13.3
Impact Factor
Theranostics 2025; 15(18):9508-9532. doi:10.7150/thno.120300 This issue Cite
Review
Advances and challenges in decellularized adipose tissue based composite hydrogels for adipose tissue regeneration: a review over the last fifteen years
1. College of Life Sciences, Institute of Life Science and Green Development, Hebei University, Baoding, 071002, China.
2. Department of Tissue Engineering, School of Intelligent Medicine, China Medical University, No. 77 Puhe Road, Shenyang North New Area, Shenyang, Liaoning Province, 110122, China.
3. Baoding Key Laboratory of Cancer and Aging, Hebei University, Baoding, 071002, China.
4. Vascular Neurology, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Received 2025-6-25; Accepted 2025-8-13; Published 2025-8-30
Abstract
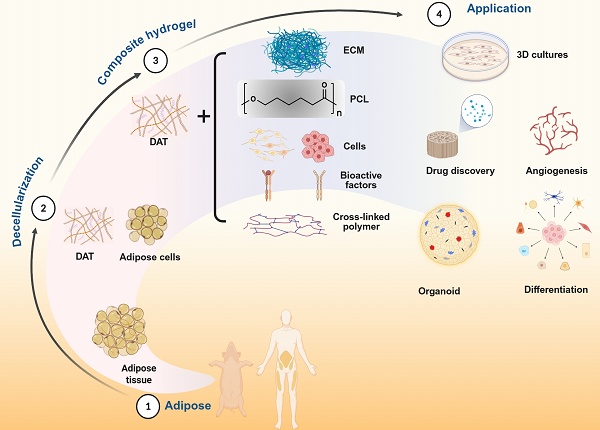
Adipose tissue regeneration has emerged as a transformative strategy for addressing soft-tissue defects resulting from trauma, oncologic resection, and burn injuries, leveraging adipose tissue's dual role as a dynamic endocrine organ that regulates systemic metabolism. Decellularized adipose tissue (DAT) scaffolds hold significant promise in adipose tissue regeneration due to their unique preservation of pro-adipogenic and structurally preserved extracellular matrix (ECM) components. However, their clinical translation faces bottlenecks, including inadequate compressive modulus, unpredictable biodegradation kinetics and limited neovascularization capacity. This review critically synthesizes methodological advancements in DAT processing, systematically evaluating protocol efficacy in DAT preservation versus immunogenic residue elimination while assessing their translational potential as implant materials. Building upon methodological innovations in DAT composite hydrogel engineering since 2013, this overview concurrently elucidates mechanobiological regulation paradigms governing hydrogel functionality and evaluates crosslinking strategies that optimize structural fidelity. Critical challenges and emerging frontiers are also discussed. The current comparative assessment of material performance metrics may offer new insights for further investigation and translational optimization in DAT based composite hydrogels for repair of soft tissue defects.
Keywords: Adipose tissue regeneration, Decellularized adipose tissue, Composite hydrogels, Soft-tissue defects, Translational challenges.
1. Introduction
Currently, plastic surgeons commonly use autologous adipose tissue for surgical grafts in wound repair [1-5], as well as in various cosmetic reconstructive procedures [2, 6]. However, despite its widespread application, this approach is limited by several factors, including a shortage of donor tissue, the risk of liquefaction, and the potential for necrosis [7]. To address these challenges, decellularized adipose tissue (DAT) presents a promising alternative [8, 9]. DAT represents a valuable renewable extracellular matrix (ECM) that serves as a biological scaffold for autologous and allogeneic transplantation, facilitating tissue growth and regeneration. Moreover, DAT can also facilitate the delivery of therapeutic agents, such as drugs [10], growth factors (GFs), and/or stem cells[11-15]. The application of DAT has demonstrated significant benefits in the treatment of various diseases, including genetic defects of the breast and chest wall, such as Poland syndrome, systemic adipose atrophy [16], lipodystrophy combined Crohn's disease [17], and facial deformities like Treacher Collins syndrome [18], as well as Barraquer-Simons syndrome [19]. Furthermore, DAT's clinical application potential is further enhanced by its intrinsic adipogenic properties [15, 20], along with the growing recognition of adipose tissue as a critical endocrine organ [21]. In summary, DAT not only holds significant promise for various medical applications but also presents a more sustainable solution compared to autologous adipose tissue.
Hydrogels are three-dimensional polymeric networks formed via physical or chemical crosslinking methods, exhibiting high water content that mimics biological tissues, and particularly those derived from biological sources possess inherent biodegradability and are suitable for use in minimally invasive techniques [22]. Notably, ECM-based hydrogels have gained increasing prominence in recent years [9], as ECM itself comprises a complex architecture of structural macromolecules, including collagens and fibronectin, as well as proteoglycans like glycosaminoglycans (GAGs) [23]. Collectively, these components establish a dynamic microenvironment that regulates cellular adhesion, proliferation, and phenotype maintenance through biomechanical and biochemical signaling [23], which is crucial for supporting tissue growth and regeneration. Thus, the use of ECM-based hydrogels is particularly advantageous in tissue engineering and regenerative medicine.
DAT hydrogels have shown potential in promoting the proliferation and differentiation of stem cells, with the expression of adipogenic genes, such as peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer binding protein α (C/EBPα), and lipoprotein lipase [8]. However, DAT hydrogels face several limitations that impede their efficacy in Adipose tissue regeneration. These include inadequate mechanical properties, instability, and insufficient vascularization, which collectively hinder the formation and functionality of engineered adipose tissue [24, 25]. The difficulty of large-scale adipose tissue regeneration is exacerbated by hypoxia in the core regions, which induces apoptosis. To address these issues, it is crucial to establish adequate vascularization, as this is essential for supporting long-term cell proliferation and differentiation. Furthermore, the long-standing issue of insufficient vascular supply limits its application in the treatment of severe burns, trauma, tumor resection, and soft tissue defects caused by congenital deformities, as it often leads to the resorption and necrosis of newly formed adipose tissue [26]. Therefore, overcoming the challenges related to vascularization is vital for the successful application of DAT hydrogels in adipose tissue regeneration.
DAT-based composite hydrogels, including the composite of scaffold materials, the addition of cells, and the addition of active factors such as growth factors may provide new strategies to overcome these challenges and improve the clinical applicability of adipose tissue regeneration. Further, DAT-based composite hydrogels for adipose tissue regeneration are evolving from structural repair to functional regeneration and may become a disruptive platform for metabolic disease treatment in the future. This review systematically summarizes the preparation methods of DAT and its current challenges, with a focused overview of the advances and challenges in DAT hydrogels and DAT-based composite hydrogels for adipose tissue regeneration. Building on this foundation, we propose future directions to bridge critical technological gaps, aiming to optimize the practical utility of DAT and its composite hydrogels in adipose tissue regeneration while providing theoretical foundations for clinical translation.
2. Development timeline of DAT
The development of DAT scaffolds and advancements in decellularization technologies are presented in the chronological timeline in Figure 1. Pioneering work in 2010 established fundamental protocols, with Flynn [11] and Choi et al. [27] independently developing human DAT isolation methods and elucidating its regulatory role in adipose-derived stem cells (ASCs) differentiation. The following year saw methodological diversification, particularly Brown et al.'s [28] optimization of porcine DAT processing, which expanded interspecies applicability. This era marked DAT's emergence as a multifunctional, biocompatible scaffold for adipogenesis, vascular engineering, and controlled therapeutic delivery. Notable milestones include Young et al.'s [29] 2011 demonstration of DAT hydrogel formation through in vivo validation, and Lin et al.'s [30] groundbreaking application of allogeneic DAT/ASCs composites for rat cavernous nerve regeneration - establishing neural repair as a novel application domain.
In 2012, Choi et al. [31] reported decellularized scaffolds of xenobiotic materials, i.e., porcine DAT, for tissue engineering and performed in vitro and in vivo studies. They demonstrated that the DAT had biochemical and mechanical properties conducive to human cell adhesion and growth, while maintaining biocompatibility, long-term stability, and bioinductivity qualities in vivo. Later, Choi et al. [32] studied the effects of human DAT on different types of cells and found that the cells exhibited robust propagation, proliferation, and successfully integration into the DAT. These findings indicate that DAT holds great promise as a potential substitute for defective or damaged human tissue. Concurrently, Turner et al. [33] engineered DAT microcarriers enabling spatial control of human ASCs differentiation, achieving enhanced neo-adipogenesis and vascularization in rodent models. This period also witnessed innovative crosslinking strategies, particularly Wu et al.'s use of hexamethylene diisocyanate and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) to enhance the hydrogel's resistance to enzymatic degradation [34]. Importantly, DAT's regenerative versatility was further expanded through synergistic combinations with bone marrow mesenchymal stem cells [35] and hematopoietic progenitor cells [36], establishing multimodal tissue restoration paradigms.
The year 2013 witnessed transformative innovations in DAT scaffold engineering. Pioneering work by Yu et al. employed electrospraying technology to fabricate hierarchically porous DAT foams with tunable micro/macropore architectures, demonstrating their efficacy as tunable biomimetic scaffolds for volumetric adipose restoration [37]. Importantly, matching the foam stiffness to that of natural tissue enhanced the adipogenesis. Electrospray technology has since been used to fabricate matrix-derived microcarriers using DAT [38-40]. Concurrently, Lu et al. engineered a heparin-functionalized DAT platform via 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxy-succinimide (EDC/NHS), enabling sustained basic fibroblast growth factor (bFGF) release to drive vascularized adipogenesis [41]. These advancements catalyzed DAT's emergence as a versatile delivery matrix for controlled release of small molecules, growth factors, and immunoregulatory agents, establishing foundational paradigms for precision tissue engineering.
The progressive refinement of decellularization methodologies has positioned DAT as a pivotal biomaterial source for bioink formulation in 3D bioprinting applications. DAT hydrogels have been used in 3D bioprinting since 2014 [42]. Pioneering work by Pati et al. (2015) demonstrated that encapsulating human adipose-derived stem cells (HASCs) within DAT-based bioinks significantly enhanced cell differentiation potential, and soft tissue regeneration [43]. Recent advances in proteomic profiling have systematically characterized species-specific DAT compositions (human vs. porcine), providing a molecular blueprint for rational scaffold optimization [44, 45]. Emerging evidence emphasizes the method-dependent nature of decellularized matrix proteomes, where collagenase-assisted processing improves detection sensitivity for low-abundance regulatory proteins [46, 47].
Timeline of DAT development. Decellularization technology for human and porcine adipose decellularization technology was first reported in 2010 [11, 27] and 2011 [28], respectively. Also in 2011, human-derived DAT was first studied in vivo [29]. In 2012, the first in vivo study of porcine-derived DAT was presented [31]. In 2013, electrospray technology was first applied to produce DAT microcarriers [37]. DAT hydrogels have been used in 3D bioprinting since 2014 [42], and the development of 3D printed structures containing DAT bioink with ASCs followed in 2015 [43]. Recently, an in-depth analysis of the proteomics of DAT derived from human [46, 47] and porcine [44] sources was conducted. Tumor models were 3D printed in 2022 using gelatin methacrylic anhydride compounded with DAT bioinks [60]. In 2024, Feng et al. introduced a classification scheme for DAT, distinguishing between T-DAM and F-DAM [48]. Their study findings suggest that F-DAM was more conductive to promote adipose regeneration. In 2025, Feng et al. observed significantly higher mitochondrial density and enhanced adipose regeneration in superficial DAT compared to deep DAT [49]. Adapted with permission from [46], copyright 2018 John Wiley and Sons Inc. Created in https://BioRender.com.
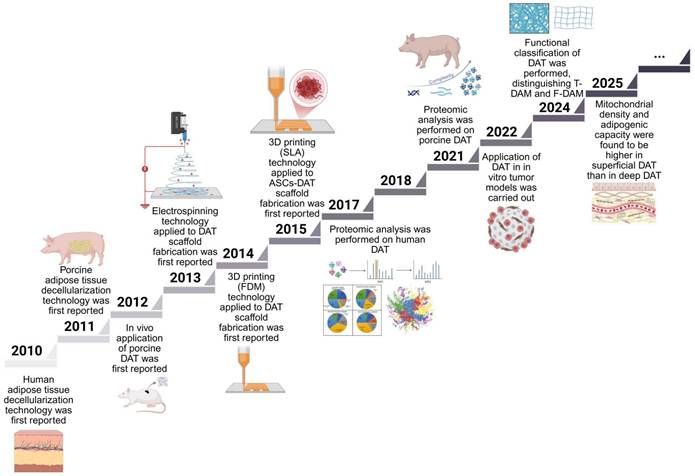
Building on these insights, Feng et al. (2024) introduced a functional classification of decellularized adipose matrix (DAM), distinguishing between tough decellularized adipose-derived matrix (T-DAM) and fine decellularized adipose-derived matrix (F-DAM) [48]. Their work demonstrated that only F-DAM, characterized by its loose fiber architecture and enrichment in matrix / adipogenic proteins, effectively supports adipose tissue regeneration. This structural dichotomy offers a compelling explanation for the previously observed heterogeneity in adipogenic outcomes following DAT implantation. Subsequently, their study (2025) demonstrated that superficial DAT exhibits a higher mitochondrial density and adipogenic capacity compared to deeper layers [49]. Critically, they showed that the regenerated fat induced by DAT remained structurally stable and biologically viable for up to one year in vivo, with a substantial proportion of adipocytes persisting long-term. These findings provide robust preclinical evidence supporting the long-term regenerative efficacy of DAT-based scaffolds.
DAT has emerged as a versatile and bioactive platform for regenerative medicine. It supports the dynamic expansion and functional maintenance of diverse cell types, including adipose stem cells [50-52], fibroblasts [40, 53], hematopoietic progenitor cells [36], hepatocarcinoma cells [54], endothelial cells [55] and others. Beyond cell culture applications, DAT has revolutionized tissue engineering strategies for the reconstruction of epidural adipose tissue [56], treatment of diabetes [57], and the study of stromal-cell metabolism [58]. Recent advances also highlight its potential as a delivery platform for controlled and sustained release of therapeutic drugs and bioactive mediators [10, 59]. Moreover, DAT provides a more physiologically relevant and human-derived matrix for the design of preclinical animal models and clinical translational studies. Moreover, DAT provides a more physiologically relevant and human-derived matrix for the design of preclinical animal models and clinical translational studies [60]. Notably, in 2022, DAT was successfully integrated with GelMA bioinks for 3D bioprinting of tumor models, marking a novel application of DAT in cancer tissue engineering [60]. In parallel, DAT-based scaffolds have been increasingly applied in the regeneration of musculoskeletal tissues, including skeletal muscle [61] and bone [24], where its extracellular matrix components and pro-regenerative signals contribute to enhanced tissue integration and functional recovery. These emerging applications point to DAT's broad translational potential across multiple disease contexts, including wound healing [1] and ischemic tissue repair [36]. Ultimately, these cumulative advances delineate DAT as a paradigm-shifting biomaterial, establishing novel pathways for human tissue-derived matrix applications in regenerative medicine.
