13.3
Impact Factor
Theranostics 2025; 15(15):7779-7801. doi:10.7150/thno.111503 This issue Cite
Review
Harnessing mRNA for heart health: a new era in cardiovascular treatment
1. Department of Cardiology, Affiliated Hospital of Zunyi Medical University, Zunyi, China.
2. Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, Shanghai, China.
3. Department of Cardiology, Affiliated Hospital of Qingdao University, Qingdao, China.
4. Department of cardiology, Chongqing University Central Hospital (Chongqing Emergency Medical Center), College of Bioengineering, Chongqing University, Chongqing, China/
*These authors contributed equally: Fengli Peng, Zhimei Qiu, Zimu Wang and Fuhai Li
Received 2025-2-4; Accepted 2025-6-3; Published 2025-7-2
Abstract
mRNA serves as a versatile platform for the expression of paracrine factors, thereby promoting cardioprotection and regeneration. In recent years, mRNA and gene editing technologies have emerged as innovative tools for tackling complex diseases. Among these, mRNA therapeutics offer distinct advantages, including favorable immunological properties, strong safety profiles, and superior flexibility compared to conventional gene-based vaccines. Specifically, they can elicit a balanced immune response—encompassing both cellular and humoral immunity—without being limited by MHC haplotypes. Furthermore, mRNA therapy represents a particularly safe treatment modality. As a minimal and transient carrier of genetic information that does not integrate into the host genome, it significantly reduces the risk of insertional mutagenesis. Importantly, mRNA can be used to express virtually any protein without requiring changes to the production process, thus offering substantial flexibility in drug development. Collectively, these attributes highlight the great potential of mRNA as a next-generation therapeutic platform, particularly for cardiovascular diseases. This review summarizes recent progress in the development and application of mRNA-based drug formulations for cardiovascular therapy.
Keywords: mRNA-based drug formulations, gene therapy, novel therapies, nanomedicine, cardiovascular diseases
Introduction
Cardiovascular disease (CVD) remains a leading global health burden, contributing substantially to both disability and mortality. According to the World Health Organization, CVD is responsible for approximately 18 million deaths annually, representing nearly one-third of all global fatalities. However, the impact of CVD extends beyond mortality [1]. It also leads to long-term disability and escalating healthcare costs, which in turn significantly diminish the quality of life for patients and their families. Although various treatment options are available—including antihypertensive medications, lipid-lowering agents, and cardiac surgical interventions—several critical challenges remain. These include delayed diagnosis, poor patient adherence, unequal access to healthcare, and a lack of innovative therapies. Collectively, these factors hinder the effective prevention, treatment, and long-term management of cardiovascular disease [2].
To address these limitations, emerging therapies—including cell-based treatments and nucleic acid therapeutics—aim to target the root causes of disease, enabling more precise and effective interventions. These strategies hold great potential for meeting unmet clinical needs, improving patient outcomes, and potentially altering the course of cardiovascular disease. Among these innovations, mRNA has emerged as a particularly versatile platform for both drug and vaccine development. It offers exceptional flexibility in molecular design, scalable production, and rapid adaptation to diverse clinical applications. By encoding virtually any protein, mRNA enables the development of both prophylactic and therapeutic vaccines for a wide range of diseases, including infections, cancers, and protein deficiency disorders. In the context of cardiovascular disease, mRNA-based therapies show promise in enhancing cardiac repair, reducing the incidence of adverse cardiovascular events, and improving patients' quality of life [3]. Furthermore, mRNA technology facilitates the advancement of personalized medicine by enabling therapies to be tailored to individual genetic and clinical profiles, thereby increasing the precision and effectiveness of treatment strategies [4].
Building on this foundation, this narrative review aims to provide a comprehensive overview of emerging therapeutic strategies for cardiovascular disease, with a particular focus on the development of mRNA-based drug formulations. It outlines key considerations such as mRNA manufacturing and quality control, structural design and formulation strategies, antigen and protein expression, and the immunological characteristics of mRNA. The review also highlights the therapeutic potential of mRNA in cardiovascular applications. Furthermore, it discusses the challenges associated with the clinical adoption of these therapies, including regulatory, economic, and ethical factors, and explores future directions for their implementation. Ultimately, this review seeks to contribute to global efforts aimed at reducing the burden of cardiovascular disease and improving cardiovascular health outcomes (Figure 1).
1. Overview of mRNA Drug Formulations
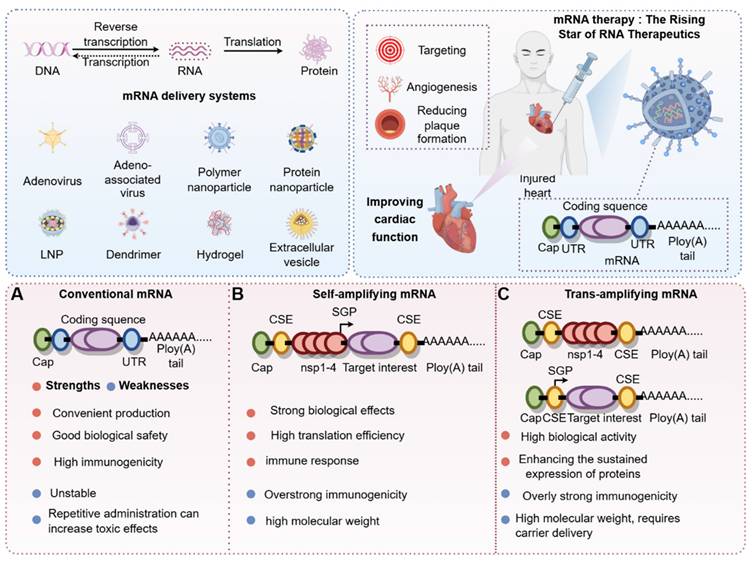
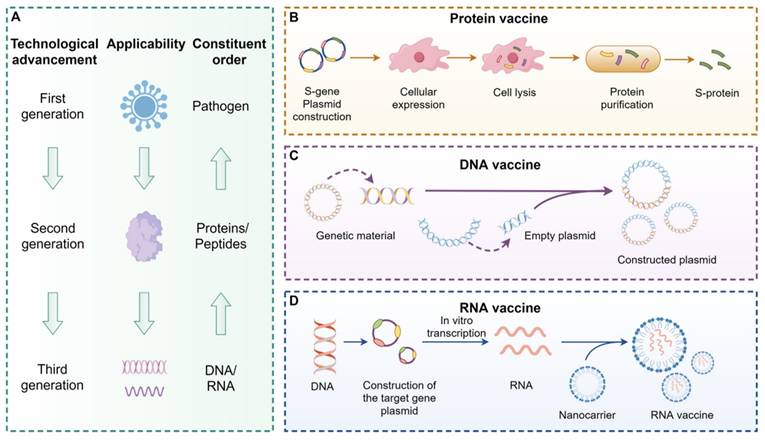
mRNA-based nucleic acid therapeutics were first proposed three decades ago as a novel strategy for developing vaccines that are safe, versatile, and easy to manufacture (Figure 2). The basic structure of an mRNA molecule comprises five key components: a 5′ cap, a 5′ untranslated region (UTR), an open reading frame (ORF) encoding the target protein, a 3′ UTR, and a poly (A) tail. Unlike traditional vaccines, mRNA does not integrate into the host genome, thereby eliminating the risk of insertional mutagenesis. Additionally, mRNA vaccines can be produced using cell-free systems, allowing for rapid, scalable, and cost-efficient manufacturing [5]. A notable advantage of mRNA technology is its ability to encode multiple antigens within a single formulation, which enhances immune responses against rapidly evolving pathogens and provides broad-spectrum protection against various microorganisms and viruses [6] (Figure 3).
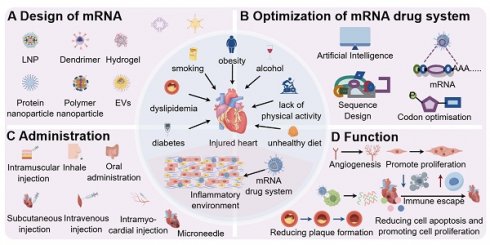
Design and application of mRNA drug system (by Figdraw). After mRNA is modified with different drug carriers, it can be targeted to the injured heart for repair, and this design mode can be used as a diagnostic medicine platform for the centralized diagnosis of the cardiovascular system. A: carrier of mRNA drugs, such as AAV, LNP, hydrogel, EVs, etc.; B: Advantages of mRNA technology, such as: targeting heart, high drug load, controlled release and long-acting; C: mRNA technology can realize gene targeting therapy and improve cardiac function through immune escape; D: mRNA technology can realize the diagnosis of cardiovascular system. Abbreviations: EVs, Extracellular vesicles; LNP, Lipid nanoparticles; AAV, Adeno-associated virus.
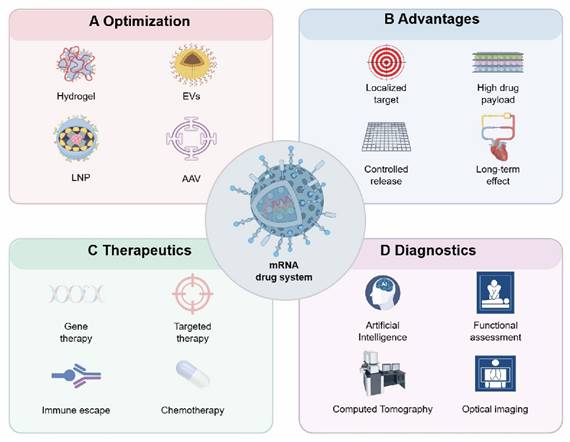
Therapeutic role of mRNA technology in different diseases (by Figdraw). mRNA technology can exert different biological effects on the heart, liver, lungs, kidneys, brain, skin, and other organs to facilitate disease repair.
Building on this foundation, vaccine development from a biological standpoint encompasses multiple strategies, including DNA vaccines, mRNA vaccines, and protein-based vaccines [7], as illustrated in Figure 4. The core mechanism of vaccination is to activate immune cells so they can recognize specific viral protein structures and initiate an immune response [5]. Among these approaches, mRNA vaccines offer several notable advantages. Compared to protein-based vaccines, they eliminate the need for in vitro antigen production, thereby significantly reducing manufacturing timelines. In contrast to DNA vaccines, mRNA does not require nuclear entry, which minimizes the risk of reverse transcription and integration into the host genome [8]. These properties position mRNA vaccines as a safer and more promising platform for infectious disease prevention.
Despite these advantages, translating mRNA into a viable therapeutic modality for both rare and common diseases remain a significant challenge [9, 10]. Major obstacles include the need for high levels of protein expression, poor tissue bioavailability, rapid degradation in circulation, and inefficient targeted delivery. Moreover, repeated administration can trigger innate immune responses, leading to diminished protein expression over time. Addressing these limitations underscores the urgent need to accelerate the research and development of mRNA-based therapeutics in the current era of biomedical innovation.
Classification of mRNA and timeline of exploration (by Figdraw). According to the central dogma, DNA is transcribed into RNA, which is then translated into protein. mRNA plays a crucial role in the RNA translation process. Through chemical modifications or carrier-mediated modifications, the stability of mRNA can be significantly enhanced to maximize its biological effects. Additionally, depending on mRNA classifications—such as conventional mRNA, self-amplifying mRNA, and trans-amplifying mRNA—we have systematically compared the advantages and disadvantages of these distinct mRNA categories. Abbreviations:DNA, Deoxyribonucleic acid; RNA, Ribonucleic acid; LNP, Lipid nanoparticles; UTR, Untranslated region.
2. Design and Optimization Strategies for mRNA Drug Formulations
2.1 Design strategies for mRNA drug formulations
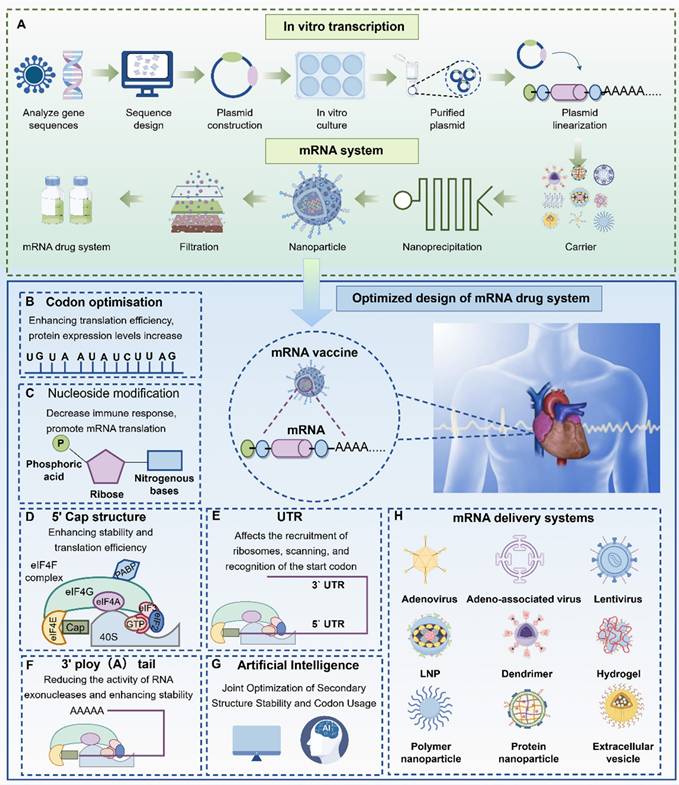
Key attributes such as vaccine efficacy and thermostability are largely determined by the intrinsic properties of the mRNA molecule [11]. To enhance these properties, researchers around the world are actively working to improve the translational efficiency and stability of mRNA. This is especially important in ischemic cardiac tissue, where improved translation can reduce the amount of mRNA required per dose and enable higher, more sustained protein expression following a single administration. These improvements can be achieved by modifying specific structural elements of the mRNA molecule, including the 5′ and 3′ untranslated regions (UTRs), the poly (A) tail, codon optimization, and chemical modification of nucleotides (Figure 5).
