13.3
Impact Factor
Theranostics 2025; 15(16):8609-8638. doi:10.7150/thno.113474 This issue Cite
Review
Synergistic integration of extracellular vesicles and metal-organic frameworks: unlocking new opportunities in disease diagnosis and therapy
1. Institute of Life Sciences & Biomedical Collaborative Innovation Center of Zhejiang Province, Wenzhou University, Wenzhou 325035, China.
2. School of Life and Environmental Science, Wenzhou University, Wenzhou 325035, China.
3. Department of Biomedical Engineering, City University of Hong Kong, Hong Kong SAR 999077, China.
4. Department of biotechnology, the University of Hong Kong, Hong Kong SAR 999077, China.
5. Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou 325001, China.
6. Key Laboratory for Biorheological Science and Technology of Ministry of Education, Bioengineering College of Chongqing University, Chongqing 400044, China.
7. Jin Feng Laboratory, Chongqing, 401329, China.
† These authors contributed equally to this work.
Received 2025-3-9; Accepted 2025-7-22; Published 2025-7-28
Abstract
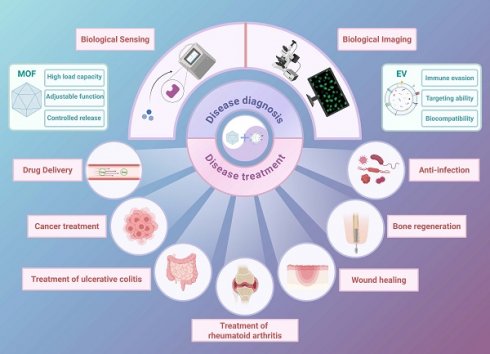
The combination of extracellular vesicles (EVs) and metal-organic frameworks (MOFs) is a cutting-edge strategy in nanomedicine, leveraging the immune evasion, targeting capabilities, and biocompatibility of EVs with the high loading capacity and tunable functionality of MOFs. This review comprehensively discusses the latest advancements in the EV-MOF collaborative system, including its combined form and preparation process, focusing on its synergistic applications in disease diagnosis and treatment. EV-MOF platforms have demonstrated enhanced sensitivity in biosensing and bioimaging, offering new avenues for early cancer detection using signal amplification and fluorescence imaging technologies. Therapeutically, EV-MOF systems have demonstrated significant promise in drug delivery, cancer treatment, rheumatoid arthritis, ulcerative colitis, wound healing, bone regeneration, and anti-infection applications, delivering targeted therapies with controlled drug release, and improved biocompatibility. Despite these advancements, challenges remain in optimizing the binding methods between EVs and MOFs, ensuring their stability, and understanding their in vivo mechanisms. Addressing these may be key to unlocking the full clinical potential of EV-MOF systems. This review provides a critical analysis of the current state of research, offering insights and guidance for future exploration in this rapidly evolving field.
Keywords: metal-organic frameworks, extracellular vesicles, synergistic systems, diagnosis, nanomedicine
Introduction
Metal-organic frameworks (MOFs), a class of hybrid porous materials composed of metal ions or clusters linked by organic ligands, have gained attention since their discovery by Yaghi et al. in 1995 [1]. MOFs are promising candidates for biomedical applications, particularly in disease diagnosis and therapy, due to their unique structural tunability, exceptionally high surface area, and versatile functionality [2]. Over the past two decades, advancements in MOF design have enabled the development of multifunctional platforms capable of precise drug delivery, biosensing, and imaging [3]. MOFs offer several advantages.
(1) Customizable functionality: The diversity of metal nodes and organic linkers enables the creation of MOFs with tailored properties, including the incorporation of active targeting molecules and stimuli-responsive components [4,5]. Attaching targeting molecules such as folic acid to the surface of MOFs enables active targeting of tumor cells [6]. This flexibility enables the construction of specialized nanoplatforms that meet specific diagnostic and therapeutic requirements.
(2) Efficient encapsulation and controlled release: Due to their high porosity and large surface area, MOFs can encapsulate numerous therapeutic agents and release them in a controlled manner in response to environmental stimuli, such as pH and enzymes, thereby enhancing therapeutic precision and minimizing off-target effects [7,8].
(3) Biocompatibility and degradability: Many MOFs exhibit high biocompatibility and biodegradability, making them suitable for in vivo applications [9]. Some zinc(Zn)-based and iron(Fe)-based MOFs can degrade gradually under physiological conditions into low-toxicity metal ions and organic ligands and do not accumulate long-term in the body [10]. Green synthetic techniques can be applied to minimize toxicity and ensure safe clearance from the body, thereby enhancing their potential for clinical translation [11].
MOFs demonstrate significant potential; however, they also face challenges, such as limited targeting ability, susceptibility to immune clearance, and suboptimal loading capacity. These limitations highlight the need for hybrid systems with enhanced biofunctional properties.
Extracellular vehicles (EVs), naturally secreted by cells and involved in intercellular communication, provide a complementary solution [12]. EVs can encapsulate a diverse array of biomolecules, including proteins, lipids, and nucleic acids, making them versatile carriers for diagnostics and therapeutics [13,14]. Moreover, their inherent ability to traverse biological barriers, coupled with their low immunogenicity, makes EVs ideal candidates for enhancing the biocompatibility and targeting precision of nanomaterials [15].
The integration of MOFs with EVs creates a synergistic system that combines diagnostic and therapeutic capabilities. Furthermore, EVs provide immune evasion and targeted delivery, while MOFs contribute to structural integrity and controlled release mechanisms. Connecting the two can also load more signal substances to achieve signal amplification [16]. This hybrid system has great potential for addressing key challenges in cancer treatment, tissue repair, regenerative medicine and cancer diagnosis (Figure 1).
Despite these promising advances, clinical translation of EV-MOF systems remains in its early stages. In this review, we provide a comprehensive overview of the EV-MOF synergistic system for the first time, focusing on its preparation methods, biomedical applications, and prospects in clinical practice. We aimed to provide a roadmap for the future development of this innovative precision medicine platform by highlighting recent breakthroughs and addressing current challenges.
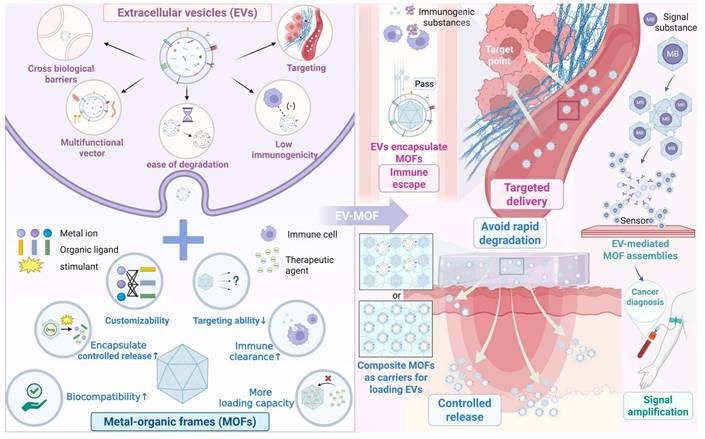
Schematic of EVs, MOFs, and EV-MOF systems. Upper left: EV characteristics include crossing biological barriers, multifunctional vector capability, ease of degradation, low immunogenicity and targeting ability. Lower left: MOF features include relatively high biocompatibility, facile drug encapsulation and controlled release, customizability, low targeting ability, susceptibility to immune clearance, and limited load-bearing capacity. Right: Three types of EV-MOF systems are presented: EVs encapsulate MOFs (pink font), which feature immune escape and targeted delivery; composite MOFs as carriers for loading EVs (blue font), which provide controlled release and avoid rapid degradation; and EV-mediated MOF assemblies (green font), which allow for signal amplification. Created using BioRender.com.
2. Synergistic Effect of EVs with MOFs
The integration of MOFs and EVs has unlocked new possibilities in biomedical applications. MOFs, with their large surface area and tunable porosity, are ideal carriers for drugs and imaging agents; however, they face challenges such as immune clearance and limited targeting [17]. EVs, as natural carriers for intercellular communication, offer high biocompatibility and precise targeting; however, their short half-life and lack of controlled release limit their efficacy [18]. The combination of MOFs and EVs creates a synergistic platform in which MOFs enhance EV stability and controlled release, while EVs provide immune evasion and tissue-specific targeting. This hybrid system demonstrated superior performance in diagnostics and therapies, particularly in cancer treatment and biosensing.
In this section, we explore the binding mechanisms and preparation techniques of these synergistic systems, highlighting their potential to overcome the limitations of individual components.
2.1 The binding form of EVs in collaboration with MOFs
2.1.1 EVs encapsulate MOFs
MOFs exhibit remarkable potential for drug delivery and diagnostic applications due to their high porosity and functional versatility. However, when applied in vivo, MOFs often encounter challenges such as the formation of a protein corona, which alters their surface properties and reduces their targeting ability [19,20]. Furthermore, immune recognition and subsequent clearance, as well as burst release effects, may limit therapeutic efficacy [21]. Surface modification with polyethylene glycol (PEG) and ligands (aptamers, liposomes, and peptides) can partially mitigate these issues. However, they often introduce immunogenic responses, increase production complexity, and raise costs [22,23].
In contrast, encapsulating MOFs in EVs offers several advantages. EVs, naturally derived from cell membranes, possess intrinsic biocompatibility and immune evasion properties [24]. Their lipid bilayer structure prevents nanoparticle aggregation and facilitates stable cargo encapsulation (studies have found an encapsulation efficiency of 76.74 ± 6.72% for MOF-loaded EVs) [25], providing a controlled release mechanism across biological barriers [26]. Compared to traditional lipid-based carriers, such as liposomes, EVs demonstrate superior immunocompatibility and targeting precision, making them ideal for use in hybrid EV-MOF systems [27].
Furthermore, EVs can better mimic the biological functions of cell membranes without requiring complex preparation processes [28]. Unlike cell membrane-coated MOFs, which require multiple steps of mechanical processing to downsize the membranes to fit the nanoscale, EVs are naturally nanosized and can easily encapsulate MOFs without compromising their structural integrity. Moreover, EVs possess inherent targeting abilities, allowing them to pinpoint specific cells or tissues with greater precision than synthetic nanoparticle systems [29].
Additionally, some EVs exhibit inherent functions, such as anti-inflammatory and immune response activation, which can synergize with MOF-loaded drugs to achieve powerful therapeutic functions [30,31]. Beyond this, EVs can alter the microenvironment at the disease site via mechanisms such as neutralizing toxins and reprogramming the tumor microenvironment (TME) [32,33], thereby accelerating and enhancing the therapeutic effect of MOFs at the disease site. Accordingly, EV-encapsulated MOFs represent a cutting-edge platform that combines "stealth + targeting + protection+ potentiation", significantly improving the biocompatibility, stability, and efficacy of MOF-based nanotherapies. EVs encapsulate MOFs; EVs serve as the outer shell, forming a core-shell MOF@EV structure, including immune nanonuts (AINUTs) (Figure 2).
2.1.2 Composite MOFs as carriers for loading EVs
EVs, as a nanomedicine, are widely recognized for their therapeutic potential in tissue repair and regeneration, primarily due to their ability to mediate intercellular communication and deliver bioactive molecules. Simultaneously, EVs, as independent units carrying enzyme activity, are attractive natural biocatalytic platforms [35]. However, when applied independently, EVs have significant limitations, including enzyme-loading capacity limited by nanoscale dimensions, rapid degradation in vivo, and insufficient accumulation at target sites [36,37]. These challenges result in suboptimal therapeutic outcomes, particularly in scenarios requiring prolonged and localized delivery.
To overcome these limitations, MOFs have emerged as promising carriers for stabilizing and sustaining release EVs. MOFs exhibit high porosity, large surface area, and tunable composition, making them ideal candidates for the adsorption and surface mineralization encapsulation of EVs via coordination bonds and electrostatic interactions [38,39]. This enables EVs to be incorporated into a controllable release system, enhancing their therapeutic efficiency by protecting them from rapid clearance while facilitating targeted and prolonged release.
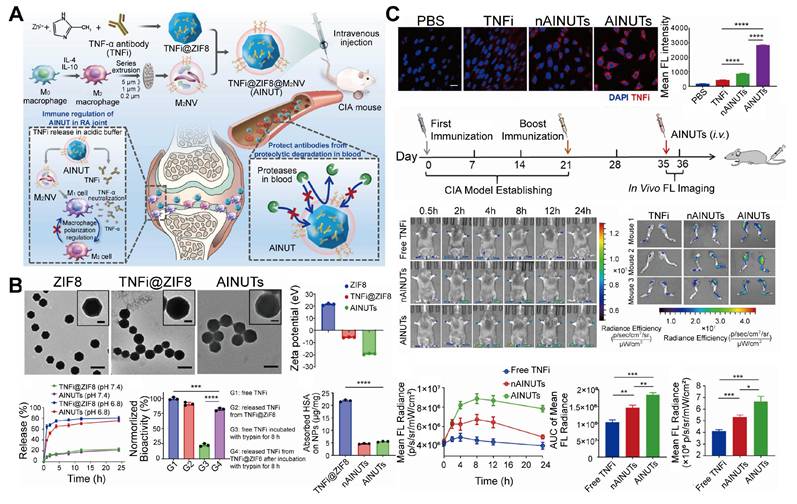
EVs encapsulate MOFs. A. Schematic representation of the preparation and anti-inflammatory mechanism of AINUTs, comprising zeolite imidazolate frameworks-8 (ZIF-8) encapsulated with anti-tumor necrosis factor-α (TNF-α) antibody (TNFi) and coated with M2 macrophage-derived vesicle (M2NV) membrane. B. Representative images of transmission electron microscopy (TEM) and zeta potential of ZIF-8, TNFi@ZIF-8, and AINUTs, and different nanoparticles release properties, biological activity, and protein adsorption properties under different conditions. AINUTs and TNFi@ZIF8 can release TNFi on demand in acidic environments. ZIF-8 can protect TNFi from protease degradation and maintain its molecular structure and biological activity. Surface coating of M2NVs can significantly reduce non-specific protein adhesion, thereby prolong the circulating half-life and reduce the clearance of the mononuclear phagocytic system (scale bar, 200 nm). The insert depicts a magnified image of a single nanoparticle (scale bar, 50 nm). C. Targeting efficiency of AINUTs in rheumatoid arthritis (RA) in vitro and in vivo. In vitro, AINUTs targeted inflammatory HUVECs more effectively, with a stronger binding affinity than TNFi@ZIF8 NPs coated with liposomes (nAINUTs) and native TNFi. In vivo, AINUTs exhibited significantly improved accumulation and sustained presence in inflamed joints compared to nAINUTs and TNFi, owing to the high binding affinity of M2NVs. Adapted with permission from [34], copyright 2022, Elsevier B.V.
In addition to offering a structural platform, MOFs enhance the functionality of EV-based therapies by incorporating metal ions, including zirconium (Zr4+), copper (Cu2+), and magnesium (Mg2+), which provide additional bioactive properties, such as antimicrobial and osteogenic activity [40,41]. These metal ions play a critical role in promoting tissue regeneration, particularly in bone healing and wound repair, by supporting cell proliferation, differentiation, and angiogenesis [42]. Furthermore, certain light-responsive MOFs can form EV@MOF core-shell structures through biomineralization on EV surfaces, thereby enhancing biocatalytic stability and enabling synergistic effects between photocatalysis and enzymatic catalysis [43]. Moreover, the high surface area of MOFs enables adsorption and controlled release of growth factors and other therapeutic agents, further amplifying the regenerative capacity of the composite system [44].
MOFs are versatile because they can be combined with hydrogels, nanofibers, and other biocompatible polymers to form composite materials with improved mechanical properties and biodegradability [45]. These hybrid structures create a three-dimensional (3D) matrix that provides mechanical support and mimics the extracellular matrix, promoting cell adhesion and nutrient exchange, thereby creating a more favorable environment for tissue regeneration [46]. This composite strategy exhibits unique advantages in payload capacity: Studies have demonstrated that MOF-polymer composites achieve an EV loading efficiency of up to 40 μg/mL, surpassing pure polymer by over two orders of magnitude, while the EV loading of pure polymers is nearly negligible [47]. The combination of structural optimization, improved drug delivery performance, and biological synergistic advantages makes EV-MOF composites ideal for applications in tissue engineering and regenerative medicine [48,49]. Composite MOFs serve as carriers for loading EVs, either in the form of scaffolds or through encapsulation. For example, a PLGA/Mg-GA MOF composite material scaffold has been used to load exosomes derived from human adipose-derived mesenchymal stem cells (hADSCs) (Figure 3).
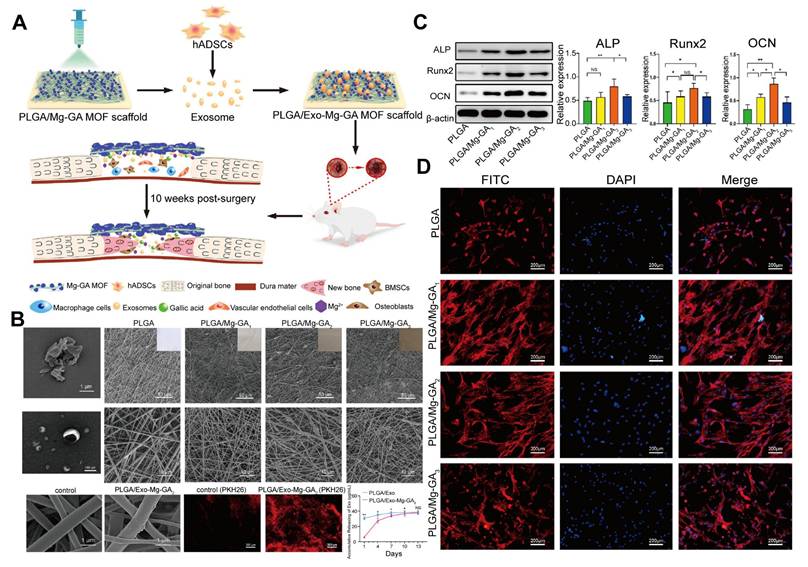
Composite MOFs as carriers for loading EVs. A. Schematic representation of the PLGA/Mg-GA MOF composite scaffold loaded with hADSC exosomes for bone tissue regeneration. B. Scanning electron microscopy (SEM) and TEM images of the EV-loaded composite scaffold and its components. The addition of Mg-GA MOF particles roughened the PLGA scaffold surface, and the EVs exhibited a disc-shaped double-layer membrane structure. The PLGA/Exo-Mg-GA2 scaffold exhibited higher EV attachment and continuous, sustained EV release than the control (PLGA). C. Enhanced expression of osteogenic proteins in hBMSCs cultured on PLGA/Exo-Mg-GA2 scaffolds. D. Immunofluorescence image of hBMSCs on the PLGA/Mg-GA MOF scaffold, showing cells exhibiting shuttle-like and polygonal morphologies, with obvious pseudopodia, along with observable intercellular filaments. Adapted with permission from [47], copyright 2022, Elsevier B.V.
2.1.3 EV-mediated MOF assemblies
MOFs have emerged as highly effective materials for biosensing applications due to their 3D porous structures, large surface area, and abundance of metal active sites [50-52]. These properties enable MOFs to serve as a platform for the development of high-performance biosensors. However, when applied independently, MOFs are limited by the surface area of the electrode interface and the size constraints of individual MOFs, which restrict their ability to capture and detect biomolecules efficiently [53].
