13.3
Impact Factor
Theranostics 2026; 16(1):516-544. doi:10.7150/thno.117949 This issue Cite
Review
Recent advances and challenges in hydrogel-based delivery of immunomodulatory strategies for diabetic wound healing
1. Department of Orthopedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
2. Hubei Key Laboratory of Regenerative Medicine and Multi-disciplinary Translational Research (Huazhong University of Science and Technology), Wuhan 430022, China.
†Longyu Du, Chuanlu Lin, and Haifeng Hu contributed equally to this work.
Received 2025-5-20; Accepted 2025-9-15; Published 2026-1-1
Abstract
Chronic wounds associated with diabetes present considerable clinical hurdles, primarily due to delayed tissue repair and dysregulated immune activity. The imbalance in immune responses, including impaired macrophage polarization, excessive neutrophil activation, and oxidative stress, further hampers the healing process. The application of immunomodulatory biologics as a novel treatment method for diabetic wounds often yields limited results due to rapid degradation and lack of targeting. Hydrogels not only prevent rapid drug degradation but also allow for conditional responsiveness and targeted delivery. Therefore, hydrogels loaded with immunomodulatory biologics emerge as a promising strategy, offering the capacity to reshape the immune milieu and promote regenerative outcomes. This review first outlines the role of immune system during the healing processes in normal and diabetic wounds. It then discusses the latest advancements in hydrogel delivery systems as part of immune-modulatory interventions, wherein hydrogels serve as pivotal carriers for (i) cell delivery, such as stem cells and macrophages; (ii) extracellular vesicles derived from both cellular and tissue sources, as well as extracellular vesicle mimetics; and (iii) bioactive substances, including oxygen-releasing microspheres, nanomaterials, and cytokines. Finally, this review focuses on the limitations of modulating immune responses in diabetic wound healing and proposes potential future directions.
Keywords: diabetic wounds, immunomodulation, hydrogels, extracellular vesicles, biomaterials
1. Introduction
Diabetes mellitus is a widespread condition hallmarked by chronic hyperglycemia and its associated range of complications, including vasculopathy, neuropathy, nephropathy, retinopathy, and chronic wounds. According to the International Diabetes Federation's 2021 report, over 500 million people worldwide are living with diabetes, and this number is projected to exceed 780 million by 2045 [1]. Among the most serious complications are diabetic wounds, particularly those affecting the lower extremities, which can lead to amputation and even death. It is estimated that nearly one-third of individuals with diabetes will develop a diabetic foot ulcer during their lifetime, and approximately 20% of these patients will require amputation [2]. Diabetic wounds impose a substantial physical, emotional, and financial burden, highlighting the urgent need for effective and affordable treatment strategies [1]. Importantly, patients with type 1 diabetes (T1D) and type 2 diabetes (T2D), as well as those with neuropathic or ischemic complications, may exhibit distinct wound pathologies and immune profiles, which can critically influence therapeutic outcomes.
A wide range of therapeutic strategies has been developed for diabetic wound management, including debridement, infection control, glycemic regulation, hyperbaric oxygen therapy, negative pressure wound therapy, energy-based interventions (e.g., pulsed electromagnetic fields, shockwaves, and lasers), and topical drug delivery. Notably, wound dressings have been a cornerstone of treatment for more than two millennia [3]. Advances in biology and materials science have led to the development of innovative dressings composed of natural polymers, synthetic polymers, or hybrid materials. Following Winter's pivotal discovery in 1962 that moist environments accelerate wound healing, there has been rapid progress in the design of moisture-retaining dressings, such as hydrocolloids and hydrogels.
An ideal wound dressing should provide adequate mechanical strength, excellent biocompatibility, and non-toxicity. It should be easy to replace, non-adherent to the wound bed, absorbent without leaking, and capable of maintaining a moist healing environment. Additionally, it should offer antimicrobial protection, reduce pain, enable real-time monitoring, support tissue regeneration, and deliver therapeutic agents in a controlled manner [4-8]. Compared to conventional dressings, hydrogels offer distinct advantages by maintaining a moist environment, reducing local temperature, and allowing customization of their physical and chemical properties to suit various wound types. Their composition and crosslinking mechanisms can be tailored to modulate biological responses and enhance healing. Furthermore, hydrogels support targeted and responsive delivery of cells, biologics, and other therapeutics [9-12]. Their physicochemical characteristics—such as composition, dimensionality, surface morphology, and porosity—can influence local cell behavior, while hydrogel-based delivery of bioactive agents has been shown to modulate immune responses, stimulate angiogenesis, and control infection. As such, hydrogels have emerged as a promising platform for diabetic wound care [12,13]. Recently, they have attracted growing interest for their anti-inflammatory, antimicrobial, antioxidant, angiogenic, stimulus-responsive, and wound-monitoring properties [8,14-16].
Diabetic wounds are hallmarked by chronic inflammation and abnormal cellular phenotypes [2]. Unlike normal wounds, they often exhibit delayed or impaired healing due to factors such as hypoxia, elevated matrix metalloproteinases (MMPs) activity, and immune dysfunction [17,18]. A dysregulated immune microenvironment is now recognized as a major barrier to effective healing [12,19,20]. Immune cells such as macrophages, neutrophils, and T lymphocytes normally orchestrate tissue repair through pathogen clearance, cytokine secretion, and resolution of inflammation. In diabetes, however, macrophage polarization skews toward the pro-inflammatory M1 phenotype, neutrophils form excessive extracellular traps, and T cell function is impaired—collectively sustaining inflammation and hindering repair [21-23].
Given these immune abnormalities, strategies to restore a balanced immune environment are urgently needed. Hydrogels, with their tunable physicochemical properties and capacity for controlled delivery, represent an ideal platform for implementing such immunomodulatory strategies. Beyond serving as moisture-retaining dressings, hydrogels can be engineered to encapsulate and release immunomodulatory agents—including cells, extracellular vesicles (EVs), cytokines, antibodies, and small molecules—in a sustained or stimuli-responsive fashion, directly targeting the immunological deficits of diabetic wounds [13]. By linking material design to the regulation of immune responses, hydrogel platforms offer a unique opportunity to integrate wound protection with active immunotherapy.
In addition to their physicochemical tunability, the degradation behavior of hydrogels is a pivotal determinant of sustained release, yet it has often been overlooked in the context of diabetic wound healing. Hydrogels degrade via hydrolytic, enzymatic, or oxidative mechanisms, with MMP and elevated reactive oxygen species (ROS) in diabetic wounds profoundly influencing their stability. Such degradation not only governs mesh size and porosity but also directly controls the kinetics and duration of therapeutic release. For instance, MMP-responsive hydrogels have been engineered to achieve on-demand growth factor delivery, synchronizing cargo release with periods of high protease activity in chronic wounds [24]. However, uncontrolled degradation in the hyperinflammatory setting may exhaust payloads too early, while overly stable hydrogels may fail to release sufficient agents when needed. Therefore, future hydrogel platforms for diabetic wound immunomodulation should incorporate tunable, environment-responsive degradation mechanisms, such as MMP-sensitive linkers or ROS-degradable crosslinks, and be assessed under standardized in-vitro models that mimic diabetic wound biochemistry (e.g., acidic pH, high ROS, and elevated protease levels) [25]. Taken together, degradation dynamics must be integrated into the broader design of immunomodulatory hydrogel platforms.
Despite extensive research on the immunomodulatory roles of hydrogels, recent systematic reviews on hydrogel-based delivery systems for immune regulation in diabetic wound treatment remain limited. This review offers a comprehensive synthesis of recent advances in the development of immunomodulatory hydrogel platforms, with a particular focus on their application in diabetic wound care. It first delineates the immunological mechanisms underlying normal wound repair and highlight the distinct immune impairments associated with diabetes. We then discuss emerging strategies that incorporate hydrogels with cells, EVs, and bioactive substances to modulate immune responses in diabetic wounds (Figure 1). The discussion then shifts to current technological progress, existing hurdles, and future perspectives in engineering immunomodulatory hydrogels for diabetic wound therapy.
2. The Role of the Immune System in Wound Healing
The healing of skin wounds typically proceeds through four overlapping but sequential phases: hemostasis, inflammation, proliferation, and remodeling. The hemostatic phase is initiated by vasoconstriction and platelet activation, followed by the inflammatory phase, which is mediated primarily by neutrophils and monocytes/macrophages. During the proliferative phase, granulation tissue forms, and neovascularization occurs. The remodeling phase then involves collagen shift and extracellular matrix (ECM) remodeling. Immune cells and the bioactive factors they secrete play indispensable roles throughout all these phases.
2.1. Hemostatic Phase
Immediately following injury, vascular disruption leads to hemorrhage. In response, the body activates a series of coagulation mechanisms, including reactive vasoconstriction, primary coagulation, and secondary coagulation. During primary coagulation, activated platelets release growth factors and cytokines that recruit neutrophils, macrophages, and other immune cells [3,20,26]. The fibrin clot formed during secondary coagulation not only halts bleeding but also provides a temporary matrix for immune cell infiltration and coagulation [7,22,27].
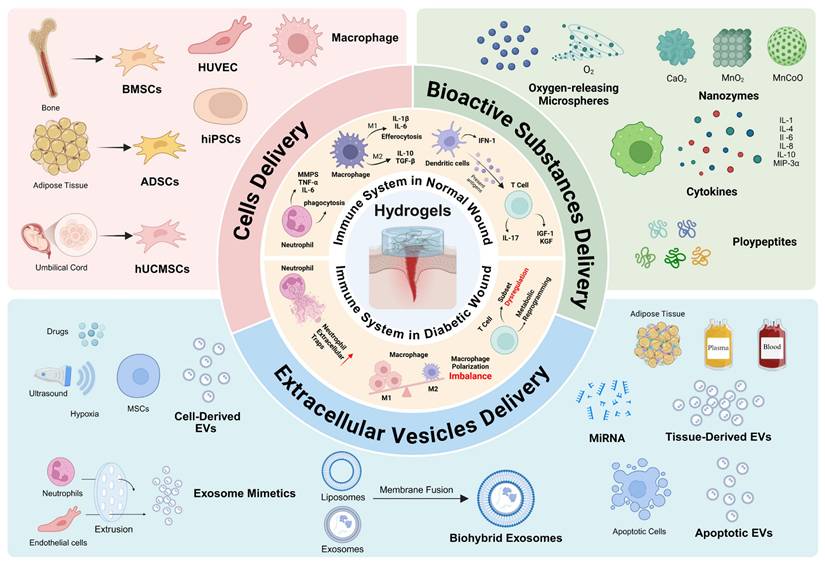
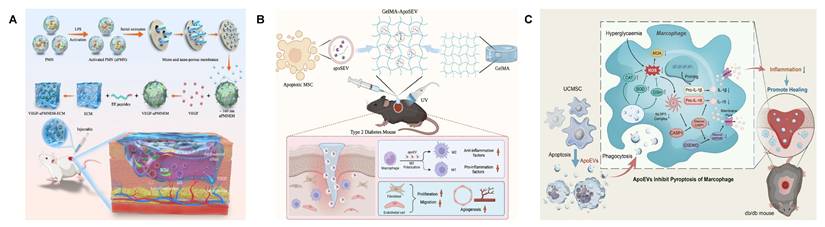
Immunomodulatory strategies of hydrogels for the treatment of diabetic wounds. Hydrogels act as versatile carriers to deliver (i) cells, including MSCs, macrophages, and iPSC-derived lineages; (ii) EVs from cellular, tissue, or other sources; and (iii) bioactive substances including oxygen-releasing microspheres, nanozymes, cytokines, peptides, and growth factors. These hydrogel-based platforms reshape the diabetic wound immune microenvironment by promoting macrophage polarization toward the M2 phenotype, reducing NETs, modulating T-cell responses, alleviating oxidative stress, and enhancing angiogenesis. Collectively, these strategies restore immune balance and accelerate tissue repair. MSC, mesenchymal stem cell; iPSC, induced pluripotent stem cell; EV, extracellular vesicle; NET, neutrophil extracellular trap. Created with https://BioRender.com.
2.2. Inflammatory Phase
In the inflammatory phase, a variety of immune cells—particularly neutrophils and macrophages—coordinate the regulation of the wound immune microenvironment. Neutrophils serve as early responders rapidly recruited to the injury site upon stimulation by damage-associated molecular patterns (DAMPs), interleukin-8 (IL-8), and other chemotactic cues. They eliminate pathogens and cellular debris via phagocytosis, protease release, neutrophil extracellular traps (NETs) formation, and secretion of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which amplify immune cell recruitment [3,28,29].
Subsequently, monocytes migrate from the circulation and differentiate into macrophages, primarily of the M1 phenotype in the early stages [7,22]. Macrophages are also activated by DAMPs to release pro-inflammatory mediators, which further recruit neutrophils and monocytes. In this phase, macrophages play a pivotal role in clearing pathogens and cellular debris [20]. Macrophages exhibit phenotypic plasticity and can be broadly classified into pro-inflammatory M1 and anti-inflammatory M2 subtypes, with M2 further subtyped into M2a, M2b, and M2c based on their functional profiles [22,28-30].
After neutrophils complete their clearance tasks, they undergo programmed cell death and are phagocytosed by M1 macrophages through efferocytosis, which triggers the reprogramming of macrophages toward the M2 phenotype [29,31]. This transition is essential for shifting the immune microenvironment from a suppressive to a reparative state.
2.3. Proliferative and Remodeling Phase
The M1-to-M2 transition of macrophages marks the beginning of the proliferative and remodeling phases. During this period, macrophages support neovascularization, stimulate fibroblast differentiation into myofibroblasts, and may even transdifferentiate into fibroblast-like cells [20,28,29,31]. In the early proliferative phase, M2a macrophages become predominant, secreting pro-fibrotic cytokines and depositing ECM components. However, prolonged retention of M2a macrophages can exacerbate scar formation [30-32]. During the remodeling phase, the macrophage population declines and becomes dominated by M2c macrophages, which release MMPs that degrade excessive ECM and contribute to matrix remodeling [22,30-32].
In addition to macrophages and neutrophils, other immune and non-immune cells are involved in coordinating the immune response during wound healing [31,33,34]. T cells and mast cells contribute by recruiting immune cells and regulating fibrosis and angiogenesis [35,36]. Dendritic cells secrete type I interferons (IFNs) and promote transforming growth factor-beta 1 (TGF-β1) production, thereby supporting granulation tissue formation and vascular regeneration during the proliferative phase [37]. Adipocytes also play a role, especially in the early phase of healing. In Drosophila, adipose-like cells migrate directionally to the wound site, assisting macrophages in clearing debris [38]. In humans, subcutaneous adipocytes secrete lipocalin and leptin to modulate macrophage polarization. Additionally, lipolysis-derived fatty acids enhance macrophage recruitment in the early wound healing stage [34,39]. Fibroblasts, which exhibit both pro-inflammatory and pro-fibrotic phenotypes, actively regulate immune cell recruitment and function throughout the wound healing process [33,34,40].