2.1.1 Optimization of coding sequences
Optimizing codon usage can enhance translational efficiency and reduce the formation of secondary structures that hinder translation [12]. However, in humans, codon usage bias is not closely correlated with tRNA abundance or gene expression, limiting the effectiveness of codon optimization—especially when the open reading frame (ORF) is derived from human or other mammalian sequences. To further improve translation, the start codon should be placed within a Kozak consensus sequence, and the sequence surrounding the stop codon can also be optimized. Additionally, upstream start codons that precede the correct initiation site should be removed to avoid aberrant translation initiation [13]. Ultimately, refining the coding sequence and minimizing innate immune activation through rational vector design can significantly improve both mRNA stability and protein expression.
Vaccine development (by Figdraw). Vaccines can activate human immune cells, enabling them to recognize specific viral protein structures, thereby achieving an immune response. Vaccines are categorized into three generations: the first generation is based on pathogens (A), the second generation is based on proteins or peptides (B), and the third generation is based on DNA (C) or RNA (D). Compared to protein vaccines, mRNA vaccines eliminate the step of culturing the antigen in vitro, significantly reducing the production cycle and accelerating the development speed. Moreover, compared to DNA vaccines, mRNA vaccines are safer because they do not involve fragment insertion. Abbreviations:DNA, Deoxyribonucleic acid; RNA, Ribonucleic acid.
2.1.2 Nucleotide modification
Nucleotide modification is a critical component in the development of mRNA therapeutics. Unmodified nucleotides can be recognized by intracellular RNA sensors, triggering innate immune responses that lead to rapid mRNA degradation and inhibition of protein translation [14]. In 2005, Katalin and colleagues [15] demonstrated that specific uridine modifications—such as m5C, m5U, and pseudouridine (Ψ)—can suppress the activation of certain human Toll-like receptors (TLRs), whereas unmodified RNA activates multiple TLRs, including TLR3, TLR7, and TLR. To investigate the effects of nucleotide modifications, researchers have utilized cardiac cells and tissues to assess immunogenicity, plasma stability in mice, and protein expression efficiency. Compared to unmodified mRNA, all modified mRNAs significantly reduced the activation of key innate immune genes, such as IFN-α, IFN-β, and RIG-I. They also demonstrated enhanced stability, greater RNA integrity in mouse plasma, and prolonged persistence. Moreover, modified mRNAs improved protein translation, exhibited extended in vitro expression kinetics, and showed favorable in vivo pharmacokinetic profiles. These features support efficient, localized, and rapid gene delivery—achieving transient expression within minutes and sustained expression for several days in adult cardiac tissue. The platform is non-immunogenic and demonstrates favorable biodistribution, with more than 20% of the administered mRNA localizing in the left ventricle. Collectively, these findings have had a significant impact on mRNA vaccine design and have further advanced the therapeutic application of mRNA.
2.1.3 5′cap design
The addition of a 5′ cap to mRNA significantly enhances translational efficiency by promoting recognition by eukaryotic initiation factor 4E (eIF4E), thereby improving mRNA stability and facilitating effective protein synthesis [16, 17]. Capping is typically performed during in vitro transcription (IVT) and can be implemented either co-transcriptionally (one-step) or post-transcriptionally (two-step). However, incorrect capping—especially in the reverse orientation—can lead to rapid mRNA degradation and substantially reduced translation efficiency.
2.1.4 3′poly-A tail
The poly(A) tail enhances mRNA stability by inhibiting ribonuclease activity and binding to poly(A)-binding protein (PABP), which subsequently recruits eIF4G and eIF4E. These interactions increase the affinity for the 5′ cap and promote the formation of a circular mRNA structure, thereby facilitating efficient translation [18, 19]. Despite its importance, the optimal length of the poly(A) tail has not been clearly defined. Therefore, determining the appropriate tail length is essential for maximizing both mRNA stability and translational efficiency.
2.1.5 Regulation by untranslated regions (UTRs)
The selection of untranslated regions (UTRs) plays a critical role in regulating mRNA stability and translational efficiency [20]. For instance [21], the combination of the β-globin 5′-UTR and α-globin 3′-UTR has been shown to enhance translation in a metastatic melanoma vaccine model. However, certain UTR features—such as upstream start codons and stable secondary structures near the 5′ end—can interfere with ribosome recruitment and start codon recognition, thereby impairing translation initiation [22, 23]. These elements should be carefully avoided during mRNA design. A recent study [24] investigated transcriptomic and proteomic changes in the left ventricle of mice at 4 and 24 hours following myocardial infarction (MI), compared with sham-operated controls. Several differentially expressed genes with distinct 5′-UTR sequences were identified. Among them, the 5′-UTR of the carboxylesterase 1D (Ces1d) gene—associated with fatty acid metabolism—was found to double the translation of luciferase (Luc) mRNA modified with N1-methyl-pseudouridine (m1Ψ) in infarcted cardiac tissue. Incorporating the Ces1d 5′-UTR into Luc or GFP reporter mRNAs resulted in both increased and prolonged protein expression. Notably, an RNA element within the Ces1d 5′-UTR, termed "Element D," enhanced translation by 2.5-fold compared with synthetic 5′-UTRs under MI conditions [24]. In a parallel development, Hadas et al. [25] established an improved in vitro mRNA synthesis protocol that yielded higher mRNA output. By optimizing the ratio of anti-reverse cap analog (ARCA) to N1-methyl-pseudouridine (N1mΨ)—favoring ARCA—they achieved increased transcriptional yield, improved protein translation, and reduced immunogenicity in vitro [25]. This protocol provides a more accessible and cost-effective method for mRNA production in both basic and translational research [24, 26].
2.1.6 Immunogenicity
Exogenous mRNA can trigger immune responses in vivo by being recognized by pattern recognition receptors (PRRs) located on the cell surface, within endosomes, or in the cytoplasm [27]. The immunogenicity of mRNA has both beneficial and detrimental effects. On the one hand, it facilitates the maturation of dendritic cells and activates T and B lymphocytes, resulting in strong adaptive immune responses. On the other hand, excessive activation of innate immunity can suppress antigen expression and impair overall vaccine efficacy. Therefore, careful evaluation and modulation of mRNA immunogenicity are essential for the successful design of mRNA vaccines.
2.1.7 AI-guided mRNA design
Due to its single-stranded nature, mRNA is inherently unstable and prone to degradation, which can compromise its immunogenicity and reduce efficacy during storage, transport, and in vivo expression [28, 29]. Enhancing mRNA stability has thus become a major challenge in the development of mRNA vaccines. Studies have shown that increasing the secondary structure of the mRNA sequence improves both its stability and translational efficiency [30, 31]. Consequently, mRNA design algorithms must be capable of simultaneously optimizing structural stability and codon usage to enhance the performance of mRNA-based vaccines and therapeutics [32-34]. To address this need, researchers developed an algorithm called LinearDesign, which jointly optimizes mRNA secondary structure and codon composition. Experimental validation demonstrated that LinearDesign significantly improved mRNA half-life and protein expression in vitro, and enhanced antibody responses in vivo by up to 128-fold compared to conventional codon optimization strategies [35]. In addition to structural features, efficient translation is also influenced by the 5′ untranslated region (5′ UTR), which plays a critical role in determining mRNA vaccine potency. However, chemical modifications such as N1-methyl-pseudouridine (m1Ψ) can alter 5′ UTR function. Zhu et al. [36] applied artificial intelligence to design nanostructured mRNA vaccines. By leveraging machine learning on datasets from various nanoparticle delivery systems, they created platforms capable of efficiently co-delivering mRNA antigens and cyclic GMP-AMP (cGAMP) to target tissues, thereby boosting immune responses while minimizing off-target immunogenicity. Given the complex interplay between RNA design, manufacturing processes, and delivery strategies, a comprehensive understanding of their impact on clinical immunogenicity and safety is essential. Integrating computational modeling and quantitative systems pharmacology into clinical trial design enables early hypothesis generation—such as identifying optimal dosing regimens or predictive immunological biomarkers—based on preclinical and early-phase clinical data, even prior to the availability of complete human trial outcomes.
Design and optimization strategies for mRNA vaccines (by Figdraw). mRNA vaccines are produced by selecting gene sequences, plasmid construction, in vitro culture, and optimizing mRNA (A). mRNA design mainly includes: codon optimization (B), nucleic acid design (C), 5' cap design (D), UTR design (E), 3' A-tail design (F), artificial intelligence algorithm design (G), and mRNA vector optimization strategy (H). Abbreviations: DNA, Deoxyribonucleic acid; RNA, Ribonucleic acid; LNP, Lipid nanoparticles; UTR, Untranslated region.
2.2 In vivo delivery of mRNA therapeutics
Synthetic mRNA provides a safe and efficient platform for gene delivery in the adult heart. Compared with viral vectors, mRNA enables transient and controllable gene expression, making it a safer alternative for delivering cardiac regenerative factors. For instance, although adenoviral delivery of stem cell factors initially supported cardiac repair, it eventually led to the development of cardiac rhabdomyosarcoma [37]. In contrast, mRNA's post-transcriptional mechanism bypasses the need for nuclear entry, allowing direct cytoplasmic translation. Furthermore, mRNA delivery permits the co-expression of multiple genes in defined ratios, enabling personalized therapies based on disease stage. Xiao et al. [37] demonstrated that synthetic mRNA encoding STEMIN and YAP5SA efficiently transfected adult cardiomyocytes both in vitro and in vivo, resulting in improved cardiac function and reduced myocardial fibrosis in infarcted mouse left ventricles.
Despite its therapeutic potential, a major challenge in mRNA therapy is achieving efficient delivery and cellular uptake [26]. As a large, negatively charged molecule, mRNA cannot freely cross the cell membrane, resulting in poor uptake when administered in its naked form. Consequently, effective intracellular delivery remains a significant barrier to therapeutic success. To overcome this, mRNA is typically encapsulated in synthetic particles composed of biocompatible materials. These carriers protect mRNA from enzymatic degradation and facilitate cytosolic release. Additionally, ligand modification of delivery systems can enable targeted delivery to specific cell types during systemic administration.
To achieve effective cardiac gene therapy, delivery platforms must satisfy several essential criteria: efficient uptake by cardiomyocytes, controlled expression kinetics, minimal immune activation, high translational efficiency and stability in cardiac tissue, and use of biocompatible carriers that do not interfere with gene function [26]. Delivery platforms such as lipid nanoparticles [38, 39], polymeric particles [40], dendrimers [41], and biomimetic nanoparticles [42] have demonstrated strong potential in protecting mRNA and enhancing its uptake by target cells.
2.2.1 Lipid nanoparticles (LNPs)
Lipid nanoparticles (LNPs) represent one of the most advanced platforms for RNA delivery, offering protection against RNase-mediated degradation and facilitating efficient cellular uptake. Their clinical relevance was first demonstrated in 2018 with the approval of Onpattro®, a double-stranded siRNA therapy for hereditary transthyretin-mediated (hATTR) amyloidosis [43-45]. More recently, during the COVID-19 pandemic, LNPs gained widespread recognition as essential carriers for mRNA vaccines, which were rapidly developed and safely administered to millions of people worldwide [46-48].
To optimize cardiac mRNA delivery, researchers [49, 50] have evaluated various LNP formulations in citrate buffer using state-of-the-art mRNA constructs. The C12-200 LNP formulation was initially selected based on its proven effectiveness in preclinical siRNA and mRNA studies. Modifying the helper lipid (DOPE or DOPC) or adjusting the molar ratio of C12-200 did not significantly alter key physicochemical parameters such as particle size, surface charge, polydispersity, or mRNA encapsulation efficiency. In vitro, enhanced transfection efficiency was observed in epicardial cells and iPSC-derived fibroblasts treated with C12-200 (40%) combined with cholesterol and DOPE (15%). However, 24 hours after intramyocardial injection, no significant differences in biodistribution or cardiomyocyte targeting were observed. Furthermore, outcomes using lipofectamine 2000-complexed luciferase mRNA in vivo did not correlate with in vitro findings, highlighting a common limitation: in vitro delivery efficiency often fails to predict in vivo performance due to the complex physiological environment, including tissue structure and biodistribution dynamics [51].
To address these challenges, Sultana et al. [52] achieved successful intramyocardial delivery of 100 µg of mRNA via three injections into the left ventricular wall in mice. Using a similar strategy, Magadum et al. [53-56] demonstrated therapeutic effects in heart failure models using mRNA constructs encoding Pip4k2c, Pkm2, or FSTL1.