3. Decellularization methods for DAT
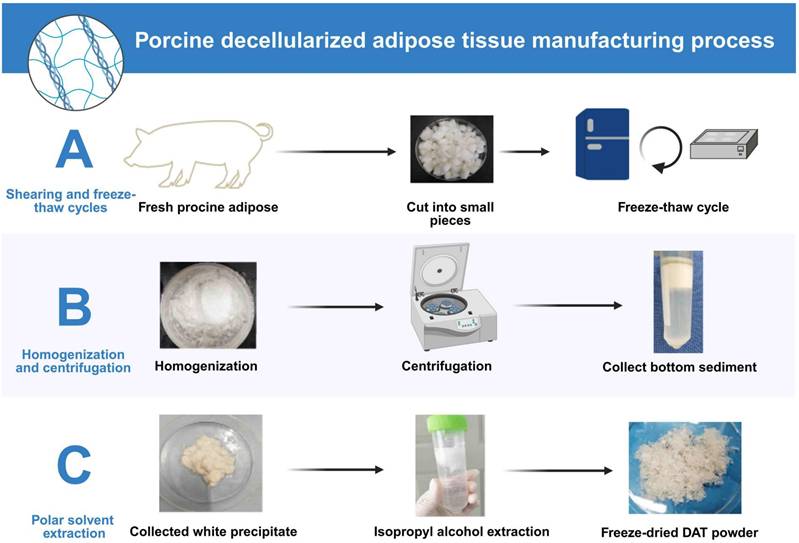
This section provides an evaluation of various decellularization protocols, some of which have been optimized to maximize the retention of functional ECM components by controlling detergent type and exposure time. Additionally, novel decellularization methods that do not rely on chemical detergents are also discussed. Current decellularization protocols for porcine and human adipose tissue exhibit methodological convergence with species-specific adaptations (Table 1). Based on a comprehensive analysis of the literature and the clinical translational potential of each technique, the most representative and widely adopted decellularization methods for porcine and human adipose tissues were selected. For porcine adipose tissue, the optimized protocol described by Brown et al. (Method A) was identified as optimal due to its thorough cell removal, excellent preservation of extracellular matrix (ECM) structure, efficient lipid removal, and strong support for ASCs adhesion and differentiation. For human adipose tissue, the method detailed in Ren et al. (Method D) was recognized as the most effective, combining enzymatic and detergent treatments to achieve efficient decellularization while maintaining essential ECM components and biocompatibility. Both protocols stand out for reproducibility, clinical relevance, and frequent application in experimental and translational research. Porcine adipose decellularization is performed at 4 °C or below 37 °C [8], and typically involves sequential steps. First, harvested porcine adipose is cut into fragments or pieces. Then, freeze-thaw cycle decellularization is performed, followed by digestion with an enzyme solution, extraction with polar solvents such as isopropanol, and washing with detergent (Fig. 2). In this process, after each step, the remaining reagent must be washed away with phosphate-buffered saline (PBS) [8, 28], especially when the lipids are extracted using polar solvents. The decellularization process for human adipose is similar to that for porcine adipose. However, in human liposuction is generally performed to obtain the adipose. Moreover, as fresh adipose should be used for decellularization [36], the adipose must be transported to the laboratory on ice, usually within 2 hours [11]. During decellularization, some active factors are lost. To minimize this loss, a mild ionic detergent should be chosen. The process is best performed on ice or at 4 °C.
Decellularization methods (Methods A‒C and D‒G is porcine and human adipose decellularization workflow, respectively).
| Step / Feature | Method A [28] | Method B [28] | Method C [28] | Method D [11, 35] | Method E [69] | Method F [46] | Method G [96] |
|---|---|---|---|---|---|---|---|
| 1 | Thaw tissue | Thaw tissue | Thaw tissue | Freeze-thaw 3 times | Wash the adipose tissue | Thaw adipose tissue in NaCl buffer at room temperature | Store the lipoaspirate at -80 °C |
| 2 | 0.02% Trypsin/0.05% ethylenediamine-tetraacetic acid (EDTA) | Digest collagenase (3 mg/g dry weight) | Digest collagenase (3 mg/g dry weight) + 0.05% EDTA | Incubate overnight in enzymatic digestion solution | 1:1 mix adipose tissue/distilled water and centrifuge | Homogenization and dispersion | Thaw frozen tissues and wash with PBS for 2 h |
| 3 | 3% Triton X-100 → 4% Deoxycholate | 0.02% trypsin/0.05% ethylenediamine-tetraacetic acid 10 U/mL deoxyribonuclease | 0.1% NP40 → 4% Deoxycholate → 1% SDS | Polar solvent extraction | Denature for 12 h in 4 M urea buffer containing protease inhibitor | Centrifugation, repeat the homogenization step and add 2M urea buffer | 1% sodium dodecyl suladiposee (SDS) for 2 h |
| 4 | 4% Ethanol + 0.1% Peracetic acid | 10 U/mL lipase | 0.9% NaCl + Tris-HCl + inhibitors | Incubate for 6 h in enzymatic digestion solution | Re-extract residues for 12 h with 4 M guanidine containing and centrifugation | Soak for 12-24 h in PBS solution containing 2.5 mM sodium deoxycholate | |
| 5 | 100% n-propanol and lyophilized | Lyophilization | Rinse three times and lyophilize | Wash 3 times (30 min each) in rinsing buffer solution | Filter and dialyze the supernatant | Centrifuge at 23, 000 ×g for 20 min at 4 °C and collect supernatant | Incubate in a solution containing DNase and RNase |
| Advan-tages | Complete decellularization, minimal lipid, high ECM retention | Simple process, efficient decellularization | Detergent-based, broad protein removal | Simple, strong lipid removal, injectable hydrogel | Good decellularization, hydrogel potential | Broad pathogen removal, low toxicity | Effective lipid removal, ECM retention |
| Disadvan-tages | More complex steps, multi-reagent, time-consuming | Lipid residue remains, lower GAG/growth factors | High DNA/lipid residue, poor ECM preservation | Harsh detergents, long duration | SDS may damage ECM | Lipid residue remains, ECM alteration | Long process, multi-enzyme, cost |
The decellularization process of porcine adipose. (A) Fresh porcine adipose tissue is cut into small pieces and subjected to freeze-thaw cycles to lyse cells. (B) The tissue is then homogenized and centrifuged to isolate ECM-rich sediment. (C) The precipitate undergoes isopropyl alcohol extraction to remove lipids, followed by freeze-drying to obtain DAT powder. This protocol efficiently removes cellular components while preserving ECM integrity. Created in https://BioRender.com.
Current standards mandate residual DNA fragments <200 bp with total content <50 ng dsDNA/mg ECM (dry weight) to ensure biocompatibility [62]. While enzymatic and chemical decellularization protocols effectively eliminate cellular components without inducing macrophage-mediated pro-inflammatory responses, critical challenges persist regarding ECM component preservation [44]. Despite differences in decellular-ization methods, scanning electron microscopy-based and histochemical analyses indicate that the physical structures of the decellularization products obtained using the various decellularization methods are highly similar [44, 46]. The urea solvent method developed by Porch et al. replicates Matrigel™'s thermal stability and injectability, but proteomic differences between commercial and decellularized matrices suggest that composition variations, related to the decellularization method, may affect clinical translatability [46]. Kuljanin et al. suggested that collagenase treatment would enhance proteomic coverage of low abundance proteins in DAT [47]. Although enzymatic and chemical decellularization methods effectively remove cellular components from the ECM, these processes are often time-consuming and may involve toxic chemicals, which can negatively impact the retention of key proteins and growth factors in the ECM [63]. Wang et al. [64] recently developed a method for the debulking and decellularization of adipose aspirated tissue using supercritical carbon dioxide, which retained more GFs (such as bFGF and vascular endothelial growth factor (VEGF)) than alternative methods. Qi [65] and Yang [66] et al. developed an enzyme-free method for adipose tissue decellularization and demonstrated that DAT promoted adipocyte formation. In a related study, Feng et al. found that varying adipogenic effects observed after in vivo transplantation may be attributed to differences in the tissue source or preparation methods of DAT [48]. Specifically, DAT prepared using conventional enzymatic methods exhibited dense fibrous structures, termed "T-DAM", whereas more delicate fibers were categorized as "F-DAM " [64]. Notably, F-DAM derived through ultrasonic separation demonstrated superior capacity for adipose tissue regeneration in vivo, indicating that the stromal or F-DAM niche is more conducive to lipid differentiation [48].
4. Advantages and limitations of DAT as implant biomaterial
The selection of an appropriate decellularization method is crucial for optimizing the efficacy of DAT in adipose regeneration. Specifically, it affects the preservation of essential bioactive components within DAT, such as VEGF, fibroblast growth factor 2 (FGF-2), and stromal cell-derived factor 1α (SDF-1α). Retaining these factors is vital for enhancing the regenerative potential of DAT in adipose tissue formation, while simultaneously minimizing its inherent limitations. In this section, we examine both the advantages and limitations of DAT in adipose regeneration, including the role of enzymes in the formation of DAT hydrogels and their subsequent impact on adipogenesis. This analysis aims to deepen our understanding of how to fully harness the potential of DAT and enhance its application in adipose regeneration.
4.1 Advantages
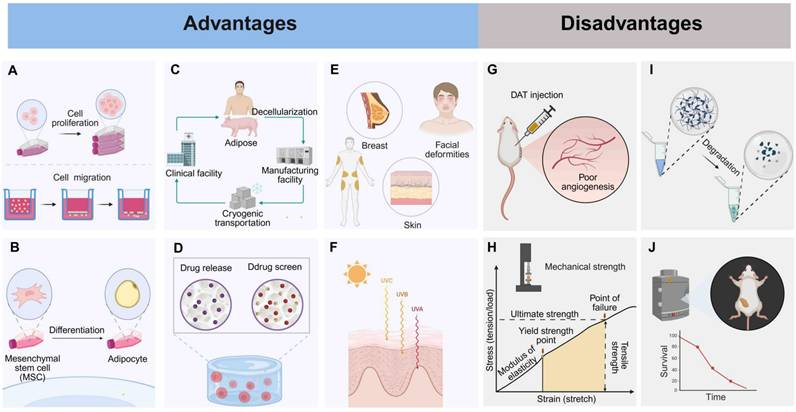
An adipose-tissue ECM provides a favorable environment for adipose formation both in vitro and in vivo [41, 67, 68]. After decellularization, DAT-derived scaffold effectively preserves the 3D structure, mechanical properties, and suitable environment of the original adipose tissue. Therefore, this scaffold possesses natural adipogenic properties and can effectively promote the proliferation and migration of adipocytes (Fig. 3A) [8]. Proteomic analysis has identified 29 ECM proteins in DAT, which are enriched in adipogenic regulatory functions. Co-culture studies have further demonstrated that DAT can guide mesenchymal stem cell differentiation into functional adipose tissue phenotypes (Fig. 3B) [35]. DAT is derived from a wide range of sources (such as medical liposuction waste or porcine adipose tissue), and its decellularization method is relatively simple (Fig. 3C) [8, 69]. Currently, a commercialized decellularized human adipose-derived product, Renuva™, is available in the U.S. market, proving that DAT can be produced in a simple and cost-effective manner [70].
Not only does DAT facilitate the delivery of therapeutic agents such as drugs, growth factors, and stem cells (Fig. 3D), but it also shows remarkable advantages in treating various soft tissue defects (Fig. 3E) [41]. For example, it has potential in the repair of genetic defects in the breast and chest wall [16], as well as in facial deformities [18, 19]. Moreover, protease-digested hydrogels derived from DAT retain essential signaling molecules, such as VEGF, FGF-2, and SDF-1α. These molecules play a key role in orchestrating wound healing by enabling spatiotemporal release that coordinates angiogenesis, fibroblast recruitment, and adipocyte progenitor differentiation [36, 71, 72].
Additionally, DAT scaffolds derived from breast tissue serve as tissue-specific platforms for breast cancer drug screening, demonstrating biomimetic functionality. These scaffolds preserve the native ECM architecture, including high porosity and a nanofibrous structure, which mimic the dynamics of the tumor microenvironment [73]. Second, DAT supports radiation damage remediation by enhancing extracellular vesicle-enriched matrix remodeling. This process results in a 65% reduction in fibrosis and restores adipose volume through mechano-transduction-mediated ASC differentiation [74]. DAT promotes therapeutic regeneration of radiation-compromised tissues in preclinical models (Fig. 3F) [20]. Furthermore, the preservation of VEGF and FGF-2 in supercritical-processed DAT synergizes with adipocyte precursors via PI3K/Akt/mTOR pathway activation, contributing to adipose volume recovery in radiation-damaged models. These multifunctional advancements position DAT as a transformative platform for precision regenerative therapies, effectively bridging the gap between adipose tissue regeneration and personalized oncology applications.
4.2 Limitations
DAT as an implant material faces several key challenges. First, DAT scaffolds exhibit limited vascular infiltration, leading to ischemic microenvironments that induce adipocyte apoptosis within 12 hours post-transplantation (Fig. 3G) [75, 76]. Surgical procedures such as liposuction worsen this problem by damaging blood vessels and inducing hypoxia-reperfusion injury, necessitating hemodynamic support to promote graft integration and tissue survival [77]. Additionally, DAT-derived hydrogels, synthesized through pepsin-mediated decellularization and digestion, exhibit inherent mechanical weaknesses, requiring crosslinking to stabilize their structure (Fig. 3H) [73]. Furthermore, unmodified DAT scaffolds degrade rapidly in vivo, necessitating controlled degradation strategies to preserve the ECM, prolong bioactivity, and facilitate cellular repopulation and tissue remodeling (Fig. 3I) [78]. The proteolytic processing of DAT alters its ultrastructure and composition, which disrupts the bioactivity necessary for effective cell-matrix interactions, leading to insufficient angiogenesis and limited bulk adipose tissue regeneration in vivo (Fig. 3J) [79, 80]. To address these challenges, DAT-based composite hydrogels have been developed to enhance their performance.
5. Cell-free composite hydrogels based on DAT for adipose tissue regeneration
Cell-free composite scaffolds are fabricated by integrating DAT matrix with natural polymers and synthetic biomaterials. Natural materials offer the advantage of superior biocompatibility but are prone to rapid absorption and limited adipogenic induction [67]. In contrast, synthetic materials exhibit better mechanical properties and greater stability over time, but they are associated with potential issues such as fibrosis and an increased risk of inflammatory reactions [81].
5.1 DAT composite with natural materials
Natural materials complexed with DAT include GAGs, mussel adhesive protein (MAP), silk fibroin (SF), methyl cellulose (MC), and other ECM (Fig. 5A).
Advantages and disadvantages of DAT. Advantages: (A) Promotes stem cell proliferation and migration. (B) Promotes adipogenesis. (C) Manufacture easily and inexpensively. (D) Provides drug delivery and screening scaffolds. (E) Fills soft tissue defects. (F) Reduces radiation-induced fibrosis. Disadvantages: (G) Poor angiogenesis. (H) Weak mechanical strength. (I) Fast degradation. (J) Limited long-term adipogenesis. Created in https://BioRender.com.
Highlights of research on DAT compounded with Glycosaminoglycan. (A) Structure of glycosaminoglycans. (B) Differential interference contrast (DIC) images of tissue particles broken down in a cyomill or homogeniser showed that the homogenized tissue appeared smaller (<1 μm) and more uniform. Adapted with permission from [23], copyright 2018 WILEY. (C) Chondroitin suladiposee molecules were modified with NHS. Extracellular matrix particle-glycosaminoglycan composite hydrogels for regenerative medicine applications. Adapted with permission from [23], copyright 2018 WILEY. (D) Macroscopic images of the MCS and MCS+DAT hydrogels at 14 and 28 days. Adapted with permission from [84], copyright 2019 FRONTIERS. (E) GPDH enzyme activity was significantly enhanced in MCS+DAT hydrogels cultured in lipid-forming medium (inducible) for 14 days. Adapted with permission from [84], copyright 2019 FRONTIERS. (F) Enhanced intracellular lipid accumulation in MCS+DAT composites induced cells to display a more mature phenotype with larger lipid droplets. Adapted with permission from [84], copyright 2019 FRONTIERS.
