X. et al. CTC-neutrophil interaction: A key driver and therapeutic target of cancer metastasis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2024;180:117474
33. Zeng W, Wang Y, Zhang Q, Hu C, Li J, Feng J. et al. Neutrophil Nanodecoys Inhibit Tumor Metastasis by Blocking the Interaction between Tumor Cells and Neutrophils. ACS nano. 2024;18:7363-78
34. De Meo ML, Spicer JD. The role of neutrophil extracellular traps in cancer progression and metastasis. Seminars in immunology. 2021;57:101595
35. Zhang G, Li M, Zhou D, Yang X, Zhang W, Gao R. Loss of endothelial EMCN drives tumor lung metastasis through the premetastatic niche. Journal of translational medicine. 2022;20:446
36. Huang YC, Chang CY, Wu YY, Wu KL, Tsai YM, Lee HC. et al. Single-Cell Transcriptomic Profiles of Lung Pre-Metastatic Niche Reveal Neutrophil and Lymphatic Endothelial Cell Roles in Breast Cancer. Cancers. 2022 15
37. Mittal M, Nepal S, Tsukasaki Y, Hecquet CM, Soni D, Rehman J. et al. Neutrophil Activation of Endothelial Cell-Expressed TRPM2 Mediates Transendothelial Neutrophil Migration and Vascular Injury. Circulation research. 2017;121:1081-91
38. Guo P, Rafii S. Dangerous Liaisons: Deviant Endothelium NOTCHes toward Tumor Metastasis. Cancer cell. 2017;31:301-3
39. Wieland E, Rodriguez-Vita J, Liebler SS, Mogler C, Moll I, Herberich SE. et al. Endothelial Notch1 Activity Facilitates Metastasis. Cancer cell. 2017;31:355-67
40. Yan L, Wang J, Cai X, Liou YC, Shen HM, Hao J. et al. Macrophage plasticity: signaling pathways, tissue repair, and regeneration. MedComm. 2024;5:e658
41. Wang H, Yung MMH, Ngan HYS, Chan KKL, Chan DW. The Impact of the Tumor Microenvironment on Macrophage Polarization in Cancer Metastatic Progression. International journal of molecular sciences. 2021 22
42. Bied M, Ho WW, Ginhoux F, Blériot C. Roles of macrophages in tumor development: a spatiotemporal perspective. Cellular & molecular immunology. 2023;20:983-92
43. Gharavi AT, Hanjani NA, Movahed E, Doroudian M. The role of macrophage subtypes and exosomes in immunomodulation. Cellular & molecular biology letters. 2022;27:83
44. Zhang Q, Sioud M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. International journal of molecular sciences. 2023 24
45. Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y. et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. Journal of hematology & oncology. 2020;13:156
46. Zhang G, Gao Z, Guo X, Ma R, Wang X, Zhou P. et al. CAP2 promotes gastric cancer metastasis by mediating the interaction between tumor cells and tumor-associated macrophages. The Journal of clinical investigation. 2023 133
47. Lu F, Ye M, Shen Y, Xu Y, Hu C, Chen J. et al. Hypoxic tumor-derived exosomal miR-4488 induces macrophage M2 polarization to promote liver metastasis of pancreatic neuroendocrine neoplasm through RTN3/FABP5 mediated fatty acid oxidation. International journal of biological sciences. 2024;20:3201-18
48. Wang Y, Li Y, Zhong J, Li M, Zhou Y, Lin Q. et al. Tumor-derived Cav-1 promotes pre-metastatic niche formation and lung metastasis in breast cancer. Theranostics. 2023;13:1684-97
49. Matusiak M, Hickey JW, van IDGP, Lu G, Kidziński L, Zhu S. et al. Spatially Segregated Macrophage Populations Predict Distinct Outcomes in Colon Cancer. Cancer discovery. 2024;14:1418-39
50. Wu K, Zhang G, Shen C, Zhu L, Yu C, Sartorius K. et al. Role of T cells in liver metastasis. Cell death & disease. 2024;15:341
51. Bai J, Ding B, Li H. Targeting TNFR2 in Cancer: All Roads Lead to Rome. Frontiers in immunology. 2022;13:844931
52. Wei Q, Ye Z, Zhong X, Li L, Wang C, Myers RE. et al. Multiregion whole-exome sequencing of matched primary and metastatic tumors revealed genomic heterogeneity and suggested polyclonal seeding in colorectal cancer metastasis. Annals of oncology: official journal of the European Society for Medical Oncology. 2017;28:2135-41
53. Jiang Y, Gao S, Sun H, Wu X, Gu J, Wu H. et al. Targeting NEDD8 suppresses surgical stress-facilitated metastasis of colon cancer via restraining regulatory T cells. Cell death & disease. 2024;15:8
54. DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N. et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer cell. 2009;16:91-102
55. Zheng Z, Li YN, Jia S, Zhu M, Cao L, Tao M. et al. Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nature communications. 2021;12:6202
56. Sun H, Meng Q, Shi C, Yang H, Li X, Wu S. et al. Hypoxia-Inducible Exosomes Facilitate Liver-Tropic Premetastatic Niche in Colorectal Cancer. Hepatology (Baltimore, Md). 2021;74:2633-51
57. Alberts E, Wall I, Calado DP, Grigoriadis A. Immune Crosstalk Between Lymph Nodes and Breast Carcinomas, With a Focus on B Cells. Frontiers in molecular biosciences. 2021;8:673051
58. Li YL, Chen CH, Chen JY, Lai YS, Wang SC, Jiang SS. et al. Single-cell analysis reveals immune modulation and metabolic switch in tumor-draining lymph nodes. Oncoimmunology. 2020;9:1830513
59. Monteiro AC, de Andrade Garcia D, Du Rocher B, Fontão A, Nogueira LP, Fidalgo G. et al. Cooperation between T and B cells reinforce the establishment of bone metastases in a mouse model of breast cancer. Bone. 2024;178:116932
60. Lin Q, Ren L, Jian M, Xu P, Li J, Zheng P. et al. The mechanism of the premetastatic niche facilitating colorectal cancer liver metastasis generated from myeloid-derived suppressor cells induced by the S1PR1-STAT3 signaling pathway. Cell death & disease. 2019;10:693
61. Pozzi S, Satchi-Fainaro R. The role of CCL2/CCR2 axis in cancer and inflammation: The next frontier in nanomedicine. Advanced drug delivery reviews. 2024;209:115318
62. Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697-710
63. Xie L, Qiu S, Lu C, Gu C, Wang J, Lv J. et al. Gastric cancer-derived LBP promotes liver metastasis by driving intrahepatic fibrotic pre-metastatic niche formation. Journal of experimental & clinical cancer research: CR. 2023;42:258
64. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer cell. 2016;30:836-48
65. Zhang C, Qin C, Dewanjee S, Bhattacharya H, Chakraborty P, Jha NK. et al. Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance. Molecular cancer. 2024;23:18
66. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature reviews Molecular cell biology. 2018;19:213-28
67. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M. et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-35
68. Liu Y, Mao D, Wang H, Che X, Chen Y. Formation of pre-metastatic niches induced by tumor extracellular vesicles in lung metastasis. Pharmacological research. 2023;188:106669
69. Dos Anjos Pultz B, Andrés Cordero da Luz F, Socorro Faria S, Peixoto Ferreira de Souza L, Cristina Brígido Tavares P, Alonso Goulart V. et al. The multifaceted role of extracellular vesicles in metastasis: Priming the soil for seeding. International journal of cancer. 2017;140:2397-407
70. Tang D, Liu S, Shen H, Deng G, Zeng S. Extracellular Vesicles Promote the Formation of Pre-Metastasis Niche in Gastric Cancer. Frontiers in immunology. 2022;13:813015
71. Ghoroghi S, Mary B, Asokan N, Goetz JG, Hyenne V. Tumor extracellular vesicles drive metastasis (it's a long way from home). FASEB bioAdvances. 2021;3:930-43
72. Giusti I, Poppa G, Di Fazio G, D'Ascenzo S, Dolo V. Metastatic Dissemination: Role of Tumor-Derived Extracellular Vesicles and Their Use as Clinical Biomarkers. International journal of molecular sciences. 2023 24
73. Hu Y, Wang C, Li Y, Zhao J, Chen C, Zhou Y. et al. MiR-21 controls in situ expansion of CCR6⁺ regulatory T cells through PTEN/AKT pathway in breast cancer. Immunology and cell biology. 2015;93:753-64
74. Zhang H, Bai M, Deng T, Liu R, Wang X, Qu Y. et al. Cell-derived microvesicles mediate the delivery of miR-29a/c to suppress angiogenesis in gastric carcinoma. Cancer letters. 2016;375:331-9
75. Zhou J, Song Q, Li H, Han Y, Pu Y, Li L. et al. Targeting circ-0034880-enriched tumor extracellular vesicles to impede SPP1(high)CD206(+) pro-tumor macrophages mediated pre-metastatic niche formation in colorectal cancer liver metastasis. Molecular cancer. 2024;23:168
76. Papiewska-Pająk I, Krzyżanowski D, Katela M, Rivet R, Michlewska S, Przygodzka P. et al. Glypican-1 Level Is Elevated in Extracellular Vesicles Released from MC38 Colon Adenocarcinoma Cells Overexpressing Snail. Cells. 2020 9
77. Blavier L, Nakata R, Neviani P, Sharma K, Shimada H, Benedicto A. et al. The capture of extracellular vesicles endogenously released by xenotransplanted tumours induces an inflammatory reaction in the premetastatic niche. Journal of extracellular vesicles. 2023;12:e12326
78. Wang Y, Jia J, Wang F, Fang Y, Yang Y, Zhou Q. et al. Pre-metastatic niche: formation, characteristics and therapeutic implication. Signal transduction and targeted therapy. 2024;9:236
79. Wang Z, Yang C, Li L, Jin X, Zhang Z, Zheng H. et al. Tumor-derived HMGB1 induces CD62L(dim) neutrophil polarization and promotes lung metastasis in triple-negative breast cancer. Oncogenesis. 2020;9:82
80. Saini M, Szczerba BM, Aceto N. Circulating Tumor Cell-Neutrophil Tango along the Metastatic Process. Cancer research. 2019;79:6067-73
81. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J. et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553-7
82. Ward MP, L EK, L AN, Mohamed BM, Kelly T, Bates M. et al. Platelets, immune cells and the coagulation cascade; friend or foe of the circulating tumour cell? Molecular cancer. 2021;20:59
83. Messina B, Lo Sardo F, Scalera S, Memeo L, Colarossi C, Mare M. et al. Hippo pathway dysregulation in gastric cancer: from Helicobacter pylori infection to tumor promotion and progression. Cell death & disease. 2023;14:21
84. Hu Y, Yang C, Yang S, Cheng F, Rao J, Wang X. MiR-665 promotes hepatocellular carcinoma cell migration, invasion, and proliferation by decreasing Hippo signaling through targeting PTPRB. Cell death & disease. 2023;14:95
85. Yu Y, Min Z, Zhou Z, Linhong M, Tao R, Yan L. et al. Hypoxia-induced exosomes promote hepatocellular carcinoma proliferation and metastasis via miR-1273f transfer. Experimental cell research. 2019;385:111649
86. Liu Z, Chen J, Ren Y, Liu S, Ba Y, Zuo A. et al. Multi-stage mechanisms of tumor metastasis and therapeutic strategies. Signal transduction and targeted therapy. 2024;9:270
87. Yu T, Tang Q, Chen X, Fan W, Zhou Z, Huang W. et al. TGF-β1 and IL-17A comediate the protumor phenotype of neutrophils to regulate the epithelial-mesenchymal transition in oral squamous cell carcinoma. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2021;50:353-61
88. Rokavec M, Öner MG, Li H, Jackstadt R, Jiang L, Lodygin D. et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. The Journal of clinical investigation. 2014;124:1853-67
89. Wu M, Ma M, Tan Z, Zheng H, Liu X. Neutrophil: A New Player in Metastatic Cancers. Frontiers in immunology. 2020;11:565165
90. Zhuang Y, Peng H, Mastej V, Chen W. MicroRNA Regulation of Endothelial Junction Proteins and Clinical Consequence. Mediators of inflammation. 2016;2016:5078627
91. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302-17
92. Dou R, Liu K, Yang C, Zheng J, Shi D, Lin X. et al. EMT-cancer cells-derived exosomal miR-27b-3p promotes circulating tumour cells-mediated metastasis by modulating vascular permeability in colorectal cancer. Clinical and translational medicine. 2021;11:e595
93. Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J. et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nature communications. 2018;9:5395
94. Li K, Xue W, Lu Z, Wang S, Zheng J, Lu K. et al. Tumor-derived exosomal ADAM17 promotes pre-metastatic niche formation by enhancing vascular permeability in colorectal cancer. Journal of experimental & clinical cancer research: CR. 2024;43:59
95. Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C. et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765-70
96. Tomita T, Kato M, Hiratsuka S. Regulation of vascular permeability in cancer metastasis. Cancer science. 2021;112:2966-74
97. Qu R, Du W, Li S, Li W, Wei G, Chen Z. et al. Destruction of vascular endothelial glycocalyx during formation of pre-metastatic niches. Heliyon. 2024;10:e29101
98. Jannaway M, Yang X, Meegan JE, Coleman DC, Yuan SY. Thrombin-cleaved syndecan-3/-4 ectodomain fragments mediate endothelial barrier dysfunction. PloS one. 2019;14:e0214737
99. Rohlenova K, Veys K, Miranda-Santos I, De Bock K, Carmeliet P. Endothelial Cell Metabolism in Health and Disease. Trends in cell biology. 2018;28:224-36
