13.3
Impact Factor
Theranostics 2025; 15(17):8840-8856. doi:10.7150/thno.120805 This issue Cite
Review
Modulation of Stem Cell Survival and Engraftment: Implications for Stem Cell-Based Therapy
1. Department of Pharmacy, the First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Wuhu 241001, China.
2. School of Pharmacy, Wannan Medical College, Wuhu 241002, China.
3. Organoid Innovation Center, CAS Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-bionics, Chinese Academy of Sciences, Suzhou 215123, China.
4. Center for Xin'an Medicine and Modernization of Traditional Chinese Medicine of IHM, Wannan Medical College, Wuhu 241002, China.
5. State Key Laboratory of Neurology and Oncology Drug Development, Nanjing, China.
6. Anhui Innovative Center for Drug Basic Research of Metabolic Diseases, Wannan Medical College, Wuhu 241002, China.
* These authors contributed equally to this work.
Received 2025-7-2; Accepted 2025-7-22; Published 2025-8-11
Abstract
Stem cell transplantation holds promise for the treatment of degenerative diseases, tissue injuries, and malignancies. Despite this potential, clinical outcomes have often fallen short, largely due to limited survival and engraftment of transplanted cells at target sites. Recent research efforts have focused on optimizing cell-based therapies through improved understanding of stem cell biology and responsiveness to environmental cues. Emerging evidence indicates that the hostile post-transplantation microenvironment contributes to irreversible cellular damage and death, driven by metabolic dysfunction, immune-mediated responses, reactive oxygen species (ROS), altered biomechanical rigidity, and disrupted intercellular communication. To address these challenges, various strategies have been explored, including supplementation with exogenous metabolic substrates, enhancement of vascular remodeling, administration of antioxidants, and the application of three-dimensional (3D) stem cell spheroids. This review synthesizes current approaches aimed at improving cell viability and therapeutic efficacy in regenerative medicine.
Keywords: stem cell therapy, metabolic remodeling, glucose delivery, oxidative stress injury, stem cell spheroids
Introduction
Stem cell-based therapy has emerged as a groundbreaking strategy for the treatment of malignancies, neurodegenerative diseases, organ fibrosis, and tissue injuries [1, 2]. Unlike conventional small-molecule drugs, which typically act through a single molecular pathway, stem cells (SCs) mediate therapeutic effects via multilineage differentiation and paracrine signaling mechanisms [3, 4]. Although numerous preclinical and clinical studies support the potential of SCs as a novel therapeutic modality, clinical translation remains significantly limited by poor post-transplantation cell survival and engraftment [5-7]. Studies indicate that up to 90% of transplanted SCs undergo apoptosis within the initial days post-transplantation, primarily due to the hostile microenvironment and disruption of cellular homeostasis [8-10]. This substantial cell loss compromises therapeutic efficacy, reproducibility, and stability—factors critical to the quality standards expected of any therapeutic intervention.
Transplanted SCs encounter a multitude of environmental stressors that collectively contribute to early cell death. Among the most acute is ischemia-reperfusion injury, stemming from inadequate vascular supply at the transplantation site [11]. In contrast to resident cells supported by an intact vascular network, transplanted SCs initially lack vascular connections and must survive until neovascularization occurs or integration into host vasculature is achieved [11, 12]. This interim phase induces a metabolic crisis characterized by severe hypoxia, nutrient deprivation, and the accumulation of cytotoxic waste products [13, 14]. To mitigate this, strategies such as direct oxygen and nutrient supplementation, as well as metabolic preconditioning via starvation-mimicking interventions, have shown promise in preserving short-term cell viability [15]. Long-term survival, however, requires robust vascular reconstruction—either through cytokine-mediated angiogenesis or the deployment of biomaterial scaffolds designed to support vascular network formation [16-18]. Oxidative stress represents another major obstacle to SC survival, driven by excessive ROS that exceed the cell's intrinsic antioxidant capacity [19]. The abrupt transition from optimized in vitro conditions to the pathological oxidative environment of damaged tissues renders transplanted SCs particularly susceptible to redox imbalance [20]. Countermeasures have included both genetic modifications to enhance endogenous antioxidant defenses and the delivery of ROS-scavenging components [21, 22]. Furthermore, recent advancements in three-dimensional (3D) culture techniques for SCs and hydrogel scaffolds have emerged as effective strategies to better replicate the in vivo microenvironment, thereby addressing limitations inherent to traditional two-dimensional (2D) cultures. The 3D architecture of SCs—such as spheroids, aggregates, and biomimetic microstructures constructed through biological 3D-printing—preserves their multidirectional differentiation potential and enhances both cell-cell and cell-matrix signaling. These features facilitate intercellular interactions and result in cellular characteristics that more closely resemble those observed in vivo [23, 24]. In this field, hydrogel scaffolds with defined physicochemical and biological properties have become a focal point in stem cell-based regenerative medicine. By combining structural support with the incorporation of biological factors [25], these scaffolds enable the construction of large-scale tissue constructs, offering potential for the artificial fabrication of complex organs [26-28].
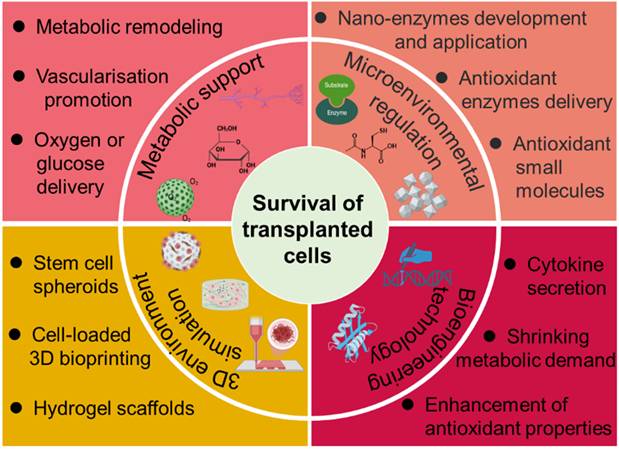
Over the years, various methods have been developed to address the challenges contributing to SC death. This review provides a comprehensive examination of current strategies aimed at improving stem cell survival post-transplantation (Figure 1), with particular focus on metabolic support, angiogenesis promotion, oxidative stress mitigation, and microenvironmental engineering. Deeper insight into the determinants of SC viability and integration may uncover novel therapeutic targets beyond those currently explored. Through continued interdisciplinary collaboration and innovation, it may be possible to overcome existing barriers and fully harness the regenerative potential of stem cell-based therapies for a wide array of debilitating conditions.
Overview of established strategies and emerging research approaches aimed at enhancing the survival and functional integration of transplanted SCs.
Factors and Strategies for Modulating the Survival of Transplanted Cells
Metabolism of SCs
Cellular metabolism serves as the biochemical foundation sustaining all life activities by generating energy through substrate utilization, synthesizing structural components, and eliminating metabolic waste to maintain homeostasis [29, 30]. Unlike resident cells, transplanted SCs face significant metabolic constraints due to the absence of vascular support at the graft site [31]. This vascular deficiency leads not only to an insufficient supply of essential substrates—including oxygen, glucose, fatty acids, and amino acids—critical for energy production via glycolysis and oxidative phosphorylation [32, 33], but also hampers the clearance of metabolic waste products. The resulting disruption in intracellular homeostasis promotes cellular stress and death. These interconnected metabolic limitations form a critical barrier to the survival of transplanted SCs.
Stem cell domestication
Abrupt changes in the microenvironment at transplantation sites significantly disrupt SC metabolism, ultimately causing cell death. According to previous research, preconditioning SCs to adapt to such adverse conditions enhances both survival and therapeutic efficacy. Extensive studies have demonstrated that hypoxic preconditioning (1-5% O₂) activates hypoxia-inducible factor (HIF-1α), which in turn upregulates pro-survival genes (e.g., VEGF, GLUT-1) and antioxidant enzymes such as SOD2, thereby strengthening the anti-apoptotic capacity of SCs [34-39]. For example, Beegle et al. found that mesenchymal stem cells (MSCs) preconditioned at 1% O₂ for 48 hours showed twice the survival rate under serum-deprived conditions compared to normoxic (20% O₂) controls [36]. Similarly, Wan et al. demonstrated that hypoxia preconditioning significantly enhances vascular endothelial growth factor (VEGF) secretion by adipose-derived stem cells (ADSCs), improving the viability, proliferation, migration, angiogenesis, and glycolysis of human umbilical vein endothelial cells (ECs) (HUVECs), ultimately promoting urethral reconstruction through the upregulation of angiogenic and glycolytic pathways [40]. This enhancement in resilience is attributed to hypoxia-induced metabolic reprogramming, characterized by a shift from oxidative phosphorylation to glycolysis, accompanied by increased glucose uptake, elevated lactate production, and reduced oxygen consumption [34]. In addition, transient serum deprivation induces autophagy, which facilitates the removal of damaged proteins and organelles while upregulating protective heat shock proteins (e.g., HSP70) to reduce stress-induced injury [41]. Moya et al. further demonstrated that reducing metabolic demand prior to transplantation enables MSCs to tolerate extreme hypoxia (2% pO₂ for 75 days) and near-anoxia (0.1% pO₂ for 14 days) [42, 43]. Importantly, this strategy preserves the proliferative capacity, differentiation potential, and paracrine function of SCs, rendering it a clinically feasible and cost-effective strategy for cell-based therapies.
Oxygen supplement
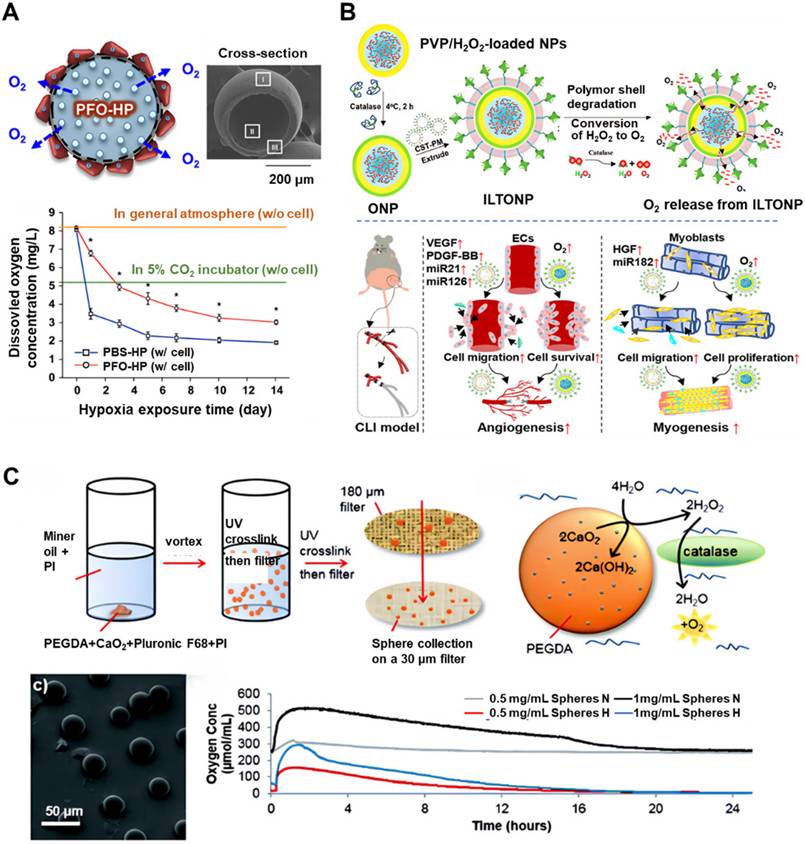
Oxygen tension has a direct effect on cellular energy metabolism, and consequently, affects both cell survival and therapeutic efficacy. Previous studies have shown that oxygen tension in rodent hearts decreases from 2.6%-3.3% to 0.9% pO2 within the first 5 minutes following arterial occlusion in rodent hearts, reaching near-anoxic conditions (0.2% pO2) after 30 minutes of ischemia [44], inevitably resulting in cellular necrosis within the ischemic tissues. To enhance SC survival and function at transplantation sites, hyperbaric oxygen therapy (HBOT) was initially employed to elevate tissue oxygen partial pressure [45]. However, this approach lacks tissue specificity, and the resulting excessive oxygen levels may induce ROS-mediated damage [46]. Hemoglobin-based oxygen carriers (HBOCs) have demonstrated potential in boosting MSC proliferation by 25% [47], yet their clinical utility remains limited due to ROS generation during oxygen release [48]. To overcome these drawbacks, Cook et al. developed a novel oxygen delivery strategy involving hyperbaric oxygen loading and subsequent controlled gas release into the surrounding environment to support cell viability [49]. Nevertheless, this method exhibits limited oxygen-loading capacity and permits only short-term oxygen release.