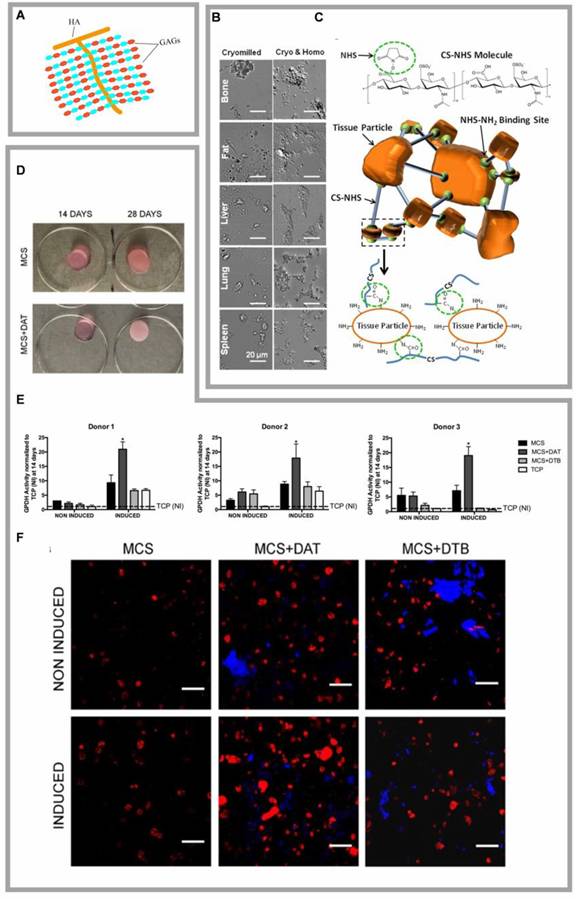
5.1.1 DAT complexed with GAGs
GAGs play crucial roles in cellular signaling cascades and tissue homeostasis by modulating the dynamics of bioactive factors through molecular sequestration and controlled release mechanisms (Fig. 4A) [82]. The GAG family includes hyaluronan, heparan sulfate/heparin (HS/HP), chondroitin/dermatan sulfate (CS/DS), and keratan sulfate (KS) [83]. GAG-ECM composite hydrogels exhibit tunable mechanical properties and programmable ECM particle integration, allowing for tailored performance in both in vitro and in vivo applications [23]. Vince et al. engineered an DAT-derived hydrogel scaffold by covalently crosslinking functionalized GAGs with different ECM particulates, circumventing matrix fragmentation and growth factor denaturation caused by enzymatic digestion, while preserving the native DAT's biochemical and ultrastructural features (Fig. 4B, C) [23]. Arthi et al. demonstrated synergistic adipose formation by combining methacrylated chondroitin sulfate (MCS) with DAT particles, where the MCS-DAT composite microenvironment enhanced adipogenic differentiation of HASCs through tissue-specific matrix signaling (Fig. 4D-F) [14, 84]. Cheung et al. further validated this approach by encapsulating ASCs in methacrylated glycol chitosan (MGC) or MCS matrices incorporating DAT, achieving enhanced adipogenesis post-implantation [67]. Chitosan is a linear polysaccharide derived from the deacetylation of chitin, known for its wound-healing and antimicrobial properties. However, it exhibits limited solubility in water at physiological pH. Glycol chitosan, formed by the reaction of ethylene oxide with chitosan, enhances its solubility in aqueous media across a pH range of 2 to 12. Both MGC and MCS lack inherent biosignals that promote cell adhesion and long-term cell viability. However, when complexed with DAT, the composite hydrogel has been shown to enhance stem cell proliferation and adipogenic differentiation in vitro, as well as support adipose tissue regeneration in vivo [67]. Despite these promising developments, challenges remain in the use of GAGs due to their structural heterogeneity, batch variability, and contamination risks, which limit reproducibility in tissue engineering applications [85]. Furthermore, while composite scaffolds incorporating CS have been shown to enhance mechanical properties, mechanical mismatch in soft tissue reconstruction may lead to complications such as scar tissue formation, poor integration, and implant failure [86].
5.1.2 DAT complexed with MAP
MAP, a natural gel secreted by mussels, contains diverse adhesive proteins, making it an ideal biomedical adhesive. MAP is biocompatible, chemically cross-linkable, and integrates long-term with host tissue [87]. Additionally, MAP prevents the formation of abnormal collagen protofibrils by binding to collagen molecules. Its lysine/tyrosine residues adsorb cells/factors, enhance platelet adhesion, and induce paracrine effects for healing [88]. MAP also inhibits collagenase activity, protecting collagen from degradation [89]. Eun et al. engineered an adipose regeneration system by integrating DAT with a thermoresponsive bioadhesive hydrogel, synthesized through covalent grafting of bioengineered MAP [90]. This MAP modified hydrogels, exhibiting superior water retention capacity, tissue adhesion, and porosity compared to unmodified hydrogels, effectively promoted adipose production by incorporating DAT as a source of adipose-induced and adipose-conducting ECM [90]. However, despite its excellent adhesive properties, MAP is prone to oxidation, exhibits low mechanical strength, suffers from poor long-term stability in air, incurs high material costs, and exhibits suboptimal biosafety and biodegradability [91].
5.1.3 DAT complexed with SF
SF is a lightweight (1.3 g/cm³), high-strength (up to 4.8 GPa) protein featuring repetitive hydrophobic blocks and small hydrophilic groups [92], exhibiting biocompatibility and biodegradability [93] for widespread use in tissue engineering applications such as cartilage regeneration [94] and wound healing [95]. Alisan et al. developed a vascularized adipose tissue regeneration scaffold to promote cell growth and tissue regeneration by incorporating DAT with SF [96]. ASCs were differentiated into preadipocytes and preendothelial cells, which were subsequently embedded in a DAT-SF hydrogel at a 1:3 ratio. The preadipocytes showed lipid vesicles formation, while the preendothelial cells were able to form tubular structures within the gel after just 3 days of in vitro culture. Angiogenesis was observed after one week of in vivo implantation. Hydrogels with a 1:3 (v/v) DAT:SF ratio exhibited superior cell viability [96]. However, SF alone demonstrates poor adhesion and proliferation for certain cell types (e.g., neuronal cells) and exhibits low in vivo retention efficiency. Therefore, improving the mechanical properties of the DAT-SF composite hydrogels, which are closely linked to cell adhesion, as well as optimizing their degradation rate, remain critical areas for further development (Table 2) [97, 98]. In a separate study, Hong et al. synthesized SF-GMA hydrogels by introducing glycidyl methacrylate (GMA) to the amine residues of SF and encapsulated cells within these hydrogels [97]. The results showed that the mechanical strength of the SF-GMA hydrogels was significantly improved, offering excellent stiffness and durability. Moreover, the degradation time of the hydrogel could be tailored by adjusting the degree and location of methacrylation, offering a customizable approach for hydrogel design [99]. These findings offer new insights into the potential of DAT-SF composite hydrogels.
Highlights of research on DAT composites with natural materials. (A) Schematic illustration of composite hydrogels formed by combining DAT with various natural materials to promote adipose tissue regeneration. DAT composite with MC enhances adipose regeneration. Created in https://BioRender.com. DAT composite with MC enhances adipose regeneration. (B)Typical macroscopic images of in vivo grafts observed at 1-, 2-, and 3-weeks post-injection. Adapted with permission from [69], copyright 2017 AMERICAN CHEMICAL SOCIETY. (C) In vivo grafts at 1, 2, and 3 weeks stained with oil red O. Adapted with permission from [69], copyright 2017 AMERICAN CHEMICAL SOCIETY. DAT composite with ECM achieves adipose regeneration and angiogenesis. (D)Typical Oil Red O staining images of lipid formation in ASCs cultured in various DAT-based composite hydrogels. Adapted with permission from [106], copyright 2023 ELSEVIER. (E) DAT composite with ECM achieves adipose regeneration and angiogenesis. Typical tube network images of endothelial cells cultured in various hydrogels. Adapted with permission from [106], copyright 2023 ELSEVIER.
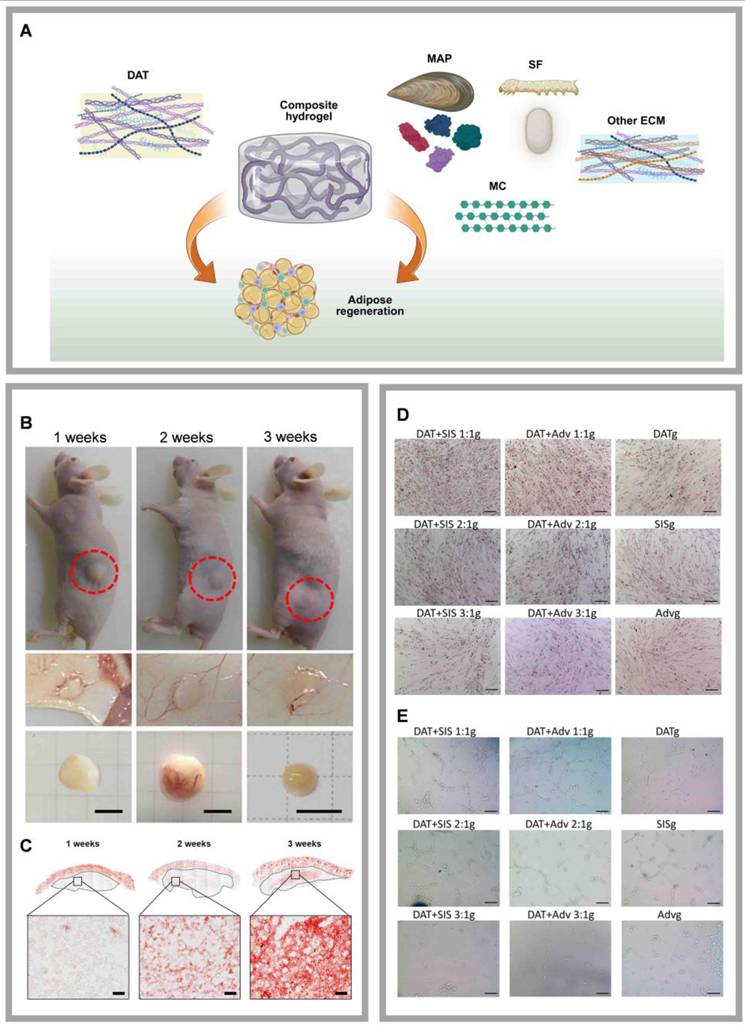
5.1.4 DAT compounded with MC
Cellulose, an abundant and cost-effective natural polymer, exhibits significant swelling capacity, cytocompatibility, and high viscosity even at low concentrations [100]. In contrast, MC, a carbohydrate polymer, is commonly used as a binder or thickener in pharmaceutical formulations due to its ability to effectively maintain the shape of implants [101-103]. Jun et al. combined DAT with MC to develop a cell-free and scaffold-free adipose tissue regeneration system (Fig. 5B, C) [69]. The DAT-MC hydrogel provided an optimal mechanical (3.8 kPa soft hydrogel) and biochemical microenvironment that induced the infiltration and differentiation of host ASCs and adipose tissue macrophages (ATMs), ultimately promoting the formation of new adipose tissue and directly facilitating adipose tissue regeneration. The results suggest that angiogenic factors, such as VEGF and Platelet-Derived Growth Factor BB (PDGF-BB), present in the DAT-MC hydrogel can contribute to angiogenesis by recruiting and activating host endothelial cells. Endothelial cells cultured on soft hydrogels stimulated angiogenesis and network formation more effectively than those cultured on stiff gels [69]. Additionally, by modulating the hydrogel stiffness through varying concentrations of MC, the migration of ASCs and post-transplant angiogenesis can be enhanced, effectively promoting adipose regeneration in vivo [95]. However, while MC offers many advantages, it also demonstrates thermal reversibility, with its viscosity being highly dependent on temperature [104]. This characteristic necessitates the inclusion of natural or synthetic modifiers when using MC or its derivatives in 3D printing, to improve the quality and consistency of the final products [105].
5.1.5 DAT composite with other ECM
ECM provides essential structural support and plays a critical role in regulating cellular behavior within tissues. The early development and long-term survival of adipose tissue require the support of an extensive vascular network [106]. Decellularized small intestine submucosa (SIS) retains numerous components that promote vascular regeneration, such as connective tissue growth factor (CTGF), bFGF, and transforming growth factor (TGF), all of which facilitate cell proliferation, attachment, and migration, thereby enhancing angiogenesis [107]. Similarly, the aortic adventitia (Adv) contains angiogenic and vasoactive factors, including bFGF, which promote endothelial cell proliferation and support the growth of new endothelium [108]. We combined DAT with decellularized SIS or Adv and incorporated genipin as a crosslinking agent to develop a decellularized matrix composite hydrogel (Fig. 5D, E) [106]. Genipin is a low-toxicity, natural crosslinking agent derived from plants. Crosslinking with genipin enhances the hydrogel's mechanical properties and reduces the inflammatory response [109]. The composite hydrogel, fabricated by mixing DAT with SIS or Adv at a 1:1 ratio and crosslinked with genipin, promotes adipose tissue regeneration and angiogenesis by inducing macrophage polarization. Although biocompatible ECMs like SIS and Adv are widely accessible, their inherent degradability limits their functional lifespan, which presents a significant challenge for long-term applications (Table 2). Collagen I, the most abundant ECM protein and a primary product of fibroblasts, provides a structural scaffold that facilitates cell attachment. In addition to promoting the differentiation of ASCs, collagen I also modulates lipogenesis and adiponectin expression [110]. However, excessive cross-linking or high concentrations of collagen I may induce a pro-fibrotic response. Notably, Lee et al. demonstrate DAT-collagen I-PLCL composites effectively enhance adipogenic differentiation and vascularization [78].
Although natural materials like gelatin, agarose and sodium alginate (SA) have not yet been reported to be compounded with DAT specifically for adipose tissue regeneration, evidence suggests their potential in this and related fields. For instance, Fu et al. combined DAT with methacrylated gelatin (GelMA) and methacrylated hyaluronic acid for wound healing [111], Dai et al. developed a DAT-GelMA composite bio-ink for colorectal cancer-like organ culture [112], and Grilli et al. utilized a GelMA and decellularized plant composite for adipose tissue regeneration [113]. These studies highlight the versatility and efficacy of gelatin-based composites in tissue engineering applications. Additionally, the well-established use of agarose as a filler in clinical settings, such as facial aesthetics, further underscores its promising potential for adipose tissue regeneration [114]. Comple-mentarily, Rijal et al. demonstrated that embedding the antioxidant enzyme catalase within DAT-SA scaffolds can effectively mitigate H2O2-mediated oxidative stress and hypoxia in large-sized scaffolds [39].
5.2 DAT composites with synthetic materials
In addition to compounding with nature materials, DAT has also been compounded with several synthetic materials to enhance adipose tissue regeneration, as discussed following (Table 2).
5.2.1 DAT composite with polyethylene glycol (PEG)
PEG is one of the most effective mediators of cell fusion, as it facilitates this process by inducing cell recruitment and modifying the cell membrane [115]. The DAT-PEG hydrogel is formed by mixing DAT with synthetic materials through Michael's addition reaction. This hydrogel has been shown to promote the proliferation of HASCs and enhance their lipid differentiation [116]. PEG hydrogels offer several advantages, including low protein adsorption, minimal inflammatory response, a well-established safety profile in biological applications, and the ease of incorporating various functional groups. Moreover, the mechanical properties of the DAT-PEG hydrogel, such as its hardness, can be modulated by adjusting the PEG thiol-acrylate content. Liu et al. further developed a PEG-modified DAT, positioning it as an ideal biomaterial for allogeneic adipose tissue regeneration (Fig. 6A) [117]. This modification significantly improved the M2/M1 macrophage ratio by promoting the secretion of interleukin-10 (IL-10), IL-13, and transforming growth factor β1. Consequently, it reduced immunogenicity and enhanced adipogenesis compared to DAT alone. However, despite the numerous advantages of PEG, it is not without its limitations. At high concentrations, PEG may be toxic [117] and could exhibit immunogenic and sensitizing properties [118]. These considerations highlight the need for careful optimization when using PEG-based hydrogels in tissue engineering applications.
