13.3
Impact Factor
Theranostics 2025; 15(13):6572-6592. doi:10.7150/thno.110866 This issue Cite
Research Paper
Activatable biomimetic probe with aggregation-induced emission characteristics for non-invasive monitoring of allograft rejection
1. Department of Ultrasound Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China;
2. Hubei Province Clinical Research Center for Medical Imaging, Wuhan 430022, China;
3. Hubei Province Key Laboratory of Molecular Imaging, Wuhan 430022, China.
*These authors contributed equally to this work
Received 2025-1-21; Accepted 2025-5-8; Published 2025-5-30
Abstract
Abstract: Background: Allograft rejection remains a major barrier to the long-term success of organ transplantation. The current gold standard for diagnosis-tissue biopsy is invasive and carries inherent risks, including sampling errors, procedural complications, and high costs. There is a pressing need for an efficient, non-invasive strategy for the early detection and monitoring of transplant rejection.
Methods: We developed a macrophage-targeted, activatable imaging probe (MTBPB/GPs) by encapsulating the H₂O₂-responsive aggregation-induced emission (AIE) molecule MTBPB into glucan particles (GPs) via electrostatic and hydrophobic interactions. The probe's responsiveness to H₂O₂ was characterized using UV-vis and fluorescence spectroscopy. Biocompatibility was evaluated through hemolysis assays, immunogenicity testing, biochemical analysis, and histopathology. Macrophage polarization and probe specificity were assessed using confocal laser scanning microscopy (CLSM), flow cytometry (FCM), and ELISA. A murine dorsal skin transplantation model was established to dynamically monitor graft rejection and the therapeutic efficacy of FK506, using in vivo fluorescence imaging at postoperative days (POD) 1, 3, 5, and 7. Pathological validation was performed via H&E staining and immunofluorescence.
Results: MTBPB/GPs exhibited excellent biosafety, with low cytotoxicity, minimal hemolytic activity, low immunogenicity, and negligible organ toxicity. Upon oral administration, the fluorescence signal of MTBPB/GPs was selectively activated by M1 macrophages, enabling early and sensitive detection of transplant rejection. Moreover, a single oral dose allowed real-time tracking of immunosuppressive therapy with FK506.
Conclusion: MTBPB/GPs represent a promising non-invasive platform for early diagnosis and longitudinal monitoring of transplant rejection and therapeutic response, with strong translational potential in solid organ transplantation.
Keywords: macrophages, transplant rejection, activatable probe, fluorescence imaging, aggregation-Induced emission.
Introduction
Organ transplantation remains the most effective and widely adopted therapeutic strategy for patients with end-stage organ failure [1, 2]. Despite substantial advancements in surgical techniques and immunosuppressive regimens, transplant rejection (TR) continues to be the leading cause of graft failure [3, 4]. Currently, the clinical gold standard for diagnosing rejection is histopathological biopsy [5, 6]. However, biopsy is inherently invasive, posing risks such as bleeding, infection, and sampling errors, and may lead to false-negative results [7, 8]. Therefore, the development of noninvasive approaches for the early detection of TR is urgently needed and holds substantial clinical value for improving graft prognosis and patient outcomes.
Given the limitations of current diagnostic approaches, there is growing interest in identifying early cellular and molecular events that contribute to transplant rejection. Among these, macrophages have emerged as key immune effectors in graft outcomes. Recent advances in transplant immunology have highlighted the functional heterogeneity of macrophages, particularly the distinct roles of M1 and M2 phenotypes in graft rejection. M1 macrophages, also known as classically activated macrophages, are induced by pro-inflammatory stimuli such as interferon-γ (IFN-γ) and lipopolysaccharide (LPS) [9, 10]. These cells play a pivotal role in the early stages of acute rejection by secreting high levels of pro-inflammatory cytokines (e.g., TNF-α, IL-6) and reactive oxygen species (e.g., H₂O₂), thereby exacerbating graft inflammation and contributing to tissue injury [11, 12]. In contrast, M2 macrophages are alternatively activated and are primarily involved in anti-inflammatory responses, tissue remodeling, and graft accommodation during the resolution or chronic phases of transplantation [13].
Clinical studies have further demonstrated that macrophage infiltration in biopsy samples correlates with transplant prognosis [14, 15]. Notably, in renal transplant recipients with graft dysfunction, over 70% of infiltrating macrophages have been identified as the M1 subtype [16]. Moreover, M1 macrophage accumulation has been implicated in a variety of adverse post-transplant events, including antibody-mediated rejection, ischemia-reperfusion injury, and chronic fibrosis [17-19]. Collectively, these findings suggest that M1 macrophage infiltration serves as a critical immunological marker and a potential early indicator of acute transplant rejection.
Several molecular imaging strategies have been developed to monitor macrophage activity for the early diagnosis of graft rejection [20-23]. For instance, macrophages readily internalize ¹⁸F-fluorodeoxy-glucose (¹⁸F-FDG), enabling positron emission tomography (PET) to visualize immune cell infiltration at transplant sites [24]. Similarly, superparamagnetic iron oxide nanoparticles (SPIONs) serve as effective magnetic resonance imaging (MRI) contrast agents that accumulate within macrophages, providing non-invasive indicators of rejection severity [20, 21, 25]. However, these techniques generally lack active targeting specificity and are often limited by background interference due to passive uptake and nonspecific biodistribution.
Beyond conventional PET and MRI probes, advances in nanomedicine have introduced a wide range of non-invasive imaging platforms with improved resolution, depth, and molecular specificity. These include second near-infrared window (NIR-II) nanoprobes based on thulium or erbium complexes for deep-tissue and real-time imaging [26, 27]; activatable fluorescent reporters that respond to enzymatic activity or inflammatory stimuli [28]; and multifunctional theranostic nanoplatforms capable of simultaneous diagnosis and therapy [29, 30]. Additionally, emerging photonic imaging technologies continue to enhance spatial resolution and signal-to-background ratios in vivo [31]. While these approaches represent important progress, many still rely on intravenous administration and lack specificity for macrophage subtypes or inflammatory microenvironments.
Glucan particles (GPs), derived from yeast cell walls, were first reported by Ostroff et al. as oral delivery vehicles that mimic the natural infection pathway of yeast [32]. Upon oral administration, GPs are transcytosed by M cells in the intestinal epithelium and delivered to gut-associated lymphoid tissues, where they are preferentially phagocytosed by macrophages [33]. This foundational work paved the way for macrophage-targeted disease diagnosis and therapy via oral delivery. Building on this concept, Zhang et al. successfully encapsulated quantum dots and paclitaxel into GPs for the oral treatment and imaging of subcutaneous breast cancer xenografts [34]. Further extending this strategy, Sung and colleagues developed doxorubicin (DOX)-loaded GPs that not only crossed the intestinal barrier but also enabled macrophage-mediated delivery across the blood-brain barrier, achieving oral “gut-to-brain” glioblastoma targeting [35]. These studies position GPs as promising biomimetic carriers for oral macrophage targeting. However, direct conjugation of imaging agents or therapeutics to GPs remains technically challenging. Most approaches require pre-formulation of positively charged nanoparticles or polymeric encapsulation, adding complexity to probe design and limiting scalability.
Fluorescence imaging, owing to its high spatiotemporal resolution, noninvasiveness, and ease of use, has been widely applied for real-time tracking of macrophage dynamics in vivo [36]. Near-infrared (NIR) fluorophores, especially in the 650-900 nm range, offer improved tissue penetration and reduced autofluorescence, making them well-suited for deep-tissue imaging [37]. However, many traditional NIR probes suffer from aggregation-caused quenching (ACQ) when used at high concentrations or when incorporated into delivery platforms, significantly impairing fluorescence intensity and imaging sensitivity [38]. Aggregation-induced emission (AIE) luminogens overcome this limitation by exhibiting enhanced fluorescence in the aggregated state. In our previous work, we demonstrated that cationic AIE molecules could stably bind to GPs in aqueous media [39]. Based on this platform, we constructed AIE-based biomimetic probes that were orally administered, phagocytosed by macrophages, and trafficked to allograft sites for the detection of transplant rejection. However, the probes exhibited a fluorescence “always-on” behavior, generating background signals in non-rejecting tissues and thereby reducing diagnostic contrast.
To improve imaging specificity, activatable probes have emerged as a preferred solution. These probes remain in an “off” state under physiological conditions and are selectively turned “on” upon encountering disease-specific stimuli such as enzymes, pH shifts, or oxidative stress [40, 41]. In the context of transplant rejection, macrophages infiltrating the graft undergo polarization toward the pro-inflammatory M1 phenotype under the influence of cytokines such as TNF-α and IL-2 [42-44]. M1 macrophages significantly upregulate inducible nitric oxide synthase (iNOS) and NADPH oxidase, leading to elevated production of reactive oxygen species (ROS), particularly hydrogen peroxide (H₂O₂), a key mediator of oxidative stress and tissue injury [45-49].
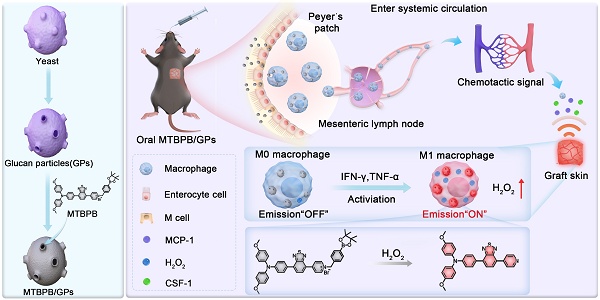
Given the central role of M1 macrophages in acute transplant rejection, we hypothesized that selectively imaging their activity through H₂O₂-responsive activation could provide a noninvasive and sensitive strategy for early diagnosis. To this end, we designed a biomimetic, activatable probe by encapsulating an H₂O₂-sensitive AIE molecule, MTBPB, into GPs (Scheme 1A). MTBPB contains a boronate ester moiety that reacts selectively with H₂O₂ [50], triggering a fluorescence turn-on response in oxidative inflammatory environments. Upon oral administration, MTBPB/GPs are taken up by intestinal macrophages and transported to the graft. In the presence of inflammatory stimuli, these macrophages polarize to the M1 phenotype, generating H₂O₂ that activates MTBPB fluorescence. This inflammation-dependent activation ensures minimal background fluorescence in non-rejecting tissues and enables precise visualization of rejection-specific macrophage infiltration (Scheme 1B). By integrating macrophage-mediated delivery with ROS-triggered activation, our system achieves high target specificity, low background interference, and fully noninvasive imaging. Thus, MTBPB/GPs offer a powerful platform for the early diagnosis and longitudinal monitoring of transplant rejection, with strong translational potential as a clinically viable alternative to invasive biopsy.