With advancements in materials science, perfluorocarbons (PFCs) have been identified as promising oxygen carriers due to their oxygen solubility being 15-20 times greater than that of water [50]. Their compatibility with microspheres and hydrogels presents opportunities for enhancing oxygen-carrying capacity and prolonging oxygen release duration [51, 52]. For instance, PFC-hydrogel systems have been shown to enhance osteoblast viability under hypoxic conditions [53], while PFC-laden scaffolds have increased bone formation by 2.5-fold in defect models (Figure 2A) [54]. However, the clinical application of low-molecular-weight PFCs is hindered by their rapid clearance and short in vivo retention time. This has prompted the development of modified PFCs through conjugation with hydrogels [55] or graphene oxide [56]. Niu et al. designed fast-gelling hydrogels synthesized from N-isopropylacrylamide (NIPAAm) copolymers and methacrylate-poly(ethylene glycol)-perfluorooctane (MAPEGPFC) [55], which demonstrated significantly higher oxygen partial pressure when transitioning from 21% O₂ to 1% O₂ environments. When bone marrow-derived MSCs (BM-MSCs) were encapsulated in these hydrogels and cultured under 1% O₂, the cells survived and proliferated over a 14-day period, with markedly improved outcomes compared to hydrogels with lower oxygen retention capacity.
To ensure continuous oxygen supply, peroxide-based systems have emerged as a promising solution. These systems primarily generate oxygen through the decomposition of peroxides. Encapsulating hydrogen peroxide (H₂O₂) within PLGA/catalase microspheres facilitates on-demand oxygen release while mitigating ROS toxicity [57]. Zhong et al. developed H₂O₂-releasing oxygen-generating nanoparticles that promote the recruitment of endothelial and skeletal muscle cells, enhance cell survival under ischemic conditions prior to neovascularization, and restore morphogenic function in high-glucose ischemic environments (Figure 2B) [15]. Despite these benefits, precise modulation of H₂O₂ release kinetics remains a technical challenge. Among solid peroxides, calcium peroxide (CaO₂) is particularly promising due to its high oxygen yield (0.0069 mol O₂/g) and sustained release profile [58]. Newland et al. introduced polyethylene glycol diacrylate (PEGDA)/CaO₂-based oxygen-producing microspheres capable of elevating the dissolved oxygen content of culture media for 16-20 hours (Figure 2C) [59]. These spheres effectively preserved the viability of SH-SY5Y cells and MSCs under oxygen- and glucose-deprived conditions, indicating that oxygen-generating biomaterials can improve post-transplantation cell survival. Furthermore, when integrated into PLGA scaffolds, CaO₂-based systems enabled continuous oxygen release for over 10 days [60], with further extensions up to 7 weeks achieved through hydrophobic surface modifications [61] and adjustments to scaffold thickness [62]. To enhance the controllability of release kinetics, Fu et al. developed an ultrasound (US)-activated oxygen-generating nanosystem. This platform consisted of CaO₂ encapsulated within mesoporous silica nanoparticles, combined with thermosensitive materials such as heneicosane and polyethylene glycol. Mild hyperthermia induced by targeted US irradiation initiated the phase transition of heneicosane, facilitating US-responsive water diffusion and oxygen release [63]. This intelligent, stimulus-responsive nanosystem serves as a reference model for future development of controllable, on-demand oxygen release technologies.
Despite the promise of oxygen delivery strategies for SC transplantation, substantial technical and biological challenges remain. One major limitation is the mismatch between the oxygen release kinetics of current systems and the dynamic oxygen demands of transplanted tissues. Most conventional delivery systems—such as CaO₂ or H₂O₂-based platforms—exhibit binary, “all-or-nothing” release behavior, which fails to recapitulate physiological oxygen gradients and may induce cyclic hypoxia-reoxygenation injury. Furthermore, alkaline byproducts produced from peroxide decomposition can disrupt local pH homeostasis [64], while residual H₂O₂, if not adequately degraded due to insufficient catalase, may induce oxidative damage [57]. To address these limitations, next-generation oxygen delivery systems are being developed to incorporate microenvironment-responsive feedback mechanisms. These advanced systems facilitate precisely regulated oxygen release in response to local pathophysiological signals, including oxygen tension, ROS levels, enzyme activity, and temperature fluctuations.
Glucose supplement
While hypoxia has long been recognized as a major contributor to the death of transplanted SCs, emerging evidence suggests that glucose deprivation is an equally critical limiting factor. Under hypoxic conditions, where oxidative phosphorylation is severely compromised, anaerobic glycolysis becomes the primary pathway for ATP generation, rendering glucose essential for cell survival [65]. Multiple studies have demonstrated that various types of SCs, including human BM-MSCs [65], rat BM-MSCs, and sheep BM-MSCs [66], can adapt to and survive in near-anoxic environments (≤ 0.5% O₂), provided that glucose is adequately available. Moya et al. provided compelling evidence that human MSCs rely almost exclusively on glucose-driven anaerobic glycolysis for ATP production, showing minimal capacity to metabolize alternative energy substrates such as glutamine, serine, or pyruvate [65]. Their work revealed that ensuring sufficient glucose supply significantly improves cell survival, reducing 14-day in vitro mortality of hMSCs from 93% to 40%. Beyond its fundamental role in energy metabolism, glucose serves as a vital signaling molecule that regulates critical cellular processes through pathways such as Akt/mTOR [67]. This dual function underscores the importance of maintaining effective glucose delivery not only for sustaining cell viability but also for preserving the therapeutic functionality of transplanted cells during the critical pre-vascularization phase post-transplantation.
Effective glucose delivery to transplanted cells encounters three primary challenges. First, the physicochemical properties of glucose, such as low molecular weight, neutral charge, and high aqueous solubility, make stable integration into scaffold matrices difficult. Second, the biological activity of glucose depends on preserving its native structure, thereby limiting options for chemical modification. Third, localized accumulation of glucose may lead to hyperosmotic stress, which can damage cellular membranes and induce lysis. In response to these challenges, Fois et al. developed nanofunctionalized microparticles to prevent necrotic core formation in SC spheroids [68]. Glucose-loaded mesoporous silica nanoparticles (MSNs) were coated onto the surface of poly(lactic-co-glycolic acid) (PLGA) microparticles, resulting in the formation of cell-nanofunctionalized microparticle spheroids (Figure 3A). Sustained local glucose release within hMSC spheroids significantly enhanced cell viability under short-term hypoxic culture conditions.
Representative examples of oxygen delivery and release system developed to support SC survival. (A) Schematic representation of perfluorooctane emulsion (PFO)-loaded hollow microparticles (HPs) designed for oxygen delivery under hypoxic conditions. The accompanying graph illustrates the concentration of dissolved oxygen in the cell culture medium surrounding the HPs during incubation in a hypoxic environment. Adapted with permission from [54], Copyright 2015 Elsevier B.V. (B) Schematic representation of the fabrication process and mechanism of action of ischemic limb-targeting oxygen-releasing nanoparticles (ILTONP) for the treatment of critical limb ischemia. Adapted with permission from [15], Copyright 2023 American Chemical Society. (C) Schematic depiction of PEGDA/CaO₂ microspheres and their corresponding oxygen release profiles at various concentrations under normoxic (N) and hypoxic (H) conditions. Adapted with permission from [59], Copyright 2018 The Royal Society of Chemistry.
Representative examples of glucose delivery and release systems developed to support SC survival. (A) Schematic illustration of functionalized microparticles designed for glucose delivery in 3D cell assemblies. The accompanying graph depicts the glucose release profile from PLGA-MSNs and its effect on cell death. Adapted with permission from [68], Copyright 2024 American Chemical Society. (B) Schematic depiction of self-feeding hydrogels. The accompanying graphs present the enzymatic degradation profiles of various forms of laminaran and the proliferation of hMSCs encapsulated in laminaran hydrogels over a two-week period. Adapted with permission from [69], Copyright 2023 Nature Portfolio. (C) Schematic illustration of the potential application of glucose-releasing alginate microgels encapsulating starch and AMG. Adapted with permission from [72], Copyright 2024 Wiley-VCH GmbH.
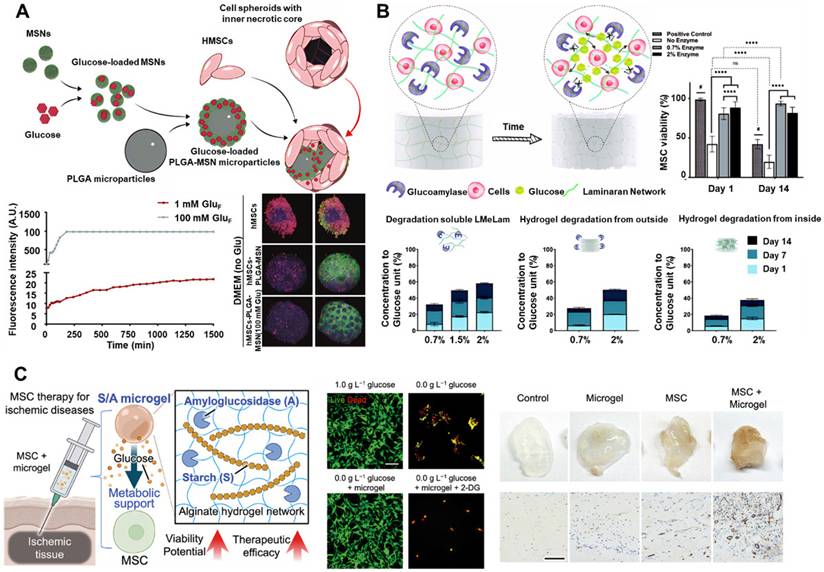
Nevertheless, to achieve durable and stable glucose release, enzymatic glucose-release platforms have become a focal point of research in recent years. Denoeud et al. proposed a composite hydrogel consisting of fibrin, starch, and amyloglucosidase (AMG), enabling sustained glucose release via AMG-mediated starch hydrolysis (Figure 3B) [69]. hMSCs encapsulated within these hydrogels, when exposed to near-anoxic conditions (0.1% pO₂) in vitro, showed improved viability and enhanced angio-inductive function for up to 14 days. Paquet et al. developed a fibrin-based hydrogel containing α-amylase, which enzymatically cleaves starch into glucose, supporting hMSC viability at levels 115 times greater than those observed with fibrin-only controls under near-hypoxic conditions after two weeks [70]. Similarly, Zargarzadeh et al. utilized glucoamylase-immobilized laminaran hydrogels, which maintained > 60% viability in both A549 cells and MSCs throughout a 14-day glucose-free culture period [71]. To extend the applicability of such nutritive platforms, Huang et al. developed an injectable glucose delivery system comprising starch and AMG encapsulated within alginate microgels (Figure 3C). These starch/AMG (S/A) microgels enabled sustained glucose release for up to 7 days via enzymatic hydrolysis, supporting MSC survival and function under ischemic conditions. In vitro studies under combined oxygen and glucose deprivation demonstrated that S/A microgels not only preserved cell viability and intracellular energy levels but also enhanced the pro-angiogenic and immunomodulatory functions of MSCs [72].
