13.3
Impact Factor
Theranostics 2025; 15(16):8068-8095. doi:10.7150/thno.115988 This issue Cite
Review
Mucus-derived biomaterial dressings: a novel approach to accelerate wound healing
1. The State Key Laboratory of Mechanism and Quality of Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau 999078, China.
2. Kitsilano Secondary School, 2706 Trafalgar Street, Vancouver, V6K 2J6, Canada.
3. Department of Gastroenterology, Shanghai Institute of Pancreatic Diseases, National Key Laboratory of Immunity and Inflammation, Changhai Clinical Research Unit, Changhai Hospital, Naval Medical University, Shanghai, 200433, China.
4. Shanghai Collaborative Innovation Center of Endoscopy, Endoscopy Center and Endoscopy Research Institute Zhongshan Hospital, Fudan University, Shanghai, 200433, China.
#These authors contributed equally.
Received 2025-4-17; Accepted 2025-6-30; Published 2025-7-24
Abstract
Wound management remains a clinical challenge due to the complexity of healing processes. Traditional dressings with passive protection mechanisms and modern synthetic alternatives often fail to recapitulate the dynamic biological interactions in the wound microenvironment. Mucus is a naturally widely available biomaterial, exhibiting superior bioactive properties as a viscoelastic gel-like substance. Notably, natural mucus derived from diverse biological sources has garnered significant attention as advanced wound dressings. This review explores the potential of natural mucus from animals, plants, microorganisms, and other complex sources as multifunctional wound healing platforms. By analyzing the therapeutic effects of natural mucus, we evaluate its key molecular mechanisms and performance metrics against clinical wound dressings. This establishes a scientific framework for mucus-inspired biomaterials design. The comprehensive assessment not only reveals the untapped potential of renewable biological resources in developing eco-friendly, high-performance wound care alternatives but also provides theoretical guidance for developing next-generation dressings with bioactive, self-adaptive, and environmentally responsive characteristics.
Keywords: mucus, wound healing, adhesion, natural biomaterial, regeneration
1. Introduction
Skin tissue injuries spanning acute trauma to chronic pathologies constitute a global healthcare crisis, with over 5 million annual deaths attributed to wound-related complications [1]. Particularly alarming is their status as a leading mortality factor for individuals under 45 years, surpassing many infectious diseases in socioeconomic impact [2]. Traditional wound management strategies often lead to fibrotic scarring, surgical site contracture, prolonged healing time and high infection risk [3]. An optimal wound dressing should orchestrate all healing phases and maintain physiological homeostasis [4]. Current clinical adhesives have some critical limitations. The strong adhesive cyanoacrylates (CAs) have slow degradation and cytotoxic side effects. Fibrin adhesives show weaker adhesion, restricting their application [5]. Thus, novel wound dressings must combine adequate adhesion, high biocompatibility and optimal therapeutic efficacy.
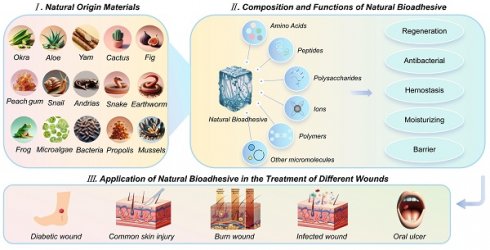
Natural mucus is a viscoelastic bio-secretion typically composed of water, mucin, polysaccharides, lipids, and other bioactive components. Its nonlinear rheological behavior mainly stems from entangled mucin glycoproteins forming transient polymer networks [6]. Serving as a multifunctional interface, it can mediate adhesion, lubrication, hydration, and antimicrobial defense in vital movement. Animal mucus is typically secreted by goblet cells in the mucosal layer. In contrast, plant mucilage is generally secreted by seed coats or specialized mucilage glands [7]. Microorganisms in nature, such as myxobacteria and microalgae, can also secrete mucus, which holds broad application potential in industrial and biomedical fields [8]. Over the past 30 years, research into natural mucus from diverse sources and its therapeutic potential has grown significantly. Various animals and plants secrete sticky tissue fluids, aiding in self-defense, locomotion, and prey capture. For instance, marine mussels anchor themselves to surfaces by secreting viscous proteins, withstanding the enormous shear forces of ocean waves [9]. When encountering predators, slugs secrete mucus to adhere on rocky surface to crawl against gravity [10]. Geckos possess sticky toe pads to crawl against gravity [11]. Snails secrete mucus with lubricating and adhesive properties, maintaining contact with smooth surfaces while crawling [12]. Okra mucilage prevents borer insects from entering the interior and consuming the seeds, also providing essential nutrients for growth [13]. Cacti mucilage forms a protective film to prevent water evaporation and store nutrients, hence enduring harsh environmental conditions [14]. The advantageous properties of natural mucus stem from its molecular composition. Most biomacromolecules in natural mucus are polymers characterized by long linear chains of repeating units, such as chitosan, alginate, hyaluronic acid, dextran, and natural proteins like fibrin and elastin [15]. After extraction and freeze-drying, natural mucus can be rehydrated to form natural hydrogels. This complex adhesive system is constructed by covalent bonds and non-covalent interactions [16]. Inspired by these natural phenomena, bioadhesives derived from living organisms, natural mucus, exhibit significant potential as the substitutes of traditional wound healing dressings (Figure 1).
Recently, natural mucus has presented significant advances in the medical, cosmetic and food industries. However, comprehensive summaries and analyses on the specific roles and applications of natural mucus in the wound healing process are still lacking. In this review, we summarized the wound healing properties, cross-linking effects and biochemical functions of natural mucus sourced from various organisms (Figure 2). We also highlighted the application of natural mucus in different types of wound healing models. Furthermore, we analyzed potential issues in wound healing applications and discuss the challenges to clinical adoption, aiming to promote further research into natural biomaterials in wound healing.
Schematic illustration of natural mucus in the treatment of diverse wound healing models. Created with BioRender.com.
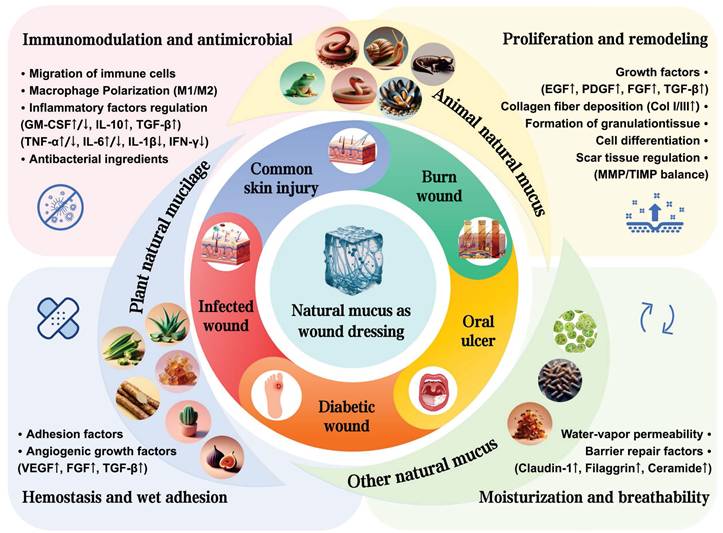
Summarizing the wound healing mechanisms of natural mucus. Created with BioRender.com.
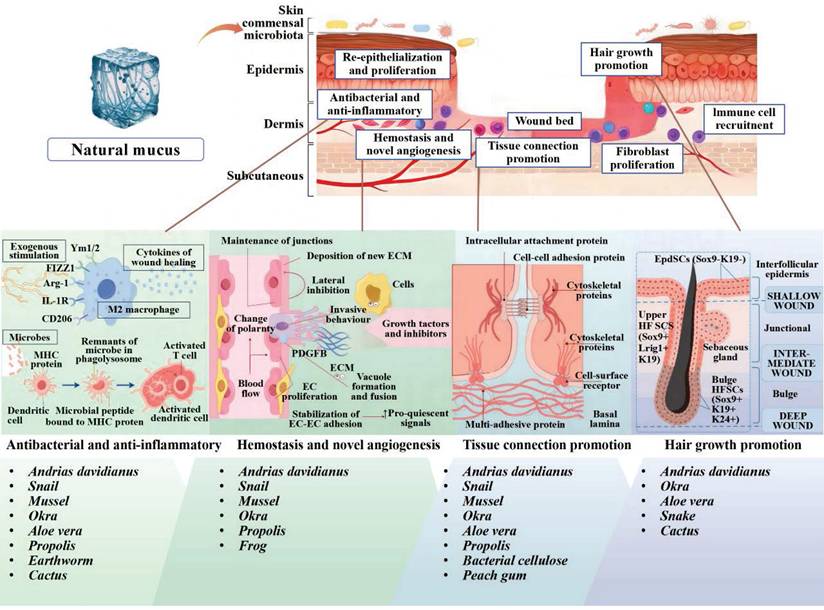
2. Natural Mucus in Wound Healing Process Regulation
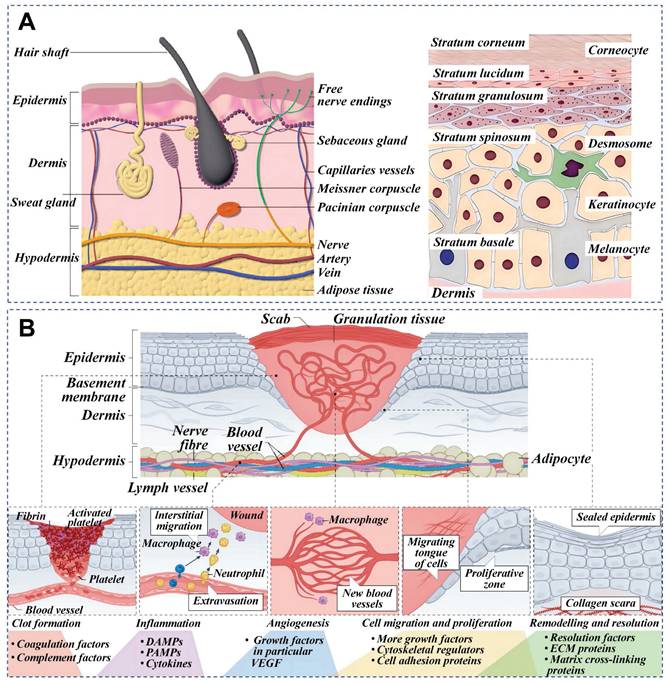
The skin is the largest organ of the human body, composed of epidermis, dermis, and subcutaneous tissue. Figure 3 depicts the epidermis, the outermost layer of the skin, which is often stratified into four distinct layers: the basal layer, spinous layer, granular layer, and horny layer. The basal layer harbors stem cells, which act as the progenitors for keratinocytes, initiating a process of epidermal renewal. As these keratinocytes ascend through the layers, they become adhered by desmosomes within the spinous layer. Keratohyalin granules in the granular layer and dead keratinocytes in the horny layer form a protective barrier against external microorganisms and substances [17]. The natural moisturizing factors in the horny layer play a significant role in skin hydration, softness, and elasticity. Natural mucus contains abundant adhesion molecules that strengthen adherent junctions. The dermal-epidermal junction plays an essential role in nutrient transport and immune isolation [18]. The dermis is primarily composed of fibroblasts accompanied by nerves, blood vessels, lymphatics, muscles, hair follicles, sebaceous glands, and sweat glands. These fibroblasts are responsible for the synthesis of collagen, elastin, and various enzymes, all of which contribute to the skin's mechanical strength, elasticity, and physiological processes [19]. Natural mucus-derived dressings interact with skin microstructure through conserved molecular mechanisms, achieving therapeutic effects via synergistic interactions between key biomolecules and dynamic adaptive mechanisms. Wound healing requires the synchronized activities of inflammation, cell migration, proliferation, matrix deposition, remodeling, and angiogenesis [20]. This reparative process can be divided into three distinct yet interwoven and successive stages: inflammation, proliferation and remodeling.
During the inflammatory phase, the evaluation criteria include the appropriateness of the inflammatory reaction, the recruitment and activity of leukocytes, and the balance of pro-inflammatory mediators. The cytokines released by the damaged blood vessel cause the inflammatory response to recruit immune cells. Neutrophils, as the first immune responders, combat infections by phagocytosis and release of antimicrobial factors [22]. Macrophages release cytokines and proteases to promote tissue repair [23]. Adhesion components like lectins in natural mucus can rapidly exert hemostatic and wound closure effects. In the initial contact with wounds, nature mucus can quickly release a variety of growth factors, enhancing the recruitment of stem cells. Cytokines like TNF, IL-1, and IL-6 are activated to regulate the inflammatory sequelae. Meanwhile, antimicrobial peptides and flavonoids in natural mucus have good antibacterial effects, reducing the risk of wound infection.
During the proliferation phase, the evaluation criteria focus on fibroblast activity, collagen and elastin synthesis and vascularization. These processes are crucial for skin toughness and elasticity. Cytokines such as TGF-β, EGF, PDGF, GM-CSF, and FGF interact with each other to promote matrix synthesis and cell proliferation [24]. Mucin in animal mucus can typically interact with dermal tissue to form a dynamic responsive network structure of reversible hydrogen bonds and disulfide bonds. This network can adaptively adjust adhesion via conformational changes in moist environments. Concurrently, proteoglycans and glycosaminoglycans are vital for cell proliferation, movement, differentiation, adhesion, and fiber formation. Plant-derived polysaccharides and pectin components are known to protonate carboxyl groups in acidic wound environments, enhancing electrostatic binding with collagen. These natural mucus substances can simulate the extracellular matrix (ECM) of dermal cells. While their self-adaptive properties can facilitate cell migration and proliferation, thereby accelerating the formation of granulation tissue [25].
Skin cross-section and wound healing phases. A) Cross-sectional anatomy of skin. Reproduced with permission [21]. Copyright 2023, John Wiley and Sons. B) Principal stages of wound healing and evaluation criteria at different wound healing stages. Reproduced with permission [19]. Copyright 2024, Springer Nature.
Main components of natural mucus associated with wound healing and their functions
| Components | Representative molecules | Primary categories | Function and mechanism | Ref. |
|---|---|---|---|---|
| Amino acids | Proline, lysine, cysteine, glutamic acid, aspartic acid | Structural molecules; Signaling molecules; Immunomodulatory molecules | Form ionic bonds with tissue surfaces via H-bonds/π-π stacking; Enhance viscosity, elasticity, and resistance to wound environmental factors | [27] |
| Protein | Mucins, enzymes, collagen, cytokines, antimicrobial peptides | Structural molecules; Signaling molecules; Hemostatic agents; Antimicrobial agents; Immunomodulatory molecules | Cross linked network provides shear-resistant scaffolds; Enhance cell adhesion and angiogenesis; Prevent infection | [28] |
| Polysaccharides | Glycosaminoglycans, pectin, heparan sulfate, hyaluronic acid | Structural molecules; Hemostatic agents; Immunomodulatory molecules | Responsive pH/redox adhesion and dynamic bond reorganization contribute to the hydration and lubrication of the wound site. | [29] |
| Lipids | Polyunsaturated fatty acids, phospholipids, | Signaling molecules; Immunomodulatory molecules | Integrate into cell membranes; Suppress pro-inflammatory mediators | [24] |
| Inorganic salts | Electrolytes, trace elements | Hemostatic agents; Immunomodulatory molecules | Form stable hemostatic plugs by electrostatic crosslinking with mucins; Enhance ionic interactions with wound exudate to stabilize the dressing and prevent maceration. | [30] |
| Other organic compounds | Phenolics, flavonoids, catechols, quinone derivatives, nucleic acids | Antimicrobial agents; Signaling molecules; Immunomodulatory molecules | Disrupt bacterial cell membranes and prevent infection; Scavenge ROS | [28] |
During the remodeling phase, focus shifts to collagen reorganization and maturation, vascular network stabilization and the reduction of cell signaling. During this stage, tissue repair is nearing completion and the function is restored. It has been confirmed that natural mucus contains a variety of growth factors associated with wound regeneration, angiogenesis, and epithelial regeneration, such as VEGF, PDGF, EGF, HGF, and bFGF. These growth factors can accelerate wound healing by meeting the remodeling needs of the wound [20]. In addition, some special components have also been proven to be effective in this stage. A novel glycosaminoglycans derived from the skin secretion of Andrias davidianus (SAGs) can modulate the gene expression related to glycolysis and lipid metabolism in macrophages via the PPARγ pathway, thereby promoting the transition of reparative macrophages. SAGs can also control the proportion of reticulum fibroblasts to curb collagen overexpression, thereby promoting hair follicle regeneration and scarless wound healing [26]. The key components of natural mucus are classified according to their biological functions in Table 1.