Experimental methods
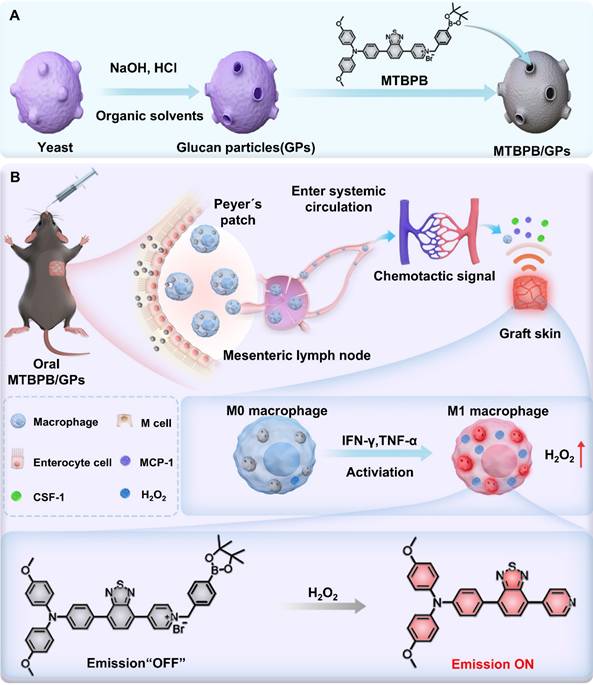
Preparation of GPs
First, 13 g of Angel yeast was added to 200 mL of 1 M sodium hydroxide (NaOH) solution. The mixture was incubated in a water bath at 80 °C with stirring for 1 h. After naturally cooling to room temperature, the solution was centrifuged at 2000 ×g for 15 min to collect the precipitate. The pH of the resulting solution was then adjusted to 4-5 using diluted hydrochloric acid (HCl), followed by incubation in a 60 °C water bath for another 1 h. After cooling to room temperature, the precipitate was washed 3 times with double-distilled water. Subsequently, the precipitate was washed 4 times with 200 mL isopropanol and twice with 200 mL acetone. The final precipitate was collected by centrifugation (2000 ×g, 15 min) and freeze-dried for two days to obtain the GPs powder.
Preparation of MTBPB/GPs
The MTBPB molecule was synthesized by modifying a borate ester reaction site on the AIE molecular probe according to the previous research method of our group [39, 51]. Next, 10 mg of GPs were dissolved in 1 mL of PBS solution and incubated for 30 min at 37 °C. Subsequently, 100 μL of 1 mM MTBPB ethanol solution was added to the reaction system and continued to be incubated at 37 °C protected from light for 6 h. Subsequently, the precipitate was collected by centrifugation (3000 rpm, 2 min) and washed 3 times with double-distilled water to obtain MTBPB/GPs that was stored at 4 °C protected from light.
Characterization of MTBPB/GPs
Yeast microcapsules (YM), GPs and MTBPB/GPs were dissolved using double-distilled water, sonicated for 30 s, and then 200 μL was taken and added dropwise to the wafer. The wafers were dried in an oven at 60 °C for 3 h and subsequently fixed on the sample stage by conductive adhesive. After spraying platinum for 5 min, the samples were removed and observed using a field emission scanning electron microscope (SEM). In addition, 10 μL of YM, GPs and MTBPB/GPs solutions were taken and added dropwise to a 400-mesh copper mesh, which was allowed to dry naturally, and then the morphological characteristics were observed by transmission electron microscopy (TEM).
The 5 mL of YM, GPs and MTBPB/GPs solutions were configured using double-distilled water. A Malvern particle size analyzer was used for detection, and each sample was repeated 3 times and averaged. Small quantities of MTBPB, GPs and MTBPB/GPs powders were taken separately and the major functional groups in the range of 4000 to 500 cm⁻¹ were measured using FT-IR. In addition, XRD spectra of GPs and MTBPB/GPs powders were measured with the scanning range set from 2θ = 3° to 2θ = 50°.
The stability of MTBPB/GPs
To assess the colloidal stability of MTBPB/GPs, the samples were stored at 4 °C, and the encapsulation efficiency and particle size were measured on days 1, 3 and 7. To simulate the highly acidic environment of the stomach, MTBPB/GPs were exposed to a simulated gastric fluid (pH 1.2), and the encapsulation efficiency was measured at different time points (10 min, 30 min, 1 h, 2 h). MTBPB/GPs were solubilized in anhydrous acetonitrile. After ultrasonic for 10 min, the MTBPB concentration in the supernatant was quantified, and the Loading Efficiency (LE) and Loading Content (LC) values were calculated.
The ROS responsiveness and sensitivity of MTBPB
The 10 μL of 1 mM MTBPB was dissolved in 1 mL of PBS. The experiment was divided into 2 groups: one group was added with 5 μM H₂O₂ and the other group was added with an equal amount of PBS. The absorbance changes of the solution before and after the addition of H₂O₂ was measured using a UV spectrophotometer. The wavelength range was set at 200-670 nm, and the scanning step was 1 nm. Next, the fluorescence absorption spectra of the solutions with different concentrations of H₂O₂ (0-6 μM) were measured using a fluorescence spectrophotometer. The excitation wavelength of the fluorescence spectrophotometer was set at 520 nm, the excitation slit at 3.0 nm, the emission slit at 5.0 nm, and the scanning speed was set at 600 nm/min.
(A) Preparation of the biomimetic probe MTBPB/GPs. (B) In an allogeneic mouse transplant model, orally administered MTBPB/GPs specifically target macrophages. Guided by chemokines, macrophages loaded with MTBPB/GPs migrate to the graft site. When stimulated by inflammatory cytokines, these macrophages generate elevated levels of H₂O₂, which activates the fluorescence MTBPB/GPs, enabling the early detection of TR.
In addition, 10 μL of 1 mM MTBPB was dissolved in different concentrations of water/DMSO (0-95%) mixtures, and the fluorescence absorption spectra were measured using the same instrumental parameters. For dynamic monitoring, 10 μL of 1 mM MTBPB solution was taken and 5 μM H₂O₂ or an equal amount of PBS was added to each solution. The parameters of the fluorescence spectrophotometer were set to an excitation wavelength of 520 nm, and the fluorescence intensity at the absorption wavelength of 670 nm was collected. The fluorescence intensity was recorded every 20 s for 6 h.
The selectivity of MTBPB
The following chemicals were dissolved in PBS solution: glucose (Glu), cysteine (Cys), glutathione (GSH), nitric oxide (NO), acetic acid (CH₃COOH), zinc chloride (ZnCl₂), copper chloride (CuCl₂), magnesium sulfate (MgSO₄), sodium chloride (NaCl), potassium chloride (KCl), peroxynitrite (ONOO-), hydroxyl radical (▪OH) and hydrogen peroxide (H₂O₂). The 10 μL of 1 mM MTBPB was dissolved in the above mixture and the fluorescence intensity at the absorption wavelength of 670 nm was measured using the same instrumental parameters.
To study the effect of pH on the reaction, the pH of MTBPB solution was adjusted to 4-10 using HCl and NaOH. 5 μM H₂O₂ was added in equal amounts at each pH and the fluorescence intensity was measured using the same instrumental parameters.
Additionally, to investigate the emission spectra of MTBPB in different organic solvents, 10 μL of 1 mM MTBPB solution was dissolved in various organic solvents, and the UV absorption and fluorescence emission spectra were measured using the same instrumental parameters.
Cell culture
The RAW264.7 macrophage cells were cultured in DMEM medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified atmosphere at 37 °C with 5% CO₂. H9C2 and 293T cells were cultured under the same conditions as the macrophages. AML12 cells were cultured in MEM complete medium (MEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin).
Experimental animals
C57BL/6 and BALB/c mice (6-8 weeks old, 20-25 g) were purchased from Beijing Viton Lihua Co. Ltd. and housed in the Animal Breeding Centre of Tongji Medical College. All experimental procedures were in accordance with Laboratory Animal Ethics (No. 4197).
Biosafety of MTBPB/GPs
Healthy C57BL/6 mice were randomly divided into healthy and MTBPB/GPs groups (n = 5). The MTBPB/GPs group received oral MTBPB/GPs (100 mg/kg) for 7 consecutive days. After 7 days, all mice were sacrificed, and peripheral blood was collected for hematology and evaluation of hepatic and renal function. Serum was analyzed for IgG and IgM levels to assess immunogenicity. The major organs were subsequently collected on days 1, 3, 7 and subjected to H&E staining.
Cytotoxicity of MTBPB/GPs
A negative control group and an MTBPB/GPs group were established (n = 5). The negative control group was treated with DMEM medium only. Macrophages were incubated with MTBPB/GPs for 24 h at ratios of 1:20, 1:50, 1:100, and 1:200. Macrophage viability was assessed using the CCK-8 kit. The cytotoxicity of MTBPB/GPs on cardiomyocytes (H9C2), hepatocytes (AML12), and renal cells (293T) were also evaluated using the CCK-8 kit (n = 5).
The phenotype of macrophage
The RAW264.7 cells (1 × 10⁵ per well) were seeded in 6-well plates and separated into two groups: LPS and control (n = 5). The LPS group was incubated with LPS (1 μg/mL) for 24 h, while the control group was treated with complete medium. Each group was incubated for 30 min with 10 μL of viability dye (FVS-AF700 channel) and washed 2 times with PBS. Then, 2 μL of F4/80 and CD86 antibodies were added to each group and incubated in the dark at 4 °C for 40 min. Finally, the expression levels of F4/80 and CD86 were measured using flow cytometry (F4/80-BV421 and CD86-PE-Cy7 channels).
The RAW 264.7 cells (8 × 10³ per well) were seeded in 96-well plates, referring to the above grouping. The concentrations of TNF-α and IFN-γ in the supernatants, both before and after LPS stimulation, were quantified using an ELISA kit (n = 5).
The RAW264.7 cells (1 × 10⁵ per well) were seeded into 6-well plates and divided into two groups: IL-4 group and control group (n = 4). The IL-4 group was cultured with IL-4 (500 ng/mL) for 48 h, while the control group was treated with complete medium. Each group was incubated with 10 μL of viability dye (FVS-AF700 channel) for 30 min and then washed 2 times with PBS. Next, 2 μL of F4/80 antibody was added to each group, followed by incubation at 4 °C in the dark for 40 min. After membrane rupture with permeabilization buffer for 1 h, 2 μL of CD206 antibody was added to each group and incubated at 4 °C in the dark for another 40 min. Finally, the expression levels of F4/80 and CD206 were measured using flow cytometry (F4/80-BV421 and CD206-AF647 channels).
Fluorescence activation of MTBPB/GPs in M1 macrophages
The RAW264.7 cells (1 × 10⁵ per well) were seeded into 6-well plates or confocal culture dishes and divided into 4 groups: M0, M1, M1 + NAC, and M2. The M0 group was left untreated, the M1 group was stimulated with 1 μg/mL LPS for 24 h, the M1 + NAC group was stimulated with 1 μg/mL LPS for 24 h, followed by the addition of 5 mM N-acetylcysteine (NAC) for an additional 24 h co-incubation, and the M2 group was stimulated with 500 ng/mL IL-4 for 48 h.
Cells were then incubated with diluted DCFH-DA (10 μM) at 37°C for 45 min, followed by 5 times washed with PBS (n = 4). ROS production was assessed using the ROS-FITC channel. After honest 3342 staining, fluorescence imaging was performed using a laser scanning confocal microscope (LSCM). The fluorescence signal was quantified by flow cytometry.
Following the same experimental groups as above, MTBPB/GPs were added to the cells, and they were cultured for an additional 24 h (n = 4). After honest 3342 staining, fluorescence intensity was observed using LSCM. Cells in the 6-well plates were washed 3 times with PBS to remove excess MTBPB/GPs, and fluorescence intensity was quantified by flow cytometry (Ex = 480 nm, Em = 620 nm).
Co-localization of MTBPB/GPs with M1 macrophage lysosomes
The RAW264.7 cells (1 × 10⁵ per well) were seeded into confocal culture dishes and incubated with LPS for 24 h. The cells were then co-incubated with MTBPB/GPs for an additional 24 h, followed by 3 times with PBS. Lysosomes were labeled by incubating the cells with a lysosomal green fluorescence probe (FITC) for 1 h, followed by 2 times washed with PBS. The cells were then incubated with 200 μL honest 3342 in the dark for 10 min, followed by 2 times washed with PBS. Co-localization of lysosomes and MTBPB/GPs was observed using LSCM.