100. Zeng H, Hou Y, Zhou X, Lang L, Luo H, Sun Y. et al. Cancer-associated fibroblasts facilitate premetastatic niche formation through lncRNA SNHG5-mediated angiogenesis and vascular permeability in breast cancer. Theranostics. 2022;12:7351-70
101. Yang Y, Guo Z, Chen W, Wang X, Cao M, Han X. et al. M2 Macrophage-Derived Exosomes Promote Angiogenesis and Growth of Pancreatic Ductal Adenocarcinoma by Targeting E2F2. Molecular therapy: the journal of the American Society of Gene Therapy. 2021;29:1226-38
102. Lu Y, Han G, Zhang Y, Zhang L, Li Z, Wang Q. et al. M2 macrophage-secreted exosomes promote metastasis and increase vascular permeability in hepatocellular carcinoma. Cell communication and signaling: CCS. 2023;21:299
103. Dror S, Lucotti S, Asao T, Li J, Wortzel I, Berger LS. et al. Tumour-derived Extracellular Vesicle and Particle Reprogramming of Interstitial Macrophages in the Lung Pre-Metastatic Niche Enhances Vascular Permeability and Metastatic Potential. Research square. 2024
104. Wen ZF, Liu H, Gao R, Zhou M, Ma J, Zhang Y. et al. Tumor cell-released autophagosomes (TRAPs) promote immunosuppression through induction of M2-like macrophages with increased expression of PD-L1. Journal for immunotherapy of cancer. 2018;6:151
105. Wang XR, Zhou XH, Sun XT, Shen YQ, Wu YY, Wu CD. et al. Tumour cell-released autophagosomes promote lung metastasis by upregulating PD-L1 expression in pulmonary vascular endothelial cells in breast cancer. Cellular oncology (Dordrecht, Netherlands). 2024
106. Suman S, Nevala WK, Leontovich AA, Ward C, Jakub JW, Kim Y. et al. Melanoma-Derived Extracellular Vesicles Induce CD36-Mediated Pre-Metastatic Niche. Biomolecules. 2024 14
107. Blanchard L, Girard JP. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis. 2021;24:719-53
108. Alsina-Sanchis E, Mülfarth R, Fischer A. Control of Tumor Progression by Angiocrine Factors. Cancers. 2021 13
109. Woodward J. Crossing the endothelium: E-selectin regulates tumor cell migration under flow conditions. Cell adhesion & migration. 2008;2:151-2
110. Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG. et al. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3725-30
111. Engleitner S, Milovanovic D, Kirisits K, Brenner S, Hong J, Ropek N. et al. Feed-back loops integrating RELA, SOX18 and FAK mediate the break-down of the lymph-endothelial barrier that is triggered by 12(S)-HETE. International journal of oncology. 2020;56:1034-44
112. Carrascal MA, Silva M, Ferreira JA, Azevedo R, Ferreira D, Silva AMN. et al. A functional glycoproteomics approach identifies CD13 as a novel E-selectin ligand in breast cancer. Biochimica et biophysica acta General subjects. 2018;1862:2069-80
113. Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok JL. Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer research. 2004;64:5261-9
114. Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H. et al. The perivascular niche regulates breast tumour dormancy. Nature cell biology. 2013;15:807-17
115. Klemke M, Weschenfelder T, Konstandin MH, Samstag Y. High affinity interaction of integrin alpha4beta1 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances migration of human melanoma cells across activated endothelial cell layers. Journal of cellular physiology. 2007;212:368-74
116. Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J. et al. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26:943-50
117. Shao Y, Saredy J, Yang WY, Sun Y, Lu Y, Saaoud F. et al. Vascular Endothelial Cells and Innate Immunity. Arteriosclerosis, thrombosis, and vascular biology. 2020;40:e138-e52
118. Jing H, Wu X, Xiang M, Wang C, Novakovic VA, Shi J. Microparticle Phosphatidylserine Mediates Coagulation: Involvement in Tumor Progression and Metastasis. Cancers. 2023 15
119. Esposito M, Mondal N, Greco TM, Wei Y, Spadazzi C, Lin SC. et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nature cell biology. 2019;21:627-39
120. Zhao R, Bei X, Yang B, Wang X, Jiang C, Shi F. et al. Endothelial cells promote metastasis of prostate cancer by enhancing autophagy. Journal of experimental & clinical cancer research: CR. 2018;37:221
121. Maishi N, Ohba Y, Akiyama K, Ohga N, Hamada J, Nagao-Kitamoto H. et al. Tumour endothelial cells in high metastatic tumours promote metastasis via epigenetic dysregulation of biglycan. Scientific reports. 2016;6:28039
122. Jiang Z, Zhou J, Li L, Liao S, He J, Zhou S. et al. Pericytes in the tumor microenvironment. Cancer letters. 2023;556:216074
123. Roblek M, Protsyuk D, Becker PF, Stefanescu C, Gorzelanny C, Glaus Garzon JF. et al. CCL2 Is a Vascular Permeability Factor Inducing CCR2-Dependent Endothelial Retraction during Lung Metastasis. Molecular cancer research: MCR. 2019;17:783-93
124. Chen X, Li Q, Fu X, Li J, Deng J, Zhang Q. et al. Tumor-derived EBV-miR-BART2-5p promotes nasopharyngeal carcinoma metastasis by inducing pre-metastatic endothelial cell pyroptosis. Molecular cancer research: MCR. 2024
125. Mu W, Gu P, Li H, Zhou J, Jian Y, Jia W. et al. Exposure of benzo[a]pyrene induces HCC exosome-circular RNA to activate lung fibroblasts and trigger organotropic metastasis. Cancer communications (London, England). 2024;44:718-38
126. Mao Y, Wang J, Wang Y, Fu Z, Dong L, Liu J. Hypoxia induced exosomal Circ-ZNF609 promotes pre-metastatic niche formation and cancer progression via miR-150-5p/VEGFA and HuR/ZO-1 axes in esophageal squamous cell carcinoma. Cell death discovery. 2024;10:133
127. Shan Y, Fan H, Chai L, Kong X, Xiao H, You M. et al. Tumor-derived exosomal miR-103a-3p promotes vascular permeability and proliferation by targeting ZO-1 and ACOX-1 in nasopharyngeal carcinoma. Translational cancer research. 2024;13:4896-912
128. Yao Y, Qian R, Gao H, Dai Y, Shi Y, An P. et al. LSD1 deficiency in breast cancer cells promotes the formation of pre-metastatic niches. NPJ precision oncology. 2024;8:260
129. Jin J, Cui Y, Niu H, Lin Y, Wu X, Qi X. et al. NSCLC Extracellular Vesicles Containing miR-374a-5p Promote Leptomeningeal Metastasis by Influencing Blood-Brain Barrier Permeability. Molecular cancer research: MCR. 2024;22:699-710
130. Xu J, Wang H, Shi B, Li N, Xu G, Yan X. et al. Exosomal MFI2-AS1 sponge miR-107 promotes non-small cell lung cancer progression through NFAT5. Cancer cell international. 2023;23:51