3. Immune Dysregulation in Diabetic Wound Healing
The impaired healing of diabetic wounds is closely associated with persistent inflammation, which stems from various immune abnormalities, including the excessive accumulation of neutrophils, macrophage phenotypic imbalances, and dysregulation of T-cell ratios and functions. Therefore, identifying and addressing these immune dysfunctions is critical for the effective treatment of diabetic wounds.
3.1. Macrophage Dysfunction in Diabetic Wounds
Macrophage dysfunction is a major contributor to the delayed healing observed in diabetic wounds. The hyperglycemic microenvironment profoundly affects macrophage polarization, leading to a functional imbalance that disrupts the wound healing process. A hallmark of this dysfunction is the skewed polarization toward the proinflammatory M1 phenotype, with a concomitant reduction in the reparative M2 phenotype. M1 macrophages continuously secrete proinflammatory cytokines such as TNF-α and interleukin-1β (IL-1β), which intensify inflammation and stimulate the production of MMPs. These enzymes degrade the ECM, exacerbating wound chronicity. Meanwhile, the deficiency of M2 macrophages impairs the resolution of inflammation and tissue regeneration [41,42].
In the diabetic microenvironment, advanced glycation end products (AGEs) interact with macrophage receptors and activate the NF-κB signaling pathway. This activation enhances the proinflammatory activity of M1 macrophages, suppresses M2 macrophage functions, and increases the release of inflammatory mediators [29]. AGEs also significantly impair macrophage phagocytosis, particularly the clearance of apoptotic cells and pathogens. This results in the accumulation of cellular debris and prolongs the inflammatory phase. Moreover, impaired efferocytosis hampers the macrophage phenotypic switch from M1 to M2 [29,43-45].
Notably, local insulin application has been shown to promote the M1-to-M2 phenotypic transition in diabetic wounds. Insulin signaling abnormalities, a hallmark of the diabetic state, have also been identified as key drivers of macrophage dysfunction [46]. Additionally, the diabetic environment disrupts macrophage interactions with other immune cells. For instance, diminished efferocytosis allows neutrophil accumulation and excessive ROS production, which can prematurely impair fibroblast functions [20,43-45].
In summary, macrophage dysfunction in diabetic wounds is characterized by phenotypic imbalance, impaired phagocytosis, excessive proinflammatory cytokine production, and disrupted cellular crosstalk. Restoring macrophage function, especially promoting their polarization toward the M2 phenotype, has become a central strategy in diabetic wound therapy. For example, PPAR-γ agonists have been shown to enhance neutrophil clearance and promote M2 polarization, thereby facilitating wound healing [47].
3.2. Neutrophil Dysfunction in Diabetic Wounds
Neutrophil dysfunction is another important factor contributing to poor healing in diabetic wounds. Under diabetic conditions, excessive neutrophil accumulation and activation lead to increased oxidative stress and overproduction of ROS, which in turn activate proteases that damage the ECM and aggravate local inflammation [20,31]. Moreover, proteases such as neutrophil elastase degrade critical growth factors necessary for tissue repair, including platelet-derived growth factor (PDGF) and TGF-β1 [20,21].
The dysregulation of neutrophil apoptosis also contributes to persistent inflammation. Apoptotic neutrophils are frequently observed in diabetic wounds, and AGEs have been shown to impair their clearance by macrophages [43,48]. Recently, attention has focused on NETs, which are web-like structures composed of chromatin and antimicrobial proteins that trap and kill pathogens [49]. In diabetes, increased activity of enzymes such as peptidyl arginine deiminase 4 (PAD4), neutrophil elastase, and protein kinase C leads to excessive NET formation. This exacerbates inflammation, damages the ECM, and impairs the wound healing environment [50].
Mechanistically, NETs activate the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome and promote IL-1β secretion through Toll-like receptor (TLR) and NF-κB pathways [49]. They also inhibit angiogenesis via dysregulation of the Hippo-YAP signaling pathway and interfere with fibroblast function, reducing collagen and ECM protein synthesis [49,51]. The overproduction of NETs thus hinders both vascularization and fibroblast-mediated tissue repair. Consequently, the use of NET inhibitors or other NET-targeting strategies has emerged as a promising approach to improving the immune microenvironment of diabetic wounds [49,52-54].
3.3. T Cell Dysfunction in Diabetic Wounds
T cell dysfunction in diabetic wounds represents a complex interplay of metabolic reprogramming and altered subset dynamics, driven primarily by hyperglycemia. Elevated glucose levels have been shown to induce mitochondrial dysfunction in CD4+ T cells, resulting in increased oxidative stress and lipid accumulation. This metabolic stress promotes lipid peroxidation (LPO) and STAT4 carbonylation, ultimately impairing T cell function [55].
In addition, T cell subset imbalance is a prominent feature of diabetic wound immunopathology. The diabetic milieu promotes the expansion of effector T cells in circulation while reducing the diversity of the TCR-β pool. These effector T cells secrete high levels of IFN-γ and TNF-α, exacerbating the inflammatory response [21,56]. Meanwhile, regulatory T cells (Tregs), which are essential for immune tolerance and inflammation resolution, are reduced in number or functionally impaired in diabetic wounds. In T2D, hyperinsulinemia has been found to further suppress Treg functions, amplifying immune dysregulation [57-59].
Insulin-like growth factor 1 (IGF-1) plays a crucial role in wound healing by promoting cell proliferation, migration, collagen synthesis, and angiogenesis. It also modulates immune responses to facilitate repair [60]. However, IGF-1 signaling is impaired in diabetic patients, and T cells from diabetic wounds often lack the ability to produce IGF-1, contributing to delayed wound healing and immune dysfunction [21,56,61].
3.4. Adipocyte and Fibroblast Dysfunction in Diabetic Wounds
Although adipocytes and fibroblasts are not immune cells, they play pivotal roles in the immunomodulation of diabetic wounds. In diabetic patients, adipocytes exhibit increased secretion of pro-inflammatory factors, which promote macrophage recruitment while inhibiting the polarization toward M2 phenotypes. Additionally, diabetic fibroblasts undergo premature senescence and develop a senescence-associated secretory phenotype (SASP), characterized by elevated expression of pro-inflammatory cytokines, chemokines, and MMPs. These factors collectively contribute to impaired wound healing and tissue regeneration [34].
3.5. Crosstalk Among Immune Cells in Diabetic Wounds
Beyond individual cellular dysfunctions, diabetic wounds are sustained by complex crosstalk among macrophages, neutrophils, and T cells [28,31]. Pro-inflammatory M1 macrophages secrete IL-1β and TNF-α, which enhance neutrophil recruitment and survival [21,22,32]. Neutrophils, in turn, release excessive NETs that activate the macrophage NLRP3 inflammasome, amplifying inflammatory signaling [21]. Macrophage-derived IL-12 and IL-23 further drive Th1/Th17 polarization, while Th17-derived IL-17 reinforces neutrophil infiltration [22,32]. Conversely, Tregs release IL-10 and TGF-β to restrain M1 macrophage activity and restore immune balance, but their numbers and function are markedly reduced in diabetes [27]. These multidirectional interactions indicate that chronic inflammation in diabetic wounds arises not from isolated defects but from interconnected networks of immune dysregulation, underscoring the need for therapeutic strategies that intervene at multiple cellular nodes simultaneously.
3.6. Potential Therapeutic Targets
Although hyperglycemia-induced immune dysfunction involves multiple cellular populations, recent evidence suggests that certain targets may be more tractable than others. Among these, macrophage polarization has emerged as the most promising therapeutic node. Driving macrophages from a pro-inflammatory M1 phenotype toward a reparative M2 phenotype not only suppresses excessive cytokine release but also promotes angiogenesis and matrix remodeling, thereby playing a central role in coordinating inflammation resolution and tissue repair. In addition, neutrophil hyperactivation and excessive formation of NETs have increasingly been recognized as pathological hallmarks of diabetic wounds, contributing to persistent inflammation, proteolytic tissue damage, and delayed re-epithelialization. By contrast, strategies aimed at harnessing T cells or non-immune cell types for immunomodulation remain in earlier stages, with limited available interventions and insufficient preclinical validation. Taken together, targeting macrophage polarization and NET clearance provides two promising avenues for restoring immune homeostasis and accelerating repair in diabetic wounds.
4. Application of the Immunomodulatory Hydrogel Delivery System
Hydrogels have garnered significant attention in diabetic wound therapy owing to their outstanding biocompatibility, customizable mechanical properties, and superior capacity for sustained delivery. These attributes make them ideal platforms for encapsulating and delivering immunomodulatory agents. Although hydrogels themselves can influence immune responses through their intrinsic physicochemical properties—such as stiffness, porosity, degradation kinetics, and composition—the magnitude of these effects is generally modest compared to the potent bioactivity of the encapsulated cargo. Therefore, this review primarily focuses on the immunomodulatory effects mediated by hydrogel-delivered agents, rather than the intrinsic immunomodulatory capacity of the hydrogels alone. Hydrogels can be engineered to achieve controlled release of therapeutic cargos, such as cells, EVs, anti-inflammatory drugs, and other bioactive substances, thereby modulating the immune microenvironment and enhancing tissue repair. This section highlights on the application of immunomodulatory hydrogels in diabetic wound healing.
4.1. Cells
Cell-based therapies, particularly those involving mesenchymal stem cells (MSCs), have shown considerable potential for diabetic wound repair. MSCs not only possess self-renewal capabilities but also differentiate into fibroblasts, endothelial cells, and other regenerative cells. Importantly, they exhibit potent immunomodulatory functions by anti-inflammatory mediators, growth factors, and other paracrine signals [12,62]. During tissue repair, MSCs have been shown to facilitate the macrophage phenotypic switching of from M1 to M2 through the secretion of factors like TGF-β, IL-6 [63,64]. Moreover, MSCs can suppress TNF-α secretion from M1 macrophages and promote a shift in T cell from the pro-inflammatory Th1 to the anti-inflammatory Th2 subtype [65,66]. These findings underscore the therapeutic relevance of MSCs in ameliorating chronic inflammation in diabetic wounds, providing a scientific rationale for their use in clinical treatment (Table 1).
4.1.1. Bone Marrow Mesenchymal Stem Cell
The safety and efficacy of bone marrow mesenchymal stem cells (BMSCs) have been extensively validated in clinical settings. Chen et al. developed a BMSC-based hydrogel delivery system that enhanced fibroblast, endothelial cell, and keratinocyte functions via the secretion of TGF-β1 and basic fibroblast growth factor (bFGF) [67]. The hydrogel also suppressed M1 macrophage activity, thereby ameliorating the inflammatory milieu and accelerating wound healing. However, the study did not directly demonstrate BMSC-mediated immunomodulation. In contrast, Bai et al. utilized a self-healing hydrogel to deliver BMSCs and reported not only suppression of M1 macrophage activation but also promotion of M2 polarization [68]. Notably, this enhanced M2 polarization was mechanistically linked to the presence of BMSCs, suggesting that their immunomodulatory potential may be context-dependent and remains a subject of ongoing debate.
4.1.2. Adipose-derived Mesenchymal Stem Cell
Adipose-derived mesenchymal stem cells (ADSCs) have garnered increasing interest for cutaneous regeneration owing to their ease of isolation, robust differentiation capacity, and strong immunomodulatory effects [78]. Shi et al. demonstrated that ADSCs can enhance macrophage polarization toward an M2-like profile [79]. However, the survival and functionality of ADSCs in the hostile diabetic wound environment remain major challenges. To address this, Dong et al. constructed an injectable hydrogel that extended ADSC survival in diabetic wounds for up to 14 days, while enhancing angiogenesis and suppressing inflammation [70]. Similarly, Xia et al. incorporated curcumin into the hydrogel matrix to reduce ADSC apoptosis, thereby improving cell viability and promoting wound repair [71].
Cells-loaded immunomodulatory hydrogels for diabetic wound healing applications.
| Cell type | Materials | Treatment | Model | Results | Days for wound healing | Refs. |
|---|---|---|---|---|---|---|
| BMSCs | NIPAM, PAA, APS, TEMED | Thermosensitive hydrogel encapsulating BMSCs | Diabetic mice (C57BL/6) full-thickness cutaneous wound | Inhibiting M1 polarization; Promoting angiogenesis | Unhealed, 35 days | [67] |
| BMSCs | N-chitosan, HA-ALD, ADH | Self-healing hydrogel encapsulating BMSCs | Diabetic rat (SD) foot skin wound | Enhancing M1-to-M2 macrophage polarization; Promoting angiogenesis | 15 days | [68] |
| BMSCs | Chitosan, PEG, Glutaraldehyde, Glycerol, Ethanol | Porous hydrogel encapsulating FGF21 pretreated BMSC | Diabetic rat (SD) full-thickness cutaneous wound | Suppressing apoptotic and inflammatory genes expression | 16 days | [69] |
| ADSCs | HB-PEGDA, Thiolated gelatin | Injectable hydrogel encapsulating allogeneic ADSCs | Diabetic mice (db/db) full-thickness cutaneous wound | Suppressing infiltration of inflammatory cells; Promoting angiogenesis | 15 days | [70] |
| ADSCs | GelMA | 3D bioprinting hydrogel encapsulating curcumin and ADSCs | Diabetic athymic nude mice (nu/nu) full thickness cutaneous wound | Suppressing AGEs-related inflammatory;Promoting angiogenesis | 21 days | [71] |
| hUCMSCs | GelMA, ZnCl2,Chitosan-catechol,DTT | Hybrid hydrogel encapsulating HUMSCs | Diabetic mice (db/db) full-thickness cutaneous wound | Downregulating inflammatory factors (TNF-α and IL-1β); Promoting angiogenesis and collagen deposition | 14 days | [72] |
| hUCMSCs | Peptide RADA16-I; Peptide KLT; Peptide RGD | Self-assembled nanopeptide hydrogels encapsulating hUCMSCs spheroids | Diabetic mice (NOD/SCID) full-thickness cutaneous wound | Downregulating inflammatory factors; Promoting angiogenesis | 10 days | [73] |
| WJMSCs | PF-127; SAP | Injectable and temperature-sensitive hydrogel encapsulating WJMSCs | Diabetic rat (SD) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; Promoting angiogenesis | Unhealed(residual wound area<10%), 14days | [74] |
| HUVECvegf165+ | γ-PGA-SH; γ-PGA-GMA; RGDC; LAP | 3D printed all-peptide hydrogel encapsulating HUVECvegf165+ | Diabetic rat (SD) full-thickness cutaneous wound | Reducing wound inflammation; Improving angiogenesis, ECM remodeling, and cell adhesion | 14 days | [75] |
| Macrophage | Alginate | Alginate hydrogels encapsulating macrophage | Diabetic mice (C57BL/6) full-thickness cutaneous wound | Any polarized macrophage subtype and their secretome similarly promotes diabetic wound healing in 10 days | 10 days(M2c) | [76] |
| hiPSC-SMCs | 3D collagen scaffolds encapsulating hiPSC-SMCs | Male athymic nude mice full-thickness cutaneous wound | Promoting angiogenesis; Increased number of total and M2 type macrophages. | 10 days | [77] |
4.1.3. Human Umbilical Cord-derived Mesenchymal Stem Cell
Comparable to BMSCs and ADSCs, human umbilical cord-derived mesenchymal stem cells (hUCMSCs) exhibit robust immunoregulatory functions and multipotency. They offer distinct advantages, such as low immunogenicity, non-invasive collection procedures, and minimal ethical concerns. Through mechanisms including multilineage differentiation, paracrine signaling, and anti-inflammatory activity, hUCMSCs significantly contribute to diabetic wound repair [80]. Encapsulation of hUCMSCs in hydrogels enhances their therapeutic efficacy, downregulates pro-inflammatory mediators, while concurrently promoting angiogenesis [72-74].