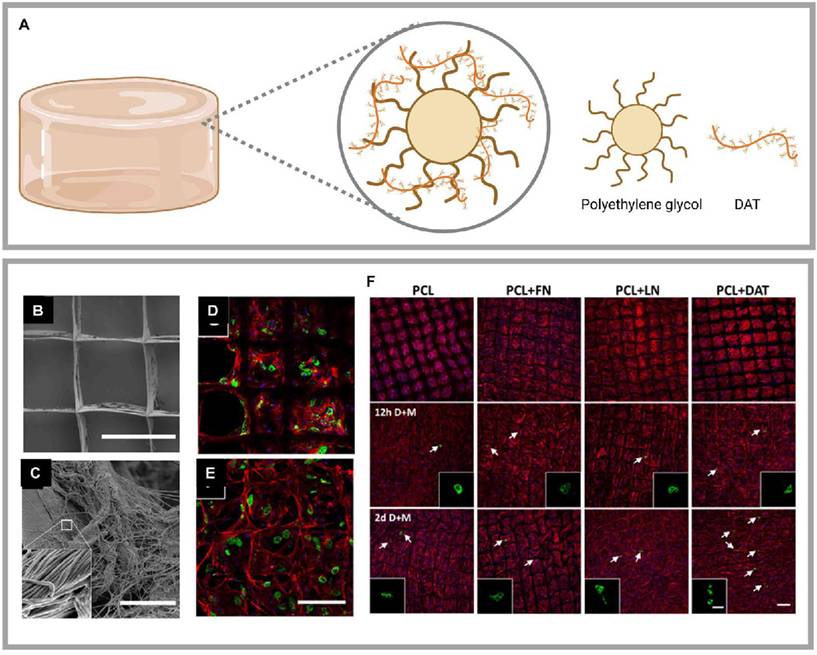
Highlights of research on DAT composite with synthetic materials. (A) DAT composite with PEG achieved regenerated soft tissues. Created in https://BioRender.com. DAT composite with PCL achieved regenerated adipose. (B) (C) Scanning electron microscopy images of PCL + DAT scaffolds. DAT was uniformly distributed on PCL fibers with a fibrous ultrastructure consistent with that of collagen-rich ECM. Confocal microscopy images of adipocytes after 21 days on (D) PCL and (E) PCL + DAT scaffolds. (F) Confocal microscopy images of the mature adipocytes detected by PLIN1 expression (green), stained actin filaments stained (red), and stained nuclei (DAPI) (blue). Adapted with permission from [120], copyright 2019 AMERICAN CHEMICAL SOCIETY.
DAT-based composite hydrogels.
| Composite hydrogel system | Advantages | Disadvantages | Reference Citations | ||
|---|---|---|---|---|---|
| Cell-free scaffolds | DAT composite with natural materials | DAT/GAGs | Promote cell proliferation, adhesion, anticoagulation and wound repair High viscoelasticity, high water absorption capacity and high biocompatibility | Heterogeneous Batch-to-batch variability Biological contamination Limited changes in mechanical properties | [23, 84] |
| DAT/MGC | Promote wound healing Increase the mechanical properties | Batch-to-batch variability Inadequately matched to soft tissue mechanical properties | [67] | ||
| DAT/MAP | An ideal bio-adhesive Biocompatible Prevents the formation of abnormal collagen protofibrils Promotes wound healing Inhibits collagenase activity | Susceptible to oxidation Poor mechanical strength Poor long-term stability in air High material cost Potential biosafety issue | [90] | ||
| DAT/SF | Good biocompatibility and degradability Adjustable structure and function | Poor adhesion and proliferation ability to neuronal cells Poor resistance to degradation Poor mechanical properties | [96] | ||
| DAT/MC | An effective adhesive or thickener Inexpensive Good swelling ability and cell affinity High viscosity High biocompatibility | Poor mechanical properties | [69, 95] | ||
| DAT composite with synthetic materials | DAT/PNIPAM | Promising thermally responsive smart polymers for biomedical applications | Non-degradable Lacks biologically active factors for cell adhesion and proliferation | [90] | |
| DAT/PEG | Mediates cell fusion Controllable mechanical properties | Inherently toxic Immunogenic Allergenicity | [117, 186] | ||
| DAT/PCL | Controllable mechanical properties | Release of compounds toxic to cells during biodegradation Poor biocompatibility Fibrous encapsulation Inflammatory reactions | [120] | ||
| DAT/PLCL | [78] | ||||
| Complexed with cells or bioactive factors | DAT complexed with cells | DAT/HASCs | The potential for multidirectional differentiation Promote adipocyte regeneration Secrete a variety of cytokines | Encapsulated cells release excess hydrogen peroxide Leading to tissue damage | [13, 124, 125, 187] |
| DAT/HUVECs | Secrete a variety of angiogenesis-promoting active factors with good vascular regeneration ability | Release excess hydrogen peroxide Limited adipose regeneration potential | [123, 125, 187] | ||
| DAT/Human subcutaneous preadipocyte cells | Proliferate and differentiate mature adipocytes and increase the volume of adipose tissue | Limited adipose regeneration potential Limited differentiation into mature adipocytes | [123] | ||
| DAT/ Dexamethasone | Dexamethasone and other glucocorticoids are necessary for the complete differentiation of adipose precursors as well as for the maintenance of key genes for glucose and lipid metabolism in cultured adipocytes and adipose tissue, and can increase adipogenesis | Short half-life | [10] | ||
| DAT complexed with bioactive factors | DAT/EVs | Induces adipogenic differentiation of HASCs and promotes aortic endothelial cell proliferation, migration, and angiogenesis | The standardization, dose control, safety, and stability of EVs need to be considered | [59, 127] | |
| DAT/Angiogenic factor | Have natural pro-angiogenic effects | Short half-life, Potential tumorigenic risk, Complex production | [41] |
5.2.2 DAT composite with poly(ε-caprolactone) (PCL) or poly(L-propylene-co-ε-caprolactone) (PLCL)
PCL is a U.S. Food and Drug Administration (FDA)-approved, biocompatible, and biodegradable synthetic aliphatic polyester, that is widely used in biomedical and pharmaceutical fields [119]. Melt electrospinning writing (MEW) is an additive manufacturing technology that can produce continuous, porous fibrous scaffolds through layer-by-layer deposition. Blum et al. utilized MEW to fabricate a PCL scaffold and combined it with DAT through non-covalent physical bonding, creating a platform for lipid cell delivery (Fig. 6B-F) [120]. However, the relatively slow degradation rate and insufficient mechanical stiffness of PCL in vivo limit its application in adipose tissue regeneration.
To overcome these limitations of PCL, researchers have turned to more advanced materials. For example, Lee et al. employed 3D printing with a dual-nozzle system to fabricate a hydrogel-PLCL composite scaffold. The hydrogel was composed of DAT and collagen I, while the PLCL component was synthesized via the ring-opening polymerization of L-lactide and ε-caprolactone (CL). This composite scaffold effectively promote adipogenic differentiation and vascularization [78]. A key advantage of PLCL is that its mechanical properties—such as flexibility, stretchability, and elasticity—as well as its degradation rate, can be precisely customized by adjusting the ratio of the two monomers. These customizable properties enable PLCL to outperform PCL in soft tissue engineering applications. Therefore, PLCL is considered an ideal alternative to address the mechanical limitations of PCL, with the potential to significantly improve the long-term stability and cell survival of DAT scaffolds [121]. However, both PCL and PLCL face common challenges, including central hypoxia and inadequate scaffold porosity that my lead to tissue necrosis. Moreover, the degradation process of both materials is complex, carrying risks such as premature loss of mechanical integrity and the release of toxic byproducts, which wuld potentially compromise the long-term biocompatibility of the implant [119]. These issues highlight the need for optimization to improve the efficacy and safety of PCL/PLCL-based scaffolds.
6. Cell-based or bioactive factor-infused composite hydrogels based on DAT for adipose tissue regeneration
Establishing functional vasculature is essential for both adipose tissue regeneration and the long-term survival of engineered scaffolds [122]. To address this critical challenge, DAT-based composite hydrogels have emerged as versatile platforms for integrating cells or bioactive factors to drive vascular network formation. These systems leverage DAT's inherent biocompatibility and pro-regenerative ECM components while addressing its limitations through strategic modifications. The advantages and challenges of DAT-cell/bioactive factor composites were summarized as following (Table 2).
6.1 DAT complexes with cells
HASCs exhibit significant adipogenic potential, while human umbilical vein endothelial cells (HUVECs) demonstrate high efficacy in vascular regeneration [123]. HUVECs are the primary cell type of vascular endothelial cells and are capable of forming vascular networks. Through interactions between cells and between cells and the ECM, HUVECs promote the formation and maturation of capillaries. Additionally, HUVECs secrete various growth factors and cytokines, such as VEGF and bFGF, which facilitate angiogenesis and endothelial cell proliferation. With excellent adhesion and migration capabilities, HUVECs respond to chemical signals, such as VEGF, guiding their migration to the site of injury and contributing to the formation of new vascular networks [123]. The proliferation and adipogenic differentiation potential of preadipocyte cells also provide possibilities for adipose tissue regeneration [123]. By forming composites with DAT, the adipogenic properties of ASCs and the angiogenic capacity of HUVECs are further enhanced. Forming composite hydrogels with DAT allows for the simulation of the natural tissue microenvironment, enhancing cell-to-cell synergy, improving oxygen and nutrient supply. This better supply supports long-term cell survival and function, significantly boosting both adipose tissue regeneration and angiogenesis. Zhao et al. developed a 3D printing technology that combines human subcutaneous preadipocyte cells with DAT, forming a novel scaffold structure. Additionally, they incorporated this scaffold with aortic decellularized matrix (dECM) and HUVECs, resulting in a composite scaffold that effectively enhances adipose regeneration and angiogenesis [123]. Kim et al. further explored a composite scaffold made from PCL scaffold, adipose dECM/heart dECM (80/20), and HASCs. This structure demonstrated superior angiogenesis, apoptosis resistance, and adipose tissue formation (Fig. 7A) [124]. Zhu et al. engineered vascularized adipose tissue by combining silica-expanded capsules with DAT, HASCs, HUVEC cell sheets, and exogenous chemokine CCL2, leading to improved adipogenesis and angiogenesis [125]. This integrated approach not only leverages the unique strengths of different cell types and biomaterials but also underscores their potential in advancing complex tissue engineering strategies.
Despite these studies demonstrating the efficacy of DAT-cell composites, challenges remain. Cells within scaffolds may release excessive hydrogen peroxide, leading to oxidative stress, hindering tissue regeneration, and causing tissue damage. To address this, Rigal et al. proposed an innovative strategy by encapsulating the antioxidant catalase (AC) in a gel scaffold composed of DAT and high-viscosity SA [39]. The DAT-SA-AC hydrogel effectively reduces oxidative damage, promotes tissue growth, and enhances angiogenesis [39]. However, despite the compelling evidence for tissue regeneration provided by these studies, the survival and integration of implanted cells remain insufficient, leading to inadequate angiogenesis. Zhao et al. improved the survival rate of ASCs using an oxygen-glucose deprivation (OGD) model and significantly upregulated the levels of VEGF and SDF-1α, improving their application in ischemic diseases [122]. Additionally, Souga et al. discovered that pre-treating ASCs in endothelial growth medium enhanced their pro-angiogenic properties, improving their therapeutic effects on ischemic diseases [126]. Nevertheless, excessive hydrogen peroxide release from cells encapsulated in DAT may lead to tissue damage, and the challenges of cell immunogenicity, survival, and integration require further resolution [39].
6.2 DAT composite with bioactive factors
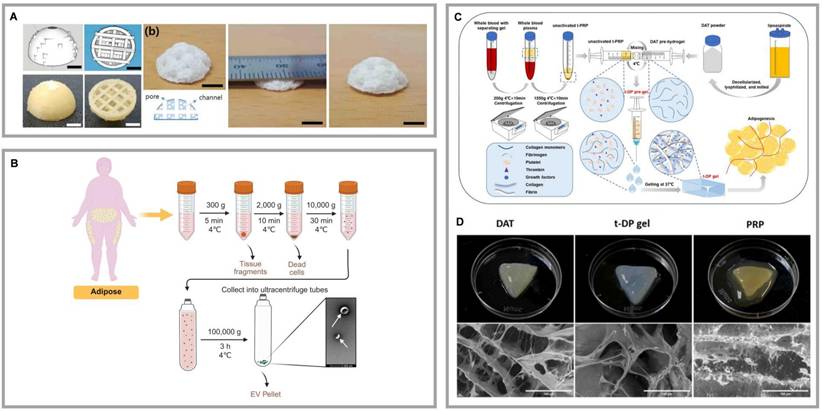
The close relationship between angiogenesis and adipogenesis suggests that the limited adipogenic potential of DAT may be due to insufficient neovascularization, which affects ASC's differen-tiation and adipose tissue regeneration. To address this bottleneck, incorporating angiogenic factors into DAT scaffolds has become a key strategy. Liu et al. encapsulated VEGF in heparinized DAT (Hep-DAT) hydrogels, significantly accelerating wound healing and angiogenesis by controlling VEGF release. Similarly, Lu et al. used EDC/NHS chemical crosslinking to immobilize bFGF on Hep-DAT, effectively promoting angiogenesis and adipose regeneration [41]. Human adipose tissue-derived extracellular vesicles (hAT-EVs) have dual regulatory functions: they induce ASC adipogenesis and activate HUVEC proliferation, migration, and angiogenesis [59]. Figure 7B demonstrates the isolation process of hAT-EVs. Nie et al. integrated hAT-EVs into DAT scaffolds for soft tissue repair, enhancing both adipogenesis and angiogenesis [59, 127].
Platelet-rich plasma (PRP) has shown promise in enhancing angiogenesis through the release of growth factors, but its application is limited by the suboptimal localization of these factors. Zhou et al. demonstrated that immobilizing PRP on gelatin microspheres improved the survival of implanted adipose tissue [128]. In further developments, Hou et al. engineered a thermoresponsive hydrogel system that integrated DAT with temperature-regulated PRP (t-PRP). This composite scaffold exhibited enhanced structural stability under physiological shear stress and sustained release of angiogenic factors (Fig. 7C, D) [129]. These studies collectively highlight the synergistic potential of integrating DAT with PRP to enhance adipogenic differentiation and vascularization. However, PRP presents limitations, including variability between donors, which can lead to inconsistent clinical outcomes. Additionally, the presence of leukocytes, platelets, and other particles in PRP may provoke unwanted immune responses. To address these issues, platelet-free plasma (PFP), which contains fibrinogen but fewer platelets, has emerged as a promising alternative. Aurora et al. developed a composite scaffold combining a polyethylene-glycolylated PFP hydrogel with a muscle-derived ECM stent, which upregulated angiopoietin-1 and Tie-2 expression, promoting vascular induction [130]. Although the combination of PFP with DAT in adipose tissue engineering has not been extensively studied, recent work by Amo et al. introduced a novel 3D-printed wound dressing made from a composite bioink consisting of DAT, human dermal fibroblasts, and low-platelet plasma or PRP [131]. This bioprinted dressing facilitated the synthesis and release of signaling proteins involved in wound healing and regeneration, creating an optimal microenvironment for tissue repair. Although these bioactivation strategies can be tailored for specific applications, significant barriers to clinical translation remain: high costs, risks of prolonged factor retention, and limitations in controlled release technologies.
7. Mechanical properties and structural stability of DAT-based composite hydrogels
DAT hydrogel is a temperature-sensitive gel. Lyophilized DAT exhibits a porous sponge-like structure with a mesh size ranging from 20 to 50 μm and an average porosity of approximately 90% [132]. Yu et al. reported that at -20°C, the Young's modulus for 50 mg/mL DAT was 3.67 ± 0.38 kPa, and for 100 mg/mL foam, it was 4.00 ± 0.38 kPa. At -80°C, the Young's modulus for 50 mg/mL DAT was 2.42 ± 0.65 kPa, and for 100 mg/mL DAT, it was 4.01 ± 0.46 kPa [37]. Wenderott et al. reported that the Young's modulus of normal human visceral adipose tissue was 4.48 ± 4.81 kPa, while that of diabetic visceral adipose tissue was 11.50 ± 16.79 kPa [133]. In comparison, Kayabolen et al. provided data for subcutaneous adipose tissue, reporting an initial modulus (10% strain) of 6.6 ± 1.8 kPa, a transition modulus (20% strain) of 24.5 ± 3.6 kPa, and a final modulus (30% strain) of 87.1 ± 8.7 kPa [96]. Furthermore, Lee et al. reported the compressive modulus of scaffolds, which were approximately 3.6 MPa for PCL, 122 kPa for PLCL, and 40 kPa for adipose tissue [78]. Notably, the Young's modulus of adipose tissue can vary significantly depending on the anatomical location and measurement methods, such as using a universal testing machine or atomic force microscopy [133]. This varations highlight that a comprehensive understanding of the tissue's mechanical properties requires consideration of both the specific adipose tissue type and the evaluation technique used. The interactions between cells and hydrogels are influenced by the hydrogel's mesh size and mechanical properties, which are determined by factors such as stiffness, stress relaxation, and the degradability of the surrounding polymer. Specifically, the mesh size affects cell spreading, growth, migration, and the development of the ECM. The mechanical properties of the hydrogel are particularly crucial when cells are encapsulated within it, as they require direct mechanical support to induce phenotypic changes, including migration and other cellular behaviors [134]. Consequently, both the mesh size and the mechanical properties of DAT and DAT composite hydrogels are pivotal factors to consider for successful tissue engineering. For example, Table 3 summarizes the Young's modulus and respective advantages of hydrogels composed of DAT and natural materials.
