13.3
Impact Factor
Theranostics 2025; 15(16):7973-7989. doi:10.7150/thno.112649 This issue Cite
Research Paper
Reduction-responsive RNAi nanoplatform for enhanced cancer sonoimmunotherapy via dual inhibition of mitophagy and Nrf2 pathways
1. Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Medical Research Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, P. R. China.
2. Guangzhou Key Laboratory of Medical Nanomaterials, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, P. R. China.
3. Nanhai Translational Innovation Center of Precision Immunology, Sun Yat-sen Memorial Hospital, Foshan 528200, P. R. China.
4. Cellular and Molecular Diagnostics Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, P. R. China.
5. Department of Dermatology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, P. R. China.
6. Department of Orthopedics and Department of Sports Medicine, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, P. R. China.
7. Department of General Medicine, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, P. R. China.
Abstract
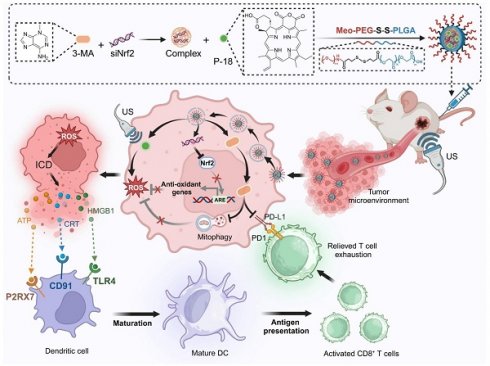
Rationale: Sonodynamic therapy (SDT) has emerged as a promising non-invasive modality with deeper tissue penetration than photodynamic or chemodynamic therapies. However, its therapeutic efficacy remains limited due to inadequate reactive oxygen species (ROS) generation, largely attributed to tumor-intrinsic antioxidant systems and mitophagy. Existing combinations of SDT with immunotherapy are primarily additive and fail to address the mechanistic interplay between ROS suppression and immune evasion.
Methods: To overcome these limitations, we developed a redox-responsive RNA interference (RNAi) nanoplatform (NP) for the co-delivery of Nrf2 siRNA, the mitophagy inhibitor 3-Methyladenine (3-MA), and the sonosensitizer purpurin-18 (P-18). This NP enables tumor-specific release in high-glutathione environments and facilitates dual-pathway inhibition upon ultrasound activation.
Results: This synergistic platform simultaneously disrupted Nrf2-mediated antioxidant defenses and mitophagy-dependent mitochondrial clearance, resulting in enhanced intracellular ROS accumulation. Elevated ROS levels triggered immunogenic cell death (ICD), promoting dendritic cells maturation and antigen presentation. Concurrently, 3-MA inhibited NF-κB signaling, downregulating PD-L1 expression and mitigating T cell exhaustion. In murine breast cancer models, this dual-action approach elicited robust CD8⁺ T cell responses and significantly suppressed tumor growth and metastasis.
Conclusions: This study introduces a mechanistically integrated sonoimmunotherapeutic strategy that concurrently overcomes ROS suppression and immune checkpoint resistance. By orchestrating redox disruption and immune reprogramming, our nanoplatform provides a compelling framework for next-generation SDT-based immunotherapy.
Keywords: sonoimmunotherapy, mitophagy inhibition, immune checkpoint blockade, reactive oxygen species, redox-responsive nanoplatform
 Global reach, higher impact
Global reach, higher impact