131. Xie L, Zhang K, You B, Yin H, Zhang P, Shan Y. et al. Hypoxic nasopharyngeal carcinoma-derived exosomal miR-455 increases vascular permeability by targeting ZO-1 to promote metastasis. Molecular carcinogenesis. 2023;62:803-19
132. Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J. et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. Journal of experimental & clinical cancer research: CR. 2022;41:296
133. Ma Z, Wei K, Yang F, Guo Z, Pan C, He Y. et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell death & disease. 2021;12:840
134. Yokota Y, Noda T, Okumura Y, Kobayashi S, Iwagami Y, Yamada D. et al. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer science. 2021;112:1275-88
135. Kim DH, Park H, Choi YJ, Kang MH, Kim TK, Pack CG. et al. Exosomal miR-1260b derived from non-small cell lung cancer promotes tumor metastasis through the inhibition of HIPK2. Cell death & disease. 2021;12:747
136. Kim DH, Park S, Kim H, Choi YJ, Kim SY, Sung KJ. et al. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1.4. Cancer letters. 2020;475:2-13
137. Fang JH, Zhang ZJ, Shang LR, Luo YW, Lin YF, Yuan Y. et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology (Baltimore, Md). 2018;68:1459-75
138. Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH. et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929-42
139. Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR. et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer cell. 2014;25:501-15
140. Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748-57
141. Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC. et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer research. 2011;71:5346-56
142. Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481:190-4
143. Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D. et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nature cell biology. 2010;12:247-56
144. Xu Y, Yao Y, Yu L, Zhang X, Mao X, Tey SK. et al. Clathrin light chain A-enriched small extracellular vesicles remodel microvascular niche to induce hepatocellular carcinoma metastasis. Journal of extracellular vesicles. 2023;12:e12359
145. Wong SWK, Tey SK, Mao X, Fung HL, Xiao ZJ, Wong DKH. et al. Small Extracellular Vesicle-Derived vWF Induces a Positive Feedback Loop between Tumor and Endothelial Cells to Promote Angiogenesis and Metastasis in Hepatocellular Carcinoma. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2023;10:e2302677
146. Mu W, Provaznik J, Hackert T, Zöller M. Tspan8-Tumor Extracellular Vesicle-Induced Endothelial Cell and Fibroblast Remodeling Relies on the Target Cell-Selective Response. Cells. 2020 9
147. Duan S, Nordmeier S, Byrnes AE, Buxton ILO. Extracellular Vesicle-Mediated Purinergic Signaling Contributes to Host Microenvironment Plasticity and Metastasis in Triple Negative Breast Cancer. International journal of molecular sciences. 2021 22
148. Mao X, Tey SK, Yeung CLS, Kwong EML, Fung YME, Chung CYS. et al. Nidogen 1-Enriched Extracellular Vesicles Facilitate Extrahepatic Metastasis of Liver Cancer by Activating Pulmonary Fibroblasts to Secrete Tumor Necrosis Factor Receptor 1. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2020;7:2002157
149. Biagioni A, Laurenzana A, Menicacci B, Peppicelli S, Andreucci E, Bianchini F. et al. uPAR-expressing melanoma exosomes promote angiogenesis by VE-Cadherin, EGFR and uPAR overexpression and rise of ERK1,2 signaling in endothelial cells. Cellular and molecular life sciences: CMLS. 2021;78:3057-72
150. Yoshida K, Tsuda M, Matsumoto R, Semba S, Wang L, Sugino H. et al. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer science. 2019;110:2119-32
151. Che SPY, Park JY, Stokol T. Tissue Factor-Expressing Tumor-Derived Extracellular Vesicles Activate Quiescent Endothelial Cells via Protease-Activated Receptor-1. Frontiers in oncology. 2017;7:261
152. Chen Y, Lei Y, Li J, Wang X, Li G. Macrophage-derived exosomal microRNAs promote metastasis in pancreatic ductal adenocarcinoma. International immunopharmacology. 2024;129:111590
153. Chen K, Wang Q, Liu X, Wang F, Yang Y, Tian X. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. International journal of biological sciences. 2022;18:1220-37
154. Jiang W, Shi X, Sun L, Zhang Y, Kong X, Yang X. et al. Exosomal miR-30a-5p promoted intrahepatic cholangiocarcinoma progression by increasing angiogenesis and vascular permeability in PDCD10 dependent manner. International journal of biological sciences. 2023;19:4571-87
155. Yang S, Sun B, Li J, Li N, Zhang A, Zhang X. et al. Neutrophil extracellular traps promote angiogenesis in gastric cancer. Cell communication and signaling: CCS. 2023;21:176
156. De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457-74
157. Jiang K, Chen H, Fang Y, Chen L, Zhong C, Bu T. et al. Exosomal ANGPTL1 attenuates colorectal cancer liver metastasis by regulating Kupffer cell secretion pattern and impeding MMP9 induced vascular leakiness. Journal of experimental & clinical cancer research: CR. 2021;40:21
158. Kuhlencordt PJ, Rosel E, Gerszten RE, Morales-Ruiz M, Dombkowski D, Atkinson WJ. et al. Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. American journal of physiology Cell physiology. 2004;286:C1195-202
159. Wang Q, He Z, Huang M, Liu T, Wang Y, Xu H. et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nature communications. 2018;9:559
160. Lu Y, Liu Y, Zuo X, Li G, Wang J, Liu J. et al. CXCL12(+) Tumor-associated Endothelial Cells Promote Immune Resistance in Hepatocellular Carcinoma. Journal of hepatology. 2024
161. Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. The Journal of experimental medicine. 2015;212:139-48
162. Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Experimental & molecular medicine. 2020;52:1475-85
163. Kim HJ, Ji YR, Lee YM. Crosstalk between angiogenesis and immune regulation in the tumor microenvironment. Archives of pharmacal research. 2022;45:401-16
164. Park HR, Shiva A, Cummings P, Kim S, Kim S, Lee E. et al. Angiopoietin-2-Dependent Spatial Vascular Destabilization Promotes T-cell Exclusion and Limits Immunotherapy in Melanoma. Cancer research. 2023;83:1968-83