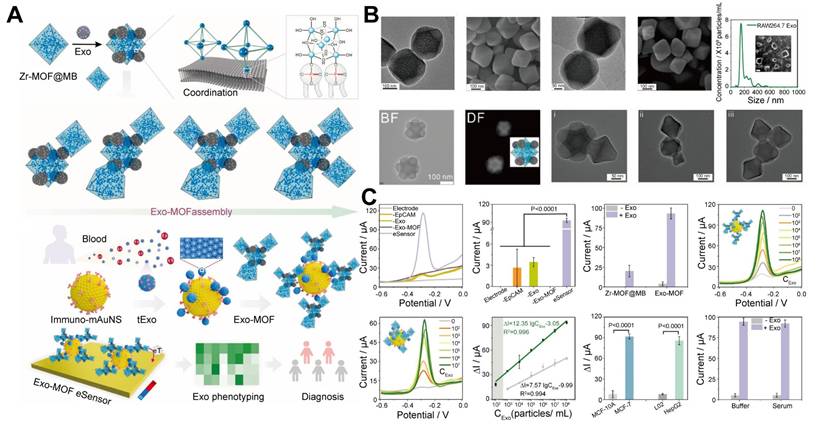
The incorporation of EVs into MOF assemblies is a promising solution to these limitations. EVs are naturally rich in hydrophilic phosphate groups on their surface [54], which can form strong coordination bonds with metal ions, Zr4+ and titanium (Ti4+), found in MOFs [55,56]. This metal-phosphate coordination facilitates the assembly of EVs with MOFs, enabling the creation of functional nanomaterials or nanostructures that act as highly efficient signal amplifiers in biosensing. Moreover, the phosphate heads on EVs act as "molecular glue", enabling the assembly of MOFs loading electroactive molecules (methylene blue, MB), into superstructures, with a loading efficiency of 78.7% loading MB into individual MOFs. Experimental studies revealed that increasing the EV-to-Zr-MOF@MB ratio from 1:2 to 1:4 triggers a morphological evolution from discrete Zr-MOF@MB complexes (monomers) to dimeric or trimeric clusters, ultimately forming multi-cluster integrated architectures. This structural progression directly enhances functional performance. Compared to monomeric MOF probes (detection range: 1×103-1×108 particles/mL), the multi-cluster exhibited at least a 10-fold improvement in sensitivity (detection threshold as low as 1×102 particles/mL) while maintaining high specificity. The single-particle level (1 particle per 100 μL sample) exceeded that of most of the reported EV sensors. Additionally, the sensor exhibited a signal difference of up to 11 times when distinguishing between cancerous and benign EVs, indicating excellent specificity. Based on this sensitive and specific strategy, the EV-MOF sensor can analyze EVs for seven cancer types and accurately distinguish between cancer patients and healthy individuals in a clinical cohort [57]. This superassembly amplifies the signal detection capabilities of the sensor by increasing the density of MOFs, which capture and identify biomolecules, thereby improving the sensitivity and accuracy of the biosensing platform.
This superassembly approach, known as EV-mediated MOF assemblies (where EVs serve as molecular glue facilitating MOF assemblies), represents a significant advancement in biosensor design, allowing for more efficient and robust detection of biomolecules in complex biological environments (Figure 4). This system provides an innovative platform that leverages the natural properties of EVs and the structural flexibility of MOFs to enhance the performance of biosensors in medical diagnostics and other applications.
EV-mediated MOFs assemblies. A. Schematic of the Exo-MOF eSensor for exosome analysis and cancer diagnosis, highlighting the Zr-phosphate coordination-driven assembly of the Exo-MOF signal amplifier and its coupling to immunomagnetic gold (Au) nanostars. B. SEM, TEM, and nanoparticle tracking analysis (NTA) images of Zr-MOF, Zr-MOF@MB, Exo, Zr-MOF@Exo core-satellite structure, and the Exo-MOF super redox signal amplifier, illustrate the formation of a Zr-MOF@Exo nanostructure as Exo assembles on the Zr-MOF octahedron. The morphological transitions with increasing Exo/Zr-MOF@MB ratio, resulted in the formation of a super redox signal amplifier. C. The Exo-MOF eSensor demonstrates ultra-sensitive and specific detection of tExo, with enhanced signal strength, signal gain, and broader dynamic range compared to other systems, along with excellent specificity and anti-interference capabilities. Adapted with permission from [57], copyright 2024, Elsevier B.V.
2.1.4 Comparison with other organic or inorganic nanocarriers
Various delivery systems, including liposomes, polymer nanoparticles, and inorganic nanomaterials have recently emerged as promising drug carriers in nanomedicine. These delivery systems provide several advantages, including mature production processes and high yields [58]. The United States Food and Drug Administration (FDA) has approved some nanocarriers for use in clinical practice [59]. However, each system has limitations; for example, liposomes are prone to leakage, polymer nanoparticles have insufficient drug loading, and inorganic materials have poor biocompatibility [60,61]. The EV-MOF system, is a "biosynthetic" hybrid system that combines the natural biological activity of EVs with the structural tunability of MOFs, endowing it with unique performance advantages (Table 1).
Regarding drug loading efficiency, the EV-MOF system leverages the high porosity characteristics of MOFs, enabling them to capture active molecules and drugs. Some inorganic nanomaterials, such as mesoporous silica, have larger pore sizes (2-50 nm), increasing their loading capacity. The drug loading capacity of polymer nanoparticles achieved via adsorption or coupling is limited [62]. Traditional liposomes use phospholipid membranes to encapsulate lipophilic drugs or water nuclei to load hydrophilic drugs; however, drug retention is insufficient [63].
Regarding in vivo stability, the MOFs of the EV-MOF system are easily recognized and removed by the immune system [64]. However, the natural phospholipid bilayer membrane of EVs resists enzymatic hydrolysis, thereby prolonging their circulating half-life [65,66]. Traditional liposomes are easily adsorbed by serum proteins, triggering complement activation and leading to rapid clearance. PEG modification can extend the half-life [67]. Polymer materials such as PLGA can slowly release drugs via hydrolysis [68]. Silica and Au nanoparticles degrade slowly in the body or hardly degrade at all, and are chemically inert [69]. The stability of the carrier must maintain a delicate balance with its biocompatibility. Excessive stability can easily lead to long-term retention of the carrier in the body and cause toxicity [70].
From the immunogenicity perspective, EVs can escape immune clearance through the surface CD47 protein. Although synthetic liposomes can reduce immunogenicity through surface modification, such as PEGylation, repeated administration of PEGylated liposomes may induce the production of anti-PEG IgM antibodies and complement activation, thereby accelerating blood clearance [71]. Most polymers exhibit excellent immunogenicity, but hydrophobic polymers, such as polycaprolactone, may trigger stronger immune responses due to hydrophobic interactions (increased cytokine levels) [72]. Some inorganic nanomaterials directly induce immune responses; although their immunomodulatory properties offer potential for developing new therapies in fields such as oncology, excessive immune reactions can directly lead to toxicity [73].
The EV-MOF system inherits the intrinsic homing capacity of EVs while leveraging the tunable size of MOFs to achieve tumor accumulation through the enhanced permeability and retention (EPR) effect, thereby establishing a "dual-targeting" mechanism [74,75]. In contrast, conventional drug carriers, such as liposomes, without ligand modification, predominantly rely on passive targeting, including the EPR effect, and exhibit limited efficacy in complex physiological environments [76].
Although direct comparative data remain to be systematically validated, current studies demonstrate that the modular design of the EV-MOF system enables the simultaneous achievement of both high drug loading capacity in MOFs and EV-mediated targeting capabilities, significantly surpassing the single-function limitations of conventional carriers [77]. Their biomimetic membrane structure confers prolonged circulation with an extended half-life compared to PEGylated liposomes and immune evasion via EV surface proteins, such as CD47 [71]. Furthermore, the nanoscale size of EV-MOF, combined with the pH-responsive degradation of MOFs, enhances tumor penetration and enables spatiotemporally controlled drug release [78]. Future studies should prioritize comparative experiments to quantitatively assess these advantages. However, the "biosynthetic synergy" strategy of EV-MOF has already established a novel paradigm for addressing critical challenges in nanomedicine, including drug loading efficiency, immune clearance, and tissue distribution.
2.2 Preparation of EVs synergistic MOFs
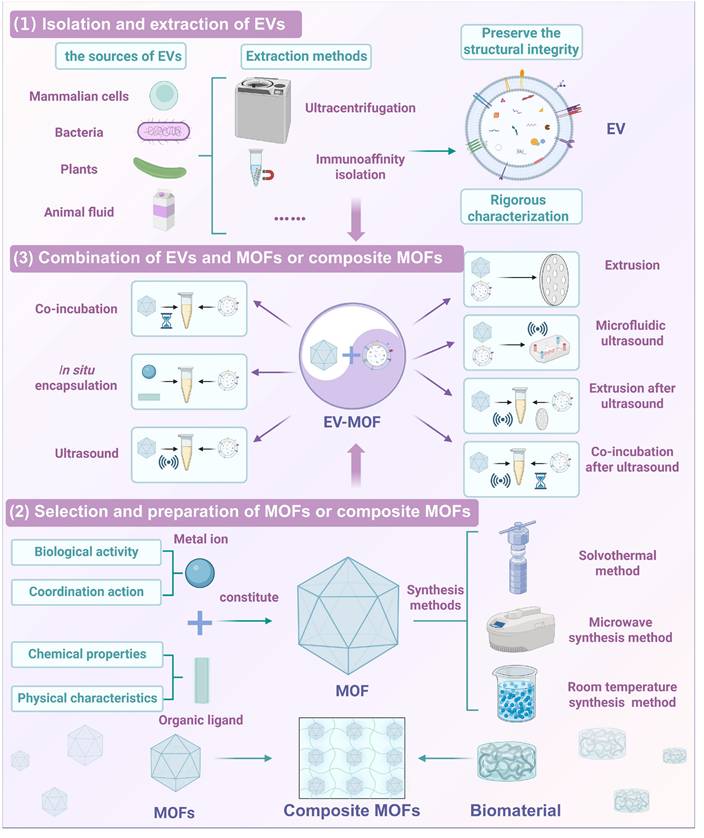
EV-MOF preparation involves three critical steps (Figure 5) [79]. First, EVs must be carefully extracted and purified to retain their biological functions. Second, the synthesis of MOFs is tailored to meet biomedical requirements by optimizing factors, including size, porosity, and surface chemistry. Third, the fusion of EVs with MOFs ensures structural stability and functionality typically achieved via coordination bonds or electrostatic interactions. These steps collectively determine the efficiency and effectiveness of the resulting EV-MOF system, which is vital for applications in drug delivery and biosensing.
Performance comparison of EV-MOF systems and traditional nanomedicine carriers.
| Comparison parameters | EV-MOF | Liposomes | Polymer nanoparticles | Inorganic nanoparticles | References |
|---|---|---|---|---|---|
| Drug loading efficiency | High (high MOF porosity) | Medium (depends on phospholipid membrane) | Medium (dependent on adsorption or coupling) | High (part of the materials are mesoporous structures with large pore sizes) | [62,63] |
| In vivo stability | Medium (some MOFs are prone to hydrolysis; EV encapsulation can improve) | Medium (easily removable, PEGylation can improve) | Medium (biodegradability) | High (chemical inertness) | [64-69] |
| Immunogenicity | Low (CD47 and other membrane proteins of EV evade immune clearance) | Medium (PEG coating can extend circulation time but may induce anti-PEG antibodies) | Low (most have good immunogenicity; hydrophobic polymers have high immunogenicity) | High (directly induces immune responses) | [71-73] |
| Targeting specificity | High (passive targeting and natural homing) | Low (passive targeting) | Low (passive targeting) | Low (passive targeting) | [74-76] |
Schematic of the preparation of EVs synergistic MOFs. Top: (1) Isolation and extraction of EVs: including the source of EVs, extraction methods, and obtained EVs must maintain structural integrity and undergo rigorous characterization. Bottom: (2) Selection and preparation of MOFs or composite MOFs: MOFs are formed by metal ions with biological activity and coordination action, and organic ligands with chemical properties and physical characteristics. The preparation methods include solvothermal synthesis, etc. MOFs can be combined with biomaterials to form composite MOFs. Middle: (3) Combination of EVs and MOFs or composite MOFs: describes seven methods for combining EVs with MOFs or composite MOFs. Created using BioRender.com.
2.2.1 Isolation and extraction of EVs
Isolation and extraction of EVs are critical steps in the development of EV-MOF systems because the integrity and functionality of EVs directly affect their therapeutic efficacy. EVs are typically derived from mammalian cells, bacteria, plants, and animal fluids are commonly extracted from cell culture media, bacterial media, plant juice or animal fluids [80-82].
Ultracentrifugation is the most commonly used method for EV isolation because it can efficiently separate EVs based on their size and density [83]. Moreover, other classical EVs separation methods based on the physical and biomolecular properties of EVs, including size exclusion chromatography, ultrafiltration, polymer precipitation, and immunoaffinity isolation have been developed to improve purity and yield [84]. Beyond classical techniques, several advanced EV isolation strategies have recently emerged, marrying functional specificity to high efficiency. MOF-based capture platforms leverage the high surface area and tunable porosity of MOFs to selectively bind EVs through surface chemistry modulation. This approach enables simultaneous isolation and high-throughput analysis, making it particularly advantageous for biosensor integration and microvolume diagnostics [85]. Meanwhile, molecular imprinting technologies have enabled the fabrication of synthetic recognition sites that mimic natural EV surface markers. These platforms demonstrate high selectivity, operational stability, and reusability, demonstrating potential for high-throughput EV screening and enrichment [86]. Another innovative approach utilizes supramolecular chemical probes based on host-guest interactions. These probes offer reversible and tunable binding dynamics, facilitating controlled EVs capture and release. This feature renders such systems promising tools for downstream EV proteomics or transcriptomics analysis [87]. Although these emerging techniques have yet to see widespread adoption in clinical workflows, their modularity and design flexibility demonstrate strong potential for integration into future diagnostic and therapeutic systems.
Preserving the structural integrity of EVs is particularly important for their immune escape and targeting properties. It ensures the presence of key membrane proteins such as CD47 ("do not eat me" signal) and tetraspanins, which are vital for immune evasion and targeted delivery [88,89].
Furthermore, rigorous characterization of isolated EVs is essential. Some commonly used methods include TEM and SEM to resolve the size and goblet morphology of EVs, NTA and dynamic light scattering (DLS) to analyze the particle size distribution of EVs, and Western blotting (WB) and proteomics to detect proteins on the surface of EVs [90].
2.2.2 Selection and preparation of MOFs or composite MOFs
The design and synthesis of MOFs for biomedical applications requires careful consideration of multiple factors, including metal ion selection, structural characteristics, and biocompatibility. MOFs typically consist of metal nodes linked by organic ligands, forming highly porous structures ideal for drug delivery, tissue regeneration, and diagnostic applications [91-93].
The choice of metal ions is important because it determines the biological activity and functionality of the framework. Metal ions including Zr2+, Cu2+, and Zn2+, provide structural stability and confer additional therapeutic properties, including antimicrobial or osteogenic activity. These ions can interact with EVs via coordination bonds, enhancing the ability of MOFs to encapsulate and deliver bioactive molecules [94].
The organic ligands in MOFs determine their specific surface area, pore size, and chemical properties, directly influencing their capacity to carry therapeutic agents or diagnostic probes [95]. Additionally, the selection of special organic ligands can confer MOF with unique properties, including light, electricity, and sound, allowing them to be directly used as therapeutic agents [96,97]. To ensure safety and efficiency, MOFs are frequently synthesized using solvothermal or microwave methods, while green solvents, such as water and ethanol, are increasingly used to improve biocompatibility [98]. Some MOFs, such as ZIF-8, can be synthesized at room temperature, offering a simpler and more sustainable production route [99].
For composite materials, integrating MOFs with biomaterials, such as hydrogels or nanofibers, enhances mechanical properties, improves biocompatibility, and promotes tissue regeneration. The porous structure of MOFs facilitates cell infiltration and growth, while their controlled degradation releases metal ions that support the repair of damaged tissues [100]. This combination creates multifunctional scaffolds that are ideal for use in tissue engineering and regenerative medicine applications.
2.2.3 Combination of EVs and MOFs or composite MOFs
The combination of EVs and MOFs creates a synergistic system that enhances the structural stability and functional efficacy of the resulting nanostructures. Several methods have been developed to integrate these two components, each offering unique advantages and challenges.
(1) Co-incubation: This method involves incubating EVs with MOFs, to allow them to interact and form composite structures. Although this method is simple and protects the structural integrity of EVs, it frequently yields low loading efficiency [101].
(2) In situ encapsulation: EVs are mixed with MOF precursors to form MOFs on the EV surface in situ, thereby encapsulating the EVs. This one-step approach enhances preparation efficiency, but may have an impact on the active material of EVs [39].
(3) Ultrasound: EV membranes (EM) are transiently disrupted by applying ultrasonic energy, enabling the entry of MOFs to form core-shell structures. This approach improves the loading efficiency but may compromise the stability of EVs [102].
(4) Extrusion: When EVs and MOFs repeatedly pass through porous membranes, mechanical disruption occurs, causing the EVs to reassemble around the MOFs. This method improves the loading efficiency but risks damaging EVs [103].
(5) Microfluidic ultrasound: In this advanced method, EVs and MOFs flow through a microfluidic device under acoustic pressure, thereby facilitating efficient fusion. This approach is fast, scalable, and conducive to mass production [104].
Additionally, hybrid methods such as co-incubation after ultrasound and extrusion after ultrasound, combine the strengths of multiple techniques [25,105], further optimizing loading efficiency while balancing the preservation of EVs structural integrity. Several methods for combining EVs with MOFs or composite MOFs are summarized in Table 2.
3. Application of the MOF and EV Collaborative System in Disease Diagnosis
The EV-MOF collaborative system offers a promising solution for early and accurate disease diagnosis by combining the immune evasion and targeting capabilities of EVs with the high surface area and tunable chemistry of MOFs. This synergy enhances the sensitivity and specificity of biosensors and bioimaging platforms, enabling the more precise detection of disease biomarkers and improving diagnostic outcomes.
3.1 Biological sensing
The accurate and efficient detection of biological substances is a critical challenge in disease diagnosis [107]. MOFs, with their large surface area and tunable pore structure, provide an ideal platform for biosensing, but their individual efficiencies are limited [108,109]. The limitations hindering MOFs in biosensors can be addressed by assembling individual MOFs into MOF clusters and using EVs as molecular glue for MOFs.
Recent studies have demonstrated that many tumor-derived EV, serving as natural biomarkers, their surface proteins are closely related to carcinogenic mechanisms. These EVs can be non-invasively obtained from body fluids, dynamically reflect disease progression, and are ideal sensing targets for cancer diagnostics [110]; however, circulating tumor EVs still face significant challenges in clinical applications. The low abundance of EVs in body fluids of early cancer patients [111], their wide distribution across heterogeneous sizes (30 nm to 10 μm) [112], complex molecular composition and the high dispersion in biological fluids make it challenging to achieve efficient isolation and detection using conventional methods [113].