Endocytosis pathway of MTBPB/GPs by M1 macrophages
RAW264.7 cells (1 × 10⁵ cells per well) were seeded in 6-well plates, using LPS stimulus 24h. One group was pretreated with the laminarin (1 mg/mL) for 2 h, while the other group received no treatment (n = 4). MTBPB/GPs (1 × 10⁵ per well) were then added and incubated for 12 h at 37 °C. Cell suspensions from both the control and MTBPB/GPs groups were collected, which phagocytosis rates were quantified by flow cytometry (Ex = 480 nm, Em = 620 nm).
Macrophage migratory activity following endocytosis of MTBPB/GPs
RAW264.7 cells (1 × 10⁵ per well) were inoculated into 6 wells and cultured for 12 h (n = 4), then MTBPB/GPs were added and cultured for another 12 h. The upper layer of the chamber was filled with 200 μL of serum-free medium. The lower layer was injected with 20% medium containing 10 ng of MCP-1. 12 h later, the cells in the upper layer were wiped off with a moistened cotton swab, and the cells on the back of the upper layer were stained with DAPI. Finally, 3 randomly selected fields of view were observed under the microscope and the number of cells was counted.
MTBPB/GPs in vivo responsiveness
The 24 allograft mice were randomly assigned to 6 groups (n = 4). Small animal live imaging was conducted on the grafted skins at 0, 6, 12, 24, 36, and 48 h following oral MTBPB/GPs (200 μL, 0.8 mg/kg MTBPB) on postoperative day (POD) 6. Imaging was performed using a Perkin Elmer IVIS Lumina system (Ex = 480 nm, Em = 620 nm). Regions of interest (ROI) were outlined for quantitative fluorescence intensity analysis.
Fluorescence imaging of TR
Allograft and isograft mice were orally administered MTBPB/GPs. (n = 5) Whole-body imaging was performed using a small-animal in vivo imaging system at 36 h after oral administration on POD 0, 2, 4, and 6, respectively. Imaging was conducted with a Perkin Elmer IVIS Lumina system (Ex = 480 nm, Em = 620 nm).
Under the same modeling conditions as described above, MTBPB/GPs were administered orally to both groups on POD 6 (n = 5). Mice were sacrificed 24 and 36 h after oral administration, and major organs were harvested from the groups, and then were performed using a small animal live imaging system.
Immunosuppressive therapy efficacy evaluation in real time
An allogeneic mouse model was established with continuous daily subcutaneous injections of FK506 (3.0 mg/kg) or PBS, starting on the day of surgery and continuing until POD 7 (n = 5). Both group received oral MTBPB/GPs (n = 4) on the day of surgery, and in vivo imaging was carried out on POD 1, 3, 5 and 7 to monitor the real-time effectiveness of the immunosuppressive treatment (Perkin Elmer IVIS Lumina: Ex = 480 nm, Em = 620 nm).
In vivo transport mechanism of MTBPB/GPs
Allograft mice on POD6 were randomly assigned to two groups, one group was orally administered MTBPB/GPs and the other group was orally administered an equal amount of MTBPB (n = 5). In vivo imaging was performed on the 36th h after oral administration and quantitative fluorescence analysis was performed. After the mice were executed, the gastrointestinal tract was used to for ex vivo imaging. (Perkin Elmer IVIS Lumina: Ex = 480nm, Em = 620nm).
Subsequently, allograft mice on POD 6were randomly divided into 5 groups (n = 4). The gastrointestinal tract, Peyer's lymph nodes, mesenteric lymph nodes, draining lymph nodes, and isolated skin of the mice were removed after necropsy. Ex vivo imaging was performed after oral administration of MTBPB/GPs and at 0, 6, 12, 24, and 36 h, respectively. Imaging parameters were consistent with those described above. At the same time points, the mesenteric lymph nodes, Peyer's patches, draining lymph nodes, and excised skin were harvested from the mice. The tissues were subjected to fluorescence sectioning, DAPI staining, and the examination of the spontaneous fluorescence of MTBPB/GPs to track the migration pathway. Chemokine levels in the grafted skin were semi-quantified in healthy mice, allografts and syngeneic grafts at POD3 and POD7 (n = 4). The mRNA expression levels of MCP-1 and CSF-1 were quantified using real-time PCR.
Histopathology and immunofluorescence analysis of graft skin
Mice were randomly assigned to 2 groups: one group received BALB/c skin grafts on the back, and the other received C57 skin grafts (n = 4). Skin were collected from both groups on POD 1, 3, 5, and 7. Hematoxylin-eosin (H&E) staining was performed, and the graft histology was evaluated by professional pathologists. The histological assessment was quantified.
Specific staining of skin tissue nuclei, CD68, iNOS using DAPI, FITC and Cy3 dyes, respectively. 3 fields of view were randomly selected for semi-quantitative the count of pro-inflammatory macrophages by fluorescence microscopy. The histopathology and immunofluorescence were evaluated in mice after 7 consecutive days of FK506 or PBS treatment using the same methods (n = 4).
Allograft and isograft skin grafts were collected from the dorsal of mice. The tissues were digested for 1 h with collagenase and dispase (2 mg/mL), followed by termination of digestion and filtration through a 70 μm filter. Each group was incubated with 10 μL of viability dye (FVS-AF700 channel) for 30 min, then washed twice with PBS. Subsequently, 2 μL of F4/80 and CD86 antibodies were added to each group and incubated at 4 °C in the dark for 40 min. Finally, the expression levels of F4/80 and CD86 were measured using flow cytometry (n = 4).
Statistical Analysis
The results are presented as mean ± SD. Continuous data following a normal distribution were analyzed using one-way ANOVA. Unpaired t-tests were applied for comparisons between two groups. All analyses were conducted using GraphPad Prism version 9.0 and Origin 2020. Statistical significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Results and discussion
Design and photophysical property of H2O2-responsive probe MTBPB
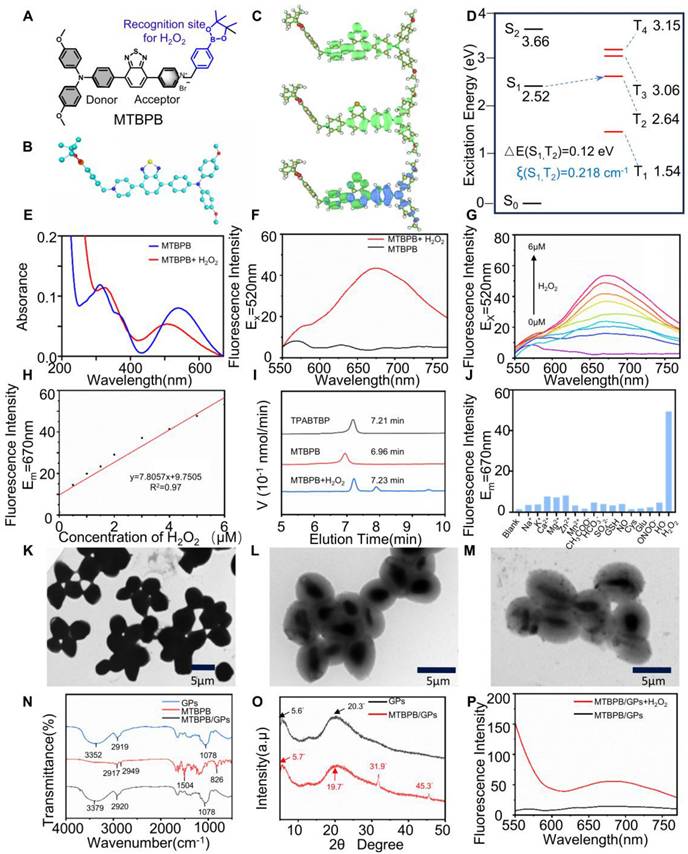
In our previous research, we successfully developed an advanced AIE molecule, TPABTBP [52]. As in Scheme S1, the molecular structure of TPABTBP features typical donor-acceptor (D-A) configuration, with triphenylamine serving as the electron donor (D) and benzothiadiazol`e and pyridine as electron acceptors (A) (Figure 1A,B). The rotational flexibility between the donor and acceptor units endows the molecule with both aggregation-induced emission (AIE) and intramolecular charge transfer (ICT) properties. The compound TPABTBP underwent a salinization reaction with benzyl bromide borate, resulting in the formation of MTBPB. Designed with an amphiphilic structure and a positively charged pyridinium group, MTBPB effectively aggregates in the confined spaces of glucan particles through electrostatic interactions [39]. The quinolinium unit's p-pinacolborylbenzyl group functions as a recognition moiety for H₂O₂ in biological systems. The molecular structure of MTBPB was analyzed using 1HNMR, 13CNMR, and spectrometry (Figure S2-S9).
First, the photophysical properties of MTBPB were studied. the AIE feature of MTBPB was studied in DMSO/water solutions with varying water concentrations (fW). As shown in Figure S10A and 10B, MTBPB exhibited weak fluorescence emission consistently as the water content increased from 0% to 95%. The weak fluorescence of MTBPB can be ascribed to the following factors: (1) The pyridinium group enhances the water solubility of MTBPB; (2) The incorporation of heavy halide ions (Br-) efficiently strengthens spin-orbit coupling (SOC), thereby promoting the efficient intersystem crossing (ISC) process and electron transfer [53]; (3) The strong ICT effect makes it prone to intersystem crossing. To confirm the ICT characteristics of MTBPB, Multiwfn software was employed to perform hole-electron analysis, providing a comprehensive investigation of the electronic excitation features [54]. As illustrated in the Figure 1C, the results revealed that the holes were predominantly localized in the electron-donating triphenylamine region, while the electrons are primarily distributed in the electron-accepting pyridinium group. The significant spatial separation between the hole and electron distributions strongly supports the conger conclusion that the MTBPB molecule exhibits pronounced ICT behavior. Strong ICT effects are typically accompanied by significant changes in the dipole moment, which may alter the nature of the excited state, bringing it closer to a triplet-state character (e.g., through the mixing of locally excited states). This, in turn, facilitates intersystem crossing (ISC) and contributes to fluorescence quenching. Therefore, we employed Time-Dependent Density Functional Theory (TD-DFT) calculations at the CAM-B3LYP/def2-TZVP level to calculate the energy gap between the singlet and triplet states of the MTBPB molecule. The energy transitions from S1 to T2 has been assumed due to the narrow energy gap of 0.12 eV. Moreover, the spin-orbit coupling (SOC) values for this transition was calculated to be 0.218 cm-1. The dense energy-level distributions and favorable SOC values provided a solid foundation for intersystem crossing (Figure 1D).
To further elucidate the photophysical behavior of MTBPB under different solvent environments, we investigated its absorption and emission charac-teristics in a series of organic solvents with varying polarity and proticity. As shown in Figure S11A and S11B, MTBPB exhibited a strong fluorescence emission peak at ~670 nm in non-polar solvents such as toluene, whereas its fluorescence intensity was significantly quenched in polar solvents such as DMF, CH₃OH, and H₂O. This behavior aligns with the classical AIE phenomenon, in which MTBPB molecules aggregate in low-polarity solvents, restricting intramolecular rotations and promoting radiative decay. In contrast, polar and protic solvents enhance solubility and allow for free intramolecular motion, thus favoring non-radiative pathways and quenching fluorescence emission. Additionally, the strong solvent-dependent photophysical response can be ascribed to the molecule's donor-acceptor (D-A) structure, comprising a triphenylamine donor and a pyridinium acceptor. In polar environments, this structure exhibits a pronounced intramolecular charge transfer (ICT) state, leading to charge-separated excited states and facilitating intersystem crossing (ISC) to triplet states. The presence of a bromide moiety further enhances spin-orbit coupling (SOC), reinforcing ISC and leading to further suppression of fluorescence in aqueous environments. These features confirm that MTBPB remains nearly non-emissive in physiological conditions but becomes selectively activated in environments with restricted solvation and elevated oxidative stress, such as those found in transplant rejection sites. Such AIE-ICT-ISC synergy ensures minimal background signal and high target-to-background contrast, making MTBPB an ideal candidate for activatable imaging of inflammatory microenvironments.