165. Siddhartha R, Garg M. Interplay Between Extracellular Matrix Remodeling and Angiogenesis in Tumor Ecosystem. Molecular cancer therapeutics. 2023;22:291-305
166. Wang YH, Dong YY, Wang WM, Xie XY, Wang ZM, Chen RX. et al. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-κB pathways induced by paracrine cytokines. Journal of experimental & clinical cancer research: CR. 2013;32:51
167. Huang QB, Ma X, Zhang X, Liu SW, Ai Q, Shi TP. et al. Down-Regulated miR-30a in Clear Cell Renal Cell Carcinoma Correlated with Tumor Hematogenous Metastasis by Targeting Angiogenesis-Specific DLL4. PloS one. 2013;8:e67294
168. Hermant B, Bibert S, Concord E, Dublet B, Weidenhaupt M, Vernet T. et al. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. The Journal of biological chemistry. 2003;278:14002-12
169. Wragg JW, Finnity JP, Anderson JA, Ferguson HJ, Porfiri E, Bhatt RI. et al. MCAM and LAMA4 Are Highly Enriched in Tumor Blood Vessels of Renal Cell Carcinoma and Predict Patient Outcome. Cancer research. 2016;76:2314-26
170. Tang M, Tiwari SK, Agrawal K, Tan M, Dang J, Tam T. et al. Rapid 3D Bioprinting of Glioblastoma Model Mimicking Native Biophysical Heterogeneity. Small (Weinheim an der Bergstrasse, Germany). 2021;17:e2006050
171. Wong BW, Marsch E, Treps L, Baes M, Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. The EMBO journal. 2017;36:2187-203
172. Benedicto A, Herrero A, Romayor I, Marquez J, Smedsrød B, Olaso E. et al. Liver sinusoidal endothelial cell ICAM-1 mediated tumor/endothelial crosstalk drives the development of liver metastasis by initiating inflammatory and angiogenic responses. Scientific reports. 2019;9:13111
173. Ma Y, Li Y, Guo P, Zhao J, Qin Q, Wang J. et al. Endothelial Cells Potentially Participate in the Metastasis of Triple-Negative Breast Cancer. Journal of immunology research. 2022;2022:5412007
174. Delprat V, Huart C, Feron O, Soncin F, Michiels C. The impact of macrophages on endothelial cells is potentiated by cycling hypoxia: Enhanced tumor inflammation and metastasis. Frontiers in oncology. 2022;12:961753
175. Li Q, Shao S, Zhu Z, Chen J, Hao J, Bai Y. et al. An IGFBP7hi endothelial cell subset drives T cell extravasation in psoriasis via endothelial glycocalyx degradation. The Journal of clinical investigation. 2023 133
176. Evans CE, Branco-Price C, Johnson RS. HIF-mediated endothelial response during cancer progression. International journal of hematology. 2012;95:471-7
177. Reiterer M, Colaço R, Emrouznejad P, Jensen A, Rundqvist H, Johnson RS. et al. Acute and chronic hypoxia differentially predispose lungs for metastases. Scientific reports. 2019;9:10246
178. Zhang L, Xu J, Zhou S, Yao F, Zhang R, You W. et al. Endothelial DGKG promotes tumor angiogenesis and immune evasion in hepatocellular carcinoma. Journal of hepatology. 2024;80:82-98
179. Liu Y, Murazzi I, Fuller AM, Pan H, Irizarry-Negron VM, Devine A. et al. Sarcoma Cells Secrete Hypoxia-Modified Collagen VI to Weaken the Lung Endothelial Barrier and Promote Metastasis. Cancer research. 2024;84:977-93
180. Yuzhalin AE, Yu D. Brain Metastasis Organotropism. Cold Spring Harbor perspectives in medicine. 2020 10
181. Carrolo M, Miranda JAI, Vilhais G, Quintela A, Sousa MFE, Costa DA. et al. Metastatic organotropism: a brief overview. Frontiers in oncology. 2024;14:1358786
182. Gao Y, Bado I, Wang H, Zhang W, Rosen JM, Zhang XH. Metastasis Organotropism: Redefining the Congenial Soil. Developmental cell. 2019;49:375-91
183. Wang C, Luo D. The metabolic adaptation mechanism of metastatic organotropism. Experimental hematology & oncology. 2021;10:30
184. Xie X, Li Y, Lian S, Lu Y, Jia L. Cancer metastasis chemoprevention prevents circulating tumour cells from germination. Signal transduction and targeted therapy. 2022;7:341
185. Azubuike UF, Tanner K. Biophysical determinants of cancer organotropism. Trends in cancer. 2023;9:188-97
186. Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N. et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. Journal of translational medicine. 2013;11:94
187. Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal transduction and targeted therapy. 2020;5:28
188. Gillot L, Baudin L, Rouaud L, Kridelka F, Noël A. The pre-metastatic niche in lymph nodes: formation and characteristics. Cellular and molecular life sciences: CMLS. 2021;78:5987-6002
189. García-Silva S, Benito-Martín A, Nogués L, Hernández-Barranco A, Mazariegos MS, Santos V. et al. Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism. Nature cancer. 2021;2:1387-405
190. Lin Y, Zheng H, Jia L, Luo Y, Zhang D, An M. et al. Integrin α6-containing extracellular vesicles promote lymphatic remodelling for pre-metastatic niche formation in lymph nodes via interplay with CD151. Journal of extracellular vesicles. 2024;13:e12518
191. Leary N, Walser S, He Y, Cousin N, Pereira P, Gallo A. et al. Melanoma-derived extracellular vesicles mediate lymphatic remodelling and impair tumour immunity in draining lymph nodes. Journal of extracellular vesicles. 2022;11:e12197
192. Su X, Brassard A, Bartolomucci A, Dhoparee-Doomah I, Qiu Q, Tsering T. et al. Tumour extracellular vesicles induce neutrophil extracellular traps to promote lymph node metastasis. Journal of extracellular vesicles. 2023;12:e12341
193. Gillot L, Lebeau A, Baudin L, Pottier C, Louis T, Durré T. et al. Periostin in lymph node pre-metastatic niches governs lymphatic endothelial cell functions and metastatic colonization. Cellular and molecular life sciences: CMLS. 2022;79:295
194. Weichand B, Popp R, Dziumbla S, Mora J, Strack E, Elwakeel E. et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. The Journal of experimental medicine. 2017;214:2695-713
195. Ogawa F, Amano H, Eshima K, Ito Y, Matsui Y, Hosono K. et al. Prostanoid induces premetastatic niche in regional lymph nodes. The Journal of clinical investigation. 2014;124:4882-94
196. Keklikoglou I, Cianciaruso C, Güç E, Squadrito ML, Spring LM, Tazzyman S. et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nature cell biology. 2019;21:190-202
197. Suehiro JI, Kimura T, Fukutomi T, Naito H, Kanki Y, Wada Y. et al. Endothelial cell-specific LAT1 ablation normalizes tumor vasculature. JCI insight. 2024 9