Summary of methods for combining EVs with MOFs or composite MOFs.
| Convergence technology | Technical principle | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Co-incubation | MOFs and EVs are incubated together at a specific temperature. | Simple operation; less structure and integrity damage to EVs. | Low loading efficiency; limited mass production. | [101] |
| In situ encapsulation | Through mixing EVs with MOF precursors, MOFs are formed in situ on the EV surface, resulting in the encapsulation of EVs. | High preparation efficiency; one-step sequential coating. | Possible EV bioactivity impairment. | [39] |
| Ultrasound | Ultrasonic energy acts on EVs to form transient channels, allowing MOFs to enter and form core-shell nanostructures. | High loading efficiency; less material loss. | More structure and integrity damage to EVs; limited mass production. | [102] |
| Extrusion | EVs and MOFs are extruded by porous membranes, and EVs are cracked and reassembled around the surface of MOFs to form core-shell nanostructures. | High loading efficiency. | More structure and integrity damage to EVs; limited mass production. | [103] |
| Microfluidic ultrasound | Microfluidics combined with ultrasound to cause batch EVs to rupture and recombine around MOFs to form core-shell nanostructures. | Streamlined operation; mass-production capability. | Specialized equipment requirement; elevated cost. | [104] |
| Extrusion after ultrasound | Ultrasonic energy and the mechanical force of extrusion cause the EVs to wrap on the surface of the MOFs. | Enhanced loading efficiency. | More structure and integrity damage to EVs; limited mass production. | [105] |
| Co-incubation after ultrasound | After ultrasonic treatment, when MOFs enter EVs, incubation at 37°C can restore the integrity of the EM. | Enhanced loading efficiency; increased restoration of EV structure and membrane integrity. | Limited mass production. | [106] |
The integration of EVs into MOF-based sensors to form a new generation of signal amplifiers can enhance sensitivity and specificity. Qin et al. [57] developed a superassembly strategy to coordinate EVs with MOFs, optimizing MOF-based signal amplification for ultrasensitive detection of tumor-derived exosomes (tExo). They assembled EV-MOF clusters to significantly amplify electrochemical signals using specific interaction recognition between Zr4+ ions in Zr-MOFs and phosphate groups in cancer exosomes. The inclusion of MB in the Zr-MOFs further enhanced the signal strength. To boost signal gain, magnetic nanoparticles with Au nanostars (mAuNSs) functionalized with antibodies were synthesized to capture exosomes more efficiently. These mAuNSs enhanced exosome capture by increasing their interaction with the electrode surface. The captured tExo contains phosphate groups that coordinate with EV-MOF, amplifying the signal and improving sensitivity. This EV-MOF sensor allows for precise tExo quantification, effectively distinguishing patients with breast cancer from healthy individuals and highlighting its potential for early cancer diagnosis.
3.2 Biological imaging
Advances in medical imaging technologies, including X-rays, computed tomography (CT), magnetic resonance imaging (MRI), and photoacoustic imaging, have contributed to enhancing disease diagnosis and monitoring [114,115]. Biological imaging enables the real-time visualization of physiological and pathological processes, making it critical for early diagnosis and treatment [116]. Fluorescence imaging stands out for its non-invasiveness, high sensitivity, and ease of operation [117]. MOFs, with their customizable emission properties, have emerged as promising materials for bioimaging due to their ability to encapsulate fluorescent dyes and their high tunability [118,119]. MOF-based biosensors have significantly contributed to disease research and diagnosis, especially when combined with EVs, which enhance targeting and immune evasion.
Adenosine triphosphate (ATP), the primary energy source for cellular processes, is a critical marker of cell function, viability, and metabolism, and its fluctuations are closely linked to various diseases [120,121]. The sensitive detection of intracellular ATP is essential for biochemical research and clinical diagnosis. MOF-based sensors have demonstrated exceptional capabilities for ATP detection [122], particularly in live cells. EV-coated MOFs have been applied for this purpose. Lv et al. [123] developed a microfluidic sonication method to create EM-coated ZIF-8 nanoparticles that demonstrated enhanced phagocytosis evasion compared to uncoated ZIF-8 nanoparticles. Additionally, in situ imaging of ATP with EM-ZIF-8 loaded with rhodamine B (RhB) revealed a unique mechanism: ATP disrupts the ZIF-8 structure via competitive coordination with Zn2+ ions, thereby restoring the RhB fluorescence. This ATP-triggered fluorescence recovery demonstrates that biomimetic EM-ZIF-8 nanoparticles are a promising platform for intracellular drug delivery and real-time ATP sensing in live cancer cells.
4. Application of the MOF and EV collaborative System in Disease Treatment
EV-coated MOFs offer a novel approach to overcoming the limitations of traditional nanotherapy, including poor targeting and immune clearance. EVs enhance biocompatibility and immune evasion, while MOFs provide efficient drug loading and controlled release. This EV-MOF system improves therapeutic efficacy and reduces toxicity by delivering drugs more precisely to target cells. Additionally, MOFs stabilize EVs via coordination bonds and electrostatic interactions, prolonging their therapeutic effects. Simultaneously, MOF + EV produced a 1 + 1> 2 effect. This synergistic system offers precise drug targeting, enhanced biocompatibility, and controlled release, making it a promising strategy for more effective and safe treatments.
4.1 Drug delivery
Nanomedicine has become a pivotal approach in modern biomedical research, offering new avenues for treating various diseases via targeted and controlled drug delivery [124]. EVs, with their intrinsic role in intercellular communication, have garnered attention as promising therapeutic carriers, particularly in treating cancer and cardiovascular disease [125]. Additionally, conventional nanoparticles, such as liposomes, have been extensively employed in clinical settings. However, the effective delivery of these agents remains a persistent challenge [126]. Current nanocarriers, such as liposomes and exosomes are frequently cleared rapidly after systemic administration and struggle to maintain effective concentrations at target sites, potentially limiting their therapeutic efficacy [127,128].
To address these limitations, advanced nanocarrier designs that can stabilize and protect therapeutic agents are crucial. An innovative approach by Li et al. [129] used nanogrid particles (NGPs), which integrate supramolecular carriers capable of encapsulating larger nanoparticles, including liposomes and exosomes. The NGP system uses cyclodextrin-based MOFs (CD-MOFs) crosslinked with boron linkers, leveraging the high porosity and biocompatibility of CD-MOFs, along with the dual pH and H2O2 responsiveness provided by boron. This design significantly enhances the distribution and retention of liposomes in lung tissues, while the encapsulated exosomes exhibit protective and sustained-release properties, offering a multifaceted platform for the effective delivery of numerous therapeutic molecules and nanoparticles.
4.2 Cancer treatment
Cancer is a leading cause of morbidity and mortality globally [130]. Although chemotherapy, protein therapy, gene therapy, immunization therapy and sonodynamic therapy have made significant progress, they still face challenges, including poor targeting, immune clearance, and side effects. Chemotherapy is widely used, but has low delivery efficiency and systemic toxicity [131]. Similarly, barriers to protein, immunization and gene therapies include low cellular uptake, enzymatic degradation, and insufficient tumor penetration [132]. Gene therapy nucleic acid drugs can specifically act on target cells at the gene level. However, naked nucleic acid drugs do not achieve desired results when administered directly due to their non-specific biodistribution, low cellular uptake, rapid clearance, and enzymatic degradation [133]. Despite encouraging progress in sonodynamic therapy, several challenges remain in its clinical application, including insufficient tumor accumulation, high tumor heat tolerance, and distant metastasis, which impede effective tumor treatment [134]. As a cutting-edge platform for cancer treatment, EV-MOF demonstrates significant potential to improve the efficacy of current therapies, offering a more targeted and biocompatible approach.
4.2.1 Chemotherapy
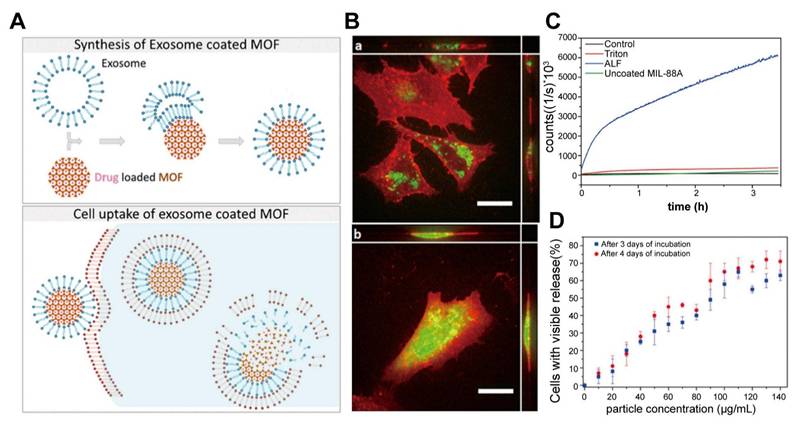
Traditional chemotherapy often faces challenges such as low targeting efficiency, immune clearance, and systemic toxicity. Bernhard Illes et al. [135] first proposed a promising solution: EV-coated MOF nanoparticles, as an intelligent and efficient drug delivery system with an "on-board trigger" (Figure 6). This system combines the chemically tunable properties of MOF nanoparticles with the biocompatibility and targeting capabilities of EVs to create an innovative drug delivery platform with efficient loading and minimal premature leakage. In this study, MIL-88A nanoparticles, composed of Fe and fumaric acid, were synthesized using the microwave method and loaded with chemotherapy drugs. 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide (MTT) cytotoxicity experiments revealed that MIL-88A exhibited good biocompatibility, even at high concentrations (8 µL/well) with cell viability maintained at > 70%, providing a safe basis for in vivo applications. Simultaneously, an in vivo study found that after intravenous injection, MIL-88A nanoparticles are primarily captured by the reticuloendothelial system of the liver and spleen, and are gradually hydrolyzed and degraded into Fe ions and fumaric acid ligands. These degradation products are safely metabolized through a homeostatic mechanism, with no significant toxicity, confirming the metabolic safety of the carrier system [136].
Then, these nanoparticles were coated with EVs derived from HeLa cells, significantly enhancing their cellular uptake and therapeutic efficacy. The EV coating enabled controlled, intracellular drug release, as verified by fluorescence release experiments using a membrane-impermeable calcein model. The results demonstrated that this EV-MOF system prevented premature drug release and improved the transmission and release of chemotherapeutic agents within tumor cells. Although some studies have indicated that delayed release of the encapsulated drug in the body may reduce its anti-cancer activity and raise the half maximal inhibitory concentration (IC50) value for cancer cells. This phenomenon could be explained by the slower rate at which the nanoparticles release chemotherapy drugs, which prevents the drug from reaching sufficient concentrations quickly enough thereby delaying the response to cytotoxic effects [137]. Nevertheless, other studies have suggested that free drugs exhibit greater cytotoxicity in the short term, while their rapid release may limit their sustained action within tumor cells. In contrast, sustained-release drugs, through sustained release and nuclear targeting, can interfere with tumor cell proliferation over a longer period, inhibiting the growth of rapidly dividing tumor cells [138]. This slow-release mechanism compensates for the delayed toxicity response in the short term and provides a more lasting effect for tumor therapy by prolonging the duration of drug action. Therefore, the system leverages endogenous exosome pathways and nanocarrier degradation to enhance drug delivery while maintaining cell viability, making it a highly effective platform for cancer treatment.
Chemotherapy. A. Schematic illustration of exosome-coated MOF synthesis, cellular uptake, and cargo release mechanisms. B. Uptake and release of exosome-coated calcein-laden MOF NPs (green) in HeLa cells (red). After two days (a), the particles were internalized with no release. Calcein was released into cells after four days (b) (scale bar, 20 μm). C. Fluorescence release assay of calcein from exosome-coated MOF nanoparticles in different solutions. No release in water was indicated, suggesting a tight exosome coating, the uncoated particles leaked steadily into the water. Calcein was released after Triton X-100 treatment. Fluorescence increases in artificial lysosomal fluid (ALF), suggesting MOF breakdown and exosome rupture. D. Intracellular calcein release efficiency after incubation with exosome-coated MOF at varying concentrations. Higher nanoparticle concentrations were correlated with increased calcein release. No significant differences were observed between days three and four of incubation. Adapted with permission from [135], copyright 2017, American Chemical Society.
4.2.2 Protein therapy
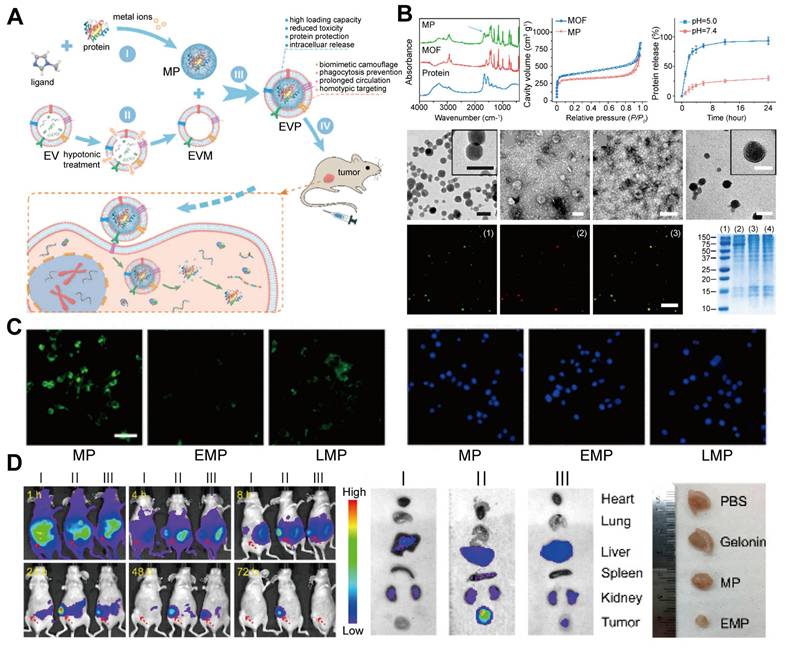
Protein therapy, a highly promising treatment approach, faces numerous challenges. Proteases easily degrade therapeutic proteins, significantly reducing their bioavailability and therapeutic efficacy. Additionally, a key challenge is the selective delivery of therapeutic proteins to target tissues while avoiding off-target effects in non-target tissues. Even if proteins are successfully delivered to target cells, achieving controlled release after intracellular internalization remains challenging, because it may cause excessively high or low intracellular drug concentrations, affecting therapeutic efficacy and increasing potential toxicity. To address these issues, Gong Cheng et al. [139] developed a biomimetic nanosystem using EM-ZIF-8 for protein delivery (Figure 7). In this system, proteins are encapsulated within pH-responsive MOF nanoparticles, protecting them from degradation and clearance while enabling efficient delivery to target cells. Zn2+ and 2-methylimidazole self-assemble to form a ZIF-8 framework that cages therapeutic proteins, such as gelonin, an rRNA-disrupting cancer therapeutic protein. This structure achieves high protein loading efficiency (~94%) and enables the controlled release of proteins in the acidic environment of endosomes and lysosomes. Additionally, coating the nanoparticles with EM helps them evade phagocytosis and prolongs their circulation time, enhancing their targeting of specific tumor cells [140,141].
In vitro and in vivo studies demonstrated that EM-coated MOF-protein nanoparticles protected protein cargos, reduced uptake by mononuclear phagocytic systems, and effectively delivered to tumors. This approach has the potential to enhance the efficacy of treatment by improving the delivery and protection of therapeutic proteins.
4.2.3 Gene therapy
MicroRNAs (miRNAs) are non-coding RNAs with a length of 18-25 nucleotides that regulate gene expression and play critical roles in processes, including cell survival, proliferation, and tumor growth [142]. Aberrant miRNA expression is closely associated with cancer progression, making miRNA-based therapies a promising approach in oncology [143,144]. For instance, miR-34a has demonstrated potential in treating breast cancer by inhibiting silencing information regulator 1 expression [145,146]. However, miRNAs face challenges, including poor stability, short half-life, and non-specific biodistribution, limiting their therapeutic efficacy [147].
Protein therapy. A. Schematic for bionic EMP nanoparticle preparation and delivery. The MOF protein (MP) nanoparticles encapsulate the protein, and the extracellular vesicle membrane (EVM) further encloses it to form EMP nanoparticles for protein delivery. B. Assembly and characterization of biomimetic nanoparticles: Fourier transform infrared (FTIR) spectroscopy and N₂ adsorption-desorption isotherms confirmed the protein assembly in MP. The release efficiency of the guest protein was higher at pH 5.0. TEM images exhibited the MP, EV, EVM, and EMP morphologies (scale bar, 100 nm). Confocal microscopy revealed green fluorescence for encapsulated protein in MP and red fluorescence for the EVM (scale bar, 2 μm). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) revealed the protein composition of (1) markers, (2) EVs, (3) EVM, and (4) bionic nanoparticles without loading cargo proteins. C. Laser scanning fluorescence microscopy of RAW264.7 cells incubated with MP, EMP, and liposomal-enveloped MP (LMP) nanoparticles for 2 h (nucleus: blue, nanoparticles: green; scale bar, 50 μm). EVM significantly reduced the internalization of the nanoparticles. D. Through systemic administration, EMP nanoparticles increased gelonin accumulation in tumors and inhibited growth. (I) Gelonin, (II) EMP, and (III) MP. Adapted with permission from [139], copyright 2018, American Chemical Society.
To address these limitations, ZIF-8, a biocompatible MOF, has been used for miRNA delivery [148]. Its porous structure and pH sensitivity allow it to encapsulate miRNAs and release them into the acidic environment of tumor cells, promoting endosomal escape and cytosolic delivery [149,150]. However, the ability of ZIF-8 to non-specifically adsorb biomolecules in the bloodstream can lead to immune clearance, thereby reducing its effectiveness [151].
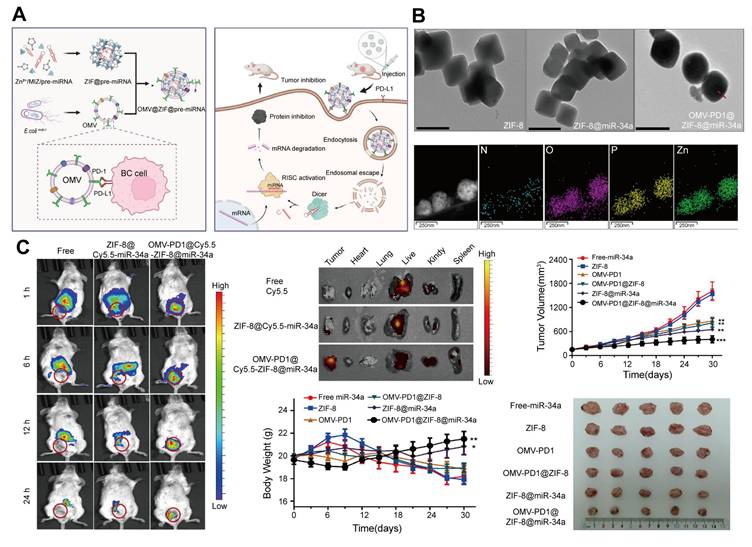
A solution to this challenge involves coating ZIF-8 nanoparticles with 20-300 nm outer membrane vesicles (OMVs) derived from gram-negative bacteria [152]. OMVs enhance the biocompatibility, targeting specificity, and immune evasion of the system while enabling synergistic miRNA and immunotherapy effects [153]. Cui et al. [154] developed an OMV-coated ZIF-8 nanodelivery system for miRNA therapy. This platform efficiently delivered miR-34a to tumor cells, while OMVs displaying programmed death receptor 1 (PD1) on their surface provided targeted tumor recognition and immune activation. This combined effect enhances miRNA delivery and immune responses, leading to improved therapeutic outcomes in breast cancer. The OMV-ZIF-8 system represents a powerful tool for miRNA-based cancer therapies, offering enhanced stability, targeted delivery, and potential for synergistic immunotherapy (Figure 8).