Dynamic interactions between these components in nature mucus are critical for wound healing (Table 1). Wound healing process could encompass a complex interplay among various cell types, biomolecules and tissues. A comprehensive grasp of the dynamic mechanisms governing cellular and molecular crosstalk provides the foundation for next-generation dressing design [31]. Functionally, natural mucus components can be categorized into several categories that work together to coordinate repair processes: structural molecules to providing mechanical support, signaling molecules to mediating cellular responses, hemostatic agents to controlling bleeding, antimicrobial agents to preventing infection, and immunomodulatory molecules to regulating inflammatory cascades. Given the challenges related to clinical wound dressings, nature mucus dressings with innate bioactivity and environmental responsiveness might be a viable and promising alternative.
3. Animal-Derived Natural Mucus and Their Properties
Animal mucus, a complex aqueous fluid secreted by goblet or mucous-producing cells lining the epithelial surfaces of organs exposed to the external environment, exhibits viscoelastic, lubricating, and hydration properties due to its composition and structure. These properties are attributed to the glycoprotein mucin, combined with electrolytes, lipids, and other smaller proteins. The structural and functional components of natural mucus offer a robust framework for wound healing [32]. And the chemical constituents, functionalities, and properties of animal mucus are influenced by the species of origin, tissue type, secretion method, and environmental conditions [33]. Water is the primary component, accounting for approximately 95% of the total mass. This elevated water content endows the mucus with fluidity and lubricative properties [34]. Highly branched mucus glycoproteins typically constitute less than 5% of the mucus. The mucin granules, which fuse with the plasma membrane and release upon activation, provide a dynamic and responsive system for wound dressing applications [35]. Other components, including lipids, inorganic salts, electrolytes, and antimicrobial substances such as lysozyme and immunoglobulins, account for about 1%. These components regulate the osmotic pressure, pH, antimicrobial and anti-inflammatory properties of the mucus [36].
Main mechanisms of wound healing and therapeutic applications of natural mucus from diverse sources: A comprehensive overview
| Type | Extraction method | Therapeutic mechanism | Representative composition | Full-thickness skin defects | Skin incision | Diabetic wound | Mucosal injury | Burn wound | Infected wound | Current progress | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Andrias davidianus | Non-invasive abrade skin, lyophilize, grind | Hemostasis, moisturization, barrier function, epithelialization, neovascularization, stem cell recruitment | Mucins, polysaccharides, growth factors, antimicrobial peptides, phenolic compounds, electrolytes | + | + | + | + | Laboratory research phase | [37-40] | ||
| Snail | Harvest mucus, lyophilize | Hydrophilic adhesion, M2 macrophage polarization, anti-inflammation, antioxidant, antibacterial, EGF promotion | Glycosaminoglycans, mucins, allantoin, ethanolamine, EGF-like peptides, | + | + | + | + | Laboratory research phase | [12, 41-44] | ||
| Mussel | Chemical/enzymatic extraction | Stable adhesive, cell adhesion/proliferation, coagulation, antioxidant, ECM synthesis | Mussel foot proteins, dopamine, polysaccharides, polyunsaturated fatty acids | + | + | + | + | Laboratory research phase | [45-50] | ||
| Okra | Soak seeds, filter, lyophilize | Hemostasis, antioxidant, M2 macrophage promotion, epithelial regeneration, collagen deposition | Pectin, acidic polysaccharides (galacturonic acid), flavonoids, phenolic acids | + | + | Laboratory research phase | [51-55] | ||||
| Aloe vera | Separate inner gel, filter, concentrate | Fibroblast proliferation, epidermal/vascular regeneration, anti-inflammatory, antioxidant, analgesic, antibacterial | Acetylated mannans, polysaccharides, aloin, lupeol, salicylic acid, gibberellins, vitamins E/C, amino acids | + | + | + | + | Partial clinical application | [56-61] | ||
| Propolis | Solvent extraction | Collagen expression, ECM remodeling, antioxidant, anti-inflammatory, antimicrobial | Resin acids, flavonoids, terpenes, enzymes, Caffeic acid derivatives, minerals | + | + | + | + | + | Partial clinical application | [62-73] | |
| Bacterial cellulose (BC) | Bacterial fermentation, collect, purify | Moisture retention, exudate absorption, physical barrier, nano-porous structure | β-1,4-glucan, trace proteins, organic acids, Exopolysaccharide matrix | + | + | + | + | Routine clinical use | [74-82] |
In this section, the design concepts of mucus from different animal sources are discussed as natural wound-healing dressings (Table 2). We emphasize the adhesion mechanisms and preparation methods of mucus derived from Andrias davidianus, snails, and mussels for natural wound-healing dressings, such as innate adhesiveness, anti-inflammatory, and biocompatible properties.
3.1. Andrias davidianus mucus
Andrias davidianus belongs to the Cryptobranchidae family originating from China. This species can attain lengths of 1 to 2 meters and typically weigh between 20 and 25 kilograms [83]. Its huge skin surface is uniformly populated with numerous granular glands and mucous glands. These skin glands secrete a mixture of milky-white and watery transparent liquids when stimulated, forming the skin secretions Andrias davidianus (SSAD), a mucus composite with distinctive bioactive properties. Contemporary research indicates that non-invasive sampling methods, such as mechanical or electrical stimulation, can enhance the efficiency of SSAD [84]. This natural advantage provides a pathway for green development and sustainable utilization in harnessing the biological resources of Andrias davidianus. Utilizing a combination of two-dimensional gel electrophoresis and mass spectrometry techniques, 155 proteins have been identified in the Andrias davidianus mucus [85]. Subsequent gene ontology analysis indicates that these proteins are implicated in ECM organization, defense responses, immune reactions, wound healing, and respiratory processes.
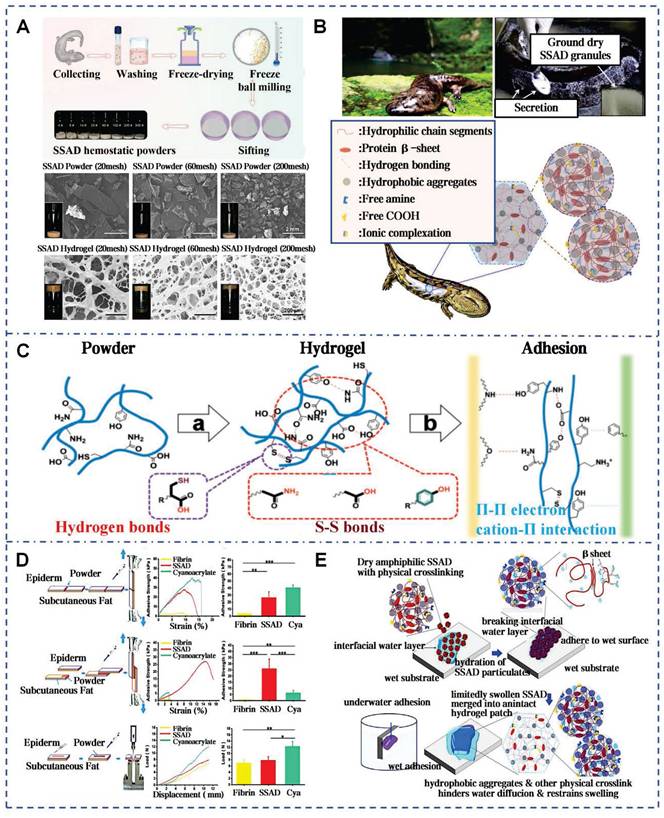
Zhang et al. pioneered the extraction of SSAD through freeze-drying and grinding, yielding approximately 2 g of powder per adult salamander monthly (Figure 4A) [40]. SSAD exhibits rapid hydration kinetics, forming a porous structure with an average pore size of 107.08±9.1 μm, which enhances stability through progressive densification of cavity walls. The natural adhesiveness of SSAD is attributed to functional groups that facilitate bonding (Figure 4B). Phenolic hydroxyl groups and amino acids donate hydrogen bonds, enhancing biological adhesion via hydrogen bonds and van der Waals forces. Benzene rings form strong substrate interactions through π-π electronic or cation-π interactions on hydrophobic surfaces. Additionally, S-S bonds reinforce the 3D structure of the hydrogel (Figure 4C). In vitro tests on pig skin showed that for edge-to-edge bonding, the shear bonding strength of SSAD is 26.66±8.22 kPa, which is similar to that of CAs (40.71±3.71 kPa), and is significantly higher than the bonding strength of fibrin glue (3.76±0.16 kPa) (Figure 4D). The presence of growth factors VEGF, PDGF, EGF, HGF, and bFGF in SSAD enhances wound healing by promoting re-epithelialization, neovascularization, and stem cells recruitment via MAPK pathway activation. In vivo studies showed that SSAD reduced healing time and promoted scarless healing, with complete biodegradation in 3 weeks [40]. Comparative analyses with Yunnan Baiyao revealed superior performance in both in vitro and in vivo models [38]. Stability studies confirmed that SSAD exhibits instantaneous adhesion, supporting 50 g within 20 seconds and sustained this performance over 7 days (Figure 4E). This attributed to the amphiphilic protein components that eliminate hydration layers and form hydrophobic cross-links [86]. The SSAD offered a multifunctional platform that integrates rapid adhesion, controlled biodegradation, and growth factor delivery.
Self-assembled SSAD hydrogel: mechanism and adhesion. A) Preparation process of the SSAD powders and the porous structures of the corresponding hydrogels. Reproduced with permission [38]. Copyright 2021, John Wiley and Sons. B) Self-assembled amphiphilic granular SSAD with strong wet adhesion. Reproduced with permission [86]. Copyright 2022, Elsevier. C) The schematic mechanism interpretation of hydrogel formation and adhesion of SSAD [40, 86]. Reproduced with permission [40]. Copyright 2019, John Wiley and Sons. Reproduced with permission [86]. Copyright 2022, Elsevier. D) Ex vivo adhesive properties of SSAD with CAs and fibrin glues. Reproduced with permission [40]. Copyright 2019, John Wiley and Sons. E) Schematic illustration of dry SSAD particulates' self-assembly and adhesion mechanism in water. Reproduced with permission [86]. Copyright 2022, Elsevier.
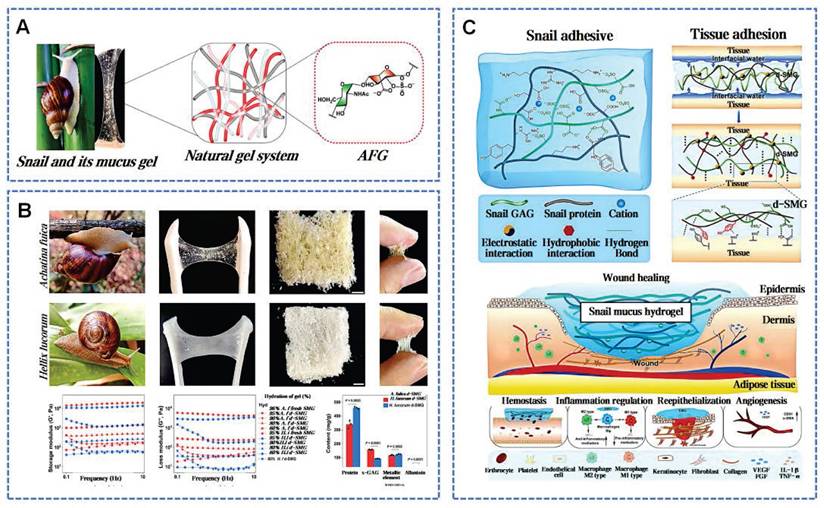
Snail mucus bioactivity and wound healing mechanism. A) Snail mucus and main bioactive glycosaminoglycan. Reproduced with permission [94]. Copyright 2023, Elsevier. B) d-SMG derived from two snail species. Reproduced with permission [41]. Copyright 2023, Springer Nature. C) Schematic interpretation of the mechanism d-SMG in wound healing. Reproduced with permission [41]. Copyright 2023, Springer Nature.
3.2. Snail mucus
Terrestrial gastropod snails secrete snail mucus (SNM) to preserve surface skin humidity and reduce the intake of contaminants. Snail pedal mucus exhibits robust interfacial adhesion, enabling resistance to detachment forces up to about 22 times body weight across varied substrate angles [87]. Snail mucus, a wondrous byproduct of the snail's instinct for survival, has also garnered human fascination for its unique therapeutic applications. In recent years, SNM is widely known for its rich bioactive components and broad application prospects. Existing research has explored the key pharmacological properties of snail mucus: enhancing cell proliferation and migration, angiogenesis, antimicrobial activity, free radical neutralization, and tumor growth inhibition [88]. Particularly, the key components of snail mucus have been a focus of research. Glycosaminoglycans (GAGs) were first extracted from the African giant snail A. fulica in 1996 [89]. GAGs not only participate directly in the construction of the ECM but also regulate the activity and inflammation-mediated functions of immune cells by intervening in cell signaling pathways (Figure 5A) [44]. The protein concentration in SNM is close to 4.8 mg/mL [90]. These proteins contain mucins, lectins, antimicrobial peptides, and growth factors, similar to human EGF and FGF, contributing to wound healing by reducing infection and inflammation [12]. The lectins in SNM are 70 kDa glycoproteins, and about 10% of their composition is carbohydrates, primarily N-acetylglucosamine [91]. Allantoin is a highly osmotic molecule in SNM, which can significantly enhance tissue absorption and retention of moisture. Ethanolamine in SNM inhibits key inflammatory enzymes by binding their active sites [88].
Assays based on cellular models revealed that SNM enhances cellular proliferation and migration, as well as increases the expression of adhesion factors in HaCaT and Hair Follicle cells [92]. Wu et al. prepared dried snail mucin gel (d-SMG) from Achatina fulica and Helix lucorum (Figure 5B) [41]. The d-SMG can rapidly form a strong adhesive on damp surfaces, suitable as natural wound adhesives. Positively charged amino or guanidino groups interact electrostatically with negatively charged sulfate and carboxyl groups in the sulfated GAGs, forming stable gel-like structures. Hydroxy, aromatic, and aliphatic amino acids in SNM facilitate extensive hydrogen bonding, π-π interactions, and hydrophobic interactions during gel formation. Divalent cations like Ca²⁺ and Mg²⁺ enhance the elasticity of the SMG through chelation and electrostatic interactions, providing structural support for adhesion. The supramolecular synergy within SMG is responsible for creating strong cohesive forces and excellent tenacity (Figure 5C) [93]. Animal studies confirm d-SMG's hemostatic properties, biocompatibility, and biodegradability. SNM notably accelerates the healing in normal and diabetic rats, with superior results in granulation tissue, collagen deposition, and neovascularization compared to alginate dressings. Further analysis indicated that d-SMG could facilitate the polarization shift from pro-inflammatory M1 to anti-inflammatory M2 macrophages [41]. These insights provide theoretical and material guidance for the design of bio-inspired tissue adhesives and bioengineering scaffolds.
3.3. Mussel mucus
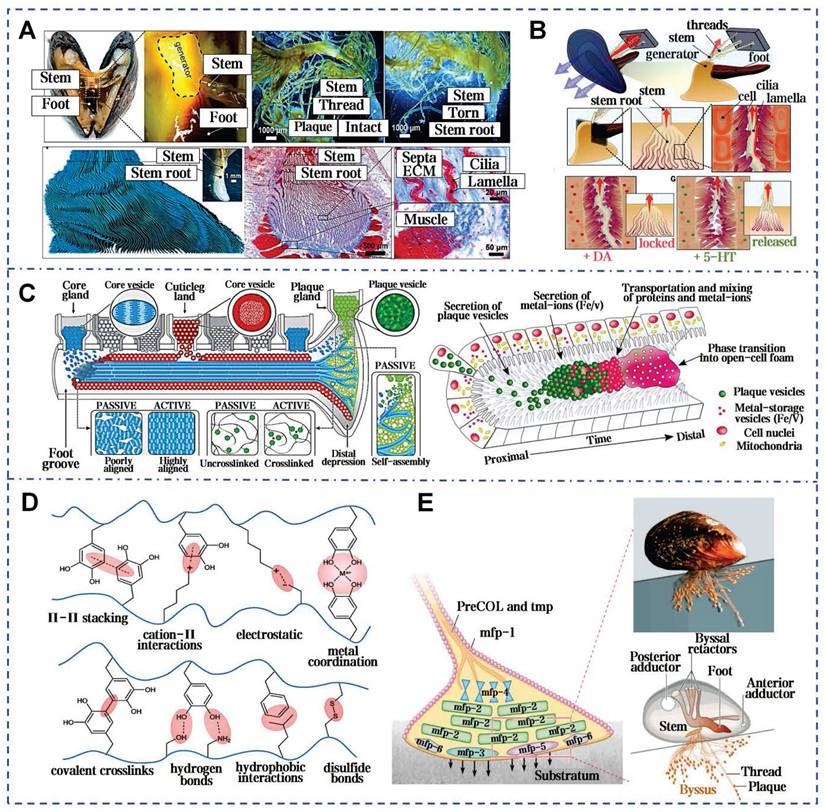
Surge, storm, salinity erosion, temperature fluctuations, biofouling, and other natural factors all contribute to the complexity of the marine environment [95]. However, numerous marine organisms, mussels in particular, still adhere strongly to substrates under ocean currents and maintain long-term stability. Mussels, dwelling in the intertidal zone, rank among the strongest natural adhesive sources in the wild [96]. When encountering seawater, the natural mucus secreted by the mussel's foot glands rapidly solidifies into byssal threads and forms byssal plaques at the point of attachment to substrates (Figure 6A). Mussel mucus exhibits extremely strong adhesion and outstanding water resistance, solidifying rapidly to firmly attach to surfaces such as rocks, ships, glass, and corals (Figure 6B) [97]. Additionally, mussel mucus also adheres strongly to inert anti-adhesive materials. In the shipbuilding industry, it can even replace conventional fastening methods like screws and rivet welding. Pujol et al. initially elucidated the composition and structure of mussel mucus (byssus), identifying mussel foot proteins (Mfps) and other 3 main categories proteins (Figure 6C) [98]. All Mfps contain dihydroxyphenylalanine (Dopa), a hydroxylated tyrosine derivative critical for adhesion. Dopa contributes to the waterproof and versatile adhesive properties of Mfps through interactions such as hydrogen bonding, π-π interactions, cation-π interactions, metal ion chelation, and electrostatic attraction (Figure 6D) [99]. The reduced catechol group adheres strongly to inorganic surfaces, but weakening upon oxidation. To counteract oxidation, mfp-6 at the adhesive plaque interface provides antioxidative protection, preserving catechol's reduced state and enhancing adhesion (Figure 6E) [100].