Among available lipid-based transfection reagents, RNAiMAX has shown robust and stable transfection efficiency in vitro in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). Other reagents such as TransIT (<90%), JetMESSENGER (<90%), and MessengerMax (<80%) have also enabled stable delivery of modified mRNA (m1Ψ) in cardiomyocytes [52].
In vivo, Zangi et al. [57] delivered mRNA encoding VEGFa, β-galactosidase, or luciferase into the myocardium using RNAiMAX, achieving sustained mRNA (m1Ψ) translation for up to one week and facilitating therapeutic benefits after MI. Similarly, Huang et al. [58] used RNAiMAX to deliver IGF1 into infarcted mouse hearts, demonstrating cardioprotective effects. However, increased apoptosis was observed near the injection site, underscoring the need for safe and precisely targeted delivery approaches for cardiac applications.
To improve delivery specificity and translational efficiency, Turnbull et al. [59, 60] developed functionalized lipid nanoparticles (FLNPs) and evaluated the delivery of chemically modified mRNA (m1Ψ + m5C). Their study demonstrated that FLNP-delivered GFP mRNA was successfully translated into protein within 20 minutes in the myocardium of both rats and pigs. Building upon these findings, the EPICCURE clinical trial became the first to assess the feasibility and safety of direct intramyocardial mRNA injection in patients undergoing elective coronary artery bypass grafting [26]. Despite the small sample size, the study confirmed the safety and tolerability of this delivery approach [55, 61]. Despite these advances, naked mRNA remains inherently unstable under physiological conditions and suffers from poor cellular uptake and inefficient endosomal escape. As a result, high doses are typically required to achieve therapeutic protein expression, which increases the risk of immune activation. In contrast, LNP-mediated delivery enables significant dose reduction, improving both safety and translational efficiency. Supporting this, Turnbull et al. demonstrated that relatively low doses of LNP-formulated mRNA were sufficient to induce cardiac protein expression in both small and large animal models [60].
The clinical success of mRNA-LNP-based COVID-19 vaccines has prompted broader interest in LNPs for other therapeutic applications, including targeted tissue regeneration. Cardiac nucleic acid delivery represents a particularly promising approach for myocardial repair. Optimizing LNP composition for heart-specific delivery could open new therapeutic frontiers in cardiovascular medicine. However, off-target delivery—particularly to the liver and spleen—remains a key limitation. Continued refinement of LNP design is therefore essential to minimize immunogenicity and enhance organ-specific delivery [54].
2.2.2 Polymeric nanoparticles
Polymeric nanoparticles were initially developed in combination with polyethyleneimine (PEI) for nucleic acid delivery. These carriers enhance mRNA stability, allow for sustained release, and help reduce immune activation. Studies have shown that PEI-mediated delivery of RNA targeting leukocyte recruitment-related genes can suppress inflammation and preserve cardiac function in murine models of MI. However, a major limitation of polymeric nanoparticles is their cytotoxicity. For instance, PEI has been reported to disrupt cell membrane integrity and activate mitochondria-mediated apoptosis in human cell lines. Therefore, further research is needed to determine safe and effective dosage levels for polymer-based vectors. Rodness et al. [62] used chitosan-encapsulated VEGF nanoparticles for intramyocardial delivery in rats with myocardial ischemia. The treatment enhanced angiogenesis and promoted cardiac tissue repair while preventing the onset of heart failure. Iron oxide nanoparticles, noted for their high biocompatibility and superparamagnetic properties, offer additional benefits by enabling controlled localization and aggregation under external magnetic fields [63]. These properties allow for targeted and tunable vaccine release, enhancing both efficacy and safety. In addition, iron oxide functions as an effective adjuvant by promoting the polarization of proinflammatory macrophages, enhancing immune cell activation, and stimulating cytokine production [64]. However, excessive generation of reactive oxygen species (ROS) by iron oxide nanoparticles can damage DNA, proteins, and membrane lipids, potentially harming healthy cells [65]. Therefore, minimizing the cytotoxic effects of polymeric nanoparticles remains a critical barrier to their clinical application.
2.2.3 Peptide and protein-based nanoparticles
Peptide- and protein-based bionanomaterials have been extensively employed in drug delivery, disease diagnostics, and vaccine development due to their excellent biocompatibility and ease of production. Among them, protein nanoparticles—typically ranging from 20 to 200 nm in diameter—are particularly suitable for targeting lymph nodes. One effective and safe strategy for vaccine delivery involves the use of self-assembling protein carriers. Chen et al. [66] developed P-MSN/miR199a-5p nanoparticles using the CSTSMLKAC peptide. Intravenous administration of these nanoparticles improved myocardial contractility and reduced apoptosis, demonstrating therapeutic potential in MI repair. In another study, Douglas et al. [67] engineered a protein-lipid vehicle (PLV) by incorporating fusion-associated small transmembrane (FAST) proteins—derived from fusogenic reoviruses—into a biocompatible lipid formulation. The results showed that FAST-PLVs can efficiently deliver mRNA or DNA vaccines while avoiding the immunogenicity and toxicity commonly associated with cationic lipid nanoparticles, as well as the necrosis and tissue damage often observed with electroporation. Overall, protein-based carriers offer a promising platform for the design and development of next-generation mRNA vaccines.
2.2.4 Dendrimers
Dendrimers [68] are synthetic polymers with highly branched, well-defined structures and multivalent surface functionalities, making them attractive candidates for mRNA delivery. In cardiac applications [69], coronary infusion of dendrimers carrying the β-galactosidase gene has been shown to support protein expression in cardiomyocytes for 7 to 14 days. Likewise, intramyocardial injection of dendrimers loaded with the relaxin gene in MI rat models improved cardiac function [70]. However, prolonged gene delivery was associated with adverse effects, including impaired cardiac repair, highlighting a potential safety concern. Collectively, these findings underscore the potential of dendrimers for targeted mRNA delivery to the heart, while also emphasizing the need for further investigation into their long-term safety and optimal dosing strategies.
2.2.5 Biomimetic nanoparticles
Biomimetic nanoparticles are created by integrating biological materials onto the surface of synthetic nanoparticles, enabling them to replicate the structural and functional properties of native cells [71]. Among these, extracellular vesicles (EVs)—nanoscale vesicles enclosed by lipid bilayers—are considered “natural lipid nanoparticles.” Michael E. et al. [72] developed EV-like vesicles (ELVs) and loaded them with miR-126. Their study demonstrated that miR-126-loaded ELVs significantly reduced infarct size, fibrosis, and cardiomyocyte hypertrophy following MI. Building on this, Wang et al. [73] engineered targeted EVs (TeEVs) by attaching a cardiac-targeting peptide to miR-222-loaded EVs. These TeEVs were encapsulated in a mechanically engineered hydrogel to form an injectable cardiac patch, allowing for minimally invasive delivery. The TeEVs precisely targeted the injured myocardium and promoted the survival of damaged cardiomyocytes. Continuous delivery of TeEVs to the infarcted area alleviated ischemia-reperfusion injury and reduced ventricular remodeling.
Together, these findings underscore the enhanced biological activity and therapeutic efficacy of modified EVs. In addition to natural EVs, extracellular vesicle mimics (EVMs) [74]—produced through physical or chemical disruption of cells—offer further advantages. EVMs can be manufactured at yields hundreds of times greater than natural EVs through methods such as direct mechanical fragmentation, removal of cytoplasmic and nuclear contents, or fusion of liposomes with secreted EVs. The method of preparation significantly influences EVM surface markers and internal cargo composition, allowing researchers to tailor them for specific biological functions, therapeutic applications, or drug delivery strategies.
3. Routes of Administration for mRNA Formulations in Cardiovascular Diseases
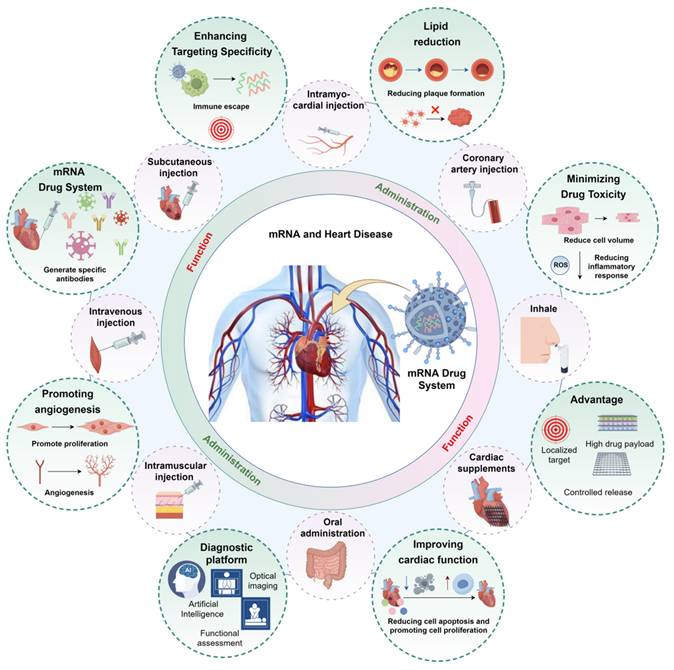
In addition to carrier-related properties, such as particle size, surface charge, and hydrophilicity, the route of administration plays a crucial role in determining the biodistribution and therapeutic efficacy of mRNA [75]. Intravenous and intramuscular injections remain the most widely used methods for delivering polynucleotides to the heart [76]. Subcutaneous and intravenous routes are also effective for targeting biomolecules in the bloodstream. For example, antisense oligonucleotides (ASOs), which are used to reduce blood lipoprotein levels, are typically administered subcutaneously. However, subcutaneous and intramuscular injections present several limitations, such as the risk of needle-induced injury, limited vaccine stability, the need for skilled healthcare professionals, and increased logistical costs due to cold-chain storage and transportation [77, 78]. To address these challenges, researchers have explored transdermal drug delivery systems as non-invasive and painless alternatives to oral or injectable methods [75, 76]. Despite these advances, the stratum corneum, the outermost layer of the skin, poses a significant barrier to the transdermal delivery of high-molecular-weight biomolecules like mRNA [79]. To overcome this challenge, several enhancement techniques have been developed, including ultrasound [80], thermal ablation [81], electroporation [82], and microneedle (MN) systems [83]. Among these, MN technology has garnered particular attention. MNs enable localized and controlled drug delivery, maintaining therapeutic concentrations over extended periods, and can be engineered to deliver mRNA directly into the dermis for targeted therapeutic action [84, 85]. Moreover, MNs offer advantages in scalability and logistics, such as lower manufacturing costs and the potential to eliminate cold-chain dependencies. These benefits position microneedles as a promising strategy for both vaccine administration and broader mRNA-based therapeutic applications [86].
Currently, intramyocardial and intracoronary injections are the primary strategies for cardiac-specific drug delivery [87]. Intracoronary injection involves delivering polynucleotides via a catheter inserted into the coronary artery, while intramyocardial injection requires direct catheter insertion into the heart muscle. Although both methods are effective, their invasive nature poses risks of serious complications, such as MI and even death. While the incidence of major adverse events remains low, catheter-based procedures increase the risk of complications. To address these limitations, hydrogels have emerged as a promising alternative for localized mRNA delivery. These biomaterials can be implanted into the heart via surgical or interventional methods, providing sustained, site-specific release of mRNA. This prolonged local delivery enhances the biological activity of the therapeutic agent and optimizes its efficacy in cardiac tissue.
Aerosolized drug delivery for cardiovascular diseases remains in the exploratory phase, but its potential is receiving increasing attention. The lungs, with their extensive capillary network, allow for rapid drug absorption through the alveoli. This route bypasses the hepatic first-pass effect, potentially offering faster onset of action compared to oral administration. Liu et al. [88] developed inhalable exosomes loaded with IL-12 mRNA (IL-12-Exo) and demonstrated that IL-12-Exo enhanced IFNγ-mediated immune activation, systemic immunity, and immunological memory in a murine lung cancer model, resulting in effective tumor suppression and prevention of recurrence. In a related study [88], the same team introduced a charge-assisted stabilization (CAS) strategy, which induces electrostatic repulsion between lipid nanoparticles (LNPs) to improve colloidal stability. By optimizing surface charge using peptide-lipid conjugates, CAS-LNPs maintained high stability during nebulization and enabled efficient pulmonary mRNA delivery in mice, dogs, and pigs.
These findings suggest that aerosolized delivery can effectively target pulmonary tissues and potentially facilitate secondary drug distribution to the heart through the circulatory system, offering a promising strategy for future heart-targeted therapies. In clinical settings, nebulized furosemide has been experimentally used in emergency departments to treat acute pulmonary edema, often combined with intravenous therapies to accelerate symptom relief in heart failure. Inhaled prostacyclins, such as iloprost, have also been used to reduce pulmonary arterial pressure in patients with pulmonary hypertension [89]. Collectively, these studies provide a theoretical foundation for the application of inhaled therapeutics in cardiovascular care. However, current clinical applications are still limited to specific scenarios, such as emergency administration of nitroglycerin [90], or are under investigation in clinical trials. At present, inhalation-based therapies should only be administered under medical supervision and are not a substitute for conventional methods. Nevertheless, with continued advancements in delivery systems and precision-targeting technologies, aerosolized mRNA delivery may represent a new frontier in cardiovascular therapeutics. Therefore, selecting the appropriate administration route for each mRNA formulation is essential to optimize drug retention and therapeutic outcomes (see Figure 6).