These biocompatible, self-nourishing platforms represent a significant advancement in cell transplantation technology. By enabling controlled and sustained glucose release, they transform passive cell delivery scaffolds into active metabolic support systems. Functioning as closed culture systems, these methods offer considerable promise for applications requiring extended cell viability before vascularization is established. Furthermore, combining glucose-delivery systems with oxygen-supplying strategies may greatly improve the efficacy of SC transplantation therapies. Future research should prioritize optimization of release kinetics, enhancement of scaffold integration, and development of clinically translatable formulations to maximize therapeutic outcomes.
Vascularization
Angiogenesis
While the temporary supply of external nutrients can extend the survival of transplanted SCs, the establishment of a functional vascular system is essential for sustaining SC viability in vivo. This vascular system enables efficient exchange of nutrients and metabolic waste, which is crucial for long-term cellular function and homeostasis [73]. Physiologically, blood vessel formation is governed by a series of tightly regulated molecular and cellular events [74, 75]. Angiogenic activation is typically initiated by hypoxia-induced signaling, inflammatory signals, or extracellular matrix (ECM) degradation, leading to the binding of VEGF to its receptor, VEGFR2, on the surface of ECs [76, 77]. This interaction activates multiple downstream pathways, including PI3K/Akt and MAPK/ERK, which promote EC proliferation and migration. Subsequently, the activated and differentiated ECs form neovascular sprouts that extend toward gradients of angiogenic factors, ultimately fusing with adjacent vascular sprouts to establish looped vascular structures. Neovascular maturation is then achieved through signaling mediated by platelet-derived growth factor (PDGF) and its receptor, PDGFRβ, which facilitates the recruitment of pericytes to encapsulate the newly formed vessels. Concurrently, ECs secrete components such as type IV collagen and laminin, contributing to the formation of a basement membrane and further reinforcing vascular integrity [78]. The induction of artificial vascularization at this stage is therefore a key strategy for ensuring the long-term survival, engraftment, and functional integration of transplanted SCs at the target site.
Among the various angiogenic regulators, VEGF is widely recognized as the primary mediator of neovascularization due to its potent effects on EC proliferation, vascular permeability enhancement, and directional guidance of vessel formation [79-81]. Consequently, contemporary research efforts have been directed toward optimizing VEGF delivery strategies to modulate SC fate and promote vascular development. For example, Park et al. developed a delivery system that co-encapsulates an angiogenesis-related peptide, apelin, with plasmid DNA to upregulate VEGF expression [82]. Transfection using VEGF-coated, apelin-loaded PLGA nanoparticles induced differentiation of hMSCs into ECs and stimulated vascular formation both in vitro and in vivo. In another approach, Ullah et al. proposed that decellularized ECM retains structural and biochemical properties suitable for VEGF delivery [83]. Supplementing VEGF with decellularized ECM enhanced the differentiation of human induced pluripotent stem cells (hiPSCs) into ECs, thereby facilitating the formation of functional vascular niches by promoting selective cell adhesion and survival. Furthermore, Lee et al. engineered a chitosan-based hydrogel embedded with VEGF-releasing microtubes [84]. This cellular construct exhibited high cell viability and minimal cytotoxicity in vitro. Upon implantation into a mouse hindlimb ischemia model, it led to significant cell retention and neovascularization through both vasculogenesis and angiogenesis, ultimately contributing to the restoration of blood flow in ischemic tissue.
Construction of blood vessels
The application of 3D bioprinting technology in SC vascularization markedly enhances the efficiency of constructing functional vascular networks by enabling precise spatial control over the distribution of cells, biomaterials, and growth factors [85, 86]. A key advantage of 3D bioprinting is its ability to fabricate vascular channels with a resolution of 10-50 μm, thereby allowing replication of the hierarchical structure found in natural capillary networks [87]. For instance, Gou et al. developed a microfiber-templated porogel (μFTP) bioink for 3D printing of tubular interfaces and microvascular structures [88]. This 3D tubular architecture exhibited up to 55% greater porosity than conventional block bioinks. The μFTP bioink effectively supported EC proliferation and spreading within the matrix and around sacrificial fibers. Scaffolds bioprinted with μFTP bioink significantly promoted inward growth of blood vessels and native tissues in vivo, demonstrating the feasibility of constructing tubular biointerfaces using customizable modular components. Additionally, 3D bioprinting enables synergistic multi-cell printing [89]. For example, co-printing techniques involving ECs and MSCs have been employed to form the inner wall of blood vessels, facilitating lumen formation and vascular integrity [90-92]. Cheng et al. implemented a one-step 3D bioprinting approach to create a physiologically relevant metastatic model incorporating tumor tissue, hollow vascular structures, and bone components [93]. By utilizing a gelatin methacryloyl-based photo-crosslinkable bioink containing three distinct cell populations—MDA-MB-231 breast cancer cells, HUVECs, and osteoblasts (hOBs)—the researchers successfully recapitulated key features of the metastatic niche, including functional vasculature, vascularized tumor masses, and vascularized bone tissue. Moreover, 3D bioprinting allows for the mimicry of SC ecological niches. Paracrine signals secreted by blood vessels, including VEGF and stromal-derived factor 1 (SDF-1), can be integrated into SC-hydrogel complexes printed around blood vessels to sustain SC viability and function [94, 95]. Consequently, the integration of 3D bioprinting for direct construction of vascularized structures with active material exchange capacity not only facilitates continuous nutrient delivery and prolongs SC survival while maintaining therapeutic efficacy but also lays the groundwork for future regeneration of large-scale organs, such as the heart and liver [96].
Despite these advancements, several critical challenges persist in the field of vascular engineering. The scientific community continues to grapple with issues such as uncontrolled vessel proliferation, incomplete vascular maturation, and progressive loss of perfusion efficiency over time. Addressing these limitations will require focused efforts to refine growth factor delivery methods, develop advanced biomimetic strategies that more accurately replicate physiological angiogenesis, and explore innovative approaches for modulating the immune microenvironment surrounding newly formed vasculature.
Relieving oxidative stress damage
Reactive oxygen species (ROS) are natural byproducts of cellular oxygen metabolism that play crucial, yet dual, roles in SC physiology. These chemically reactive molecules, which include superoxide anion (O₂•⁻), H₂O₂, singlet oxygen (¹O₂), and hydroxyl radical (•OH), are generated through the sequential reduction of molecular oxygen [97, 98]. Under physiological conditions, redox homeostasis is maintained through a network of enzymatic antioxidant systems. Specifically, superoxide dismutase (SOD) catalyzes the conversion of O₂•⁻ into H₂O₂, which is subsequently decomposed into water by catalase (CAT), peroxidase (POD), and glutathione peroxidase (GPX) [99]. These antioxidant enzymes also modulate redox-sensitive signaling pathways that involve cytokines, growth factors, protein kinases and phosphatases, and nuclear transcription factors [100, 101]. Nevertheless, when ROS production exceeds the cellular antioxidant defense capacity, oxidative stress ensues, resulting in damage to vital cellular components. In the context of SC transplantation, abnormal metabolic activity at the lesion site often leads to ROS overproduction. This is further exacerbated by inflammatory and immune responses that generate excessive ROS, which can infiltrate and compromise the viability of transplanted SCs. This aggressive influx of ROS, along with associated oxidative damage, is commonly observed in pathological conditions such as atherosclerosis, ischemic tissue necrosis, pulmonary and hepatic fibrosis, and neurodegenerative diseases [102-104]. To mitigate the oxidative stress associated with these conditions, various antioxidant-based strategies have been developed to protect transplanted SCs from ROS-induced injury. The following section provides an overview of recent advances in antioxidant compound development and their efficacy in enhancing SC resistance to oxidative stress in transplantation settings.
Antioxidant enzymes
Enhancing the activity of endogenous antioxidant enzymes through gene editing represents an effective strategy for mitigating oxidative stress in transplanted SCs. For instance, Baldari et al. demonstrated that adipose-derived stromal and vascular cells engineered to overexpress SOD2 exhibited significantly enhanced survival under hypoxic conditions, highlighting the protective role of SOD2[105]. In a more comprehensive approach, Zhu et al. engineered bone marrow-derived MSCs (bMSCs) to co-express both SOD1 and GPX, establishing a synergistic antioxidant defense system [106]. These genetically modified bMSCs showed improved survival following transplantation and contributed to enhanced circulatory and functional recovery in a diabetic critical limb ischemia model. However, gene editing technologies remain primarily limited to preclinical research, as the accumulation of genetic modifications may pose risks and potentially impair SC function.
Alternatively, recent advances in delivery platforms have enabled the direct intracellular transport of antioxidant enzymes, offering a promising strategy to alleviate oxidative stress [107-109]. For instance, Chen et al. developed a nanoparticle-based SOD delivery system (FMSN-TAT-SOD), which consists of mesoporous silica nanoparticles combined with a cell-penetrating peptide [109]. This system significantly increased intracellular SOD levels in HeLa cells, enhancing resistance to free radicals and superoxide anions induced by lipopolysaccharide (LPS) and paraquat. In our previous work, SOD-conjugated gold nanoparticles (SOD@Au NS) were engineered as ROS scavengers for MSCs in the treatment of idiopathic pulmonary fibrosis (IPF) [22]. In this approach, SOD was immobilized on the surface of gold nanoparticles (AuNPs) and encapsulated within polyphosphazene nanospheres to improve cell membrane permeability and chemical stability. In addition to endogenous antioxidant enzymes, exogenous proteins such as Wnt3a—which modulates oxidative stress via the Wnt/β-catenin signaling pathway—have also been utilized. Qi et al. demonstrated the use of cell-penetrating peptide-conjugated porous silicon nanoparticles (TPSi NPs) for efficient Wnt3a loading and sustained release, which enhanced the antioxidative capacity of MSCs [107]. This strategy holds significant promise for improving outcomes in SC therapy for myocardial infarction by protecting transplanted cells from ROS-induced damage.