4.1.4. Macrophage
Beyond stem cell delivery, recent investigations have also explored macrophage transplantation via hydrogel scaffolds as a strategy to expedite diabetic wound repair. Given the pivotal role of macrophages in shaping the immune milieu of chronic wounds, targeted delivery of these cells represents a promising immunotherapeutic avenue [76,81,82]. For example, Georgios et al. loaded M0, M1, M2a, and M2c macrophages, along with their secretome, into an alginate dressing, and found that macrophages, regardless of their polarization state, can improve wound healing, while the delivered M2 macrophages were able to maintain or promote similar polarization states [76].
4.1.5. Induced Pluripotent Stem Cell-Derived Cells
Human induced pluripotent stem cells (hiPSCs) represent a renewable and patient-compatible source for multiple cell lineages, including endothelial cells (hiPSC-ECs), smooth muscle cells (hiPSC-SMCs), fibroblasts (hiPSC-FBs), and mesenchymal stem cells (hiPSC-MSCs) [83]. In addition to their regenerative potential, hiPSC-derived cells have shown emerging immunomodulatory activity, such as suppression of pro-inflammatory cytokine production and promotion of macrophage polarization. These dual properties address two critical barriers to diabetic wound healing: chronic inflammation and ischemia [84,85].
4.1.5.1. hiPSC-ECs
Among hiPSC-derived lineages, hiPSC-ECs are the most extensively studied in diabetic wounds, where they have been shown to accelerate angiogenesis and restore endothelial function [86]. For instance, nanovesicles derived from hiPSC-ECs and loaded with dapagliflozin enhanced HIF-1α/VEGFA signaling, promoted neovascularization, and improved wound closure, suggesting a paracrine or gene-regulatory mechanism rather than direct engraftment [87]. While most work highlights vascular repair, endothelial cells are known to crosstalk with innate immune populations, including neutrophils and macrophages, implying that future hiPSC-EC strategies could exploit a vascular-immune axis to integrate angiogenesis with immunomodulation.
4.1.5.2. hiPSC-FBs
hiPSC-FBs recapitulate the transcriptional profiles of primary dermal fibroblasts and provide a scalable source for individualized dermal reconstruction. Although their direct immunoregulatory role in diabetic wounds is less defined, evidence from cutaneous and mucosal repair demonstrates that certain fibroblast subsets can modulate immune responses and support regeneration [88]. Moreover, iPSC-derived extracellular matrix has been shown to reshape the wound environment, indicating that fibroblasts may contribute to both structural and immunological aspects of repair [89].
4.1.5.3. hiPSC-SMCs
hiPSC-SMCs support vessel maturation and secrete pro-angiogenic mediators. Importantly, in diabetic wound models, the delivery of hiPSC-SMCs not only accelerated healing but also increased the prevalence of M2 macrophages, suggesting that their therapeutic effect extends beyond angiogenesis to active immunomodulation. This positions hiPSC-SMCs as a lineage capable of bridging vascular stabilization with immune rebalancing [77].
4.1.5.4. hiPSC-MSCs
hiPSC-derived MSCs combine the regenerative and immunomodulatory features of conventional MSCs with the scalability and autologous compatibility of hiPSCs. While direct data on their use in diabetic wounds remains limited, accumulating evidence indicates that hiPSC-MSCs, particularly through extracellular vesicles, can suppress macrophage-derived pro-inflammatory cytokines, enhance angiogenesis, and accelerate wound repair. These paracrine effects underscore their promise as an immunoregulatory therapy, although head-to-head comparisons with conventional MSCs are still needed [90].
4.1.5.5. hiPSC-Derived Immune Cells
As an emerging avenue, standardized platforms now allow mid-scale production of functionally mature hiPSC-derived immune cells. Notably, hiPSC-macrophages exhibit transcriptional and chromatin landscapes comparable to peripheral blood-derived macrophages during M1/M2 polarization. This raises the possibility of engineering hiPSC-derived macrophages for therapeutic use, such as promoting M2 polarization or enhancing the clearance of NETs. Incorporating such cells into hydrogel systems could represent a next-generation strategy for direct immune cell-based therapy in diabetic wounds [91,92].
Overall, hiPSC-ECs and hiPSC-SMCs primarily act through vascular regeneration, with hiPSC-SMCs already showing immunomodulatory effects via M2 polarization. hiPSC-FBs and their ECM remodeling capacity may further shape the immune milieu, while hiPSC-MSCs and their EVs provide paracrine immunomodulation. Future studies should incorporate immune endpoints—such as macrophage polarization, T cell balance, and cytokine profiles—to establish immunoregulatory efficacy. Nonetheless, several challenges remain: (i) safety and phenotypic stability, given the risks of residual pluripotency and phenotypic drift; (ii) survival and functional persistence in the hostile diabetic wound microenvironment, which necessitate protective hydrogel formulations with antioxidant or protease-adaptive properties; and (iii) scalability and GMP-standardized differentiation, as batch-to-batch variation in iPSC-derived products and hydrogel manufacturing remain bottlenecks for translation. Addressing these limitations will be essential to realize the full potential of hiPSC-derived cells as dual regenerative and immunomodulatory agents in hydrogel-based diabetic wound therapy.
4.1.6. Strategies to Improve Cell Delivery Efficiency
Despite the promising results of cell-based therapies, several obstacles remain, particularly the limited survival and activity of transplanted cells in the inflammatory, hypoxic, and hyperglycemic wound microenvironment. These conditions attenuate cytokine secretion and impair therapeutic outcomes [93]. While hydrogel encapsulation improves cell viability and retention, additional strategies are required to optimize therapeutic benefits.
Pre-conditioning cells before transplantation has shown the potential to improve survival and function [94]. For example, Anisa et al. demonstrated that FGF21 pretreatment enhanced BMSC viability in a hyperglycemic context, thereby promoting wound healing [69]. Similarly, melatonin preconditioning was reported to boost the immunomodulatory effects of hUCMSCs, particularly in regulating macrophage polarization [95]. Genetic engineering of therapeutic cells is another emerging approach. Modifying cellular gene expression not only enhances cell survival but also tailors their secretory profiles for targeted therapy [96]. Huang et al. developed a hydrogel platform encapsulating human umbilical vein endothelial cells (HUVECs) overexpressing VEGF165, which mitigated mitochondrial damage and downregulated multiple pro-inflammatory cytokines, ultimately improving the immune environment and facilitating diabetic wound healing [75].
4.2. Extracellular Vesicles
Despite extensive efforts by researchers, ranging from the development of hydrogel systems, preconditioned cells, and gene editing strategies to the exploration of alternative cell types beyond stem cells, cell-based therapies for diabetic wounds remain suboptimal.
The notable regenerative potential of the cellular secretome, particularly that of stem cells, has redirected attention toward the use of EVs for diabetic wound healing [76]. Compared to their cellular origins, EVs offer lower immunogenicity, improved safety, greater stability, and enhanced drug-loading capacity, granting them a competitive edge in clinical applications, especially in tissue repair. Furthermore, mounting evidence indicates that the immunomodulatory capacity of MSCs is predominantly mediated by their secreted EVs [97,98].
However, the rapid clearance of EVs when administered alone remains a major limitation. To address this, hydrogels have gained widespread application as sustained-release matrices to improve their retention (Table 2). The relationship between hydrogels and EVs is mutually beneficial: hydrogels protect EVs from immune clearance and enable their sustained release, while EVs enhance the immunomodulatory properties of hydrogels, suppress inflammation, and promote cellular proliferation and migration. Besides, EVs can be engineered as carriers for therapeutic molecules involved in wound repair, offering a versatile strategy for enhanced healing outcomes [99,100].
4.2.1. Cell-Derived Extracellular Vesicles
4.2.1.1. BMSCs-Derived Extracellular Vesicles
BMSCs-derived EVs (BMSC-EVs) have been shown to inhibit M1 macrophage polarization and foster M2 polarization. These vesicles suppress the NF-κB/p65 signaling pathway and downregulate pro-inflammatory cytokines [62,117,118]. Additionally, BMSC-EVs support wound healing by enhancing cell proliferation, migration, and angiogenesis [62,119]. Wang et al. engineered a BMSC-EV-laden hydrogel, which modulated IL-6 expression in diabetic wounds [101]. Moreover, Geng et al. incorporated BMSC-EVs into a chitosan hydrogel to shift macrophage phenotype from M1 to M2, thereby reshaping the local immune microenvironment [102].
EVs-loaded immunomodulatory hydrogels for diabetic wound healing applications.
| EVs source | Materials | Treatment | Model | Results | Days for wound healing | Refs |
|---|---|---|---|---|---|---|
| BMSCs-EVs | GelMA, DOPA, EDC, NHS | Adhesive in situ photo-crosslinked hydrogel encapsulating BMSCs-EVs | Diabetic rat (SD) full-thickness cutaneous wound | Downregulating inflammatory factors (IL-6); Promoting angiogenesis | 14 days | [101] |
| BMSCs-EVs | Chitosan, CMC, acrylic acid | Antibacterial and self-healing hydrogel encapsulating BMSCs-EVs | Diabetic rat (SD) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; Promoting angiogenesis | 14 days | [102] |
| ADSCs-EVs | 4-Arm-PEG-Thiol, AgNO3, MWCNTs | Injectable, adhesive, self-healing hydrogels encapsulating ADSCs-EVs | Diabetic mice (Balb/c) full-thickness cutaneous wound | Downregulating inflammatory factors (IL-6, TNF-α); Promoting angiogenesis | 14 days | [103] |
| ADSCs-EVs | Porcine cardiac tissue | ECM hydrogel encapsulating ADSCs-EVs | Diabetic mice (ICR) full-thickness cutaneous wound | Downregulating inflammatory factors (IL-6, TNF-α);Promoting angiogenesis | 14 days | [104] |
| ADSCs-EVs | HB-PEG, HA-SH | In situ formed hydrogel encapsulating hypoxic pretreated ADSCs-EVs | Diabetic rat (SD) full-thickness cutaneous wound | Downregulating TNF-α; Preventing scar formation | Unhealed(residual wound area<10%), 21 days | [105] |
| hUCMSCs-EVs | Alginate, HDAC7-derived 7-amino-acid peptide, CaCl2 | Alginate hydrogel encapsulating hUCMSCs-EVs and 7A | Diabetic rat full-thickness cutaneous wound | Downregulating inflammatory factors (IL-6, IL-1β, TNF-α);Promoting angiogenesis | Unhealed(residual wound area<10%), 18 days | [106] |
| FSMSCs-EVs | PVP, SiW | Self-healing, adhesive, and antibacterial hydrogel encapsulating FSMSCs-EVs | Diabetic mouse full-thickness excisional wound | Enhancing M1-to-M2 macrophage polarization;Promoting angiogenesis | 12 days | [107] |
| M2-EVs | HAMA, PVA, LAP, PDA, Dil | Photosensitive hydrogel microneedles encapsulating M2-EVs and PDA nanoparticles | Diabetic rat (SD) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization;Promoting angiogenesis | 14 days | [108] |
| M0-EVs | CMC, HA, NBT, DMF | Hydrogel with M0-EVs | Diabetic rat (SD) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization; Releasing VEGF | 14 days | [109] |
| Treg-EVs | PF-127 | Thermoresponsive hydrogel encapsulating Treg-EVs | Diabetic mice (C57BL/6) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization;Reducing IL-6 production;Enhancing skin and vascular endothelial cell migration | Unhealed(residual wound area>60%), 7 days | [110] |
| AT-EVs | Egg whites | Hydrogel with both radial topological and biological cues encapsulating AT-EVs | Diabetic mice (C57BL/6) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization;Promoting angiogenesis | 14 days | [111] |
| PRP-EVs | PF-127 | Thermoresponsive hydrogel encapsulating PRP-EVs | Diabetic mice (db/db) full-thickness cutaneous wound | Inhibiting fibroblast ferroptosis; Increasing M2 macrophage | Unhealed(residual wound area≈20%), 14 days | [112] |
| PLT-EVs | Gelatin, alginate, Reduced graphene oxide | Photothermally responsive hydrogel encapsulating PLT-EVs | Diabetic rat (Wistar) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization | 14 days | [113] |
| PLT-EVs | ADM, MA | Photo-crosslinking and fast-gelling hydrogel encapsulating PLT-EVs | Diabetic rat (SD) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization;Promoting angiogenesis | 21 days | [114] |
| G-EVs | HA, MES, EDC, HOBT, ADH, OSA, Mg2+ | Injectable hydrogel encapsulating G-EVsDM | Diabetic mice full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization;Promoting angiogenesis | 14 days | [115] |
| Apoptotic EVs | GelMA, LAP | Photo-crosslinking hydrogel encapsulating apoptotic EVs | Diabetic mice (db/db) full-thickness cutaneous wound | Enhancing M1-to-M2 macrophage polarization;Enhancing the endothelial cell and fibroblast function | 14 days | [116] |
4.2.1.2. ADSCs-derived Extracellular Vesicles
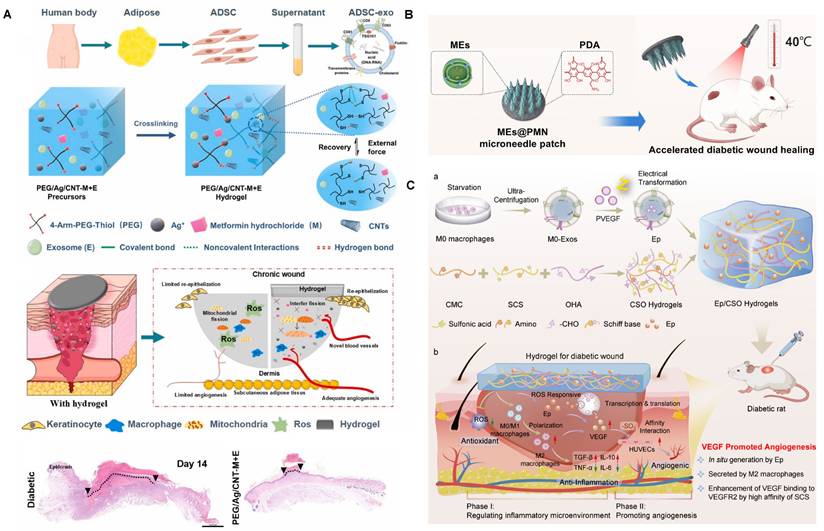
Adipose-derived stem cell EVs (ADSC-EVs) also exhibit potent immunomodulatory effects [119]. Zhang et al. co-loaded ADSC-EVs and metformin into an injectable conductive hydrogel that suppressed vascular inflammation and reduced IL-6 and TNF-α secretion (Figure 2A). The hydrogel also mitigated mitochondrial fission and ROS generation in hyperglycemic HUVECs, thereby promoting angiogenesis [103]. Similarly, Song et al. developed an ECM-based hydrogel loaded with ADSC-EVs, which significantly downregulated IL-6 and TNF-α levels at the wound site [104]. To achieve precision delivery, Jiang et al. employed MMP-responsive smart hydrogels to release ADSC-EVs upon MMP-2 stimulation, reactivating the suppressed AKT signaling pathway in diabetic wounds and promoting cell proliferation and migration [120].