The adhesive properties of mussel mucus are not limited to mechanical bonding, also extend to wound healing and tissue repair. This is attributed to Mfps facilitating cell adhesion and migration [101]. Furthermore, Dopa in Mfps is linked to anti-inflammatory properties, reducing the expression of pro-inflammatory cytokines. The oxidation of Dopa to Dopa quinone and its subsequent reactions form a cross-linked network, potentially strengthening the ECM for damaged tissues rebuilding [102]. The catechol groups in mussel mucus are recognized for their multifunctional role in supporting coagulation, anti-inflammatory, antioxidant effects and promoting cell adhesion in a moist environment. A catechol-modified levan hydrogel demonstrated low immunogenicity, biocompatibility. This bioadhesive demonstrates an adhesive strength of up to 42.17±0.24 kPa under moist conditions, about 3 times higher than fibrin glue and accelerates the growth and migration of NIH3T3 fibroblasts and HaCaT keratinocytes [103]. Lee et al. innovated a dopamine-based surface modification technique by oxidative self-polymerization, forming polydopamine layers of tunable thickness on various substrates [104]. The detachment mechanism of mussel byssus from living tissues reveals an interface with controllable adhesion, crucial for clinical applications like implantable biomaterials and detachable biosensors.
3.4. Other animal mucus
Similar to snail mucus, slug mucus is also a terrestrial gastropod secretion that showed comparable efficacy in promoting wound repair. Slug mucus possesses large content of bioactive components, such as hemocyanin beta, SSD domain-containing protein, calcium-transporting ATPase and phospholipase C. These components work together to achieve a lap-shear force of approximately 1.1 N and enable rapid hemostasis in liver trauma in less than 15 seconds [106]. Polysaccharides derived from natural loach mucus can significantly inhibit leukocyte migration, showing superior anti-inflammatory activity compared to the dexamethasone sodium phosphate [107]. Frog skin mucus enhanced wound healing through TGF-β1 pathway activation, while radiation injury models further validate the efficacy of amphibian cutaneous mucus peptides in resolving complex tissue damage [108]. Earthworm mucus extract (EE) orchestrated wound healing by stimulating cell proliferation, collagen synthesis and increasing the number of early white blood cells, neutrophilic granulocytes, and platelets [109]. EE-mediated fibroblast cycle regulation specifically demonstrated mitochondrial membrane potential restoration in diabetic wounds [110]. Furthermore, as a snake extract from Bothrops atrox, hemocoagulase can rapidly convert fibrinogen into fibrin for hemostasis and promotes wound healing through the synergistic effects of platelet activation and fibrin mesh formation. This unique rapid hemostasis property provides a foundation for its application in advanced wound dressings [111]. These comparisons underscore the evolutionary conservation of bioactive mucus components, particularly in species subjected to frequent cutaneous injuries, suggesting phylogenetic patterns could guide future bioprospecting strategies for precision wound therapeutics.
Mussel byssus attachment system: structure, function, and molecular interactions. A) The intricate structure of a mussel's byssus attachment system, including the generator region that produces the stem and its root. Reproduced with permission [100]. Copyright 2023, The American Association for the Advancement of Science. B) How wave forces acting on the mussel are transmitted through the byssus into the stem and generator. Reproduced with permission [100]. Copyright 2023, The American Association for the Advancement of Science. C) Schematic model of the secretion process during plaque formation [97, 105]. Reproduced with permission [97]. Copyright 2017, Springer Nature. Reproduced with permission [105]. Copyright 2021, The American Association for the Advancement of Science. D) Overview of the proposed different adhesive and cohesive molecular interactions found in mussels. Reproduced with permission [99]. Copyright 2018, John Wiley and Sons. E) The mussel byssal adhesion manifests through a dense protein framework. Mfp-1 functions as an outer cuticle for thread protection. Reproduced with permission [99]. Copyright 2018, John Wiley and Sons.
4. Plant-Derived Natural Mucilage and Their Properties
Plant mucilage is generally biosynthesized by specialized cells or tissues, including mucilage glands, seed coat cells, or special structures on the leaf surface [112]. For example, the Golgi apparatus of testa in angiosperms can secrete a special pectic complex polysaccharide, whose seeds are known as myxospermy [113]. The mucilage of myxospermy is often in a dehydrated state. However, when myxospermy is exposed to water, the mucilage absorbs water and swells, breaking through the primary wall, completely wrapping around the seed's periphery and forming a gelatinous layer of mucilage on the surface of the seed [114]. The plant mucilage is composed of polysaccharides, proteins, and other bioactive compounds. Plant mucilage plays multiple roles in plant physiological processes, including environmental adaptation, seed protection and dispersal, growth promotion, aiding in moisture and nutrient absorption, as well as capturing and digesting prey in carnivorous plants [115]. Moreover, plant mucilage holds extensive uses in daily life and industrial applications. Carob seeds mucilage can be used as a thickener and emulsifier in food industry [116]. Okra mucilage is rich in soluble dietary fibers, which can promote gastrointestinal motility, protect gastric mucosa, reduce cholesterol absorption, and facilitate lipid-lowering and laxative effects [117]. Similar to flocculant, the polysaccharides present in okra mucilage can agglomerate and carry away microplastics in the water [118]. This section mainly focuses on the adhesion effects, preparatory methods, and potential in promoting wound healing of natural plant mucilage derived from okra and aloe.
4.1. Okra mucilage
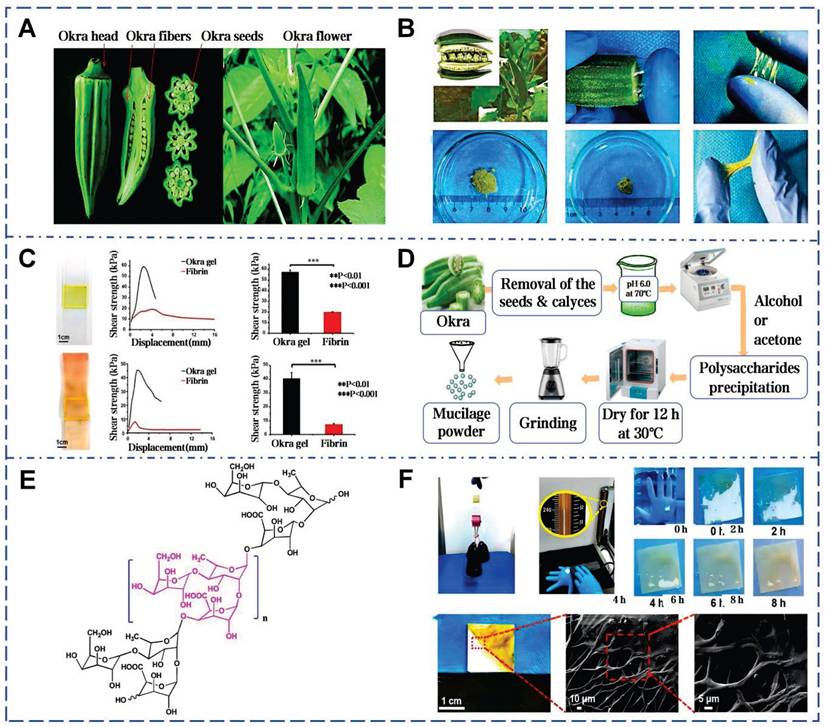
Okra is an herbaceous annual plant belonging to the genus Abelmoschus in the Malvaceae family (Figure 7A). Conrad et al. first isolated mucilage from okra pods, beginning the identification of its acidic polysaccharides (Figure 7B) [119]. Subsequent research established a direct correlation between the polysaccharide content and the viscosity of okra mucilage (Figure 7C) [120]. Okra mucilage is primarily composed of pectin, polysaccharides, and glycoproteins, with pectin being the major constituent [54]. Galacturonic acid, galactose, rhamnose, glucose and other monosaccharides are interconnected, forming a robust structure. High content of galactose and galacturonic acid indicate that okra polysaccharides (OPS) possess characteristics akin to pectin, supported by the RG-I region [121]. OPS can be used as an adhesive in Naproxen sodium tablets, surpassing traditional starch in adhesive strength [122]. In addition, different extraction conditions can also affect the molecular structure within okra mucilage, thereby impacting its rheological properties and adhesive effectiveness (Figure 7D) [123].
Glycosidic bonds and ionic groups on okra polysaccharide chains can form intermolecular and intramolecular hydrogen bond networks, causing chain expansion and increasing viscosity (Figure 7E) [124]. These high molecular weight polysaccharides offer abundant interaction sites to form a robust 3D network, which is beneficial for wound coverage and protection (Figure 7F) [125]. According to traditional medicinal practices, the mucilage derived from pounded okra fruits can be used directly to heal skin wounds and subcutaneous abscesses [126]. When powder of freeze-dried okra mucilage rehydrated, it forms a highly viscous natural okra hydrogel (OHG) [55]. OHG can substantially reduce the levels of TGF-β and IL-1β in the damaged tissue, and enhances collagen deposition and tissue maturation. The adhesive strength of OHG (glass: 57.6±1.9 kPa, pigskin: 40.6±4.3 kPa) is about 3 times higher than that of fibrin glue on glass substrates and about 6 times higher on porcine skin. Compared with chitosan hemostatic agents, OHG has a shorter coagulation time. Notably, this work firstly elucidates the potential of okra mucilage as an innovative natural biomaterial in stimulating platelet polarization and promoting tissue regeneration.
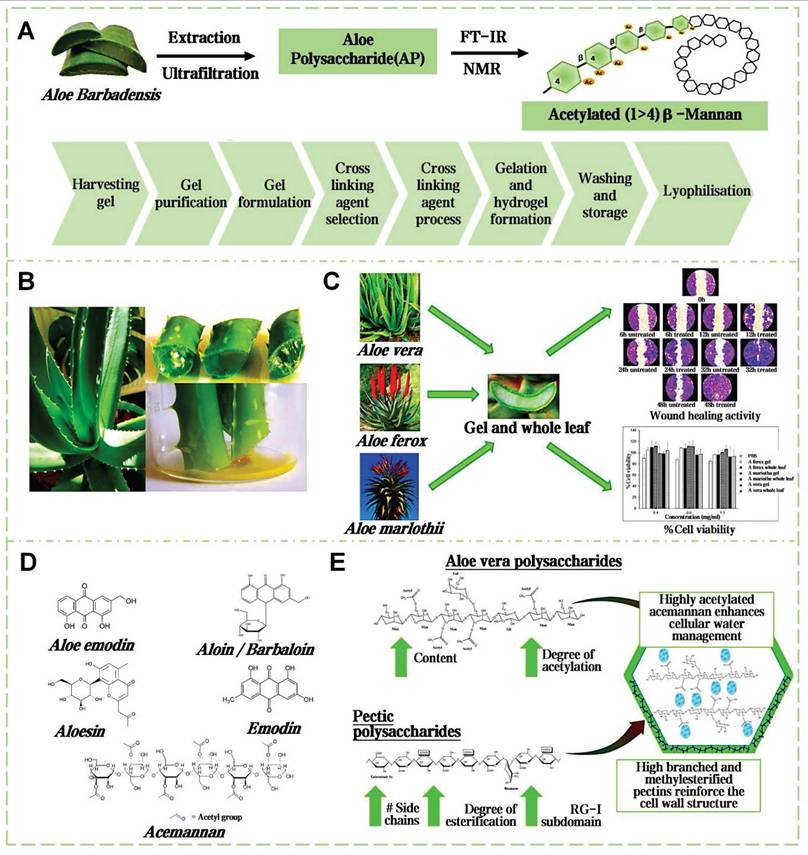
4.2. Aloe mucilage
Aloe vera, a perennial herb of the Liliaceae family, has globally medicinal applications for over 23 centuries [127]. The structure of the aloe leaf is tri-layered. Outermost layer is fibrous epidermis, preventing excessive moisture evaporation and external environmental damage (Figure 8A). The outer leaf area secretes a yellowish latex. The inner leaf's thin-walled tubular cells secrete a colorless, tasteless mucilage, known as aloe gel (Figure 8B) [128]. Dry weight analysis shows the key components of aloe gel are polysaccharides, followed sequentially by minerals, proteins, lipids, and phenolic compounds [129]. The acetylated mannans and polymannoses can establish hydrogen and ionic bonding with polar entities on the surface encountered. The carboxyl groups can ionically bond with skin cations, the hydroxyl groups of phenolic compounds can form covalent bonds with thiol groups in skin proteins, further stabilizing the adhesive interface [130]. Concurrently, the moisture in aloe gel aids in the relative movement and reorientation of polysaccharide chains, thus improving the adhesion effect [131]. Gao et al. delineated the pharmacological activity and clinical utilities of aloe gel, including its roles in tissue repair, radiation recovery, burn healing, acne, antioxidation, antiviral, antibacterial, anti-inflammatory, antidiabetic, anticancer, skin protection and immunity boosting (Figure 8C) [132].
Okra mucilage: structural, chemical, and mechanical evaluations. A) Picture of okra plant and okra internal structure. Reproduced with permission [126]. Copyright 2021, Springer Nature. B) Okra mucilage and mucilage freeze-dried powder. Reproduced with permission [55]. Copyright 2022, John Wiley and Sons. C) Comparison of adhesion between okra mucilage and fibrin glue on glass and pig skin. Reproduced with permission [55]. Copyright 2022, John Wiley and Sons. D) Extraction and isolation of mucilage from okra pods. Reproduced with permission [118]. Copyright 2020, Elsevier. E) Chemical structure of okra mucilage polysaccharide. Reproduced with permission [118]. Copyright 2020, Elsevier. F) Load-bearing test, underwater adhesion test and SEM images of okra mucilage. Reproduced with permission [55]. Copyright 2022, John Wiley and Sons.
Aloe gel enhances wound healing via synergistic mechanisms (Figure 8D) [133]. Acemannan polysaccharides activate macrophage phagocytosis and stimulate TGF-β-mediated collagen synthesis. Interlinked polysaccharide chains create a 3D framework, which can augment the oxygen concentration and microcirculation within the wound vicinity (Figure 8E). Lupeol, saponins, salicylic acid, urea nitrogen, cinnamic acid, dihydroxyanthraquinone, anthraquinone derivatives, as well as those containing phenolic and sulfur elements, together form a complex system with antibacterial and anti-inflammatory properties [134]. The experiment results show that aloe mucilage at a 40% (w/v) concentration was effective in inhibiting the growth of gram-negative bacteria [135]. Moreover, essential amino acids provide the necessary substrate for tissue repair and cell regeneration. Vitamins, organic acids, minerals, and other various trace elements are crucial in shielding wounds from oxidative stress. Lectins, gibberellins, and growth factors can fortify the body's innate reparative capabilities [136]. Aloe mucilage can enhance fibroblast and blood vessel counts in burn wound healing [137]. In addition, aloe mucilage has demonstrated efficacy in remedying insect stings and ringworm, also applied to treat various viral skin lesions [138]. Blending freeze-dried aloe mucilage powder with varying quantities of water, which can serve as a coating for bamboo fiber sutures [139]. This composite dressing can be customized to the size and shape of the wound. Pan et al. mixed an aloe hydrogel matrix with aloe-derived exosome nanoparticles to create a new wound dressing (ADENHs) [140]. ADENHs treatment can significantly reduce serum IgE levels in atopic dermatitis model, decrease the expression of inflammatory cytokines in diabetic wound. Furthermore, the integration of aloe mucilage into the E-skin architecture can be used as an advanced wound dressing, that can monitor and respond to the healing process [141].