Small molecule antioxidants
Unlike biologically active macromolecules, small-molecule antioxidants typically exhibit superior physicochemical stability. Compounds such as vitamin C (ascorbic acid), vitamin E (tocopherol), vitamin A (β-carotene), selenium, glutathione (GSH), and N-acetylcysteine (NAC) have shown efficacy in counteracting oxidative damage [110-112]. These antioxidants play a crucial role in protecting SCs from oxidative stress and enhancing post-transplantation survival. N-acetylcysteine, for example, has been shown to safeguard MSCs against oxidative stress and metabolic dysfunction by mitigating DNA damage and apoptosis through the Nrf2/Sirt3 signaling pathway [112]. In one study, Zhu et al. administered NAC via drinking water in a diabetic mouse model of critical limb ischemia. This intervention significantly reduced tissue ROS levels and improved the survival rate of bMSCs by nearly five-fold, increasing from 7.63% to 35.66% compared to untreated controls [106]. However, a major challenge in the clinical application of small-molecule antioxidants lies in achieving effective concentrations at the transplantation site following systemic administration. This is primarily due to low bioavailability, which results from high water solubility and poor absorption. Therefore, the development of optimized delivery methods remains essential to maximize the antioxidative potential of these compounds in transplanted SCs.
In addition to direct delivery, pretreating SCs with antioxidants prior to transplantation has emerged as an effective strategy for enhancing resistance to ROS-mediated damage. Deferoxamine (DEF), a clinically approved iron chelator, has been shown to improve MSC viability by enhancing antioxidant defenses and upregulating neurotrophic and anti-inflammatory factors [113]. For example, Soleimani Asl et al. reported that DEF-preconditioned adipose-derived MSCs exhibited increased antioxidant activity, proliferation, and overall viability. In an Alzheimer's disease model, DEF-treated AMSCs significantly improved cognitive function compared to untreated cells [114]. While in vitro preconditioning offers advantages such as procedural simplicity and enhanced controllability, several challenges persist. These include achieving sufficient intracellular drug accumulation and establishing reliable stimulus-responsive release mechanisms to sustain antioxidant protection after transplantation.
Nanozymes
Nanozymes are a distinct class of engineered nanomaterials that exhibit catalytic properties similar to those of natural enzymes [115]. These synthetic catalysts offer significant advantages over conventional protein-based enzymes, including a broader catalytic spectrum, sustained activity across diverse temperature and pH ranges [116], and enhanced cost-effectiveness in terms of production and storage stability [117]. Recent studies have identified a wide range of nanozyme materials, including metal and metal oxide nanoparticles (e.g., Au, Ag, Pt, CuxO, CeO₂, V₂O₅, Fe₃O₄, and MoO₃), carbon-based nanostructures (such as metal-doped carbon dots and graphene quantum dots), and metal-organic framework (MOF) nanoparticles. While existing reviews have comprehensively summarized nanozyme classifications, mechanisms of action, and biomedical applications [99, 118, 119], the present discussion focuses specifically on the emerging role of nanozymes in enhancing SC therapy through ROS scavenging.
Cerium oxide nanoparticles are particularly notable for their exceptional antioxidant properties, which arise from reversible redox cycling between the Ce⁴⁺ and Ce³⁺ oxidation states [118, 120-123]. Periodontitis, a chronic inflammatory disease, significantly impairs the function of periodontal ligament stem cells (PDLSCs) due to excessive ROS accumulation, which undermines their regenerative capacity and hinders periodontal tissue repair. To address this issue, Ren et al. developed PEGylated mesoporous silica nanoparticles loaded with cerium oxide (MSN@Ce@PEG) [124]. These nanoparticles effectively protected PDLSCs from oxidative damage, as evidenced by experimental data indicating a reduction in H₂O₂-induced apoptosis from 65.4% in untreated controls to 5.16% following treatment. In vivo studies conducted on rat models of periodontitis further confirmed the anti-inflammatory properties of these nanoparticles and their ability to enhance the periodontal microenvironment, ultimately improving PDLSC survival and function. In the context of neurodegenerative diseases, neural stem cell (NSC) transplantation presents promising therapeutic prospects for addressing cognitive impairment. To support NSC viability, Yu et al. developed cerium-based nanoparticles encapsulated within MOFs [125], which revealed efficient ROS scavenging and neuroprotective effects. Quantitative analyses demonstrated significantly improved neuronal survival rates and enhanced neurite outgrowth, underscoring the therapeutic potential of this antioxidant strategy in promoting neural regeneration.
Manganese dioxide (MnO₂)-based nanozymes have shown considerable potential in protecting SCs from oxidative damage [126, 127]. For instance, Peng et al. developed an injectable hydrogel microsphere system (LMGDNPs) by incorporating MnO₂ nanozymes into glucose-enriched decellularized nucleus pulposus hydrogel microspheres [128]. MSCs cultured on LMGDNPs exhibited improved cell viability and ECM synthesis, thereby promoting intervertebral disc regeneration. In a related approach, Chen et al. dispersed MnO₂ nanoparticles into a gelatin/κ-carrageenan hydrogel to enhance its ROS scavenging ability. The resulting hydrogel mitigated oxidative stress, improved SC viability, and boosted paracrine function, ultimately promoting angiogenesis in a skin flap repair model [129]. Similarly, Yang et al. fabricated MnO₂-containing granular hydrogel matrices capable of supporting MSC adhesion, preserving stemness, and modulating the ROS microenvironment by catalyzing the conversion of H₂O₂ into oxygen. This approach promoted MSC viability and osteogenic differentiation [130]. In a two-week diabetic rat model, the granular hydrogel also demonstrated enhanced in vivo cell retention and anti-inflammatory immunomodulation effects, highlighting its therapeutic potential for SC transplantation and immune regulation in diabetic conditions. In the context of spinal cord injury (SCI), Li et al. introduced a nanoparticle-dotted hydrogel by incorporating MnO₂ nanoparticles into a hyaluronic acid-based matrix [131]. This system effectively alleviated oxidative stress at the lesion site, improved MSC viability, and promoted the regeneration of central nervous spinal cord tissue.
Copper-based nanoparticles represent another promising class of nanozymes [132], exhibiting broad and potent catalytic capabilities. Liu et al. demonstrated that Cu₅.₄O nanoparticles could degrade up to 80% of three major ROS—H₂O₂, O₂•⁻, and •OH—at a concentration of 150 ng/mL [133]. In the context of SC therapy, Chen et al. fabricated a continuous nanozyme coating by sequentially depositing copper phosphate nanosheets (Cu NS) and the phenolic ligand epigallocatechin gallate (EGCG) onto titanium (Ti) implants [134]. These metal-phenolic nanozyme bio-interfaces exhibited robust free radical scavenging activity and facilitated SC adhesion, migration, and osteogenic differentiation, thus enhancing implant osseointegration in diabetic rat models. In our previous work, ROS-scavenging nanocomposites (RSNPs) were developed by encapsulating CuxO nanoparticles and AuNPs to serve both as antioxidance agents and computed tomography (CT) imaging tracers [135]. Upon internalization by MSCs, RSNPs released CuxO nanoparticles in response to oxidative stress, thereby protecting the cells and improving therapeutic efficacy in IPF models by enhancing SC survival.
In addition to metal-based nanozymes, non-metal nanozymes—particularly those derived from carbon-based materials—have also demonstrated significant success in the field of SC therapy [136, 137]. Among these, reduced graphene oxide (rGO) has been shown to mitigate excessive ROS through its ability to scavenge free radicals via electron transfer mechanisms [138, 139]. For instance, Choe et al. encapsulated MSCs in rGO/alginate composite microgels using an electrospraying technique [140]. The encapsulated MSCs exhibited increased viability under oxidative stress conditions induced by H2O2. Furthermore, when cardiomyocytes (CMs) were co-cultured with the encapsulated MSCs in a transwell system and exposed to H2O2, the CMs showed enhanced survival and greater degrees of cardiac maturation compared to those cultured in monolayers. In a related study, Zhou et al. confirmed that rGO-embedded antioxidant hydrogels possess significant ROS-scavenging capacity and promote both the osteogenic differentiation of bMSCs and angiogenesis in HUVECs. These hydrogels neutralized local ROS at the site of skull defects and stimulated the ZEB1/Notch1 signaling pathway, thereby facilitating coordinated osteogenesis and angiogenesis [137].
Nanozymes hold significant potential for improving the efficacy of SC therapy by mimicking the activity of natural antioxidant enzymes and effectively neutralizing deleterious ROS. However, several critical challenges must be addressed to fully realize their therapeutic potential. One major challenge lies in the precise modulation of ROS levels. It is imperative that nanozymes are meticulously engineered to selectively eliminate pathological concentrations of ROS while preserving basal ROS levels necessary for redox signaling, which governs a variety of cellular processes. To achieve this, the development of sophisticated ROS-recognition systems capable of enabling accurate and controlled ROS elimination is urgently needed [135, 141, 142]. In addition, long-term biocompatibility remains a significant concern, particularly for metal-based nanozyme formulations. The accumulation of exogenous nanomaterials may trigger chronic inflammatory responses, unintended immune activation, or organ-specific toxicity. Consequently, comprehensive preclinical assessments—including extensive toxicological profiling and thorough pharmacokinetic analyses—are imperative before clinical translation can be considered [143, 144]. Moreover, the therapeutic challenges in SC transplantation extend beyond ROS-related injury [6]. Integrating multifunctional capabilities into nanozymes represents a promising direction for future research. For example, combining ROS-scavenging activity with functionalities such as SC tracking or controlled drug delivery could maximize the benefits of nanoparticles, offering a platform for more effective and versatile regenerative strategies.
Stem cell spheroids
SCs isolated from human tissues are typically cultured in two-dimensional (2D) monolayer systems to produce sufficient quantities for biological research and therapeutic applications [145-147]. However, a growing body of evidence suggests that prolonged culture under 2D conditions results in the gradual loss of stemness characteristics. This decline is primarily attributed to inadequate interaction with the ECM and the absence of physiological relevance, resulting in abnormal cellular metabolism and altered protein expression profiles [148, 149]. These suboptimal culture conditions contribute to accelerated cellular aging, diminished paracrine signaling, and reduced post-transplantation survival, ultimately compromising therapeutic efficacy [150, 151]. To overcome these limitations, 3D cell culture systems have gained significant attention for their ability to mimic the in vivo microenvironment more accurately, including dynamic cell-cell and cell-ECM interactions [152]. 3D cell formats—such as aggregates, spheroids, and organoids—exhibit unique properties that influence specific cellular functions and growth processes, including embryogenesis, morphogenesis, and organogenesis. These advantages stem from the preservation of natural cytoskeletal organization, maintenance of apical-basal polarity, provision of appropriate matrix stiffness, and the establishment of physiological cytokine gradients [153, 154]. Spheroids derived from 3D culture demonstrate enhanced ECM production, which plays a protective role by mitigating inflammatory damage, reducing macrophage-mediated clearance, and improving post-transplantation migration and survival [155, 156]. For instance, sphere-forming cultures maintained under hypoxic conditions upregulate SDF-1α and HIF-1α expression, thereby increasing stress resistance, survival, homing, and angiogenic capacity of MSCs in vivo [157]. Furthermore, MSC spheroids display increased expression of SOD2, which reduces oxidative stress and apoptosis, while activating the PI3K/AKT/NRF2 and ERK/NRF2 signaling pathways to promote cell survival [158]. Thus, the development and utilization of SC spheroids and organoids represent promising alternatives for advancing regenerative medicine and enhancing the efficacy of SC-based therapies.