Application of mRNA drug system in cardiovascular diseases (by Figdraw). Based on the varying mRNA therapeutic formulations, different administration routes should be selected, such as: inhalation, intravenous, oral, intramuscular injection, subcutaneous injection, intramyocardial injection, cardiac patch, and intracoronary administration (The red picture is the mode of administration of mRNA drugs in cardiovascular diseases). After targeting the heart, mRNA drugs can improve cardiomyocyte function, promote angiogenesis, reduce inflammatory responses, and decrease plaque formation, thereby improving cardiac function in damaged hearts. mRNA is also expected to become a means of diagnosis of cardiovascular system diseases (The green picture shows the biological role of mRNA drugs in cardiovascular diseases).
The application of mRNA technology in cardiovascular diseases.
| Disease | Carrier | Payload | Admini-stration | Therapeutic effects | Model / Phase | Refer-ences |
|---|---|---|---|---|---|---|
| MI | \ | VEGF-A modRNA | Intramyocardial injection | Promote vascular and heart regeneration | Mouse | [57] |
| LNP | modRNA | Intravenous infusion | Targeting of the cardiac infarct zone | Mouse | [178] | |
| RGD-PEG-PLGA NPs | mRNA | Intravenous infusion | Inhibition of cardiomyocyte apoptosis, and inflammation | Rat | [139] | |
| Hep@PGEA NPs | miRNA-499 | Intravenous infusion | Targeted damage to the heart | Mouse | [140] | |
| LNP | modRNA | Intravenous infusion | Improving cardiac regeneration | Rat, Pig | [60] | |
| LNP | VEGF-mRNA | Epicardial injection | Demonstrated safety and tolerability. | Phase II clinical trial | [124] | |
| \ | miR-146b | Intravenous infusion | Protect heart function | Rat | [179] | |
| Atherosclerosis | NPs | m1Ψ mRNA | Intravenous infusion | Reduce the chronic inflammatory response | Mouse | [136] |
| LNP | mRNA | Intravenous infusion | The PCSK9 levels were significantly reduced | Mouse | [180] | |
| \ | ASO | Subcutaneous injection | Reduce triglycerides | Advanced clinical | [181] | |
| NPs | ABC1 mRNA | Co-culture | Reduce the cholesterol efflux | Human monocyte cell line-U937 | [182] | |
| NPs | Atorvastatin | Intravenous infusion | Reduce the inflammatory response | Mouse | [183] | |
| NPs | IL-10 mRNA | Intravenous infusion | Modulate inflammation in advanced atherosclerotic lesions | Mouse | [184] | |
| Heart failure | LNP | FAP mRNA | Intravenous infusion | Reduction of cardiac fibrosis | Mouse | [84] |
| LNP | mRNA | Intravenous infusion | Reduction of cardiac fibrosis | Mouse | [153] | |
| \ | mRNA-0184 | Intravenous infusion | Hormone replacement therapy | Phase I clinical trial | [137] | |
| \ | VEGF-A mRNA | Intramyocardial injection | Promote angiogenesis and reduce fibrosis | Pig | [185] | |
| NPs | TP-10 | Inhalation | Inhibit cardiac myocyte hypertrophy and reduce fibrosis | Mouse | [186] | |
| Myocardiosis | rBV | CVB3 | Intravenous infusion | Anti-inflammatory action | Mouse | [148] |
| AAV-9 | MYBPC3 mRNA | Intravenous infusion | Prevent the development of cardiac hypertrophy | Mouse | [159] | |
| AAV-9 | BAG3 | Intravenous infusion | Prevent the development of a dilated cardiomyopathy | Mouse | [163] | |
| AAV-9 | BAG3 | Coronary sinus infusion | Diffuse myocardial transduction | Pig | [162] | |
| LNP | NTLA-2001 | Intravenous infusion | The concentration of reducing TTR in the serum | Phase III clinical trial | [187] | |
| CHD | LNP | mRNA | Intrauterine injection | Reduce cardiac toxicity | Mouse | [144] |
Abbreviations: modRNA, modified RNA; VEGF-A, human vascular endothelial growth factor-A; NPs, nanoparticles; ASCs, Adipose-derived stem cells; EVs, Extracellular vesicles; rBV, recombinant baculovirus; CHD, Congenital Heart Disease; TTR, Transthyretin; I/R, Ischemia-reperfusion; MI, Myocardial infarction.
4. Application of mRNA Formulations in Cardiovascular Diseases
Inflammation plays a critical role in the development and progression of cardiovascular diseases [91]. As a result, many researchers are focusing on modulating inflammation to reduce the risk of these conditions. However, traditional anti-inflammatory strategies have shown limited effectiveness in cardiovascular disease treatment. Upon administration, mRNA formulations can trigger complex immune responses, engaging both innate and adaptive immunity, potentially improving cardiovascular health through immunomodulation. Despite this potential, research in this area remains limited. In 2020, the phase III clinical trial of the small interfering RNA (siRNA) drug, inclisiran, demonstrated its efficacy and safety, showing potential benefits for cardiovascular patients by reducing plasma levels of low-density lipoprotein cholesterol (LDL-C) [92]. As a "close relative" of siRNA drugs, the application of mRNA vaccines in cardiovascular diseases raises important questions about their potential, which warrants further investigation (Figure 6). Table 1 details the application of mRNA technology in cardiovascular diseases.
4.1 Atherosclerosis
Extensive evidence from human genetics, basic research, and clinical epidemiology has established low-density lipoprotein cholesterol (LDL-C) as a central pathogenic driver of atherosclerosis [93, 94]. Meta-analyses of randomized clinical trials further support a linear association between absolute LDL-C reduction and decreased cardiovascular events across diverse patient populations [95, 96]. Because the risk and progression of atherosclerotic cardiovascular disease (ASCVD) are directly proportional to cumulative LDL-C exposure over time (i.e., LDL-years), sustained and effective LDL-C lowering is critical to improving clinical outcomes [97]. In line with this evidence, current clinical guidelines recommend both lifestyle modifications and pharmacologic interventions to reduce LDL-C levels, with particular focus on two high-risk groups: individuals with familial hypercholesterolemia and those with established ASCVD [98, 99]. However, despite strong supporting data and multiple approved lipid-lowering therapies, most high-risk patients still fail to achieve target LDL-C levels under current chronic care models. For instance, registry data indicate that only 22% of U.S. patients and 2.7% of patients globally with heterozygous familial hypercholesterolemia attain LDL-C levels below 70 mg/dL. Similarly, only 11% to 27% of patients with ASCVD meet guideline-recommended targets [100-102]. One major contributing factor is the burden imposed by chronic treatment regimens, which often require daily oral medications or periodic injections. Achieving therapeutic goals typically relies on multiple factors, including the clinician's willingness to intensify treatment [101, 102], consistent insurance coverage [103, 104], drug affordability [105], and routine access to lipid monitoring [106]—all of which are frequently inadequate [107]. To address these limitations, novel therapeutic paradigms such as one-time treatments are being explored. These approaches may offer more sustainable solutions for achieving long-term LDL-C control in patients with familial hypercholesterolemia and ASCVD [97].
In this context, in vivo gene editing has emerged as a promising therapeutic strategy that enables the permanent modification of disease-associated genes within specific organs, such as the liver [108]. This approach is particularly relevant to ASCVD, as natural loss-of-function mutations in the PCSK9 gene are associated with lifelong reductions in LDL-C, favorable safety profiles, and significantly lower ASCVD risk [109, 110]. These observations have driven the development of gene-editing therapies targeting PCSK9. Pharmacologic inhibition of PCSK9 (proprotein convertase subtilisin/kexin type 9) has further validated the therapeutic relevance of this pathway [92, 111-114]. Multiple preclinical studies using gene-editing tools—including nucleases and CRISPR base editors—have demonstrated successful PCSK9 disruption in the livers of non-human primates [115-117]. Foundational research supporting the development of VERVE-101, a CRISPR-based therapy, has involved primary human cells, murine models, and four non-human primate species, with follow-up durations extending up to eight months after treatment [115]. Despite these encouraging results, several critical concerns remain. These include the long-term durability of gene editing, potential off-target effects in non-hepatic tissues, and the possibility of unintended germline modifications [94].
Building on this foundation, VERVE-101 is an investigational CRISPR base-editing therapy that consists of mRNA encoding an adenine base editor and guide RNA targeting the PCSK9 gene. These components are encapsulated in lipid nanoparticles (LNPs) and delivered via a single intravenous infusion. In parallel, researchers [115] developed an epigenetic editor specifically targeting human PCSK9. In preclinical studies involving 36 non-human primates monitored for at least one year, as well as germline editing assessments in sexually mature male primates and female mice, a single dose of the LNP-encapsulated epigenetic editor achieved near-complete silencing of PCSK9 expression in transgenic mice [94]. This silencing effect persisted for at least one year and was maintained even after liver regeneration following partial hepatectomy. Notably, the gene silencing was reversible upon treatment with targeted epigenetic activators that demethylate the PCSK9 locus. In crab-eating macaques, a single administration of the epigenetic editor reduced circulating PCSK9 protein levels by approximately 90% and LDL-C levels by 70%, with durable effects [118].
These findings underscore the broader therapeutic potential of in vivo gene and epigenetic editing for cardiovascular risk reduction. Supporting this concept, a phase II clinical trial of the antisense oligonucleotide AKCEA-APO(a)-L—designed to target LPA mRNA—successfully reduced lipoprotein(a) [Lp(a)] concentrations to below 50 mg/dL, a clinically safe threshold, in over 90% of treated patients [26]. Lp(a), encoded by the LPA gene, is a genetically determined and independent risk factor for cardiovascular disease. Elevated Lp(a) levels are associated with increased cardiovascular risk and are largely unresponsive to conventional lipid-lowering therapies. Most Lp(a)-lowering treatments currently in development require repeated dosing. In contrast, gene-editing strategies offer the promise of a single-dose, long-lasting solution [119].
To this end, Ramon and colleagues developed a gene-editing approach using mRNA encoding transcription activator-like effector nucleases (TALENs) to disrupt the LPA gene. In vitro screening identified TALEN constructs capable of precise gene editing with minimal off-target effects. The TALEN mRNA was subsequently encapsulated in a proprietary lipid nanoparticle platform (LUNAR) and administered to transgenic mice expressing human LPA. A single injection resulted in an over 80% reduction in plasma Lp(a) levels, with sustained effects lasting at least five weeks. These findings highlight the feasibility of gene-editing-based therapies to reduce cardiovascular risk and offer a potential long-term treatment for patients with elevated Lp(a) [119].
In parallel with genetic approaches, recent attention has turned to immune-mediated mechanisms of plaque destabilization, which represent another promising avenue for therapeutic intervention in atherosclerosis [120]. An increasing accumulation of bone marrow-derived cells and lymphocytes within atherosclerotic plaques has been associated with fibrous cap thinning and plaque destabilization—key features of vulnerable plaques that are prone to rupture and can lead to thromboembolic events. Mechanistically, activated CD4⁺ T cells and elevated levels of interferon-γ (IFN-γ) suppress collagen synthesis, compromising the structural integrity of the fibrous cap. Simultaneously, activated macrophages secrete cathepsins that degrade collagen and elastin, further weakening plaque stability [121]. Among immune cell subsets, Th1 cells play a particularly important role in promoting atherogenesis by producing pro-inflammatory cytokines such as IFN-γ, TNF-α, and IL-2. These cytokines amplify the inflammatory cascade by activating macrophages and additional T cells, thereby intensifying plaque inflammation [122].
mRNA vaccines may help counteract these pro-atherogenic processes. By suppressing Th1 cell activity, reducing the secretion of inflammatory cytokines (e.g., IFN-γ), and disrupting Th1-macrophage interactions, these vaccines may reduce local immune activation. Collectively, these actions could attenuate inflammation within plaques and enhance fibrous cap stability. Overall, mRNA vaccines represent a promising therapeutic strategy for both the prevention and treatment of atherosclerosis. They offer the potential to inhibit the formation of new plaques and stabilize existing ones, thereby reducing the risk of clinical complications such as coronary embolism.
4.2 Ischemic heart disease
Acute myocardial ischemia initiates a pro-inflammatory response aimed at removing necrotic cellular debris from the infarcted myocardium. However, myocardial reperfusion following percutaneous coronary intervention often amplifies this inflammatory cascade, leading to additional cardiomyocyte death and tissue injury, typically within 6 to 24 hours post-reperfusion. This initial inflammatory phase is followed by an anti-inflammatory reparative phase that promotes wound healing and scar formation, thereby preventing cardiac rupture. The transition between these phases is tightly regulated by complex interactions between cardiac-resident cells (such as cardiomyocytes, endothelial cells, fibroblasts, and stromal cells) and immune cells (including neutrophils, monocytes, macrophages, dendritic cells, and lymphocytes) [123]. Considering the harmful effects of an exaggerated and prolonged pro-inflammatory response and the beneficial role of the subsequent reparative phase, therapeutic strategies that suppress early inflammation while promoting tissue repair may help reduce infarct size and prevent adverse left ventricular remodeling.