Characterization of probe MTBPB in response to H₂O₂
We explored the fluorescent properties of MTBPB in the presence of H₂O₂. MTBPB displayed distinct absorptions at 310 and 540 nm, along with weak emission upon excitation at 520 nm. Upon the addition of H₂O₂ (5 μM), the absorption peak at 540 nm decreased in intensity, while a new peak emerged at 506 nm (Figure 1E). The observed blue shift in the absorption spectrum was due to the formation of TPABTBP, which decreased the intramolecular electron transfer. Furthermore, the fluorescence intensity at 670 nm increased markedly following the introduction of H₂O₂ (5 μM) (Figure 1F). Fluorescence titration studies showed a continuous amplification of the signal at 670 nm as the concentration of H₂O₂ was elevated (Figure 1G). Additionally, the fluorescence intensity at 670 nm demonstrated a robust linear relationship (R2 = 0.97) with the concentration of H₂O₂ (0-6.0 μM) (Figure 1H). According to the reported method, the detection limit of H₂O₂ for MTBPB was determined to be 0.11 μM [55], demonstrating the high sensitivity of MTBPB toward H₂O₂. To verify the formation of TPABTBP upon incubation of MTBPB with H₂O₂, we conducted high-performance liquid chromatography (HPLC) analysis. As shown in Figure 1I, the retention times for TPABTBP and MTBPB were 7.21 and 6.96 min, respectively. When exposed to H₂O₂ (5 μM), a new peak at 7.23 min with a mass-to-charge ratio (m/z) of 517.1689, confirming that the probe underwent oxidation-induced cleavage of the p-boratebenzyl group to generate TPABTBP (Figure S12).
Next, to determine the optimal reaction time for H₂O₂ detection, time-dependent absorption and fluorescence emission experiments were conducted using MTBPB in the presence of 5 μM H₂O₂ in PBS buffer (10.0 mM, pH 7.4, containing 5% DMSO). As shown in Figure S10C, in the absence of H₂O₂, the fluorescence intensity remains relatively stable over time, showing minimal variation. However, upon the introduction of 5 μM H₂O₂, a noticeable increase in fluorescence intensity is observed reached equilibrium within 6 h. The appropriate reaction rate between MTBPB and H₂O₂ ensures that the probe remains stable and unreactive in the background conditions of the gastrointestinal tract, reducing interference from low levels of H₂O₂. At the same time, it maintains high sensitivity and specificity toward higher H₂O₂ concentrations found in allograft rejection. The effect of pH on the MTBPB probe's ability to detect H₂O₂ was also explored. As shown in Figure S10D, the probe exhibits negligible fluorescence between pH 4 and 10. However, upon the addition of 5 μM H₂O₂, the MTBPB fluorescence signal was activated and enhanced within the pH range of 7-10. Since the reaction between MTBPB and H₂O₂ was a nucleophilic substitution process, these results indicated that highly acidic conditions were not conducive to the nucleophilic substitution, while the probe exhibited strong fluorescence respon-siveness under physiological pH conditions. To evaluate the selectivity of the MTBPB probe and exclude interference from other molecules and ions present in biological systems, a range of including metal cations (Na+, K+, Ca2+, Mg2+, Zn2+), ainions (CH3COO-, HCO3-, SO42- ), as well as biothiols such as GSH, Cys, Glu and other ROS like NO, peroxynitrite (ONOO-), hydroxyl radical (⋅OH). As shown in Figure 1J, the results demonstrated that the probe MTBPB produced negligible fluorescence when reacted with these potential interferents. In contrast, a pronounced fluorescence response was detected solely in the presence of H₂O₂. This selective fluorescence activation confirmed that the MTBPB probe displayed high specificity for H₂O₂ detection, with minimal cross-reactivity to other commonly encountered molecules or ROS.
Characterization activatable biomimetic probe MTBPB/GPs
We synthesized glucan particles (GPs) using an acid-base organic solvent extraction method, followed by the encapsulation of the cationic H₂O₂-responsive AIE molecule MTBPB via electrostatic and hydrophobic interactions [32]. Owing to the hollow and porous architecture of GPs, MTBPB is hypothesized to be effectively retained within the internal β-glucan matrix. Dynamic light scattering (DLS) analysis revealed that the average hydrodynamic diameter of MTBPB/GPs was approximately 3847 nm, which is comparable to native GPs but smaller than intact yeast shells (Figure S13A). The zeta potential of MTBPB/GPs was slightly negative (-4.8 mV), closely matching that of GPs and significantly reduced compared to the highly cationic MTBPB precursor (Figure S13B). This neutral surface charge is advantageous for reducing nonspecific protein adsorption and enhancing in vivo biocompatibility. Compared to the smooth surface of intact yeast (Figure S14A), the GPs exhibited a roughened exterior with pronounced wrinkles and pores measuring approximately 500-600 nm in diameter, consistent with the presence of yeast bud scars [35, 51, 56] (Figure S14B). Transmission electron microscopy further confirmed the successful formation of spherical, hollow particles and the efficient internalization of MTBPB within GPs (Figure 1K-M). The encapsulation efficiency of MTBPB reached 99.2%, with a drug loading content of 0.7%, supporting the strong affinity between MTBPB and the inner cavity of the GPs and validating the robustness of the loading strategy.
To further explore the interaction between MTBPB and GPs, FT-IR spectroscopy and XRD analyses were performed. In the FT-IR spectrum of GPs and MTBPB/GPs (Figure 1N), the peak at 1078 cm⁻¹ was attributed to the polysaccharide component. The peak at 1078 cm was ascribed to the polysaccharide component, while the one at 2919 cm⁻¹ was linked to the vibration of the -CH- groups. Additionally, the peak at 3352 cm⁻¹ corresponded to the stretching vibration of -OH groups. MTBPB exhibited alkyl C-H stretching vibrations at 2917 and 2949 cm⁻¹, along with the C=C stretching vibration of the aromatic ring at 1504 cm⁻¹. A peak between 700-900 cm⁻¹ range was attributed to the C-H bending vibrations on the benzene ring. In the MTBPB/GPs spectrum, the absence of the C-H bending peaks of the benzene ring and the C=C stretching vibration of the aromatic ring indicated the successful encapsulation of MTBPB within the GPs cavity. In the XRD spectra of GPs (Figure 1O), a diffraction peak at 5.6° verified the existence of the triple helical configuration of β-glucan [57]. Notably, the XRD spectrum of MTBPB/GPs showed distinct crystalline peaks at 31.9°, and 45.3°, suggesting that MTBPB formed crystalline states within the GPs. To further confirm the H₂O₂-activated properties of MTBPB/GPs, we anlyzed the fluorescence emission spectra of MTBPB/GPs before and after exposure to H₂O₂. As depicted in Figure 1P , MTBPB/GPs exhibited weak fluorescence in the absence of H₂O₂. Upon addition of H₂O₂, a significant fluorescence enhancement was observed at 670 nm, indicating that H₂O₂ effectively activated the MTBPB/GPs.
Characterization of probe MTBPB/GPs in response to H2O2. (A) structure and design of MTBPB. (B) The optimal configuration of the probe MTBPB in the ground state. (C) Electron-hole analysis of MTBPB. (D) The energy gap and associated spin-orbit coupling constants (𝜉) of MTBPB were calculated based on the S0 structure using the CAM-B3LYP/def2-TZVP method. (E) The UV absorption and (F) fluorescence emission spectra of MTBPB (10 μM) with or without H2O2 (5 μM) in PBS. (G) Fluorescence emission spectra of MTBPB (10 μM) after incubation with different amounts of H2O2 (0-6 μM). (H) Correlation between the fluorescence intensity of MTBPB and the concentration of H2O2. (I) HPLC assays of MTBPB in the presence or absence of H2O2 and pure TPABTBP. (J) Fluorescence intensity (Em = 670 nm) after mixture of MTBPB (10 μM) with the Na+, K+, Ca2+, Mg2+, Zn2+, CH3COO-, HCO3-, SO42-, GSH, Cys, Glu, NO, ONOO-, ⋅OH and H2O2. (K) TEM image of yeast. (L) TEM image of GPs and (M) MTBPB/GPs. (N) FT-IR spectra of MTBPB, GPs, MTBPB/GPs. (O) XRD spectra of GPs, MTBPB/GPs. (P) Fluorescence emission spectra of MTBPB/GPs with or without H2O2 (5 μM) in PBS.
To evaluate the stability of MTBPB/GPs under both physiological and storage conditions, we investigated their behavior in simulated gastric fluid and under refrigerated storage. First, to assess acid stability, MTBPB/GPs were incubated in a buffer at pH 1.2 to mimic the gastric environment. After 2 h, the cumulative release of MTBPB remained below 25%, indicating minimal premature leakage and strong structural integrity under harsh gastrointestinal conditions (Figure S15). Next, the colloidal stability of MTBPB/GPs was assessed by monitoring particle size changes in PBS at 4 °C over a 7-day period. The average hydrodynamic diameter remained consistently around 3.7 μm, with no evidence of aggregation or degradation (Figure S16A). In addition, loading efficiency (LE) remained above 95% on days 1, 3, and 7, suggesting strong encapsulation retention and negligible payload loss during refrigerated storage (Figure S16B). Together, these findings confirm the excellent structural and colloidal stability of MTBPB/GPs under both gastrointestinal and storage conditions, supporting their suitability for oral delivery and in vivo biomedical applications.