Gene therapy. A. Schematic of the preparation and targeted delivery of the OMV-PD1@ZIF-8@miRNA nanodelivery system and its therapeutic effect. B. TEM images of ZIF-8, ZIF-8@miR-34a, OMV-PD1@ZIF-8@miR-34a, and elemental mapping of OMV-PD1@ZIF-8@miR-34a (scale bar, 200 nm); arrows indicate OMV membranes. C. In vivo tumor-targeting and antitumor effects. The OMV-PD1@ZIF-8@Cy5.5-miR-34a exhibited rapid tumor accumulation, and enhanced tumor targeting, whereas the control group of free Cy5.5 and ZIF-8@Cy5.5-miR-34a preferentially accumulated in the liver and kidneys. The treatment group also exhibited significant inhibition of tumor growth and weight gain. Adapted with permission from [154], copyright 2023, Elsevier B.V.
4.2.4 Immunization therapy
Ascorbic acid (AA)-mediated T cell-dependent therapy has gained attention in tumor immunology due to its potential to enhance immune responses against tumors [155]. However, the rapid clearance of AA from the bloodstream and its limited efficacy in "cold" tumors, which lack immune cell infiltration, present significant challenges [156,157]. Integrating AA with immunogenic cell death (ICD) inducers, such as bortezomib (BTZ), offers a strategy to stimulate immune response effectively [158,159]. Nonetheless, achieving tumor-targeted delivery and controlled release of BTZ and AA requires an advanced delivery system capable of high payload stability, immune evasion, and selective release within the TME.
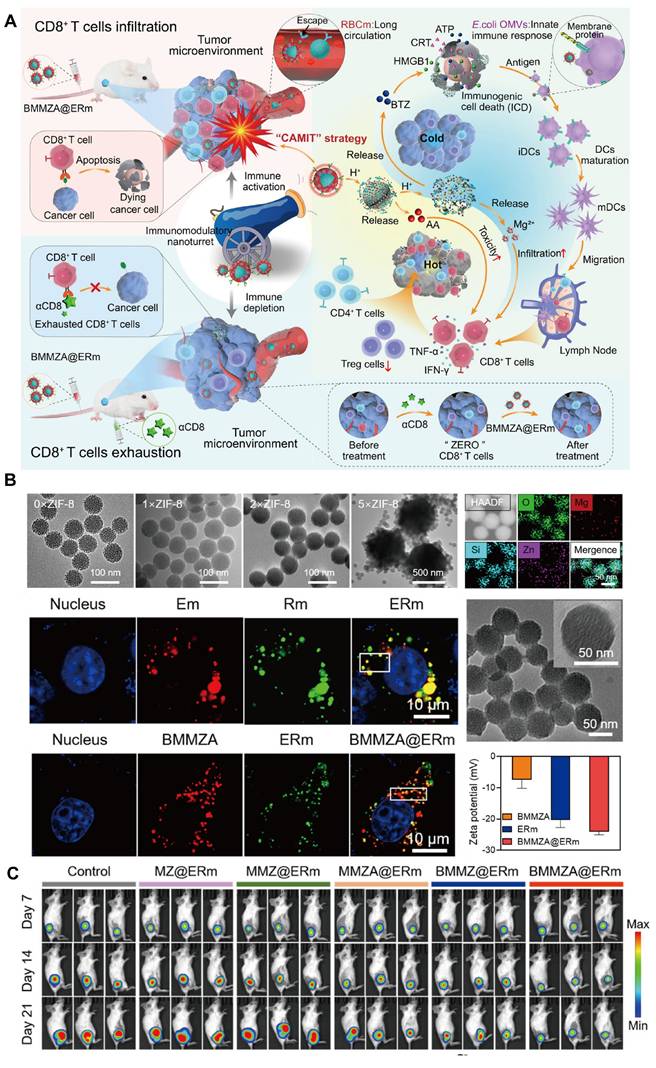
To address these challenges, Yao et al. [160] developed a biomimetic nanocarrier, BMMZA@ERm, designed for the co-delivery of AA and BTZ (Figure 9). This system employs magnesium-doped mesoporous silica (MMS) nanocarriers with AA immobilized within a ZIF-MOF matrix, and MOF served as a pH-sensitive gate for BTZ-loaded MMS (BMMS). The nanocarrier was further camouflaged with a hybrid membrane derived from Escherichia coli OMVs and erythrocyte membranes (ERm), extending circulation time and enhancing immune compatibility. The MOF gate degrades in the acidic TME, enabling controlled and sequential release of AA and BTZ. This cascade facilitates potent ICD induction via BTZ, which promotes dendritic cell maturation and T-cell infiltration with the immunomodulatory effects of OMVs and Mg2+. Subsequently, AA subsequently activates T cells, converting "cold" tumors into "hot" ones with significant anti-tumor and antimetastatic effects.
This innovative AA-based immunotherapy platform demonstrates a T cell-dependent mechanism, as confirmed by CD8-deficient models, highlighting the potential of the system in personalized cancer immunotherapy. The BMMZA@ERm platform underscores the promise of integrating MOFs, ICD inducers, and AA in a multifaceted nanocarrier, offering new avenues for immunotherapy and overcoming immunological barriers within the TME.
Immunization therapy. A. Schematic of an immunomodulatory nano-turret based on biodegradable nanocarriers camouflaged with hybrid Escherichia coli OMV-ERm, for chemotherapy-assisted ascorbic acid-mediated immunotherapy (CAMIT). B. TEM images of MMS@ZIF-8 synthesized with varied feeding concentrations of ZIF-8 precursors; element mapping of MMS@ZIF-8; the confocal laser scanning microscopy images of Em, Rm, hybrid ERm and BMMZA, ERm, BMMZA@ERm; TEM of BMMZA@ERm; and zeta potentials of BMMZA, ERm, and BMMZA@ERm. C. In vivo tumor therapy. Bioluminescence imaging revealed the BMMZA@ERm-treated mice demonstrated the strongest tumor inhibition. Adapted with permission from [160], copyright 2024, American Chemical Society.
4.2.5 Sonodynamic therapy
Sonodynamic therapy uses low-intensity ultrasound to generate reactive oxygen species (ROS) at tumor sites, offering a non-invasive treatment with high tissue penetration [161]. Despite these advantages, the accumulation of sonosensitizers in tumors is frequently hindered by biological barriers in the TME [162]. Furthermore, many sonosensitizers, such as porphyrins and pyrroles, suffer from short half-lives and poor targeting, limiting their effectiveness in sonodynamic therapy [163,164].
To overcome these challenges, Zhang et al. [25] constructed an EV-coated MOF system for targeted delivery of sonosensitizers. In this approach, ZIF-8 nanoparticles were loaded with the sonosensitizer Ce6 and coated with OMVs derived from Escherichia coli MG1655. This coating enhanced the tumor-targeting ability and immune activation of the nanoplatform. In vivo studies using a mouse model of breast cancer demonstrated that this OMV-modified MOF system improved sonosensitizer accumulation in tumors, triggered effective immune responses in the TME, and enhanced the therapeutic efficacy of sonodynamic therapy. This platform represents a promising direction for future sonodynamic therapy applications in cancer therapy.
4.3 Treatment of rheumatoid arthritis (RA)
RA is a chronic inflammatory disease driven by dysregulated immune responses that cause joint destruction and systemic comorbidities [165]. Conventional therapies, including non-steroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying antirheumatic drugs, are frequently associated with severe side effects, such as osteoporosis, liver and kidney toxicity, and heightened infection risks [166,167]. These limitations necessitate the development of advanced therapies to improve drug efficacy and minimize adverse effects.
To address these challenges, Liu et al. [34] designed a biomimetic nanoplatform by encapsulating TNFi within ZIF-8 nanoparticles, coated with membranes from M2NV. This AINUT platform protected TNFi from protease degradation and enhanced its targeted delivery to inflamed joints. In vivo studies have demonstrated that AINUT effectively reprogrammed inflammatory macrophages into anti-inflammatory subtypes and preferentially accumulated in the acidic RA joint environment, leading to significant therapeutic benefits.
Additionally, Wang et al. [106] developed a dexamethasone (Dex)-loaded ZIF-8 nanoplatform coated with macrophage-derived microvesicles (MVs) to improve Dex bioavailability. The resulting MV/Dex/ZIF-8 system prolonged drug circulation, enhanced stability, and allowed sustained release at inflamed joints, reducing the need for repeated dosing and minimizing side effects.
Furthermore, Wang et al. [168] developed a similar strategy to deliver methotrexate (MTX), a common RA treatment. They developed MV/MTX@ZIF-8 by encapsulating MTX in ZIF-8 nanoparticles and coating them with MVs, which targeted inflamed joints by overexpressing folic acid receptors on macrophages. This system enhanced drug accumulation in arthritic tissues, reduced hepatotoxicity, and improved overall therapeutic outcomes.
4.4 Treatment of ulcerative colitis (UC)
UC is a chronic inflammatory bowel disease characterized by recurrent inflammation and bleeding that profoundly affects a patient's quality of life [169]. The global incidence and prevalence of UC have risen dramatically, with approximately five million cases reported worldwide as of 2023 [170]. Current therapies, including aminosalicylates, corticosteroids, and biological treatments such as anti-TNF agents [171], offer limited efficacy due to high dosage requirements, toxicity, and systemic side effects [172]. Consequently, there is an urgent need for targeted therapies with high specificity and minimal systemic impact.
To address this therapeutic gap dilemma, Cui et al. [173] developed a novel TNF-α siRNA delivery platform (EVs@ZIF-8@siRNA) targeting UC. This design uses ZIF-8 to encapsulate and stabilize TNF-α siRNA, providing a robust delivery vehicle. To enhance targeting and bioavailability, the ZIF-8 nanocarrier was coated with ginger-derived EVs, which improved the acid stability in the gastrointestinal tract and promoted colon-specific targeting. Furthermore, 6-shogaol, an EV component of ginger, exerts anti-inflammatory effects, creating a synergistic interaction with TNF-α siRNA that amplifies therapeutic efficacy. This system also supports intestinal barrier integrity and modulates gut microbiota, fostering an environment conducive to mucosal healing and inflammation control in UC models.
4.5 Wound healing
Wound healing, particularly for chronic wounds, oral ulcers, and hypertrophic scars, is a major healthcare challenge [174]. The increasing global incidence of diabetes, obesity, and aging populations has exacerbated the prevalence of chronic wounds, such as diabetic ulcers, resulting in high healthcare costs [175]. In addition, more than 25% of the global population has experienced or is experiencing oral ulcers, seriously affecting the quality of life of patients [176]. Similarly, hypertrophic scars caused by burns, trauma, or surgery frequently cause severe physical discomfort and psychological distress [177].
Mesenchymal stromal cell-derived EVs (MSC-EVs) have demonstrated great promise in promoting wound healing due to their involvement in angiogenesis, immunomodulation, and tissue regeneration [178,179]. MSC-EVs are rich in miRNAs and cytokines that facilitate these processes [180]; however, their rapid degradation and short lifespan limit their efficacy [181].
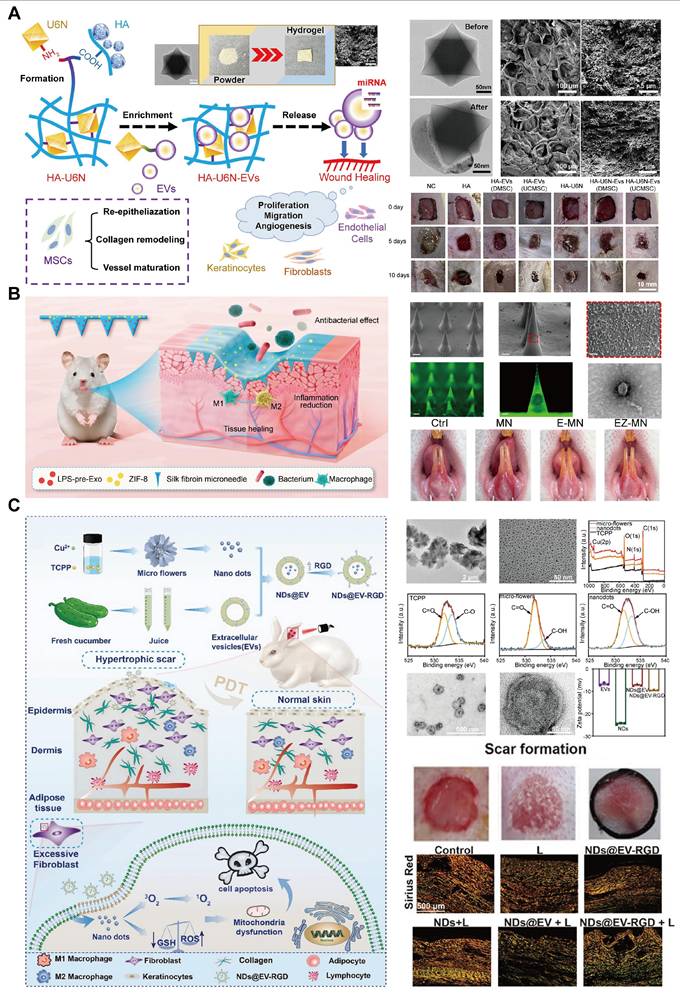
To address this issue, MOF-based delivery systems have been developed to protect EVs, prolong their release, and enhance their therapeutic effects. Wang et al. [182] developed a UiO-66-NH2-based hydrogel that effectively adsorbs MSC-EVs via strong Zr-O-P coordination bonds. This hydrogel protects the EVs and enables their sustained release at wound sites, thereby accelerating tissue regeneration. In diabetic wound models, the hydrogel system significantly enhanced wound closure and tissue repair (Figure 10A).
Ge et al. [183] developed a silk fibroin microneedle patch to treat oral ulcers that included lipopolysaccharide (LPS)-preconditioned bone marrow mesenchymal stem cells and their secreted exosomes (LPS-pre-Exos) alongside ZIF-8 for the treatment of oral ulcers (Figure 10B). This microneedle (MN) patch design facilitates painless, minimally invasive, and targeted transdermal exosome delivery, enabling sustained release of LPS-pre-Exos and promoting macrophage polarization toward a pro-healing phenotype. Furthermore, the controlled release of Zn2+ from ZIF-8 contributes to the anti-inflammatory and antimicrobial properties of the patch, fostering an environment conducive to rapid tissue regeneration and ulcer healing. This innovative MN patch offers a promising localized, and sustained-release approach for enhancing mucosal wound healing.
Photodynamic therapy (PDT) has gained attention for treating hypertrophic scars due to its ability to soften scar tissue and reduce pain [184]. However, poor transdermal delivery of photosensitizers frequently limits the PDT efficacy [185]. To overcome this, Kong et al. [186] developed a copper-based MOF nanoplatform, functionalized with EVs and arginine-glycine-aspartic acid (RGD) peptides for targeted delivery. This system enhances skin penetration and ROS generation under near-infrared light, promoting fibroblast apoptosis and collagen remodeling in hypertrophic scar tissues [187]. EVs can overcome the barrier of the dense stratum corneum (SC) due to their high affinity for SC, which is primarily composed of lipids, as well as their superdeformation ability, allowing them to penetrate the skin surface layer [188,189]. These studies highlight the potential of EV-MOF hybrid systems in addressing the challenges of chronic wounds and hypertrophic scars, providing innovative solutions for effective and sustained wound healing therapies (Figure 10C).
4.6 Bone regeneration
Reconstructing large bone defects caused by trauma, inflammation, or tumors is a significant challenge in orthopedics [190]. In addition to promoting osteogenesis, early vascularization is critical for supplying nutrients and removing waste during bone formation [191-193]. Accordingly, regulating osteogenesis and angiogenesis is essential for bone regeneration.
EVs derived from hADSCs have demonstrated promise in enhancing bone regeneration [194]. However, their rapid degradation and short half-life limit their efficacy [195,196]. To address this, researchers have used MOFs as scaffolds, which offer tunable porosity, biocompatibility, and controlled release properties. For example, ZIF-8-modified hydrogels have been demonstrated to promote angiogenesis and osteogenesis [197].
Mg2+ has also proven beneficial in bone regeneration by enhancing autophagic activity and promoting calcium deposition, essential for bone growth [198,199]. Moreover, Mg2+ exhibits angiogenic and anti-inflammatory effects [200,201], making it an ideal component in bone tissue engineering. Kang et al. [47] developed a PLGA/Mg-GA MOF scaffold, incorporating hADSCs-EVs, Mg2+, and gallic acid (GA) to enhance osteogenesis, angiogenesis, and anti-inflammatory reduction. In vitro experiments have demonstrated that this matrix can significantly promote osteogenesis of hBMSCs and angiogenesis of HUVECs. The slowly released hADSC-EVs provided a stable growth environment for the cells and accelerated bone regeneration. In vivo studies have revealed that this scaffold accelerates bone remodeling and promotes successful osseointegration, indicating its promising clinical application prospects.
Cu2+ has also been recognized for its role in promoting osteogenesis and angiogenesis [202-204]. Xu et al. [205] combined Cu-based MOFs with EVs from human bone mesenchymal stem cells to create a scaffold that provided sustained release of bioactive ions and EVs, upregulated the expression of osteogenic-related proteins in hBMSCs, promoted angiogenesis and formation, and significantly enhanced bone regeneration in a rat model. These findings highlight the potential of EV-MOF scaffolds as powerful tools for promoting vascularized bone regeneration, offering a promising strategy for the treating of large bone defects.
Wound healing. A. Schematic and characterization of MOF-based hydrogels (HA-U6N-EVs) for chronic wound healing. TEM images of MOFs and MOF-adsorbed EVs, SEM images of HA-U6N hydrogels before and after swelling, and wound closure progression in diabetic rats at 0, 5, and 10 days. MSC-EV-treated rats exhibited significant wound healing by day 5, whereas controls healed slowly. B. Schematic and characterization of MNs loaded with LPS-pre-Exos and ZIF-8 treating for oral ulcers. SEM images of normal MNs and images of MNs with green fluorescent substance added, along with TEM images of LPS-pre-Exos. Representative images displayed near-complete ulcer healing in the EZ-MN group with red mucosa, while the control group displayed slower healing. C. Schematic and characterization of NDs@EV-RGD nanoparticles for PDT-mediated hypertrophic scar treatment. TEM images of Cu-MOF microflowers, Cu-MOF NDs, EVs, and NDs@EV-RGD, along with the X-ray photoelectron spectroscopy (XPS) of TCPP, Cu-MOFs microflowers and Cu-MOFs NDs, and zeta potential measurements of EVs, NDs, NDs@EV, and NDs@EV-RGD. The efficacy of the treatment was demonstrated through scar imaging and three-color staining of the treated scars. (A) Adapted with permission from [182], copyright 2024, Elsevier B.V. (B) Adapted with permission from [183], copyright 2024, American Chemical Society. (C) Adapted with permission from [186], copyright 2024, Wiley-VCH GmbH.