Highlights of research on DAT composite cell or bioactive factor. (A) DAT composite PLCL scaffolds enabled regenerated soft tissues. 3D-printed PVA molds and PLCL scaffolds with a porous structure. Adapted with permission from [124], copyright 2021 MDPI. (B) Process for the isolation of adipose tissue-derived extracellular vesicles from adipose liquid extracts. Created in https://BioRender.com. (C) The process of preparing PRP from whole blood. Adapted with permission from [129], copyright 2025 WILEY-BLACKWELL PUBLISHING LTD. (D) Upper panel: photographs of PRP, DAT, and t-DP (t-PRP and DAT gels were mixed at low temperature to obtain t-DP) hydrogels at 37°C. Lower panel: the typical microstructural images of PRP, DAT, and t-DP hydrogels observed under scanning electron microscopy. Adapted with permission from [129], copyright 2025 WILEY-BLACKWELL PUBLISHING LTD.
Modifications to the structural stability of a bio-scaffold need to meet stringent criteria, including biocompatibility, porosity, biodegradability, and mechanical suitability [135]. Cell infiltration is crucial for enhancing structural stability [90], and hydrogels with high porosity are essential for promoting this, while their mechanical suitability is critical for cell function, as poor stability can lead to cell detachment and death [136]. Fibrillar ECM proteins like fibronectin, collagen, and elastin form a fibrous network that displays mechanical asymmetry—stiffening under shear or tensile strain, and softening under compression. This property protects tissues from excessive deformation while regulating cell contraction, migration, proliferation, and differentiation [137]. Therefore, maintaining optimal mechanical conditions is crucial for effective use of DAT and DAT-based composite hydrogels in adipose tissue regeneration.
To enhance the mechanical stability of composite hydrogels, strategies such as crosslinking are commonly employed, including chemical, physical, and biological crosslinking (Fig. 8). Crosslinking can occur through intermolecular or intramolecular bonds, depending on the reaction conditions. This process improves mechanical properties, such as degradation resistance, elasticity and other properties of the polymer characteristics [138]. For example, intra-fiber crosslinking can control the stiffness of fibers, resulting in changes to the hydrogel's stiffness without altering its overall structure [137]. The following sections disscuss various crosslinking methods for enhancing the mechanical properties of DAT-based composite hydrogels.
Synthetic chemical crosslinkers, such as glutaraldehyde [139] and and 1-ethyl-3-(3-dimethy-laminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) [140, 141], are commonly used but are associated with certain limitations, including cytotoxicity and the risk of calcification. Additionally, unreacted crosslinkers must be removed prior to implantation, which complicates their practical use [142]. In contrast, naturally derived crosslinkers—such as alginate aldehyde from brown algae [143, 144], genipin from gardenia fruit [106, 145], polyphenols from natural sources [146, 147], dialdehyde CMC [148], and phytic acid (PA) from plant seeds [149]—are generally more biocompatible and less toxic than their synthetic counterparts [150]. However, these natural crosslinkers often suffer from instability [151], poor crosslinking efficiency, and the potential for product staining [152, 153]. Despite the development of low-toxicity chemical crosslinkers, their residual byproducts may still present risks. As a result, physical crosslinking is gaining popularity as a simpler and safer alternative.
Radiation techniques, such as gamma [154], and electron beam irradiation [155], have been investigated as physical crosslinking methods. Notably, ultraviolet (UV) irradiation has been shown to induce covalent bond formation between collagen fibers [138, 156, 157]. For instance, baking a scaffold at 105 °C for 24 hours in the presence of UV light has been demonstrated to achieve covalent crosslinking of collagen chains, thereby enhancing the scaffold's structural strength [135]. Physical crosslinking is advantageous in producing highly biocompatible materials while avoiding the introduction of exogenous toxic chemicals into the tissue [158]. As a result, physical crosslinking is increasingly recognized as a method for manufacturing biomaterials. However, these techniques may lead to protein denaturation and environmental contamination. Radiation-based crosslinking methods, however, face limitations including the need for specialized facilities, longer waiting times (due to the radiation half-life), and logistical challenges such as transportation [159]. Furthermore, the crosslinking process may be uneven, leading to inconsistent results with each application [154].
Young's modulus of composite hydrogels formed by DAT and natural materials.
| Composite hydrogels | Young's modulus | Advantage |
|---|---|---|
| DAT+GAG | DAT+MGC/MCS 30-40 kPa [67] | The overall mechanical properties of the composite hydrogel are adjustable, allowing it to simulate the physical characteristics of natural soft tissues and effectively prevent frictional irritation after implantation |
| DAT+MCS 123 ±17 kPa [84] | Enhancement of adipogenesis of MCS+DAT (8wt%DAT) composites | |
| DAT+ Fibrin | 22.4 ± 3.5 kPa [96] | 1:13 (v/v) DAT: Fibrin hydrogels with mechanical properties similar to natural adipose tissue enhanced vascularization both in vitro and in vivo |
| DAT+MC | 3.8 kPa [69] | The stiffness of DAT (6 wt%) and MC (6 wt%) hydrogels, similar to that of adipose tissue, promotes adipogenic differentiation of stem cells |
Stability of the DAT composite hydrogel.
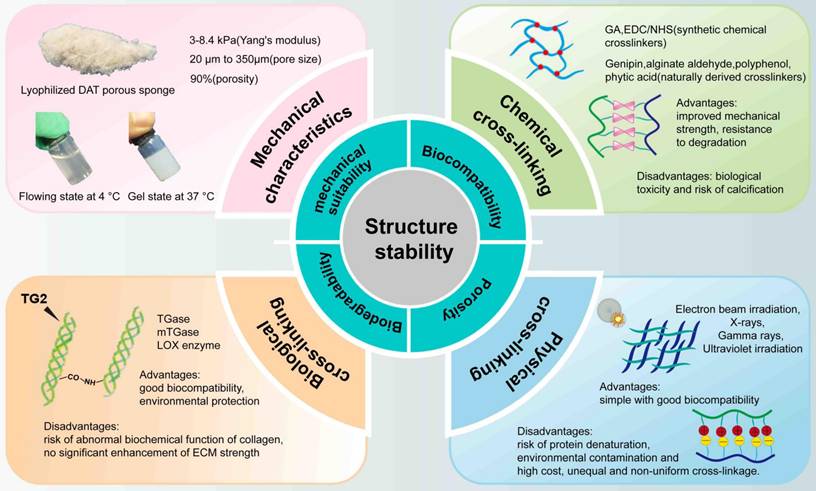
Finally, biological crosslinking, particularly enzymatic crosslinking, has emerged as a promising alternative. Enzymatic crosslinking reactions are highly specific, rapid, and can be performed under physiological conditions, without introducing toxic or contaminating substances. Transglutaminase (TG), a multifunctional enzyme [160] found in animals, plants, and human tissues, has been widely used to crosslink ECM proteins [161]. TG selectively mediates the formation of amide bonds between glutamine and lysine residues, facilitating cell adhesion through interactions with fibronectin and integrins [162]. Another example is lysyl oxidase, which catalyzes the oxidative deamination of lysine and hydroxylysine residues in collagen and elastin, initiating the covalent crosslinking of ECM proteins [163]. However, enzymatic crosslinking can sometimes lead to abnormal biochemical functions in collagen, with unpredictable consequences, such as those seen in diseases like diabetes [164]. In addition, another study found that at low crosslinking concentrations, ECM treated with TG exhibited no significant strength increase [165].
8. Recent advances in DAT applications
DAT demonstrates promising adipogenic properties. However, it also faces several challenges, including limited angiogenic potential, rapid degradation, and insufficient mechanical properties. In contrast, composite hydrogels present distinct advantages over DAT hydrogels, such as the ability to incorporate pro-angiogenic cytokines or endothelial cells to enhance angiogenesis [123, 166], as well as the use of various crosslinking methods or the addition of other materials to improve mechanical strength [73, 144]. However, the direct addition of bioactive factors presents several challenges, including high costs and susceptibility to inactivation. In contrast, the integration of these factors from the ECM helps to mitigate these issues. Additionally, John et al. demonstrated that DAT scaffolds seeded with ASCs induced early modulation of macrophage polarization compared to non-seeded DAT controls. However, longitudinal analysis revealed no significant differences between the groups in terms of macrophage phenotypic profiles, vascular perfusion, implant remodeling, or long-term host tissue integration [167]. This study highlights the inherent challenges of cell-seeding therapies, particularly the rapid clearance of therapeutic cells, underscoring the need for strategies aimed at sustaining cell retention or protocols for replenishing cells to support therapeutic supplementation.
Recent studies on DAT-induced adipose tissue regeneration have shown heterogeneous adipogenesis following in vivo grafting, with some areas failing to undergo adipogenesis [64]. Esteve et al. discovered that the adipose tissue ECM comprises septa and stroma. The stroma fibers, which are small and sparse, are enriched in the MSCA1+ adipogenic subpopulation, while the septal fibers are densely structured and contain MSCA1-/CD271- and MSCA1-/CD271high progenitors, which contribute to the differentiation and activation of myofibroblasts [65]. Furthermore, a recent comparative analysis of abdominal adipose tissue layers revealed that superficial DAT had significantly higher mitochondrial density and enhanced adipogenic regenerative capacity compared to deep DAT, providing new insights into the adipogenesis effects of DAT and generating new directions for future research in adipose regeneration [49].
Adipose tissue has a highly developed vascular system that is essential for nutrient transport, waste metabolism, and the provision of paracrine signals for intercellular communication [21]. Extracellular vesicles (EVs), as crucial paracrine mediators, play a key role in this process [168]. Adipose tissue, functioning as an endocrine organ, also relies on EVs as carriers for intercellular signaling [169-171]. However, previous studies have suggested that the decellularization process inevitably results in the loss of these vesicles. Consequently, future research should focus on improving cellular integration in implanted materials and restoring paracrine signaling functions.
In 2016, Luai et al. identified matrix-bound nanovesicles (MBVs) within the ECM and reported their presence in decellularized biological scaffolds [172]. This groundbreaking discovery revealed that ECM scaffolds derived from tissues such as the urethral bladder, SIS, and dermis contained MBVs ranging in size from 10 to 1000 nm. These MBVs were resistant to various cell removal protocols, including chemical, enzymatic, and detergent treatments, and were shown to significantly alter cellular behavior. Remarkably, MBVs promoted macrophage polarization toward the anti-inflammatory M2 phenotype and influenced stem cell differentiation, phenomena previously linked to ECM-mediated structural remodeling. While MBVs share some similarities with exosomes (30-150nm) and microvesicles (150-1000nm), such as nanoscale size and miRNA content. While they lack typical molecular markers such as CD63, CD81, CD9, and Hsp70. Furthermore, Luai et al. demonstrated that MBVs from porcine urinary bladder matrix (UBM) and SIS were enriched in specific miRNAs [173]. When these MBVs were applied to bone marrow-derived macrophages, the cells exhibited an anti-inflammatory phenotype, characterized by upregulation of M2 markers such as Fizz1 and Arg1, along with enhanced phagocytic activity and nitric oxide production. In contrast, inhibition of these miRNAs led to a shift toward a pro-inflammatory (M1-like) phenotype, marked by increased expression of iNOS and TNF-α. These findings suggest that MBVs mediate ECM-induced macrophage phenotype switching through their specific miRNA cargo, shedding new light on the immunomodulatory mechanisms by which ECM-based biomaterials support tissue repair. Further research by Van der Merwe et al. showed that MBVs from porcine bladder ECM could protect retinal ganglion cells from ischemic injury by reducing inflammation and providing neuroprotection, suggesting their potential in retinal and central nervous system diseases [174]. Similarly, Anne et al. found that ECM hydrogels from porcine bladder ECM promoted the survival of hippocampal neurons and enhanced axonal growth. Additionally, MBVs from bladder ECM were taken up by neurons and promoted axon growth and branching, providing valuable insights for central nervous system injury repair [175]. Although no studies have yet been conducted on MBVs derived from DAT, it is plausible that DAT-derived MBVs may also facilitate tissue regeneration and offer insights into ECM-mediated cellular behavior.
George et al. compared liquid-phase EVs with MBVs, finding that, although both had similar morphology and size, their miRNA profiles differed significantly [176]. Specifically, 28 miRNAs were differentially expressed, with miRNAs associated with tissue development markedly enriched in MBVs. Additionally, MBVs exhibited a lipid composition rich in phospholipids associated with polyunsaturated fatty acids, lysophospholipids, and oxidized lipids, contrasting with the phosphatidylcholine-dominated composition of liquid-phase EVs. These differences suggest that MBVs play a significant role in ECM-mediated tissue repair and offer novel insights into ECM-based biomaterial design for minimally invasive therapies. Dake et al. employed click chemistry to conjugate the integrin α4β1 ligand LLP2A to the surface of electrospun scaffolds, facilitating the specific binding of EVs derived from placental mesenchymal stem cells [177]. This functionalized scaffold mimicked natural ECM-EV complexes and was shown to enhance the migration of HUVECs, promote vascular sprouting, and upregulate angiogenesis-related genes such as KDR and TIE2. Additionally, LLP2A modification significantly improved EV adhesion to the scaffold surface, increased endothelial cell survival under hypoxic conditions, and inhibited apoptosis markers such as caspase 3 and caspase 9. These findings highlight the potential of integrin-mediated EV immobilization strategies in enhancing the vascularization capacity of tissue-engineered scaffolds, with promising applications in ischemic tissue repair. Dake's research provides important insights into adipose tissue regeneration, suggesting that future efforts in this field could benefit from the modification of DAT-derived MBVs to enhance adipose structural repair and functional regeneration.
9. Challenges and perspectives
Although the clinical application of DAT composite hydrogels is still in its early stages, their potential as acellular scaffold for adipose tissue regeneration or as a therapeutic delivery vehicle is significant [178]. The manufacturing of DAT hydrogels as a biomaterial is relatively simple and does not raise significant ethical concerns. For instance, MTF Biologics currently markets Renuva™ in the U.S. as a decellularized human adipose-derived product for soft tissue augmentation. However, the exact mechanisms by which DAT hydrogel components influence cellular behavior are not fully understood. Current understanding the mechanisms proposes that ECM mediates cellular behavior through inherent surface morphology [179], biomechanical influences [180], and the impact of signaling peptides and proteins [181]. However, the clinical translation of DAT hydrogels confronts numerous challenges [70]. Primary among these is the complex decellularization process, which is difficult to standardize, leading to significant batch-to-batch variations that fail to meet ISO 13485 medical device quality control standards [182]. Additionally, precise control over material properties, including mechanical performance, degradation rate, and drug release kinetics, remains chanllenges. Moreover, residual decellularization reagents may trigger immune reactions, while chemical crosslinking methods could generate toxic residues that impact biocompatibility [62]. In large-scale production, crosslinking consistency and minimizing batch varations remain prominent issues, driving up production costs and complicating the manufacturing process. In terms of standardization and quality control, there is a lack of dedicated evaluation standards for DAT scaffolds. While multiple decellularization methods exist, low production efficiency impedes the progress of large-scale production. How to pre-screen adipose donors to collect clinically acceptable adipose tissue for raw materials, as well as addressing issues related to the use of xenogeneic adipose tissue-derived DAT, remain critical problems that need to be solved [70]. Despite the therapeutic potential of DAT composite hydrogels, overcoming a range of technical, cost-related, and standardization challenges is essential to achieve a clinical-grade product comparable to Renuva™.