Specific Targeting and Imaging of M1 Macrophages with MTBPB/GPs
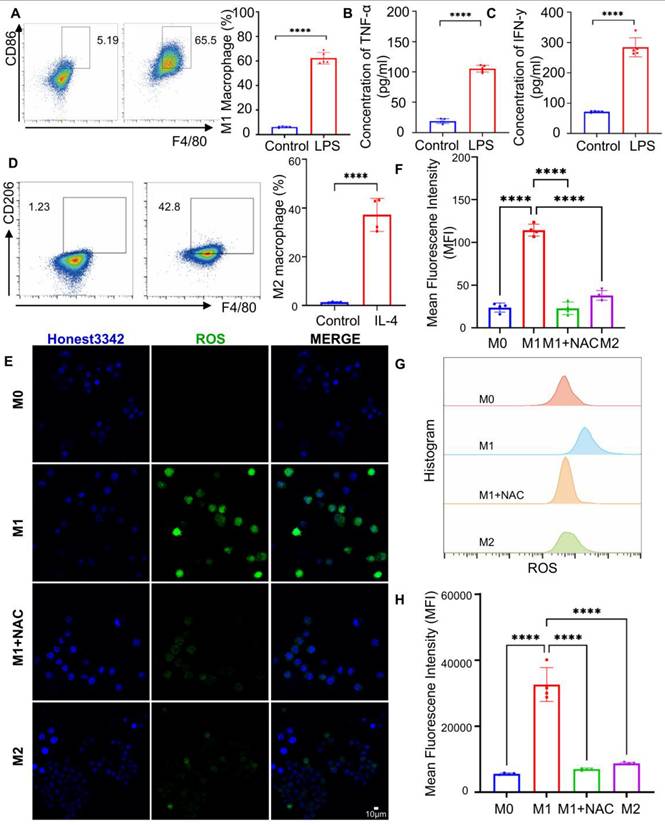
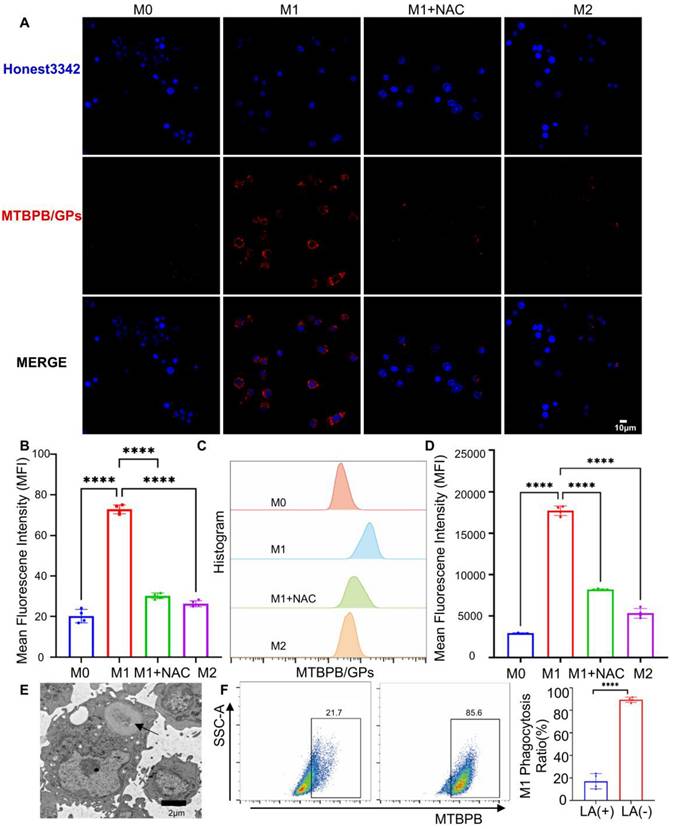
Macrophages can be polarized into the pro-inflammatory M1 phenotype upon stimulation with LPS [58]. This polarization is characterized by the upregulation of membrane CD86 and the secretion of pro-inflammatory cytokines such as TNF-α and IFN-γ [59, 60]. To verify successful M1 induction, macrophages were treated with 1 μg/mL LPS for 24 h, and CD86 expression was analyzed via flow cytometry. Compared to unstimulated controls, CD86 levels increased nearly 10-fold (Figure 2A). ELISA analysis of the culture supernatant further confirmed polarization, revealing 5.5- and 3.9-fold increases in TNF-α and IFN-γ secretion, respectively (Figure 2B, C).
M1 macrophage polarization is also accompanied by elevated intracellular reactive oxygen species (ROS) levels [60, 61], which serve as key markers of oxidative stress during inflammation. To characterize ROS profiles across macrophage phenotypes, IL-4 stimulation was used to induce M2 polarization, as confirmed by CD206 upregulation via flow cytometry (Figure 2D). Confocal microscopy and flow cytometric analysis using ROS-sensitive fluorescent dyes revealed that M1 macrophages exhibited significantly higher ROS levels-4.8- and 5.8-fold increases relative to M0 controls, and 3.0- and 3.7-fold increases compared to M2 macrophages (Figure 2E, F). Treatment with the ROS scavenger N-acetylcysteine (NAC) substantially reduced ROS fluorescence intensity, confirming the oxidative profile of M1 macrophages (Figure 2G, H). These results collectively validate the successful induction of M1 polarization and underscore the distinct ROS-rich microenvironment associated with M1 macrophages.
Next, we evaluated the fluorescence activation of MTBPB/GPs in different macrophage subtypes. Confocal imaging revealed that M1 macrophages exhibited a fluorescence signal 3.6 times higher than M0 and 2.8 times higher than M2 cells, aligning with their elevated ROS levels (Figure 3A, B). Flow cytometry analysis further validated this trend, with M1 macrophages displaying 6.0- and 3.3-fold higher fluorescence compared to M0 and M2 cells, respectively. Notably, treatment with the ROS scavenger N-acetylcysteine (NAC) significantly attenuated the fluorescence signal in M1 macrophages, reducing it to less than 50% of the original level (Figure 3C, D). These findings confirm that fluorescence activation of MTBPB/GPs is tightly correlated with intracellular oxidative stress and is selectively triggered by the ROS-enriched environment of M1 macrophages. Mechanistically, the boronate ester moiety on MTBPB is cleaved by H₂O₂, which is abundantly produced in M1 macrophages upon stimulation with LPS and IFN-γ, leading to fluorescence activation. In contrast, M0 macrophages under homeostatic conditions exhibit low ROS levels, insufficient to activate the probe.
Previous studies have shown that GPs are internalized by macrophages via the dectin-1 receptor-mediated endocytic pathway [39]. Transmission electron microscopy confirmed that MTBPB/GPs were successfully phagocytosed by macrophages (Figure 3E). To determine whether this mechanism applies to M1 macrophages, we pretreated cells with laminin (LA), a known dectin-1 receptor inhibitor. LA treatment reduced the uptake of MTBPB/GPs to approximately 20% of the control group, highlighting the pivotal role of dectin-1-mediated endocytosis in probe internalization (Figure 3F).
To investigate the subcellular localization of the probe, we performed colocalization analysis of MTBPB/GPs and lysosomes in LPS-stimulated M1 macrophages using confocal microscopy. The Manders' overlap coefficient was calculated to be 0.58, indicating moderate colocalization (Figure S17). These results suggest that while part of the probe localizes to acidic lysosomes, a substantial fraction escapes into the cytosol, where the pH is more favorable for H₂O₂-mediated activation. Moreover, during M1 polarization, cytosolic H₂O₂-primarily derived from NADPH oxidase and iNOS signaling-plays a central role in mediating oxidative stress and thus enables efficient intracellular activation of MTBPB/GPs [55, 62].
Biocompatibility evaluation of MTBPB/GPs
The biosafety of MTBPB/GPs is essential for their translational potential in in vivo applications. We first evaluated the cytocompatibility of MTBPB/GPs using RAW264.7 macrophages. Cells were incubated with varying concentrations of MTBPB/GPs for 24 h, and viability remained above 90% across all tested doses, indicating minimal cytotoxicity (Figure S18A). Moreover, the phagocytosis of MTBPB/GPs did not impair macrophage migration, further supporting their cellular biocompatibility (Figure S19). To assess potential cytotoxic effects on non-immune cells, primary cardiomyocytes, hepatocytes, and renal tubular epithelial cells were treated with MTBPB/GPs under the same conditions. All tested cell types exhibited high viability (> 90%), suggesting favorable biosafety across major organ-derived cells (Figure S18B-D).
Macrophage polarization phenotype validation. (A) Representative images and quantitative analysis of CD86 expression in macrophages before and after LPS (1 µg/mL) stimulation by flow analysis (n = 5). (B) Measurement of TNF-α concentration and (C) IFN-γ in cell supernatant before and after LPS stimulation (n = 5). (D) Representative and quantitative analysis plots of CD206 measured by flow assay before and after 48 h stimulation with the addition of IL-4 (500 ng/ml) (n = 4). (E) Confocal representative images of intracellular ROS in M0, M1, M2, and M1 + NAC macrophages. (F) Fluorescence quantification plots (n = 4). (G) Flow representative images of intracellular ROS in M0, M1, M2, and M1 + NAC macrophages flow representative images. (H) flow quantitative analysis map (n = 4). ***P < 0.001, **** P < 0.0001
M1 macrophages activate MTBPB/GPs fluorescent signals. (A) Confocal representative images of MTBPB/GPs in M0, M1, M2, and M1+NAC macrophages. (B) Fluorescence quantification plots (n = 4). (C) Flow representative images of MTBPB/GPs in M0, M1, M2, and M1+NAC macrophages. (D) Flow quantitative map analysis of mean fluorescence intensity (n = 4). (E) TEM of MTBPB/GPs phagocytosis by macrophages. (F) Flow cytometry-based quantitative analysis plot of the phagocytosis of MTBPB/GPs by M1 macrophages, both before and after 12 hours of LA treatment (n = 4). ***P < 0.001, **** P < 0.0001.
The in vivo biosafety profile of MTBPB/GPs was further assessed in C57BL/6 mice following daily oral administration for seven consecutive days. Hematological analysis showed no significant changes in leukocyte, erythrocyte, hemoglobin, or differential blood counts compared with untreated controls (Figure S20). Liver and kidney function markers, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine (CR), remained within normal ranges (Figure S21A, B), suggesting weak hepatotoxicity or nephrotoxicity. Histological examination of the heart, liver, spleen, lungs, and kidneys was performed at days 1, 3, and 7 after daily oral administration of a high dose (100 mg/kg) of MTBPB/GPs. Hematoxylin and eosin (H&E) staining revealed no evident tissue damage, inflammatory infiltration, or pathological abnormalities in any of the major organs (Figure S22), indicating excellent systemic biocompatibility. Given the exogenous nature of MTBPB/GPs, their immunogenicity was also assessed. No apparent systemic immune activation was observed following repeated oral administration, as evidenced by stable levels of inflammatory cytokines (Figure S23A, B). Collectively, these results demonstrate that MTBPB/GPs possess excellent cytocompatibility, systemic biosafety, and low immunogenicity, supporting their suitability for oral administration and translational application in vivo.
Oral MTBPB/GPs for monitoring transplant rejection
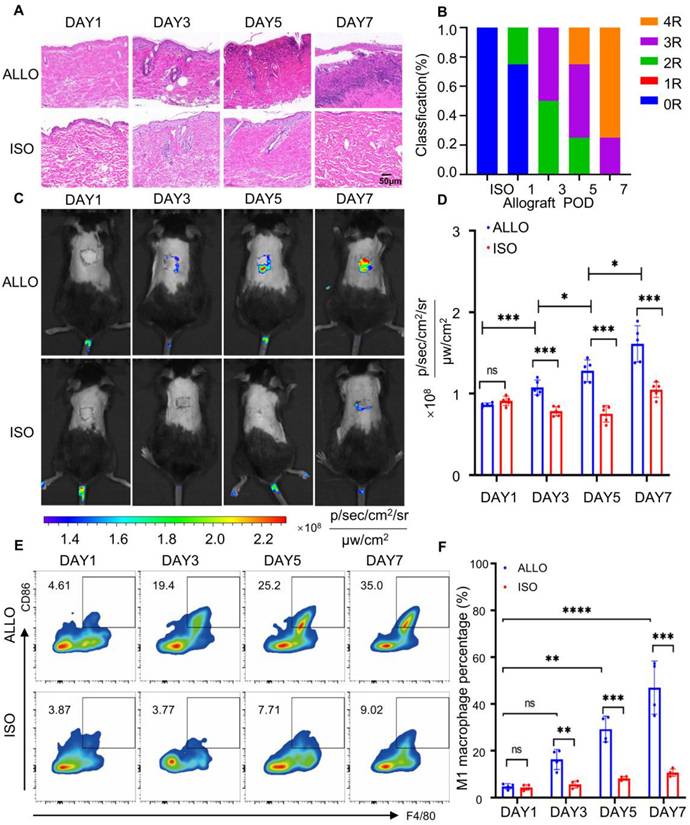
A murine skin transplantation model was established to evaluate allograft rejection and probe performance. C57BL/6 mice served as recipients, with donor skin obtained from either syngeneic C57BL/6 (isograft) or allogeneic BALB/c mice (allograft). In the isograft group, the transplanted skin remained structurally intact and gradually integrated with the host tissue. In contrast, the allograft displayed progressive wrinkling and eventual detachment (Figure S24). Histopathological evaluation was conducted based on the Banff classification system [63, 64]. On postoperative day 1 (POD1), both groups exhibited a rejection grade of 0R, characterized by intact epidermal and dermal architecture with minimal inflammatory infiltration. By POD7, the allograft skin showed massive leukocyte infiltration and extensive epidermal and dermal necrosis, reaching a rejection grade of ≥ 3R (Figure 4A, B). In comparison, the isograft maintained normal architecture with only mild inflammatory changes. These results confirm the occurrence and progression of acute rejection in the allograft group.