4.7 Anti-infection
The rise of multidrug-resistant (MDR) and extensively drug-resistant (XDR) bacterial strains has raised serious global health concerns [206,207]. Conventional antibiotics are becoming increasingly ineffective, prompting the need for novel antimicrobial strategies [208]. MOFs and EVs have emerged as promising platforms for combating infections by providing new approaches for targeted delivery, enhanced drug efficacy, and controlled release [209].
MOFs can encapsulate and protect antimicrobial agents, prevent degradation and enhance their delivery to infected sites [210]. Furthermore, the ability of MOFs to bypass bacterial efflux pumps and resist bacterial enzymatic degradation makes them a potent solution against MDR pathogens [211]. Combining EVs with MOFs further enhances targeting efficiency and antimicrobial effects, enabling the precise delivery of antimicrobial agents to infection sites [212]. This synergy between MOFs and EVs opens up new possibilities for treating numerous infections, including biofilm-associated and systemic bacterial infections.
A notable example is the development of the ZnBq/Ce6@ZIF-8@OMV nanoplatform that integrates a Zn-based metal complex (ZnBq) with Ce6 as a photosensitizer and is encapsulated within ZIF-8 MOF nanoparticles [213]. These nanoparticles were further coated with genetically engineered OMVs to enable precise targeting and enhanced antimicrobial action. The ZnBq/Ce6@ZIF-8@OMV system demonstrated exceptional efficacy in treating infections caused by Acinetobacter baumannii, a leading MDR pathogen in healthcare settings. The MOF component stabilizes and protects the antimicrobial agents, while the OMV coating ensures targeted delivery, allowing the nanoplatform to penetrate biofilms and effectively eliminate bacteria. This system also offers the added benefit of PDT, in which Ce6 is activated by light to generate ROS, further enhancing its bactericidal activity. The success of the ZnBq/Ce6@ZIF-8@OMV platform underscores the potential of EV-MOF hybrid systems as next-generation anti-infective therapies, that can address the limitations of current antibiotics and offer new hope against resistant infections.
In addition to traditional MOF-based antibacterial agents, porphyrin MOFs with conjugated systems and electrocatalytic properties have emerged as potent electrosensitizers in electrodynamic therapy (EDT) for bacterial infections. EDT, an innovative non-antibiotic approach, leverages an electric field to generate ROS and provides a particularly advantageous targeted bactericidal effect combating bacterial biofilms. Concurrently, plant-derived EVs are promising for neutralizing bacterial toxins due to their phospholipid membrane structure, which mimics host cell membranes and interacts with bacterial toxins. Based on this, Wang et al. [214] developed a bionic nano-sponge (MOF@EV) camouflaged with ginger-derived EVs to camouflage and functionalize with nano-porphyrin as an electrical sensitizer. This system effectively absorbs toxins and generates ROS under electrical stimulation, achieving potent anti-biofilm and antibacterial effects. In both in vitro and in vivo experiments, MOF@EV exhibited superior biocompatibility and effectively cleared Staphylococcus aureus-induced subcutaneous abscesses, demonstrating its potential in clinical anti-infective therapies.
Currently, biocatalytic therapy targeting bacterial pathogens has emerged as a novel therapeutic strategy for antimicrobial treatment [215]. Studies have demonstrated that in the presence of nitric oxide synthase (iNOS), L-arginine undergoes biocatalysis to generate nitric oxide (NO) [216]. NO can be toxic to bacteria by inhibiting bacterial DNA recombination and causing severe peroxidative stress in biofilms [217]. Wan et al. [218] constructed a nanobiohybrid nanoreactor EV@ZIF, mimicking the natural biomineralization process to deposit a ZIF layer on the EV surface, thereby enhancing the reactor stability. Besides, EVs inherently possess iNOS activity that can catalytically generate NO, while the ZIF, as a photocatalyst, can absorb light energy under illumination to produce photogenerated electron-hole pairs. The photogenerated electrons transfer to the EVs, bind with NADP+, and promote its reduction to NADPH, a key coenzyme in the iNOS enzymatic reaction, thereby accelerating the conversion of NO. This enables EV@ZIF to efficiently generate NO under illumination, with a yield far exceeding that of unmodified EVs. The system exhibited strong bactericidal activity (~70%) and the ability to penetrate the biofilm in antibacterial experiments, significantly inhibited the inflammatory response of gingival tissue and promoted periodontal tissue repair in animal experiments, suggesting its potential for treating infectious diseases such as periodontitis.
Additionally, the challenges of implant-associated infections (IAIs), particularly for orthopedic implants, are increasingly pressing due to the global demand for effective, infection-resistant implants [219]. IAIs are especially resistant to conventional treatments due to the anaerobic biofilms that form on implant surfaces, protecting bacterial cells from immune responses and reducing antibiotic efficacy [220]. Therefore, non-surgical surface engineering is thus critical for mitigating IAIs by directly enhancing antibacterial efficacy at the implant surface while promoting bone regeneration [221]. Li et al. [222] introduced a novel coating strategy that combines an ultrasonically-activated copper-based MOF (Cu-TCPP), probiotic-derived OMVs, hypoxia-induced antibiotic tinidazole (TNZ), and poly-tannic acid (pTA) to form an antimicrobial coating layer for titanium implants. Under ultrasonication, Cu-TCPP catalyzes the production of singlet oxygen (1O2) and generates a hypoxic environment to enhance the antibacterial effects of TNZ. Simultaneously, Cu (II) ions from Cu-TCPP were reduced to Cu (I), disrupting bacterial metabolism, thereby causing cell death. The OMVs layer modulates immune response by promoting macrophage polarization to an M2 phenotype, reducing inflammation, and enhancing osteogenic factor expression, thereby fostering an infection-resistant bone-regenerative microenvironment. This multifunctional coating displays promise for effective biofilm prevention, improved bone integration, and long-term protection against reinfection.
4.8 Analysis of signal path mechanism
The EV-MOF system has demonstrated therapeutic effects in various diseases, including inhibition of the proliferation and metastasis of tumor cells, alleviation of inflammation in RA, improvement of the intestinal microenvironment in UC, promotion of wound healing, and acceleration of bone tissue regeneration. However, current research lacks a systematic analysis of the regulatory mechanisms of core signaling pathways, limiting a comprehensive understanding of its mechanism of action and hindering the clinical translation of EV-MOF [223].
Research on RA, has already yielded EV-MOF studies that use omics technologies to explore signaling pathway mechanisms. The study employed a global RNA profiling approach to investigate primary M1 macrophages and M1 macrophages treated with M2NVs. The pathways altered after treatment with M2NVs were concentrated in the nuclear factor-kappa B (NF-κB), Toll-like receptors (TLRs), and mitogen-activated protein kinase (MAPK) signaling pathways, which play dominant roles in macrophage polarization. In M1 macrophages treated with M2NVs, the expression of anti-inflammatory regulatory genes (Foxp3, MapK11, and Gdf15) was upregulated, while the expression of pro-inflammatory genes was downregulated. M2NVs demonstrate a robust immunoregulatory capacity by modulating pathways related to macrophage polarization [34].
However, in other diseases, such as cancer, abnormal activation of signaling pathways, including phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and extracellular signal-regulated kinase (ERK) is closely related to tumor malignancy [224]. There is still a lack of conclusive evidence on whether and how the EV-MOF system achieves anti-cancer effects by regulating these key signaling pathways. Similarly, in UC, the regulatory mechanisms of signaling pathways involved in intestinal inflammation and repair, such as Wnt/β-catenin and transforming growth factor-β (TGF-β) pathways, require in-depth study [225]. Further research into the regulatory mechanisms of signaling pathways, such as TGF pathway, and vascular endothelial growth factor (VEGF) signaling pathway, which involve cell proliferation, migration, and angiogenesis, is required for wound healing [226,227]. In terms of bone regeneration, the regulatory mechanisms of signaling pathways, such as bone morphogenetic protein (BMP) and Wnt pathways, that control bone formation and resorption remain poorly understood [228].
Most current studies use only traditional molecular biology methods, such as WB, quantitative polymerase chain reaction (qPCR) to detect changes in the expression levels of a small number of key proteins or genes, making it difficult to fully understand the complex dynamic processes in signaling pathways. The limitations of this research method result in a limited understanding of the overall mechanism of the EV-MOF system in intracellular signal regulation [154].
To systematically address this knowledge gap, it is recommended that comprehensive multi-omics research be conducted. Proteomics techniques can be used to comprehensively analyze the expression and modification profiles of hundreds or thousands of proteins within cells, potentially revealing the modulatory effects of the EV-MOF system on multiple nodes of signaling pathways. Dynamic changes in the phosphorylation levels of key proteins in signaling pathways can be monitored using quantitative proteomics combined with the analysis of phosphorylation modifications, thereby inferring the regulatory role of the EV-MOF system [229]. Transcriptomic techniques can comprehensively analyze changes in gene expression within cells, helping to trace upstream regulatory events in signaling pathways and when integrated with proteomic data, can provide a complete regulatory network from the transcriptional to protein level [230]. Additionally, metabolomics research can reveal whether the EV-MOF system indirectly affects the activity of signaling pathways by regulating intracellular metabolomic pathways [231]. Integrating these multi-omics data may provide a new perspective to understand the role and mechanism of the EV-MOF system in different diseases [232].
In-depth mechanistic research is important to guide the rational design of next-generation EV-MOF systems. Only by clarifying the regulatory mechanisms of EV-MOF systems in signaling pathways can these systems be precisely optimized based on the pathological characteristics and signaling pathway abnormalities of different diseases. For example, if it is found that a certain EV-MOF system can inhibit the PI3K-AKT signaling pathway in tumor cells, its inhibitory effect can be further enhanced by chemically modifying MOF materials or genetically engineering EV components, thereby improving cancer treatment efficacy [233,234]. Similarly, mechanistic research based on corresponding signaling pathways for RA, UC, wound healing, bone regeneration, and anti-infection may provide a theoretical foundation for developing EV-MOF systems with improved bioactivity.
In summary, integrated systems research helps to reveal the mechanisms by which the EV-MOF system regulates intracellular signaling pathways, laying a solid theoretical foundation for the rational design of next-generation EV-MOF systems. This may promote the clinical translation of EV-MOF systems treating various diseases, opening up new avenues for future precision medicine and regenerative medicine research.
5. Challenges and Prospects
The synergistic application of EVs and MOFs presents transformative potential in nanomedicine; however, several key challenges must be addressed for clinical translation.
(1) Large-scale production and material integration: Scalability, large-scale production, and EV-MOF integration are the major barriers. Current traditional separation technologies for EVs, such as ultracentrifugation, have low yields and significant batch-to-batch quality variability, limiting the stable supply of therapeutic-grade exosomes [235]. To overcome this, advances in bioreactors and microfluidics are critical for the continuous, large-scale production of EVs [236,237]. The traditional MOFs synthesis process requires high temperatures, high pressures, or organic solvent environments and are highly sensitive to minor changes in synthetic conditions, frequently resulting in poor batch consistency of nanostructures, high laboratory costs, and commercialization barriers. Optimizing synthetic conditions, such as low-temperature continuous flow processes and green solvent alternatives, and tuning crystal structures, such as ZIF-8 and MIL-101, can reduce costs [238]. Furthermore, the methods used to couple EVs with MOFs, such as ultrasound, extrusion, or co-incubation, vary in efficiency and consistency [239]. Standardizing these methods is necessary to ensure high binding efficiency and structural integrity across different EV-MOF systems. Evaluating these approaches using metrics, such as loading capacity and purity, is crucial for optimizing therapeutic outcomes. Furthermore, artificial or engineered EVs can be designed to optimize their compatibility with MOFs [240,241], providing a more standardized system for clinical applications.
(2) Stability and functional integrity: EVs are susceptible to degradation, limiting their therapeutic window [242]. Developing cryopreservation and lyophilization techniques that maintain the structural and functional integrity of EV-MOF systems is essential [243,244]. Currently, cryoprotectants, such as trehalose have been developed to stabilize the structure of EVs [245]. Simultaneously, novel EV preservation methods have been developed, such as the non-destructive encapsulation of EV via ZIF-8, which, while developing as well as new EV preservation methods, provides a new perspective on the combination form of the EV-MOF system by achieving MOF synthesis and EV encapsulation in one step [39]. Additionally, the consistency of biological functions must be ensured for functional integrity by real-time monitoring of key parameters of EVs, such as particle size and surface markers [246]. Moreover, the design of MOF scaffolds must ensure controlled degradation and sustained release of EV cargo while maintaining bioactivity over longer periods.
(3) Safety and immunogenicity: Although EVs improve the biocompatibility of MOFs, safety concerns arise when using EVs derived from cancer cells, which may promote tumor growth if not carefully engineered [247]. Alternatives such as non-cancer cell-derived EVs or engineered can mitigate this risk, while maintaining effective targeting and immune evasion [248]. Establishing safe EVs sources, such as mesenchymal stem cells (MSCs) or macrophages, may be pivotal in future applications. Although the dissociation of the structure and release of metal ions in MOFs can result in the release of drugs and therapeutic factors by drug carriers reaching targets in the body, excessive and highly toxic metal ions and ligands can cause immunocompatibility issues [249,250]. Choosing endogenous metal ions, such as Zn2+ and Fe3+ and natural ligands, such as cyclodextrin, can reduce immunotoxicity due to their specific metabolic pathways and biodegradability [251,252].
(4) Mechanistic understanding and preclinical research: A deeper understanding of EV-MOF interactions in vivo is necessary. Key questions regarding biodistribution, biotransformation, and cellular uptake remain unresolved [253]. Developing tracers to track EV-MOF in real time, along with extensive preclinical studies in relevant disease models, helps elucidate their mechanism of action and optimize therapeutic delivery. Furthermore, comprehensive pharmacokinetic and pharmacodynamic studies are vital for assessing the effectiveness and safety of EV-MOF systems before clinical trials [254]. Yao et al. [160] reported that the circulation half-life of BMMZA@ERm (11.78 h) was significantly prolonged compared to BMMZA (3.42 h), and it could be completely excreted through urine and feces within 48 h.
(5) Quality monitoring and regulatory review: The clinical transformation of EV-MOF composite products must overcome the dual regulatory challenges posed by agencies such as the FDA for biological products and nanomaterials [255]. Although the Pharmaceuticals and Medical Devices Agency of Japan's Scientific Committee issued the first official guideline for EV treatment products by an official regulatory body [256], there is still a global lack of a specialized regulatory framework for MOF and EV-MOF composite products. Their review needs to integrate existing standards for biological products, such as traceability of EV sources and batch-to-batch consistency requirements, and nanomaterials standards, such as MOF cytotoxicity thresholds and degradation kinetics [257,258], and, using artificial intelligence (AI)-driven tools and global platforms such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) platform, promote the development of adaptive regulatory models, to achieve cross-innovation transformation in regenerative medicine and nanotechnology [259,260].
The EV-MOF platform holds promise for theranostics by combining diagnostics and therapeutics in a single system for future clinical applications. Beyond cancer, they have potential applications in cardiovascular, neurodegenerative, and viral diseases, where precise targeting and controlled drug release are critical. As AI and machine learning are integrated into the design and optimization of EV-MOF systems [261,262], these platforms may become highly personalized, further advancing precision medicine (Figure 11).
Challenges in the clinical transformation of EV-MOF, corresponding solutions, and prospects for EV-MOF systems. The circular outer ring and adjacent purple text depicts the challenges in the clinical transformation of EV-MOF. The inner circle and adjacent green font, along with the graphics inside the sector, display the solutions for the corresponding challenges. The pentagon on the right presents the prospects for the EV-MOF system. Created using BioRender.com.
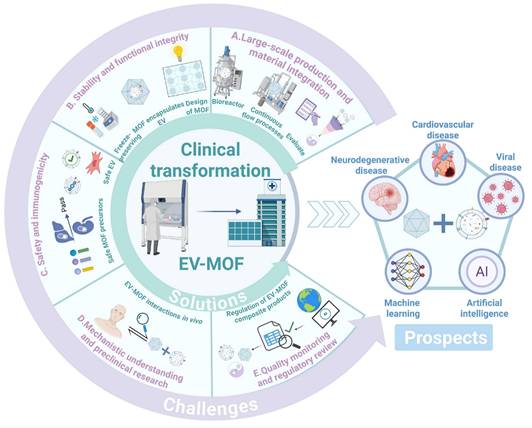
6. Conclusion
The synergistic application of EVs and MOFs has introduced a transformative approach to nanomedicine, offering advanced solutions for diagnostics and therapeutics. By leveraging the unique properties of MOFs, such as high porosity and tunable release, alongside the inherent biocompatibility and targeting capabilities of EVs, EV-MOF systems have demonstrated significant potential in enhancing disease detection, particularly through improved sensitivity in biomarker identification and imaging technologies. In the therapeutic context, EV-MOF platforms offer efficient drug delivery systems that ensure controlled release and reduced toxicity, as evidenced by their promising applications in cancer treatment, RA, and bone regeneration. However, challenges remain in scaling production, ensuring long-term stability, and understanding the in vivo mechanisms of EV-MOF interactions. Addressing these issues through continued research is crucial for realizing the full clinical potential of EV-MOF systems. As nanomedicine advances toward more personalized and precise treatments, EV-MOF systems are poised to play a crucial role, offering innovative pathways for overcoming the current limitations in diagnosis and therapy. Their integration into clinical practice can advance this field by offering safer and more effective therapeutic strategies and enhancing early disease detection.