The formation of spheroids arises from the self-assembly behavior of single cells in suspension, facilitated by engineered platforms that provide environments conducive to cell aggregation and self-organization of the resulting multicellular structures [159-161]. Based on the engineering tools employed, 3D cell culture systems can be classified into scaffold-based systems (e.g., hydrogel matrices, well plates, and microfluidic chips) and scaffold-free systems (e.g., hanging drop, rotation methods, and magnetic force) [152]. In the scaffold-free category, He et al. prepared MSC spheroids using the hanging drop method [159]. The enhanced formation of ECM receptor interactions, gap junctions, and tight junctions within the spheroids activated neuroactive ligand-receptor interactions, which in turn triggered downstream PI3K-Akt signaling, resulting in superior anti-inflammatory and neurogenic effects. However, the absence of ECM-like materials in scaffold-free systems raises concerns regarding cell-cell adhesion and spheroid structural integrity. To address this, advances in biomedical engineering have introduced a variety of biological and synthetic scaffold materials with tunable porosity, permeability, surface chemistry, and stiffness to better replicate tissue-specific microenvironments and promote cell growth and migration. Inspired by lotus seedpod-structured hydrogels with temperature-responsive properties, Kim et al. developed a novel strategy for the culture and delivery of hADMSC spheroids using a microwell platform [162]. Spheroids cultured within these microwells demonstrated a high transfer efficiency of 93.78 ± 2.30% to target substrates. In a full-thickness murine skin wound model, a significant increase in SMA-positive vessel formation was observed by day 21. In addition, Rossen et al. introduced a sacrificial scaffold that allows ECs and MSCs to form self-organized clusters. Their results indicated that this facilitated the self-assembly of ECs and MSCs into prevascular units, which rapidly developed into functional, perfusing vasculature upon injection into mic [163]. This microplate array-based approach leverages the dynamic properties of hydrogels and offers scalability and reproducibility in producing self-organized cell clusters. Beyond single-cell-type spheroids, multicellular spheroid systems have further advanced SC therapy by more effectively mimicking native cellular interactions. Sung et al. created self-assembled 3D spheroids composed of umbilical cord MSCs and HUVECs [164, 165]. These constructs rapidly formed primitive vascular networks that matured into functional vasculature, supported by MSC-derived trophic and proangiogenic factors [164, 166]. When co-transplanted with islet cells, the spheroids yielded remarkable therapeutic benefits, including a 78% improvement in hypoxia resistance under 1% O₂, a 2.3-fold increase in insulin secretion, and a 62% enhancement in in vivo survival rates [166].
While 3D SC spheroids offer substantial advantages over traditional 2D cultures, several critical challenges must be addressed before their widespread clinical adoption. First, the inherent heterogeneity of the cellular microenvironment within spheroids presents a significant limitation. As cells with varying metabolic states aggregate into 3D structures, uneven oxygen and nutrient gradients emerge, coupled with inefficient waste removal. These suboptimal conditions result in spatial variations in gene expression patterns and metabolic activity, ultimately compromising the quality and functional consistency of the resulting spheroids [167]. Second, the processing of 3D spheroids introduces substantial risks of cellular damage. Larger spheroids require prolonged enzymatic dissociation, which subjects cells to increased mechanical stress and potential damage. Such processing-induced injury is particularly concerning, as apoptotic or compromised cells may elicit undesirable immune responses following transplantation, potentially undermining therapeutic outcomes [168, 169]. Third, standardization remains a formidable obstacle in spheroid production. Current fabrication methods exhibit considerable batch-to-batch variability in both spheroid size and quality, posing challenges for research reproducibility, limiting the reliability of preclinical validation in animal models, and ultimately hindering the clinical translation of MSC-based therapies [170, 171]. These technical challenges collectively underscore the need for continued innovation in 3D culture technologies. Systematic resolution of these issues through advanced bioengineering approaches and rigorous quality control measures will be essential to establish a robust foundation for clinical applications of 3D SC spheroids. Only through comprehensive optimization can the full therapeutic potential of this promising technology be effectively realized in clinical settings.
Summary and Prospects
The therapeutic efficacy of transplanted SCs is closely linked to their in vivo survival rate, with higher survival rates typically correlating with more consistent and durable therapeutic outcomes. However, SC survival is influenced by a range of factors, including intrinsic cellular states (e.g., metabolic disorders and substrate deficiencies), microenvironmental conditions at the transplantation sites (e.g., immune responses, oxidative stress, and suboptimal physicochemical microenvironments), as well as the biomechanical properties of the host matrix. In response to these challenges, researchers have developed various strategies to enhance SC viability, including the supplementation of oxygen and glucose, promotion of neovascularization, scavenging of excessive ROS, and the use of SC spheroids and hydrogel scaffolds to better mimic physiological microenvironments. These advances have collectively provided valuable research tools and technical support for preclinical SC research and clinical translation of SC-based therapies.
Nevertheless, several key issues remain that warrant systematic investigation to guide future developments. (1) Biocompatibility remains a fundamental prerequisite for clinical translation. A comprehensive assessment should include not only the inherent toxicity of biomaterials but also the safety profile of their degradation byproducts. For instance, although sodium peroxide and calcium peroxide exhibit minimal direct effects on SC growth, their byproducts—sodium hydroxide and calcium hydroxide—may disrupt local pH homeostasis [62]. Similarly, metal-based nanozymes such as MnO₂ and CuₓO nanoparticles demonstrate promising application potential due to their low cytotoxicity. However, as exogenous substances, their in vivo metabolism and pharmacokinetics must be thoroughly characterized before clinical application. (2) Spatiotemporal regulation of the transplantation microenvironment is essential to meet the dynamic needs of SC growth and differentiation. For example, while ROS at low concentrations serve critical physiological signaling functions, excessive ROS can lead to oxidative stress and cellular damage. Therefore, the use of antioxidant materials alone may be insufficient to maintain normal cellular function. The development of intelligent delivery systems—featuring stimuli-responsive activation, microenvironment-sensitive behavior, and tunable therapeutic release kinetics—is essential for achieving precise, on-demand modulation of therapeutic agents. (3) Integration of multifunctional biomaterials is necessary to address the multifaceted challenges encountered in SC therapy systematically. For example, a multicomponent system using hydrogel scaffolds to support the formation of HUVEC-lined vascular structures, while concurrently incorporating antioxidant molecules, could synergistically address key factors limiting graft survival, including biomechanical stress, insufficient angiogenesis, and oxidative damage. In addition, persistent obstacles such as immune rejection and the inability to achieve non-invasive, real-time tracking of transplanted cells continue to hinder the clinical advancement of cell therapies. The incorporation of multifunctional capabilities—such as immunomodulation, imaging contrast enhancement, and survival-promoting agents—within a single, integrated system represents a promising direction. Such holistic strategies not only enhance SC survival post-transplantation but also expand the applicability and clinical utility of SC-based therapeutic interventions [172].
Overall, SC research is not solely a biological endeavor but inherently intersects with tissue engineering, nanotechnology, drug delivery, and molecular imaging. These interdisciplinary connections present both substantial opportunities and complex challenges. Realizing the full therapeutic potential of SC-based therapies will require ongoing collaboration and communication among researchers from diverse fields, including chemistry, engineering, biology, and clinical medicine. Through such integrative efforts, rapid development and breakthroughs in clinical applications are anticipated, transforming the understanding of fundamental physiological processes and delivering meaningful benefits to human health in the near future.
Acknowledgements
Funding
This work was financially supported by the Anhui Provincial Natural Science Foundation (2308085QH243, 2408085QH232), Natural Science Foundation of Jiangsu Province (Grants No BK20230236), the Major Project of Natural Science Foundation of the Department of Education of Anhui Province (2024AH040235), the Key Project of Natural Science Foundation of the Department of Education of Anhui Province (2023AH051753), Center for Xin'an Medicine and Modernization of Traditional Chinese Medicine of IHM (2023CXMMTCM007), Municipal Science and Technology Project of Wuhu Science and Technology Bureau (2023yf090), Opening Foundation of State Key Laboratory of Neurology and Oncology Drug Development (SKLSIM-F-202457), the PhD Research Startup Foundation of Wannan Medical College (WYRCQD2024013), and the Talent Introduction Science Foundation of Yijishan Hospital, Wannan Medical College (YR20230101).