Comprehensive analysis of aloe mucilage: from extraction to biological properties. A) Extraction and isolation routes of natural aloe mucilage. Reproduced with permission [142]. Copyright 2020, Elsevier. B) Picture of aloe plant and aloe internal structure. Reproduced with permission [134]. Copyright 2023, MDPI. C) Three species of aloe mucilages for cell growth and wound healing promotion. Reproduced with permission [143]. Copyright 2017, Elsevier. D) The main chemical components of aloe vera mucilage. Reproduced with permission [134]. Copyright 2023, MDPI. E) The molecular mechanism of moisture retention properties of aloe mucilage. Reproduced with permission [131]. Copyright 2024, Elsevier.
4.3. Other botanical mucilages
There are also many unexplored natural plant mucilages with potential to promote wound healing. Yam mucilage facilitates effective hemostatic management in complex tissues and organs with its blood-activated gelation and robust hemostatic adhesion capabilities [144]. The glial mucus in Tunisian cactus has been shown to have antibacterial and antifungal properties [145]. Mustard mucilage contains glucosinolates, which possesses antimicrobial properties [146]. The bark of the peach tree can secrete a type of natural plant mucilage, known as peach gum [147]. Peach gum is rich in various polysaccharides and phenolic compounds, which endow it with certain anti-inflammatory and antioxidant properties. Currently, peach gum has been successfully applied in the preparation of adhesives and hydrogel materials. Oliveira et al. summarized the application of mucilage from flaxseed, Brazilian cactus pear, and chia seeds in wound treatment [133]. They found that these plant-derived mucilages are rich in bioactive components, such as Omega-3 fatty acids and vitamin E, which show potential in accelerating wound healing and reducing infection risk.
5. Complex-Sourced Natural Mucus and Their Properties
The ability to secrete mucus with distinctive properties is not confined to just fauna and flora. In fact, the kingdom of biological mucus pervades every species in nature, demonstrating remarkable diversity in morphology and function. Complex-sourced natural mucus is not a single substance, but a general term for a class of dynamic biohydrogels. Depending on their origin and function, they are often referred to by various terms such as "slime, hydrogel, biofilm, mucilage, glycocalyx exopolysaccharides and extracellular polymeric substances (EPS)". Complex-sourced natural mucus can also be found in ecological contexts, such as the EPS of biological soil crusts or the organic aggregates of marine snow [8]. These mucous substances present great application potential in biomedicine and material science. This section focuses on two typical types of mucus with natural biopolymer characteristics: Propolis (a complex substance derived from both animal and plant components) and microbial EPS (exemplified by BC, a structurally defined exopolysaccharide for clinical application). We discussed the biological origins, chemical compositions, and properties and their prospective applications in enhancing wound healing. By deeply exploring the properties and functions of these unconventional bioadhesives, we can discover more natural resources, providing innovative ideas and methodologies for the advancement of biomaterials treatment methods.
5.1. Propolis
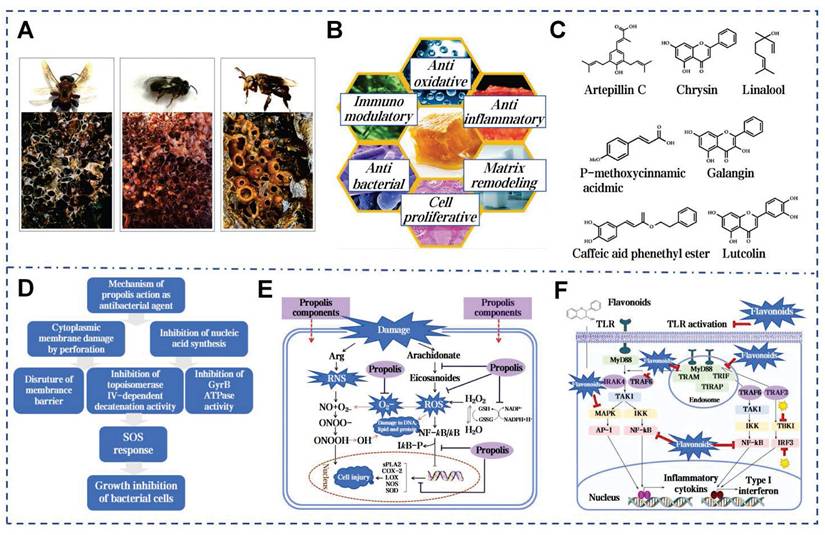
Propolis, a fragrant resinous substance, is synthesized by worker bees that mixing plant exudates such as buds, leaves, and sap from tree wounds with secretions from their own glands (Figure 9A). The medicinal value of propolis has been documented in ancient texts worldwide and dates back 3,000 years (Figure 9B) [148]. Bees utilize propolis to fortify their hives against environmental hazards and intruders, or to encase carcasses and prevent decay and microbial growth. The adhesiveness of propolis arises from its complex chemical composition, primarily consisting of resin, beeswax, essential oils and pollen [149]. Resin acids and gum form hydrogen and ionic bonds. Flavonoids enhance adhesion to nonpolar surfaces and terpenes promote π-π stacking. Beeswax components contribute through hydrophobic and Van der Waals forces. Additionally, enzymes secreted by bees during processing catalyze cross-linking reactions, further strengthening the network and enhancing propolis's mechanical properties and adhesion on various surfaces (Figure 9D) [150].
Propolis contains over 600 compounds, with coffee acid phenethyl ester (CAPE) being the most studied [151]. CAPE can reduce histamine release and inflammatory cytokine production, while also acting as a potent inhibitor of the NF-κB pathway [152]. Emodin and Kaempferol are key antiallergic components [153]. The ethanol extract of propolis shows stronger antioxidant activity than vitamin C and E (Figure 9E) [154]. Propolis has been confirmed to inhibit various bacteria. The active components of propolis can attach to the bacterial cytoplasmic membrane, leading to membrane perforation. Its flavonoid compounds inhibit the activity of topoisomerase IV, suppressing bacterial growth (Figure 9F) [155]. Propolis contains over 12 mineral elements. The Zn2+ can aid skin follicle regeneration and suppress bacterial growth [155]. Martinotti et al. outlined the role of propolis in stimulating wound matrix remodeling and increasing the components of the ECM in the early stages of wound repair [72]. Studies indicate that Brazilian red propolis enhances wound healing by reducing neutrophils and macrophages at the wound site [156]. The ethanol extract of Chinese propolis reduced the buildup of reactive oxygen in fibroblasts by modulating antioxidants gene expression [157]. Propolis nanoparticles (PNPs) are synthesized from propolis extract using a pH differential method. PNPs significantly enhance the levels of antioxidant enzymes SOD and glutathione in wound tissue and upregulate TGF-β. These effects indicate their potential in clinical skin wound treatment by promoting collagen formation and angiogenesis [71].
Multifaceted biological activities and molecular mechanisms of propolis. A) The images of stingless bees and their propolis. Reproduced with permission [153]. Copyright 2020, Elsevier. B) The main mechanisms of propolis in promoting wound healing. Reproduced with permission [72]. Copyright 2015, Oxford University Press. C) Main functional compounds of propolis. Reproduced with permission [63]. Copyright 2022, John Wiley and Sons. D) Mechanism of propolis action as anti-bacterial agent Reproduced with permission [73]. Copyright 2018, Elsevier. E) The molecular mechanism of the propolis-mediated protective effect during the oxidative stress Reproduced with permission [73]. Copyright 2018, Elsevier. F) Antibacterial mechanism of flavonoids in propolis Reproduced with permission [73]. Copyright 2018, Elsevier.
5.2. Microbial EPS
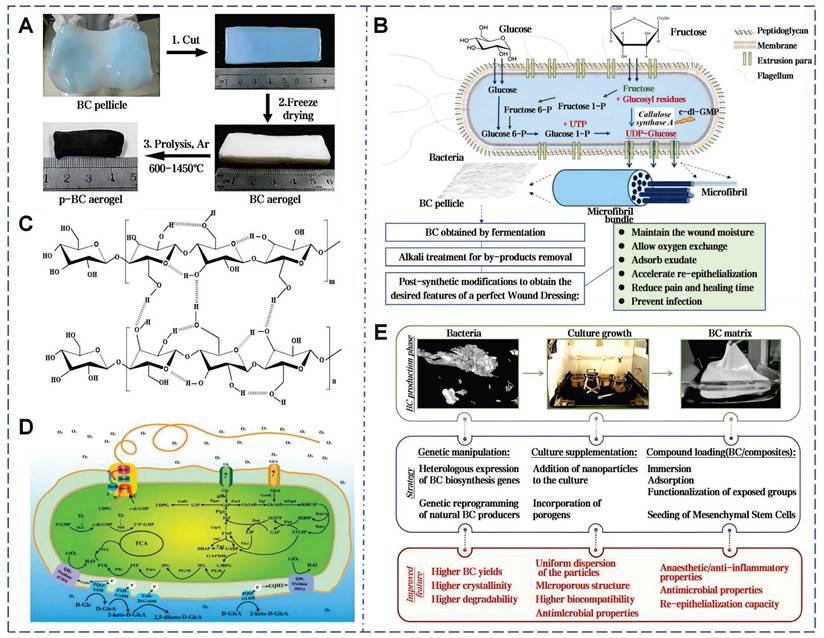
As the most primitive and proficient mucus producers in nature, microorganisms lack a unified nomenclature for their secreted mucus. Due to their unique growth characteristics, microorganisms can bind self-secreted extracellular polysaccharides with water, sediments, metabolites, and other matrices in the surroundings to form specialized EPS [158]. BC is a natural polysaccharide hydrogel generated through metabolic fermentation using sugars as the main carbon source (Figure 10A) [159]. In 1886, Brown first isolated BC from acetic acid fermentation tanks, and this bacterium was later named Komagataeibacter xylinus [160]. This bacterium links β-D-glucopyranose units via β-1,4-glycosidic bonds to form nanoscale glucose polymers (Figure 10B). During secretion, the bacteria move randomly in the culture medium, resulting in an ultra-delicate 3D porous network structure. (Figure 10C) [75]. The nanoscale thickness fiber network of BC provides high-density hydrophilic groups and an expanded internal surface area, enabling hydrogen bonding with water molecules and enhancing adhesion to moist surfaces [161]. Compared to plant cellulose, BC exhibits greater mechanical strength, with high degrees of polymerization and crystallinity [162].
Studies have shown that EPS derived from bacteria (Bacillus subtilis) and microalgae (Chlorella zofingiensis) can accelerate wound healing. This microalgae-probiotics biogenic dressing establishes a 3D harmonized microbial community at wound sites, delivering dissolved oxygen while suppressing pathogenic colonization and modulating healing dynamics [163]. BC exhibits flexibility, high purity, non-toxicity, non-irritation to skin, high biocompatibility, degradability, and renewability, making it an ideal candidate for natural wound dressings (Figure 10D) [164]. Over the last century, BC-based commercial wound dressings have been marketed. They showed excellent wound adhesion, healing acceleration, and infection risk reduction in over 300 clinical trials for skin damage and burns [165]. To date, various BC products are widely available. The ultrafine fibers of BC can granulation tissue adhesion, avoiding epithelium stripping during dressing changes and facilitating wound monitoring (Figure 10E) [74]. The unique structure of BC can continuously absorb exudate, maintaining optimal moisture and inflammatory levels at the wound site [166]. In addition, the chemical formula of BC is (C6H10O5)n, with each glucose ring featuring hydroxyl groups. These hydroxyl groups in BC molecular chains can be readily modified by functional groups such as aldehydes, carboxylic acids, and amines, resulting in different properties [167]. The allylation modification to BC promoted its water absorption capacity after drying [168]. Hollow BC microspheres, fabricated using microfluidic technology, can be served as innovative injectable porous scaffolds in 3D cell culture and tissue regeneration. After 48 h of culture, cells in the hollow BC microsphere scaffold proliferated to 95 µm depth, versus 10 µm in the bulk BC scaffold (within 100 µm framework) [77].
Comprehensive fabrication and properties of BC-based wound dressings. A) Various steps involved in the fabrication of BC-based materials. Reproduced with permission [169]. Copyright 2021, John Wiley and Sons. B) The steps involved in the production of a BC-based wound dressing. Reproduced with permission [75]. Copyright 2019, John Wiley and Sons. C) Chemical structure of BC. Reproduced with permission [74]. Copyright 2021, Elsevier. D) Metabolic diagram of BC produced by Acetobacter. Reproduced with permission [74]. Copyright 2021, Elsevier. E) Summary of the processes involved in BC production. Reproduced with permission [75]. Copyright 2019, John Wiley and Sons.
6. Pre-Clinical Studies for Natural Mucus in Diverse Wound Models
Wound is defined as injury to the structure of tissues or organs, customarily divided into acute and chronic wounds. Uncontrolled bleeding from acute wound is the second leading cause of pre-hospital deaths, and about 50% combat-related deaths linked to acute wounds [170]. Chronic wounds could not revert to normal anatomical and functional states via the organism's innate reparative mechanisms. 1%-2% of the global population could experience chronic wounds during their lifetime [171]. Given the limitations of self-healing capabilities, medical intervention is often required to facilitate treatment. In 1962, Dr. Winter found that the healing rate in a moist milieu is over twice as quick as in a dry setting, hence mitigating scar formation. This revelation prompted the FDA to establish the protocol of moist wound as a standard approach in wound management [172]. Based on this concept, a lot of natural mucus has been frequently applied in wound healing. This chapter summarizes the characteristics of various wound models and their clinical treatment challenges, further analyzes the therapeutic effects of natural mucus in these models.
6.1. Acute skin traumas
The integumentary system acts as the first line of defense against external stimuli and injuries. Skin is one of the largest organs of the human body, with the surface area reaching 1.5 to 2.0 m2 in adults and about 0.21 m2 for newborns [173]. Acute skin traumas can rely on the skin's self-repair capabilities to fully heal within 2 to 4 weeks. However, due to factors such as metabolic diseases, impaired wound microcirculation, or microbial infections, common skin injuries may develop into chronic wounds. Therefore, promoting the speed of wound healing is crucial for acute skin traumas [174].
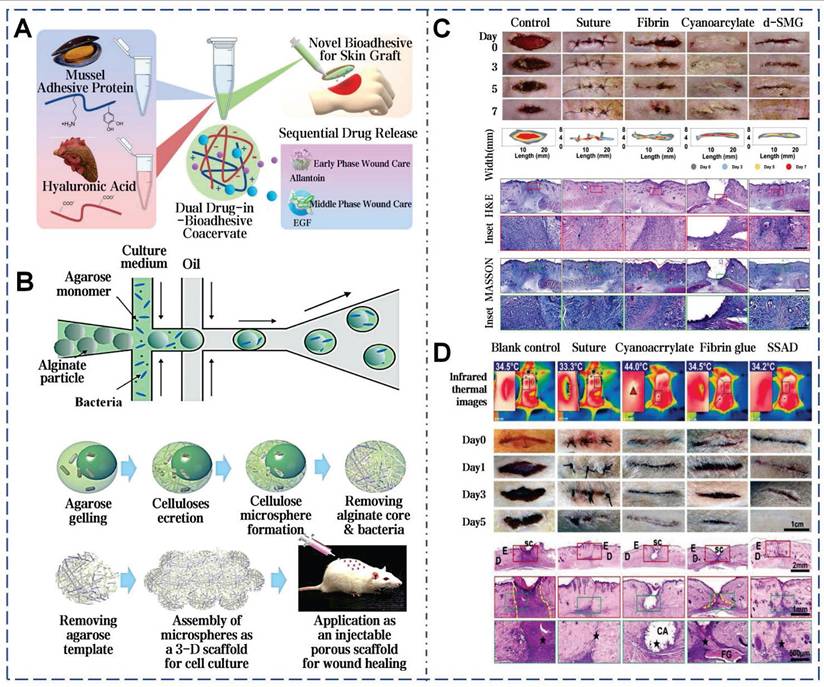
Adhesives developed from mussel adhesive proteins still exhibit high adhesion strength under wet conditions. The dopamine residues in these adhesives can interact with other molecules and accommodate the dynamic progression of wounds, thereby enhancing wound healing following full-thickness skin transplants (Figure 11A) [175]. Additionally, BC without any modification is also demonstrated effective promotion of wound healing. Compared with traditional BC membranes and solid BC microsphere scaffolds, cells on the scaffold assembled by hollow BC microspheres show a deeper penetration depth and higher proliferation rate (Figure 11B) [77]. Parallel to previous findings, snail mucus is known for having unique bioactive properties and enhancing wound healing [41]. After treatment with snail mucus, the epidermis is completely regenerated, with distinct hair follicles and sebaceous glands near the incision (Figure 11C). The efficacy of SSAD in wound management is also demonstrated. Zhang et al. compared SSAD to traditional suture, α-cyanoacrylate, and fibrin glue [40]. Application of 5 mg of SSAD powder to a 2-cm full-thickness skin incision can achieve rapid hemostasis and wound closure within 30 seconds. Furthermore, SSAD modulated acute inflammatory cell recruitment to the wound site and promoted continuous basal membrane integration which resolves completely within 21 days to minimize scarring (Figure 11D). SSAD can inhibit excessive TGF-β1 and TGFB1 secretion, while enhancing FGF2-mediated intercellular signaling. This scarless regeneration was further validated by near-absent expression of EN-1 (a fibrosis-associated fibroblast marker) in SSAD-treated wounds. Transcriptomic analyses revealed upregulated genes for ECM remodeling and downregulated fibrosis-related pathways. The scar ratio in the SSAD group was only 18.08%±6.64% significantly lower than 63.87%±6.46% in the blank control group [176].