4.2.1.3. hUCMSCs-derived Extracellular Vesicles
Human umbilical cord MSC-derived EVs (hUCMSC-EVs) accelerate diabetic wound closure by enhancing angiogenesis and promoting fibroblast proliferation and migration [121,122]. Their immunomodulatory functions have also attracted increasing attention. For instance, hUCMSC-EVs suppress monocyte chemoattractant protein-1 (MCP-1) in retinal injury, thereby attenuating inflammation [123]. Moreover, they reduce the expression of IL-6, TNF-α, iNOS, IL-1β, IL-7, and other pro-inflammatory cytokines; ameliorate insulin resistance; enhance glucose metabolism; and inhibit pancreatic β cell apoptosis under hyperglycemic conditions, offering promise for type 2 diabetes therapy [124,125]. Injecting hUCMSC-EVs into type 1 diabetic mice via the tail vein improved insulin secretion and lowered the mice's blood sugar levels. At the same time, the wounds of the mice were treated with a hydrogel with anti-inflammatory effects. The synergistic effect of the two further improved the wound healing of type 1 diabetic mice [126]. Co-loading hUCMSC-EVs with a histone deacetylase 7 (HDAC7)-derived peptide into alginate hydrogels significantly reduced pro-inflammatory cytokine production from macrophages in diabetic wounds [106].
4.2.1.4. Other cell-Derived Extracellular Vesicles
Beyond the common stem cell sources, other cell types also yield EVs with immunomodulatory potential. Foreskin-derived MSCs (FSMSCs), which are ethically favorable and possess stronger proliferative and immunoregulatory properties than hUCMSCs, are one such example [107,127]. Xu et al. isolated FSMSCs and embedded FSMSC EVs (FM-EVs) into a polyvinylpyrrolidone/silicotungstic acid-based hydrogel. These FM-EVs promoted angiogenesis and induced M2 macrophage polarization via let-7b-5p, thereby accelerating diabetic wound healing [107].
Immune cell-derived EVs also hold promise. For example, M2 macrophage-derived EVs (M2-EVs) promote M2 polarization through the delivery of CCL22, CCL24, and MFG-E8 [128,129]. Zeng et al. co-delivered M2-EVs and polydopamine (PDA) nanoparticles via hydrogel microneedles [108]. These systems not only enhanced M2 polarization but also boosted angiogenesis through the photothermal effects of PDA (Figure 2B). In addition, M0 macrophage-derived EVs embedded in immunomodulatory hydrogels have been shown to attenuate inflammation, and engineered loading of VEGF plasmids into these EVs further enhanced angiogenesis, demonstrating synergistic promotion of diabetic wound repair (Figure 2C) [109]. Tregs also secrete EVs with strong immunomodulatory potential [130]. Treg-derived EVs (Treg-EVs) modulate macrophages, dendritic cells, and T cells via miRNAs, enzymes, and surface proteins [131]. Cord blood-derived Treg EVs promoted M2 polarization in monocytes and decreased local inflammatory cytokines in diabetic wounds [110].
4.2.1.5. Strategies for Enhanced Efficacy of Cell-Derived Extracellular Vesicles
As with cell therapies, EVs' function can be enhanced via preconditioning strategies. Environmental stimuli significantly influence the bioactivity and cargo of EVs. For example, hypoxia-preconditioned ADSC-EVs demonstrate enhanced pro-angiogenic and anti-inflammatory effects, thereby improving diabetic wound repair [132-135]. Wang et al. loaded hypoxia-preconditioned ADSC-Evs into in situ formed injectable hydrogels and verified their promoting effects on the immune microenvironment and angiogenesis of diabetic wounds both in vivo and in vitro [105]. Similarly, hypoxia-treated hUCMSC-EVs inhibit excessive NET formation in diabetic wounds via miR-17-5p [54]. Pharmacologic interventions are also employed to modulate EVs' function. Melatonin-treated BMSC-EVs promote M2 macrophage polarization by upregulating PTEN and inhibiting AKT phosphorylation [136]. However, the impact of melatonin on EV yield, cargo, and stability remains debated [136,137].
Low EV yield and technical barriers to isolation still hinder clinical translation. CHIR99021, a Wnt/β-catenin pathway agonist, has been shown to increase hUCMSC-EV yield by 1.5-fold while modulating EV-related genes involved in TGF-β and PI3K-AKT signaling, reducing inflammation at wound sites [138-140]. Astragaloside IV (ASIV) also enhances EV production from endothelial progenitor cells and improves diabetic wound inflammation [141,142]. Moreover, low-intensity ultrasound stimulation effectively boosts EV secretion from ADSCs [143].
4.2.2. Tissue-Derived Extracellular Vesicles
Compared to cell-derived EVs, adipose and plasma-derived EVs offer a more cost-effective and scalable alternative, while retaining comparable immunomodulatory capabilities [111,113,144]. Adipose tissue consists of a heterogeneous mix of adipocytes, endothelial cells, fibroblasts, immune cells, and other cell types. EVs secreted by these various cells exert distinct effects on wound healing. Notably, EVs derived from whole adipose tissue, without enzymatic removal of specific cell populations, have shown superior therapeutic efficacy in promoting wound repair [145]. For instance, Ma et al. incorporated adipose tissue-derived EVs (AT-EVs) into an egg white-based hydrogel, leveraging the combined antioxidant and anti-inflammatory properties of both components [111]. This formulation effectively reduced excess ROS levels and encouraged macrophage polarization toward a reparative M2 phenotype, thereby significantly enhancing diabetic wound healing.
Plasma-derived EVs, particularly those originating from platelets, also contribute meaningfully to tissue repair. Platelets not only play a critical role in hemostasis and coagulation but also release a range of cytokines upon activation that modulate the wound healing process [3,20,26]. Studies have demonstrated that platelet-rich plasma (PRP) accelerates diabetic wound healing, and EVs isolated from PRP exhibit similar biological functions [146,147]. Wang et al. delivered PRP-EVs to diabetic wounds via hydrogels, which not only increased the proportion of M2 macrophages but also reduced the number of neutrophils, thereby improving excessive inflammation [112].
Immunomodulatory hydrogel systems for delivering cell-derived EVs. (A) Preparation and application of the PEG/Ag/CNT-M+E hydrogel for diabetic wounds. Adapted with permission from [103]. Copyright 2023, Elsevier. (B) Immunomodulation of the M2 macrophage-derived EV-encapsulated microneedles with PDA (MEs@PMN) for diabetic wound healing. Adapted with permission from [108]. Copyright 2023, Elsevier. (C) Fabrication and therapeutic mechanisms of regulating the inflammatory microenvironment and promoting angiogenesis in diabetic wound healing by Ep/CSO hydrogels. Adapted with permission from [109]. Copyright 2025, American Chemical Society.
Platelet-derived EVs (pEVs) help neutralize intracellular ROS, upregulate IL-10 expression via the TGF-β pathway, and drive macrophage polarization in favor of M2-like behavior—collectively facilitating the shift from inflammation to tissue regeneration [113]. When delivered via hydrogels, pEVs not only stimulate local angiogenesis but also establish a low-inflammatory, pro-regenerative microenvironment conducive to healing (Figure 3A) [113,114]. Additionally, EVs derived from umbilical cord blood and peripheral blood have demonstrated angiogenic and cell migration-promoting properties, though their potential to modulate immune responses in diabetic wounds remains underexplored [148,149].
4.2.3. Plant-Derived and Non-Mammalian Animals-Derived Extracellular Vesicles
Although cell- and mammalian tissue-derived EVs have shown remarkable immunomodulatory efficacy in diabetic wound healing, their broader application is still hampered by low yield, manufacturing difficulties, and potential immunogenicity. To address these limitations, EVs derived from plants and non-mammalian animals have emerged as attractive alternative therapeutic candidates.
EVs derived from plant and non-mammalian animal sources offer unique biological activities and are increasingly being explored for diabetic wound therapy [115,150]. For example, Liao et al. isolated EVs from Periplaneta americana, identifying a panel of miRNAs associated with Hedgehog, TGF-β, autophagy, and mTOR signaling pathways (Figure 3B) [150]. These EVs mitigated excessive autophagy and MMP-9 overexpression, alleviating tissue damage and chronic inflammation in diabetic wounds. Similarly, EVs extracted from ginseng sap have been employed to deliver didymin—a compound known to promote M2 macrophage polarization—via Mg²⁺-incorporated hydrogels. This strategy supported wound healing by orchestrating neurogenesis, immune regulation, and angiogenesis (Figure 3C) [115].
Beyond ginseng-derived EVs, recent studies have explored additional plant-derived exosome-laden hydrogels. For instance, Zaffar et al. encapsulated rose petal-derived EVs, which possess intrinsic antibacterial properties, into an injectable hydrogel [151]. This formulation effectively cleared Gram-negative bacteria from the wound surface and reduced local inflammation. However, in the diabetic context, antimicrobial activity alone is insufficient, as persistent immune imbalance—particularly the prolonged retention of M1 macrophages—remains a critical barrier. Notably, many plant-derived EVs exhibit intrinsic anti-inflammatory effects. Jin et al. demonstrated that lemon-derived EVs, when incorporated into hydrogels, significantly suppressed local inflammation and facilitated wound closure [152]. Similarly, EVs from Houttuynia cordata Thunb. showed immunomodulatory potential in diabetic wound models [153]. Liu et al. further advanced this concept by loading caffeic acid into Saccharina japonica-derived EVs and integrating them with electroconductive microneedles. This combined system promoted diabetic wound healing through multiple mechanisms, including neuroregulation, immunomodulation, angiogenesis, and inhibition of AGEs (Figure 3D) [154]. In addition to antimicrobial and anti-inflammatory functions, certain plant-derived EVs also provide metabolic benefits specific to diabetes. Weng et al. reported that EVs from Momordica charantia (MC), a plant with hypoglycemic activity, not only improved glycemic control but also alleviated chronic inflammation and promoted healing in diabetic wounds [155].
Collectively, these findings highlight the translational promise of hydrogel systems incorporating plant- and non-mammalian animal-derived EVs. Owing to their low cost, scalability, and intrinsic bioactivities, such EVs represent a compelling next-generation strategy for diabetic wound immunotherapy.
4.2.4. EV Mimetics
Despite the therapeutic promise of EVs, challenges related to low yield and limited modifiability constrain their broader application. EV mimetics, which mimic the structure and function of natural EVs, have emerged as a scalable and customizable alternative for drug delivery [156,157]. These can be produced via methods such as extrusion, chemical induction, nitrogen cavitation, or liposome-based engineering.
Yu et al. employed extrusion to generate mimetic EVs from polymorphonuclear neutrophils (PMNs), leveraging their inherent antimicrobial activity to deliver vascular endothelial growth factor (VEGF) and enhance healing (Figure 4A) [158]. Similarly, Zhu et al. fabricated mimetic EVs from hUCMSCs via extrusion and validated their wound-healing efficacy [159]. In another approach, mimetic EVs derived from induced pluripotent stem cells were used to deliver dapagliflozin (DA), a selective SGLT2 inhibitor with anti-inflammatory and angiogenic effects, to diabetic wounds [160]. Such strategies open avenues for targeted delivery of immune-regulatory agents and growth factors.
Hybrid EVs, formed by fusing natural EVs with liposomes, offer an additional platform for engineering [157]. For instance, hybrid vesicles generated by combining M2 macrophage EVs with M1-derived membranes exhibited anti-inflammatory activity while neutralizing pro-inflammatory cytokines through membrane receptor interactions. This technique, already tested in arthritis models, remains in early-stage exploration for diabetic wounds [161]. However, the application of this method in diabetic wound management remains in the preliminary phase.
In parallel, researchers are investigating controlled-release systems to improve the spatial and temporal delivery of EVs from hydrogels. Traditional hydrogels often release EVs in an uncontrolled manner, resulting in therapeutic inefficiency. Ma et al. addressed this by designing a hydrogel with radial physical and biochemical gradients, facilitating both directional EV release and enhanced M2 macrophage polarization [111].
Immunomodulatory hydrogel systems for delivering tissue-derived, plant-derived, and animals-derived EVs. (A) Preparation, merits, and mechanisms of dissolvable MN-based wound dressing (PLT-EVs@ADMMA-MN). Adapted with permission from [114]. Copyright 2024, Elsevier. (B) Isolation and application of PA-EVs. Adapted with permission from [150]. Copyright 2023, Springer Nature. (C) Beneficial role of HA-ADH/OSA@Mg@sEVs hydrogel. Adapted with permission from [115]. Copyright 2023, Wiley. (D) Fabrication and therapeutic mechanisms of Saccharina japonica-derived EVs-functionalized conductive microneedles. Adapted with permission from [154]. Copyright 2025, Wiley.