Author contributions
All authors revised and approved the manuscript. Qingwen Xu, Xinyu Liu, Chaochao Zhang, and Yan Wang participated in the manuscript writing. Xinyu Liu, Yuyao Sun, and Bowei Li prepared the figures. Chenggong Yu designed the review, supervised all figures, and cowrote the manuscript. Qingwen Xu, Chenggong Yu, and Sihui Nian acquired the fundings.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Acharya S, Shaha S, Bibbey MG, Mukherji M, Zhao Z, Mitragotri S. Stem cell therapies in the clinic. Bioeng Transl Med. 2025;10:e70000
2. Wang B, Zhao J, Cai W, Wang G, Li Y, Wang J. et al. Progress in transdifferentiation of autologous alternative cell sources into corneal epithelial cells. Stem Cell Rev Rep. 2025;21:226-35
3. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85-100
4. Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6:402-20
5. Li Q, Yu H, Zhao F, Cao C, Wu T, Fan Y. et al. 3D printing of microenvironment-specific bioinspired and exosome-reinforced hydrogel scaffolds for efficient cartilage and subchondral bone regeneration. Adv Sci. 2023;10:e2303650
6. Rahimi Darehbagh R, Seyedoshohadaei SA, Ramezani R, Rezaei N. Stem cell therapies for neurological disorders: current progress, challenges, and future perspectives. Eur J Med Res. 2024;29:386
7. Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9:17
8. Styczyński J, Tridello G, Koster L, Iacobelli S, van Biezen A, van der Werf S. et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020;55:126-36
9. Salazar-Noratto GE, Luo G, Denoeud C, Padrona M, Moya A, Bensidhoum M. et al. Concise review: understanding and ieveraging cell metabolism to enhance mesenchymal stem cell transplantation survival in tissue engineering and regenerative medicine applications. Stem Cells. 2020;38:22-33
10. An CF, Zhao Y, Guo LP, Zhang ZJ, Yan CX, Zhang SY. et al. Innovative approaches to boost mesenchymal stem cells efficacy in myocardial infarction therapy. Mater Today Bio. 2025;31:101476
11. Hyun JS, Tran MC, Wong VW, Chung MT, Lo DD, Montoro DT. et al. Enhancing stem cell survival in vivo for tissue repair. Biotechnol Adv. 2013;31:736-43
12. Rigaud VOC, Hoy R, Mohsin S, Khan M. Stem cell metabolism: powering cell-based therapeutics. Cells. 2020;9:2490
13. Chen Y, Liang Y, Luo X, Hu Q. Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy. Cell Death Dis. 2020;11:291
14. Hu XM, Zhang Q, Zhou RX, Wu YL, Li ZX, Zhang DY. et al. Programmed cell death in stem cell-based therapy: mechanisms and clinical applications. World J Stem Cells. 2021;13:386-415
15. Zhong T, Gao N, Guan Y, Liu Z, Guan J. Co-delivery of bioengineered exosomes and oxygen for treating critical limb ischemia in diabetic mice. ACS Nano. 2023;17:25157-74
16. O'Connor C, Brady E, Zheng Y, Moore E, Stevens KR. Engineering the multiscale complexity of vascular networks. Nat Rev Mater. 2022;7:702-16
17. Diomede F, Marconi GD, Fonticoli L, Pizzicanella J, Merciaro I, Bramanti P. et al. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int J Mol Sci. 2020;21:3242
18. Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16:178
19. Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE. et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23:499-515
20. Denu RA, Hematti P. Optimization of oxidative stress for mesenchymal stromal/stem cell engraftment, function and longevity. Free Radic Biol Med. 2021;167:193-200
21. Bao H, Wu M, Xing J, Li Z, Zhang Y, Wu A. et al. Enzyme-like nanoparticle-engineered mesenchymal stem cell secreting HGF promotes visualized therapy for idiopathic pulmonary fibrosis in vivo. Sci Adv. 2024;10:eadq0703
22. Yu C, Lv Y, Li X, Bao H, Cao X, Huang J. et al. SOD-Functionalized gold nanoparticles as ROS scavenger and CT contrast agent for protection and imaging tracking of mesenchymal stem cells in Idiopathic pulmonary fibrosis treatment. Chem Eng J. 2023;459:141603
23. Kang Y, Na J, Karima G, Amirthalingam S, Hwang NS, Kim HD. Mesenchymal stem cell spheroids: a promising tool for vascularized tissue regeneration. Tissue Eng Regener Med. 2024;21:673-93
24. Hazrati A, Malekpour K, Soudi S, Hashemi SM. Mesenchymal stromal/stem cells spheroid culture effect on the therapeutic efficacy of these cells and their exosomes: a new strategy to overcome cell therapy limitations. Biomed Pharmacother. 2022;152:113211
25. Nishiguchi A. Advances in injectable hydrogels with biological and physicochemical functions for cell delivery. Polym J. 2024;56:895-903
26. Li R, Xu J, Rao Z, Deng R, Xu Y, Qiu S. et al. Facilitate angiogenesis and neurogenesis by growth factors integrated decellularized matrix hydrogel. Tissue Eng Part A. 2021;27:771-87
27. Piard C, Jeyaram A, Liu Y, Caccamese J, Jay SM, Chen Y. et al. 3D printed HUVECs/MSCs cocultures impact cellular interactions and angiogenesis depending on cell-cell distance. Biomaterials. 2019;222:119423
28. Heo DN, Hospodiuk M, Ozbolat IT. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019;95:348-56
29. Jiang X, Peng Q, Peng M, Oyang L, Wang H, Liu Q. et al. Cellular metabolism: a key player in cancer ferroptosis. Cancer Commun. 2024;44:185-204
30. Wiley CD, Campisi J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat Metab. 2021;3:1290-301
31. Zhang L, Bhaloo SI, Chen T, Zhou B, Xu QB. Role of resident stem cells in vessel formation and arteriosclerosis. Circ Res. 2018;122:1608-24
32. Folmes CDL, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596-606
33. Bahat A, Gross A. Mitochondrial plasticity in cell fate regulation. J Biol Chem. 2019;294:13852-63
34. Tan SC, Gomes RSM, Yeoh KK, Perbellini F, Malandraki-Miller S, Ambrose L. et al. Preconditioning of cardiosphere-derived cells with hypoxia or prolyl-4-hydroxylase inhibitors increases sternness and decreasesreliance on oxidative metabolism. Cell Transplant. 2016;25:35-53
35. Liu XB, Wang JA, Ji XY, Yu SP, Wei L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res Ther. 2014;5:111
36. Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA. et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015;33:1818-28
37. Ho SS, Hung B, Heyrani N, Lee MA, Leach JK. Hypoxic preconditioning of mesenchymal stem cells with subsequent spheroid formation accelerates repair of segmental bone defects. Stem Cells. 2018;36:1393-403
38. Gugliandolo A, Diomede F, Scionti D, Bramanti P, Trubiani O, Mazzon E. The role of hypoxia on the neuronal differentiation of gingival mesenchymal stem cells: a transcriptional study. Cell Transplant. 2019;28:538-52
39. Li LX, Liu YQ, Zhi N, Ji YX, Xu JL, Mao GY. et al. Hypoxic preconditioning accelerates the healing of ischemic intestinal injury by activating HIF-1α/PPARα pathway-mediated fatty acid oxidation. Cell Death Discov. 2024;10:164
40. Wan X, Xie MK, Xu H, Wei ZW, Yao HJ, Wang Z. et al. Hypoxia-preconditioned adipose-derived stem cells combined with scaffold promote urethral reconstruction by upregulation of angiogenesis and glycolysis. Stem Cell Res Ther. 2020;11:535
41. Goyal U, Ta M. A novel role of vitronectin in promoting survival of mesenchymal stem cells under serum deprivation stress. Stem Cell Res Ther. 2020;11:181
42. Moya A, Larochette N, Paquet J, Deschepper M, Bensidhoum M, Izzo V. et al. Quiescence preconditioned human multipotent stromal cells adopt a metabolic profile favorable for enhanced survival under ischemia. Stem Cells. 2017;35:181-96
43. Ferro F, Spelat R, Shaw G, Duffy N, Islam MN, O'Shea PM. et al. Survival/adaptation of bone marrow-derived mesenchymal stem cells after long-term starvation through selective processes. Stem Cells. 2019;37:813-27
44. Khan N, Hou H, Swartz HM, Kuppusamy P. Direct and repeated measurement of heart and brain oxygenation using In vivo EPR oximetry. Methods Enzymol. 2015;564:529-52
45. Londahl M. Hyperbaric oxygen therapy as adjunctive treatment for diabetic foot ulcers. Int J Low Extrem Wounds. 2013;12:152-7
46. Zhou D, Shao L, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1-67
47. Le Pape F, Cosnuau-Kemmat L, Richard G, Dubrana F, Ferec C, Zal F. et al. HEMOXCell, a new oxygen carrier usable as an additive for mesenchymal stem cell culture in platelet lysate-supplemented media. Artif Organs. 2017;41:359-71
48. Alayash AI. Blood substitutes: why haven't we been more successful? Trends Biotechnol. 2014;32:177-85
49. Cook CA, Hahn KC, Morrissette-McAlmon JBF, Grayson WL. Oxygen delivery from hyperbarically loaded microtanks extends cell viability in anoxic environments. Biomaterials. 2015;52:376-84
50. Day RA, Sletten EM. Perfluorocarbon nanomaterials for photodynamic therapy. Curr Opin Colloid Interface Sci. 2021;54:101454
51. Zhuang J, Ying M, Spiekermann K, Holay M, Zhang Y, Chen F. et al. Biomimetic nanoemulsions for oxygen delivery in vivo. Adv Mater. 2018;30:1804693
52. Lee AL, Gee CT, Weegman BP, Einstein SA, Juelfs AR, Ring HL. et al. Oxygen sensing with perfluorocarbon-loaded ultraporous mesostructured silica nanoparticles. ACS Nano. 2017;11:5623-32
53. Kimelman-Bleich N, Pelled G, Sheyn D, Kallai I, Zilberman Y, Mizrahi O. et al. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials. 2009;30:4639-48
54. Bae JS, Park BL, Cheong HS, Kim JH, Kim JY, Namgoong S. et al. Association analysis of PDE4B polymorphisms with schizophrenia and smooth pursuit eye movement abnormality in a Korean population. Gen Physiol Biophys. 2015;34:277-84
55. Niu H, Li C, Guan Y, Dang Y, Li XF, Fan ZB. et al. High oxygen preservation hydrogels to augment cell survival under hypoxic condition. Acta Biomater. 2020;105:56-67
56. Maio A, Scaffaro R, Lentini L, Piccionello AP, Pibiri I. Perfluorocarbons-graphene oxide nanoplatforms as biocompatible oxygen reservoirs. Chem Eng J. 2018;334:54-65
57. Dhar R, Rana MN, Zhang L, Li Y, Li N, Hu Z. et al. Phosphodiesterase 4B is required for NLRP3 inflammasome activation by positive feedback with Nrf2 in the early phase of LPS- induced acute lung injury. Free Radic Biol Med. 2021;176:378-91
58. Hassan S, Cecen B, Pena-Garcia R, Marciano FR, Miri AK, Fattahi A. et al. Survival and proliferation under severely hypoxic microenvironments using cell-laden oxygenating hydrogels. J Funct Biomater. 2021;12:30
59. Newland H, Eigel D, Rosser AE, Werner C, Newland B. Oxygen producing microscale spheres affect cell survival in conditions of oxygen-glucose deprivation in a cell specific manner: implications for cell transplantation. Biomater Sci. 2018;6:2571-7
60. Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30:757-62
61. Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A. 2012;109:4245-50
62. Liu L, Wan J, Dai M, Ye X, Liu C, Tang C. et al. Effects of oxygen generating scaffolds on cell survival and functional recovery following acute spinal cord injury in rats. J Mater Sci:Mater Med. 2020;31:115