6.2. Diabetic wounds
Diabetic wounds are among the most challenging diseases globally, with approximately 600 million people worldwide affected by diabetes. At present, over 80 million people with diabetes worldwide are struggling with diabetic wounds and diabetic foot ulcers, resulting in amputation rates as high as 24% [177]. Regrettably, the 5-year survival rate of patients who undergo amputations due to diabetes is lower than that of most cancer patients, and the treatment costs associated with diabetes-related amputations often exceed those of common cancers [178]. The main etiologies of chronic diabetic wounds include difficulties in vascular reconstruction, peripheral neuropathy, and continuous activation of inflammation. Elevated blood glucose levels lead to excessive generation of ROS in HUVECs, activating pathways like protein kinase C, which induces cell damage and ultimately delaying wound healing [179].
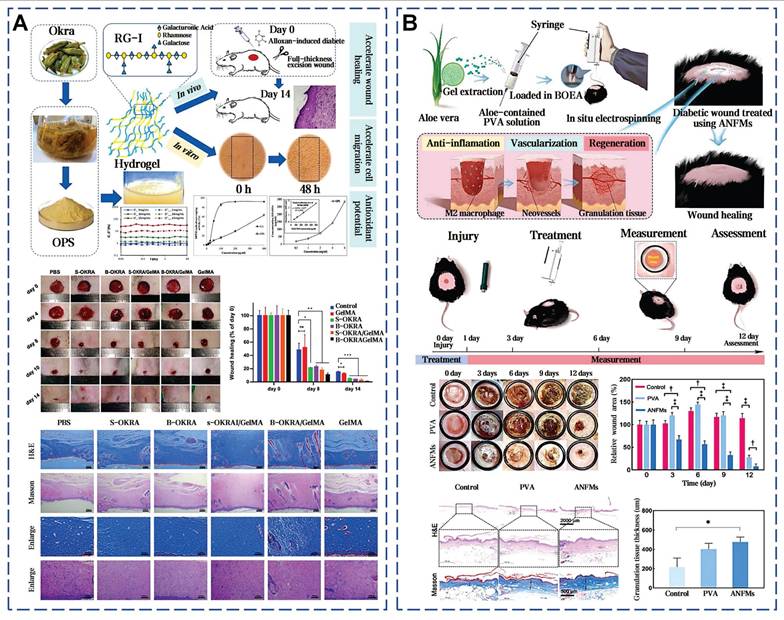
Wu et al. delved into the application of snail mucus for diabetic wounds, demonstrating its ability to increase granulation tissue thickness and collagen deposition, as well as promote angiogenesis and epithelialization [41]. The presence of GAG in d-SMG is associated with the promotion of M2 macrophage polarization through STAT3 phosphorylation upregulation. SSAD also has the significant potential in the treatment of diabetic wounds by stimulating angiogenesis and reduces inflammation [40]. Notably, full-skin diabetic defect treated with SSAD showed minimal scarring. In addition, okra mucilage has demonstrated the ability to markedly enhance the healing process in diabetic wounds (Figure 12A) [52]. These effects are attributed to the continuous release of various bioactive components in okra mucilage, such as okra polysaccharides, flavonoids, phenolic acids, and small molecular weight nutrients. Aloe mucilage was fabricated into aloe nanofiber membranes (ANFMs) to promote chronic wound healing. ANFMs promoted granulation tissue thickening and neovascularization, and the increase in the proportion of Ki-67 positive cells further confirms the potential to accelerate cell proliferation (Figure 12B) [58].
Selected cases of natural mucus utilization in routine skin wound healing. A). MAP-derived bioadhesive coacervates are utilized to facilitate wound healing following full-thickness skin transplants. Reproduced with permission [49]. Copyright 2022, Elsevier. B). BC microspheres promote wound healing. Reproduced with permission [77]. Copyright 2016, John Wiley and Sons. C). In vivo adhesion and healing effects of SNM. Reproduced with permission [41]. Copyright 2023, Springer Nature. D). In vivo adhesion and healing effects of SSAD. Reproduced with permission [40]. Copyright 2019, John Wiley and Sons.
6.3. Burn injuries
Over 11 million burn cases are reported globally annually, with 180,000 fatalities attributed to burn-related complications [180]. Burn injuries represent a special kind of skin injury. First-degree burns are similar to common skin injury and typically heal within one week. Second and third-degree burns extend into the dermis and the full thickness of the skin, resulting in significant immunological and barrier dysfunction [181]. Infection remains a leading cause of mortality, with systemic infections occurring in 17.73% of burn patients and rising to 39.96% in third-degree cases. Moreover, the total body surface area is also employed to assess the severity of burns. Burn wound healing process represents the body's protective and adaptive responses to burn-damage tissue. During this process, macrophages stimulate fibroblasts to proliferate and secrete collagen and other ECM components, forming granulation tissue and supporting epithelial coverage [182].
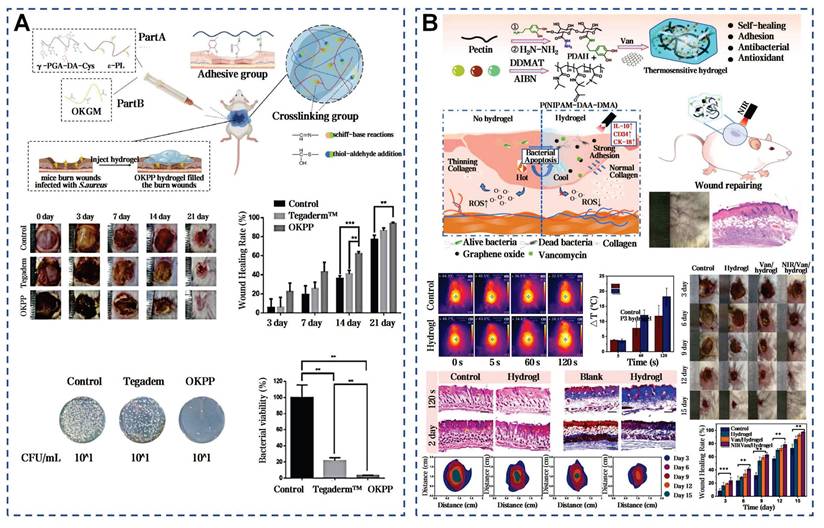
Aloe vera mucilage has shown significant application prospect in burn wound healing. In patients with first or second degree burns, the healing success rate treated with aloe vera mucilage reached 95%, outperforming the sulfadiazine cream group and the framycetin cream group [60]. Gauze soaked in aloe vera mucilage was more effective than petroleum jelly gauze in partial-thickness burns, with minor side effects like irritation or itching. Recent research further supported that aloe mucilage mixtures can enhance cell proliferation and migration via AKT and ERK pathway phosphorylation [183]. The mussel mucus contains a high concentration of PUFAs, vitamins E and D, and omega-3 fatty acids. These lipids can significantly shorten the healing time of burn wounds [184]. Due to low yield and high costs of natural mussel mucus, a range of synthetic mussel-inspired dressings have been developed [185]. Chemical cross-linking can mimic the wet adhesion and self-healing abilities of mussel mucus. The mussel-inspired hydrogel reduced the healing time from 20-22 days to 12-16 days, better than the 3M Tegaderm commercial dressing (Figure 13A) [46]. Another approach used dopamine functionalization to enhance antibacterial properties under near-infrared (NIR) irradiation, achieving a 97.8±0.5% wound closure rate in 15 days (Figure 13B) [47]. The modified mussel-inspired mucus can be designed to enhance specific functionalities, such as antimicrobial effects, making it a promising candidate for clinical burn treatment.
Selected cases of natural mucus utilization in diabetic wound healing. A). Okra mucilage-loaded gel promotes diabetic wound healing [52, 54]. Reproduced with permission [52]. Copyright 2023, Elsevier. Reproduced with permission [54]. Copyright 2023, Elsevier. B). Aloe vera mucilage-derived antimicrobial nanofiber mats to promote chronic wound healing. Reproduced with permission [58]. Copyright 2023, Elsevier.
6.4. Infected wounds
Bacterial infection is an inevitable issue during the wound healing process. Up to now, infected wounds remain the most challenging and costly wound problems globally, with severe manifestations potentially culminating in sepsis, osteomyelitis, or amputation [186]. Invasive bacteria secrete various polymers to form a protective biofilm, thereby eluding the host's immune defenses and resisting antibiotic therapies. The exudates and necrotic tissues further impede the deep infiltration of antimicrobial medications. Infected wounds are accompanied by sustained, low-level inflammation and excess inflammatory cytokines release. This traps the wound healing process in the inflammatory phase, impeding the transition to the proliferation and remodeling. Even if the bacteria at the trauma site are killed by external intervention, the remaining dead bacteria and toxins in the wound area can still hinder the healing process of the wound [187]. Therapeutic interventions for bacterial wound infections are generally classified into two main parts: antimicrobial therapy and facilitation of wound healing. Antimicrobial strategies now include alternatives to conventional antibiotics [188]. The overuse of antibiotics has accelerated the increase in bacterial drug resistance. Additionally, metallic antimicrobial agents are frequently associated with latent toxicity concerns, environmental contamination risks, and discoloration [189]. Consequently, the development of biocompatible and environmentally friendly natural mucus is expected to offer a novel solution to this problem.
Selected cases of natural mucus utilization in burn wound healing. A) Mussel-inspired dopamine-mediated adhesive and antioxidant hydrogel for advanced burn wound healing. Reproduced with permission [46]. Copyright 2022, Springer Nature. B) Mussel-inspired catechol-functionalized pectin hydrogel: a NIR-enhanced thermo-responsive composite for accelerated burn wound healing. Reproduced with permission [47]. Copyright 2024, Elsevier.
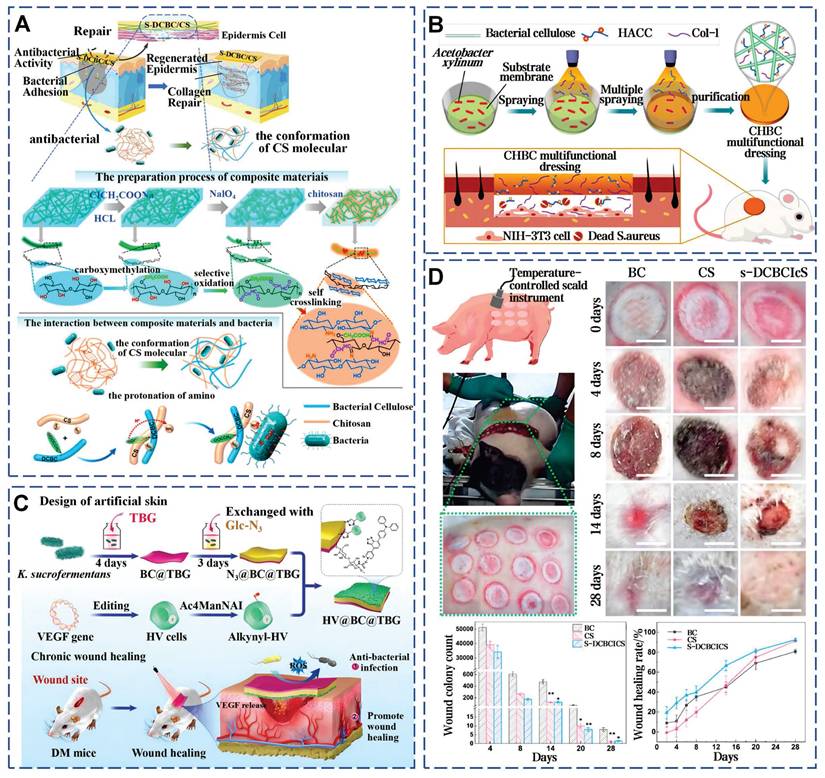
Propolis is a natural viscous substance with multiple antimicrobial components. By incorporating Water Extract of Propolis into high-porosity polyurethane foam dressings, the antimicrobial activity of the dressings is significantly enhanced [190]. The Film-forming System (FFS) is a non-solid topical drug delivery system that enables sustained release of medication, rapid drying, and good film adaptation, making it suitable for local wound healing applications. A novel FFS using propolis from the stingless bee as the active ingredient, effectively inhibits S. aureus and S. epidermidis, highlighting propolis's role in infected wound healing [191]. BC has been enhanced through innovative modifications to overcome its natural antibacterial limitations. Chemical modification strategies can introduce functional groups into the side chains of BC, enhancing mechanical properties and antibacterial performance. Carboxymethylated and selectively oxidized introduced aldehyde and carboxyl groups into BC chains, achieving over 95% in vitro antibacterial efficacy against E. coli and S. aureus through active antibacterial effects. In a deep second-degree infected burn model of Bama miniature pigs, this material attains an 80% healing rate within three weeks, surpassing traditional chitosan dressings (Figure 14A) [81]. Secondly, the addition of antimicrobial agents can enhance the functionality of BC. Hydroxypropyl trimethyl ammonium chloride chitosan (HACC), an antimicrobial agent derived from chitosan, exhibits superior antibacterial performance due to its cationic quaternary ammonium groups and enhanced water solubility. Through the membrane-liquid Interface culture technique, collagen I and HACC can be integrated into the network structure of BC (Figure 14B) [82]. Additionally, combining with Usnic acid and Sanxan gel [78] or embedding Ag-MOF and curcumin [79] can also efficiently control bacterial infections and mitigate inflammatory reactions through the controlled release of drugs. Thirdly, combined with photothermal therapy, a novel living artificial skin HV@BC@TBG has been prepared by sandwiching the photosensitizer TBG and functional living cells HV on both sides of BC (Figure 14C) [76]. This design leverages the TBG layer's ability to generate ROS under light exposure to effectively eradicate bacteria, while the HV layer functions as a living cell factory that continuously secretes VEGF, facilitating wound repair. These findings highlight that the unique microarchitecture of BC not only promotes efficient material exchange and cell penetration but also establishes a solid foundation for the tailored design and functional modification of complex wound healing strategies through precise interfacial engineering.
Strategies for advanced modifications of BC for infected wound healing. A) Chemical crosslinking and functional group integration in BC for enhanced wound healing. Reproduced with permission [81]. Copyright 2022, Elsevier. B) HACC-infused BC dressings: integration of antimicrobial agents and bioactive compounds in BC. Reproduced with permission [82]. Copyright 2021, Elsevier. C) Photothermal therapy and functional modifications of BC for complex wound healing strategies. Reproduced with permission [76]. Copyright 2024, John Wiley and Sons. D) The efficacy of modified BC dressings on deep second-degree scald wounds in bama miniature pigs. Reproduced with permission [81]. Copyright 2022, Elsevier.
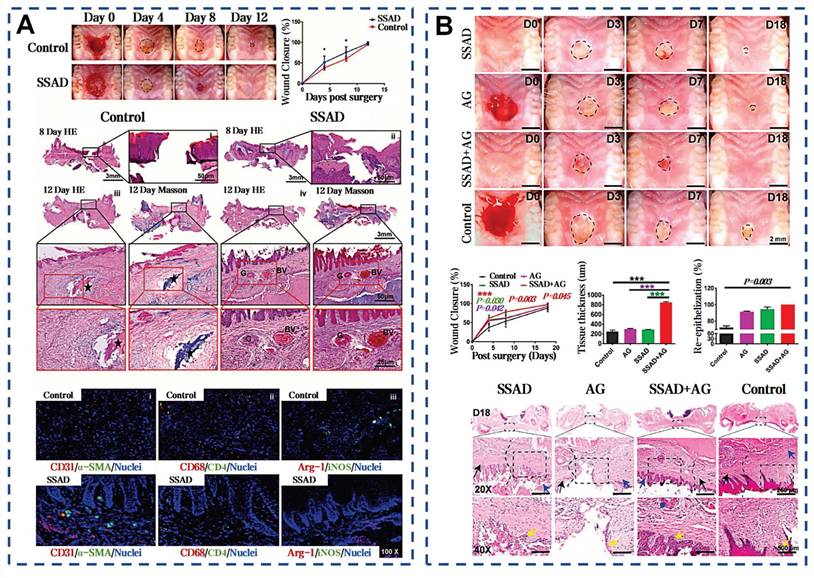
Selected cases of natural mucus utilization in oral ulcer. A) Effect of SSAD on the healing of oral palate wounds. Reproduced with permission [38]. Copyright 2021, John Wiley and Sons. B) Effects of SSAD and SSAD+AG on oral palate mucosal injury in SD diabetic rats. Reproduced with permission [39]. Copyright 2022, Elsevier.