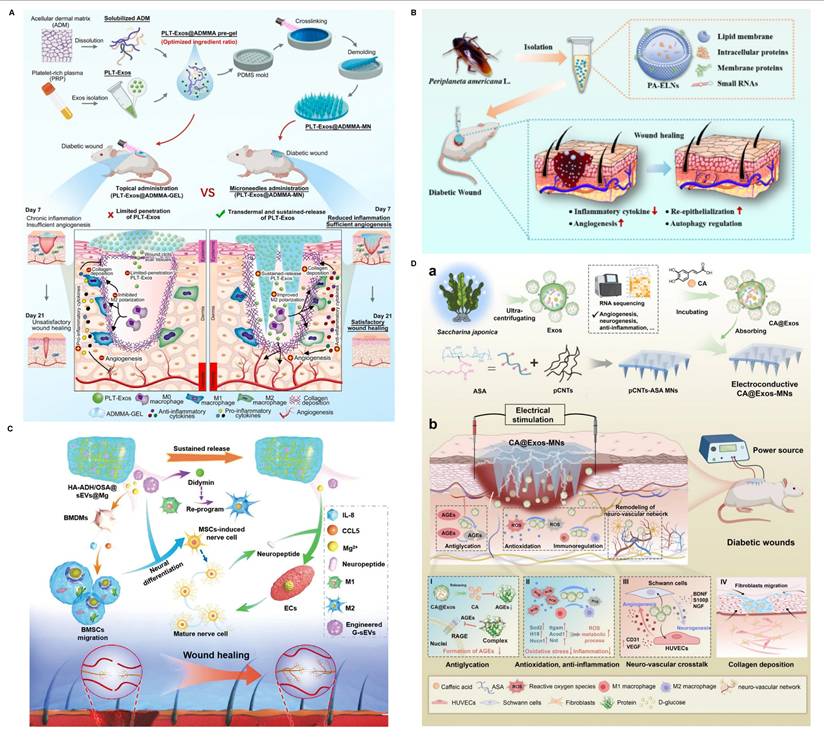
Immunomodulatory hydrogel systems for delivering EV mimetics and apoptotic EVs. (A) The fabrication of VEGF-aPMNEM-ECM hybrid hydrogel: preparation of activated PMN EV mimetics and vascular endothelial growth factor wrapping into aPMNEM. Adapted with permission from [158]. Copyright 2023, Springer Nature. (B) MSC-derived apoptotic EVs converting macrophages towards the M2 phenotype and improving the functions of fibroblasts and endothelial cells. Adapted with permission from [116]. Copyright 2020, Springer Nature. (C) The mechanisms of hUCMSC-derived apoptotic EVs inhibiting macrophage pyroptosis. Adapted with permission from [167]. Copyright 2023, Springer Nature.
4.2.5. Apoptotic Extracellular Vesicles
Apoptotic EVs, released during programmed cell death, are gaining recognition as potent mediators of tissue repair. Although apoptosis is often considered detrimental in cell therapies, recent studies highlight that apoptotic EVs modulate macrophages, endothelial cells, and fibroblasts to support healing—while the production of apoptotic EVs is greater than that of EVs under similar conditions [116,162,163]. Yang et al. encapsulated ADSC-derived apoptotic EVs in GelMA hydrogels, observing enhanced M2 macrophage polarization and protection of fibroblasts and endothelial cells from high-glucose-induced damage (Figure 4B) [116].
Persistent inflammation in diabetic wounds is closely linked to hyperactivation of the NLRP3 inflammasome—a key player in innate immunity and inflammatory signaling [164]. Chronic hyperglycemia exacerbates NLRP3 activity, perpetuating the release of pro-inflammatory cytokines like IL-1β and IL-18, and inhibiting angiogenesis [165,166]. Wang et al. harvested apoptotic EVs from apoptotic hUCMSCs and showed that macrophages engulfing these vesicles exhibited reduced NLRP3 activation and pyroptosis, ultimately improving the inflammatory microenvironment (Figure 4C) [167]. Harnessing apoptotic EVs thus represents a novel strategy for immunomodulation in diabetic wounds.
4.2.6. miRNAs in Extracellular Vesicles
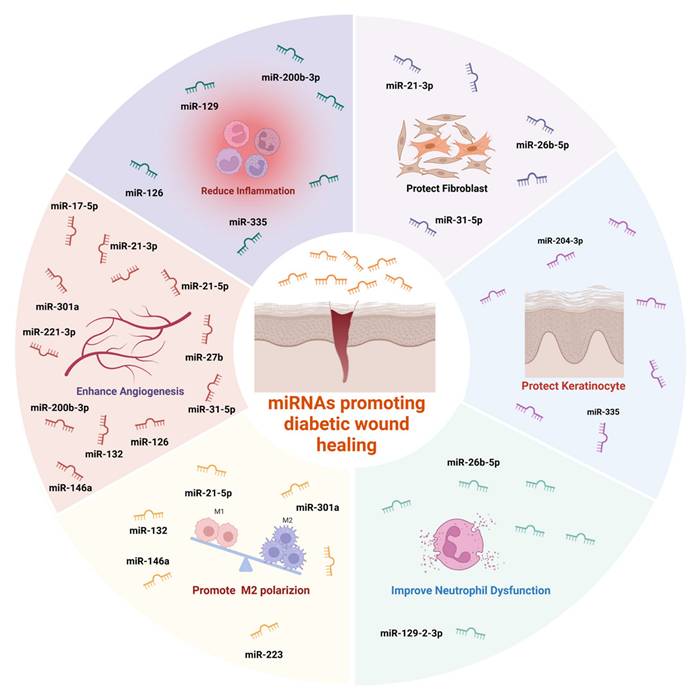
The functions of EVs are predominantly governed by their cargo, particularly microRNAs (miRNAs), which modulate gene expression by binding to target mRNAs [168]. A growing body of evidence indicates that various miRNAs play promotive roles in diabetic wound repair (Figure 5) [169-177]. The potential of EVs in this context continues to garner significant interest [176,177].
4.2.6.1. miRNA in Stem Cell-Derived Extracellular Vesicles
ADSC-EVs are especially rich in diverse miRNAs compared to BMSC-EVs, endowing them with broader regulatory capabilities [119,178]. These miRNAs promote cell proliferation, migration, angiogenesis, and immune modulation [119,179]. For instance, miR-451a, highly expressed in ADSC-EVs, suppresses macrophage migration inhibitory factor (MIF), facilitating M2 polarization [179]. Additionally, circRNAs and lncRNAs within ADSC-EVs modulate miRNA activity. mmu_circ_0001542, for example, upregulates miR-124-3p to enhance M2 polarization [180,181]. Long non-coding RNA (lncRNA) H19, a competing endogenous RNA (ceRNA), modulates gene expression by sequestering miR-130b-3p, upregulating PPARγ and STAT3, and promoting an anti-inflammatory macrophage phenotype [182,183].
4.2.6.2. Strategies for Regulating MiRNA in Extracellular Vesicles
Cell pre-conditioning offers a method to modify miRNA content in EVs. Hypoxia, for example, enhances mmu_circ_0001542 and miR-22-3p expression in ADSC-EVs, both of which regulate macrophage polarization [181,184,185]. Selenium treatment has been shown to induce ADSCs to release EVs enriched in anti-inflammatory and pro-angiogenic miRNAs [186,187]. Similarly, LPS stimulation upregulates miR-150-5p and let-7b, which promote M2 polarization [188,189]. Additionally, Insulin-induced gene 1 (Insig1), a regulator of lipid metabolism, indirectly modulates the immunoregulatory properties of BMSC-EVs [190]. Other stimuli, such as specific wavelength monochromatic light, may also influence EV-mediated immune functions, though this remains underexplored [191].
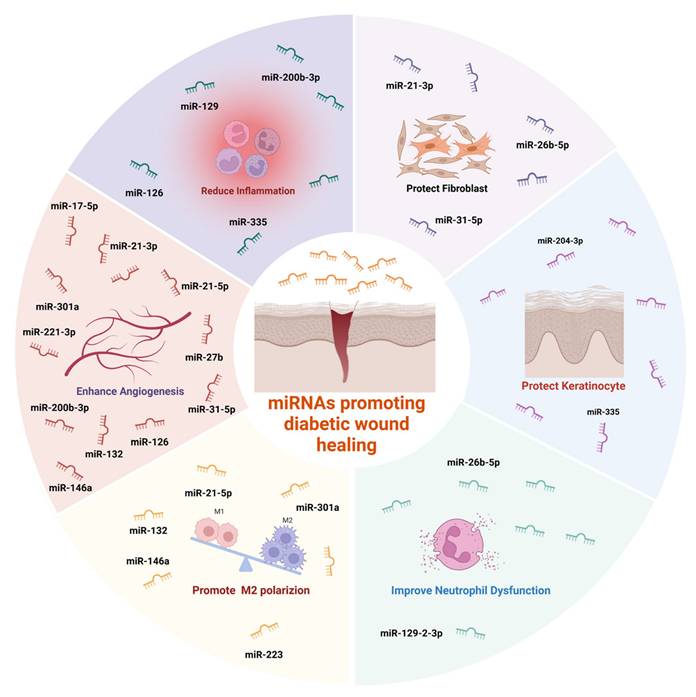
miRNAs promoting diabetic wound healing. Created with https://BioRender.com.
4.2.6.3. Direct miRNA Delivery
Beyond cell-derived EVs, miRNAs can be directly incorporated into hydrogels for therapeutic use. Wu et al. embedded miR-301a into hydrogels, enhancing M2 polarization via the mTOR pathway [173]. Similarly, Small interfering RNA (siRNA)—such as those targeting MMP9—have been used to adjust the M1/M2 macrophage ratio and reduce inflammation [192]. Meanwhile, most studies on the immune modulation characteristics of post-intervention EVs are still in the basic research stage or directly applied to diabetic wounds, and research on EV-integrated hydrogels for diabetic wound applications remain relatively scarce.
4.3. Bioactive Substances
Although EVs have been widely applied in hydrogel-based delivery, their extraction and quality control are time-consuming and labor-intensive. Therefore, hydrogels loaded with bioactive substances that possess immune-regulatory capabilities have emerged as a focal point in diabetic wound research. Loading bioactive substances into hydrogels can prevent their rapid degradation in vivo while minimizing the waste or off-target effect caused by rapid release.
4.3.1. Oxygen-Releasing Microspheres
Hypoxia is also a significant contributor to immune dysregulation within diabetic wounds. However, oxygen therapy has not yielded satisfactory results in diabetic wound healing, and even the explosive oxygen supply to the wound may exacerbate oxidative stress in the wound microenvironment. Therefore, providing a persistent and appropriate amount of oxygen to the wound is key to solving this issue [193,194]. Guan et al. developed hydrogen peroxide-based oxygen-releasing microspheres and loaded them into ROS-scavenging hydrogels (Figure 6A) [195]. This approach was shown to suppress M1 macrophage polarization and dampen pro-inflammatory signaling, thereby expediting wound resolution in diabetic models. However, even when loaded in ROS-scavenging hydrogels, the hydrogen peroxide-based oxygen release mechanism carries a certain risk of increasing oxidative stress in the diabetic microenvironment. Additionally, the uniform distribution of oxygen in wound tissue remains challenging, reducing therapeutic efficacy.
To overcome the limitations of H₂O₂-based oxygen release systems—which risk oxidative stress and uneven oxygenation—emerging strategies such as oxygen nanobubble-embedded hydrogels and hemoglobin-based oxygen carriers offer significant advantages. For instance, Han et al. developed an EV-coated oxygen nanobubble-laden hydrogel that effectively alleviated hypoxia while enhancing EV delivery, angiogenesis, and immunomodulation in a wound-healing model (Figure 6B) [196]. Similarly, oxygen nanobubble-embedded Carbopol hydrogels have demonstrated stable and prolonged oxygen delivery—up to three weeks—improving wound healing without provoking oxidative injury [197].
Several studies have demonstrated the therapeutic potential of hemoglobin-based hydrogels in diabetic wound healing. For example, integrating hemoglobin with black phosphorus quantum dots into hydrogel microneedles enables near-infrared-triggered, on-demand oxygen release, leveraging the photothermal properties of BP to meet the dynamic oxygen demands of the diabetic wound microenvironment [198]. Other approaches have modified red blood cells into Mn-based mineralized carriers, endowing them with sustained oxygen delivery and immunomodulatory capacity tailored for diabetic wounds [199]. Nevertheless, hemoglobin-based systems still face important limitations. Hemoglobin is prone to oxidation and denaturation, which diminishes its oxygen-carrying capacity. Immunogenicity also remains a concern, particularly when hemoglobin is derived from xenogeneic sources. Moreover, environmental factors such as pH and temperature strongly affect hemoglobin stability, creating significant challenges for storage and long-term use [200].
4.3.2. ROS-Scavenging Nanozymes
Nanozymes—synthetic enzyme mimetics—have drawn substantial interest owing to their distinctive enzyme-like functionalities. They not only efficiently scavenge ROS to alleviate oxidative stress but also catalyze the release of oxygen, improving hypoxic conditions and the immune microenvironment. This dual-regulatory function makes nanozymes highly promising tools for diabetic wound therapy. Li et al. developed a CaO2-based sustainable oxygen-releasing hydrogel, which regulates both angiogenesis and inflammation in diabetic wounds [201]. MnO2 nanosheets, which are widely known for their ability to effectively scavenge ROS and generate oxygen, were incorporated into hydrogels by Tu et al. [202]. This approach not only scavenged ROS in the diabetic wound environment, improving the hypoxic state, but also alleviated the dysregulation between neutrophils and macrophages. However, MnO2 and CaO2 exhibit pH-responsive rapid degradation, limiting the sustained effect of ROS clearance and oxygen release. Moreover, traditional nanozymes, while capable of scavenging existing ROS in diabetic wounds, cannot inhibit the ongoing generation of ROS. Therefore, the development of new nanozymes capable of continuously catalyzing ROS into oxygen has become a research hotspot. Li et al. and Zhao et al. utilized the peroxide-based MnCoO nanozyme properties to develop hydrogels that achieve this goal [203,204]. These hydrogels not only continuously capture ROS in diabetic wounds but also generate oxygen via hydrogen peroxide while significantly inducing macrophage M2 polarization.
4.3.3. Cytokines
Among the various delivery strategies mentioned above, whether regulating macrophage polarization or using other approaches, the focus is often on the changes modulating the balance between pro- and anti-inflammatory cytokine secretion. The network structure of hydrogels can stabilize cytokines, slow their release rate, and promote their prolonged action. Therefore, using hydrogels for the delivery of cytokines to reshape the immune landscape of diabetic wounds and enhance tissue regeneration is also a promising strategy.
Interleukins are a group of cytokines with highly dynamic and complex functions. They often interact through positive or negative regulation, playing pivotal roles in inflammation resolution and tissue repair. For example, IL-1, IL-6, and IL-17 act as potent inflammatory drivers, while IL-4 and IL-10 serve as hallmark anti-inflammatory mediators [205-208]. In most cases, hydrogels are used to deliver anti-inflammatory cytokines like IL-4 and IL-33 to improve chronic inflammation in diabetic wounds [207,209]. However, Yoon et al. loaded pro-inflammatory cytokines like IL-8 and macrophage inflammatory protein-3α into hydrogels to improve the delayed infiltration of inflammatory cells and promote wound healing [206].