Abbreviations
3D: three-dimensional; AA: ascorbic acid; AI: artificial intelligence; AKT: protein kinase B; ALF: artificial lysosomal fluid; ATP: adenosine triphosphate; Au: gold; BMMS: BTZ-loaded MMS; BMP: bone morphogenetic protein; BTZ: bortezomib; CAMIT: chemotherapy-assisted ascorbic acid-mediated immunotherapy; CD-MOFs: cyclodextrin-based MOFs; CT: computed tomography; Cu-TCPP: copper-based MOF; Dex: dexamethasone; DLS: dynamic light scattering; EDT: electrodynamic therapy; EM: EV membranes; ERK: extracellular signal-regulated kinase; EPR: enhanced permeability and retention; ERm: erythrocyte membranes; EVM: extracellular vesicle membrane; EV-MOF: extracellular vesicle-metal organic framework; EVs: extracellular vesicles; FDA: U.S. Food and Drug Administration; Fe: iron; FTIR: Fourier transform infrared; GA: gallic acid; hADSCs: human adipose-derived mesenchymal stem cells; IAIs: implant-associated infections; IC50: half maximal inhibitory concentration; ICD: immunogenic cell death; ICH: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; iNOS: nitric oxide synthase; LPS: lipopolysaccharide; LPS-pre-Exos: lipopolysaccharide-preconditioned bone marrow mesenchymal stem cells and their secreted exosomes; LMP: liposomal-enveloped MP; M2NV: M2 macrophage-derived vesicle; mAuNSs: Au nanostars; MB: methylene blue; MDR: multidrug-resistant; Mg: magnesium; miRNAs: microRNAs; MMS: magnesium-doped mesoporous silica; MN: microneedles; MOFs: metal-organic frameworks; MP: MOF-protein; MRI: magnetic resonance imaging; MSCs: mesenchymal stem cells; MSC-EVs: mesenchymal stromal cells-derived EVs; MTT: 3- (4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide; MTX: methotrexate; MVs: macrophage-derived microvesicles; NGPs: nanolecular particles; NO: nitric oxide; NTA: nanoparticle tracking analysis; 1O2: singlet oxygen; OMVs: outer membrane vesicles; PD1: programmed death receptor 1; PDT: photodynamic therapy; PEG: polyethylene glycol; PI3K: phosphatidylinositol 3-kinase; pTA: poly-tannic acid; qPCR: quantitative polymerase chain reaction; RA: rheumatoid arthritis; RhB: rhodamine B; ROS: reactive oxygen species; SDS-PAGE: sodium dodecyl sulfate-polyacrylamide gel electrophoresis; SEM: scanning electron microscopy; TEM: transmission electron microscopy; tExo: tumor-derived exosomes; TGF-β: transforming growth factor-β; Ti: titanium; TME: tumor microenvironment; TNF-α: tumor necrosis factor-α; TNFi: TNF-α inhibitors; TNZ: tinidazole; UC: ulcerative colitis; VEGF: vascular endothelial growth factor; WB: western blotting; XDR: extensively drug-resistant; XPS: X-ray photoelectron spectroscopy; ZIF-8: zeolite imidazolate frameworks-8; Zn: zinc; Zr: zirconium.
Acknowledgements
This work was supported by the Scientific Research Cultivation Project of the College of Life and Environmental Sciences, Wenzhou University (SHPY2025010), National Natural Science Foundation of China (51901160), JinFeng Laboratory of Chongqing (JFLKYXM202303AZ-204).
Author contributions
Peiye Xu: Writing - original draft, Formal analysis and Visualization. Jiaxuan He: Writing - original draft; Ting Xu: Writing - original draft. Weijie Wang: Visualization. Baihui Wu: Visualization. Rongbing Chen: Software. Hanbing Wang: Software. Qinsi Yang: Conceptualization, Supervision and Project administration. Wei Wu: Conceptualization, Funding acquisition and Project administration. Da Sun: Conceptualization, Funding acquisition and Writing - review & editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yaghi OM, Li G, Li H. Selective binding and removal of guests in a microporous metal-organic framework. Nature. 1995;378:703-6
2. Abánades Lázaro I, Chen X, Ding M, Eskandari A, Fairen-Jimenez D, Giménez-Marqués M. et al. Metal-organic frameworks for biological applications. Nat Rev Methods Primer. 2024;4:1-20
3. Peterson GW, Lee DT, Barton HF, Epps TH, Parsons GN. Fibre-based composites from the integration of metal-organic frameworks and polymers. Nat Rev Mater. 2021;6:605-21
4. Wang S, McGuirk CM, d'Aquino A, Mason JA, Mirkin CA. Metal-Organic Framework Nanoparticles. Adv Mater. 2018;30:1800202
5. He Q-T, Qian P, Yang X-Y, Kuang Q, Lin Y-T, Yi W. et al. Rational design of Bacteria-Targeted and Photo-Responsive MOF gel with antibacterial and anti-inflammatory function for infected wound healing. Chem Eng J. 2024;493:152760
6. Chiang M-R, Shen W-T, Huang P-X, Wang K-L, Weng W-H, Chang C-W. et al. Programmed T cells infiltration into lung metastases with harnessing dendritic cells in cancer immunotherapies by catalytic antigen-capture sponges. J Controlled Release. 2023;360:260-73
7. Wang Y, Yan J, Wen N, Xiong H, Cai S, He Q. et al. Metal-organic frameworks for stimuli-responsive drug delivery. Biomaterials. 2020;230:119619
8. Lu K, Aung T, Guo N, Weichselbaum R, Lin W. Nanoscale Metal-Organic Frameworks for Therapeutic, Imaging, and Sensing Applications. Adv Mater. 2018;30:1707634
9. Wu M-X, Yang Y-W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv Mater. 2017;29:1606134
10. Dutta S, Noh S, Gual RS, Chen X, Pané S, Nelson BJ. et al. Recent Developments in Metallic Degradable Micromotors for Biomedical and Environmental Remediation Applications. Nano-Micro Lett. 2023;16:41
11. Wiśniewska P, Haponiuk J, Saeb MR, Rabiee N, Bencherif SA. Mitigating metal-organic framework (MOF) toxicity for biomedical applications. Chem Eng J. 2023;471:144400
12. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-79
13. Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat Rev Bioeng. 2023;1:608-9
14. Hu D, Yang R, Wang G, Li H, Fan X, Liang G. Emerging Strategies to Overcome Current CAR-T Therapy Dilemmas - Exosomes Derived from CAR-T Cells. Int J Nanomedicine. 2024;19:2773-91
15. Chan M-H, Chang Z-X, Huang C-YF, Lee LJ, Liu R-S, Hsiao M. Integrated therapy platform of exosomal system: hybrid inorganic/organic nanoparticles with exosomes for cancer treatment. Nanoscale Horiz. 2022;7:352-67
16. Song H, Chen X, Hao Y, Wang J, Xie Q, Wang X. Nanoengineering facilitating the target mission: targeted extracellular vesicles delivery systems design. J Nanobiotechnology. 2022;20:431
17. Liu W, Yan Q, Xia C, Wang X, Kumar A, Wang Y. et al. Recent advances in cell membrane coated metal-organic frameworks (MOFs) for tumor therapy. J Mater Chem B. 2021;9:4459-74
18. Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T. et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77-84
19. Fan W, Yung B, Huang P, Chen X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem Rev. 2017;117:13566-638
20. Jiang Z, Chu Y, Zhan C. Protein corona: challenges and opportunities for targeted delivery of nanomedicines. Expert Opin Drug Deliv. 2022;19:833-46
21. Bieniek A, Terzyk AP, Wiśniewski M, Roszek K, Kowalczyk P, Sarkisov L. et al. MOF materials as therapeutic agents, drug carriers, imaging agents and biosensors in cancer biomedicine: Recent advances and perspectives. Prog Mater Sci. 2021;117:100743
22. Cai M, Liang W, Wang K, Yin D, Fu T, Zhu R. et al. Aperture Modulation of Isoreticular Metal Organic Frameworks for Targeted Antitumor Drug Delivery. ACS Appl Mater Interfaces. 2022;14:36366-78
23. Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T. et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9:172-8
24. Ding H, Xia Q, Shen J, Zhu C, Zhang Y, Feng N. Advances and prospects of tumor immunotherapy mediated by immune cell-derived biomimetic metal-organic frameworks. Colloids Surf B Biointerfaces. 2023;232:113607
25. Zhang Z, Tu J, Kuang X, Shi M, Zhang Y, Li H. et al. Bacterial outer membrane vesicle-modified metal-organic frameworks for sonodynamic therapy-immunotherapy of breast cancer. New J Chem. 2023;48:367-76
26. Gao Y, Zhou R, Wang Q, Qi S, Lv Y, Liu S. et al. Natural killer cell membrane doped supramolecular nanoplatform with immuno-modulatory functions for immuno-enhanced tumor phototherapy. Chin Chem Lett. 2024 109521
27. Guan J, Shen Q, Zhang Z, Jiang Z, Yang Y, Lou M. et al. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat Commun. 2018;9:2982
28. Shao M, Lopes D, Lopes J, Yousefiasl S, Macário-Soares A, Peixoto D. et al. Exosome membrane-coated nanosystems: Exploring biomedical applications in cancer diagnosis and therapy. Matter. 2023;6:761-99
29. Oveili E, Vafaei S, Bazavar H, Eslami Y, Mamaghanizadeh E, Yasamineh S. et al. The potential use of mesenchymal stem cells-derived exosomes as microRNAs delivery systems in different diseases. Cell Commun Signal. 2023;21:20
30. Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z. et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321-40
31. Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH. et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 2017;8:626
32. Keller MD, Ching KL, Liang F-X, Dhabaria A, Tam K, Ueberheide BM. et al. Decoy exosomes provide protection against bacterial toxins. Nature. 2020;579:260-4
33. Tan S, Liu Z, Cong M, Zhong X, Mao Y, Fan M. et al. Dandelion-derived vesicles-laden hydrogel dressings capable of neutralizing Staphylococcus aureus exotoxins for the care of invasive wounds. J Controlled Release. 2024;368:355-71
34. Liu L, Zhang Y, Mao C, Chen H, Zhang Y, Wang J. et al. Antibody-sheltered immunological nanonut (AINUT) for rheumatoid arthritis-targeted efficient alleviation. Nano Today. 2022;47:101640
35. Iraci N, Gaude E, Leonardi T, Costa ASH, Cossetti C, Peruzzotti-Jametti L. et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat Chem Biol. 2017;13:951-5
36. György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic Applications of Extracellular Vesicles: Clinical Promise and Open Questions. Annu Rev Pharmacol Toxicol. 2015;55:439-64
37. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28
38. Fan Y, Wu W, Xie N, Huang Y, Wu H, Zhang J. et al. Biocompatible engineered erythrocytes as plasmonic sensor initiators for high-sensitive screening of non-small cell lung cancer-derived exosomal miRNA in an integrated system. Biosens Bioelectron. 2023;219:114802
39. Jia J, Kong D, Liu Y, Zhang H, Liang X, Li Q. A Biomimetic Mineralization Strategy for the Long-Term Preservation of Exosomes Through Non-Destructive Encapsulation Within Zeolite Imidazolate Frameworks-8. Small. 2025;21:2412264
40. Yan B, Tan J, Chen L, Zhang H, Ma X, Qiao Y. et al. Stimuli-responsive doxorubicin-loading Zr-MOF film for time-ordered tumor therapy and bone regeneration. Compos Part B Eng. 2023;250:110452
41. Liu J, Tan Y, Shen E, Liu B, Tian Y, Liang L. et al. Highly water-stable bimetallic organic framework MgCu-MOF74 for inhibiting bacterial infection and promoting bone regeneration. Biomed Mater. 2022;17:065026
42. Li X, Shu X, Shi Y, Li H, Pei X. MOFs and bone: Application of MOFs in bone tissue engineering and bone diseases. Chin Chem Lett. 2023;34:107986
43. Doonan C, Riccò R, Liang K, Bradshaw D, Falcaro P. Metal-Organic Frameworks at the Biointerface: Synthetic Strategies and Applications. Acc Chem Res. 2017;50:1423-32
44. Mi B, Xiong Y, Zhao Y, Lin Z, Lu L, Liu G. et al. Metal-Organic Framework-Integrated Composites for Bone Tissue Regeneration. Adv Funct Mater. 2024;34:2308656
45. Li J, Yan Y, Chen Y, Fang Q, Hussain MI, Wang L-N. Flexible Curcumin-Loaded Zn-MOF Hydrogel for Long-Term Drug Release and Antibacterial Activities. Int J Mol Sci. 2023;24:11439
46. Ma J, Yu H, Zhang X, Xu Z, Hu H, Liu J. et al. Dual-Targeted Metal Ion Network Hydrogel Scaffold for Promoting the Integrated Repair of Tendon-Bone Interfaces. ACS Appl Mater Interfaces. 2024;16:5582-97
47. Kang Y, Xu C, Meng L, Dong X, Qi M, Jiang D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact Mater. 2022;18:26-41
48. Shuai C, Zan J, Deng F, Yang Y, Peng S, Zhao Z. Core-shell-Structured ZIF-8@PDA-HA with Controllable Zinc Ion Release and Superior Bioactivity for Improving a Poly-l-lactic Acid Scaffold. ACS Sustain Chem Eng. 2021;9:1814-25
49. Karakeçili A, Topuz B, Ersoy FŞ, Şahin T, Günyakti A, Demirtaş TT. UiO-66 metal-organic framework as a double actor in chitosan scaffolds: Antibiotic carrier and osteogenesis promoter. Biomater Adv. 2022;136:212757
50. Mohanty B, Kumari S, Yadav P, Kanoo P, Chakraborty A. Metal-organic frameworks (MOFs) and MOF composites based biosensors. Coord Chem Rev. 2024;519:216102
51. Jiang Q, Xiao Y, Hong AN, Shen Y, Li Z, Feng P. et al. Highly Stable Fe/Co-TPY-MIL-88(NH2) Metal-Organic Framework (MOF) in Enzymatic Cascade Reactions for Chemiluminescence-Based Detection of Extracellular Vesicles. ACS Sens. 2023;8:1658-66
52. Bi W, Cao X, Li J, Gao Y, Song Y, He B. Ultrasensitive Detection of Extracellular Vesicles Based on Metal-Organic Framework DNA Biobarcodes Triggered G-Quadruplex Coupled with Rolling Circle Amplification Assay. ACS Sens. 2025;10:2136-46
53. Xia Q, Mu Z, Qing M, Zhou J, Bai L. A sandwich-type electrochemical immunosensor for sensitive detection of HER2 based on dual signal amplification of La-MOF-PbO and PEI-MoSNFs composites. Bioelectrochemistry. 2023;152:108431
54. Gu C, Bai L, Pu L, Gai P, Li F. Highly sensitive and stable self-powered biosensing for exosomes based on dual metal-organic frameworks nanocarriers. Biosens Bioelectron. 2021;176:112907
55. Qin X, Xiang Y, Mao L, Yang Y, Wei B, Lu H. et al. Buoyant Metal-Organic Framework Corona-Driven Fast Isolation and Ultrasensitive Profiling of Circulating Extracellular Vesicles. ACS Nano. 2024;18:14569-82
56. Sun Z, Wang L, Wu S, Pan Y, Dong Y, Zhu S. et al. An Electrochemical Biosensor Designed by Using Zr-Based Metal-Organic Frameworks for the Detection of Glioblastoma-Derived Exosomes with Practical Application. Anal Chem. 2020;92:3819-26
57. Qin X, Wei B, Xiang Y, Lu H, Liu F, Li X. et al. Exosome-tuned MOF signal amplifier boosting tumor exosome phenotyping with high-affinity nanostars. Biosens Bioelectron. 2024;245:115828
58. Jiao L, Sun Z, Sun Z, Liu J, Deng G, Wang X. Nanotechnology-based non-viral vectors for gene delivery in cardiovascular diseases. Front Bioeng Biotechnol. 2024;12:1349077
59. Han L, Zhang X-Y, Wang Y-L, Li X, Yang X-H, Huang M. et al. Redox-responsive theranostic nanoplatforms based on inorganic nanomaterials. J Controlled Release. 2017;259:40-52
60. Mohammadpour R, Dobrovolskaia MA, Cheney DL, Greish KF, Ghandehari H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv Drug Deliv Rev. 2019;144:112-32
61. Zhao L, Shen G, Ma G, Yan X. Engineering and delivery of nanocolloids of hydrophobic drugs. Adv Colloid Interface Sci. 2017;249:308-20
62. Wuttke S, Lismont M, Escudero A, Rungtaweevoranit B, Parak WJ. Positioning metal-organic framework nanoparticles within the context of drug delivery - A comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials. 2017;123:172-83
63. Eugster R, Luciani P. Liposomes: Bridging the gap from lab to pharmaceuticals. Curr Opin Colloid Interface Sci. 2025;75:101875
64. Cutrone G, Qiu J, Menendez-Miranda M, Casas-Solvas JM, Aykaç A, Li X. et al. Comb-like dextran copolymers: A versatile strategy to coat highly porous MOF nanoparticles with a PEG shell. Carbohydr Polym. 2019;223:115085
65. Salomon C, Das S, Erdbrügger U, Kalluri R, Kiang Lim S, Olefsky JM. et al. Extracellular Vesicles and Their Emerging Roles as Cellular Messengers in Endocrinology: An Endocrine Society Scientific Statement. Endocr Rev. 2022;43:441-68
66. Du Y, Wu L, Wang L, Reiter RJ, Lip GYH, Ren J. Extracellular vesicles in cardiovascular diseases: From pathophysiology to diagnosis and therapy. Cytokine Growth Factor Rev. 2023;74:40-55
67. Li P, Li J, Cheng J, Huang J, Li J, Xiao J. et al. Hypoxia-responsive liposome enhances intracellular delivery of photosensitizer for effective photodynamic therapy. J Controlled Release. 2025;377:277-87
68. Wang X, Wang R, Roy M, Wang Y, Qin B, Burgess DJ. Understanding drug release mechanisms of risperidone in situ forming implant depots in rabbits. J Controlled Release. 2025;383:113785
69. Liao X, Liu M, He M, Yuan C, Zhang Q, Wan Q. et al. Damage-Free Silica Coating for Colloidal Nanocrystals Through a Proactively Water-Generating Amidation Reaction at High Temperature. Small. 2024;20:2309902
70. Jia Y-P, Ma B-Y, Wei X-W, Qian Z-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin Chem Lett. 2017;28:691-702
71. Chen Z-Q, Tang T-T, Tang R-N, Zhang Y, Zhang Y-L, Yang H-B. et al. A comprehensive evaluation of stability and safety for HEK293F-derived extracellular vesicles as promising drug delivery vehicles. J Controlled Release. 2025;382:113673
72. Maiti S, Manna S, Shen J, Esser-Kahn AP, Du W. Mitigation of Hydrophobicity-Induced Immunotoxicity by Sugar Poly(orthoesters). J Am Chem Soc. 2019;141:4510-4
73. Pizzoli G, Gargaro M, Drava G, Voliani V. Inorganic Nanomaterials Meet the Immune System: An Intricate Balance. Adv Healthc Mater. 2025;14:2404795
74. Setua S, Shabir S, Shaji P, Bulnes AM, Dhasmana A, Holla S. et al. Exosomes derived from tumor adjacent fibroblasts efficiently target pancreatic tumors. Acta Pharm Sin B. 2024;14:3009-26