6.5. Oral mucosal defects
Oral mucosal defects, characterized by circular mountain-like defects and ulcerations, affect eating and speaking when getting severe. Among the most common oral conditions, oral mucosal defects affect over 20% of the general population [192]. Oral mucosal defects are notorious for their recurrence and resistance to healing, particularly recurrent oral ulceration (ROU). Notably, no existing pharmaceuticals can fully eradicate ROU. The etiology remains unclear, with potential factors including genetics, allergies, infections, immune dysregulation, systemic diseases, microbial imbalances, nutrient deficiencies, and psychological stress [193]. Current treatments, including growth factor gels, antibiotics, corticosteroids, trichloroacetic acid, and metronidazole patches, are limited by issues like short duration, secondary infections, immune suppression, mucosal damage, and side effects [194]. Consequently, there is an urgent need to develop oral ulcer treatments with natural drugs that have minimal side effects and enhanced therapeutic efficacy.
SSAD powder accelerates intraoral wound healing by forming a protective barrier and promoting tissue repair. The underlying alveolar bone has no noticeable signs of necrosis, and the surface is partially covered by blood clots and degraded tissue mass in the SSAD groups (Figure 15A) [40]. Its porous structure facilitates sustained drug release, enhancing therapeutic outcomes [38]. Fresh aloe vera mucilage extract enhanced oral mucosal healing through immune modulation, increasing CD8+ cells and a notable increase in IL-2 and IFN-γ levels [195]. The aloe mucilage also demonstrates significant antioxidant effects by increasing the activity of SOD in both plasma and mucosa and reducing malondialdehyde levels. These findings provide the first evidence of the potential of aloe mucilage to bolster innate immunity and mitigate oxidative harm, presenting a novel approach for oral ulcer therapy. Yang et al. performed a Meta-analysis to evaluate the clinical effectiveness and safety of propolis for treating oral mucosal defects, encompassing 23 randomized controlled trials with a total of 2467 participants [196].
Summary of main critical properties for natural mucus wound dressing and their advantages and limitations
| Crucial properties | Definition & clinical importance | Advantages & limitations | ||
|---|---|---|---|---|
| Animal mucus | Plant mucilage | Complex-sourced natural mucus | ||
| Wet adhesion and absorption capacity | Adhere to moist wound bed, absorb exudate, maintain optimal hydration and prevent maceration | Hydrophobic molecules enable direct tissue adhesion in high-exudate environment; Sensitive to temperature/pH fluctuations | High absorption of polysaccharides facilitates wet adhesion and water retention; Excessive swelling weakens adhesion; Reduced adhesion to dry and necrotic tissues | Nanofiber-wound interlock; Crosslinking reduces capacity; Require modification for enhanced adhesion |
| Moisture retention and oxygen permeability | Maintain hydration for autolytic debridement and cell migration, enable oxygen exchange for aerobic healing and angiogenesis | Moderate absorption for protein and lipid; Susceptible to enzymatic degradation; Long-term retention may cause collapse; High-protein exudate clogs pores | Hydrophilic matrix sustains moisture and oxygen exchange; Over-hydration risk in high-exudate environment; Reduced water retention during degradation | Elevated concentration induces hypoxia; Unsuitable for anaerobic infection control; Synthetic additives impede gas exchange and breakdown |
| Mechanical resilience | Resists deformation and fracture during movement | Require prolonged in situ gelation time; Relatively low strength tears during movement; Shrink upon drying, pulling wound edges | Excellent contour adaptation; High ductility when hydrated but weak tensile strength; Brittle when dehydrated | Rigid structure limits deformation |
| Antimicrobial activity | Inherent capacity to kill/inhibit pathogens or incorporate antimicrobial agents | Defensin and immunoglobulins provide activity; Reduced by proteases in chronic wounds | Anthraquinones and acemannan disrupt biofilms; Limited efficacy against Gram-negative strains | Species-dependent |
| Immunomodulation | Modulates inflammatory cytokines to prevent chronic inflammation | Risk of allergy and immune reactions from exogenous factors | Effects may be inconsistent or paradoxical at high concentrations | Potential endotoxin contamination |
| Biocompatibility | Non-toxic, non-irritating, non-bioaccumulative, and non-allergenic to surrounding tissue | Zoonotic pathogen transmission risk; Potential allergens | Generally low immunogenicity; Residual pesticides and extraction solvents may cause reactions | Context-dependent |
| Ease of application and removal | Applicable and removable without causing pain or tissue damage | Require temperature control; Residue risk from fragments; | Brittle films may fragment during removal; potential residue | Without concentration processing; Complex applications require professional handling |
| Production feasibility | Sustainable source, cost-effectiveness, scalability, sterilization compatibility, regulatory pathway | High-cost live harvesting; Expensive medical-grade purification; Sterilization and storage escalate expenses | Renewable farming sources; Seasonal batch variability impedes standardization | Require equipment investment; Rigorous filtration needed for particulate residues |
| Other considerations | Odor control, pain management, eco-friendliness | Sulfur and amine components yield distinctive odors; Neuroinflammatory reaction risk | Cooling sensation reduces burning; Peculiar odor when improperly preserved | Degradation often requires multiple enzymes; Added complexity affects degradation |
The propolis treatment group exhibited a significantly higher total effectiveness rate compared to the control group. Additionally, no adverse reactions related to propolis were observed in these experiments. These findings underscore the potential of natural mucus in addressing the complex mechanisms of oral ulceration, offering promising strategies for clinical application.
7. Future Perspectives
Despite natural mucus demonstrates inherent therapeutic superiority, there are still many challenges demand resolution. 1) In terms of composition, fresh natural mucus often contains high water content, complicating active ingredient concentration control and posing preservation risks. 2) The abundant proteins in natural mucus can affect its viscosity and bioactivity. However, their conformations are sensitive to temperature and enzymatic degradation thereby restricting its stable application. 3) Although natural mucus shows anti-inflammatory and antimicrobial properties, the relative contributions of specific bioactive compounds remain uncharacterized. The crudely extracted natural mucus might still be unable to substitute specialized antimicrobial dressings. 4) Many animals secrete different types of mucus in response to various stimuli or different diets. Plant mucilages might also vary with seasons and environmental changes. Some microbe-derived mucus has also been proven to have its properties altered by artificially adding components. All these factors can lead to changes in the structure and composition of natural mucus, further affecting the control of standardization. We summarized the crucial properties of advanced wound dressings and the advantages and limitations of natural mucus under these indicators in Table 3.
In the future, we can envision a new era of highly personalized and precise wound treatment by integrating artificial intelligence (AI), 3D printing, and advanced bioengineering techniques. Research on natural mucus may focus on the following areas: 1) Raw material selection: Explore more biological resources, ensure sustainability, improve yield and maximize the retention of active ingredients. For natural mucus with excellent effects, it is necessary to clarify the composition ratio, structure and standardization parameters of its key components. Conduct long-term immunological and in-depth toxicological assessments to accelerate its commercialization process. 2) Structure-Function Optimization: Gene editing may be used to enhance the yield of effective ingredients in raw materials. With specialized design, the stability and processability of natural mucus can be improved through chemical modification and physical processing. Machine learning algorithms can predict optimal component ratios, accelerating rational material design. 3) Preparation method optimization: Techniques like electrospinning, nanoneedles, 3D bioprinting, and layer-by-layer assembly can be customized according to the shape of the wound. Methods like microfluidic patterning and biomimetic templating can further improve biological interaction, integration, adaptability, thereby enabling precise wound management. 4) Multi-module integrated design: Multifunctional integration without effect on the inherent therapeutic properties of natural bioadhesives represents a critical advancement. When integrated with biosensors and AI-based wound assessment platforms, wound dressings with wound monitoring, data transmission, smart response and personalized therapy may become a trend.
8. Conclusions
In recent years, due to the rapid progress in regenerative medicine and bioengineering technology, the high-efficiency wound healing attracts widespread attention. Natural mucus has emerged as a promising wound dressing by its innate biocompatibility, adhesion and multifaceted bioactivity. It can replicate the ECM structure while enabling physiological moisture-oxygen exchange and localized bioactive molecule delivery. Advanced fabrication technologies can enable the standing limitations to accelerate the development of natural bioadhesive. In this review, we summarized several representative natural mucus substances from animal, plant, and other complex sources. We further analyzed their applications in different types of wound management, including skin injuries, diabetic wounds, burns, infected wounds, and oral mucosal defects. These mucous substances have shown significant therapeutic effects, such as hemostasis, anti-inflammatory and antioxidant, cell growth, angiogenesis, wound closure and antibacterial properties.
Abbreviations
ANFMs: Aloe nanofiber membranes
ADENHs: Aloe-derived exosome nanoparticles
BC: Bacterial cellulose
bFGF: Basic fibroblast growth factor
CAPE: Coffee acid phenethyl ester
CAs: Cyanoacrylates
d-SMG: Dried snail mucin gel
ECM: Extracellular matrix
EE: Earthworm mucus extract
EGF: Epidermal growth factor
EPS: Extracellular polymeric substances
FGF: Fibroblast growth factor
FFS: Film-forming System
GAGs: Glycosaminoglycans
GM-CSF: Granulocyte-macrophage colony-stimulating factor
HACC: Hydroxypropyl trimethyl ammonium chloride chitosan
HGF: Hepatocyte growth factor
HUVECs: Human umbilical vein endothelial cells
IL-1: Interleukin-1
IL-6: Interleukin-6
Mfps: Mussel foot proteins
NIR: Near-infrared
NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells
OPS: Okra polysaccharides
OHG: Okra hydrogel
PDGF: Platelet-derived growth factor
PNPs: Propolis nanoparticles
PUFAs: Polyunsaturated fatty acids
ROU: Recurrent oral ulceration
ROS: Reactive oxygen species
SAGs: Skin secretion-derived glycosaminoglycans of Andrias davidianus
SNM: Snail mucus
SOD: Superoxide dismutase
SSAD: Skin secretions of Andrias davidianus
TGF-β: Transforming growth factor-β
TNF: Tumor necrosis factor
VEGF: Vascular endothelial growth factor
Acknowledgements
Xuanqi Peng and Ziyi Wang are contributed equally to this work, they acknowledge the support from the Science and Technology Development Fund (FDCT) of Macau SAR (No.005/2023/SKL, Macao). Weiliang Hou acknowledges support from Shanghai Collaborative Innovation Center of Endoscopy and Foundation Shanghai Magnolia Talent Plan Pujiang Project (No.24PJD135, China).
Author contributions
Xuanqi Peng: Conceptualization, Investigation, Writing-review & editing, Writing-original draft, Conceptualization. Ziyi Wang: Writing-review & editing. Leo Wang: Methodology, Visualization. Weiliang Hou: Writing-review & editing, Supervision, Project administration, Funding acquisition.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dobson GP. Trauma of major surgery: A global problem that is not going away. Int J Surg. 2020;81:47-54
2. Carter MJ, DaVanzo J, Haught R, Nusgart M, Cartwright D, Fife CE. Chronic wound prevalence and the associated cost of treatment in Medicare beneficiaries: changes between 2014 and 2019. J Med Econ. 2023;26:894-901
3. Dong Y, Fu S, Yu J, Li X, Ding B. Emerging Smart Micro/Nanofiber-Based Materials for Next-Generation Wound Dressings. Adv Funct Mater. 2024;34:2311199
4. Joshi S, Maan M, Barman P, Sharely I, Verma K, Preet S. et al. Advances in biomaterials for wound care management: Insights from recent developments. Adv Colloid Interface Sci. 2025;343:103563
5. Ren H, Zhang Z, Cheng X, Zou Z, Chen X, He C. Injectable, self-healing hydrogel adhesives with firm tissue adhesion and on-demand biodegradation for sutureless wound closure. Sci Adv. 2023;9:eadh4327
6. Cerullo AR, McDermott MB, Pepi LE, Liu Z-L, Barry D, Zhang S. et al. Comparative mucomic analysis of three functionally distinct Cornu aspersum Secretions. Nat Commun. 2023;14:5361
7. Cerullo AR, Lai TY, Allam B, Baer A, Barnes WJP, Barrientos Z. et al. Comparative Animal Mucomics: Inspiration for Functional Materials from Ubiquitous and Understudied Biopolymers. ACS Biomater Sci Eng. 2020;6:5377-5398
8. Bansil R, Turner BS. The biology of mucus: Composition, synthesis and organization. Adv Drug Deliv Rev. 2018;124:3-15
9. Li Y, Cao Y. The molecular mechanisms underlying mussel adhesion. Nanoscale Adv. 2019;1:4246-4257
10. Leśków A, Tarnowska M, Szczuka I, Diakowska D. The effect of biologically active compounds in the mucus of slugs Limax maximus and Arion rufus on human skin cells. Sci Rep. 2021;11:18660
11. Liu Y, Zhou Q, Wang Y, Luo L, Yang J, Yang L. et al. Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration. Nat Commun. 2015;6:10033
12. Singh N, Brown AN, Gold MH. Snail extract for skin: A review of uses, projections, and limitations. J Cosmet Dermatol. 2024;23:1113-1121
13. Messing J, Thöle C, Niehues M, Shevtsova A, Glocker E, Borén T. et al. Antiadhesive Properties of Abelmoschus esculentus (Okra) Immature Fruit Extract against Helicobacter pylori Adhesion. PLoS ONE. 2014;9:e84836
14. De Andrade Vieira É, Alves Alcântara M, Albuquerque Dos Santos N, Duarte Gondim A, Iacomini M, Mellinger C. et al. Mucilages of cacti from Brazilian biodiversity: Extraction, physicochemical and technological properties. Food Chem. 2021;346:128892
15. Zhao X, Li S, Du X, Li W, Wang Q, He D. et al. Natural polymer-derived photocurable bioadhesive hydrogels for sutureless keratoplasty. Bioact Mater. 2022;8:196-209
16. Li S, Chen N, Li X, Li Y, Xie Z, Ma Z. et al. Bioinspired Double-Dynamic-Bond Crosslinked Bioadhesive Enables Post-Wound Closure Care. Adv Funct Mater. 2020;30:2000130
17. Pignet A-L, Schellnegger M, Hecker A, Kamolz L-P, Kotzbeck P. Modeling Wound Chronicity In Vivo: The Translational Challenge to Capture the Complexity of Chronic Wounds. J Invest Dermatol. 2024;144:1454-1470
18. Bäsler K, Bergmann S, Heisig M, Naegel A, Zorn-Kruppa M, Brandner JM. The role of tight junctions in skin barrier function and dermal absorption. J Controlled Release. 2016;242:105-118