Therefore, both pro-inflammatory and anti-inflammatory cytokines can promote healing in diabetic wounds, highlighting the importance of spatial-temporal-specific delivery of immune-regulatory agents during the physiological healing process of the wound. To achieve this, Tolouei et al. engineered a magnetically responsive delivery platform capable of orchestrating the early-stage recruitment of M1 macrophages via localized pro-inflammatory cytokine release, followed by the delayed administration of IL-4 and IL-10 under magnetic induction to steer macrophage polarization toward the M2 phenotype [205]. However, simply time-specific immune regulation does not fully satisfy the need, therefore, researchers have combined IL-10 with VEGF and Ag nanoparticles cluster for layered packaging in hydrogels to achieve sequential release. This approach not only regulates immune modulation but also addresses infection and angiogenesis [208,210]. Similar to using cationic polyethyleneimine-functionalized mesoporous polydopamine to remove excessive NETs from the wound, hydrogels can also be used to clear inflammatory chemokines from the wound (Figure 6C) [52,211]. Recently, Emiroglu et al. developed a particulate hydrogel that can isolate IL-6 while simultaneously releasing VEGF, representing a novel strategy. In the future, hydrogels with spatiotemporal delivery and isolation capabilities hold great potential to further promote the healing of diabetic wounds [212].
4.3.4. Peptides
In addition, bioactive peptides can also modulate the immune microenvironment. Netrin-1 has immune-regulatory properties, not only promoting inflammation in atherosclerosis but also alleviating inflammation following liver ischemia and reperfusion injury. Therefore, Shu et al. attempted to load Netrin-1 into GelMA hydrogels to modulate macrophage heterogeneity, improving the chronic inflammatory stage associated with low expression of Netrin-1 in diabetic wounds (Figure 6C) [213]. The mitochondrial-targeted peptide SS31 can alleviate mitochondrial oxidative stress, promoting M2 macrophage polarization. To protect this bioactive peptide from rapid hydrolysis and ensure sustained release, Deng et al. loaded SS31 onto mesoporous polydopamine nanoparticles, which were further incorporated into hydrogels, achieving sustained immune reprogramming within the diabetic wound [214].
4.3.5. Growth Factor
Growth factor-loaded hydrogels remain a cornerstone of bioactive wound dressings, aiming to restore impaired angiogenesis and immune balance in diabetic wounds. Among them, PDGF is the most clinically advanced; hydrogel-based delivery can overcome its short half-life and achieve sustained, localized release. For example, a hydrogel co-delivering PDGF and tannic acid promotes macrophage polarization toward M2 and enhanced angiogenesis in diabetic mouse wounds (Figure 6D) [215]. Similarly, hierarchical hydrogels co-loaded with PDGF-BB and DNase I demonstrated dual benefits of promoting endothelial migration and degrading excessive neutrophil extracellular traps, thereby achieving dual-axis intervention in chronic inflammation and vascular insufficiency [216].
Fibroblast growth factors (FGF) have also shown promise. FGF-21-loaded heparin-poloxamer hydrogels significantly improved wound closure and angiogenesis compared with free FGF-21 in diabetic mice [217]. In addition, recombinant human acidic FGF hydrogels mitigated NLRP3 inflammasome activation and promoted regenerative remodeling in diabetic ulcers [218].
Collectively, these studies demonstrate that growth factor-loaded hydrogels can both stimulate pro-regenerative pathways (e.g., VEGF/FGF signaling) and modulate immune responses, underscoring their dual role in correcting the ischemic and inflammatory barriers characteristic of diabetic wound healing.
5. Diabetes-Specific Efficacy of Immunomodulatory Hydrogel Strategies
Emerging evidence indicates that not all hydrogel-based immunomodulatory therapies exert uniform effects across wound types; rather, certain strategies confer diabetes-specific advantages by addressing pathologies unique to the diabetic milieu (Table 3). For example, MSC-derived EVs mitigate hyperglycemia-induced inflammation through miRNA-mediated suppression of NF-κB and NLRP3, while PDGF-BB hydrogels compensate for impaired PDGF signaling observed in diabetic wounds. Similarly, oxygen nanobubble and hemoglobin-based hydrogels overcome chronic hypoxia without aggravating oxidative stress, and DNase I-containing formulations directly target excessive NET formation—a pathological hallmark of diabetic ulcers. Other agents such as FGF21 offer additional metabolic benefits by improving insulin sensitivity and vascular function. These distinct features underscore that hydrogel therapies for diabetic wounds should not be evaluated solely by generic regenerative outcomes, but also by their capacity to address diabetes-specific immune, metabolic, and microenvironmental deficits.
6. Challenges and Future Perspectives
Diabetic wound healing is profoundly modulated by immune dynamics. Immune system imbalance, such as macrophage polarization dysfunction, excessive neutrophil activation, T cell dysfunction, and oxidative stress dysregulation, significantly impedes wound repair under conditions of persistent chronic inflammation. This review systematically summarizes the key immunological abnormalities within diabetic wounds and discusses the immunoregulatory potential of hydrogels loaded with various biological components. It focuses on how hydrogels can enhance the regenerative niche through cell-based delivery strategies, extracellular vesicles, and bioactive factors. Despite the remarkable potential of immune-regulatory hydrogels in diabetic wound-related research, their clinical translation still faces several challenges:
6.1. Translational Barriers in Hydrogel
Scalability of hydrogel fabrication remains one of the most critical obstacles for clinical translation. Although hydrogels have shown considerable efficacy as carriers for immunomodulatory agents in preclinical models, few formulations have been produced under conditions compatible with clinical-scale production. Laboratory preparation typically relies on small-batch, manually prepared hydrogels, which are poorly aligned with industrial requirements for reproducibility, cost-effectiveness, and regulatory compliance. Advanced fabrication approaches, such as microfluidics or 3D printing, further complicate scalability by introducing challenges of process reproducibility and material standardization [225].
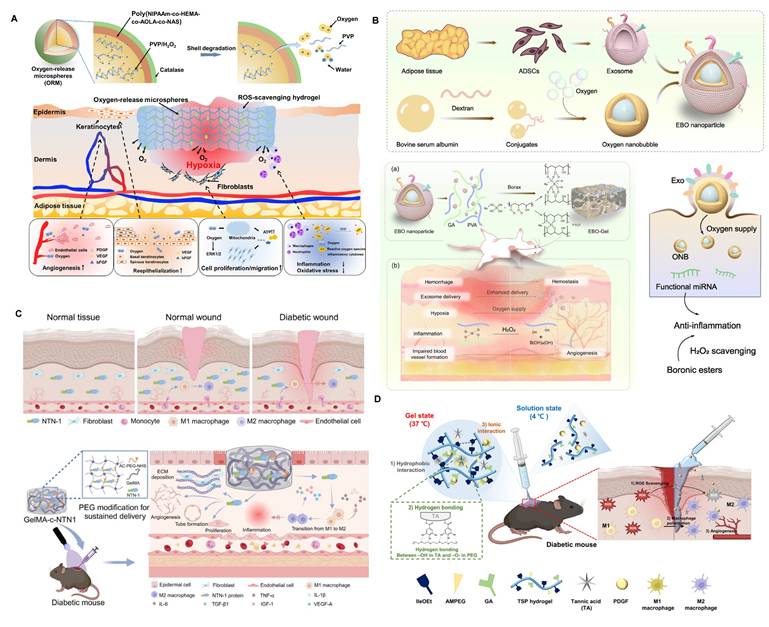
Immunomodulatory hydrogel systems for delivering bioactive substances. (A) The mechanisms of oxygen-release microspheres (ORMs) and accelerated wound healing by ORMs encapsulated in hydrogel. Adapted with permission from [195]. Copyright 2021, American Association for the Advancement of Science. (B) The preparation and mechanisms of ADSC-derived exosome coated BSA-based oxygen nanobubbles. Adapted with permission from [196]. Copyright 2024, Springer Nature. (C) The mechanisms of netrin-1 co-crosslinked hydrogel accelerated diabetic wound healing. Adapted with permission from [213]. Copyright 2024, Elsevier. (D) The mechanisms of a thermo-responsive hydrogel and the wound healing process. Adapted with permission from [215]. Copyright 2025, Royal Society of Chemistry.
Diabetes-specific efficacy of hydrogel-based delivery of immunomodulatory strategies for diabetic wound healing.
| Therapy / Cargo | Diabetes-Specific Features | Key Mechanisms | Refs. |
|---|---|---|---|
| BMSCs/ADSCs/hUCMSCs | Dual action on inflammation and angiogenesis tailored to hyperglycemic milieu | M1→M2 polarization, endothelial protection | [219] |
| BMSC-EVs | Suppress NF-κB/NLRP3 activation; promote M1→M2 polarization even under hyperglycemia | miR-146a-5p, miR-223 regulate inflammatory pathways | [220,221] |
| hUCMSC-EVs | Enhance angiogenesis and immune regulation in diabetic models | miR-17-5p promotes angiogenesis and anti-inflammation | [221,222] |
| O₂ Nanobubbles/Hb Hydrogels | Continuous oxygen release; lower oxidative stress risk in hyper-ROS environment | ROS/MMP-responsive O₂ release | [197,198] |
| EV-O₂ Nanobubbles | Relieve hypoxia and improve EV uptake under diabetic wound conditions | Sustained oxygen supply + enhanced EV stability | [196] |
| ROS-Scavenging Nanozymes | Specifically mitigate oxidative stress in diabetic wounds | Promote M2 polarization, anti-inflammatory effects | [201-204] |
| IL-1Ra (matrix-bound) | Provides long-acting local immunoregulation; suppresses persistent IL-1 activity | IL-1 pathway blockade | [223] |
| FGF21 | Corrects metabolic imbalance and vascular inflammation unique to diabetes | Corrects metabolic imbalance and vascular inflammation unique to diabetes | [217,218] |
| PDGF-BB | Counteracts suppressed PDGF signaling in diabetes | Counteracts suppressed PDGF signaling in diabetes | [216,224] |
| DNase I | Degrades excess NETs, targeting diabetes-specific high NETosis | Degrades excess NETs, targeting diabetes-specific high NETosis | [216] |
An essential yet under-discussed aspect of advancing hydrogel-based immunomodulatory platforms toward clinical application is the necessity for comprehensive safety evaluations. First, while numerous hydrogels show promise in preclinical studies, translating these materials safely to humans mandates rigorous assessment of biocompatibility, immunotoxicity, and off-target effects. For instance, a recent review on clinical hydrogel applications identified 139 clinical trials involving hydrogel dressings or implants, yet many failed to report on long-term immune responses or systemic safety outcomes [226]. Second, beyond acute toxicity, hydrogels must undergo extended observation for potential chronic inflammation, immunogenic reactions, or unintended immune activation, especially when delivering cells, biologics, or immune-active cargo. Materials designed for immune modulation may induce unexpected feedback responses if not carefully characterized in relevant large-animal or human-like models.
Regulatory approval constitutes another major bottleneck. Clinical translation of hydrogels must comply with international standards (e.g., ISO 10993), ensuring biocompatibility, sterility, and biosafety. In addition, hydrogel systems delivering immunologically active substances require rigorous evaluation under investigational new drug applications (INDAs) and subsequent regulatory pathways, such as new drug applications (NDAs) or biologics license applications (BLAs) [227]. This multilayered regulatory framework, involving multiple agencies, substantially prolongs the time required for bench-to-bedside translation.
Physicochemical composition further impacts manufacturability. Polymer selection, crosslinking chemistry, and water content not only determine therapeutic properties but also affect sterilization compatibility and batch-to-batch reproducibility. For instance, conventional sterilization processes can partially degrade hydrogel networks, while high water content complicates storage and shelf life [228,229].
Encouragingly, technological solutions are beginning to emerge. For example, Del Río et al. demonstrated that 3D printing can be leveraged to scale up biohybrid hydrogel structures for T cell culture, enhancing reproducibility, cell infiltration, and nutrient transport. Such approaches may provide a blueprint for future immunomodulatory hydrogel platforms applicable to diabetic wound healing [230].
In addition to scalability, safety, and regulatory compliance, hydrogel systems for diabetic wound healing must also meet disease-specific design requirements. The diabetic wound microenvironment is characterized by persistent hyperglycemia, high levels of AGEs, excessive ROS, and protease overactivity, all of which can compromise the stability and function of hydrogel dressings. For example, glycation can alter polymer crosslinking and mechanical integrity, while elevated ROS and MMPs may accelerate premature degradation, leading to uncontrolled release of therapeutic payloads. To address these challenges, new generations of hydrogels are being developed with anti-glycation modifications, ROS- and MMP-responsive linkers, and multimodal protective chemistries that allow spatiotemporal control of degradation.
Collectively, these challenges underscore that the successful translation of hydrogels will require not only advances in scalable and standardized manufacturing, but also the integration of regulatory compliance and material innovations to ensure clinical feasibility.
6.2. Barriers to Delivering Cells
Hydrogel-based delivery systems for cells, particularly stem cells, hold considerable therapeutic promise but face substantial translational hurdles. First, cell viability and retention in the harsh diabetic wound microenvironment (characterized by oxidative stress, high protease activity, and hypoxia) remain problematic. Hydrogels must be engineered not only to protect embedded cells but also to support their paracrine or immunomodulatory functionality over time. Second, regulatory and clinical translation face intensified scrutiny when viable cells are delivered: formulations must ensure cell identity, biosafety, absence of tumorigenicity, and functional consistency under GLP/GMP conditions. This complexity increases both development timelines and regulatory burden.
Taken together, although cell delivery via hydrogels enhances therapeutic potential, clinical translation demands robust solutions for scalable production, functional stability, and regulatory compliance.
6.3. Challenges in Clinical-Grade Preparation and Translation of EVs
Despite extensive research on EVs, no EV-based therapeutics have yet received regulatory approval for clinical use, largely due to challenges in production scale, product consistency, safety, and cost. Several major barriers remain to be addressed before EVs can be translated into clinical-grade products.
Achieving high-yield, bioactive EV production remains a persistent difficulty. Conventional cells secrete limited amounts of EVs, and while external stimulation can increase output, it often alters the molecular composition and biological activity of EVs. For instance, silicate stimulation of endothelial progenitor cells enhances EV secretion but simultaneously modifies angiogenic factor content [231]. At present, there is no consensus on standardized interventions for boosting EV yield without compromising functionality [231-233].
At the same time, laboratory ultracentrifugation methods are inadequate for Good Manufacturing Practice (GMP) standards. Efforts to scale up have focused on combining tangential flow filtration, ultracentrifugation with density gradients, and size exclusion chromatography to generate high-quality EVs [234]. However, reproducibility and cost remain limiting factors for industrial application.
The cellular origin of EVs poses another challenge. For mesenchymal stem cell-derived EVs, batch-to-batch variability and serum-derived impurities hinder reproducibility, while EVs derived from tumor cell lines raise concerns regarding oncogenicity and immune modulation. Standardization of EV potency and quality control—including particle number, size distribution, total protein, lipid, RNA content, and marker profiling—remains an unmet requirement for regulatory approval [235,236].
EVs are vulnerable to damage even at -80 °C, as ice crystal formation can impair biological activity. Although cryoprotectants such as phosphate-buffered saline with sucrose or trehalose have been explored, stability varies across EV sources, highlighting the absence of unified strategies for long-term preservation [232]. Following administration, EVs suffer from rapid clearance and suboptimal tissue targeting. Engineering approaches, including surface modification with targeting peptides, represent promising strategies to improve biodistribution and functional delivery. While most preclinical studies confirm EV safety in animal models, comprehensive data on biodistribution, pharmacokinetics, and immunological effects in humans are still lacking, necessitating further clinical investigation.