198. Quan L, Ohgaki R, Hara S, Okuda S, Wei L, Okanishi H. et al. Amino acid transporter LAT1 in tumor-associated vascular endothelium promotes angiogenesis by regulating cell proliferation and VEGF-A-dependent mTORC1 activation. Journal of experimental & clinical cancer research: CR. 2020;39:266
199. Wang Z, Zhu J, Liu Y, Wang Z, Cao X, Gu Y. Tumor-polarized GPX3(+) AT2 lung epithelial cells promote premetastatic niche formation. Proceedings of the National Academy of Sciences of the United States of America. 2022;119:e2201899119
200. Gong Z, Li Q, Shi J, Wei J, Li P, Chang CH. et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity. 2022;55:1483-500.e9
201. Zhang C, Li Q, Xu Q, Dong W, Li C, Deng B. et al. Pulmonary interleukin 1 beta/serum amyloid A3 axis promotes lung metastasis of hepatocellular carcinoma by facilitating the pre-metastatic niche formation. Journal of experimental & clinical cancer research: CR. 2023;42:166
202. Ramachandran P, Matchett KP, Dobie R, Wilson-Kanamori JR, Henderson NC. Single-cell technologies in hepatology: new insights into liver biology and disease pathogenesis. Nature reviews Gastroenterology & hepatology. 2020;17:457-72
203. Yazdani HO, Kaltenmeier C, Morder K, Moon J, Traczek M, Loughran P. et al. Exercise Training Decreases Hepatic Injury and Metastases Through Changes in Immune Response to Liver Ischemia/Reperfusion in Mice. Hepatology (Baltimore, Md). 2021;73:2494-509
204. Heo W, Lee W, Cheun JH, Lee ES, Li S, Kim HS. et al. Triple-Negative Breast Cancer-Derived Extracellular Vesicles Promote a Hepatic Premetastatic Niche via a Cascade of Microenvironment Remodeling. Molecular cancer research: MCR. 2023;21:726-40
205. Pei W, Wei K, Wu Y, Qiu Q, Zhu H, Mao L. et al. Colorectal cancer tumor cell-derived exosomal miR-203a-3p promotes CRC metastasis by targeting PTEN-induced macrophage polarization. Gene. 2023;885:147692
206. Bocchi M, de Sousa Pereira N, de Oliveira KB, Amarante MK. Involvement of CXCL12/CXCR4 axis in colorectal cancer: a mini-review. Molecular biology reports. 2023;50:6233-9
207. Yang M, Zhang C. The role of liver sinusoidal endothelial cells in cancer liver metastasis. American journal of cancer research. 2021;11:1845-60
208. Im HJ, Kim HG, Lee JS, Kim HS, Cho JH, Jo IJ. et al. A Preclinical Model of Chronic Alcohol Consumption Reveals Increased Metastatic Seeding of Colon Cancer Cells in the Liver. Cancer research. 2016;76:1698-704
209. Cheng P, Wu J, Zong G, Wang F, Deng R, Tao R. et al. Capsaicin shapes gut microbiota and pre-metastatic niche to facilitate cancer metastasis to liver. Pharmacological research. 2023;188:106643
210. Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A. et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer cell. 2021;39:708-24.e11
211. Arnold J, Schattschneider J, Blechner C, Krisp C, Schlüter H, Schweizer M. et al. Tubulin Tyrosine Ligase Like 4 (TTLL4) overexpression in breast cancer cells is associated with brain metastasis and alters exosome biogenesis. Journal of experimental & clinical cancer research: CR. 2020;39:205
212. Vasco C, Rizzo A, Cordiglieri C, Corsini E, Maderna E, Ciusani E. et al. The Role of Adhesion Molecules and Extracellular Vesicles in an In Vitro Model of the Blood-Brain Barrier for Metastatic Disease. Cancers. 2023 15
213. Bejarano L, Kauzlaric A, Lamprou E, Lourenco J, Fournier N, Ballabio M. et al. Interrogation of endothelial and mural cells in brain metastasis reveals key immune-regulatory mechanisms. Cancer cell. 2024;42:378-95.e10
214. Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100-4
215. Gan DX, Wang YB, He MY, Chen ZY, Qin XX, Miao ZW. et al. Lung Cancer Cells-Controlled Dkk-1 Production in Brain Metastatic Cascade Drive Microglia to Acquire a Pro-tumorigenic Phenotype. Frontiers in cell and developmental biology. 2020;8:591405
216. Kang SA, Hasan N, Mann AP, Zheng W, Zhao L, Morris L. et al. Blocking the adhesion cascade at the premetastatic niche for prevention of breast cancer metastasis. Molecular therapy: the journal of the American Society of Gene Therapy. 2015;23:1044-54
217. Morita Y, Kamal M, Kang SA, Zhang R, Lokesh GL, Thiviyanathan V. et al. E-selectin Targeting PEGylated-thioaptamer Prevents Breast Cancer Metastases. Molecular therapy Nucleic acids. 2016;5:e399
218. Shenker RF, McTyre ER, Ruiz J, Weaver KE, Cramer C, Alphonse-Sullivan NK. et al. The Effects of smoking status and smoking history on patients with brain metastases from lung cancer. Cancer medicine. 2017;6:944-52
219. Prasadam I, Zhou Y, Du Z, Chen J, Crawford R, Xiao Y. Osteocyte-induced angiogenesis via VEGF-MAPK-dependent pathways in endothelial cells. Molecular and cellular biochemistry. 2014;386:15-25
220. Briggs EN, Lynch ME. The Role of Osteocytes in Pre-metastatic Niche Formation. Current osteoporosis reports. 2024;22:105-14
221. Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M. et al. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:2117-24
222. Min JK, Cho YL, Choi JH, Kim Y, Kim JH, Yu YS. et al. Receptor activator of nuclear factor (NF)-kappaB ligand (RANKL) increases vascular permeability: impaired permeability and angiogenesis in eNOS-deficient mice. Blood. 2007;109:1495-502
223. Ma YV, Xu L, Mei X, Middleton K, You L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. Journal of cellular biochemistry. 2019;120:7590-601
224. Krohn-Grimberghe M, Mitchell MJ, Schloss MJ, Khan OF, Courties G, Guimaraes PPG. et al. Nanoparticle-encapsulated siRNAs for gene silencing in the haematopoietic stem-cell niche. Nature biomedical engineering. 2020;4:1076-89
225. Li XQ, Zhang R, Lu H, Yue XM, Huang YF. Extracellular Vesicle-Packaged CDH11 and ITGA5 Induce the Premetastatic Niche for Bone Colonization of Breast Cancer Cells. Cancer research. 2022;82:1560-74
226. Mulcrone PL, Campbell JP, Clément-Demange L, Anbinder AL, Merkel AR, Brekken RA. et al. Skeletal Colonization by Breast Cancer Cells Is Stimulated by an Osteoblast and β2AR-Dependent Neo-Angiogenic Switch. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2017;32:1442-54
227. Yue Z, Niu X, Yuan Z, Qin Q, Jiang W, He L. et al. RSPO2 and RANKL signal through LGR4 to regulate osteoclastic premetastatic niche formation and bone metastasis. The Journal of clinical investigation. 2022 132