Over a decade of research by Kenneth Chien's team at the Karolinska Institute has shown that chemically modified mRNA encoding vascular endothelial growth factor (VEGF) can induce stable yet transient VEGF protein expression within days [124-126]. This transient expression minimizes the risk of toxicity associated with prolonged VEGF exposure. In a phase 2a clinical trial involving patients undergoing cardiac surgery, direct intramyocardial injection of VEGF-mRNA was shown to be safe and improved cardiac function. These findings highlight the therapeutic potential of mRNA-based approaches for tissue repair, particularly in cardiac regeneration.
Beyond angiogenesis, immunomodulation has also emerged as a promising therapeutic avenue. Studies [127] have demonstrated that CD4⁺ T lymphocyte infiltration exacerbates early reperfusion injury following myocardial ischemia. Mice deficient in CD4⁺ T cells exhibit significantly smaller infarcts during the acute phase [128]. However, during the healing phase, these mice display impaired collagen matrix formation, greater left ventricular dilation, increased cardiac rupture, and higher mortality compared to wild-type controls. In contrast, CD4⁺CD25⁺FOXP3⁺ regulatory T cells (Tregs) exert protective effects by secreting anti-inflammatory cytokines such as TGF-β and IL-10 [129]. These Tregs suppress the recruitment of inflammatory cells—including neutrophils, monocytes, and CD4⁺ T cells—promote macrophage polarization from a pro-inflammatory M1 to an anti-inflammatory M2 phenotype, and inhibit fibroblast-to-myofibroblast differentiation, thereby limiting fibrotic remodeling and adverse structural changes [130-132].
Given the pivotal role of immune responses in ischemic heart disease, mRNA-based therapeutics targeting immune pathways offer considerable promise. Mays et al. [133] developed a chemically modified mRNA encoding FOXP3. When delivered to the lungs, it upregulated FOXP3 expression and, via an IL-10-dependent mechanism, modulated immune cell recruitment and maintained balance among Tregs, Th2, and Th17 cells, preventing asthma onset. Drawing on this concept, a similar strategy applied early after myocardial ischemia could promote Treg expansion and IL-10 secretion, attenuating pro-inflammatory responses and reducing myocardial injury.
Ischemic damage also promotes activation of antigen-presenting cells (APCs) through damage-associated molecular patterns (DAMPs) released by necrotic tissue, leading to the presentation of cardiac self-antigens and activation of T and B lymphocytes [134]. Autoantibodies against cardiac troponin I (cTnI-Ab) have been detected in patients with acute MI and correlate with clinical outcomes, suggesting that ischemia can disrupt immune tolerance [135]. As previously noted, nucleoside-modified mRNA vaccines reduce nonspecific inflammation during antigen presentation and induce immune tolerance to self-antigens [136]. These vaccines also promote Treg expansion while inhibiting Th1 responses, offering a potential strategy to mitigate post-ischemic autoimmune injury.
Importantly, CD4⁺ T cells and other immune cells, such as M2 macrophages, also contribute to the reparative phase of ischemic injury. Since anti-inflammatory mRNA therapies might interfere with this process, it is essential to restrict their activity to the early pro-inflammatory window. This could be achieved by leveraging the transient nature of mRNA through chemical modifications that reduce its half-life and confine its action to the acute phase.
Encouragingly, efforts to translate mRNA-based therapies into clinical applications have already begun. In 2017 [137], AstraZeneca and Moderna Therapeutics jointly developed AZD7970, an mRNA vaccine encoding relaxin, for the treatment of heart failure. Preclinical studies showed that liposome-modified mRNA could recruit chemotactic monocytes to sites of cardiac injury, reducing inflammation, further supporting the therapeutic potential of mRNA in cardiovascular disease.
Because the generation of new cardiomyocytes after birth is minimal, preventing cardiomyocyte death is essential for effective cardiac repair. Researchers from Harvard and the Karolinska Institute [57] showed that chemically modified mRNA, when injected into infarcted mice, promoted the differentiation of cardiac stem cells into functional cardiovascular cells rather than fibrotic tissue. Notably, mRNA administered within 48 hours of infarction significantly improved cardiac function.
These findings have been supported by complementary studies. Liu et al. [138] demonstrated that miR-141, delivered via tail vein injection, downregulated ICAM-1 expression in the heart, reduced serum cTnI and LDH levels, and mitigated ischemia/reperfusion injury. Sun et al. [139] used RGD-PEG-PLGA nanoparticles to deliver mRNA targeting the SIRT3/AMPK pathway, reducing apoptosis, inflammation, and oxidative stress. Nie et al. [140] developed a Hep@PGEA-based delivery system for miRNA-499, achieving cardiac-specific targeting and functional improvement. Turnbull further showed that lipid-like nanoparticles could efficiently deliver mRNA to the heart and induce transient protein expression.
Taken together, these studies underscore the promise of mRNA-based interventions for myocardial repair. When applied prophylactically in high-risk populations with acute coronary syndrome, such therapies may reduce the incidence of MI-related disability and mortality, ultimately improving long-term patient outcomes.
4.3 Myocarditis
mRNA vaccines [141], as a novel class of nucleic acid-based therapeutics, offer distinct advantages and show considerable potential in the prevention and treatment of viral myocarditis. By designing mRNA sequences that encode viral antigenic epitopes, these vaccines enable in vivo expression of target proteins. Such proteins serve as effective alternatives to inactivated or attenuated vaccines, as their degradation products are non-toxic amino acids, and their structurally defined, purified antigens effectively induce both cellular and humoral immune responses. Unlike DNA vaccines, mRNA is translated directly in the cytoplasm without needing to cross the nuclear membrane, resulting in greater intracellular flexibility, higher translational efficiency, and reduced safety risks—such as insertional mutagenesis [142]. Additionally, naturally occurring RNA sequences from coxsackievirus B3 (CVB3) may serve as templates for mRNA vaccine design, supporting scalable production [143].
Beyond general immunogenic benefits, mRNA vaccines have shown particular advantages in minimizing antigenic cross-reactivity. For example, a 2017 study published in Cell by Richner et al. [143] described a modified mRNA vaccine encoding a mutated prM-E gene of Zika virus (ZIKV). By disrupting a conserved fusion loop epitope in the E protein, the vaccine protected mice from ZIKV infection while reducing cross-reactive antibodies that could otherwise enhance dengue virus infection. A similar strategy applied to CVB3 could potentially elicit robust neutralizing antibody responses to control viral replication and inflammation, while also reducing autoimmune activation and myocardial injury.
Although the pathogenesis of viral myocarditis remains incompletely understood, it is most frequently associated with infections caused by CVB3, adenoviruses, echoviruses, and more recently, SARS-CoV-2 [144]. Several preclinical studies have explored CVB3-targeted vaccination strategies, including inactivated, live attenuated, DNA, and virus-like particle (VLP) vaccines. For instance, Park et al. [145] developed a highly attenuated CVB3 mutant (YYFF) by substituting two conserved tyrosine residues in the VP2 C-terminal region with phenylalanine, which induced high titers of neutralizing antibodies and CD8⁺ T cell responses in mice. Kim et al. [146] constructed recombinant plasmids encoding CVB3 VP1 and VP3 proteins, which elicited strong neutralizing antibody responses and improved survival in immunized mice. Similarly, Zhang et al. [147] developed a recombinant baculovirus expressing the full-length CVB3 genome, which conferred protective immunity comparable to that of attenuated vaccines.
Despite these promising findings, none of these vaccine candidates have advanced to clinical application. Major limitations include the risk of reversion to virulence or incomplete inactivation for live attenuated and inactivated vaccines, the potential for genomic integration and oncogenic transformation with DNA vaccines, and the high production costs associated with certain platforms. These barriers continue to hinder clinical translation and underscore the need for novel vaccine designs and efficient delivery systems.
Complicating vaccine development further is the role of autoimmunity in CVB3-induced myocarditis. Following infection, cardiac-resident cells—including cardiomyocytes, phagocytes, and fibroblasts—secrete pro-inflammatory cytokines such as IL-1, IL-6, TNF-α, and IL-18, initiating acute inflammation [148]. As the adaptive immune system is activated, antigen-specific lymphocyte responses are triggered. B cells produce neutralizing antibodies, while T cells release cytokines such as IFN-γ to suppress viral replication [149].
However, certain regions of the CVB3 proteome share sequence similarity with cardiac self-antigens, leading to molecular mimicry that activates cross-reactive T cells and promotes autoantibody production [150]. Moreover, the cytolytic nature of CVB3 can result in the release of intracellular and membrane-bound cardiac antigens, further driving the activation of autoreactive lymphocytes and antibodies. This dysregulated immune response may underlie the progression from acute to chronic myocarditis and eventually to dilated cardiomyopathy, presenting additional challenges in the development of safe and effective vaccines [151].
4.4 Heart failure
Heart failure remains a major unmet clinical need, as current therapies provide limited benefit in halting or reversing disease progression [152]. One key pathological feature of heart failure is myocardial fibrosis, driven largely by activated fibroblasts that contribute to adverse tissue remodeling. Consequently, therapeutic strategies that specifically target these pathological fibroblasts are urgently needed [152]. Among emerging approaches, lipid nanoparticle (LNP)-encapsulated mRNA has shown promise. This platform, which has demonstrated clinical success in mRNA-based COVID-19 vaccines, offers a flexible and efficient means of in vivo protein expression. In a murine model of cardiac injury, researchers [153] utilized T cell-targeted LNPs to generate anti-fibroblast chimeric antigen receptor (CAR) T cells in vivo. These engineered cells not only reduced cardiac fibrosis but also migrated to fibrotic regions, where they exerted paracrine effects to suppress local inflammation. This suggests a potential immunomodulatory role in addition to direct cytotoxicity. In a separate study using a mouse model of heart disease, CD5-targeted mRNA-LNPs were employed to induce CAR T cell generation in vivo. The resulting T cells reduced myocardial fibrosis and restored cardiac function, further highlighting the therapeutic potential of mRNA-based cell reprogramming. Complementing these findings, another research [154] group optimized the delivery of human VEGF-A mRNA to the left ventricular myocardium in pigs. This intervention led to a marked reduction in myocardial fibrosis. Notably, intravenous and localized administration of mRNA in rodent and non-human primate models did not induce innate immune activation, supporting the safety profile of this approach. However, the invasive routes currently required for mRNA delivery may hinder the broad adoption of such therapies. To address this limitation, AstraZeneca recently developed an mRNA-LNP formulation suitable for subcutaneous administration. By incorporating a steroidal prodrug, this formulation significantly enhanced protein expression and prolonged translation duration. Such innovations are expected to improve the feasibility, scalability, and accessibility of mRNA therapeutics for treating heart failure [155].
4.5 Congenital heart disease
Nanoparticle-based drug delivery systems hold significant potential for advancing medical therapies [156]. However, their clinical utility is often hindered by limited vascular permeability and rapid clearance by phagocytic cells. Interestingly, the fetal environment—characterized by accelerated angiogenesis, active cell proliferation, and an underdeveloped immune system—may circumvent these limitations, making in utero nanoparticle delivery a promising alternative [157]. Nevertheless, current understanding of nanoparticle-mediated drug delivery during fetal development remains limited. To explore this approach, researchers [144] used Ai9 CRE reporter mice and demonstrated that lipid nanoparticle (LNP)-mRNA complexes can be delivered in utero with high efficiency and low toxicity. These complexes successfully transfected major fetal organs, including the heart, liver, kidneys, lungs, and gastrointestinal tract. At four weeks postnatally, sustained transfection was observed in 50.99% ± 5.05% of diaphragm myofibers, 36.62% ± 3.42% in the heart, and 23.7% ± 3.21% in skeletal muscle. These findings confirm that Cas9 mRNA and sgRNA delivered via LNPs can mediate genome editing in fetal organs in utero. Overall, this study establishes the feasibility of non-viral mRNA delivery to extrahepatic fetal tissues and underscores its potential as a novel therapeutic strategy for the treatment of congenital heart disease.
4.6 Cardiomyopathies
Gene therapy may present a potential opportunity for the treatment of various cardiomyopathies, yet alternative treatment options for some cardiomyopathies are very limited [158]. Mearini et al. [159] have shown that a single administration of AAV9-MYBPC3 in neonatal homozygous MYBPC3-targeted knock-in mice can prevent the development of cardiac hypertrophy and lead to a dose-dependent increase in MYBPC3 protein expression. BAG3 [160] is highly expressed in the heart, skeletal muscle, and central nervous system. Loss-of-function variants of BAG3 leading to decreased BAG3 levels were associated with dilated cardiomyopathy. Researchers [161] have demonstrated that intravenous injection of AAV9-BAG3 in mice can prevent the onset of dilated cardiomyopathy. Additionally, studies [162] have found that catheter-based retrograde coronary sinus infusion for the delivery of low doses of AAV9-BAG3 in healthy mini pigs can result in diffuse myocardial transduction. Furthermore, PRKAG2 [163] mutations cause glycogen storage disease of the heart, characterized by a hypertrophic phenotype, supraventricular arrhythmias, and atrioventricular block. The CRISPR-Cas9 system combined with AAV9 has been used to disrupt the mutated PRKAG2 alleles encoding the H503 mutation in knock-in mice, and a single systemic injection of this product on day 4 or day 42 can restore morphology and function in the mouse model, thereby reducing left ventricular thickness and myocardial glycogen content. Additionally, treatments for cardiac amyloidosis [164] include transthyretin amyloidosis (ATTR) stabilizers that prevent tetramer dissociation and amyloid formation, such as tafamidis [165], which can reduce TTR production through RNA silencing.