In recent years, significant progress has been made in the application of DAT composite hydrogels in adipose tissue regeneration, presenting new opportunities for clinical treatment. Currently, research on DAT composite hydrogels in tissue engineering mainly focuses on structural repair. However, future studies should place greater emphasis on promoting the functional regeneration of adipose tissue, particularly in terms of energy storage, vascularization, and the secretion of hormones and cytokines. In addition to direct incorporation of bioactive factors or cells to enhance adipose functional regeneration, 3D bioprinting technology has also provided important technical support for functional regeneration. 3D bioprinting technology includes two methods: Fused Deposition Modeling (FDM) and Stereolithography (SLA) [183]. FDM works by heating thermoplastic filament to a semi-molten state, extruding it layer by layer, and allowing it to cool and solidify, making it suitable for printing high-strength scaffolds. For example, Temple et al. fabricated PCL scaffolds via FDM with 250 μm pores, populated them with HASCs, and demonstrated robust adipogenesis both in vitro and in vivo [184]. FDM's high-temperature process prevents live cell printing, requiring scaffolds to be printed and subsequently seeded with cells. In contrast, SLA uses ultraviolet lasers to irradiate photosensitive resin layer by layer, providing higher precision and lower temperature, which allows the direct printing of cell-laden structures. For example, Pati et al. used SLA technology to print DAT hydrogel scaffolds containing HASCs, achieving excellent soft tissue regeneration and promoting adipogenic differentiation of stem cells [43]. The advantage of FDM lies in its lower equipment cost and rich material options, although it has lower precision. SLA, on the other hand, has significant advantages in detailed structure and direct cell printing, though the equipment is expensive and requires post-processing [185]. Both methods offer distinct advantages in adipose tissue engineering: FDM allows for printing scaffolds followed by cell seeding, while SLA can process cells and their microenvironment in a single step. In addition to these technologies, organoids based on DAT composite hydrogels hold promise for the treatment of metabolic diseases and adipose regeneration [168].
Future research should focus on the following key directions: (I) Optimizing decellularization methods, achieving efficient and non-toxic removal of cellular components while supporting large-scale production; (II) Extending DAT composite hydrogels degradation time, ensuring long-term cell survival and effective adipogenesis in vivo. This can be achieved by adjusting the crosslinking degree and material composition to design composites with controllable degradation rates that meet clinical demands; (III) Improving the mechanical properties of DAT composite scaffolds, ensuring they better simulate the biomechanical properties of soft tissue and prevent premature degradation or functional failure after implantation. This can be achieved by optimizing the molecular structure of the material and utilizing appropriate crosslinking technologies to enhance the mechanical properties and ensure its compatibility with the desired clinical applications; (IV) Investigating the impact of processing methods on DAT hydrogel properties, exploring how different decellularization methods influence the physical and chemical properties of hydrogels, and optimizing the preparation process to meet specific clinical applications; (V) Exploring the impact of DAT composite hydrogel components on cell behavior and tissue remodeling, utilizing organoid models to deeply investigate the cellular and molecular processes involved in adipogenesis and tissue repair, thereby providing a theoretical basis for improving therapeutic strategies; (VI) Ensuring the safety of DAT composite hydrogels, focusing on chemical composition, crosslinker residues, and immune responses to ensure their biocompatibility and clinical applicability, reducing potential risks in clinical applications; (VII) Strengthening the integration of functional regeneration and structural repair, optimizing the physical properties and biological functions of DAT composite hydrogels to enhance their multifunctional roles in soft tissue regeneration, such as angiogenesis and the secretion of bioactive molecules (MBVs), achieving comprehensive tissue regeneration effects; (VIII) Designing novel DAT composite hydrogels to address the evolving clinical needs, combining cutting-edge technologies and new challenges in clinical practice to develop more efficient, personalized therapeutic strategies that cater to the needs of different patient populations.
10. Conclusion
This review comprehensively presents fifteen years of advances in DAT composite hydrogels for adipose regeneration, critically evaluating historical developments, decellularization methodologies, biomaterial efficacy, and mechanical optimization strategies—particularly crosslinking-enhanced structural stability. While current research prioritizes structural repair, the critical gap in functional regeneration (metabolic activity, vascularization, endocrine signaling) fundamentally limits physiological relevance. Future innovation should concurrently advance structural fidelity and functional restoration to unlock the full therapeutic potential of DAT composite hydrogels platforms.
Acknowledgements
This work was supported by the Liaoning Provincial Central Guide local science and technology development fund plan, China [no. 2024JH6/ 100800021]; the National Natural Science Foundation, China [No. 81571919]; the Scientific Research Fund of Educational Department of Liaoning Province of China [No. LJKMZ20221203]; the National Natural Science Foundation of China [No.32171181, R.G.]; and Hebei Natural Science Foundation [No.H2023201901, R.G.].
Author contributions
Lu Cui: Writing - Original draft, Conceptualization. Tian-Jie Lyu: Writing - original draft. Bin Fu: Writing - review & editing. Yubo Wang: Writing - review & editing. Qing Qu: Writing - review & editing. Fanglin Wang: Writing - review & editing. Rui Guo: Writing - review & editing, Funding acquisition. Jun Fan: Writing - review & editing, Conceptualization, Funding acquisition.
Competing interests
The authors have declared that no competing interest exists.
References
1. Xia Z, Guo X, Yu N, Zeng A, Si L, Long F. et al. The Application of Decellularized Adipose Tissue Promotes Wound Healing. Tissue Eng Regen Med. 2020;17:863-74
2. Zhou Z-Q, Chen Y, Chai M, Tao R, Lei Y-H, Jia Y-Q. et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose-derived stem cells into fibroblasts. Int J Mol Med. 2019;43:890-900
3. He Y, Xia J, Chen H, Wang L, Deng C, Lu F. Human adipose liquid extract induces angiogenesis and adipogenesis: a novel cell-free therapeutic agent. Stem Cell Res Ther. 2019;10:252
4. Gold JS. Early Clinical Experience with the Use of a New Allograft Adipose Matrix for Foot Fat Pad Restoration. J Am Podiatr Med Assoc. 2023 113
5. Gold MH, Kinney BM, Kaminer MS, Rohrich RJ, D'Amico RA. A multi-center, open-label, pilot study of allograft adipose matrix for the correction of atrophic temples. J Cosmet Dermatol. 2020;19:1044-56
6. Giatsidis G, Succar J, Haddad A, Lago G, Schaffer C, Wang X. et al. Preclinical Optimization of a Shelf-Ready, Injectable, Human-Derived, Decellularized Allograft Adipose Matrix. Tissue Eng Part A. 2019;25:271-87
7. Xu M, He Y, Li Y, Liu K, Zhang Y, Su T. et al. Combined Use of Autologous Sustained-Release Scaffold of Adipokines and Acellular Adipose Matrix to Construct Vascularized Adipose Tissue. Plast Reconstr Surg. 2024;153:348e-60e
8. Tan Q-W, Zhang Y, Luo J-C, Zhang D, Xiong B-J, Yang J-Q. et al. Hydrogel derived from decellularized porcine adipose tissue as a promising biomaterial for soft tissue augmentation. J Biomed Mater Res A. 2017;105:1756-64
9. Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017 49
10. Mahoney CM, Kelmindi-Doko A, Snowden MJ, Peter Rubin J, Marra KG. Adipose derived delivery vehicle for encapsulated adipogenic factors. Acta Biomater. 2017;58:26-33
11. Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715-24
12. Han TTY, Toutounji S, Amsden BG, Flynn LE. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials. 2015;72:125-37
13. Zhao Y, Fan J, Bai S. Biocompatibility of injectable hydrogel from decellularized human adipose tissue in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2019;107:1684-94
14. Shridhar A, Gillies E, Amsden BG, Flynn LE. Composite Bioscaffolds Incorporating Decellularized ECM as a Cell-Instructive Component Within Hydrogels as In Vitro Models and Cell Delivery Systems. Methods Mol Biol. 2018;1577:183-208
15. Yang J-Z, Qiu L-H, Xiong S-H, Dang J-L, Rong X-K, Hou M-M. et al. Decellularized adipose matrix provides an inductive microenvironment for stem cells in tissue regeneration. World J Stem Cells. 2020;12:585-603
16. Yüce E, Şirvan SS, Demir IA, Eren HI, Karşıdağ S. Coexistance of Gynecomastia and Poland Syndrome: Case Report. Sisli Etfal Hastan Tip Bul. 2019;53:314-7
17. Ziegler JF, Böttcher C, Letizia M, Yerinde C, Wu H, Freise I. et al. Leptin induces TNFα-dependent inflammation in acquired generalized lipodystrophy and combined Crohn's disease. Nat Commun. 2019;10:5629
18. Mitsukawa N, Saiga A, Satoh K. Changing the facial features of patients with Treacher Collins syndrome: protocol for 3-stage treatment of hard and soft tissue hypoplasia in the upper half of the face. Ann Plast Surg. 2014;73:39-42
19. Velter C, Messaddeq N, Levy E, Morruzzi C, Cribier B, Dali-Youcef N. et al. Abnormal lipid storage related to adipocyte shrinkage in acquired partial lipodystrophy (Barraquer-Simons syndrome). J Eur Acad Dermatol Venereol. 2019;33:2188-91
20. Adem S, Abbas DB, Lavin CV, Fahy EJ, Griffin M, Diaz Deleon NM. et al. Decellularized Adipose Matrices Can Alleviate Radiation-Induced Skin Fibrosis. Adv Wound Care (New Rochelle). 2021
21. Cypess AM. Reassessing Human Adipose Tissue. N Engl J Med. 2022;386:768-79
22. Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337-51
23. Beachley V, Ma G, Papadimitriou C, Gibson M, Corvelli M, Elisseeff J. Extracellular matrix particle-glycosaminoglycan composite hydrogels for regenerative medicine applications. J Biomed Mater Res A. 2018;106:147-59
24. Mohiuddin OA, Campbell B, Poche JN, Ma M, Rogers E, Gaupp D. et al. Decellularized Adipose Tissue Hydrogel Promotes Bone Regeneration in Critical-Sized Mouse Femoral Defect Model. Front Bioeng Biotechnol. 2019;7:211
25. Porzionato A, Stocco E, Barbon S, Grandi F, Macchi V, De Caro R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int J Mol Sci. 2018 19
26. Wang X, Gao L, Han Y, Xing M, Zhao C, Peng J. et al. Silicon-Enhanced Adipogenesis and Angiogenesis for Vascularized Adipose Tissue Engineering. Adv Sci (Weinh). 2018;5:1800776
27. Choi JS, Yang H-J, Kim BS, Kim JD, Lee SH, Lee EK. et al. Fabrication of porous extracellular matrix scaffolds from human adipose tissue. Tissue Eng Part C Methods. 2010;16:387-96
28. Brown BN, Freund JM, Han L, Rubin JP, Reing JE, Jeffries EM. et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods. 2011;17:411-21
29. Young DA, Ibrahim DO, Hu D, Christman KL. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2011;7:1040-9
30. Lin G, Albersen M, Harraz AM, Fandel TM, Garcia M, McGrath MH. et al. Cavernous nerve repair with allogenic adipose matrix and autologous adipose-derived stem cells. Urology. 2011;77:1509.e1-e8
31. Choi YC, Choi JS, Kim BS, Kim JD, Yoon HI, Cho YW. Decellularized extracellular matrix derived from porcine adipose tissue as a xenogeneic biomaterial for tissue engineering. Tissue Eng Part C Methods. 2012;18:866-76
32. Kim BS, Choi JS, Kim JD, Choi YC, Cho YW. Recellularization of decellularized human adipose-tissue-derived extracellular matrix sheets with other human cell types. Cell Tissue Res. 2012;348:559-67
33. Turner AEB, Yu C, Bianco J, Watkins JF, Flynn LE. The performance of decellularized adipose tissue microcarriers as an inductive substrate for human adipose-derived stem cells. Biomaterials. 2012;33:4490-9
34. Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff JH. An injectable adipose matrix for soft-tissue reconstruction. Plast Reconstr Surg. 2012;129:1247-57
35. Jiang X, Lai X-R, Lu J-Q, Tang L-Z, Zhang J-R, Liu H-W. Decellularized adipose tissue: A key factor in promoting fat regeneration by recruiting and inducing mesenchymal stem cells. Biochem Biophys Res Commun. 2021;541:63-9
36. Leclerc CJ, Cooper TT, Bell GI, Lajoie GA, Flynn LE, Hess DA. Decellularized adipose tissue scaffolds guide hematopoietic differentiation and stimulate vascular regeneration in a hindlimb ischemia model. Biomaterials. 2021;274:120867
37. Yu C, Bianco J, Brown C, Fuetterer L, Watkins JF, Samani A. et al. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials. 2013;34:3290-302
38. Kornmuller A, Flynn LE. Development and characterization of matrix-derived microcarriers from decellularized tissues using electrospraying techniques. J Biomed Mater Res A. 2022;110:559-75
39. Rijal G, Kim BS, Pati F, Ha D-H, Kim SW, Cho D-W. Robust tissue growth and angiogenesis in large-sized scaffold by reducing HO-mediated oxidative stress. Biofabrication. 2017;9:015013
40. Morissette Martin P, Grant A, Hamilton DW, Flynn LE. Matrix composition in 3-D collagenous bioscaffolds modulates the survival and angiogenic phenotype of human chronic wound dermal fibroblasts. Acta Biomater. 2019;83:199-210
41. Lu Q, Li M, Zou Y, Cao T. Delivery of basic fibroblast growth factors from heparinized decellularized adipose tissue stimulates potent de novo adipogenesis. J Control Release. 2014;174:43-50
42. Pati F, Jang J, Ha D-H, Won Kim S, Rhie J-W, Shim J-H. et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935
43. Pati F, Ha D-H, Jang J, Han HH, Rhie J-W, Cho D-W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 2015;62:164-75
44. Cicuéndez M, Casarrubios L, Feito MJ, Madarieta I, Garcia-Urkia N, Murua O. et al. Effects of Human and Porcine Adipose Extracellular Matrices Decellularized by Enzymatic or Chemical Methods on Macrophage Polarization and Immunocompetence. Int J Mol Sci. 2021 22