75. Zhao D, Zhang W, Yu S, Xia S-L, Liu Y-N, Yang G-J. Application of MOF-based nanotherapeutics in light-mediated cancer diagnosis and therapy. J Nanobiotechnology. 2022;20:421
76. Park J, Choi Y, Chang H, Um W, Ryu JH, Kwon IC. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics. 2019;9:8073-90
77. Guo H, Li X, Mao D, Wang H, Wei L, Qu D. et al. Homologous-magnetic dual-targeted metal-organic framework to improve the anti-hepatocellular carcinoma efficacy of PD-1 inhibitor. J Nanobiotechnology. 2024;22:206
78. Du H, Meng S, Geng M, Zhao P, Gong L, Zheng X. et al. Detachable MOF-Based Core/Shell Nanoreactor for Cancer Dual-Starvation Therapy With Reversing Glucose and Glutamine Metabolisms. Small. 2023;19:2303253
79. Sancho-Albero M, Encabo-Berzosa M del M, Beltrán-Visiedo M, Fernández-Messina L, Sebastián V, Sánchez-Madrid F. et al. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale. 2019;11:18825-36
80. Cao M, Diao N, Cai X, Chen X, Xiao Y, Guo C. et al. Plant exosome nanovesicles (PENs): green delivery platforms. Mater Horiz. 2023;10:3879-94
81. Cocozza F, Grisard E, Martin-Jaular L, Mathieu M, Théry C. SnapShot: Extracellular Vesicles. Cell. 2020;182:262-262.e1
82. Xie J, Li Q, Haesebrouck F, Van Hoecke L, Vandenbroucke RE. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 2022;40:1173-94
83. Zhu L, Sun H-T, Wang S, Huang S-L, Zheng Y, Wang C-Q. et al. Isolation and characterization of exosomes for cancer research. J Hematol OncolJ Hematol Oncol. 2020;13:152
84. Ding L, Yang X, Gao Z, Effah CY, Zhang X, Wu Y. et al. A Holistic Review of the State-of-the-Art Microfluidics for Exosome Separation: An Overview of the Current Status, Existing Obstacles, and Future Outlook. Small. 2021;17:2007174
85. Wu G, Zhang Y, Jia S, Qi X, Feng X, Ren Y. et al. Preparation of Dysprosium(III)-Metal Organic Framework Nanofiber for Exosome Capture and Biomarker Discovery toward Liver Disease. ACS Appl Mater Interfaces. 2024;16:56874-83
86. Zhou J, Cheng X, Guo Z, Ali MM, Zhang G, Tao WA. et al. Epitope Imprinting of Phospholipids by Oriented Assembly at the Oil/Water Interface for the Selective Recognition of Plasma Membranes. Angew Chem Int Ed. 2023;62:e202213938
87. Feng X, Shen A, Zhang W, Jia S, Iliuk A, Wang Y. et al. High-throughput capture and in situ protein analysis of extracellular vesicles by chemical probe-based array. Nat Protoc. 2025;20:1057-81
88. Liu S, Xiao X, Zhang L, Wang J, Zhao W, Liu H. et al. Reprogramming Exosomes to Escape from Immune Surveillance for Mitochondrial Protection in Hepatic Ischemia-Reperfusion Injury. Theranostics. 2024;14:116-32
89. Magoling BJA, Wu AY-T, Chen Y-J, Wong WW-T, Chuo ST-Y, Huang H-C. et al. Membrane Protein Modification Modulates Big and Small Extracellular Vesicle Biodistribution and Tumorigenic Potential in Breast Cancers In Vivo. Adv Mater. 2023;35:2208966
90. Altıntaş Ö, Saylan Y. Exploring the Versatility of Exosomes: A Review on Isolation, Characterization, Detection Methods, and Diverse Applications. Anal Chem. 2023;95:16029-48
91. Zheng W, Meng Z, Zhu Z, Wang X, Xu X, Zhang Y. et al. Metal-Organic Framework-Based Nanomaterials for Regulation of the Osteogenic Microenvironment. Small. 2024;20:2310622
92. Lv M, Zhou W, Tavakoli H, Bautista C, Xia J, Wang Z. et al. Aptamer-functionalized metal-organic frameworks (MOFs) for biosensing. Biosens Bioelectron. 2021;176:112947
93. Wang Z, Chen J, Gao R, Jiang L, Zhang G, Zhao Y. et al. Spatiotemporal manipulation metal-organic frameworks as oral drug delivery systems for precision medicine. Coord Chem Rev. 2024;502:215615
94. Kuang J, Zhao L, Ruan S, Sun Y, Wu Z, Zhang H. et al. The integration platform for exosome capture and colorimetric detection: Site occupying effect-modulated MOF-aptamer interaction and aptamer-Au NPs-dopamine interaction. Anal Chim Acta. 2024;1329:343234
95. Wang D, Yao H, Ye J, Gao Y, Cong H, Yu B. Metal-Organic Frameworks (MOFs): Classification, Synthesis, Modification, and Biomedical Applications. Small. 2024;20:2404350
96. Chen J, Zhou Q, Cao W. Multifunctional Porphyrin-Based Sonosensitizers for Sonodynamic Therapy. Adv Funct Mater. 2024;34:2405844
97. Bottari G, de la Torre G, Guldi DM, Torres T. An exciting twenty-year journey exploring porphyrinoid-based photo- and electro-active systems. Coord Chem Rev. 2021;428:213605
98. Kong X-J, Li J-R. An Overview of Metal-Organic Frameworks for Green Chemical Engineering. Engineering. 2021;7:1115-39
99. Bustamante EL, Fernández JL, Zamaro JM. Influence of the solvent in the synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals at room temperature. J Colloid Interface Sci. 2014;424:37-43
100. Zhao C, Shu C, Yu J, Zhu Y. Metal-organic frameworks functionalized biomaterials for promoting bone repair. Mater Today Bio. 2023;21:100717
101. Sun Z, Yang J, Li H, Wang C, Fletcher C, Li J. et al. Progress in the research of nanomaterial-based exosome bioanalysis and exosome-based nanomaterials tumor therapy. Biomaterials. 2021;274:120873
102. Fathi P, Rao L, Chen X. Extracellular vesicle-coated nanoparticles. VIEW. 2021;2:20200187
103. Lu M, Huang Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials. 2020;242:119925
104. Wang Z, Rich J, Hao N, Gu Y, Chen C, Yang S. et al. Acoustofluidics for simultaneous nanoparticle-based drug loading and exosome encapsulation. Microsyst Nanoeng. 2022;8:45
105. Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL. et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine Nanotechnol Biol Med. 2016;12:655-64
106. Wang Y, Zhang D, Jia M, Zheng X, Liu Y, Wang C. et al. ZIF-8 nanoparticles coated with macrophage-derived microvesicles for sustained, targeted delivery of dexamethasone to arthritic joints. J Drug Target. 2022;30:1006-16
107. Wang X, Ding Q, Groleau RR, Wu L, Mao Y, Che F. et al. Fluorescent Probes for Disease Diagnosis. Chem Rev. 2024;124:7106-64
108. Singh R, Musameh M, Gao Y, Ozcelik B, Mulet X, Doherty CM. Stable MOF@enzyme composites for electrochemical biosensing devices. J Mater Chem C. 2021;9:7677-88
109. Hira SA, Nagappan S, Annas D, Kumar YA, Park KH. NO2-functionalized metal-organic framework incorporating bimetallic alloy nanoparticles as a sensor for efficient electrochemical detection of dopamine. Electrochem Commun. 2021;125:107012
110. Liu Y-G, Jiang S-T, Zhang J-W, Zheng H, Zhang L, Zhao H-T. et al. Role of extracellular vesicle-associated proteins in the progression, diagnosis, and treatment of hepatocellular carcinoma. Cell Biosci. 2024;14:113
111. Amrhein K, Taylor ML, Wilson R, Gallops CE, Annamer A, Vinduska V. et al. Dual Imaging Single Vesicle Surface Protein Profiling and Early Cancer Detection. ACS Appl Mater Interfaces. 2023;15:2679-92
112. Zhang X, Cui H, Zhang W, Li Z, Gao J. Engineered tumor cell-derived vaccines against cancer: The art of combating poison with poison. Bioact Mater. 2023;22:491-517
113. Pan W-L, Feng J-J, Luo T-T, Tan Y, Situ B, Nieuwland R. et al. Rapid and efficient isolation platform for plasma extracellular vesicles: EV-FISHER. J Extracell Vesicles. 2022;11:e12281
114. Diekhoff T, Greese J, Krohn M, Huppertz A, Hamm B, Hermann K-G. OP0050 Erosion Detection on the Si-Joints - A Comparison between X-Ray, Low Dose CT and MRI Including High Resolution Sequences. Ann Rheum Dis. 2014;73:79-80
115. Lin L, Wang LV. The emerging role of photoacoustic imaging in clinical oncology. Nat Rev Clin Oncol. 2022;19:365-84
116. Tan B, Zhao C, Wang J, Tiemuer A, Zhang Y, Yu H. et al. Rational design of pH-activated upconversion luminescent nanoprobes for bioimaging of tumor acidic microenvironment and the enhancement of photothermal therapy. Acta Biomater. 2023;155:554-63
117. Jiang X, Zhou Q, Du B, Li S, Huang Y, Chi Z. et al. Noninvasive monitoring of hepatic glutathione depletion through fluorescence imaging and blood testing. Sci Adv. 2021;7:eabd9847
118. Kong X-J, Ji X, He T, Xie L-H, Zhang Y-Z, Lv H. et al. A Green-Emission Metal-Organic Framework-Based Nanoprobe for Imaging Dual Tumor Biomarkers in Living Cells. ACS Appl Mater Interfaces. 2020;12:35375-84
119. Zeng L, Huang L, Han G. Dye Doped Metal-Organic Frameworks for Enhanced Phototherapy. Adv Drug Deliv Rev. 2022;189:114479
120. Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108-11
121. Mo R, Jiang T, DiSanto R, Tai W, Gu Z. ATP-triggered anticancer drug delivery. Nat Commun. 2014;5:3364
122. Zhao H, Dou L, Ren J, Cui M, Li N, Ji X. et al. MOF-derived porous Co3O4 coupled with AuNPs and nucleic acids as electrocatalysis signal probe for sensitive electrochemical aptasensing of adenosine triphosphate. Sens Actuators B Chem. 2022;362:131753
123. Lv W, Han Z, Li Y, Huang Y, Sun J, Lu X. et al. Exosome-Coated Zeolitic Imidazolate Framework Nanoparticles for Intracellular Detection of ATP†. Chin J Chem. 2021;39:2107-12
124. Liu Q, Zou J, Chen Z, He W, Wu W. Current research trends of nanomedicines. Acta Pharm Sin B. 2023;13:4391-416
125. Cheng L, Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022;21:379-99
126. Liu J, Xi Z, Fan C, Mei Y, Zhao J, Jiang Y. et al. Hydrogels for Nucleic Acid Drugs Delivery. Adv Healthc Mater. 2024;13:2401895
127. Scherphof GL, Kamps JAAM. Receptor versus non-receptor mediated clearance of liposomes. Adv Drug Deliv Rev. 1998;32:81-97
128. Li L, Zhang Y, Mu J, Chen J, Zhang C, Cao H. et al. Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury. Nano Lett. 2020;20:4298-305
129. Li J, Ma W, Ren X, Guo T, Yang B, Huang C. et al. Supramolecular Nano-Grid Platform to Load and Deliver Liposomes and Exosomes. Small Struct. 2024;5:2300487
130. Song H, Liu D, Dong S, Zeng L, Wu Z, Zhao P. et al. Epitranscriptomics and epiproteomics in cancer drug resistance: therapeutic implications. Signal Transduct Target Ther. 2020;5:193
131. Goydel RS, Rader C. Antibody-based cancer therapy. Oncogene. 2021;40:3655-64
132. Cheng L, Yang L, Meng F, Zhong Z. Protein Nanotherapeutics as an Emerging Modality for Cancer Therapy. Adv Healthc Mater. 2018;7:1800685
133. Zhang Q, Kuang G, Li W, Wang J, Ren H, Zhao Y. Stimuli-Responsive Gene Delivery Nanocarriers for Cancer Therapy. Nano-Micro Lett. 2023;15:44
134. Yang M, Wang X, Peng M, Wang F, Hou S, Xing R. et al. Nanomaterials Enhanced Sonodynamic Therapy for Multiple Tumor Treatment. Nano-Micro Lett. 2025;17:157
135. Illes B, Hirschle P, Barnert S, Cauda V, Wuttke S, Engelke H. Exosome-Coated Metal-Organic Framework Nanoparticles: An Efficient Drug Delivery Platform. Chem Mater. 2017;29:8042-6
136. Baati T, Njim L, Neffati F, Kerkeni A, Bouttemi M, Gref R. et al. In depth analysis of the in vivo toxicity of nanoparticles of porous iron(III) metal-organic frameworks. Chem Sci. 2013;4:1597-607
137. Anees M, Tiwari S, Mehrotra N, Kharbanda S, Singh H. Development and evaluation of PLA based hybrid block copolymeric nanoparticles for systemic delivery of pirarubicin as an anti-cancer agent. Int J Pharm. 2022;620:121761
138. Phungula A, Zuffi S, Thongsom S, Gianvincenzo PD, Reyes SG, Valle ABC dos S. et al. Lauryl-NrTP6 lipopeptide self-assembled nanorods for nuclear-targeted delivery of doxorubicin. Nanoscale. 2025;17:4659-69
139. Cheng G, Li W, Ha L, Han X, Hao S, Wan Y. et al. Self-Assembly of Extracellular Vesicle-like Metal-Organic Framework Nanoparticles for Protection and Intracellular Delivery of Biofunctional Proteins. J Am Chem Soc. 2018;140:7282-91
140. Belhadj Z, He B, Deng H, Song S, Zhang H, Wang X. et al. A combined "eat me/don't eat me" strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9:1806444
141. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503
142. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522-31
143. Sengupta D, Deb M, Kar S, Pradhan N, Parbin S, Kirtana R. et al. Dissecting miRNA facilitated physiology and function in human breast cancer for therapeutic intervention. Semin Cancer Biol. 2021;72:46-64
144. Cuciniello R, Filosa S, Crispi S. Novel approaches in cancer treatment: preclinical and clinical development of small non-coding RNA therapeutics. J Exp Clin Cancer Res. 2021;40:383
145. Adams BD, Wali VB, Cheng CJ, Inukai S, Booth CJ, Agarwal S. et al. miR-34a Silences c-SRC to Attenuate Tumor Growth in Triple-Negative Breast Cancer. Cancer Res. 2016;76:927-39
146. Bayraktar R, Ivan C, Bayraktar E, Kanlikilicer P, Kabil NN, Kahraman N. et al. Dual Suppressive Effect of miR-34a on the FOXM1/eEF2-Kinase Axis Regulates Triple-Negative Breast Cancer Growth and Invasion. Clin Cancer Res. 2018;24:4225-41
147. Ganju A, Khan S, Hafeez BB, Behrman SW, Yallapu MM, Chauhan SC. et al. miRNA nanotherapeutics for cancer. Drug Discov Today. 2017;22:424-32
148. Shou X, Chen C, Ying H, Liu Z, Zeng L, Li Q. et al. Biomimetic MOF Nanocarrier-Mediated Synergistic Delivery of Mitochondria and Anti-Inflammatory miRNA to Alleviate Acute Lung Injury. Adv Sci. 2025;12:2416594
149. Angelos S, Khashab NM, Yang Y-W, Trabolsi A, Khatib HA, Stoddart JF. et al. pH Clock-Operated Mechanized Nanoparticles. J Am Chem Soc. 2009;131:12912-4
150. Xu C, Haque F, Jasinski DL, Binzel DW, Shu D, Guo P. Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy. Cancer Lett. 2018;414:57-70
151. Peng S, Liu J, Qin Y, Wang H, Cao B, Lu L. et al. Metal-Organic Framework Encapsulating Hemoglobin as a High-Stable and Long-Circulating Oxygen Carriers to Treat Hemorrhagic Shock. ACS Appl Mater Interfaces. 2019;11:35604-12
152. Li Y, Zhao R, Cheng K, Zhang K, Wang Y, Zhang Y. et al. Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition. ACS Nano. 2020;14:16698-711
153. Gujrati V, Kim S, Kim S-H, Min JJ, Choy HE, Kim SC. et al. Bioengineered Bacterial Outer Membrane Vesicles as Cell-Specific Drug-Delivery Vehicles for Cancer Therapy. ACS Nano. 2014;8:1525-37
154. Cui C, He Q, Wang J, Kang J, Ma W, Nian Y. et al. Targeted miR-34a delivery with PD1 displayed bacterial outer membrane vesicles-coated zeolitic imidazolate framework nanoparticles for enhanced tumor therapy. Int J Biol Macromol. 2023;247:125692
155. Lv H, Zong Q, Chen C, Lv G, Xiang W, Xing F. et al. TET2-mediated tumor cGAS triggers endothelial STING activation to regulate vasculature remodeling and anti-tumor immunity in liver cancer. Nat Commun. 2024;15:6
156. Zhang H, Liu K, Gong Y, Zhu W, Zhu J, Pan F. et al. Vitamin C supramolecular hydrogel for enhanced cancer immunotherapy. Biomaterials. 2022;287:121673
157. Ma Z, Yang M, Foda MF, Zhang K, Li S, Liang H. et al. Polarization of Tumor-Associated Macrophages Promoted by Vitamin C-Loaded Liposomes for Cancer Immunotherapy. ACS Nano. 2022;16:17389-401
158. Shenoy N, Creagan E, Witzig T, Levine M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell. 2018;34:700-6
159. Gulla A, Morelli E, Hideshima T, Samur MK, Bianchi G, Fulciniti M. et al. Optimizing Mechanisms for Induction of Immunogenic Cell Death to Improve Patient Outcome in Multiple Myeloma. Blood. 2018;132:1932
160. Yao W, Liu W, Su F, Wang J, Li H, Sun M. et al. Hybrid Membrane-Camouflaged Biomimetic Immunomodulatory Nanoturrets with Sequential Multidrug Release for Potentiating T Cell-Mediated Anticancer Immunity. J Am Chem Soc. 2024;146:18592-605
161. Zhang Y, Zhang X, Yang H, Yu L, Xu Y, Sharma A. et al. Advanced biotechnology-assisted precise sonodynamic therapy. Chem Soc Rev. 2021;50:11227-48
162. Zhou X-Q, Wang P, Ramu V, Zhang L, Jiang S, Li X. et al. In vivo metallophilic self-assembly of a light-activated anticancer drug. Nat Chem. 2023;15:980-7
163. Guo Q-L, Dai X-L, Yin M-Y, Cheng H-W, Qian H-S, Wang H. et al. Nanosensitizers for sonodynamic therapy for glioblastoma multiforme: current progress and future perspectives. Mil Med Res. 2022;9:26
164. Wu T, Liu Y, Cao Y, Liu Z. Engineering Macrophage Exosome Disguised Biodegradable Nanoplatform for Enhanced Sonodynamic Therapy of Glioblastoma. Adv Mater. 2022;34:2110364
165. McInnes IB, Schett G. The Pathogenesis of Rheumatoid Arthritis. N Engl J Med. 2011;365:2205-19
166. Bullock J, Rizvi SAA, Saleh AM, Ahmed SS, Do DP, Ansari RA. et al. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med Princ Pract. 2018;27:501-7
167. Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky DS, Naredo E. et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016;75:1428-37
168. Wang Y, Jia M, Zheng X, Wang C, Zhou Y, Pan H. et al. Microvesicle-camouflaged biomimetic nanoparticles encapsulating a metal-organic framework for targeted rheumatoid arthritis therapy. J Nanobiotechnology. 2022;20:253
169. Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B. et al. Ulcerative colitis. Nat Rev Dis Primer. 2020;6:74
170. Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. The Lancet. 2023;402:571-84
171. Eisenstein M. Gut reaction. Nature. 2018;563:S34-5
172. Hirten RP, Sands BE. New Therapeutics for Ulcerative Colitis. Annu Rev Med. 2021;72:199-213
173. Cui C, Du M, Zhao Y, Tang J, Liu M, Min G. et al. Functional Ginger-Derived Extracellular Vesicles-Coated ZIF-8 Containing TNF-α siRNA for Ulcerative Colitis Therapy by Modulating Gut Microbiota. ACS Appl Mater Interfaces. 2024;16:53460-73
174. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314-21
175. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK. et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-71
176. Xiang Y, Zhuge P, Qi X, Ge X, Xiang J, Xu H. et al. A cuttlefish ink nanoparticle-reinforced biopolymer hydrogel with robust adhesive and immunomodulatory features for treating oral ulcers in diabetes. Bioact Mater. 2024;39:562-81
177. Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R. et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6:15
178. Ha DH, Kim H, Lee J, Kwon HH, Park G-H, Yang SH. et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells. 2020;9:1157
179. Meng Z, Zhou D, Gao Y, Zeng M, Wang W. miRNA delivery for skin wound healing. Adv Drug Deliv Rev. 2018;129:308-18
180. An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y. et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54:e12993
181. Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW. et al. Dynamic Biodistribution of Extracellular Vesicles in Vivo Using a Multimodal Imaging Reporter. ACS Nano. 2014;8:483-94
182. Pan W, Wang W, Wang P, Chen D, Liu S, Zhang L. et al. Sustained delivery of extracellular vesicles using UiO-66-NH2 crosslinked hydrogel for accelerating chronic diabetic wound-healing. Mater Des. 2024;238:112688
183. Ge W, Gao Y, Zeng Y, Yu Y, Xie X, Liu L. Silk Fibroin Microneedles Loaded with Lipopolysaccharide-Pretreated Bone Marrow Mesenchymal Stem Cell-Derived Exosomes for Oral Ulcer Treatment. ACS Appl Mater Interfaces. 2024;16:37486-96
184. Huang Y, Peng T, Hu W, Gao X, Chen Y, Zhang Q. et al. Fully armed photodynamic therapy with spear and shear for topical deep hypertrophic scar treatment. J Controlled Release. 2022;343:408-19
185. Han XB, Li HX, Jiang YQ, Wang H, Li XS, Kou JY. et al. Upconversion nanoparticle-mediated photodynamic therapy induces autophagy and cholesterol efflux of macrophage-derived foam cells via ROS generation. Cell Death Dis. 2017;8:e2864-e2864
186. Kong T, Zhang K, Wang Y, Ye Y, Hou J, Xu C. et al. Cucumber-Derived Extracellular Vesicle-Functionalized Metal-Organic Frameworks for Enhanced Photodynamic Therapy of Hypertrophic Scars. Adv Funct Mater. 2024;34:2400379
187. Chen S, Lu J, You T, Sun D. Metal-organic frameworks for improving wound healing. Coord Chem Rev. 2021;439:213929
188. Barry BW. Breaching the skin's barrier to drugs. Nat Biotechnol. 2004;22:165-7
189. Zhai Y, Zhai G. Advances in lipid-based colloid systems as drug carrier for topic delivery. J Controlled Release. 2014;193:90-9
190. Zhang J, Tong D, Song H, Ruan R, Sun Y, Lin Y. et al. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv Mater. 2022;34:2202044
191. Stegen S, Carmeliet G. The skeletal vascular system - Breathing life into bone tissue. Bone. 2018;115:50-8
192. Diomede F, Marconi GD, Fonticoli L, Pizzicanella J, Merciaro I, Bramanti P. et al. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int J Mol Sci. 2020;21:3242
193. Li Y, Zhu J, Zhang X, Li Y, Zhang S, Yang L. et al. Drug-Delivery Nanoplatform with Synergistic Regulation of Angiogenesis-Osteogenesis Coupling for Promoting Vascularized Bone Regeneration. ACS Appl Mater Interfaces. 2023;15:17543-61
194. Nagelkerke A, Ojansivu M, van der Koog L, Whittaker TE, Cunnane EM, Silva AM. et al. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv Drug Deliv Rev. 2021;175:113775
195. Xin L, Lin X, Zhou F, Li C, Wang X, Yu H. et al. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020;113:252-66
196. Zhou Y, Liu S, Zhao M, Wang C, Li L, Yuan Y. et al. Injectable extracellular vesicle-released self-assembling peptide nanofiber hydrogel as an enhanced cell-free therapy for tissue regeneration. J Controlled Release. 2019;316:93-104
197. Liu Y, Zhu Z, Pei X, Zhang X, Cheng X, Hu S. et al. ZIF-8-Modified Multifunctional Bone-Adhesive Hydrogels Promoting Angiogenesis and Osteogenesis for Bone Regeneration. ACS Appl Mater Interfaces. 2020;12:36978-95
198. Yoshizawa S, Brown A, Barchowsky A, Sfeir C. Role of magnesium ions on osteogenic response in bone marrow stromal cells. Connect Tissue Res. 2014;55:155-9
199. Qi T, Weng J, Yu F, Zhang W, Li G, Qin H. et al. Insights into the Role of Magnesium Ions in Affecting Osteogenic Differentiation of Mesenchymal Stem Cells. Biol Trace Elem Res. 2021;199:559-67
200. Lin Z, Shen D, Zhou W, Zheng Y, Kong T, Liu X. et al. Regulation of extracellular bioactive cations in bone tissue microenvironment induces favorable osteoimmune conditions to accelerate in situ bone regeneration. Bioact Mater. 2021;6:2315-30
201. Liu C, Yang G, Zhou M, Zhang X, Wu X, Wu P. et al. Magnesium Ammonium Phosphate Composite Cell-Laden Hydrogel Promotes Osteogenesis and Angiogenesis In Vitro. ACS Omega. 2021;6:9449-59
202. Zhang X, Wang Y, Liu J, Shi J, Mao D, Midgley AC. et al. A metal-organic-framework incorporated vascular graft for sustained nitric oxide generation and long-term vascular patency. Chem Eng J. 2021;421:129577
203. Yang J, Huang Z, Tan J, Pan J, Chen S, Wan W. Copper ion/gallic acid MOFs-laden adhesive pomelo peel sponge effectively treats biofilm-infected skin wounds and improves healing quality. Bioact Mater. 2024;32:260-76
204. Hao C, Huang L, Zhang H, Xu L, Sun M, Kuang H. et al. Chiral CuxOS@Fe-MOFs for Enhanced Cancer Therapy. Adv Funct Mater. 2024;34:2312795
205. Xu C, Kang Y, Dong X, Jiang D, Qi M. Integration exosomes with MOF-modified multifunctional scaffold for accelerating vascularized bone regeneration. Chin Chem Lett. 2023;34:107528
206. Ayoub Moubareck C, Hammoudi Halat D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics. 2020;9:119
207. Antunes LCS, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71:292-301
208. Fiester SE, Arivett BA, Beckett AC, Wagner BR, Ohneck EJ, Schmidt RE. et al. Miltefosine Reduces the Cytolytic Activity and Virulence of Acinetobacter baumannii. Antimicrob Agents Chemother. 2018;63:10.1128 /aac.01409-18
209. Frei A, Verderosa AD, Elliott AG, Zuegg J, Blaskovich MAT. Metals to combat antimicrobial resistance. Nat Rev Chem. 2023;7:202-24
210. Kumari S, Howlett TS, Ehrman RN, Koirala S, Trashi O, Trashi I. et al. In vivo biocompatibility of ZIF-8 for slow release via intranasal administration. Chem Sci. 2023;14:5774-82
211. Frei A, Zuegg J, Elliott AG, Baker M, Braese S, Brown C. et al. Metal complexes as a promising source for new antibiotics. Chem Sci. 2020;11:2627-39
212. Yuan S, Zhu Y, Dai Y, Wang Y, Jin D, Liu M. et al. 19F NMR Allows the Investigation of the Fate of Platinum(IV) Prodrugs in Physiological Conditions. Angew Chem Int Ed. 2022;61:e202114250
213. Wei X, Xue B, Ruan S, Guo J, Huang Y, Geng X. et al. Supercharged precision killers: Genetically engineered biomimetic drugs of screened metalloantibiotics against Acinetobacter baumanni. Sci Adv. 2024;10:eadk6331
214. Wang Y, Guo W, Zhang K, Liu Z, Dai X, Qiao Z. et al. Biomimetic Electrodynamic Metal-Organic Framework Nanosponges for Augmented Treatment of Biofilm Infections. Adv Sci. 2024;11:2408442
215. Fan X, Yang F, Nie C, Ma L, Cheng C, Haag R. Biocatalytic Nanomaterials: A New Pathway for Bacterial Disinfection. Adv Mater. 2021;33:2100637
216. Tan H, Wang S, He X, Yang G, Zhu Y, Yang S. et al. Microneedles Loaded with Nitric-Oxide Driven Nanomotors Improve Force-Induced Efferocytosis Impairment and Sterile Inflammation by Revitalizing Macrophage Energy Metabolism. ACS Nano. 2025;19:9390-411
217. Wu X, Qi M, Liu C, Yang Q, Li S, Shi F. et al. Near-infrared light-triggered nitric oxide nanocomposites for photodynamic/photothermal complementary therapy against periodontal biofilm in an animal model. Theranostics. 2023;13:2350-67
218. Wan S, Liu W, Wu Q, Wang K, Li Y, Huang P. et al. Nanobiohybrid Extracellular Vesicle Nanoreactor with Improving Metabolical Activity for Biocatalytic Therapy. ACS Nano. 2024;18:32899-909
219. Wu S, Liu X, Yeung KWK, Liu C, Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater Sci Eng R Rep. 2014;80:1-36
220. Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16:397-409
221. Amin Yavari S, Castenmiller SM, van Strijp JAG, Croes M. Combating Implant Infections: Shifting Focus from Bacteria to Host. Adv Mater. 2020;32:2002962
222. Li S, Yue Y, Wang W, Han M, Wan X, Li Q. et al. Ultrasound-Activated Probiotics Vesicles Coating for Titanium Implant Infections Through Bacterial Cuproptosis-Like Death and Immunoregulation. Adv Mater. 2024;36:2405953
223. Liang G, Cao W, Tang D, Zhang H, Yu Y, Ding J. et al. Nanomedomics. ACS Nano. 2024;18:10979-1024
224. Wang X, Jiang W, Du Y, Zhu D, Zhang J, Fang C. et al. Targeting feedback activation of signaling transduction pathways to overcome drug resistance in cancer. Drug Resist Updat. 2022;65:100884
225. Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M. et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. 2022;21:144
226. Miscianinov V, Martello A, Rose L, Parish E, Cathcart B, Mitić T. et al. MicroRNA-148b Targets the TGF-β Pathway to Regulate Angiogenesis and Endothelial-to-Mesenchymal Transition during Skin Wound Healing. Mol Ther. 2018;26:1996-2007
227. Zhao Y, Liu J, Sun L, Liu H, Chen X, Deng X. et al. Zinc-doped curcumin carbon dots promote infected wound healing with photodynamic via the VEGF signaling pathway. J Nanobiotechnology. 2025;23:424
228. Vermeulen S, Tahmasebi Birgani Z, Habibovic P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials. 2022;283:121431
229. Lin Y, Deng H, Deng F, Yao S, Deng X, Cheng Y. et al. Remodeling of intestinal epithelium derived extracellular vesicles by nanoparticles and its bioeffect on tumor cell migration. J Controlled Release. 2024;365:60-73
230. Wan C, Mahara S, Sun C, Doan A, Chua HK, Xu D. et al. Genome-scale CRISPR-Cas9 screen of Wnt/β-catenin signaling identifies therapeutic targets for colorectal cancer. Sci Adv. 2021;7:eabf2567
231. Pan S, Yin L, Liu J, Tong J, Wang Z, Zhao J. et al. Metabolomics-driven approaches for identifying therapeutic targets in drug discovery. MedComm. 2024;5:e792
232. Han B. et al. Integrated multi-omics reveal gut microbiota-mediated bile acid metabolism alteration regulating immunotherapy responses to anti-α4β7-integrin in Crohn's disease. Gut Microbes. 2024;16:2310894
233. Wu Q, Tan L, Ren X, Fu C, Chen Z, Ren J. et al. Metal-Organic Framework-Based Nano-Activators Facilitating Microwave Combined Therapy via a Divide-and-Conquer Tactic for Triple-Negative Breast Cancer. ACS Nano. 2023;17:25575-90
234. Qin Q, Li M, Fan L, Zeng X, Zheng D, Wang H. et al. RVG engineered extracellular vesicles-transmitted miR-137 improves autism by modulating glucose metabolism and neuroinflammation. Mol Psychiatry. 2025:1-13
235. Ren J, Jing X, Liu Y, Liu J, Ning X, Zong M. et al. Exosome-based engineering strategies for the diagnosis and treatment of oral and maxillofacial diseases. J Nanobiotechnology. 2023;21:501
236. Watson DC, Yung BC, Bergamaschi C, Chowdhury B, Bear J, Stellas D. et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles. 2018;7:1442088
237. Bai J, Wei X, Zhang X, Wu C, Wang Z, Chen M. et al. Microfluidic strategies for the isolation and profiling of exosomes. TrAC Trends Anal Chem. 2023;158:116834
238. Achenbach B, Yurdusen A, Stock N, Maurin G, Serre C. Synthetic Aspects and Characterization Needs in MOF Chemistry - from Discovery to Applications. Adv Mater. 2025 2411359
239. Zhuo Z, Wang J, Luo Y, Zeng R, Zhang C, Zhou W. et al. Targeted extracellular vesicle delivery systems employing superparamagnetic iron oxide nanoparticles. Acta Biomater. 2021;134:13-31
240. García-Manrique P, Gutiérrez G, Blanco-López MC. Fully Artificial Exosomes: Towards New Theranostic Biomaterials. Trends Biotechnol. 2018;36:10-4
241. Mondal J, Pillarisetti S, Junnuthula V, Saha M, Hwang SR, Park I. et al. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J Controlled Release. 2023;353:1127-49
242. Sun J, Li G, Wu S, Zou Y, Weng W, Gai T. et al. Engineering preparation and sustained delivery of bone functional exosomes-laden biodegradable hydrogel for in situ bone regeneration. Compos Part B Eng. 2023;261:110803
243. Zhou H, Yuen PST, Pisitkun T, Gonzales PA, Yasuda H, Dear JW. et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006;69:1471-6
244. Liu A, Lin D, Zhao H, Chen L, Cai B, Lin K. et al. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272:120718
245. Görgens A, Corso G, Hagey DW, Jawad Wiklander R, Gustafsson MO, Felldin U. et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J Extracell Vesicles. 2022;11:e12238
246. Adamo G, Picciotto S, Gargano P, Paterna A, Raccosta S, Rao E. et al. DetectEV: A functional enzymatic assay to assess integrity and bioactivity of extracellular vesicles. J Extracell Vesicles. 2025;14:e70030
247. McAndrews KM, Kalluri R. Nischarin Regulates Secretion of Exosomes and Cancer Progression. Cancer Res. 2019;79:2099-101
248. Namee NM, O'Driscoll L. Extracellular vesicles and anti-cancer drug resistance. Biochim Biophys Acta BBA - Rev Cancer. 2018;1870:123-36
249. Gao P, Chen Y, Pan W, Li N, Liu Z, Tang B. Antitumor Agents Based on Metal-Organic Frameworks. Angew Chem Int Ed. 2021;60:16763-76
250. Raza A, Wu W. Metal-organic frameworks in oral drug delivery. Asian J Pharm Sci. 2024;19:100951
251. Mendes RF, Figueira F, Leite JP, Gales L, Paz FAA. Metal-organic frameworks: a future toolbox for biomedicine? Chem Soc Rev. 2020;49:9121-53
252. Si Y, Luo H, Zhang P, Zhang C, Li J, Jiang P. et al. CD-MOFs: From preparation to drug delivery and therapeutic application. Carbohydr Polym. 2024;323:121424
253. Oh JY, Sim Y, Yang G, Park M-H, Kim K, Ryu J-H. Surface functionalization of metal-organic framework nanoparticle for overcoming biological barrier in cancer therapy. Inorg Chem Front. 2024;11:3119-35
254. Tanziela T, Shaikh S, Jiang H, Lu Z, Wang X. Efficient encapsulation of biocompatible nanoparticles in exosomes for cancer theranostics. Nano Today. 2020;35:100964
255. Tian J, Song X, Wang Y, Cheng M, Lu S, Xu W. et al. Regulatory perspectives of combination products. Bioact Mater. 2022;10:492-503
256. Takakura Y, Hanayama R, Akiyoshi K, Futaki S, Hida K, Ichiki T. et al. Quality and Safety Considerations for Therapeutic Products Based on Extracellular Vesicles. Pharm Res. 2024;41:1573-94
257. Giovannelli L, Bari E, Jommi C, Tartara F, Armocida D, Garbossa D. et al. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioact Mater. 2023;29:16-35
258. Sharifi S, Mahmoud NN, Voke E, Landry MP, Mahmoudi M. Importance of Standardizing Analytical Characterization Methodology for Improved Reliability of the Nanomedicine Literature. Nano-Micro Lett. 2022;14:172
259. Beetler DJ, Di Florio DN, Law EW, Groen CM, Windebank AJ, Peterson QP. et al. The evolving regulatory landscape in regenerative medicine. Mol Aspects Med. 2023;91:101138
260. Warraich HJ, Tazbaz T, Califf RM. FDA Perspective on the Regulation of Artificial Intelligence in Health Care and Biomedicine. JAMA. 2025;333:241-7
261. Lin X, Zhu J, Shen J, Zhang Y, Zhu J. Advances in exosome plasmonic sensing: Device integration strategies and AI-aided diagnosis. Biosens Bioelectron. 2024;266:116718
262. Wu Y, Qiao Y, Yang C, Chen Y, Shen X, Deng C. et al. Accelerated Exosomal Metabolic Profiling Enabled by Robust On-Target Array Sintering with Metal-Organic Frameworks. Small Methods. 2025;9:2401238
Author Biographies
 Assoc. Prof. Da Sun: Sun Da received his Ph.D. degree in biomedical engineering from Chongqing University, China, in 2016. He is currently an associate professor at Wenzhou University. He has good research foundation and experience in exploring therapeutic strategies for diseases including wounds and diabetes based on natural bioactive substances.
Assoc. Prof. Da Sun: Sun Da received his Ph.D. degree in biomedical engineering from Chongqing University, China, in 2016. He is currently an associate professor at Wenzhou University. He has good research foundation and experience in exploring therapeutic strategies for diseases including wounds and diabetes based on natural bioactive substances.
 Prof. Wei Wu received his Ph.D. in biomedical engineering at Sichuan University, China (2015). He is currently a professor at Chongqing University. He has broad experience in the fields of stimuli-responsive polymeric nanocarriers and cell-membrane based biomimetic/intelligent nanocarriers for cardiovascular and antitumor applications.
Prof. Wei Wu received his Ph.D. in biomedical engineering at Sichuan University, China (2015). He is currently a professor at Chongqing University. He has broad experience in the fields of stimuli-responsive polymeric nanocarriers and cell-membrane based biomimetic/intelligent nanocarriers for cardiovascular and antitumor applications.
![]() Corresponding author: Da Sun, sundayedu.cn; Wei Wu, david2015edu.cn; Qinsi Yang, yangqsac.cn.
Corresponding author: Da Sun, sundayedu.cn; Wei Wu, david2015edu.cn; Qinsi Yang, yangqsac.cn.
 Global reach, higher impact
Global reach, higher impact