Desmocollin-2 (DSC2), a desmosomal membrane-anchored protein, has long been recognized as a central pathogenic factor in arrhythmogenic right ventricular cardiomyopathy (ARVC) [166]. Despite the well-established role of its mutations in disease pathogenesis, effective therapeutic strategies for DSC2 mutation-induced cardiomyopathy have remained elusive. Ge et al. [166] proposed a highly targeted, non-viral, and repeatable therapeutic approach by encapsulating DSC2 mRNA within lipid nanoparticles (LNPs) and administering the mRNA-based drug via echocardiography-guided intraventricular septal injection. Their findings demonstrated that a single injection of modified DSC2 mRNA effectively reversed cardiomyopathy, restored right ventricular function, and significantly prolonged survival in vivo. Notably, this therapeutic efficacy was observed not only in early-stage disease models using young mice but also in aged mice with advanced disease progression. Furthermore, repeated dosing in late-stage disease models reactivated therapeutic benefits and extended survival, highlighting the controllable and sustained potential of the mRNA platform. Compared to conventional gene therapies such as adeno-associated virus (AAV) vectors, this strategy enables rapid protein translation while circumventing risks associated with viral vectors, including immunogenicity and genomic integration.
Overall, these therapies have the potential to fundamentally alter the pathogenesis of diseases, but drug toxicity is dose-dependent, making the development of vectors with improved myocardial tropism and transduction efficiency crucial.
5. Discussion and Perspectives
mRNA-based therapeutics offer several notable advantages, including the ability to encode a wide range of proteins, induce strong immune responses, and support rapid, scalable manufacturing. These platforms also enable targeted delivery via various nanocarrier systems while minimizing systemic side effects. Moreover, because mRNA does not integrate into the host genome, it avoids the risk of insertional mutagenesis, contributing to a favorable safety profile. These attributes have sparked growing interest in applying mRNA technologies to cardiovascular diseases.
Despite these benefits, several limitations remain. A major challenge is the intrinsic instability of mRNA. Due to the widespread presence of RNases in tissues and cells, mRNA is prone to rapid degradation, compromising antigen expression and therapeutic efficacy. Another concern is the high immunogenicity of exogenous mRNA. It can be detected by multiple pattern recognition receptors (PRRs) on the cell surface and within the cytoplasm, activating innate immune pathways [167]. While this immunostimulatory effect may serve as a natural adjuvant—promoting dendritic cell maturation and enhancing T and B cell responses—overactivation can result in excessive type I interferon (IFN) production, which suppresses mRNA translation and impairs antigen-specific immunity [168]. In addition, the large molecular size and negative charge of naked mRNA limit its ability to cross the phospholipid bilayer of cell membranes, making intracellular delivery a critical bottleneck in the development of mRNA-based therapeutics. These limitations underscore the importance of advancing delivery systems, particularly lipid-based carriers that can protect mRNA and promote cellular uptake.
To address these challenges and enhance the clinical utility of mRNA vaccines, several molecular engineering strategies have been explored. Modifications such as locked nucleic acids (LNAs) in the 5′ cap structure can improve mRNA stability by up to 1.6-fold [169]. Similarly, adding stabilizing elements to the 3′ untranslated region (3′UTR) further increases transcript half-life [170]. To reduce immunogenicity, nucleoside analogs like pseudouridine (Ψ), 5-methylcytidine (m5C), and 2-thiouridine (s2U) have been incorporated to blunt Toll-like receptor (TLR) signaling and attenuate innate immune activation [15]. Translation efficiency can be optimized using anti-reverse cap analogs (ARCA) and by minimizing stable secondary structures in the 5′UTR, which can impede ribosome binding [171, 172]. Lipid nanoparticles (LNPs) remain the leading delivery platform due to their protective, biocompatible, and membrane-fusogenic properties [173]. For example, Kranz et al. [174] developed mRNA-LPX, a liposomal system that successfully delivers mRNA to CD11c⁺ antigen-presenting cells in lymphoid tissues, triggering robust antigen-specific immune responses. These findings highlight the need for a systems-level understanding of how RNA design, delivery technology, and immune signaling intersect to influence clinical outcomes. Computational modeling and systems pharmacology may provide critical insights into optimal dosing, immune biomarker identification, and trial design—particularly in the preclinical and early clinical stages.
Alongside molecular optimization, the safety of mRNA-based therapeutics must be thoroughly assessed. Data from 3.644 million COVID-19 mRNA vaccine recipients (BNT162b2 and mRNA-1273), published in JAMA [175], showed that the most common adverse reactions—such as injection site pain, fatigue, headache, myalgia, and chills—generally resolved within five days and affected fewer than 10% of individuals thereafter. These results suggest an overall favorable safety profile. However, some studies have raised concerns regarding spike protein-associated cardiac toxicity, with evidence pointing to potential cardiomyocyte dysfunction and inflammation. Separately, adeno-associated virus (AAV)-mediated gene therapy in a pig model of MI led to sudden death by the seventh week post-treatment. These findings emphasize the need for rigorous safety evaluations, particularly concerning dose and route of administration, before proceeding to large-scale trials in patients with preexisting cardiac conditions.
Another critical limitation is the suboptimal therapeutic efficacy observed in many gene therapy trials targeting cardiovascular diseases. Despite six clinical trials, the gene therapy MYDICAR failed to improve cardiac function in phase II trials [176, 177]. Similarly, BioBypass—an adenoviral vector encoding VEGF-121—was safe but failed to restore myocardial function in refractory ischemia patients. Other trials involving VEGF-A165, bFGF, HGF, SDF-1, and FGF4 have also demonstrated limited clinical benefit. These outcomes reflect broader translational hurdles in gene therapy for cardiovascular medicine. As such, improving efficacy while ensuring safety remains a key unmet need. Overcoming these challenges will be critical for realizing the full therapeutic potential of mRNA vaccines in cardiovascular disease.
Finally, mRNA therapy introduces a new paradigm for gene expression-based treatment that may circumvent the complications seen with DNA- and protein-based modalities. Its capacity for transient expression is particularly well-suited for regenerative applications such as myocardial repair. Nevertheless, this feature poses a barrier for long-term protein replacement therapies. If challenges related to stability, delivery, and duration of expression can be addressed, mRNA therapeutics may become a cornerstone of next-generation cardiovascular interventions—complementing existing modalities such as AAV vectors, small molecules, recombinant proteins, monoclonal antibodies, and peptide carriers [26].
6. Conclusion
mRNA-based formulations represent a highly promising and potentially disruptive platform for both therapeutic and preventive applications. While the scientific community eagerly awaits the first definitive clinical efficacy data, substantial opportunities remain for further development and optimization. As previously discussed, the structural configuration and cellular uptake of mRNA are key determinants of antigen expression efficiency. These factors can be strongly influenced by advances in RNA sequence design, formulation chemistry, and delivery methods. Any modifications to these parameters may significantly affect mRNA stability, translational output, or interactions with intracellular RNA sensors, and should therefore be carefully evaluated during early-stage development. For example, beyond nucleotide modifications, novel delivery strategies can greatly influence the adjuvanticity of mRNA vaccines. Although direct cytosolic delivery can enhance antigen expression, it may bypass endosomal pathways necessary for interaction with RNA-sensing receptors, potentially weakening the desired immunostimulatory response. This issue may need to be addressed through tailored design. In more challenging therapeutic scenarios, the inclusion of auxiliary mRNA molecules may serve to modulate immune responses and improve therapeutic outcomes. Furthermore, combining mRNA-based therapies with other treatment modalities may produce synergistic effects. However, such combination strategies inevitably increase the complexity of both vaccine development and clinical implementation, posing additional regulatory and manufacturing challenges. In conclusion, mRNA therapeutics offer a flexible, potent, and safe treatment platform with the potential to revolutionize cardiovascular medicine. With continued advancements in molecular design and delivery systems, mRNA technologies are well-positioned to become a central component of next-generation precision therapies.
Acknowledgements
We gratefully acknowledge the support provided by the following research grants: Chongqing Key Laboratory of Emergency Medicine (2024KFKTYB01), a key project co-organized by the Health Commission and the Science & Technology Bureau of Chongqing Province (2024DXM024). We are grateful to the anonymous reviewers for their constructive comments and suggestions, which have significantly improved the quality of our paper.
Author contributions
Fengli Peng, Zhimei Qiu, Zimu Wang and Fuhai Li: Writing - original draft. Bei Shi and Yongchao Zhao: Supervision. Chaofu Li: Writing - review & editing, Writing - original draft.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J Am Coll Cardiol. 2022;80:2361-71
2. Global regional, national burden of disorders affecting the nervous system 1990-2021. a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23:344-81
3. Argiro A, Bui Q, Hong KN, Ammirati E, Olivotto I, Adler E. Applications of Gene Therapy in Cardiomyopathies. JACC Heart Fail. 2024;12:248-60
4. Singh R, Chandi SK, Sran S, Aulakh SK, Nijjar GS, Singh K. et al. Emerging Therapeutic Strategies in Cardiovascular Diseases. Cureus. 2024;16:e64388
5. Canouï E, Launay O. [History and principles of vaccination]. Rev Mal Respir. 2019;36:74-81
6. Van Lint S, Renmans D, Broos K, Dewitte H, Lentacker I, Heirman C. et al. The ReNAissanCe of mRNA-based cancer therapy. Expert Rev Vaccines. 2015;14:235-51
7. Hammershaimb EAD, Campbell JD. Vaccine Development. Pediatr Clin North Am. 2024;71:529-49
8. Liu MA. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines (Basel). 2019;7:37
9. Tahtinen S, Tong AJ, Himmels P, Oh J, Paler-Martinez A, Kim L. et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol. 2022;23:532-42
10. Szabó GT, Mahiny AJ, Vlatkovic I. COVID-19 mRNA vaccines: Platforms and current developments. Mol Ther. 2022;30:1850-68
11. Zhou L, Yi W, Zhang Z, Shan X, Zhao Z, Sun X. et al. STING agonist-boosted mRNA immunization via intelligent design of nanovaccines for enhancing cancer immunotherapy. Natl Sci Rev. 2023;10:nwad214
12. Presnyak V, Alhusaini N, Chen YH, Martin S, Morris N, Kline N. et al. Codon optimality is a major determinant of mRNA stability. Cell. 2015;160:1111-24
13. Kondratov O, Zolotukhin S. Exploring the Comprehensive Kozak Sequence Landscape for AAV Production in Sf9 System. Viruses. 2023;15:1983
14. Gu Y, Duan J, Yang N, Yang Y, Zhao X. mRNA vaccines in the prevention and treatment of diseases. MedComm (2020). 2022;3:e167
15. Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165-75
16. Lim J, Ha M, Chang H, Kwon SC, Simanshu DK, Patel DJ. et al. Uridylation by TUT4 and TUT7 marks mRNA for degradation. Cell. 2014;159:1365-76
17. Kajjo S, Sharma S, Chen S, Brothers WR, Cott M, Hasaj B. et al. PABP prevents the untimely decay of select mRNA populations in human cells. Embo j. 2022;41:e108650
18. Passmore LA, Coller J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat Rev Mol Cell Biol. 2022;23:93-106
19. Kim SC, Sekhon SS, Shin WR, Ahn G, Cho BK, Ahn JY. et al. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol Cell Toxicol. 2022;18:1-8
20. Tang X, Huo M, Chen Y, Huang H, Qin S, Luo J. et al. A novel deep generative model for mRNA vaccine development: Designing 5' UTRs with N1-methyl-pseudouridine modification. Acta Pharm Sin B. 2024;14:1814-26
21. Targholi S, Noormohammadi Z, Tafsiri E, Karimipoor M. Evaluation of the Function of a Rare Variant in the 3'-Untranslated Region of the β-Globin Gene. Hemoglobin. 2022;46:312-6
22. Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA. et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567-71
23. Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M. et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283-9