To monitor probe distribution and activation, in vivo fluorescence imaging was performed at 0, 6, 12, 24, 36, and 48 h following oral administration of MTBPB/GPs (Figure S25A, B). Fluorescence intensity at the graft site peaked at 36 h, which was subsequently selected as the optimal imaging time point. Quantitative analysis revealed that the fluorescence signal in allograft skin on POD7 was 1.87-fold higher than on POD1. Notably, fluorescence in the allograft group began to increase as early as POD3, reaching a 1.54-fold elevation compared to the isograft by POD7 (Figure 4C, D), indicating probe activation in response to progressive rejection (Figure S26).
To evaluate the biodistribution of MTBPB/GPs, ex vivo fluorescence imaging of major organs (heart, liver, spleen, lungs, kidneys) was performed at 24 and 36 h post-administration on POD6. Strong fluorescence signals were observed in the liver and kidneys (Figure S27A-D), consistent with previous reports indicating that these are the primary clearance organs for orally administered GPs [65-67].
To confirm that the fluorescence signals originated from probe activation by pro-inflammatory M1 macrophages, we performed immunofluorescence staining of graft sections using CD68 and iNOS as markers for M1 macrophages [59, 60]. Immuno-staining on POD1, 3, 5, and 7 revealed progressively increasing M1 macrophage infiltration in the allograft, which became significantly elevated from POD3 onward. By POD7, the number of CD68⁺ iNOS⁺ cells in the allograft was 9.24-fold higher than in the isograft group (Figure S28). These results were further validated by flow cytometry, which showed a 2.9-fold increase in M1 macrophages at POD3 and a 4.2-fold increase by POD7 (Figure 4E, F). Collectively, these data demonstrate that MTBPB/GPs preferentially accumulate and activate at sites of allograft rejection, where M1 macrophages are enriched. The observed correlation between fluorescence intensity and M1 macrophage infiltration confirms the utility of MTBPB/GPs for noninvasive, real-time monitoring and early diagnosis of transplant rejection.
Oral MTBPB/GPs to monitor immunosuppressant efficacy
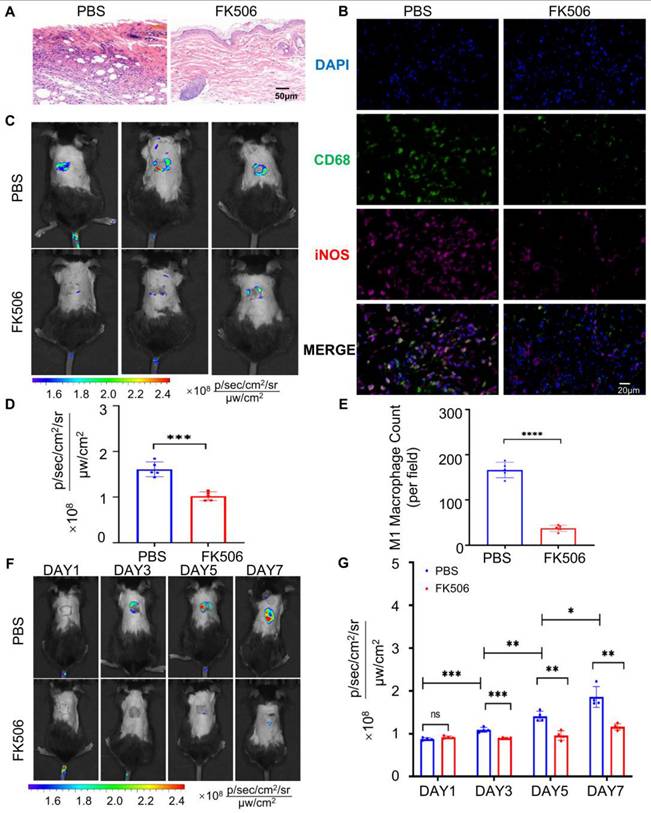
Real-time assessment of immunotherapy efficacy is essential for managing transplant rejection and minimizing post-transplant complications. To this end, we investigated the utility of orally administered MTBPB/GPs for monitoring the effectiveness of immunosuppressive treatments. Tacrolimus (FK506), a first-line immunosuppressant, primarily inhibits the calcineurin pathway in T cell, thereby suppressing interleukin-2 production and T-cell proliferation [68, 69]. Additionally, FK506 effectively inhibits macrophage proliferation and the secretion of TNF-α [70, 71]. We established an allograft rejection model in mice and administered FK506 subcutaneously at a dosage of 3 mg/kg daily for 7 days. Histopathological analysis of grafted skin on POD 7 revealed that the FK506-treated group maintained intact skin architecture, whereas the control group exhibited extensive necrosis and significant inflammatory cell infiltration (Figure 5A). Immunofluorescence analysis quantified CD68⁺ iNOS⁺ M1 macrophages within the grafted skin, showing that the control group had approximately 4.4 times more M1 macrophages than the FK506-treated group (Figure 5B and 5E). This indicates that FK506 effectively reduces M1 macrophage infiltration into the graft.
Subsequently, we investigated whether oral administration of MTBPB/GPs could facilitate imaging of M1 macrophages within the graft, thereby enabling evaluation of therapeutic efficacy. After 7 days of FK506 treatment, the fluorescence signal decreased to approximately half of the pre-treatment level (Figure 5C, D), suggesting that oral MTBPB/GPs can reflect the efficacy of FK506. To assess the potential of MTBPB/GPs for monitoring immunosuppressant efficacy, we orally administered MTBPB/GPs on the day after surgery and conducted imaging on POD 1, 3, 5, and 7 (Figure S29). In the FK506-treated group, fluorescence intensity began to decrease on POD3 and was reduced by 1.6-fold compared to the control group by POD7 (Figure 5F, G). These findings demonstrate that MTBPB/GPs can monitor M1 macrophage dynamics over time, offering a non-invasive approach for real-time evaluation of immunosuppressive therapy efficacy.
Establishment of a skin graft rejection model and monitoring of graft rejection using oral administration of MTBPB/GPs. (A) Histologic analysis of grafted skin at POD1, 3, 5, and 7 (n = 4). (B) Grade of rejection of allografts and isografts assessed by Banff classification. (C) Representative images of oral MTBPB/GPs at different time points after transplantation (POD1, 3, 5, and 7). (D) Fluorescence quantification image of the transplanted skin (n = 5). (E) Representative flow cytometry contour plots show F4/80⁺ CD86⁺ double-positive M1 macrophage populations in graft tissues. (F) Quantification of flow cytometry. *P < 0.05, ***P < 0.001, **** P < 0.0001.
MTBPB/GPs were administered orally to assess the effectiveness of the immunosuppressive therapy. (A) Pathology results of transplanted skin after 7 days of subcutaneous injection of FK506 or PBS (n = 4). (B) Immunofluorescence staining of CD68⁺ iNOS⁺ M1 macrophages in allografted skin after 7 days of subcutaneous injection of FK506 or PBS; FITC-labeled for staining CD68, Cy3-labeled for staining iNOS; nuclei were stained with DAPI. (C) Whole-body fluorescence imaging was conducted on FK506- or PBS-treated allograft mice over a period of 7 consecutive days. (D) Quantification of the transplanted skin (n = 5). (E) Quantification of CD68+ iNOS+ M1 macrophages in transplanted skin (n = 4). (F) Whole-body fluorescence imaging was carried out on allograft subcutaneous injection of FK506 or PBS over a span of 7 consecutive days (POD 1, 3, 5, and 7). (G) Quantification of transplanted skin (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001.
Mechanism of oral MTBPB/GPs targeting graft tissue
Following oral administration, GPs are internalized by intestinal macrophages via M cells in Peyer's patches, after which they migrate through the lymphatic system to peripheral tissues [72, 73]. This macrophage-mediated delivery route is critical for enabling specific probe accumulation at inflammatory sites [74, 75]. To evaluate the necessity of macrophage targeting for effective graft localization, we compared the biodistribution of free MTBPB versus MTBPB/GPs. As shown in Figure S30A-C, free MTBPB failed to accumulate at the graft, while MTBPB/GPs exhibited a 1.67-fold higher fluorescence signal at the transplant site, confirming the pivotal role of GPs in enabling efficient macrophage-mediated delivery.
In our previous work [39], we demonstrated that orally administered GPs are taken up by M cells in Peyer's patches and phagocytosed by intestinal macrophages. These macrophages then migrate through gut-associated lymphoid tissues (GALT), including the mesenteric lymph nodes (MLNs) and draining lymph nodes (DLNs), and ultimately home to sites of inflammation. This process was confirmed by time-resolved fluorescence imaging, showing sequential probe accumulation in Peyer's patches, MLNs, DLNs, and the graft. In this study, we employed the same GP-based delivery platform for the H₂O₂-activatable AIE probe MTBPB. First, we performed ex vivo fluorescence imaging of the gastrointestinal tract at 0, 6, 12, 24, and 36 h post-oral administration. Across all time points, the GI tract exhibited minimal fluorescence signal (Figure 6A), suggesting that MTBPB/GPs remain in an “off” state in the gastrointestinal environment, likely due to the lack of sufficient ROS, thus preserving imaging specificity.
In vivo transport of MTBPB/GPs to grafted skin. (A) Distribution of MTBPB/GPs in the ex vivo gastrointestinal tract at various time intervals (0, 6, 12, 24, 36 h) of allografted mice on POD 6 (n = 4). (B) Representative images of MTBPB/GPs in lymph nodes (PP, MLNs, DLNs) and graft skin and (C) quantitative fluorescence intensity analysis graph (n = 4). (D) Immunofluorescence staining of allografted skin on POD6 after oral administration of MTBPB/GPs for 36 h. DAPI stained nuclei, FITC labeled CD68, Cy5 labeled iNOS, MTBPB/GPs autofluoresced in red. (E) expression levels of MCP-1 and (F) CSF-1 in both healthy and transplanted skin (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001.
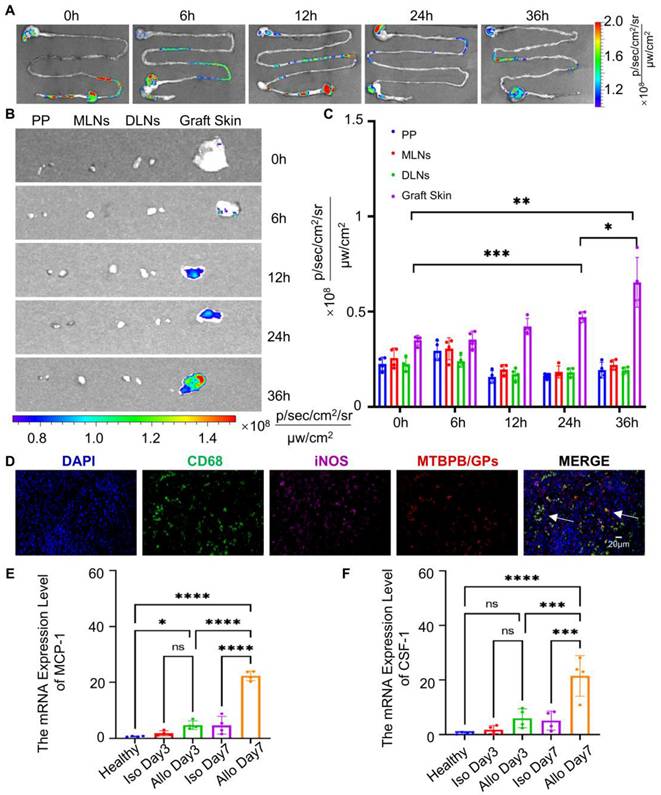
To further elucidate the spatiotemporal behavior of the probe, we conducted a time-course analysis (0-36 h) using ex vivo organ imaging and fluorescence histology. Fluorescence signals in lymphoid tissues remained low, consistent with probe quiescence during systemic trafficking (Figure S31). In contrast, graft-specific fluorescence progressively increased over time, indicating selective probe activation within the inflammatory microenvironment enriched in H₂O₂ due to M1 macrophage infiltration (Figure 6B, C). Immunofluorescence analysis on POD7 confirmed significant colocalization between MTBPB/GPs and CD68⁺ iNOS⁺ M1 macrophages in the allograft (Figure 6D). These findings support a mechanism in which intestinal macrophages engulf MTBPB/GPs and migrate via the lymphatic system to the graft, where they undergo M1 polarization in response to inflammatory stimuli, thereby generating elevated ROS levels that activate the probe.