Together, these barriers underscore the urgent need for standardized production protocols, improved storage and delivery strategies, and rigorous safety assessments to accelerate the clinical translation of EV-based therapeutics. When combined with hydrogel platforms, these challenges in EV standardization, storage, and delivery become even more complex, underscoring the need for integrated solutions that address both material and biological barriers in clinical translation.
6.4. Limitations of Cytokines, Peptides, Growth Factors, and Antibodies
Diabetic wounds are characterized by elevated levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α, which collectively hinder the healing process. Anti-inflammatory cytokine therapies can act through mechanisms including direct neutralization, receptor blockade, receptor modulation, or selective depletion of target cells. Among these, cytokine-targeting antibodies neutralize inflammatory mediators without binding to cell-surface receptors, offering longer-lasting effects with potentially fewer off-target consequences [237]. However, compared with cytokines, peptides, and growth factors, antibodies are large proteins with limited tissue penetration and diffusion capacity. In contrast, small-molecule inhibitors can achieve similar immunomodulatory effects while exhibiting superior tissue permeability. For example, blockade of the IL-1 pathway using IL-1 receptor antagonists has been shown to markedly restore the immune microenvironment and promote healing in diabetic and chronic wound models [223].
Many current hydrogel formulations suffer from limited spatiotemporal precision in therapeutic release, often resulting in transient or poorly timed immune modulation. In addition, these bioactive molecules are prone to rapid degradation and typically have short half-lives in vivo, resulting in transient or unstable immunomodulatory effects. Their delivery also carries the risk of systemic diffusion beyond the wound site, which may trigger off-target effects or systemic immunosuppression. This represents a significant safety concern that remains insufficiently investigated in current preclinical models.
6.5. Limited Precision in Delivery
Many current hydrogel formulations suffer from limited spatiotemporal precision in therapeutic release, often resulting in transient or poorly timed immune modulation. Although smart hydrogels responsive to pH, temperature, enzymes, or ROS offer enhanced precision, their performance can be inconsistent due to patient heterogeneity and dynamic changes in wound conditions. Developing patient-specific, adaptive hydrogel systems remains a critical priority.
6.6. Immunogenicity Risks of Xenogeneic Hydrogel Materials
The immunogenicity of xenogeneic hydrogel materials represents a critical yet frequently overlooked barrier to clinical translation. Natural polymers such as alginate, collagen, and chitosan are widely used in hydrogel systems due to their biocompatibility and gel-forming properties. However, their biological origin inherently carries the risk of undesirable immune activation. For instance, alginate formulations vary in purity and may contain endotoxins or protein contaminants that stimulate macrophages via TLR4 signaling, thereby initiating a pro-inflammatory cascade [238]. Similarly, collagen and decellularized ECM hydrogels can retain residual antigens or DAMPs, triggering both cellular and humoral immune responses even after decellularization [239].
Chitosan illustrates the complexity of immunogenicity, as its effects vary depending on molecular weight, degree of deacetylation, and residual endotoxin levels. These parameters can determine whether chitosan elicits an inflammatory or reparative response [240]. To mitigate such risks, advanced purification, chemical modification, and endotoxin removal technologies are increasingly being applied to natural polymer systems such as alginate and chitosan [241]. In parallel, synthetic or semi-synthetic alternatives—including PEG-based and peptide-derived hydrogels—offer more predictable immunological profiles and align more closely with regulatory expectations [242].
Nevertheless, the hyperinflammatory environment of diabetic wounds amplifies the consequences of even low-level immune stimulation by xenogeneic matrices, potentially undermining the therapeutic efficacy of encapsulated immunomodulatory agents. Future research should therefore prioritize the development of standardized immunogenicity assays—such as in vitro human immune cell co-culture and in vivo diabetic wound models—to systematically evaluate both hydrogel carriers and their therapeutic cargo. Ensuring that the delivery vehicle itself does not exacerbate inflammation is essential for the successful clinical translation of hydrogel-based immunotherapies.
6.7. Complexity and Target Selection in Immune Modulation
Immune responses in wound healing shift from early activation to later resolution, yet static immunomodulatory approaches cannot accommodate this transition. Although staged-release hydrogels have been proposed, standardized protocols for timing and dosing remain undeveloped. In diabetic wounds, immune dysregulation involves multiple cell types, but most studies disproportionately target macrophage polarization, while other pathways—such as neutrophil extracellular trap clearance or T cell regulation—are rarely explored. Moreover, few comparative studies evaluate different strategies to identify the most relevant immune targets. Future efforts should therefore integrate spatiotemporal delivery control with broader and more systematic target selection to optimize hydrogel-based immunotherapies for diabetic wound healing.
6.8. Patient Heterogeneity and Limitations of Preclinical Models
A major limitation of current research lies in the insufficient consideration of patient heterogeneity and the reliance on inadequate preclinical models. Most studies employ rodent models such as mice and rats, which differ markedly from humans in wound healing mechanisms, while porcine models offer closer approximation but remain logistically difficult to manipulate. In contrast, clinical patients with T1D and T2D display distinct metabolic and immune profiles that shape wound healing responses differently. Furthermore, diabetic foot ulcers can be stratified into neuropathic, ischemic, or mixed phenotypes, each driven by distinct pathological mechanisms. For instance, hydrogels engineered with neuroregenerative properties may be preferentially effective in neuropathic ulcers but show limited benefit in ischemic wounds, where angiogenic factor-loaded formulations such as VEGF- or angiopoietin-based hydrogels may be more suitable. Addressing this heterogeneity requires both the development of more predictive and manipulable platforms—such as organ-on-a-chip systems—and the incorporation of patient stratification into preclinical and clinical trial design. Ultimately, modular hydrogel systems tailored to wound-specific phenotypes will be essential for ensuring clinical efficacy of hydrogel-based immunotherapies.
6.9. Limited Exploration of Immune Cell Interactions
Most research has focused on macrophage polarization, while the roles of additional immune populations—including T lymphocytes, neutrophils, and so on- remain underexplored. Future studies should aim to understand how hydrogels influence the broader cell populations to achieve more precise immune modulation. Additionally, a novel immune modulation strategy involves the crosstalk between non-immune cells and immune cells that play pivotal roles in wound resolution to regulate immune responses in diabetic wounds. For instance, fibroblasts can release Macrophage Colony-Stimulating Factor (M-CSF) to enhance macrophage phagocytosis.
6.10. Future Research Directions
To better promote the application of immunomodulatory hydrogels in diabetic wounds, we proposed three feasible scientific research hypotheses: immune sensing, artificial intelligence drive, and modular hydrogel platform, which may affect the next generation of hydrogel-based diabetic wound immunotherapy.
6.10.1. Real-Time Immunosensing Hydrogels
To move beyond general observations, future research must focus on testable hypotheses that integrate hydrogel design with dynamic immune regulation and patient-specific needs. One promising avenue is the development of hydrogels capable of real-time immune sensing, extending beyond current systems that mainly monitor pH or glucose. By incorporating biosensors for inflammatory cytokines (e.g., IL-1β, TNF-α), such platforms could provide direct insights into the immunological status of diabetic wounds and guide timely therapeutic interventions [243,244].
6.10.2. Integration with AI-Driven Wearable Devices
Another direction involves coupling hydrogels with AI-driven wearable devices. These integrated systems would not only monitor biochemical and biophysical indicators but could also trigger hydrogels to release immunomodulatory cargos in response to detected abnormalities. Such “closed-loop” wound management would allow both predictive monitoring and on-demand therapy, thereby improving therapeutic precision [245,246].
6.10.3. Modular and Patient-specific Hydrogel Design
Finally, advances in modular and customizable hydrogel design are expected to enable therapies tailored to specific wound phenotypes. Hydrogels could be engineered with interchangeable modules that address neuropathic versus ischemic ulcers, or T1D versus T2D immune profiles, thus aligning with patient stratification strategies. Combining modularity with intelligent sensing and AI integration could ultimately establish personalized hydrogel platforms that restore immune balance and accelerate diabetic wound healing.
7. Conclusion
Diabetic wounds present persistent clinical challenges due to immune dysregulation and chronic inflammation. Engineered immunomodulatory hydrogel delivery systems hold significant promise by enabling targeted and sustained release of therapeutics—including cells, EVs, and bioactive molecules—to restore immune balance. These platforms regulate macrophage polarization, suppress neutrophil overactivation, and promote angiogenesis, thereby accelerating wound healing. Among them, MSC-EVs and immune cell-based therapies have shown particular potential, offering low immunogenicity, high stability, and versatile immunoregulatory functions.
Despite encouraging progress, several obstacles remain. Clinical translation is hampered by scalability, regulatory hurdles, and safety concerns, while limitations of delivered cargos, the need for precise spatiotemporal control, and complex hydrogel-cargo interactions further complicate application. Moreover, although most strategies demonstrate broad regenerative efficacy, their diabetes-specific advantages are not fully understood. For example, MSC-EVs may exert unique immunomodulatory effects in diabetic wounds, but the mechanisms and wound subtype specificity require clarification. Future studies should focus on treatment strategies targeting such diabetes-specific mechanisms—such as immune microenvironment modulation, glycemic regulation, AGEs resistance, and stability under hyperinflammatory or high-MMP conditions—and integrating them into hydrogel design. Addressing these challenges will be critical for advancing hydrogel-based immunotherapies into effective clinical treatments for diabetic wound management.
Acknowledgements
This work was supported by grants from the National Key Research and Development Program (2024YFC2510603), National Science Foundation of Hubei Province (2025AFB283), Hubei Key Laboratory of Regenerative Medicine and Multi-disciplinary Translational Research (2022zsyx009), and Award Program for outstanding recent Ph.D. Graduates at Wuhan Union Hospital (2022).
Consent for publication
All authors have given their consent for publication.
Author contributions
LYD and YQH conceptualized and supervised the review. CLL and YZZ conducted literature collection and data interpretation. LYD and HFH contributed to main manuscript text writing and FAS prepared figure 1 and figure 5. JWL participated in other figures. BBM and GHL revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Magliano DJ, Boyko EJ, IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS [Internet]. 10th ed. Brussels: International Diabetes Federation. 2021 (IDF Diabetes Atlas)
2. Armstrong DG, Tan T-W, Boulton AJM, Bus SA. Diabetic Foot Ulcers: A Review. JAMA. 2023;330:62-75
3. Las Heras K, Igartua M, Santos-Vizcaino E, Hernandez RM. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J Control Release. 2020;328:532-50
4. Zeng Q, Qi X, Shi G, Zhang M, Haick H. Wound Dressing: From Nanomaterials to Diagnostic Dressings and Healing Evaluations. ACS Nano. 2022;16:1708-33
5. Farahani M, Shafiee A. Wound Healing: From Passive to Smart Dressings. Adv Healthc Mater. 2021;10:2100477
6. Naseri-Nosar M, Ziora ZM. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr Polym. 2018;189:379-98
7. Kim HS, Sun X, Lee J-H, Kim H-W, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209-39
8. Chen Y, Wang X, Tao S, Wang Q, Ma P-Q, Li Z-B. et al. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil Med Res. 2023;10:37
9. Ghobril C, Grinstaff MW. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chem Soc Rev. 2015;44:1820-35
10. Oliva N, Conde J, Wang K, Artzi N. Designing Hydrogels for On-Demand Therapy. Acc Chem Res. 2017;50:669-79
11. da Silva LP, Reis RL, Correlo VM, Marques AP. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu Rev Biomed Eng. 2019;21:145-69
12. Kharaziha M, Baidya A, Annabi N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv Mater. 2021;33:e2100176
13. Bu W, Wu Y, Ghaemmaghami AM, Sun H, Mata A. Rational design of hydrogels for immunomodulation. Regen Biomater. 2022;9:rbac009
14. Wang H, Xu Z, Zhao M, Liu G, Wu J. Advances of hydrogel dressings in diabetic wounds. Biomater Sci. 2021;9:1530-46
15. Qi L, Zhang C, Wang B, Yin J, Yan S. Progress in Hydrogels for Skin Wound Repair. Macromol Biosci. 2022;22:2100475
16. Tehrany PM, Rahmanian P, Rezaee A, Ranjbarpazuki G, Sohrabi Fard F, Asadollah salmanpour Y. et al. Multifunctional and theranostic hydrogels for wound healing acceleration: An emphasis on diabetic-related chronic wounds. Environ Res. 2023;238:117087
17. Wang Z, Sun L, Wang W, Wang Z, Shi G, Dai H. et al. A double-network porous hydrogel based on high internal phase emulsions as a vehicle for potassium sucrose octasulfate delivery accelerates diabetic wound healing. Regen Biomater. 2024;11:rbae024