24. Sultana N, Hadas Y, Sharkar MTK, Kaur K, Magadum A, Kurian AA. et al. Optimization of 5' Untranslated Region of Modified mRNA for Use in Cardiac or Hepatic Ischemic Injury. Mol Ther Methods Clin Dev. 2020;17:622-33
25. Singh RD, Hillestad ML, Livia C, Li M, Alekseev AE, Witt TA. et al. M(3)RNA Drives Targeted Gene Delivery in Acute Myocardial Infarction. Tissue Eng Part A. 2019;25:145-58
26. Magadum A. Modified mRNA Therapeutics for Heart Diseases. Int J Mol Sci. 2022;23:15514
27. Qian Y, Ding J, Zhao R, Song Y, Yoo J, Moon H. et al. Intrinsic immunomodulatory hydrogels for chronic inflammation. Chem Soc Rev. 2025;54:33-61
28. Crommelin DJA, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E. Addressing the Cold Reality of mRNA Vaccine Stability. J Pharm Sci. 2021;110:997-1001
29. Naik GG, Jagtap VA. Two heads are better than one: Unravelling the potential Impact of Artificial Intelligence in nanotechnology. Nano TransMed. 2024;3:100041
30. Mauger DM, Cabral BJ, Presnyak V, Su SV, Reid DW, Goodman B. et al. mRNA structure regulates protein expression through changes in functional half-life. Proc Natl Acad Sci U S A. 2019;116:24075-83
31. Lu Y, Zeng T, Zhang H, Li Y, Zhu X, Liu H. et al. Nano-immunotherapy for lung cancer. Nano TransMed. 2023;2:e9130018
32. Mauro VP, Chappell SA. A critical analysis of codon optimization in human therapeutics. Trends Mol Med. 2014;20:604-13
33. Gustafsson C, Govindarajan S, Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol. 2004;22:346-53
34. Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics-developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759-80
35. Zhang H, Zhang L, Lin A, Xu C, Li Z, Liu K. et al. Algorithm for optimized mRNA design improves stability and immunogenicity. Nature. 2023;621:396-403
36. Zhu W, Wei L, Dong C, Wang Y, Kim J, Ma Y. et al. cGAMP-adjuvanted multivalent influenza mRNA vaccines induce broadly protective immunity through cutaneous vaccination in mice. Mol Ther Nucleic Acids. 2022;30:421-37
37. Xiao S, Liang R, Lucero E, McConnell BK, Chen Z, Chang J. et al. STEMIN and YAP5SA synthetic modified mRNAs regenerate and repair infarcted mouse hearts. J Cardiovasc Aging. 2022;2:31
38. Chen J, Ye Z, Huang C, Qiu M, Song D, Li Y. et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc Natl Acad Sci U S A. 2022;119:e2207841119
39. Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319-34
40. Han J, Zhao D, Li D, Wang X, Jin Z, Zhao K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers (Basel). 2018;10:31
41. Perche F, Benvegnu T, Berchel M, Lebegue L, Pichon C, Jaffrès PA. et al. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine. 2011;7:445-53
42. Wu H, Li H, Liu Y, Liang J, Liu Q, Xu Z. et al. Blockading a new NSCLC immunosuppressive target by pluripotent autologous tumor vaccines magnifies sequential immunotherapy. Bioact Mater. 2022;13:223-38
43. Thi TTH, Suys EJA, Lee JS, Nguyen DH, Park KD, Truong NP. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines (Basel). 2021;9:359
44. Hoy SM. Patisiran: First Global Approval. Drugs. 2018;78:1625-31
45. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155-65
46. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA. et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384:1412-23
47. Bajema KL, Dahl RM, Prill MM, Meites E, Rodriguez-Barradas MC, Marconi VC. et al. Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalization - Five Veterans Affairs Medical Centers, United States, February 1-August 6, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1294-9
48. Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE. et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. Jama. 2021;326:1390-9
49. Novobrantseva TI, Borodovsky A, Wong J, Klebanov B, Zafari M, Yucius K. et al. Systemic RNAi-mediated Gene Silencing in Nonhuman Primate and Rodent Myeloid Cells. Mol Ther Nucleic Acids. 2012;1:e4
50. Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005-10
51. Paunovska K, Sago CD, Monaco CM, Hudson WH, Castro MG, Rudoltz TG. et al. A Direct Comparison of in Vitro and in Vivo Nucleic Acid Delivery Mediated by Hundreds of Nanoparticles Reveals a Weak Correlation. Nano Lett. 2018;18:2148-57
52. Sultana N, Magadum A, Hadas Y, Kondrat J, Singh N, Youssef E. et al. Optimizing Cardiac Delivery of Modified mRNA. Mol Ther. 2017;25:1306-15
53. Magadum A, Singh N, Kurian AA, Sharkar MTK, Chepurko E, Zangi L. Ablation of a Single N-Glycosylation Site in Human FSTL 1 Induces Cardiomyocyte Proliferation and Cardiac Regeneration. Mol Ther Nucleic Acids. 2018;13:133-43
54. Labonia MCI, Estapé Senti M, van der Kraak PH, Brans MAD, Dokter I, Streef TJ. et al. Cardiac delivery of modified mRNA using lipid nanoparticles: Cellular targets and biodistribution after intramyocardial administration. J Control Release. 2024;369:734-45
55. Magadum A, Singh N, Kurian AA, Munir I, Mehmood T, Brown K. et al. Pkm2 Regulates Cardiomyocyte Cell Cycle and Promotes Cardiac Regeneration. Circulation. 2020;141:1249-65
56. Magadum A, Singh N, Kurian AA, Sharkar MTK, Sultana N, Chepurko E. et al. Therapeutic Delivery of Pip4k2c-Modified mRNA Attenuates Cardiac Hypertrophy and Fibrosis in the Failing Heart. Adv Sci (Weinh). 2021;8:2004661
57. Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM. et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898-907
58. Huang CL, Leblond AL, Turner EC, Kumar AH, Martin K, Whelan D. et al. Synthetic chemically modified mrna-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Mol Pharm. 2015;12:991-6
59. Turnbull IC, Eltoukhy AA, Anderson DG, Costa KD. Lipidoid mRNA Nanoparticles for Myocardial Delivery in Rodents. Methods Mol Biol. 2017;1521:153-66
60. Turnbull IC, Eltoukhy AA, Fish KM, Nonnenmacher M, Ishikawa K, Chen J. et al. Myocardial Delivery of Lipidoid Nanoparticle Carrying modRNA Induces Rapid and Transient Expression. Mol Ther. 2016;24:66-75
61. Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL. et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458-61
62. Rodness J, Mihic A, Miyagi Y, Wu J, Weisel RD, Li RK. VEGF-loaded microsphere patch for local protein delivery to the ischemic heart. Acta Biomater. 2016;45:169-81
63. Zhao Y, Zhao X, Cheng Y, Guo X, Yuan W. Iron Oxide Nanoparticles-Based Vaccine Delivery for Cancer Treatment. Mol Pharm. 2018;15:1791-9
64. Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A. et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986-94
65. Liu G, Gao J, Ai H, Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013;9:1533-45
66. Chen Y, Liu S, Liang Y, He Y, Li Q, Zhan J. et al. Single dose of intravenous miR199a-5p delivery targeting ischemic heart for long-term repair of myocardial infarction. Nat Commun. 2024;15:5565
67. Brown DW, Wee P, Bhandari P, Bukhari A, Grin L, Vega H. et al. Safe and effective in vivo delivery of DNA and RNA using proteolipid vehicles. Cell. 2024;187:5357-75.e24
68. Zhang D, Atochina-Vasserman EN, Maurya DS, Huang N, Xiao Q, Ona N. et al. One-Component Multifunctional Sequence-Defined Ionizable Amphiphilic Janus Dendrimer Delivery Systems for mRNA. J Am Chem Soc. 2021;143:12315-27
69. Chen Y, Fu W, Zheng Y, Yang J, Liu Y, Qi Z. et al. Galectin 3 enhances platelet aggregation and thrombosis via Dectin-1 activation: a translational study. Eur Heart J. 2022;43:3556-74
70. Lee YS, Choi JW, Oh JE, Yun CO, Kim SW. Human relaxin gene expression delivered by bioreducible dendrimer polymer for post-infarct cardiac remodeling in rats. Biomaterials. 2016;97:164-75
71. Beh CY, Prajnamitra RP, Chen LL, Hsieh PC. Advances in Biomimetic Nanoparticles for Targeted Cancer Therapy and Diagnosis. Molecules. 2021;26:5052
72. Li X, La Salvia S, Liang Y, Adamiak M, Kohlbrenner E, Jeong D. et al. Extracellular Vesicle-Encapsulated Adeno-Associated Viruses for Therapeutic Gene Delivery to the Heart. Circulation. 2023;148:405-25
73. Wang Y, Meng D, Shi X, Hou Y, Zang S, Chen L. et al. Injectable hydrogel with miR-222-engineered extracellular vesicles ameliorates myocardial ischemic reperfusion injury via mechanotransduction. Cell Rep Med. 2025;6:101987
74. Li Q, Song Y, Wang Q, Chen J, Gao J, Tan H. et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics. 2021;11:3916-31
75. Ma G, Wu C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J Control Release. 2017;251:11-23
76. Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-68
77. Songane M. Challenges for nationwide vaccine delivery in African countries. Int J Health Econ Manag. 2018;18:197-219
78. Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y. et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397:1023-34
79. Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, Gu Z. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51-70
80. Daftardar S, Neupane R, Boddu Sai HS, Renukuntla J, Tiwari AK. Advances in Ultrasound Mediated Transdermal Drug Delivery. Curr Pharm Des. 2019;25:413-23
81. Alkilani AZ, McCrudden MT, Donnelly RF. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics. 2015;7:438-70
82. Anirudhan TS, Nair SS. Development of voltage gated transdermal drug delivery platform to impose synergistic enhancement in skin permeation using electroporation and gold nanoparticle. Mater Sci Eng C Mater Biol Appl. 2019;102:437-46
83. Chen J, Huang W, Huang Z, Liu S, Ye Y, Li Q. et al. Fabrication of Tip-Dissolving Microneedles for Transdermal Drug Delivery of Meloxicam. AAPS PharmSciTech. 2018;19:1141-51
84. Tang J, Wang J, Huang K, Ye Y, Su T, Qiao L. et al. Cardiac cell-integrated microneedle patch for treating myocardial infarction. Sci Adv. 2018;4:eaat9365
85. Liu GS, Kong Y, Wang Y, Luo Y, Fan X, Xie X. et al. Microneedles for transdermal diagnostics: Recent advances and new horizons. Biomaterials. 2020;232:119740
86. Sheng T, Luo B, Zhang W, Ge X, Yu J, Zhang Y. et al. Microneedle-Mediated Vaccination: Innovation and Translation. Adv Drug Deliv Rev. 2021;179:113919
87. Kobayashi H, Tohyama S, Kanazawa H, Ichimura H, Chino S, Tanaka Y. et al. Intracoronary transplantation of pluripotent stem cell-derived cardiomyocytes: Inefficient procedure for cardiac regeneration. J Mol Cell Cardiol. 2023;174:77-87
88. Liu S, Wen Y, Shan X, Ma X, Yang C, Cheng X. et al. Charge-assisted stabilization of lipid nanoparticles enables inhaled mRNA delivery for mucosal vaccination. Nat Commun. 2024;15:9471
89. Ghaysouri A, Basati G, Shams M, Tavan H. Efficiency of Nebulizing Furosemide in the Treatment of Chronic Pulmonary Obstructive Disease: A Systematic Review and Meta-Analysis of Clinical Trials. Tanaffos. 2020;19:340-9
90. Long B, Brady WJ, Gottlieb M. Emergency medicine updates: Sympathetic crashing acute pulmonary edema. Am J Emerg Med. 2025;90:35-40
91. Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14:133-44
92. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ. et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N Engl J Med. 2020;382:1507-19
93. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459-72
94. Lee RG, Mazzola AM, Braun MC, Platt C, Vafai SB, Kathiresan S. et al. Efficacy and Safety of an Investigational Single-Course CRISPR Base-Editing Therapy Targeting PCSK9 in Nonhuman Primate and Mouse Models. Circulation. 2023;147:242-53
95. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM. et al. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. Jama. 2016;316:1289-97
96. Efficacy and safety of statin therapy in older people. a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-15
97. Braunwald E. How to live to 100 before developing clinical coronary artery disease: a suggestion. Eur Heart J. 2022;43:249-50
98. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-209
99. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-88
100. Gu J, Sanchez R, Chauhan A, Fazio S, Wong N. Lipid treatment status and goal attainment among patients with atherosclerotic cardiovascular disease in the United States: A 2019 update. Am J Prev Cardiol. 2022;10:100336
101. Cannon CP, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Gao Q. et al. Use of Lipid-Lowering Therapies Over 2 Years in GOULD, a Registry of Patients With Atherosclerotic Cardiovascular Disease in the US. JAMA Cardiol. 2021;6:1060-8
102. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G. et al. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: the DA VINCI study. Eur J Prev Cardiol. 2021;28:1279-89
103. Zafrir B, Egbaria A, Stein N, Elis A, Saliba W. PCSK9 inhibition in clinical practice: Treatment patterns and attainment of lipid goals in a large health maintenance organization. J Clin Lipidol. 2021;15:202-11.e2
104. Hess GP, Natarajan P, Faridi KF, Fievitz A, Valsdottir L, Yeh RW. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor Therapy: Payer Approvals and Rejections, and Patient Characteristics for Successful Prescribing. Circulation. 2017;136:2210-9
105. Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M. et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088-97
106. Dayoub EJ, Eberly LA, Nathan AS, Khatana SAM, Adusumalli S, Navar AM. et al. Adoption of PCSK9 Inhibitors Among Patients With Atherosclerotic Disease. J Am Heart Assoc. 2021;10:e019331