63. Alaaeldin E, Abu Lila AS, Ando H, Fukushima M, Huang C, Wada H. et al. Co-administration of liposomal l-OHP and PEGylated TS shRNA-lipoplex: a novel approach to enhance anti-tumor efficacy and reduce the immunogenic response to RNAi molecules. J Control Release. 2017;255:210-7
64. Gao S, Fan M, Li Z, Ge K, Liang X-J, Zhang J. Smart calcium peroxide with self-sufficience for biomedicine. Sci China Life Sci. 2020;63:152-6
65. Moya A, Paquet J, Deschepper M, Larochette N, Oudina K, Denoeud C. et al. Human mesenchymal stem cell failure to adapt to glucose shortage and rapidly use intracellular energy reserves through glycolysis explains poor cell survival after implantation. Stem Cells. 2018;36:363-76
66. Deschepper M, Oudina K, David B, Myrtil V, Collet C, Bensidhoum M. et al. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med. 2011;15:1505-14
67. Mylotte LA, Duffy AM, Murphy M, O'Brien T, Samali A, Barry F. et al. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. 2008;26:1325-36
68. Fois MG, Zengin A, Song K, Giselbrecht S, Habibović P, Truckenmüller RK. et al. Nanofunctionalized microparticles for glucose delivery in three-dimensional cell assemblies. ACS Appl Mater Interfaces. 2024;16:17347-60
69. Denoeud C, Luo G, Paquet J, Boisselier J, Wosinski P, Moya A. et al. Enzyme-controlled, nutritive hydrogel for mesenchymal stromal cell survival and paracrine functions. Commun Biol. 2023;6:1266
70. Paquet J, Boisselier J, Bidault L, Deschepper M, Lefebvre E, Gossard C. et al. Glucose delivery system based-hydrogel composite scaffold for enhancing MSC survival. Tissue Eng Part A. 2015;21:S115
71. Zargarzadeh M, Silva AS, Nunes C, Coimbra MA, Custodio CA, Mano JF. Self-glucose feeding hydrogels by enzyme empowered degradation for 3D cell culture. Mater Horiz. 2022;9:694-707
72. Huang CC, Chang CK, Yang PC, Chiu H, Chen SH, Hsu LW. Injectable glucose-releasing microgels enhance the survival and therapeutic potential of transplanted MSCs under ischemic conditions. Adv Healthc Mater. 2025;14:e2401724
73. Bautch VL. Stem cells and the vasculature. Nat Med. 2011;17:1437-43
74. Sun X, Altalhi W, Nunes SS. Vascularization strategies of engineered tissues and their application in cardiac regeneration. Adv Drug Deliv Rev. 2016;96:183-94
75. Hashemi G, Dight J, Khosrotehrani K, Sormani L. Melanoma tumour vascularization and tissue-resident endothelial progenitor cells. Cancers. 2022;14:4216
76. Son J, Mohamed HJ, Ha W, Naren A, Choi C, Kwon YH. et al. Bioprinting of pre-vascularized constructs for enhanced in vivo neo-vascularization. Biofabrication. 2023;15:034101
77. Li ZR, Zeng L, Huang W, Zhang XX, Zhang L, Xie Q. Angiogenic factors and Inflammatory bowel diseases. Biomedicines. 2025;13:1154
78. Lu W, Li X. Vascular stem/progenitor cells: functions and signaling pathways. Cell Mol Life Sci. 2018;75:859-69
79. Faircloth TU, Temple S, Parr RN, Tucker AB, Rajan D, Hematti P. et al. Vascular endothelial growth factor secretion and immunosuppression are distinct potency mechanisms of human bone marrow mesenchymal stromal cells. Stem Cells. 2024;42:736-51
80. Fitzpatrick V, Martín-Moldes Z, Deck A, Torres-Sanchez R, Valat A, Cairns D. et al. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials. 2021;276:120995
81. Pérez-Gutiérrez L, Ferrara N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat Rev Mol Cell Biol. 2023;24:816-34
82. Park J, Yang H, Yi SW, Kim J, Park K. Neoangiogenesis of human mesenchymal stem cells transfected with peptide-loaded and gene-coated PLGA nanoparticles. Biomaterials. 2016;76:226-37
83. Ullah I, Abu-Dawud R, Busch JF, Rabien A, Erguen B, Fischer I. et al. VEGF - Supplemented extracellular matrix is sufficient to induce endothelial differentiation of human iPSC. Biomaterials. 2019;216:119283
84. Lee S, Valmikinathan CM, Byun J, Kim S, Lee G, Mokarram N. et al. Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes. Biomaterials. 2015;63:158-67
85. Kant RJ, Coulombe KLK. Integrated approaches to spatiotemporally directing angiogenesis in host and engineered tissues. Acta Biomater. 2018;69:42-62
86. Ye T, Chai M, Wang Z, Shao T, Liu J, Shi X. 3D-printed hydrogels with engineered nanocrystalline domains as functional vascular constructs. ACS Nano. 2024;18:25765-77
87. Richards D, Jia J, Yost M, Markwald R, Mei Y. 3D bioprinting for vascularized tissue fabrication. Ann Biomed Eng. 2017;45:132-47
88. Guo Y, Wang Z, Zhang X, Li J, Gao S, Lv Y. et al. Microfiber-templated porogel bioinks enable tubular interfaces and microvascularization down to the building blocks for 3D bioprinting. Small. 2025: 2501594.
89. Tomasina C, Bodet T, Mota C, Moroni L, Camarero-Espinosa S. Bioprinting vasculature: materials, cells and emergent techniques. Materials. 2019;12:2701
90. Rofaani E, Mardani MW, Yutiana PN, Amanda O, Darmawan N. Differentiation of mesenchymal stem cells into vascular endothelial cells in 3D culture: a mini review. Mol Biol Rep. 2024;51:781
91. Chiesa I, De Maria C, Lapomarda A, Fortunato GM, Montemurro F, Di Gesù R. et al. Endothelial cells support osteogenesis in an in vitro vascularized bone model developed by 3D bioprinting. Biofabrication. 2020;12:025013
92. Kuss MA, Wu SH, Wang Y, Untrauer JB, Li WL, Lim JY. et al. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J Biomed Mater Res, Part B. 2018;106:1788-98
93. Cheng S, Li Y, Yu C, Deng Z, Huang J, Zhang Z. 3D bioprinted tumor-vessel-bone co-culture scaffold for breast cancer bone metastasis modeling and drug testing. Chem Eng J. 2023;476:146685
94. Mir A, Lee E, Shih W, Koljaka S, Wang A, Jorgensen C. et al. 3D bioprinting for vascularization. Bioengineering. 2023;10:606
95. Grottkau BE, Hui Z, Ye C, Pang Y. 3D-printed insert-array and 3D-coculture-array for high-throughput screening of cell migration and application to study molecular and cellular influences. Biomed Mater. 2020;15:055028
96. Fang YC, Guo YH, Wu BY, Liu ZB, Ye M, Xu YY. et al. Expanding embedded 3D bioprinting capability for engineering complex organs with freeform vascular networks. Adv Mater. 2023;35:202205082
97. Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev. 2019;119:4881-985
98. D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813-24
99. Zhao TJ, Wu W, Sui LH, Huang Q, Nan YY, Liu JH. et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 2022;7:47-72
100. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122:877-902
101. Abot A, Fried S, Cani PD, Knauf C. Reactive oxygen species/reactive nitrogen species as messengers in the gut: impact on physiology and metabolic disorders. Antioxid Redox Signal. 2021;37:394-415
102. Korovesis D, Rubio-Tomas T, Tavernarakis N. Oxidative stress in age-related neurodegenerative diseases: an overview of recent tools and findings. Antioxidants. 2023;12:131
103. Klemmensen MM, Borrowman SH, Pearce C, Pyles B, Chandra B. Mitochondrial dysfunction in neurodegenerative disorders. Neurotherapeutics. 2024;21:e00292
104. Yutani R, Venketaraman V, Sheren N. Treatment of acute and long-COVID, diabetes, myocardial infarction, and alzheimer's disease: the potential role of a novel nano-compound-the transdermal glutathione-cyclodextrin complex. Antioxidants. 2024;13:1106
105. Cho I, Jeong KH, Zhu J, Choi YH, Cho KH, Heo K. et al. Sirtuin3 protected against neuronal damage and cycled into nucleus in status epilepticus model. Mol Neurobiol. 2019;56:4894-903
106. Zhu Q, Hao H, Xu HF, Fichman Y, Cui YQ, Yang CL. et al. Combination of antioxidant enzyme overexpression and n-acetylcysteine treatment enhances the survival of bone marrow mesenchymal stromal cells in ischemic limb in mice with type 2 diabetes. J Am Heart Assoc. 2021;10:e023491
107. Qi S, Zhang P, Ma M, Yao M, Wu J, Makila E. et al. Cellular internalization-induced aggregation of porous silicon nanoparticles for ultrasound imaging and protein-mediated protection of stem cells. Small. 2019;15:201804332
108. Petro M, Jaffer H, Yang J, Kabu S, Morris VB, Labhasetwar V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials. 2016;81:169-80
109. Balachandran YL, Wang W, Yang H, Tong H, Wang L, Liu F. et al. Heterogeneous iron oxide/dysprosium oxide nanoparticles target liver for precise magnetic resonance imaging of liver fibrosis. ACS Nano. 2022;16:5647-59
110. Morry J, Ngamcherdtrakul W, Yantasee W. Oxidative stress in cancer and fibrosis: opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017;11:240-53
111. Tong L, Chuang C-C, Wu S, Zuo L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015;367:18-25
112. Barzegari A, Omidi Y, Landon R, Gueguen V, Parvizpour S, Meddahi-Pelle A. et al. The protective effect of N-acetylcysteine on antimycin A-induced respiratory chain deficiency in mesenchymal stem cells. Chem Biol Interact. 2022;360:109937
113. Khoshlahni N, Sagha M, Mirzapour T, Zarif MN, Mohammadzadeh-Vardin M. Iron depletion with deferoxamine protects bone marrow-derived mesenchymal stem cells against oxidative stress-induced apoptosis. Cell Stress Chaperones. 2020;25:1059-69
114. Asl SS, Gharebaghi A, Shahidi S, Afshar S, Kalhori F, Amiri K. et al. Deferoxamine preconditioning enhances the protective effects of stem cells in streptozotocin-induced Alzheimer's disease. Life Sci. 2021;287:120093
115. Ghorbani M, Derakhshankhah H, Jafari S, Salatin S, Dehghanian M, Falahati M. et al. Nanozyme antioxidants as emerging alternatives for natural antioxidants: achievements and challenges in perspective. Nano Today. 2019;29:100775
116. Han L, Li C, Zhang T, Lang Q, Liu A. Au@Ag heterogeneous nanorods as nanozyme interfaces with peroxidase-like activity and their application for one-pot analysis of glucose at nearly neutral pH. ACS Appl Mater Interfaces. 2015;7:14463-70
117. Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y. et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev. 2019;48:1004-76
118. Huang Y, Ren J, Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev. 2019;119:4357-412
119. Chong Y, Liu Q, Ge C. Advances in oxidase-mimicking nanozymes: classification, activity regulation and biomedical applications. Nano Today. 2021;37:101076
120. Kim J, Kim HY, Song SY, Go SH, Sohn HS, Baik S. et al. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano. 2019;13:3206-17
121. Zhao JW, Wang YG, Wang WJ, Tian Y, Gan ZD, Wang YL. et al. In situ growth of nano-antioxidants on cellular vesicles for efficient reactive oxygen species elimination in acute inflammatory diseases. Nano Today. 2021;40:101282
122. Pagliari F, Mandoli C, Forte G, Magnani E, Pagliari S, Nardone G. et al. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6:3767-75
123. Amani H, Habibey R, Hajmiresmail SJ, Latifi S, Pazoki-Toroudi H, Akhavan O. Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries. J Mater Chem B. 2017;5:9452-76