19. Peña OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25:599-616
20. Mamun AA, Shao C, Geng P, Wang S, Xiao J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front Immunol. 2024;15:1395479
21. Lee DH, Lim S, Kwak SS, Kim J. Advancements in Skin-Mediated Drug Delivery: Mechanisms, Techniques, and Applications. Adv Healthc Mater. 2024;13:2302375
22. Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97-125
23. Zhang Q, Gong H, Gao W, Zhang L. Recent Progress in Capturing and Neutralizing Inflammatory Cytokines. CCS Chem. 2020;2:376-389
24. Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019;20:207-217
25. Narkar AR, Tong Z, Soman P, Henderson JH. Smart biomaterial platforms: Controlling and being controlled by cells. Biomaterials. 2022;283:121450
26. Yang P, Lu Y, Gou W, Qin Y, Zhang X, Li J. et al. Andrias davidianus Derived Glycosaminoglycans Direct Diabetic Wound Repair by Reprogramming Reparative Macrophage Glucolipid Metabolism. Adv Mater. 2025;37:2417801
27. Wang ZJ, Li W, Li X, Nakajima T, Rubinstein M, Gong JP. Rapid self-strengthening in double-network hydrogels triggered by bond scission. Nat Mater. 2025;24:607-614
28. Sarkar P, Iyengar D, Mukhopadhyay K. Emergence of snail mucus as a multifunctional biogenic material for biomedical applications. Acta Biomater. 2025;200:21-46
29. Teng P, Cai Y, Liu X, Tuo Y, Wu S, Wang Q. et al. Inspiration of plant-related adhesion for plant wearable sensor interface design. Nanoscale. 2025;17:13057-13075
30. Liegertová M, Malý J. Gastropod Mucus: Interdisciplinary Perspectives on Biological Activities, Applications, and Strategic Priorities. ACS Biomater Sci Eng. 2023;9:5567-5579
31. Zeng Q, Qi X, Shi G, Zhang M, Haick H. Wound Dressing: From Nanomaterials to Diagnostic Dressings and Healing Evaluations. ACS Nano. 2022;16:1708-1733
32. Degen GD, Stevens CA, Cárcamo-Oyarce G, Song J, Bej R, Tang P. et al. Mussel-inspired cross-linking mechanisms enhance gelation and adhesion of multifunctional mucin-derived hydrogels. Proc Natl Acad Sci. 2025;122:e2415927122
33. Lai SK, Wang Y-Y, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61:86-100
34. Ma X, Bian Q, Hu J, Gao J. Stem from nature: Bioinspired adhesive formulations for wound healing. J Controlled Release. 2022;345:292-305
35. Dhanisha SS, Guruvayoorappan C, Drishya S, Abeesh P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit Rev Oncol Hematol. 2018;122:98-122
36. Katija K, Troni G, Daniels J, Lance K, Sherlock RE, Sherman AD. et al. Revealing enigmatic mucus structures in the deep sea using DeepPIV. Nature. 2020;583:78-82
37. Liang H, Wang X-T, Ge W-Y, Zhang R, Liu J, Chen L-L. et al. Andrias Davidianus Mucus-Based Bioadhesive with Enhanced Adhesion and Wound Healing Properties. ACS Appl Mater Inter. 2023;15:49931-49942
38. Zhang X, Jiang L, Li X, Zheng L, Dang R, Liu X. et al. A Bioinspired Hemostatic Powder Derived from the Skin Secretion of Andrias davidianus for Rapid Hemostasis and Intraoral Wound Healing. Small. 2022;18:2101699
39. Liu X, Mao X, Ye G, Wang M, Xue K, Zhang Y. et al. Bioinspired Andrias davidianus-Derived wound dressings for localized drug-elution. Bioact Mater. 2022;15:482-494
40. Deng J, Tang Y, Zhang Q, Wang C, Liao M, Ji P. et al. A Bioinspired Medical Adhesive Derived from Skin Secretion of Andrias davidianus for Wound Healing. Adv Funct Mater. 2019;29:1809110
41. Deng T, Gao D, Song X, Zhou Z, Zhou L, Tao M. et al. A natural biological adhesive from snail mucus for wound repair. Nat Commun. 2023;14:396
42. Song Y, Cui Y, Hao L, Zhu J, Yi J, Kang Q. et al. Wound-healing activity of glycoproteins from white jade snail (Achatina fulica) on experimentally burned mice. Int J Biol Macromol. 2021;175:313-321
43. Cilia G, Fratini F. Antimicrobial properties of terrestrial snail and slug mucus. J Complement Integr Med. 2018;15:20170168
44. Zhu K, Zhang Z, Li G, Sun J, Gu T, Ain NU. et al. Extraction, structure, pharmacological activities and applications of polysaccharides and proteins isolated from snail mucus. Int J Biol Macromol. 2024;258:128878
45. Yang Y, Liang Y, Chen J, Duan X, Guo B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact Mater. 2022;8:341-354
46. Sun A, Hu D, He X, Ji X, Li T, Wei X. et al. Mussel-inspired hydrogel with injectable self-healing and antibacterial properties promotes wound healing in burn wound infection. NPG Asia Mater. 2022;14:86
47. Chen Y, Chang L, Zhang Z, Zhou M, Gao Y, Wang Y. et al. Biodegradable pectin-based thermo-responsive composite GO/hydrogel with mussel inspired tissue adhesion for NIR enhanced burn wound healing. Chem Eng J. 2024;480:148067
48. Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials. 2012;33:7972-7983
49. Park WH, Lee J, Kim HJ, Joo KI, Cha HJ. Sutureless full-thickness skin grafting using a dual drug-in-bioadhesive coacervate. Chem Eng J. 2022;446:137272
50. Mou X, Zhang H, Qiu H, Zhang W, Wang Y, Xiong K. et al. Mussel-Inspired and Bioclickable Peptide Engineered Surface to Combat Thrombosis and Infection. Research. 2022;2022:2022 /9780879
51. Zhu X, Xu R, Wang H, Chen J, Tu Z. Structural Properties, Bioactivities, and Applications of Polysaccharides from Okra [ Abelmoschus esculentus (L.) Moench]: A Review. J Agric Food Chem. 2020;68:14091-14103
52. Xin P, Han S, Huang J, Zhou C, Zhang J, You X. et al. Natural okra-based hydrogel for chronic diabetic wound healing. Chin Chem Lett. 2023;34:108125
53. Sipahi H, Orak D, Reis R, Yalman K, Şenol O, Palabiyik-Yücelik SS. et al. A comprehensive study to evaluate the wound healing potential of okra (Abelmoschus esculentus) fruit. J Ethnopharmacol. 2022;287:114843
54. Maalej H, Maalej A, Bayach A, Zykwinska A, Colliec-Jouault S, Sinquin C. et al. A novel pectic polysaccharide-based hydrogel derived from okra (Abelmoschus esculentusL. Moench) for chronic diabetic wound healing. Eur Polym J. 2023;183:111763
55. Huang Y, Fan C, Liu Y, Yang L, Hu W, Liu S. et al. Nature-Derived Okra Gel as Strong Hemostatic Bioadhesive in Human Blood, Liver, and Heart Trauma of Rabbits and Dogs. Adv Healthc Mater. 2022;11:2200939
56. Kumar R, Singh AK, Gupta A, Bishayee A, Pandey AK. Therapeutic potential of Aloe vera—A miracle gift of nature. Phytomedicine. 2019;60:152996
57. Muangman P, Praditsuktavorn B, Chinaroonchai K, Chuntrasakul C. Clinical Efficacy Test of Polyester Containing Herbal Extract Dressings in Burn Wound Healing. Int J Low Extrem Wounds. 2016;15:203-212
58. Liu C, Wang Y, Wang P, Gong Y, Yi B, Ruan J. et al. In situ electrospun aloe-nanofiber membrane for chronic wound healing. Smart Mater Med. 2023;4:514-521
59. Sharma S, Alfonso AR, Gordon AJ, Kwong J, Lin LJ, Chiu ES. Second-Degree Burns and Aloe Vera: A Meta-analysis and Systematic Review. Adv Skin Wound Care. 2022;35:1-9
60. Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, Kongkaew C. The efficacy of aloe vera used for burn wound healing: A systematic review. Burns. 2007;33:713-718
61. Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J Tradit Complement Med. 2015;5:21-26
62. Da Rosa C, Bueno IL, Quaresma ACM, Longato GB. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals. 2022;15:1143
63. Yang J, Pi A, Yan L, Li J, Nan S, Zhang J. et al. Research Progress on Therapeutic Effect and Mechanism of Propolis on Wound Healing. Evid Based Complement Alternat Med. 2022;2022:1-15
64. Manginstar CO, Tallei TE, Niode NJ, Salaki CL, Hessel SS. Therapeutic potential of propolis in alleviating inflammatory response and promoting wound healing in skin burn. Phytother Res. 2024;38:856-879
65. Olczyk P, Komosińska-Vassev K, Winsz-Szczotka K, Koźma EM, Wisowski G, Stojko J. et al. Propolis modulates vitronectin, laminin, and heparan sulfate/heparin expression during experimental burn healing. J Zhejiang Univ Sci B. 2012;13:932-941
66. McLennan SV, Bonner J, Milne S, Lo L, Charlton A, Kurup S. et al. The anti-inflammatory agent Propolis improves wound healing in a rodent model of experimental diabetes. Wound Repair Regen. 2008;16:706-713
67. Abu-Seida AM. Effect of Propolis on Experimental Cutaneous Wound Healing in Dogs. Vet Med Int. 2015;2015:1-4
68. Olczyk P, Komosinska-Vassev K, Winsz-Szczotka K, Stojko J, Klimek K, Kozma EM. Propolis Induces Chondroitin/Dermatan Sulphate and Hyaluronic Acid Accumulation in the Skin of Burned Wound. Evid Based Complement Alternat Med. 2013;2013:1-8
69. Sehn E, Hernandes L, Franco SL, Gonçalves CCM, Baesso ML. Dynamics of reepithelialisation and penetration rate of a bee propolis formulation during cutaneous wounds healing. Anal Chim Acta. 2009;635:115-120
70. Romana-Souza B, Dos Santos JS, Monte-Alto-Costa A. Caffeic acid phenethyl ester promotes wound healing of mice pressure ulcers affecting NF-κB, NOS2 and NRF2 expression. Life Sci. 2018;207:158-165
71. Yang J, He Y, Nan S, Li J, Pi A, Yan L. et al. Therapeutic effect of propolis nanoparticles on wound healing. J Drug Deliv Sci Technol. 2023;82:104284
72. Martinotti S, Ranzato E. Propolis: a new frontier for wound healing? Burns Trauma. 2015;3:s41038-015 -0010-z
73. Oryan A, Alemzadeh E, Moshiri A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed Pharmacother. 2018;98:469-483
74. Wahid F, Huang L-H, Zhao X-Q, Li W-C, Wang Y-Y, Jia S-R. et al. Bacterial cellulose and its potential for biomedical applications. Biotechnol Adv. 2021;53:107856
75. Portela R, Leal CR, Almeida PL, Sobral RG. Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb Biotechnol. 2019;12:586-610
76. Liu X, Wang M, Cao L, Zhuang J, Wang D, Wu M. et al. Living Artificial Skin: Photosensitizer and Cell Sandwiched Bacterial Cellulose for Chronic Wound Healing. Adv Mater. 2024;36:2403355
77. Yu J, Huang T, Lim ZH, Luo R, Pasula RR, Liao L. et al. Production of Hollow Bacterial Cellulose Microspheres Using Microfluidics to Form an Injectable Porous Scaffold for Wound Healing. Adv Healthc Mater. 2016;5:2983-2992
78. Zhao X, Shi Y, Niu S, Wei X, Liu T, Yang M. et al. Enhancing Wound Healing and Bactericidal Efficacy: A Hydrogel Membrane of Bacterial Cellulose and Sanxan Gel for Accelerating the Healing of Infected Wounds. Adv Healthc Mater. 2024;13:2303216
79. Wang F, Sun M, Li D, Qin X, Liao Y, Liu X. et al. Multifunctional Asymmetric Bacterial Cellulose Membrane with Enhanced Anti-Bacterial and Anti-Inflammatory Activities for Promoting Infected Wound Healing. Small. 2023;19:2303591
80. Czaja W, Krystynowicz A, Bielecki S, Brownjr R. Microbial cellulose—the natural power to heal wounds. Biomaterials. 2006;27:145-151
81. Xie Y, Qiao K, Yue L, Tang T, Zheng Y, Zhu S. et al. A self-crosslinking, double-functional group modified bacterial cellulose gel used for antibacterial and healing of infected wound. Bioact Mater. 2022;17:248-260
82. Zhou C, Yang Z, Xun X, Ma L, Chen Z, Hu X. et al. De novo strategy with engineering a multifunctional bacterial cellulose-based dressing for rapid healing of infected wounds. Bioact Mater. 2022;13:212-222
83. Murphy RW, Fu J, Upton DE, De Lema T, Zhao E. Genetic variability among endangered Chinese giant salamanders, Andrias davidianus. Mol Ecol. 2000;9:1539-1547
84. Guo W, Ao M, Li W, Wang J, Yu L. Major Biological Activities of the Skin Secretion of the Chinese Giant Salamander, Andrias davidianus. Z Für Naturforschung C. 2012;67:86-92
85. Geng X, Wei H, Shang H, Zhou M, Chen B, Zhang F. et al. Proteomic analysis of the skin of Chinese giant salamander (Andrias davidianus). J Proteomics. 2015;119:196-208
86. Liu Y, Li Y, Shang H, Zhong W, Wang Q, Mequanint K, Zhu C, Xing M, Wei H. Underwater instant adhesion mechanism of self-assembled amphiphilic hemostatic granular hydrogel from Andrias davidianus skin secretion. iScience. 2022;25:105106
87. Denny MW. Mechanical Properties of Pedal Mucus and Their Consequences for Gastropod Structure and Performance. Am Zool. 1984;24:23-36
88. McDermott M, Cerullo AR, Parziale J, Achrak E, Sultana S, Ferd J. et al. Advancing Discovery of Snail Mucins Function and Application. Front Bioeng Biotechnol. 2021;9:734023
89. Kim YS, Jo YY, Chang IM, Toida T, Park Y, Linhardt RJ. A New Glycosaminoglycan from the Giant African Snail Achatina fulica. J Biol Chem. 1996;271:11750-11755
90. Pitt SJ, Graham MA, Dedi CG, Taylor-Harris PM, Gunn A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br J Biomed Sci. 2015;72:174-181
91. Ito S, Shimizu M, Nagatsuka M, Kitajima S, Honda M, Tsuchiya T. et al. High Molecular Weight Lectin Isolated from the Mucus of the Giant African Snail Achatina fulica. Biosci Biotechnol Biochem. 2011;75:20-25
92. Trapella C, Rizzo R, Gallo S, Alogna A, Bortolotti D, Casciano F. et al. HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Sci Rep. 2018;8:17665
93. Newar J, Ghatak A. Studies on the Adhesive Property of Snail Adhesive Mucus. Langmuir. 2015;31:12155-12160
94. Zhou Z, Deng T, Tao M, Lin L, Sun L, Song X. et al. Snail-inspired AFG/GelMA hydrogel accelerates diabetic wound healing via inflammatory cytokines suppression and macrophage polarization. Biomaterials. 2023;299:122141
95. Ludwigsen CB, Andersen OB, Marzeion B, Malles J-H, Müller Schmied H, Döll P. et al. Global and regional ocean mass budget closure since 2003. Nat Commun. 2024;15:1416
96. Silverman HG, Roberto FF. Understanding Marine Mussel Adhesion. Mar Biotechnol. 2007;9:661-681
97. Priemel T, Degtyar E, Dean MN, Harrington MJ. Rapid self-assembly of complex biomolecular architectures during mussel byssus biofabrication. Nat Commun. 2017;8:14539
98. Pujol JP. Formation of the Byssus in the Common Mussel (Mytilus edulis L.). Nature. 1967;214:204-205
99. Hofman AH, Van Hees IA, Yang J, Kamperman M. Bioinspired Underwater Adhesives by Using the Supramolecular Toolbox. Adv Mater. 2018;30:1704640
100. Sivasundarampillai J, Youssef L, Priemel T, Mikulin S, Eren ED, Zaslansky P. et al. A strong quick-release biointerface in mussels mediated by serotonergic cilia-based adhesion. Science. 2023;382:829-834
101. Lin Q, Gourdon D, Sun C, Holten-Andersen N, Anderson TH, Waite JH. et al. Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. Proc Natl Acad Sci. 2007;104:3782-3786
102. Suhre MH, Gertz M, Steegborn C, Scheibel T. Structural and functional features of a collagen-binding matrix protein from the mussel byssus. Nat Commun. 2014;5:3392
103. Osman A, Lin E, Hwang DS. A sticky carbohydrate meets a mussel adhesive: Catechol-conjugated levan for hemostatic and wound healing applications. Carbohydr Polym. 2023;299:120172
104. Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science. 2007;318:426-430
105. Priemel T, Palia G, Förste F, Jehle F, Sviben S, Mantouvalou I. et al. Microfluidic-like fabrication of metal ion-cured bioadhesives by mussels. Science. 2021;374:206-211
106. Yuan Z, Wu S, Fu L, Wang X, Wang Z, Shafiq M. et al. A natural biological adhesive from slug mucus for wound repair. Bioact Mater. 2025;47:513-527
107. Qin C, Huang K, Xu H. Protective effect of polysaccharide from the loach on the in vitro and in vivo peroxidative damage of hepatocyte. J Nutr Biochem. 2002;13:592-597
108. Geng F, Zhong L, Yang T, Chen J, Yang P, Jiang F. et al. A Frog Skin-Derived Peptide Targeting SCD1 Exerts Radioprotective Effects Against Skin Injury by Inhibiting STING-Mediated Inflammation. Adv Sci. 2024;11:2306253
109. Deng Z, Yin J, Luo W, Kotian RN, Gao S, Yi Z. et al. The effect of earthworm extract on promoting skin wound healing. Biosci Rep. 2018;38:BSR20171366
110. Wang W, Ye J, Guo Z, Ma Y, Yang Q, Zhong W. et al. A novel glycoprotein from earthworm extract PvE-3: Insights of their characteristics for promoting diabetic wound healing and attenuating methylglyoxal-induced cell damage. Int J Biol Macromol. 2023;239:124267