228. Catalano M, O'Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. Journal of extracellular vesicles. 2020;9:1703244
229. Kosgodage US, Mould R, Henley AB, Nunn AV, Guy GW, Thomas EL. et al. Cannabidiol (CBD) Is a Novel Inhibitor for Exosome and Microvesicle (EMV) Release in Cancer. Frontiers in pharmacology. 2018;9:889
230. Kosgodage US, Trindade RP, Thompson PR, Inal JM, Lange S. Chloramidine/Bisindolylmaleimide-I-Mediated Inhibition of Exosome and Microvesicle Release and Enhanced Efficacy of Cancer Chemotherapy. International journal of molecular sciences. 2017 18
231. Siklos M, BenAissa M, Thatcher GR. Cysteine proteases as therapeutic targets: does selectivity matter? A systematic review of calpain and cathepsin inhibitors. Acta pharmaceutica Sinica B. 2015;5:506-19
232. Jia W, Yuan J, Cheng B, Ling C. Targeting tumor-derived exosome-mediated premetastatic niche formation: The metastasis-preventive value of traditional Chinese medicine. Cancer letters. 2023;567:216261
233. Jia WT, Xiang S, Zhang JB, Yuan JY, Wang YQ, Liang SF. et al. Jiedu recipe, a compound Chinese herbal medicine, suppresses hepatocellular carcinoma metastasis by inhibiting the release of tumor-derived exosomes in a hypoxic microenvironment. Journal of integrative medicine. 2024
234. Jia W, Liang S, Jin M, Li S, Yuan J, Zhang J. et al. Oleanolic acid inhibits hypoxic tumor-derived exosomes-induced premetastatic niche formation in hepatocellular carcinoma by targeting ERK1/2-NFκB signaling. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2024;126:155208
235. Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P. et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. Journal of cell science. 2013;126:5553-65
236. Chen M, Mao A, Xu M, Weng Q, Mao J, Ji J. CRISPR-Cas9 for cancer therapy: Opportunities and challenges. Cancer letters. 2019;447:48-55
237. Li Y, Chen ZK, Duan X, Zhang HJ, Xiao BL, Wang KM. et al. Targeted inhibition of tumor-derived exosomes as a novel therapeutic option for cancer. Experimental & molecular medicine. 2022;54:1379-89
238. Dupas A, Goetz JG, Osmani N. Extravasation of immune and tumor cells from an endothelial perspective. Journal of cell science. 2024 137
239. Liu XJ, Zhao HC, Hou SJ, Zhang HJ, Cheng L, Yuan S. et al. Recent development of multi-target VEGFR-2 inhibitors for the cancer therapy. Bioorganic chemistry. 2023;133:106425
240. Wang D, Xiao F, Feng Z, Li M, Kong L, Huang L. et al. Sunitinib facilitates metastatic breast cancer spreading by inducing endothelial cell senescence. Breast cancer research: BCR. 2020;22:103
241. Chen D, Qu X, Shao J, Wang W, Dong X. Anti-vascular nano agents: a promising approach for cancer treatment. Journal of materials chemistry B. 2020;8:2990-3004
242. Jiang Z, Xiong H, Yang S, Lu Y, Deng Y, Yao J. et al. Jet-Lagged Nanoparticles Enhanced Immunotherapy Efficiency through Synergistic Reconstruction of Tumor Microenvironment and Normalized Tumor Vasculature. Advanced healthcare materials. 2020;9:e2000075
243. Guo F, Cui J. Anti-angiogenesis: Opening a new window for immunotherapy. Life sciences. 2020;258:118163
244. Xing R, Gao J, Cui Q, Wang Q. Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma. Frontiers in immunology. 2021;12:783236
245. Ribatti D, Annese T, Ruggieri S, Tamma R, Crivellato E. Limitations of Anti-Angiogenic Treatment of Tumors. Translational oncology. 2019;12:981-6
246. Michaelsen SR, Staberg M, Pedersen H, Jensen KE, Majewski W, Broholm H. et al. VEGF-C sustains VEGFR2 activation under bevacizumab therapy and promotes glioblastoma maintenance. Neuro-oncology. 2018;20:1462-74
247. Zhang M, Chu S, Zeng F, Xu H. Bevacizumab modulates the process of fibrosis in vitro. Clinical & experimental ophthalmology. 2015;43:173-9
248. Vilà N, Coblentz J, Moreira-Neto C, Bravo-Filho V, Zoroquiain P, Burnier MN Jr. Pretreatment of RPE Cells with Lutein Can Mitigate Bevacizumab-Induced Increases in Angiogenin and bFGF. Ophthalmic research. 2017;57:48-53
249. Goede V, Coutelle O, Neuneier J, Reinacher-Schick A, Schnell R, Koslowsky TC. et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. British journal of cancer. 2010;103:1407-14
250. Carbone C, Moccia T, Zhu C, Paradiso G, Budillon A, Chiao PJ. et al. Anti-VEGF treatment-resistant pancreatic cancers secrete proinflammatory factors that contribute to malignant progression by inducing an EMT cell phenotype. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:5822-32
251. Lopes-Coelho F, Martins F, Pereira SA, Serpa J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. International journal of molecular sciences. 2021 22
252. Saravanan S, Vimalraj S, Pavani K, Nikarika R, Sumantran VN. Intussusceptive angiogenesis as a key therapeutic target for cancer therapy. Life sciences. 2020;252:117670
253. Cannell IG, Sawicka K, Pearsall I, Wild SA, Deighton L, Pearsall SM. et al. FOXC2 promotes vasculogenic mimicry and resistance to anti-angiogenic therapy. Cell reports. 2023;42:112791
254. Teuwen LA, De Rooij L, Cuypers A, Rohlenova K, Dumas SJ, García-Caballero M. et al. Tumor vessel co-option probed by single-cell analysis. Cell reports. 2021;35:109253
255. Thiel KW, Devor EJ, Filiaci VL, Mutch D, Moxley K, Alvarez Secord A. et al. TP53 Sequencing and p53 Immunohistochemistry Predict Outcomes When Bevacizumab Is Added to Frontline Chemotherapy in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2022;40:3289-300
256. Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer research. 2010;70:5679-85
257. Dimova I, Karthik S, Makanya A, Hlushchuk R, Semela D, Volarevic V. et al. SDF-1/CXCR4 signalling is involved in blood vessel growth and remodelling by intussusception. Journal of cellular and molecular medicine. 2019;23:3916-26
258. Smolarczyk R, Czapla J, Jarosz-Biej M, Czerwinski K, Cichoń T. Vascular disrupting agents in cancer therapy. European journal of pharmacology. 2021;891:173692
259. Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y. et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer science. 2014;105:1334-42
260. Yang S, Fang Y, Ma Y, Wang F, Wang Y, Jia J. et al. Angiogenesis and targeted therapy in the tumour microenvironment: From basic to clinical practice. Clinical and translational medicine. 2025: e70313.