107. Piña IL, Di Palo KE, Brown MT, Choudhry NK, Cvengros J, Whalen D. et al. Medication adherence: Importance, issues and policy: A policy statement from the American Heart Association. Prog Cardiovasc Dis. 2021;64:111-20
108. Musunuru K. Moving toward genome-editing therapies for cardiovascular diseases. J Clin Invest. 2022;132:e148555
109. Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD. et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514-23
110. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264-72
111. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA. et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713-22
112. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R. et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097-107
113. Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS. et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N Engl J Med. 2020;382:1520-30
114. Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50(Suppl):S172-7
115. Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, DeNizio JE, Reiss CW. et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593:429-34
116. Wang L, Smith J, Breton C, Clark P, Zhang J, Ying L. et al. Meganuclease targeting of PCSK9 in macaque liver leads to stable reduction in serum cholesterol. Nat Biotechnol. 2018;36:717-25
117. Wang L, Breton C, Warzecha CC, Bell P, Yan H, He Z. et al. Long-term stable reduction of low-density lipoprotein in nonhuman primates following in vivo genome editing of PCSK9. Mol Ther. 2021;29:2019-29
118. Tremblay F, Xiong Q, Shah SS, Ko CW, Kelly K, Morrison MS. et al. A potent epigenetic editor targeting human PCSK9 for durable reduction of low-density lipoprotein cholesterol levels. Nat Med. 2025;31:1329-38
119. Garcia DA, Pierre AF, Quirino L, Acharya G, Vasudevan A, Pei Y. et al. Lipid nanoparticle delivery of TALEN mRNA targeting LPA causes gene disruption and plasma lipoprotein(a) reduction in transgenic mice. Mol Ther. 2025;33:90-103
120. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS. et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226-35
121. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282-92
122. Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26:2421-32
123. Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA. et al. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73-87
124. Anttila V, Saraste A, Knuuti J, Hedman M, Jaakkola P, Laugwitz KL. et al. Direct intramyocardial injection of VEGF mRNA in patients undergoing coronary artery bypass grafting. Mol Ther. 2023;31:866-74
125. Al-Saadi J, Waldén M, Sandell M, Sohlmér J, Grankvist R, Friberger I. et al. Endovascular transplantation of mRNA-enhanced mesenchymal stromal cells results in superior therapeutic protein expression in swine heart. Mol Ther Methods Clin Dev. 2024;32:101225
126. Rohner E, Yang R, Foo KS, Goedel A, Chien KR. Unlocking the promise of mRNA therapeutics. Nat Biotechnol. 2022;40:1586-600
127. Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA. et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056-64
128. Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G. et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652-63
129. Wang X, Zhou H, Liu Q, Cheng P, Zhao T, Yang T. et al. Targeting regulatory T cells for cardiovascular diseases. Front Immunol. 2023;14:1126761
130. Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N. et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232
131. Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A. et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55-67
132. Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N. et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol. 2014;307:H1233-42
133. Mays LE, Ammon-Treiber S, Mothes B, Alkhaled M, Rottenberger J, Müller-Hermelink ES. et al. Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism. J Clin Invest. 2013;123:1216-28
134. Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res. 2015;116:354-67
135. Leuschner F, Li J, Göser S, Reinhardt L, Ottl R, Bride P. et al. Absence of auto-antibodies against cardiac troponin I predicts improvement of left ventricular function after acute myocardial infarction. Eur Heart J. 2008;29:1949-55
136. Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145-53
137. Shi Y, Shi M, Wang Y, You J. Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications. Signal Transduct Target Ther. 2024;9:322
138. Liu RR, Li J, Gong JY, Kuang F, Liu JY, Zhang YS. et al. MicroRNA-141 regulates the expression level of ICAM-1 on endothelium to decrease myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;309:H1303-13
139. Sun B, Liu S, Hao R, Dong X, Fu L, Han B. RGD-PEG-PLA Delivers MiR-133 to Infarct Lesions of Acute Myocardial Infarction Model Rats for Cardiac Protection. Pharmaceutics. 2020;12:575
140. Nie JJ, Qiao B, Duan S, Xu C, Chen B, Hao W. et al. Unlockable Nanocomplexes with Self-Accelerating Nucleic Acid Release for Effective Staged Gene Therapy of Cardiovascular Diseases. Adv Mater. 2018;30:e1801570
141. Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95-114
142. Bitounis D, Jacquinet E, Rogers MA, Amiji MM. Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat Rev Drug Discov. 2024;23:281-300
143. Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM. et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017;168:1114-25.e10
144. Gao K, Li J, Song H, Han H, Wang Y, Yin B. et al. In utero delivery of mRNA to the heart, diaphragm and muscle with lipid nanoparticles. Bioact Mater. 2023;25:387-98
145. Park JH, Kim DS, Cho YJ, Kim YJ, Jeong SY, Lee SM. et al. Attenuation of coxsackievirus B3 by VP2 mutation and its application as a vaccine against virus-induced myocarditis and pancreatitis. Vaccine. 2009;27:1974-83
146. Kim JY, Jeon ES, Lim BK, Kim SM, Chung SK, Kim JM. et al. Immunogenicity of a DNA vaccine for coxsackievirus B3 in mice: protective effects of capsid proteins against viral challenge. Vaccine. 2005;23:1672-9
147. Zhang L, Parham NJ, Zhang F, Aasa-Chapman M, Gould EA, Zhang H. Vaccination with coxsackievirus B3 virus-like particles elicits humoral immune response and protects mice against myocarditis. Vaccine. 2012;30:2301-8
148. Lasrado N, Reddy J. An overview of the immune mechanisms of viral myocarditis. Rev Med Virol. 2020;30:1-14
149. Fairweather D, Stafford KA, Sung YK. Update on coxsackievirus B3 myocarditis. Curr Opin Rheumatol. 2012;24:401-7
150. Root-Bernstein R, Fairweather D. Unresolved issues in theories of autoimmune disease using myocarditis as a framework. J Theor Biol. 2015;375:101-23
151. Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411-7
152. Zhang XK, Sun AJ, Hu K, Ge JB. [Application prospects of mRNA vaccines in cardiovascular diseases]. Zhonghua Xin Xue Guan Bing Za Zhi. 2022;50:514-9
153. Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L. et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91-6
154. Cao Q, Fang H, Tian H. mRNA vaccines contribute to innate and adaptive immunity to enhance immune response in vivo. Biomaterials. 2024;310:122628
155. Ramachandran S, Satapathy SR, Dutta T. Delivery Strategies for mRNA Vaccines. Pharmaceut Med. 2022;36:11-20
156. Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41
157. Muskan M, Abeysinghe P, Cecchin R, Branscome H, Morris KV, Kashanchi F. Therapeutic potential of RNA-enriched extracellular vesicles: The next generation in RNA delivery via biogenic nanoparticles. Mol Ther. 2024;32:2939-49
158. Argirò A, Ding J, Adler E. Gene therapy for heart failure and cardiomyopathies. Rev Esp Cardiol (Engl Ed). 2023;76:1042-54
159. Mearini G, Stimpel D, Geertz B, Weinberger F, Krämer E, Schlossarek S. et al. Mybpc3 gene therapy for neonatal cardiomyopathy enables long-term disease prevention in mice. Nat Commun. 2014;5:5515
160. Domínguez F, Cuenca S, Bilińska Z, Toro R, Villard E, Barriales-Villa R. et al. Dilated Cardiomyopathy Due to BLC2-Associated Athanogene 3 (BAG3) Mutations. J Am Coll Cardiol. 2018;72:2471-81
161. Martin TG, Myers VD, Dubey P, Dubey S, Perez E, Moravec CS. et al. Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover. Nat Commun. 2021;12:2942
162. Myers VD, Landesberg GP, Bologna ML, Semigran MJ, Feldman AM. Cardiac Transduction in Mini-Pigs After Low-Dose Retrograde Coronary Sinus Infusion of AAV9-BAG3: A Pilot Study. JACC Basic Transl Sci. 2022;7:951-3
163. Xie C, Zhang YP, Song L, Luo J, Qi W, Hu J. et al. Genome editing with CRISPR/Cas9 in postnatal mice corrects PRKAG2 cardiac syndrome. Cell Res. 2016;26:1099-111
164. Gillmore JD, Judge DP, Cappelli F, Fontana M, Garcia-Pavia P, Gibbs S. et al. Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2024;390:132-42
165. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M. et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379:1007-16
166. Zou Y, Lu J, Lian Z, Jia J, Shen J, Li Q. et al. Modified mRNA Treatment Restores Cardiac Function in Desmocollin-2-Deficient Mouse Models of Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation. 2025;151:1780-1796
167. Chen N, Xia P, Li S, Zhang T, Wang TT, Zhu J. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life. 2017;69:297-304
168. Pollard C, Rejman J, De Haes W, Verrier B, Van Gulck E, Naessens T. et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol Ther. 2013;21:251-9
169. Kore AR, Shanmugasundaram M, Charles I, Vlassov AV, Barta TJ. Locked nucleic acid (LNA)-modified dinucleotide mRNA cap analogue: synthesis, enzymatic incorporation, and utilization. J Am Chem Soc. 2009;131:6364-5
170. Ferizi M, Leonhardt C, Meggle C, Aneja MK, Rudolph C, Plank C. et al. Stability analysis of chemically modified mRNA using micropattern-based single-cell arrays. Lab Chip. 2015;15:3561-71
171. Jemielity J, Fowler T, Zuberek J, Stepinski J, Lewdorowicz M, Niedzwiecka A. et al. Novel "anti-reverse" cap analogs with superior translational properties. Rna. 2003;9:1108-22
172. Sample PJ, Wang B, Reid DW, Presnyak V, McFadyen IJ, Morris DR. et al. Human 5' UTR design and variant effect prediction from a massively parallel translation assay. Nat Biotechnol. 2019;37:803-9
173. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-79
174. Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396-401
175. Chapin-Bardales J, Gee J, Myers T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. Jama. 2021;325:2201-2
176. Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A. et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355-67
177. Horowitz JD, Rosenson RS, McMurray JJ, Marx N, Remme WJ. Clinical Trials Update AHA Congress 2010. Cardiovasc Drugs Ther. 2011;25:69-76
178. Evers MJW, Du W, Yang Q, Kooijmans SAA, Vink A, van Steenbergen M. et al. Delivery of modified mRNA to damaged myocardium by systemic administration of lipid nanoparticles. J Control Release. 2022;343:207-16
179. Di YF, Li DC, Shen YQ, Wang CL, Zhang DY, Shang AQ. et al. MiR-146b protects cardiomyocytes injury in myocardial ischemia/reperfusion by targeting Smad4. Am J Transl Res. 2017;9:656-63
180. Liu J, Chang J, Jiang Y, Meng X, Sun T, Mao L. et al. Fast and Efficient CRISPR/Cas9 Genome Editing In Vivo Enabled by Bioreducible Lipid and Messenger RNA Nanoparticles. Adv Mater. 2019;31:e1902575
181. Chebli J, Larouche M, Gaudet D. APOC3 siRNA and ASO therapy for dyslipidemia. Curr Opin Endocrinol Diabetes Obes. 2024;31:70-7
182. Su XM, Wei Y, Wang Y, Zhang W. ABCA1 mRNA expression and cholesterol outflow in U937 cells. Int J Clin Exp Pathol. 2015;8:3116-21
183. Gao C, Huang Q, Liu C, Kwong CHT, Yue L, Wan JB. et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11:2622
184. Gao M, Tang M, Ho W, Teng Y, Chen Q, Bu L. et al. Modulating Plaque Inflammation via Targeted mRNA Nanoparticles for the Treatment of Atherosclerosis. ACS Nano. 2023;17:17721-39
185. Collén A, Bergenhem N, Carlsson L, Chien KR, Hoge S, Gan LM. et al. VEGFA mRNA for regenerative treatment of heart failure. Nat Rev Drug Discov. 2022;21:79-80
186. Weng H, Zou W, Tian F, Xie H, Liu A, Liu W. et al. Inhalable cardiac targeting peptide modified nanomedicine prevents pressure overload heart failure in male mice. Nat Commun. 2024;15:6058
187. Kotit S. Lessons from the first-in-human in vivo CRISPR/Cas9 editing of the TTR gene by NTLA-2001 trial in patients with transthyretin amyloidosis with cardiomyopathy. Glob Cardiol Sci Pract. 2023;2023:e202304
Author contact
![]() Corresponding authors: Bei Shi, shibedu.cn and Yongchao Zhao, yongchaozhaoedu.cn, Department of Cardiology, Affiliated Hospital of Zunyi Medical University, Zunyi, 563000, China. Chaofu Li, licf20edu.cn, Department of cardiology, Chongqing University Central Hospital (Chongqing Emergency Medical Center), College of Bioengineering, Chongqing University, Chongqing, 400014, China.
Corresponding authors: Bei Shi, shibedu.cn and Yongchao Zhao, yongchaozhaoedu.cn, Department of Cardiology, Affiliated Hospital of Zunyi Medical University, Zunyi, 563000, China. Chaofu Li, licf20edu.cn, Department of cardiology, Chongqing University Central Hospital (Chongqing Emergency Medical Center), College of Bioengineering, Chongqing University, Chongqing, 400014, China.
 Global reach, higher impact
Global reach, higher impact