During graft rejection, immune cells particularly T cells and macrophages—secrete chemokines that guide monocyte and macrophage recruitment to the graft [76-78]. Monocyte chemoattractant protein-1 (MCP-1) and colony-stimulating factor-1 (CSF-1) are key mediators of this chemotactic process [79, 80]. To investigate their involvement, we quantified mRNA expression levels of MCP-1 and CSF-1 in graft tissues at POD3 and POD7. Both chemokines were significantly upregulated by POD7 (Figure 6E, F), consistent with increased macrophage recruitment and M1 polarization at later stages of rejection. These findings confirm the mechanism of macrophage-mediated trafficking of MTBPB/GPs following oral administration. The probe remains inactive during transit and is selectively activated in situ by oxidative stress from M1 macrophages at the site of rejection. This spatially restricted activation provides high imaging specificity and supports the clinical utility of MTBPB/GPs as a noninvasive platform for early detection and monitoring of transplant rejection.
Discussion
Biopsy remains the clinical gold standard for diagnosing transplant rejection [6], yet it is invasive and carries risks such as bleeding, infection, and sampling error. In routine practice, non-invasive alternatives rely on serum biomarkers or imaging assessments [3, 4, 8]. However, commonly used biomarkers like creatinine and urea suffer from poor specificity, as their elevations may reflect various non-rejection-related causes of graft dysfunction [81, 82]. Similarly, conventional imaging modalities such as ultrasound and MRI provide only anatomical or perfusion-level information and lack sensitivity for early immune activation or real-time tracking of immune cell infiltration.
To overcome these limitations, we developed MTBPB/GPs an orally administered, H₂O₂-activatable, AIE-based biomimetic probe designed for macrophage-targeted imaging of transplant rejection. This system offers three key advantages: (i) excellent biosafety, as demonstrated by both in vitro and in vivo evaluations; (ii) high sensitivity enabled by selective fluorescence activation in M1 macrophage-rich, oxidative microenvironments; and (iii) the capability to dynamically monitor immune response and treatment efficacy with a single oral dose.
Despite these advantages, the current imaging system is limited by shallow penetration depth, restricting its utility in monitoring deeply situated organs such as kidney transplants. To address this, future iterations of the probe will be designed to operate within the second near-infrared window (NIR-II), which offers deeper tissue penetration and improved spatial resolution. In addition, combining this probe with other imaging modalities-such as PET, CT, or MRI-could enable a multimodal imaging strategy, thereby improving diagnostic accuracy and spatiotemporal resolution. In conclusion, MTBPB/GPs complement and extend current diagnostic strategies by providing a non-invasive, macrophage-responsive, and dynamically activatable platform for early detection and longitudinal monitoring of transplant rejection. This approach may significantly advance personalized transplant management and improve patient outcomes.
Conclusion
In this study, we developed an innovative platform for non-invasive immune monitoring of transplant rejection using an H₂O₂-activatable biomimetic probe based on aggregation-induced emission (AIE) glucan particles (MTBPB/GPs). The probe demonstrates excellent biocompatibility, characterized by low cytotoxicity, minimal hemolytic activity, negligible immunogenicity, and low systemic toxicity, making it a promising alternative to conventional biopsy for graft evaluation. Importantly, MTBPB/GPs enable selective fluorescence activation by pro-inflammatory M1 macrophages, allowing for early and highly sensitive detection of transplant rejection. A single oral administration facilitates dynamic tracking of the immune response and therapeutic efficacy of immunosuppressive regimens, enabling real-time monitoring without repeated interventions. By integrating inflammation-triggered activation with macrophage-mediated delivery, MTBPB/GPs offer a high signal-to-noise ratio, excellent specificity, and operational simplicity. Collectively, this approach holds strong translational potential to enhance immune surveillance, reduce dependence on invasive procedures, and support personalized post-transplant management.
Abbreviations
ALT: alanine aminotransferase; AST: aspartate aminotransferase; AIE: aggregation-induced emission; BUN: blood urea nitrogen; CR: creatinine; CSF-1: colony-.stimulating factor-1; DLNs: draining lymph nodes; FI-IR: Fourier Transform Infrared; FK506: Tacrolimus; 18F-FDG : 18F-fluorodeoxyglucose; GPs: glucan particles; H₂O₂: hydrogen peroxide; HPLC: high-performance liquid chromatography; ICT: intramolecular charge transfer; ISC: intersystem crossing; LPS: lipopolysaccharide; LA: laminin; MLNs: mesenteric lymph nodes; MCP-1: Monocyte chemoattractant protein-1; MRI: magnetic resonance imaging; NIR-II: second near-infrared window; POD: postoperative day; PP: Peyer's patches; PET: positron emission computed tomography; ROS: reactive oxygen species; SOC: spin-orbit coupling; SPIONs: superparamagnetic iron oxide nanoparticles; SEM: scanning electron microscopy; TR: transplant rejection; TEM: transmission electron microscopy; XRD: X-ray diffraction; YM: yeast microcapsules.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This study was supported by the Natural Science Foundation of China (82171964, 82230066, 82202234, 82427805, 82201408), the Natural Science Foundation of Hubei (2021CFA046 and 2023AFB753), and the Natural Science Foundation of Wuhan (2024040801020350).
Data availability
The data can be accessed upon request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kahan BD. Individuality: The barrier to optimal immunosuppression. Nat Rev Immunol. 2003;3:831-8
2. Siren EMJ, Luo HD, Tam F, Montgomery A, Enns W, Moon H. et al. Prevention of vascular-allograft rejection by protecting the endothelial glycocalyx with immunosuppressive polymers. Nat Biomed Eng. 2021;5:1202-16
3. Lo DJ, Kaplan B, Kirk AD. Biomarkers for kidney transplant rejection. Nat Rev Nephrol. 2014;10:215-25
4. Fogo AB, Lusco MA, Najafian B, Alpers CE. Ajkd atlas of renal pathology: Ischemic acute tubular injury. Am J Kidney Dis. 2016;67:e25
5. Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G. et al. 2011 consensus statement on endomyocardial biopsy from the association for european cardiovascular pathology and the society for cardiovascular pathology. Cardiovasc Pathol. 2012;21:245-74
6. Yang HM, Lai CK, Gjertson DW, Baruch-Oren T, Ra SH, Watts W. et al. Has the 2004 revision of the international society of heart and lung transplantation grading system improved the reproducibility of the diagnosis and grading of cardiac transplant rejection? Cardiovasc Pathol. 2009;18:198-204
7. Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L. et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374:333-43
8. Halloran PF, Famulski KS, Reeve J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol. 2016;12:534-48
9. Liu C, Hu F, Jiao G, Guo Y, Zhou P, Zhang Y. et al. Dental pulp stem cell-derived exosomes suppress m1 macrophage polarization through the ros-mapk-nfκb p65 signaling pathway after spinal cord injury. J Nanobiotechnology. 2022;20:65
10. Tsai C-F, Chen G-W, Chen Y-C, Shen C-K, Lu D-Y, Yang L-Y. et al. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients. 2022;14:67
11. Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. Ros play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898-914
12. Tan H-Y, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. 2016;2016:2795090
13. Zhang HW, Li ZN, Li W. M2 macrophages serve as critical executor of innate immunity in chronic allograft rejection. Front Immunol. 2021;17:648539
14. Braesen JH, Khalifa A, Schmitz J, Dai W, Einecke G, Schwarz A. et al. Macrophage density in early surveillance biopsies predicts future renal transplant function. Kidney Int. 2017;92:479-89
15. Panzer SE. Macrophages in transplantation: A matter of plasticity, polarization, and diversity. Transplantation. 2022;106:257-67
16. Devraj VM, Kalidindi K, Guditi S, Uppin M, Taduri G. Macrophage polarization in kidney transplant patients. Transpl Immunol. 2022;75:101717
17. van den Bosch TPP, Caliskan K, Kraaij MD, Constantinescu AA, Manintveld OC, Leenen PJM. et al. Cd16+monocytes and skewed macrophage polarization toward m2 type hallmark heart transplant acute cellular rejection. Front Immunol. 2017;24:346
18. Zhang M, Nakamura K, Kageyama S, Lawal AO, Gong KW, Bhetraratana M. et al. Myeloid ho-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. Jci Insight. 2018;3:e120596
19. Hang Z, Wei J, Zheng M. et al. Iguratimod attenuates macrophage polarization and antibody mediated rejection after renal transplant by regulating klf4. Front Pharmacol. 2022;9:865363
20. Chae EY, Song EJ, Sohn JY, Kim S-T, Woo CW, Gong G. et al. Allogeneic renal graft rejection in a rat model: In vivo mr imaging of the homing trait of macrophages. Radiology. 2010;256:847-54
21. Chen C-L, Zhang H, Ye Q, Hsieh W-Y, Hitchens TK, Shen H-H. et al. A new nano-sized iron oxide particle with high sensitivity for cellular magnetic resonance imaging. Mol Imaging Biol. 2011;13:825-39
22. Kanno S, Wu YJL, Lee PC, Dodd SJ, Williams M, Griffith BP. et al. Macrophage accumulation associated with rat cardiac allograft rejection detected by magnetic resonance imaging with ultrasmall superparamagnetic iron oxide particles. Circulation. 2001;104:934-8
23. O'Neill ASG, Terry SYA, Brown K, Meader L, Wong AMS, Cooper JD. et al. Non-invasive molecular imaging of inflammatory macrophages in allograft rejection. Ejnmmi Res. 2015;5:69
24. Reuter S, Schnoeckel U, Edemir B, Schroeter R, Kentrup D, Pavenstaedt H. et al. Potential of noninvasive serial assessment of acute renal allograft rejection by 18f-fdg pet to monitor treatment efficiency. J Nucl Med. 2010;51:1644-52
25. Zacharovova K, Berkova Z, Jirak D, Herynek V, Vancova M, Dovolilova E. et al. Processing of superparamagnetic iron contrast agent ferucarbotran in transplanted pancreatic islets. Contrast Media Mol Imaging. 2012;7:485-93
26. Yang YW, Chen Y, Pei P, Fan Y, Wang SF, Zhang HX. et al. Fluorescence-amplified nanocrystals in the second near-infrared window for in vivo real-time dynamic multiplexed imaging. Nat Nanotechnol. 2023;18:1195-204