261. Mensah SA, Nersesyan AA, Harding IC, Lee CI, Tan X, Banerjee S. et al. Flow-regulated endothelial glycocalyx determines metastatic cancer cell activity. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2020;34:6166-84
262. Offeddu GS, Hajal C, Foley CR, Wan Z, Ibrahim L, Coughlin MF. et al. The cancer glycocalyx mediates intravascular adhesion and extravasation during metastatic dissemination. Communications biology. 2021;4:255
263. Okorafor CC, Shastri S, Wen K, Ebong EE. Mechanisms of triple-negative breast cancer extravasation: Impact of the physical environment and endothelial glycocalyx. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2024;38:e23785
264. Li Y, Shteyman DB, Hachem Z, Ulay AA, Fan J, Fu BM. Heparan Sulfate Modulation Affects Breast Cancer Cell Adhesion and Transmigration across In Vitro Blood-Brain Barrier. Cells. 2024 13
265. Xia Y, Li Y, Fu BM. Differential effects of vascular endothelial growth factor on glycocalyx of endothelial and tumor cells and potential targets for tumor metastasis. APL bioengineering. 2022;6:016101
266. Shi SM, Suh RJ, Shon DJ, Garcia FJ, Buff JK, Atkins M. et al. Glycocalyx dysregulation impairs blood-brain barrier in ageing and disease. Nature. 2025;639:985-94
267. Jackson DG. Lymphatic trafficking of immune cells and insights for cancer metastasis. Clinical & experimental metastasis. 2024;41:381-6
268. Ikari A, Ito Y, Taniguchi K, Shibata MA, Kimura K, Iwamoto M. et al. Role of CD44-Positive Extracellular Vesicles Derived from Highly Metastatic Mouse Mammary Carcinoma Cells in Pre-Metastatic Niche Formation. International journal of molecular sciences. 2024 25
269. Zeng Y, Qiu Y, Jiang W, Fu BM. Glycocalyx Acts as a Central Player in the Development of Tumor Microenvironment by Extracellular Vesicles for Angiogenesis and Metastasis. Cancers. 2022 14
270. Singhal M, Augustin HG. Beyond Angiogenesis: Exploiting Angiocrine Factors to Restrict Tumor Progression and Metastasis. Cancer research. 2020;80:659-62
271. Dhami SPS, Patmore S, Comerford C, Byrne CM, Cavanagh B, Castle J. et al. Breast cancer cells mediate endothelial cell activation, promoting von Willebrand factor release, tumor adhesion, and transendothelial migration. Journal of thrombosis and haemostasis: JTH. 2022;20:2350-65
272. Xu W, Tan X, Li ML, Xu H, Villegas J, Fu H. Von Willebrand factor and hematogenous cancer metastasis under flow. Frontiers in cell and developmental biology. 2024;12:1435718
273. Trindade A, Duarte A. Notch Signaling Function in the Angiocrine Regulation of Tumor Development. Cells. 2020 9
274. Roy-Luzarraga M, Hodivala-Dilke K. Molecular Pathways: Endothelial Cell FAK-A Target for Cancer Treatment. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:3718-24
275. Roy-Luzarraga M, Reynolds LE, de Luxán-Delgado B, Maiques O, Wisniewski L, Newport E. et al. Suppression of Endothelial Cell FAK Expression Reduces Pancreatic Ductal Adenocarcinoma Metastasis after Gemcitabine Treatment. Cancer research. 2022;82:1909-25
276. Hanada K, Saito Y, Takagi T, Go M, Nakano Y, Inagawa T. et al. Reduced lung metastasis in endothelial cell-specific transforming growth factor β type II receptor-deficient mice with decreased CD44 expression. iScience. 2024;27:111502
277. Jacobsen A, Siebler J, Grützmann R, Stürzl M, Naschberger E. Blood Vessel-Targeted Therapy in Colorectal Cancer: Current Strategies and Future Perspectives. Cancers. 2024 16
278. Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Medical sciences (Basel, Switzerland). 2018 6
279. Heissig B, Salama Y, Takahashi S, Okumura K, Hattori K. The Multifaceted Roles of EGFL7 in Cancer and Drug Resistance. Cancers. 2021 13
280. Hayashi Y, Hashimoto M, Takaoka K, Takemoto T, Takakura N, Kidoya H. Tumor endothelial cell-derived Sfrp1 supports the maintenance of cancer stem cells via Wnt signaling. In vitro cellular & developmental biology Animal. 2024
Author contact
![]() Corresponding authors: Weitang Yuan, PhD. Department of Colorectal Surgery, The First Affiliated Hospital of Zhengzhou University, East Jianshe Road, Zhengzhou 450000, China; Tel/fax: +86 0371-67967141; E-mail: 13673384555com; Zhen Li, PhD. Department of Colorectal Surgery, The First Affiliated Hospital of Zhengzhou University, 1 East Jianshe Road, Zhengzhou 450000, China; Tel/fax: +86 0371-67967141; E-mail: doctorlizhencom; Shuaixi Yang, PhD. Department of Colorectal Surgery, The First Affiliated Hospital of Zhengzhou University, 1 East Jianshe Road, Zhengzhou 450000, China; Tel/fax: +86 0371-67967141; E-mail: sxyangedu.cn.
Corresponding authors: Weitang Yuan, PhD. Department of Colorectal Surgery, The First Affiliated Hospital of Zhengzhou University, East Jianshe Road, Zhengzhou 450000, China; Tel/fax: +86 0371-67967141; E-mail: 13673384555com; Zhen Li, PhD. Department of Colorectal Surgery, The First Affiliated Hospital of Zhengzhou University, 1 East Jianshe Road, Zhengzhou 450000, China; Tel/fax: +86 0371-67967141; E-mail: doctorlizhencom; Shuaixi Yang, PhD. Department of Colorectal Surgery, The First Affiliated Hospital of Zhengzhou University, 1 East Jianshe Road, Zhengzhou 450000, China; Tel/fax: +86 0371-67967141; E-mail: sxyangedu.cn.
 Global reach, higher impact
Global reach, higher impact