45. Lim H-J, Kwon T-Y. Thermal and Spectroscopic Analyses of Human Adipose Tissue-Derived Extracellular Matrix. J Nanosci Nanotechnol. 2021;21:3662-6
46. Thomas-Porch C, Li J, Zanata F, Martin EC, Pashos N, Genemaras K. et al. Comparative proteomic analyses of human adipose extracellular matrices decellularized using alternative procedures. J Biomed Mater Res A. 2018;106:2481-93
47. Kuljanin M, Brown CFC, Raleigh MJ, Lajoie GA, Flynn LE. Collagenase treatment enhances proteomic coverage of low-abundance proteins in decellularized matrix bioscaffolds. Biomaterials. 2017;144:130-43
48. Feng J, Fu S, Luan J. Harnessing fine fibers in decellularized adipose-derived matrix for enhanced adipose regeneration. Mater Today Bio. 2024;25:100974
49. Feng J, Fu S, Luan J. Regenerated fat induced by a decellularized adipose matrix can survive long-term in vivo. Acta Biomater. 2025;191:233-43
50. Chen Z, Zhang B, Shu J, Wang H, Han Y, Zeng Q. et al. Human decellularized adipose matrix derived hydrogel assists mesenchymal stem cells delivery and accelerates chronic wound healing. J Biomed Mater Res A. 2021;109:1418-28
51. Pu W, Han Y, Yang M. Human decellularized adipose tissue hydrogels as a culture platform for human adipose-derived stem cell delivery. J Appl Biomater Funct Mater. 2021;19:2280800020988141
52. Kornmuller A, Cooper TT, Jani A, Lajoie GA, Flynn LE. Probing the effects of matrix-derived microcarrier composition on human adipose-derived stromal cells cultured dynamically within spinner flask bioreactors. J Biomed Mater Res A. 2023;111:415-34
53. Getova VE, van Dongen JA, Brouwer LA, Harmsen MC. Adipose tissue-derived ECM hydrogels and their use as 3D culture scaffold. Artif Cells Nanomed Biotechnol. 2019;47:1693-701
54. Vafaei S, Tabaei SR, Guneta V, Choong C, Cho N-J. Hybrid Biomimetic Interfaces Integrating Supported Lipid Bilayers with Decellularized Extracellular Matrix Components. Langmuir. 2018;34:3507-16
55. Manikowski D, Andrée B, Samper E, Saint-Marc C, Olmer R, Vogt P. et al. Human adipose tissue-derived stromal cells in combination with exogenous stimuli facilitate three-dimensional network formation of human endothelial cells derived from various sources. Vascul Pharmacol. 2018;106:28-36
56. Lin C-Y, Liu T-Y, Chen M-H, Sun J-S, Chen M-H. An injectable extracellular matrix for the reconstruction of epidural fat and the prevention of epidural fibrosis. Biomed Mater. 2016;11:035010
57. Baker NA, Muir LA, Washabaugh AR, Neeley CK, Chen SY-P, Flesher CG. et al. Diabetes-Specific Regulation of Adipocyte Metabolism by the Adipose Tissue Extracellular Matrix. J Clin Endocrinol Metab. 2017;102:1032-43
58. Flesher CG, Baker NA, Strieder-Barboza C, Polsinelli D, Webster PJ, Varban OA. et al. A Human 3D Extracellular Matrix-Adipocyte Culture Model for Studying Matrix-Cell Metabolic Crosstalk. J Vis Exp. 2019
59. Nie J-Y, Zhu Y-Z, Wang J-W, Hu X, Wang Z-H, Wu S. et al. Preparing Adipogenic Hydrogel with Neo-Mechanical Isolated Adipose-Derived Extracellular Vesicles for Adipose Tissue Engineering. Plast Reconstr Surg. 2021;148:212e-22e
60. Chen Y, Xu L, Li W, Chen W, He Q, Zhang X. et al. 3D bioprinted tumor model with extracellular matrix enhanced bioinks for nanoparticle evaluation. Biofabrication. 2022 14
61. Wang Z, Liang W, Ao R, An Y. Adipose Decellularized Matrix: A Promising Skeletal Muscle Tissue Engineering Material for Volume Muscle Loss. Biomater Res. 2025;29:0174
62. Chun SY, Lim JO, Lee EH, Han M-H, Ha Y-S, Lee JN. et al. Preparation and Characterization of Human Adipose Tissue-Derived Extracellular Matrix, Growth Factors, and Stem Cells: A Concise Review. Tissue Eng Regen Med. 2019;16:385-93
63. Wang H, Sun WQ, Wang J. Complete proteomic profiling of regenerative bio-scaffolds with a two-step trypsinization method. J Biomed Mater Res B Appl Biomater. 2023;111:62-72
64. Wang JK, Luo B, Guneta V, Li L, Foo SEM, Dai Y. et al. Supercritical carbon dioxide extracted extracellular matrix material from adipose tissue. Mater Sci Eng C Mater Biol Appl. 2017;75:349-58
65. Qi J, Li Z, Li S, Fu S, Luan J. Effectiveness of a New Enzyme-Free Method for the Preparation of a Decellularized Adipose-Derived Matrix. Aesthet Surg J. 2024;44:NP184-NP92
66. Yang J, Tang J, Dang J, Rong X, Wang K, Zhang Z. et al. Bioactive decellularized adipose matrix prepared using a rapid, nonchemical/enzymatic method for adipogenesis. Biotechnol Bioeng. 2024;121:157-75
67. Cheung HK, Han TTY, Marecak DM, Watkins JF, Amsden BG, Flynn LE. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials. 2014;35:1914-23
68. Liu P-C, Tan Q-W, Zhang Y, Wang H, Zhou L, Yang Q-R. et al. Hydrogel from acellular porcine adipose tissue promotes survival of adipose tissue transplantation. Biomed Mater. 2021 16
69. Kim JS, Choi JS, Cho YW. Cell-Free Hydrogel System Based on a Tissue-Specific Extracellular Matrix for In Situ Adipose Tissue Regeneration. ACS Appl Mater Interfaces. 2017;9:8581-8
70. Hayes DJ, Gimble JM. Developing a clinical grade human adipose decellularized biomaterial. Biomater Biosyst. 2022;7:100053
71. van Dongen JA, Getova V, Brouwer LA, Liguori GR, Sharma PK, Stevens HP. et al. Adipose tissue-derived extracellular matrix hydrogels as a release platform for secreted paracrine factors. J Tissue Eng Regen Med. 2019;13:973-85
72. Mohiuddin OA, O'Donnell BT, Poche JN, Iftikhar R, Wise RM, Motherwell JM. et al. Human Adipose-Derived Hydrogel Characterization Based on ASC Biocompatibility and Differentiation. Stem Cells Int. 2019;2019:9276398
73. Nyambat B, Manga YB, Chen C-H, Gankhuyag U, Pratomo Wp A, Kumar Satapathy M. et al. New Insight into Natural Extracellular Matrix: Genipin Cross-Linked Adipose-Derived Stem Cell Extracellular Matrix Gel for Tissue Engineering. Int J Mol Sci. 2020 21
74. Zhang Z, Cai J, Li Y, He Y, Dong Z, Dai J. et al. External Volume Expansion Adjusted Adipose Stem Cell by Shifting the Ratio of Fibronectin to Laminin. Tissue Eng Part A. 2020;26:66-77
75. Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno S. et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129:1081-92
76. Zheng H, Yu Z, Deng M, Cai Y, Wang X, Xu Y. et al. Fat extract improves fat graft survival via proangiogenic, anti-apoptotic and pro-proliferative activities. Stem Cell Res Ther. 2019;10:174
77. Han Y-d, Bai Y, Yan X-L, Ren J, Zeng Q, Li X-D. et al. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497:305-12
78. Lee S, Lee HS, Chung JJ, Kim SH, Park JW, Lee K. et al. Enhanced Regeneration of Vascularized Adipose Tissue with Dual 3D-Printed Elastic Polymer/dECM Hydrogel Complex. Int J Mol Sci. 2021 22
79. Yemanyi F, Vranka J, Raghunathan VK. Crosslinked Extracellular Matrix Stiffens Human Trabecular Meshwork Cells Via Dysregulating β-catenin and YAP/TAZ Signaling Pathways. Invest Ophthalmol Vis Sci. 2020;61:41
80. Výborný K, Vallová J, Kočí Z, Kekulová K, Jiráková K, Jendelová P. et al. Genipin and EDC crosslinking of extracellular matrix hydrogel derived from human umbilical cord for neural tissue repair. Sci Rep. 2019;9:10674
81. Tian J, Yang Y, Song J. Grafting polycaprolactone onto alkaline lignin for improved compatibility and processability. Int J Biol Macromol. 2019;141:919-26
82. Pennec JL, Picart C, Vivès RR, Migliorini EJAM. Sweet but Challenging: Tackling the Complexity of GAGs with Engineered Tailor-Made Biomaterials. 36.
83. Mende M, Bednarek C, Wawryszyn M, Sauter P, Biskup MB, Schepers U. et al. Chemical Synthesis of Glycosaminoglycans. Chem Rev. 2016;116:8193-255
84. Shridhar A, Amsden BG, Gillies ER, Flynn LE. Investigating the Effects of Tissue-Specific Extracellular Matrix on the Adipogenic and Osteogenic Differentiation of Human Adipose-Derived Stromal Cells Within Composite Hydrogel Scaffolds. Front Bioeng Biotechnol. 2019;7:402
85. Menezes R, Arinzeh TL. Comparative Study of Electrospun Scaffolds Containing Native GAGs and a GAG Mimetic for Human Mesenchymal Stem Cell Chondrogenesis. Ann Biomed Eng. 2020;48:2040-52
86. Kim JC, Min K, Tae G. The effect of the surface coating of human adipose-derived stem cells by various GAGs on the biodistribution of them upon intravenous administration. Mater Sci Eng C Mater Biol Appl. 2022: 112671.
87. Lim S, Park TY, Jeon EY, Joo KI, Cha HJ. Double-layered adhesive microneedle bandage based on biofunctionalized mussel protein for cardiac tissue regeneration. Biomaterials. 2021;278:121171
88. Park TY, Maeng S-W, Jeon EY, Joo KI, Cha HJ. Adhesive protein-based angiogenesis-mimicking spatiotemporal sequential release of angiogenic factors for functional regenerative medicine. Biomaterials. 2021;272:120774
89. Fang H, Li Q-L, Han M, Mei ML, Chu CH. Anti-proteolytic property and bonding durability of mussel adhesive protein-modified dentin adhesive interface. Dent Mater. 2017;33:1075-83
90. Jeon EY, Joo KI, Cha HJ. Body temperature-activated protein-based injectable adhesive hydrogel incorporated with decellularized adipose extracellular matrix for tissue-specific regenerative stem cell therapy. Acta Biomater. 2020;114:244-55
91. Zhang C, Wu B, Zhou Y, Zhou F, Liu W, Wang Z. Mussel-inspired hydrogels: from design principles to promising applications. Chem Soc Rev. 2020;49:3605-37
92. Gholipourmalekabadi M, Sapru S, Samadikuchaksaraei A, Reis RL, Kaplan DL, Kundu SC. Silk fibroin for skin injury repair: Where do things stand? Adv Drug Deliv Rev. 2020;153:28-53
93. Oral CB, Yetiskin B, Okay O. Stretchable silk fibroin hydrogels. Int J Biol Macromol. 2020;161:1371-80
94. Gu MJ, Fan SN, Zhou GD, Ma K, Yao X, Zhang YP. Effects of dynamic mechanical stimulations on the regeneration of in vitro and in vivo cartilage tissue based on silk fibroin scaffold. Composites Part B-Engineering. 2022 235
95. Li Y, Bi X, Wu M, Chen X, Zhan W, Dong Z. et al. Adjusting the stiffness of a cell-free hydrogel system based on tissue-specific extracellular matrix to optimize adipose tissue regeneration. Burns Trauma. 2023;11:tkad002
96. Kayabolen A, Keskin D, Aykan A, Karslıoglu Y, Zor F, Tezcaner A. Native extracellular matrix/fibroin hydrogels for adipose tissue engineering with enhanced vascularization. Biomed Mater. 2017;12:035007
97. Hong H, Seo YB, Kim DY, Lee JS, Lee YJ, Lee H. et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679
98. Sun W, Gregory DA, Tomeh MA, Zhao X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int J Mol Sci. 2021 22
99. Kim SH, Yeon YK, Lee JM, Chao JR, Lee YJ, Seo YB. et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat Commun. 2018;9:1620
100. Chen N, Wang H, Ling C, Vermerris W, Wang B, Tong Z. Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohydr Polym. 2019;225:115207
101. Abbas HA, Mabrouk M, Soliman AAF, Beherei HH. Dual-function membranes based on alginate/methyl cellulose composite for control drug release and proliferation enhancement of fibroblast cells. Int J Biol Macromol. 2020;164:2831-41
102. Zhou Y, Katsou E, Fan M. Interfacial structure and property of eco-friendly carboxymethyl cellulose/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biocomposites. Int J Biol Macromol. 2021;179:550-6
103. Zhang Y, Zhu C, Zhang Z, Zhao J, Yuan Y, Wang S. Oxidation triggered formation of polydopamine-modified carboxymethyl cellulose hydrogel for anti-recurrence of tumor. Colloids Surf B Biointerfaces. 2021;207:112025
104. Kar AK, Shil A, Kar B, Dey S. Formulation development and statistical optimization of zingiberol incorporated sodium alginate-methyl cellulose blend microspheres. Int J Biol Macromol. 2020;162:1578-86
105. Mallakpour S, Tukhani M, Hussain CM. Recent advancements in 3D bioprinting technology of carboxymethyl cellulose-based hydrogels: Utilization in tissue engineering. Adv Colloid Interface Sci. 2021;292:102415
106. Cui L, Zhao Y, Zhong Y, Zhang L, Zhang X, Guo Z. et al. Combining decellularized adipose tissue with decellularized adventitia extravascular matrix or small intestinal submucosa matrix for the construction of vascularized tissue-engineered adipose. Acta Biomater. 2023;170:567-79
107. Yao W, Song Z, Ma X, Huang Y, Zhang X, Li Y. et al. Asymmetric adhesive SIS-based wound dressings for therapeutically targeting wound repair. J Nanobiotechnology. 2024;22:34
108. Fercana GR, Yerneni S, Billaud M, Hill JC, VanRyzin P, Richards TD. et al. Perivascular extracellular matrix hydrogels mimic native matrix microarchitecture and promote angiogenesis via basic fibroblast growth factor. Biomaterials. 2017;123:142-54
109. Donovan C, Sun M, Cogswell D, Margo CE, Avila MY, Espana EM. Genipin increases extracellular matrix synthesis preventing corneal perforation. Ocul Surf. 2023;28:115-23
110. Zöller N, Schreiner S, Petry L, Hoffmann S, Steinhorst K, Kleemann J. et al. Collagen I Promotes Adipocytogenesis in Adipose-Derived Stem Cells In Vitro. Cells. 2019 8
111. Fu H, Zhang D, Zeng J, Fu Q, Chen Z, Sun X. et al. Application of 3D-printed tissue-engineered skin substitute using innovative biomaterial loaded with human adipose-derived stem cells in wound healing. Int J Bioprint. 2023;9:674
112. Dai R, Chen W, Chen Y, Jin J, Zhang S, Zhang C. et al. 3D bioprinting platform development for high-throughput cancer organoid models construction and drug evaluation. Biofabrication. 2024 16
113. Grilli F, Pitton M, Altomare L, Farè S. Decellularized fennel and dill leaves as possible 3D channel network in GelMA for the development of an in vitro adipose tissue model. Front Bioeng Biotechnol. 2022;10:984805
114. Buhsem O, Kirazoglu A. Agarose Gel: An Overview of the Dermal Filler and a Clinical Experience With 700 Patients. Aesthetic surgery journal Open forum. 2023;5:ojad051
115. Paskal AM, Paskal W, Pietruski P, Wlodarski PK. Polyethylene Glycol: The Future of Posttraumatic Nerve Repair? Systemic Review. Int J Mol Sci. 2019 20
116. Li S, Liu Y, McCann J, Ravnic DJ, Gimble JM, Hayes DJ. Hybrid adipose graft materials synthesized from chemically modified adipose extracellular matrix. J Biomed Mater Res A. 2022;110:156-63
117. Liu K, He Y, Yao Y, Zhang Y, Cai Z, Ru J. et al. Methoxy polyethylene glycol modification promotes adipogenesis by inducing the production of regulatory T cells in xenogeneic acellular adipose matrix. Mater Today Bio. 2021;12:100161
118. Giavina-Bianchi P, Kalil J. Polyethylene Glycol Is a Cause of IgE-Mediated Anaphylaxis. J Allergy Clin Immunol Pract. 2019;7:1874-5