27. Wang T, Wang SF, Liu ZY, He ZY, Yu P, Zhao MY. et al. A hybrid erbium(iii)-bacteriochlorin near-infrared probe for multiplexed biomedical imaging. Nat Mater. 2021;20:1571-8
28. Chen Y, Wang SF, Zhang F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat Rev Bioeng. 2023;1:60-78
29. Fan Y, Wang PY, Lu YQ, Wang R, Zhou L, Zheng XL. et al. Lifetime-engineered nir-ii nanoparticles unlock multiplexed in vivo imaging. Nat Nanotechnol. 2018;13:941-6
30. Pei P, Chen Y, Sun CX, Fan Y, Yang YM, Liu X. et al. X-ray-activated persistent luminescence nanomaterials for nir-ii imaging. Nat Nanotechnol. 2021;16:1011-8
31. Ming J, Chen Y, Miao H, Fan Y, Wang SF, Chen ZH. et al. High-brightness transition metal-sensitized lanthanide near-infrared luminescent nanoparticles. Nat Photonics. 2024 18
32. Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E. et al. Orally delivered sirna targeting macrophage map4k4 suppresses systemic inflammation. Nature. 2009;458:1180-4
33. Miao YB, Lin YJ, Chen KH, Luo PK, Chuang SH, Yu YT. et al. Engineering nano- and microparticles as oral delivery vehicles to promote intestinal lymphatic drug transport. Adv Mater. 2021;33:e2104139
34. Zhou X, Zhang X, Han S, Dou Y, Liu M, Zhang L. et al. Yeast microcapsule-mediated targeted delivery of diverse nanoparticles for imaging and therapy via the oral route. Nano Lett. 2017;17:1056-64
35. Miao YB, Chen KH, Chen CT, Mi FL, Lin YJ, Chang Y. et al. A noninvasive gut-to-brain oral drug delivery system for treating brain tumors. Adv Mater. 2021;33:e2100701
36. Fernández A, Vendrell M. Smart fluorescent probes for imaging macrophage activity. Chem Soc Rev. 2016;45:1182-96
37. Wu LL, Li ZL, Wang K, Groleau RR, Rong XD, Liu XT. et al. Advances in organic small molecule-based fluorescent probes for precision detection of liver diseases: A perspective on emerging trends and challenges. J Am Chem Soc. 2025;147:9001-18
38. Wang WJ, Xin ZY, Su XX, Hao L, Qiu ZJ, Li K. et al. Aggregation-induced emission luminogens realizing high-contrast bioimaging. ACS Nano. 2025;19:281-306
39. Gao T, Wu Y, Wang W, Deng C, Chen Y, Yi L. et al. Biomimetic glucan particles with aggregation-induced emission characteristics for noninvasive monitoring of transplant immune response. ACS Nano. 2021;15:11908-28
40. Huang J, Chen X, Jiang Y, Zhang C, He S, Wang H. et al. Renal clearable polyfluorophore nanosensors for early diagnosis of cancer and allograft rejection. Nat Mater. 2022;21:598-607
41. Huang J, Xian S, Liu Y, Chen X, Pu K, Wang H. A renally clearable activatable polymeric nanoprobe for early detection of hepatic ischemia-reperfusion injury. Adv Mater. 2022;34:e2201357
42. Paschalidi P, Gkouveris I, Soundia A, Kalfarentzos E, Vardas E, Georgaki M. et al. The role of m1 and m2 macrophage polarization in progression of medication-related osteonecrosis of the jaw. Clin Oral Investig. 2021;25:2845-57
43. Shao X. Roles of m1 and m2 macrophage infiltration in post-renal transplant antibody-mediated rejection. Transpl Immunol. 2024;85:102076
44. Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK. et al. M1/m2 macrophages and their overlaps - myth or reality? Clin Sci (Lond). 2023;137:1067-93
45. Lu X, Xu Z, Shu F, Wang Y, Han Y, Yang X. et al. Reactive oxygen species responsive multifunctional fusion extracellular nanovesicles: Prospective treatments for acute heart transplant rejection. Adv Mater. 2024: e2406758.
46. Li Y, Liang Q, Zhou L, Cao Y, Yang J, Li J. et al. An ros-responsive artesunate prodrug nanosystem co-delivers dexamethasone for rheumatoid arthritis treatment through the hif-1α/nf-kb cascade regulation of ros scavenging and macrophage repolarization. Acta Biomater. 2022;152:406-24
47. Kalyanaraman B, Cheng G, Hardy M, Ouari O, Bennett B, Zielonka J. Teaching the basics of reactive oxygen species and their relevance to cancer biology: Mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biology. 2018;15:347-62
48. de Gracia Lux C, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N. et al. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J Am Chem Soc. 2012;134:15758-64
49. Hirao H, Dery KJ, Kageyama S, Nakamura K, Kupiec-Weglinski JW. Heme oxygenase-1 in liver transplant ischemia-reperfusion injury: From bench-to-bedside. Free Radic Biol Med. 2020;157:75-82
50. Zheng D-J, Yang Y-S, Zhu H-L. Recent progress in the development of small-molecule fluorescent probes for the detection of hydrogen peroxide. TrAC-Trends Anal Che. 2019;118:625-51
51. Wu Y, Jin Q, Chen Y, Li H, Deng C, Sun Z. et al. Bioinspired beta-glucan microcapsules deliver fk506 to lymph nodes for treatment of cardiac allograft acute rejection. Biomater Sci. 2020;8:5282-92
52. Wang Y, Song Y, Xu L, Zhou W, Wang W, Jin Q. et al. A membrane-targeting aggregation-induced emission probe for monitoring lipid droplet dynamics in ischemia/reperfusion-induced cardiomyocyte ferroptosis. Adv Sci. 2024;11:e2309907
53. Shen Y, Wang B, Wang P, Chen Y, Xu Z, Huang W. et al. Achieving dual emission of fluorescence and phosphorescence from anti-kasha's metal-organic halides for information encryption. Inorg Chem. 2024;63:12073-80
54. Lu T, Chen F. Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem. 2011;33:580-92
55. Roth A, Li H, Anorma C, Chan J. A reaction-based fluorescent probe for imaging of formaldehyde in living cells. J Am Chem Soc. 2015;137:10890-3
56. Bartholomew JW, Mittwer T. Demonstration of yeast bud scars with the electron microscope. J. Bacteriol. 1953;65:272-5
57. M Hisamatsu TM, K Teranishi, T Yamada. The correlation between adhesion of schizophyllan to yeast glucan and its effect on regeneration of yeast protoplast. Carbohydr Res. 1997;298:117-21
58. Li M, Wu J, Geng W, Gao P, Yang Y, Li X. et al. Interaction pathways of implant metal localized corrosion and macrophage inflammatory reactions. Bioact Mater. 2024;27:355-67
59. Ma C, Wang C, Zhang Y, Li Y, Fu K, Gong L. et al. Phillygenin inhibited m1 macrophage polarization and reduced hepatic stellate cell activation by inhibiting macrophage exosomal mir-125b-5p. Biomed Pharmacother. 2023;159:114264
60. Mondadori C, Chandrakar A, Lopa S, Wieringa P, Talo G, Perego S. et al. Assessing the response of human primary macrophages to defined fibrous architectures fabricated by melt electrowriting. Bioact Mater. 2023;27:209-22
61. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili S-A, Mardani F. et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425-40
62. Lambeth JD. Nox enzymes and the biology of reactive oxygen. Nature Reviews Immunology. 2004;4:181-9
63. Etra JW, Grzelak MJ, Fidder SAJ, Kolegraff K, Bonawitz S, Shores J. et al. A skin rejection grading system for vascularized composite allotransplantation in a preclinical large animal model. Transplantation. 2019;103:1385-91
64. Roy SF, Krishnan V, Trinh VQ, Collette S, Dufresne SF, Borsuk DE. et al. Lymphocytic vasculitis associated with mild rejection in a vascularized composite allograft recipient: A clinicopathological study. Transplantation. 2020;104:e208-e13
65. Hino S, Kito A, Yokoshima R, Sugino R, Oshima K, Morita T. et al. Discharge of solubilized and dectin-1-reactive β-glucan from macrophage cells phagocytizing insoluble β-glucan particles: Involvement of reactive oxygen species (ros)-driven degradation. Biochem Biophys Res Commun. 2012;421:329-34
66. Yu M, Chen Z, Guo W, Wang J, Feng Y, Kong X. et al. Specifically targeted delivery of protein to phagocytic macrophages. Int J of Nanomed. 2015;4:1743-57
67. Sun Y, Duan B, Chen H, Xu X. A novel strategy for treating inflammatory bowel disease by targeting delivery of methotrexate through glucan particles. Adv Healthc Mater. 2020;9:e1901805
68. Sano T, Nakamura Y, Matsunaga Y, Takahashi T, Azuma M, Okano Y. et al. Fk506 and cyclosporin a inhibit granulocyte/macropahge colony-stimulating factor production by mononuclear cells in asthma. Eur Respir J. 1995;8:1473-8
69. Hortelano S, López-Collazo E, Boscá L. Protective effect of cyclosporin a and fk506 from nitric oxide-dependent apoptosis in activated macrophages. Br J Pharmacol. 1999;126:1139-46
70. Setkowicz Z, Caryk M, Szafraniec M, Zmudzinska A, Janeczko K. Tacrolimus (fk506) and cyclosporin a reduce macrophage recruitment to the rat brain injured at perinatal and early postnatal periods. Neurol Res. 2009;31:1060-7
71. Vichare R, Crelli C, Liu L, Das AC, McCallin R, Zor F. et al. A reversibly thermoresponsive, theranostic nanoemulgel for tacrolimus delivery to activated macrophages: Formulation and in vitro validation. Pharmaceutics. 2023;15:2372
72. Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L. et al. Dectin-1 is a major β-glucan receptor on macrophages. J Exp Med. 2002;196:407-12
73. Tesz GJ, Aouadi M, Prot M, Nicoloro SM, Boutet E, Amano SU. et al. Glucan particles for selective delivery of sirna to phagocytic cells in mice. Biochem J. 2011;436:351-62
74. Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest. 1999;103:73-80
75. Zhang X, Zhao Y, Xu Y, Pan Y, Chen F, Kumar A. et al. In situ self-assembly of peptides in glucan particles for macrophage-targeted oral delivery. J Mater Chem B. 2014;2:5882-90
76. Sakanashi Y, Takeya M, Yoshimura T, Feng L, Morioka T, Takahashi K. Kinetics of macrophage subpopulations and expression of monocyte chemoattractant protein-1 (mcp-1) in bleomycin-induced lung injury of rats studied by a novel monoclonal antibody against rat mcp-1. J Leukocyte Biol. 1994;56:741-50
77. Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S. et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111-20
78. Yoshimura T, Imamichi T, Weiss JM, Sato M, Li L, Matsukawa A. et al. Corrigendum: Induction of monocyte chemoattractant proteins in macrophages via the production of granulocyte/macrophage colony-stimulating factor by breast cancer cells. Front Immunol. 2016;13:1524
79. Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the g1 phase of the cell cycle. Cell. 1991;65:701-13
80. Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF. et al. Csf-1r inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264-72
81. Chen YH, Zhang L, Liu JF, Zhang PY, Chen XY, Xie MX. Molecular imaging of acute cardiac transplant rejection: Animal experiments and prospects. Transplantation. 2017;101:1977-86
82. Choy JC. Granzymes and perforin in solid organ transplant rejection. Cell Death Differ. 2010;17:567-76
Author contact
![]() Corresponding author: gaotangedu.cn; xiemxedu.cn; zli429edu.cn.
Corresponding author: gaotangedu.cn; xiemxedu.cn; zli429edu.cn.
 Global reach, higher impact
Global reach, higher impact