124. Ren SS, Zhou Y, Fan RY, Peng WZ, Xu XW, Li L. et al. Constructing biocompatible MSN@Ce@PEG nanoplatform for enhancing regenerative capability of stem cell via ROS-scavenging in periodontitis. Chem Eng J. 2021;423:130207
125. Balupuri A, Lee DY, Lee MH, Chae S, Jung E, Kim Y. et al. Design, synthesis, docking and biological evaluation of 4-phenyl-thiazole derivatives as autotaxin (ATX) inhibitors. Bioorg Med Chem Lett. 2017;27:4156-64
126. Feng Q, Li Q, Lin Z, Xu X, Huang Y, Dong H. et al. Injectable microspheres with cartilage-like structure facilitate inflammation inhibition and tissue regeneration. Adv Healthc Mater. 2025;14:202404877
127. Al Sulaiman D, Chang JYH, Bennett NR, Topouzi H, Higgins CA, Irvine DJ. et al. Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano. 2019;13:9620-8
128. Peng Y, Chen X, Zhang Q, Liu S, Wu W, Li K. et al. Enzymatically bioactive nucleus pulposus matrix hydrogel microspheres for exogenous stem cells therapy and endogenous repair strategy to achieve disc regeneration. Adv Sci. 2024;11:2304761
129. Chen J, Pan C, Gao Y, Chen Q, An X, Liu Z. Reactive oxygen species scavenging injectable hydrogel potentiates the therapeutic potential of mesenchymal stem cells in skin flap regeneration. ACS Appl Mater Interfaces. 2024;16:17120-8
130. Yang Q, Miao Y, Luo J, Li C, Li X, Chen Y. et al. Nanofibril-structured granular hydrogels harness stem cell retention and immunoregulation in diabetic microenvironment. ACS Nano. 2025;19:6795-814
131. Li LM, Xiao B, Mu JF, Zhang Y, Zhang CY, Cao HC. et al. A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano. 2019;13:14283-93
132. Khodaei A, Nawaz Q, Zhu ZQ, Yavari SA, Weinans H, Boccaccini AR. Biomolecule and ion releasing mesoporous nanoparticles: nonconvergent osteogenic and osteo-immunogenic performance. ACS Appl Mater Interfaces. 2024;16:67491-503
133. Liu TF, Xiao BW, Xiang F, Tan JL, Chen Z, Zhang XR. et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11:2788
134. Chen XX, Wang WJ, Hu YH, Sun J, Zhang LG, Chen Y. et al. In situ assembled metal-phenolic nanozyme biointerfaces revitalize stem cells and optimize diabetic implant osseointegration. Adv Healthc Mater. 2025;14:2404804
135. Lv Y, Yu C, Li X, Bao H, Song S, Cao X. et al. ROS-activatable nanocomposites for CT imaging tracking and antioxidative protection of mesenchymal stem cells in idiopathic pulmonary fibrosis therapy. J Control Release. 2023;357:249-63
136. Cheng Y, Cheng S, Chen H, Hsu W. Development of injectable graphene oxide/laponite/gelatin hydrogel containing Wharton's jelly mesenchymal stem cells for treatment of oxidative stress-damaged cardiomyocytes. Colloids Surf B Biointerfaces. 2022;209:112150
137. Zhou J, Li Y, He J, Liu L, Hu S, Guo M. et al. ROS scavenging graphene-based hydrogel enhances type H vessel formation and vascularized bone regeneration via ZEB1/Notch1 mediation. Macromol Biosci. 2023;23:202200502
138. Lu F, Yang S, Song Y, Zhai C, Wang Q, Ding G. et al. Hydroxyl functionalized carbon dots with strong radical scavenging ability promote cell proliferation. Mater Res Express. 2019;6:065030
139. Lee S, Choe G, Yi J, Kim J, Lee SH, Jeon J. et al. ROS-scavenging ultrasonicated graphene oxide/alginate microgels for mesenchymal stem cell delivery and hindlimb ischemia treatment. Mater Today Bio. 2024;29:101289
140. Choe G, Kim S-W, Park J, Park J, Kim S, Kim YS. et al. Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: mesenchymal stem cell encapsulation and regeneration of infarcted hearts. Biomaterials. 2019;225:119513
141. Li YH, Li J, Zhong YX, Zhang QS, Wu YC, Huang JP. et al. pH-responsive and nanoenzyme-loaded artificial nanocells relieved osteomyelitis efficiently by synergistic chemodynamic and cuproptosis therapy. Biomaterials. 2025;313:122762
142. Wu JS, Chen MW, Xiao Y, Yang H, Wang GY, Zhang XH. et al. The bioactive interface of titanium implant with both anti-oxidative stress and immunomodulatory properties for enhancing osseointegration under diabetic condition. Adv Healthc Mater. 2024;13:e2401974
143. Higbee-Dempsey EM, Amirshaghaghi A, Case MJ, Bouché M, Kim J, Cormode DP. et al. Biodegradable gold nanoclusters with Improved excretion due to pH-triggered hydrophobic-to-hydrophilic transition. J Am Chem Soc. 2020;142:7783-94
144. Liew SS, Zeng Z, Cheng P, He S, Zhang C, Pu K. Renal-clearable molecular probe for near-infrared fluorescence imaging and urinalysis of SARS-CoV-2. J Am Chem Soc. 2021;143:18827-31
145. Li X, Li Y, Yu C, Bao H, Cheng S, Huang J. et al. ROS-responsive janus Au/mesoporous silica core/shell nanoparticles for drug delivery and long-term CT imaging tracking of MSCs in pulmonary fibrosis treatment. ACS Nano. 2023;17:6387-99
146. Yu C, Bao H, Chen Z, Li X, Liu X, Wang W. et al. Enhanced and long-term CT imaging tracking of transplanted stem cells labeled with temperature-responsive gold nanoparticles. J Mater Chem B. 2021;9:2854-65
147. Yu C, Chen Z, Li X, Bao H, Wang Y, Zhang B. et al. pH-triggered aggregation of gold nanoparticles for enhanced labeling and long-term CT imaging tracking of stem cells in pulmonary fibrosis treatment. Small. 2021;17:2101861
148. Wolff A, Frank M, Staehlke S, Springer A, Hahn O, Meyer J. et al. 3D spheroid cultivation alters the extent and progression of osteogenic differentiation of mesenchymal stem/stromal cells compared to 2D cultivation. Biomedicines. 2023;11:1049
149. Ansah IB, Kim S, Yang JY, Mun C, Jung HS, Lee S. et al. In situ electrodeposition of gold nanostructures in 3D ultra-thin hydrogel skins for direct molecular detection in complex mixtures with high sensitivity. Laser Photon Rev. 2021;15:2100316
150. Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol. 2019;98:151041
151. Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E. et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483-96
152. Kim W, Gwon Y, Park S, Kim H, Kim J. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact Mater. 2023;19:50-74
153. Meng Qiu, Dou Wang, Hao Huang, Teng Yin, Wenli Bao, Bin Zhang. et al. A regioselectively oxidized 2D Bi/BiOx lateral nano-heterostructure for hypoxic photodynamic therapy. Adv Mater. 2021;33:2102562
154. Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015-24
155. Chen L, Wang H, Huang C. Modulation of inherent niches in 3D multicellular MSC spheroids reconfigures metabolism and enhances therapeutic potential. Cells. 2021;10:2747
156. Jaukovic A, Abadjieva D, Trivanovic D, Stoyanova E, Kostadinova M, Pashova S. et al. Specificity of 3D MSC spheroids microenvironment: impact on MSC behavior and properties. Stem Cell Rev Rep. 2020;16:853-75
157. Xu Y, Shi T, Xu A, Zhang L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J Cell Mol Med. 2016;20:1203-13
158. Lee M, Song BR, Kim DH, Ha J, Lee M, Choi SJ. et al. Up-regulation of superoxide dismutase 2 in 3D spheroid formation promotes therapeutic potency of human umbilical cord blood-derived mesenchymal stem cells. Antioxidants. 2020;9:66
159. He J, Zhang N, Zhu Y, Jin R, Wu F. MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-Akt signaling pathway. Biomaterials. 2021;265:120448
160. Yu SJ, Choi G, Cho Y, Lee M, Cho Y, Shin JH. et al. Three-dimensional spheroid culture on polymer-coated surface potentiate stem cell functions via enhanced cell-extracellular matrix interactions. ACS Biomater Sci Eng. 2020;6:2240-50
161. Ohori-Morita Y, Niibe K, Limraksasin P, Nattasit P, Miao X, Yamada M. et al. Novel mesenchymal stem ell spheroids with enhanced stem cell characteristics and bone regeneration ability. Stem Cells Transl Med. 2022;11:434-49
162. Kim S, Park J, Kim EM, Choi J, Kim H, Chin IL. et al. Lotus seedpod-inspired hydrogels as an all-in-one platform for culture and delivery of stem cell spheroids. Biomaterials. 2019;225:119534
163. Rossen NS, Anandakumaran PN, zur Nieden R, Lo K, Luo W, Park C. et al. Injectable therapeutic organoids using sacrificial hydrogels. iScience. 2020;23:101052
164. Huang CC, Wei HJ, Lin KJ, Lin WW, Wang CW, Pan WY. et al. Multimodality noninvasive imaging for assessing therapeutic effects of exogenously transplanted cell aggregates capable of angiogenesis on acute myocardial infarction. Biomaterials. 2015;73:12-22
165. Huang C, Pan W, Tseng MT, Lin K, Yang Y, Tsai H. et al. Enhancement of cell adhesion, retention, and survival of HUVEC/cbMSC aggregates that are transplanted in ischemic tissues by concurrent delivery of an antioxidant for therapeutic angiogenesis. Biomaterials. 2016;74:53-63
166. Yu C, Juong J, Lin Y, Kuo C, Hsieh L, Huang C. Enhancement of subcutaneously transplanted beta cell survival using 3D stem cell spheroids with proangiogenic and prosurvival potential. Adv Biosyst. 2020;4:e1900254
167. Zhang L, Tang H, Xiahou Z, Zhang J, She Y, Zhang K. et al. Solid multifunctional granular bioink for constructing chondroid basing on stem cell spheroids and chondrocytes. Biofabrication. 2022;14:035003
168. Liang LT, Li Z, Yao B, Enhe J, Song W, Zhang C. et al. Extrusion bioprinting of cellular aggregates improves mesenchymal stem cell proliferation and differentiation. Biomater Adv. 2023;149:213369
169. Lin F, Li X, Sun SY, Li ZY, Lv CL, Bai JB. et al. Mechanically enhanced biogenesis of gut spheroids with instability-driven morphomechanics. Nat Commun. 2023;14:6016
170. Quintard C, Tubbs E, Jonsson G, Jiao J, Wang J, Werschler N. et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat Commun. 2024;15:1452
171. Acharya P, Joshi P, Shrestha S, Choi NY, Jeong S, Lee M. Uniform cerebral organoid culture on a pillar plate by simple and reproducible spheroid transfer from an ultralow attachment well plate. Biofabrication. 2024;16:1758-5090
172. Bao H, Cheng S, Li X, Li Y, Yu C, Huang J. et al. Functional Au nanoparticles for engineering and long-term CT imaging tracking of mesenchymal stem cells in idiopathic pulmonary fibrosis treatment. Biomaterials. 2022;288:121731
Author contact
![]() Corresponding authors: Chenggong Yu, School of Pharmacy, Wannan Medical College, Wuhu 241002, China; Email: cgyu2018ac.cn. Yan Wang, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Wuhu 241001, China; Email: wangyan_scucom. Sihui Nian, School of Pharmacy, Wannan Medical College, Wuhu 241002, China; Email: niansihuicom.
Corresponding authors: Chenggong Yu, School of Pharmacy, Wannan Medical College, Wuhu 241002, China; Email: cgyu2018ac.cn. Yan Wang, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Wuhu 241001, China; Email: wangyan_scucom. Sihui Nian, School of Pharmacy, Wannan Medical College, Wuhu 241002, China; Email: niansihuicom.
 Global reach, higher impact
Global reach, higher impact