119. Backes EH, Harb SV, Beatrice CAG, Shimomura KMB, Passador FR, Costa LC. et al. Polycaprolactone usage in additive manufacturing strategies for tissue engineering applications: A review. J Biomed Mater Res B Appl Biomater. 2022;110:1479-503
120. Blum C, Schlegelmilch K, Schilling T, Shridhar A, Rudert M, Jakob F. et al. Extracellular Matrix-Modified Fiber Scaffolds as a Proadipogenic Mesenchymal Stromal Cell Delivery Platform. ACS Biomater Sci Eng. 2019;5:6655-66
121. Baharlou Houreh A, Masaeli E, Nasr-Esfahani MH. Chitosan/polycaprolactone multilayer hydrogel: A sustained Kartogenin delivery model for cartilage regeneration. Int J Biol Macromol. 2021;177:589-600
122. Zhao Y, Zhang M, Lu G-L, Huang B-X, Wang D-W, Shao Y. et al. Hypoxic Preconditioning Enhances Cellular Viability and Pro-angiogenic Paracrine Activity: The Roles of VEGF-A and SDF-1a in Rat Adipose Stem Cells. Front Cell Dev Biol. 2020;8:580131
123. Cho W-W, Kim BS, Ahn M, Ryu YH, Ha D-H, Kong JS. et al. Flexible Adipose-Vascular Tissue Assembly Using Combinational 3D Printing for Volume-Stable Soft Tissue Reconstruction. Adv Healthc Mater. 2021;10:e2001693
124. Kim SH, Kim D, Cha M, Kim SH, Jung Y. The Regeneration of Large-Sized and Vascularized Adipose Tissue Using a Tailored Elastic Scaffold and dECM Hydrogels. Int J Mol Sci. 2021 22
125. Zhu Z, Yuan Z, Guo L, Nurzat Y, Xu H, Zhang Y. Construction of adipose tissue using a silica expander capsule and cell sheet-assembled of decellularized adipose tissue. Acta Biomater. 2022 141
126. Souza LEB, Beckenkamp LR, Sobral LM, Fantacini DMC, Melo FUF, Borges JS. et al. Pre-culture in endothelial growth medium enhances the angiogenic properties of adipose-derived stem/stromal cells. Angiogenesis. 2018;21:15-22
127. Nie J, Yi Y, Zhu Y. [Construction of tissue engineered adipose by human adipose tissue derived extracellular vesicle combined with decellularized adipose tissues scaffold]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34:226-33
128. Zhou S, Chang Q, Lu F, Xing M. Injectable Mussel-Inspired Immobilization of Platelet-Rich Plasma on Microspheres Bridging Adipose Micro-Tissues to Improve Autologous Fat Transplantation by Controlling Release of PDGF and VEGF, Angiogenesis, Stem Cell Migration. Adv Healthc Mater. 2017 6
129. Hou M, Tang J, Guo Y, Peng H, Liang B, Cheng Y. et al. T-PRP-DAT Gel: A Novel Material Promotes Adipose Tissue Regeneration. J Cosmet Dermatol. 2025;24:e70045
130. Aurora A, Wrice N, Walters TJ, Christy RJ, Natesan S. A PEGylated platelet free plasma hydrogel based composite scaffold enables stable vascularization and targeted cell delivery for volumetric muscle loss. Acta Biomater. 2018;65:150-62
131. Del Amo C, Fernández-San Argimiro X, Cascajo-Castresana M, Perez-Valle A, Madarieta I, Olalde B. et al. Wound-Microenvironment Engineering through Advanced-Dressing Bioprinting. Int J Mol Sci. 2022 23
132. Yu C, Kornmuller A, Brown C, Hoare T, Flynn LE. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials. 2017;120:66-80
133. Wenderott JK, Flesher CG, Baker NA, Neeley CK, Varban OA, Lumeng CN. et al. Elucidating nanoscale mechanical properties of diabetic human adipose tissue using atomic force microscopy. Sci Rep. 2020;10:20423
134. Vernerey FJ, Lalitha Sridhar S, Muralidharan A, Bryant SJ. Mechanics of 3D Cell-Hydrogel Interactions: Experiments, Models, and Mechanisms. Chem Rev. 2021;121:11085-148
135. Perez-Puyana V, Jiménez-Rosado M, Romero A, Guerrero A. Crosslinking of hybrid scaffolds produced from collagen and chitosan. Int J Biol Macromol. 2019;139:262-9
136. Sun J-Y, Zhao X, Illeperuma WRK, Chaudhuri O, Oh KH, Mooney DJ. et al. Highly stretchable and tough hydrogels. Nature. 2012;489:133-6
137. Chen Z, Ezzo M, Zondag B, Rakhshani F, Ma Y, Hinz B. et al. Intrafibrillar Crosslinking Enables Decoupling of Mechanical Properties and Structure of a Composite Fibrous Hydrogel. Adv Mater. 2024;36:e2305964
138. Zou C, Chen C. Polar-Functionalized, Crosslinkable, Self-Healing, and Photoresponsive Polyolefins. Angew Chem Int Ed Engl. 2020;59:395-402
139. Zhu Z, Niu Y, Wang S, Su M, Long Y, Sun H. et al. Magnesium hydroxide coated hollow glass microspheres/chitosan composite aerogels with excellent thermal insulation and flame retardancy. J Colloid Interface Sci. 2022;612:35-42
140. Yegappan R, Selvaprithiviraj V, Mohandas A, Jayakumar R. Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel for angiogenic drug delivery. Colloids Surf B Biointerfaces. 2019;177:41-9
141. Khajavi M, Hajimoradloo A, Zandi M, Pezeshki-Modaress M, Bonakdar S, Zamani A. Fish cartilage: A promising source of biomaterial for biological scaffold fabrication in cartilage tissue engineering. J Biomed Mater Res A. 2021;109:1737-50
142. Stone KR, Walgenbach A, Galili U. Induced Remodeling of Porcine Tendons to Human Anterior Cruciate Ligaments by α-GAL Epitope Removal and Partial Cross-Linking. Tissue Eng Part B Rev. 2017;23:412-9
143. Sánchez-Morán H, Ahmadi A, Vogler B, Roh K-H. Oxime Cross-Linked Alginate Hydrogels with Tunable Stress Relaxation. Biomacromolecules. 2019;20:4419-29
144. Hafeez S, Ooi HW, Morgan FLC, Mota C, Dettin M, Van Blitterswijk C. et al. Viscoelastic Oxidized Alginates with Reversible Imine Type Crosslinks: Self-Healing, Injectable, and Bioprintable Hydrogels. Gels. 2018 4
145. Zhou X, Tao Y, Chen E, Wang J, Fang W, Zhao T. et al. Genipin-cross-linked type II collagen scaffold promotes the differentiation of adipose-derived stem cells into nucleus pulposus-like cells. J Biomed Mater Res A. 2018;106:1258-68
146. Xue J, Ji W, Dong S, Zhang Z, Gao J, Yang P. et al. Degradable and Thermosensitive Microgels with Tannic Acid as the Sole Cross-Linker. Langmuir. 2019;35:16353-65
147. Wen J, Zhang X, Pan M, Yuan J, Jia Z, Zhu L. A Robust, Tough and Multifunctional Polyurethane/Tannic Acid Hydrogel Fabricated by Physical-Chemical Dual Crosslinking. Polymers (Basel). 2020 12
148. Wang X, Wang Y, Li L, Gu Z, Yu X. Feasibility study of the naturally occurring dialdehyde carboxymethyl cellulose for biological tissue fixation. Carbohydr Polym. 2015;115:54-61
149. Wang X, Wen K, Yang X, Li L, Yu X. Biocompatibility and anti-calcification of a biological artery immobilized with naturally-occurring phytic acid as the crosslinking agent. J Mater Chem B. 2017;5:8115-24
150. Zhou X, Wang J, Fang W, Tao Y, Zhao T, Xia K. et al. Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018;71:496-509
151. Lee J, Hong J, Kim W, Kim GH. Bone-derived dECM/alginate bioink for fabricating a 3D cell-laden mesh structure for bone tissue engineering. Carbohydr Polym. 2020;250:116914
152. García-García ÓD, El Soury M, González-Quevedo D, Sánchez-Porras D, Chato-Astrain J, Campos F. et al. Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves. Int J Mol Sci. 2021 22
153. Sundararaghavan HG, Monteiro GA, Lapin NA, Chabal YJ, Miksan JR, Shreiber DI. Genipin-induced changes in collagen gels: correlation of mechanical properties to fluorescence. J Biomed Mater Res A. 2008;87:308-20
154. Chung Y, Park D, Kim H, Kim Y, Kang S. The impact of gamma-irradiation from radioactive liquid wastewater on polymeric structures of nanofiltration (NF) membranes. J Hazard Mater. 2021;403:123578
155. Tadsen M, Friedrich RP, Riedel S, Alexiou C, Mayr SG. Contact Guidance by Microstructured Gelatin Hydrogels for Prospective Tissue Engineering Applications. ACS Appl Mater Interfaces. 2019;11:7450-8
156. Zou X, Xiao X, Zhang S, Zhong J, Hou Y, Liao L. A photo-switchable and thermal-enhanced fluorescent hydrogel prepared from N-isopropylacrylamide with water-soluble spiropyran derivative. J Biomater Sci Polym Ed. 2018;29:1579-94
157. Zhu Y, Reinach PS, Zhu H, Tan Q, Zheng Q, Qu J. et al. High-intensity corneal collagen crosslinking with riboflavin and UVA in rat cornea. PLoS One. 2017;12:e0179580
158. Zou W, Chen Y, Zhang X, Li J, Sun L, Gui Z. et al. Cytocompatible chitosan based multi-network hydrogels with antimicrobial, cell anti-adhesive and mechanical properties. Carbohydr Polym. 2018;202:246-57
159. Gomes AD, de Oliveira AAR, Houmard M, Nunes EHM. Gamma sterilization of collagen/hydroxyapatite composites: Validation and radiation effects. Appl Radiat Isot. 2021;174:109758
160. Rayavara K, Kurosky A, Hosakote YM. Respiratory syncytial virus infection induces the release of transglutaminase 2 from human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2022 322
161. Tsai C-C, Kuo S-H, Lu T-Y, Cheng N-C, Shie M-Y, Yu J. Enzyme-Cross-linked Gelatin Hydrogel Enriched with an Articular Cartilage Extracellular Matrix and Human Adipose-Derived Stem Cells for Hyaline Cartilage Regeneration of Rabbits. ACS Biomater Sci Eng. 2020;6:5110-9
162. Tempest R, Guarnerio S, Maani R, Cooper J, Peake N. The Biological and Biomechanical Role of Transglutaminase-2 in the Tumour Microenvironment. Cancers (Basel). 2021 13
163. Vallet SD, Miele AE, Uciechowska-Kaczmarzyk U, Liwo A, Duclos B, Samsonov SA. et al. Insights into the structure and dynamics of lysyl oxidase propeptide, a flexible protein with numerous partners. Sci Rep. 2018;8:11768
164. Bullock PTB, Reid DG, Ying Chow W, Lau WPW, Duer MJ. A new glycation product 'norpronyl-lysine,' and direct characterization of cross linking and other glycation adducts: NMR of model compounds and collagen. Biosci Rep. 2014 34
165. Nair M, Johal RK, Hamaia SW, Best SM, Cameron RE. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: A comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials. 2020;254:120109
166. Dashnyam K, Jin G-Z, Kim J-H, Perez R, Jang J-H, Kim H-W. Promoting angiogenesis with mesoporous microcarriers through a synergistic action of delivered silicon ion and VEGF. Biomaterials. 2017;116:145-57
167. Walker JT, Cooper TT, Dunmore-Buyze J, Serack FE, Brooks C, Grant A. et al. Syngeneic adipose-derived stromal cells modulate the immune response but have limited persistence within decellularized adipose tissue implants in C57BL/6 mice. Acta Biomater. 2025
168. Zhu C, Huang Z, Zhou H, Han X, Li L, Yin N. Purified adipose tissue-derived extracellular vesicles facilitate adipose organoid vascularization through coordinating adipogenesis and angiogenesis. Biofabrication. 2025 17
169. Wang J, Li L, Zhang Z, Zhang X, Zhu Y, Zhang C. et al. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022 34
170. Rohm TV, Castellani Gomes Dos Reis F, Isaac R, Murphy C, Cunha E Rocha K, Bandyopadhyay G. et al. Adipose tissue macrophages secrete small extracellular vesicles that mediate rosiglitazone-induced insulin sensitization. Nat Metab. 2024;6:880-98
171. Zhao H, Chen X, Hu G, Li C, Guo L, Zhang L. et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circ Res. 2022;130:1490-506
172. Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ. et al. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016;2:e1600502
173. Huleihel L, Bartolacci JG, Dziki JL, Vorobyov T, Arnold B, Scarritt ME. et al. Matrix-Bound Nanovesicles Recapitulate Extracellular Matrix Effects on Macrophage Phenotype. Tissue Eng Part A. 2017;23:1283-94
174. van der Merwe Y, Faust AE, Sakalli ET, Westrick CC, Hussey G, Chan KC. et al. Matrix-bound nanovesicles prevent ischemia-induced retinal ganglion cell axon degeneration and death and preserve visual function. Sci Rep. 2019;9:3482
175. Faust A, Kandakatla A, van der Merwe Y, Ren T, Huleihel L, Hussey G. et al. Urinary bladder extracellular matrix hydrogels and matrix-bound vesicles differentially regulate central nervous system neuron viability and axon growth and branching. J Biomater Appl. 2017;31:1277-95
176. Hussey GS, Pineda Molina C, Cramer MC, Tyurina YY, Tyurin VA, Lee YC. et al. Lipidomics and RNA sequencing reveal a novel subpopulation of nanovesicle within extracellular matrix biomaterials. Sci Adv. 2020;6:eaay4361
177. Hao D, Swindell HS, Ramasubramanian L, Liu R, Lam KS, Farmer DL. et al. Extracellular Matrix Mimicking Nanofibrous Scaffolds Modified With Mesenchymal Stem Cell-Derived Extracellular Vesicles for Improved Vascularization. Front Bioeng Biotechnol. 2020;8:633
178. Dong J, Yu M, Zhang Y, Yin Y, Tian W. Recent developments and clinical potential on decellularized adipose tissue. J Biomed Mater Res A. 2018;106:2563-74
179. Ning L-J, Zhang Y-J, Zhang Y, Qing Q, Jiang Y-L, Yang J-L. et al. The utilization of decellularized tendon slices to provide an inductive microenvironment for the proliferation and tenogenic differentiation of stem cells. Biomaterials. 2015;52:539-50
180. Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251-62
181. Mammoto T, Jiang E, Jiang A, Mammoto A. Extracellular matrix structure and tissue stiffness control postnatal lung development through the lipoprotein receptor-related protein 5/Tie2 signaling system. Am J Respir Cell Mol Biol. 2013;49:1009-18
182. Guo A, Cao Q, Fang H, Tian H. Recent advances and challenges of injectable hydrogels in drug delivery. J Control Release. 2025: 114021.
183. Wang Z, Xiang L, Lin F, Tang Y, Cui W. 3D bioprinting of emulating homeostasis regulation for regenerative medicine applications. J Control Release. 2023;353:147-65
184. Temple JP, Hutton DL, Hung BP, Huri PY, Cook CA, Kondragunta R. et al. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res A. 2014;102:4317-25
185. Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536
186. Li S, Poche JN, Liu Y, Scherr T, McCann J, Forghani A. et al. Hybrid Synthetic-Biological Hydrogel System for Adipose Tissue Regeneration. Macromol Biosci. 2018;18:e1800122
187. Zhang Q, Johnson JA, Dunne LW, Chen Y, Iyyanki T, Wu Y. et al. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater. 2016;35:166-84
Author contact
![]() Corresponding authors: Dr. Rui Guo, E-mail: rguoedu.cn. College of Life Sciences, Institute of Life Science and Green Development, Hebei University, Baoding 071002, China; Jun Fan MD, PhD, E-mail: jfanedu.cn Department of Tissue Engineering, School of Intelligent Medicine, China Medical University, No. 77 Puhe Road, Shenyang North New Area, Shenyang, Liaoning Province, 110122, P.R. China.
Corresponding authors: Dr. Rui Guo, E-mail: rguoedu.cn. College of Life Sciences, Institute of Life Science and Green Development, Hebei University, Baoding 071002, China; Jun Fan MD, PhD, E-mail: jfanedu.cn Department of Tissue Engineering, School of Intelligent Medicine, China Medical University, No. 77 Puhe Road, Shenyang North New Area, Shenyang, Liaoning Province, 110122, P.R. China.
 Global reach, higher impact
Global reach, higher impact