111. Guo Y, Wang Y, Zhao X, Li X, Wang Q, Zhong W. et al. Snake extract-laden hemostatic bioadhesive gel cross-linked by visible light. Sci Adv. 2021;7:eabf9635
112. Prajapati VD, Jani GK, Moradiya NG, Randeria NP. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr Polym. 2013;92:1685-1699
113. Samateh M, Pottackal N, Manafirasi S, Vidyasagar A, Maldarelli C, John G. Unravelling the secret of seed-based gels in water: the nanoscale 3D network formation. Sci Rep. 2018;8:7315
114. Cakmak H, Ilyasoglu-Buyukkestelli H, Sogut E, Ozyurt VH, Gumus-Bonacina CE, Simsek S. A review on recent advances of plant mucilages and their applications in food industry: Extraction, functional properties and health benefits. Food Hydrocoll Health. 2023;3:100131
115. Goksen G, Demir D, Dhama K, Kumar M, Shao P, Xie F. et al. Mucilage polysaccharide as a plant secretion: Potential trends in food and biomedical applications. Int J Biol Macromol. 2023;230:123146
116. Ben Ayache S, Reis FS, Inês Dias M, Pereira C, Glamočlija J, Soković M. et al. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021;351:129263
117. Lousinian S, Dimopoulou M, Panayiotou C, Ritzoulis C. Self-assembly of a food hydrocolloid: The case of okra mucilage. Food Hydrocoll. 2017;66:190-198
118. Raj V, Shim J-J, Lee J. Grafting modification of okra mucilage: Recent findings, applications, and future directions. Carbohydr Polym. 2020;246:116653
119. Whistler RL, Conrad HE. A Crystalline Galactobiose from Acid Hydrolysis of Okra Mucilage1. J Am Chem Soc. 1954;76:1673-1674
120. Zhu W, Obara H. Flow structure of okra mucilage in rotating wall vessel system. Heliyon. 2024;10:e36149
121. Wang C, Yu Y-B, Chen T-T, Wang Z-W, Yan J-K. Innovative preparation, physicochemical characteristics and functional properties of bioactive polysaccharides from fresh okra (Abelmoschus esculentus (L.) Moench). Food Chem. 2020;320:126647
122. Hussain A, Qureshi F, Abbas N, Arshad M, Ali E. An Evaluation of the Binding Strength of Okra Gum and the Drug Release Characteristics of Tablets Prepared from It. Pharmaceutics. 2017;9:20
123. Yuan Q, Lin S, Fu Y, Nie X-R, Liu W, Su Y. et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int J Biol Macromol. 2019;127:178-186
124. Chen J, Chen W, Duan F, Tang Q, Li X, Zeng L. et al. The synergistic gelation of okra polysaccharides with kappa-carrageenan and its influence on gel rheology, texture behaviour and microstructures. Food Hydrocoll. 2019;87:425-435
125. Zhu X, Chen J, Wang H, Tu Z, Yin J, Nie S. Mechanism of viscosity reduction of okra pectic polysaccharide by ascorbic acid. Carbohydr Polym. 2022;284:119196
126. Al-Shawi AAA, Hameed MF, Hussein KA, Thawini HK. Review on the “Biological Applications of Okra Polysaccharides and Prospective Research.” Future J Pharm Sci. 2021; 7: 102
127. Adlakha K, Koul B, Kumar A. Value-added products of Aloe species: Panacea to several maladies. South Afr J Bot. 2022;147:1124-1135
128. Sajjad A, Subhani Sajjad S. Aloe vera : An Ancient Herb for Modern Dentistry—A Literature Review. J Dent Surg. 2014;2014:1-6
129. Grace OM, Dzajic A, Jäger AK, Nyberg NT, Önder A, Rønsted N. Monosaccharide analysis of succulent leaf tissue in Aloe. Phytochemistry. 2013;93:79-87
130. Wang W, An Z, Wang Z, Wang S. Chemical Design of Supramolecular Reversible Adhesives for Promising Applications. Chem-Eur J. 2024;30:e202304349
131. Comas-Serra F, Miró JL, Umaña MM, Minjares-Fuentes R, Femenia A, Mota-Ituarte M. et al. Role of acemannan and pectic polysaccharides in saline-water stress tolerance of Aloe vera (Aloe barbadensis Miller) plant. Int J Biol Macromol. 2024;268:131601
132. Gao Y, Kuok KI, Jin Y, Wang R. Biomedical applications of Aloe vera. Crit. Rev Food Sci Nutr. 2019;59:S244-S256
133. Oliveira Filho JGD, Lira MM, Sousa TLD, Campos SB, Lemes AC, Egea MB. Plant-based mucilage with healing and anti-inflammatory actions for topical application: A review. Food Hydrocoll Health. 2021;1:100012
134. Chelu M, Musuc AM, Popa M, Calderon Moreno J. Aloe vera-Based Hydrogels for Wound Healing: Properties and Therapeutic Effects. Gels. 2023;9:539
135. Tarameshloo M, Norouzian M, Zarein-Dolab S, Dadpay M, Mohsenifar J, Gazor R. Aloe vera gel and thyroid hormone cream may improve wound healing in Wistar rats. Anat Cell Biol. 2012;45:170
136. Kudłacik-Kramarczyk S, Drabczyk A, Głąb M, Alves-Lima D, Lin H, Douglas TEL. et al. Investigations on the impact of the introduction of the Aloe vera into the hydrogel matrix on cytotoxic and hydrophilic properties of these systems considered as potential wound dressings. Mater Sci Eng C. 2021;123:111977
137. Heck E, Head M, Nowak D, Helm P, Baxter C. Aloe vera (gel) cream as a topical treatment for outpatient burns. Burns. 1981;7:291-294
138. Chen W, Van Wyk B-E, Vermaak I, Viljoen AM. Cape aloes—A review of the phytochemistry, pharmacology and commercialisation of Aloe ferox. Phytochem Lett. 2012;5:1-12
139. Pushpangadan R. et al. Development and Characterization of Bamboo Based Wound Dressing Coated with Natural Extracts of Curcumin, Aloe Vera and Chitosan Enhanced with Recombinant Human Epidermal Growth Factor and In Vivo Evaluation for Wistar Albino Wounded Rats. Int Res J Pharm. 2017;8:50-55
140. Pan Q, Bao Z, Wang Y, Wan T. RETRACTED: Nrf2 pathway activation with natural plant-derived exosome-like nanovesicle/hydrogel preparations for oxidative stress modulation in inflammation related diseases. Chem Eng J. 2024;480:148282
141. Mousa MA, Soliman M, Saleh MA, Radwan AG. Tactile sensing biohybrid soft E-skin based on bioimpedance using aloe vera pulp tissues. Sci Rep. 2021;11:3054
142. Zhang Q, Zhang M, Wang T, Chen X, Li Q, Zhao X. Preparation of aloe polysaccharide/honey/PVA composite hydrogel: Antibacterial activity and promoting wound healing. Int J Biol Macromol. 2022;211:249-258
143. Fox LT, Mazumder A, Dwivedi A, Gerber M, Du Plessis J, Hamman JH. In vitro wound healing and cytotoxic activity of the gel and whole-leaf materials from selected aloe species. J Ethnopharmacol. 2017;200:1-7
144. Huang Y, Hu W, Xu K, Dan R, Tan S, Shu Z. et al. Plant mucus-derived microgels: Blood-triggered gelation and strong hemostatic adhesion. Biomaterials. 2024;307:122535
145. Khémiri I, Essghaier Hédi B, Sadfi Zouaoui N, Ben Gdara N, Bitri L. The Antimicrobial and Wound Healing Potential of Opuntia ficus indica L. inermis Extracted Oil from Tunisia. Evid Based Complement Alternat Med. 2019;2019:1-10
146. Wu Y, Hui D, Eskin NAM, Cui SW. Water-soluble yellow mustard mucilage: A novel ingredient with potent antioxidant properties. Int J Biol Macromol. 2016;91:710-715
147. Zeng S, Long J, Sun J, Wang G, Zhou L. A review on peach gum polysaccharide: Hydrolysis, structure, properties and applications. Carbohydr Polym. 2022;279:119015
148. Rojczyk E, Klama-Baryła A, Łabuś W, Wilemska-Kucharzewska K, Kucharzewski M. Historical and modern research on propolis and its application in wound healing and other fields of medicine and contributions by Polish studies. J Ethnopharmacol. 2020;262:113159
149. Easton-Calabria A, Demary KC, Oner NJ. Beyond Pollination: Honey Bees (Apis mellifera) as Zootherapy Keystone Species. Front Ecol Evol. 2019;6:161
150. Hossain R, Quispe C, Khan RA, Saikat ASM, Ray P, Ongalbek D. et al. Propolis: An update on its chemistry and pharmacological applications. Chin Med. 2022;17:100
151. Freires IA, De Alencar SM, Rosalen PL. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur J Med Chem. 2016;110:267-279
152. Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K. et al. Antimicrobial Peptides Human β-Defensins Stimulate Epidermal Keratinocyte Migration, Proliferation and Production of Proinflammatory Cytokines and Chemokines. J Invest Dermatol. 2007;127:594-604
153. Abdullah NA, Zullkiflee N, Zaini SNZ, Taha H, Hashim F, Usman A. Phytochemicals, mineral contents, antioxidants, and antimicrobial activities of propolis produced by Brunei stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J Biol Sci. 2020;27:2902-2911
154. Shi H, Yang H, Zhang X, Yu L (Lucy). Identification and Quantification of Phytochemical Composition and Anti-inflammatory and Radical Scavenging Properties of Methanolic Extracts of Chinese Propolis. J Agric Food Chem. 2012;60:12403-12410
155. Belmehdi O, El Menyiy N, Bouyahya A, El Baaboua A, El Omari N, Gallo M. et al. Recent Advances in the Chemical Composition and Biological Activities of Propolis. Food Rev Int. 2023;39:6078-6128
156. Corrêa FRS, Schanuel FS, Moura-Nunes N, Monte-Alto-Costa A, Daleprane JB. Brazilian red propolis improves cutaneous wound healing suppressing inflammation-associated transcription factor NFκB. Biomed Pharmacother. 2017;86:162-171
157. Cao X-P, Chen Y-F, Zhang J-L, You M-M, Wang K, Hu F-L. Mechanisms underlying the wound healing potential of propolis based on its in vitro antioxidant activity. Phytomedicine. 2017;34:76-84
158. Kaur N, Dey P. Bacterial exopolysaccharides as emerging bioactive macromolecules: from fundamentals to applications. Res Microbiol. 2023;174:104024
159. Navya PV, Gayathri V, Samanta D, Sampath S. Bacterial cellulose: A promising biopolymer with interesting properties and applications. Int J Biol Macromol. 2022;220:435-461
160. Hestrin S, Aschner M, Mager J. Synthesis of Cellulose by Resting Cells of Acetobacter xylinum. Nature. 1947;159:64-65
161. Benziman M, Haigler CH, Brown RM, White AR, Cooper KM. Cellulose biogenesis: Polymerization and crystallization are coupled processes in Acetobacter xylinum. Proc Natl Acad Sci. 1980;77:6678-6682
162. Shavandi A, Hosseini S, Okoro OV, Nie L, Eghbali Babadi F, Melchels F. 3D Bioprinting of Lignocellulosic Biomaterials. Adv Healthc Mater. 2020;9:2001472
163. Liu H, Mei H, Jiang H, Jiang L, Lin K, Jiang M. et al. Bioprinted Symbiotic Dressings: A Lichen-Inspired Approach to Diabetic Wound Healing with Enhanced Bioactivity and Structural Integrity. Small. 2025;21:2407105
164. Wu Z, Chen S, Li J, Wang B, Jin M, Liang Q. et al. Insights into Hierarchical Structure-Property-Application Relationships of Advanced Bacterial Cellulose Materials. Adv Funct Mater. 2023;33:2214327
165. Halim A, Khoo T, Shah JumaatMohdY. Biologic and synthetic skin substitutes: An overview. Indian J Plast Surg. 2010;43:23
166. Gea S, Putra IB, Lindarto D, Pasaribu KM, Saraswati Y, Karina M. et al. Bacterial cellulose impregnated with andaliman (Zanthoxylum acanthopodium) microencapsulation as diabetic wound dressing. Int J Biol Macromol. 2023;253:126572
167. Chen C, Ding W, Zhang H, Zhang L, Huang Y, Fan M. et al. Bacterial cellulose-based biomaterials: From fabrication to application. Carbohydr Polym. 2022;278:118995
168. Ciecholewska-Juśko D, Żywicka A, Junka A, Drozd R, Sobolewski P, Migdał P. et al. Superabsorbent crosslinked bacterial cellulose biomaterials for chronic wound dressings. Carbohydr Polym. 2021;253:117247
169. Poddar MK, Dikshit PK. Recent development in bacterial cellulose production and synthesis of cellulose based conductive polymer nanocomposites. Nano Sel. 2021;2:1605-1628
170. Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ. et al. Trauma-induced coagulopathy. Nat. Rev. Dis. Primer. 2021;7:30
171. Evans JA, Van Wessem KJP, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of Traumatic Deaths: Comprehensive Population-Based Assessment. World J Surg. 2010;34:158-163
172. Driver VR, Gould LJ, Dotson P, Gibbons GW, Li WW, Ennis WJ. et al. Identification and content validation of wound therapy clinical endpoints relevant to clinical practice and patient values for FDA approval. Part 1. Survey of the wound care community. Wound Repair Regen. 2017;25:454-465
173. Trompette A, Ubags ND. Skin barrier immunology from early life to adulthood. Mucosal Immunol. 2023;16:194-207
174. Guo S, DiPietro LA. Factors Affecting Wound Healing. J Dent Res. 2010;89:219-229
175. Heinritz C, Ng XJ, Scheibel T. Bio-inspired Protein-Based and Activatable Adhesion Systems. Adv Funct Mater. 2024;34:2303609
176. Zhang H, Song M, Hu C, Zhang Z, Zhang S, Zhang Y. et al. Efficient scarless skin regeneration enabled by loading micronized amnion in a bioinspired adhesive wound dressing. Aggregate. 2023;4:e332
177. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843
178. Heald AH, Stedman M, Davies M, Livingston M, Alshames R, Lunt M. et al. Estimating life years lost to diabetes: outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc Endocrinol Metab. 2020;9:183-185
179. Wang Y, Shao T, Wang J, Huang X, Deng X, Cao Y. et al. An update on potential biomarkers for diagnosing diabetic foot ulcer at early stage. Biomed Pharmacother. 2021;133:110991
180. Jeschke MG, Van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primer. 2020;6:11
181. Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P. Assessment of burn depth and burn wound healing potential. Burns. 2008;34:761-769
182. Smolle C, Cambiaso-Daniel J, Forbes AA, Wurzer P, Hundeshagen G, Branski LK. et al. Recent trends in burn epidemiology worldwide: A systematic review. Burns. 2017;43:249-257
183. Razia S, Park H, Shin E, Shim K-S, Cho E, Kang MC. et al. Synergistic effect of Aloe vera flower and Aloe gel on cutaneous wound healing targeting MFAP4 and its associated signaling pathway: In-vitro study. J Ethnopharmacol. 2022;290:115096
184. Badiu DL, Balu AM, Barbes L, Luque R, Nita R, Radu M. et al. Physico-Chemical Characterisation of Lipids from Mytilus galloprovincialis (L.) and Rapana venosa and their Healing Properties on Skin Burns. Lipids. 2008;43:829
185. Wang L, Zhao Z, Dong J, Li D, Dong W, Li H. et al. Mussel-Inspired Multifunctional Hydrogels with Adhesive, Self-Healing, Antioxidative, and Antibacterial Activity for Wound Healing. ACS Appl Mater Inter. 2023;15:16515-16525
186. Armstrong DG, Tan T-W, Boulton AJM, Bus SA. Diabetic Foot Ulcers: A Review. JAMA. 2023;330:62
187. Uberoi A, McCready-Vangi A, Grice EA. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat Rev Microbiol. 2024;22:507-521
188. Liang Y, He J, Guo B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano. 2021;15:12687-12722
189. Uddin TM, Chakraborty AJ, Khusro A, Zidan BRM, Mitra S, Emran TB. et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J Infect Public Health. 2021;14:1750-1766
190. Khodabakhshi D, Eskandarinia A, Kefayat A, Rafienia M, Navid S, Karbasi S. et al. In vitro and in vivo performance of a propolis-coated polyurethane wound dressing with high porosity and antibacterial efficacy. Colloids Surf B Biointerfaces. 2019;178:177-184
191. Huanbutta K, Sittikijyothin W, Sangnim T. Development of topical natural based film forming system loaded propolis from stingless bees for wound healing application. J Pharm Investig. 2020;50:625-634
192. Thakrar P, Chaudhry SI. Oral Ulceration: An Overview of Diagnosis and Management. Prim Dent J. 2016;5:30-33
193. Lau CB, Smith GP. Recurrent aphthous stomatitis: A comprehensive review and recommendations on therapeutic options. Dermatol Ther. 2022;35:e15500
194. Wu DT, Freedman BR, Vining KH, Cuylear DL, Guastaldi FPS, Levin Y. et al. Tough Adhesive Hydrogel for Intraoral Adhesion and Drug Delivery. J Dent Res. 2023;102:497-504
195. Yu Z, Jin C, Xin M, JianMin H. Effect of Aloe vera polysaccharides on immunity and antioxidant activities in oral ulcer animal models. Carbohydr Polym. 2009;75:307-311
196. Jinlong Y, Wenhua L. Meta-analysis on the Effectiveness and Safety of Propolis Preparation in the Treatment of Aphthous Ulcers. J Pharm Res Int. 2022;34:48-57
Author contact
![]() Corresponding author: Weiliang Hou (houweiliangedu.cn).
Corresponding author: Weiliang Hou (houweiliangedu.cn).
 Global reach, higher impact
Global reach, higher impact