18. Zhou W, Duan Z, Zhao J, Fu R, Zhu C, Fan D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact Mater. 2022;17:1-17
19. Lai C-M, Chen W-J, Qin Y, Xu D, Lai Y-K, He S-H. Innovative Hydrogel Design: Tailoring Immunomodulation for Optimal Chronic Wound Recovery. Adv Sci. 2025;12:e2412360
20. Larouche J, Sheoran S, Maruyama K, Martino MM. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv Wound Care. 2018;7:209-31
21. Lin S, Wang Q, Huang X, Feng J, Wang Y, Shao T. et al. Wounds under diabetic milieu: The role of immune cellar components and signaling pathways. Biomed Pharmacother. 2023;157:114052
22. Sharifiaghdam M, Shaabani E, Faridi-Majidi R, De Smedt SC, Braeckmans K, Fraire JC. Macrophages as a therapeutic target to promote diabetic wound healing. Mol Ther. 2022;30:2891-908
23. Louiselle AE, Niemiec SM, Zgheib C, Liechty KW. Macrophage polarization and diabetic wound healing. Transl Res. 2021;236:109-16
24. Meng H, Su J, Shen Q, Hu W, Li P, Guo K. et al. A Smart MMP-9-responsive Hydrogel Releasing M2 Macrophage-derived Exosomes for Diabetic Wound Healing. Adv Healthc Mater. 2025;14:e2404966
25. He X, Wei Y, Xu K. Hydrogel-Based Treatment of Diabetic Wounds: From Smart Responsive to Smart Monitoring. Gels Basel. 2025;11:647
26. Li R, Liu K, Huang X, Li D, Ding J, Liu B. et al. Bioactive Materials Promote Wound Healing through Modulation of Cell Behaviors. Adv Sci. 2022;9:2105152
27. Knoedler S, Knoedler L, Kauke-Navarro M, Rinkevich Y, Hundeshagen G, Harhaus L. et al. Regulatory T cells in skin regeneration and wound healing. Mil Med Res. 2023;10:49
28. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223
29. Sim SL, Kumari S, Kaur S, Khosrotehrani K. Macrophages in Skin Wounds: Functions and Therapeutic Potential. Biomolecules. 2022;12:1659
30. Li M, Hou Q, Zhong L, Zhao Y, Fu X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front Immunol. 2021;12:681710
31. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019;99:665-706
32. Zheng H, Cheng X, Jin L, Shan S, Yang J, Zhou J. Recent advances in strategies to target the behavior of macrophages in wound healing. Biomed Pharmacother. 2023;165:115199
33. Liu Y, Liu Y, He W, Mu X, Wu X, Deng J. et al. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front Immunol. 2022;13:918223
34. Cooper PO, Haas MR, Noonepalle S kumar R, Shook BA. Dermal Drivers of Injury-Induced Inflammation: Contribution of Adipocytes and Fibroblasts. Int J Mol Sci. 2021;22:1933
35. Ozpinar EW, Frey AL, Cruse G, Freytes DO. Mast Cell-Biomaterial Interactions and Tissue Repair. Tissue Eng Part B Rev. 2021;27:590-603
36. Munoz LD, Sweeney MJ, Jameson JM. Skin Resident γδ T Cell Function and Regulation in Wound Repair. Int J Mol Sci. 2020;21:9286
37. Vinish M, Cui W, Stafford E, Bae L, Hawkins H, Cox R. et al. Dendritic cells modulate burn wound healing by enhancing early proliferation. Wound Repair Regen. 2016;24:6-13
38. Franz A, Wood W, Martin P. Fat Body Cells Are Motile and Actively Migrate to Wounds to Drive Repair and Prevent Infection. Dev Cell. 2018;44:460-470.e3
39. Shook BA, Wasko RR, Mano O, Rutenberg-Schoenberg M, Rudolph MC, Zirak B. et al. Dermal adipocyte lipolysis and myofibroblast conversion are required for efficient skin repair. Cell Stem Cell. 2020;26:880-895.e6
40. Rai V, Moellmer R, Agrawal DK. Role of fibroblast plasticity and heterogeneity in modulating angiogenesis and healing in the diabetic foot ulcer. Mol Biol Rep. 2023;50:1913-29
41. Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56:256-64
42. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front Physiol. 2018;9:419
43. Wang Q, Zhu G, Cao X, Dong J, Song F, Niu Y. Blocking AGE-RAGE Signaling Improved Functional Disorders of Macrophages in Diabetic Wound. J Diabetes Res. 2017;2017:1428537
44. Barman PK, Koh TJ. Macrophage Dysregulation and Impaired Skin Wound Healing in Diabetes. Front Cell Dev Biol. 2020;8:528
45. Liu BF, Miyata S, Kojima H, Uriuhara A, Kusunoki H, Suzuki K. et al. Low phagocytic activity of resident peritoneal macrophages in diabetic mice: relevance to the formation of advanced glycation end products. Diabetes. 1999;48:2074-82
46. Yu T, Gao M, Yang P, Liu D, Wang D, Song F. et al. Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing. J Cell Physiol. 2019;234:4217-31
47. Chen H, Shi R, Luo B, Yang X, Qiu L, Xiong J. et al. Macrophage peroxisome proliferator-activated receptor γ deficiency delays skin wound healing through impairing apoptotic cell clearance in mice. Cell Death Dis. 2015;6:e1597
48. Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C. et al. Macrophage Dysfunction Impairs Resolution of Inflammation in the Wounds of Diabetic Mice. PLoS ONE. 2010;5:e9539
49. Zhu Z, Zhou S, Li S, Gong S, Zhang Q. Neutrophil extracellular traps in wound healing. Trends Pharmacol Sci. 2024;45:1033-45
50. Zhu Y, Xia X, He Q, Xiao Q-A, Wang D, Huang M. et al. Diabetes-associated neutrophil NETosis: pathogenesis and interventional target of diabetic complications. Front Endocrinol. 2023;14:1202463
51. Yang S, Wang S, Chen L, Wang Z, Chen J, Ni Q. et al. Neutrophil Extracellular Traps Delay Diabetic Wound Healing by Inducing Endothelial-to-Mesenchymal Transition via the Hippo pathway. Int J Biol Sci. 2023;19:347
52. Xiao Y, Ding T, Fang H, Lin J, Chen L, Ma D. et al. Innovative Bio-based Hydrogel Microspheres Micro-Cage for Neutrophil Extracellular Traps Scavenging in Diabetic Wound Healing. Adv Sci. 2024;11:e2401195
53. Yang S, Feng Y, Chen L, Wang Z, Chen J, Ni Q. et al. Disulfiram accelerates diabetic foot ulcer healing by blocking NET formation via suppressing the NLRP3/Caspase-1/GSDMD pathway. Transl Res J Lab Clin Med. 2023;254:115-27
54. Chu Z, Huang Q, Ma K, Liu X, Zhang W, Cui S. et al. Novel neutrophil extracellular trap-related mechanisms in diabetic wounds inspire a promising treatment strategy with hypoxia-challenged small extracellular vesicles. Bioact Mater. 2023;27:257-70
55. Gray V, Chen W, Tan RJY, Teo JMN, Huang Z, Fong CH-Y. et al. Hyperglycemia-triggered lipid peroxidation destabilizes STAT4 and impairs anti-viral Th1 responses in type 2 diabetes. Cell Metab. 2024;36:2511-2527.e7
56. Ramirez K, Witherden DA, Havran WL. All hands on DE(T)C: Epithelial-resident γδ T cells respond to tissue injury. Cell Immunol. 2015;296:57-61
57. Han JM, Patterson SJ, Speck M, Ehses JA, Levings MK. Insulin inhibits IL-10-mediated regulatory T cell function: implications for obesity. J Immunol Baltim Md 1950. 2014;192:623-9
58. Qiao Y-C, Shen J, He L, Hong X-Z, Tian F, Pan Y-H. et al. Changes of Regulatory T Cells and of Proinflammatory and Immunosuppressive Cytokines in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J Diabetes Res. 2016;2016:3694957
59. Sheikh V, Zamani A, Mahabadi-Ashtiyani E, Tarokhian H, Borzouei S, Alahgholi-Hajibehzad M. Decreased regulatory function of CD4+CD25+CD45RA+ T cells and impaired IL-2 signalling pathway in patients with type 2 diabetes mellitus. Scand J Immunol. 2018;88:e12711
60. Garoufalia Z, Papadopetraki A, Karatza E, Vardakostas D, Philippou A, Kouraklis G. et al. Insulin-like growth factor-I and wound healing, a potential answer to non-healing wounds: A systematic review of the literature and future perspectives. Biomed Rep. 2021;15:66
61. Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal Homeostasis: The Role of the Growth Hormone and Insulin-Like Growth Factor Systems. Endocr Rev. 2003;24:737-64
62. Guillamat-Prats R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells. 2021;10:1729
63. Zhu M, Cao L, Melino S, Candi E, Wang Y, Shao C. et al. Orchestration of Mesenchymal Stem/Stromal Cells and Inflammation During Wound Healing. Stem Cells Transl Med. 2023;12:576-87
64. Liu F, Qiu H, Xue M, Zhang S, Zhang X, Xu J. et al. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. 2019;10:345
65. Jiang D, Qi Y, Walker NG, Sindrilaru A, Hainzl A, Wlaschek M. et al. The effect of adipose tissue derived MSCs delivered by a chemically defined carrier on full-thickness cutaneous wound healing. Biomaterials. 2013;34:2501-15
66. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-36
67. Chen S, Shi J, Zhang M, Chen Y, Wang X, Zhang L. et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5:18104
68. Bai H, Kyu-Cheol N, Wang Z, Cui Y, Liu H, Liu H. et al. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J Tissue Eng. 2020;11:2041731420947242
69. Andleeb A, Mehmood A, Tariq M, Butt H, Ahmed R, Andleeb A. et al. Hydrogel patch with pretreated stem cells accelerates wound closure in diabetic rats. Biomater Adv. 2022;142:213150
70. Dong Y, Rodrigues M, Kwon SH, Li X, A S, Brett EA. et al. Acceleration of Diabetic Wound Regeneration using an In Situ-Formed Stem-Cell-Based Skin Substitute. Adv Healthc Mater. 2018;7:e1800432
71. Xia S, Weng T, Jin R, Yang M, Yu M, Zhang W. et al. Curcumin-incorporated 3D bioprinting gelatin methacryloyl hydrogel reduces reactive oxygen species-induced adipose-derived stem cell apoptosis and improves implanting survival in diabetic wounds. Burns Trauma. 2022;10:tkac001
72. Xu H, Wang J, Wu D, Qin D. A hybrid hydrogel encapsulating human umbilical cord mesenchymal stem cells enhances diabetic wound healing. J Mater Sci Mater Med. 2022;33:60
73. Xue J, Sun N, Liu Y. Self-Assembled Nano-Peptide Hydrogels with Human Umbilical Cord Mesenchymal Stem Cell Spheroids Accelerate Diabetic Skin Wound Healing by Inhibiting Inflammation and Promoting Angiogenesis. Int J Nanomedicine. 2022;17:2459-74
74. Jiao Y, Chen X, Niu Y, Huang S, Wang J, Luo M. et al. Wharton's jelly mesenchymal stem cells embedded in PF-127 hydrogel plus sodium ascorbyl phosphate combination promote diabetic wound healing in type 2 diabetic rat. Stem Cell Res Ther. 2021;12:559
75. Huang J, Yang R, Jiao J, Li Z, Wang P, Liu Y. et al. A click chemistry-mediated all-peptide cell printing hydrogel platform for diabetic wound healing. Nat Commun. 2023;14:7856
76. Theocharidis G, Rahmani S, Lee S, Li Z, Lobao A, Kounas K. et al. Murine macrophages or their secretome delivered in alginate dressings enhance impaired wound healing in diabetic mice. Biomaterials. 2022;288:121692
77. Gorecka J, Gao X, Fereydooni A, Dash BC, Luo J, Lee SR. et al. Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen Med. 2020;15:1277-93
78. Shukla L, Yuan Y, Shayan R, Greening DW, Karnezis T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front Pharmacol. 2020;11:158
79. Shi M, Gao Y, Lee L, Song T, Zhou J, Yan L. et al. Adaptive Gelatin Microspheres Enhanced Stem Cell Delivery and Integration With Diabetic Wounds to Activate Skin Tissue Regeneration. Front Bioeng Biotechnol. 2022;10:813805
80. Li L, Li J, Guan H, Oishi H, Takahashi S, Zhang C. Human umbilical cord mesenchymal stem cells in diabetes mellitus and its complications: applications and research advances. Int J Med Sci. 2023;20:1492-507
81. Gu X, Shen S, Huang C, Liu Y, Chen Y, Luo L. et al. Effect of activated autologous monocytes/macrophages on wound healing in a rodent model of experimental diabetes. Diabetes Res Clin Pract. 2013;102:53-9
82. Hu MS, Walmsley GG, Barnes LA, Weiskopf K, Rennert RC, Duscher D. et al. Delivery of monocyte lineage cells in a biomimetic scaffold enhances tissue repair. JCI Insight. 2017;2:e96260 96260
83. Cerneckis J, Cai H, Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduct Target Ther. 2024;9:112
84. Ma K, Luo C, Du M, Wei Q, Luo Q, Zheng L. et al. Advances in stem cells treatment of diabetic wounds: A bibliometric analysis via CiteSpace. Skin Res Technol. 2024;30:e13665
85. Ho J, Yue D, Cheema U, Hsia HC, Dardik A. Innovations in Stem Cell Therapy for Diabetic Wound Healing. Adv Wound Care. 2023;12:626-43
86. Shen Y-I, Cho H, Papa AE, Burke JA, Chan XY, Duh EJ. et al. Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials. 2016;102:107-19
87. Zhang W, Wang L, Guo H, Chen L, Huang X. Dapagliflozin-Loaded Exosome Mimetics Facilitate Diabetic Wound Healing by HIF-1α-Mediated Enhancement of Angiogenesis. Adv Healthc Mater. 2023;12:e2202751
88. Ko KI, DerGarabedian BP, Chen Z, Debnath R, Ko A, Link BN. et al. Distinct fibroblast progenitor subpopulation expedites regenerative mucosal healing by immunomodulation. J Exp Med. 2023;220:e20221350
89. Dong X, Xiang H, Li J, Hao A, Wang H, Gou Y. et al. Dermal fibroblast-derived extracellular matrix (ECM) synergizes with keratinocytes in promoting re-epithelization and scarless healing of skin wounds: Towards optimized skin tissue engineering. Bioact Mater. 2025;47:1-17
90. Buitrago JC, Morris SL, Backhaus A, Kaltenecker G, Kaipa JM, Girard C. et al. Unveiling the Immunomodulatory and regenerative potential of iPSC-derived mesenchymal stromal cells and their extracellular vesicles. Sci Rep. 2024;14:24098
91. Ackermann M, Saleh F, Abdin SM, Rafiei Hashtchin A, Gensch I, Golgath J. et al. Standardized generation of human iPSC-derived hematopoietic organoids and macrophages utilizing a benchtop bioreactor platform under fully defined conditions. Stem Cell Res Ther. 2024;15:171
92. Yang Q, Barbachano-Guerrero A, Fairchild LM, Rowland TJ, Dowell RD, Allen MA. et al. Macrophages derived from human induced pluripotent stem cells (iPSCs) serve as a high-fidelity cellular model for investigating HIV-1, dengue, and influenza viruses. J Virol. 2024;98:e0156323
93. Huang J-N, Cao H, Liang K-Y, Cui L-P, Li Y. Combination therapy of hydrogel and stem cells for diabetic wound healing. World J Diabetes. 2022;13:949-61
94. Zhao Y, Wang M, Liang F, Li J. Recent strategies for enhancing the therapeutic efficacy of stem cells in wound healing. Stem Cell Res Ther. 2021;12:588
95. Qin W, Wang J, Hu Q, Qin R, Ma N, Zheng F. et al. Melatonin-pretreated human umbilical cord mesenchymal stem cells improved endometrium regeneration and fertility recovery through macrophage immunomodulation in rats with intrauterine adhesions†. Biol Reprod. 2023;109:918-37
96. Ding S, O'Banion CP, Welfare JG, Lawrence DS. Cellular Cyborgs: On the Precipice of a Drug Delivery Revolution. Cell Chem Biol. 2018;25:648-58
97. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019;8:1605
98. Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019;8:467
99. Wang Y, Cao Z, Wei Q, Ma K, Hu W, Huang Q. et al. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022;147:342-55
100. Xiong W, Zhang X, Hu J, Zou X, Huang H, Qu W. et al. PF-PEG@ASIV-EXO Hydrogel Accelerates Diabetic Wound Healing by Ferroptosis Resistance and Promoting Angiogenesis. ACS Biomater Sci Eng. 2024;10:6263-85
101. Wang Y, Song P, Wu L, Su Z, Gui X, Gao C. et al. In situ photo-crosslinked adhesive hydrogel loaded with mesenchymal stem cell-derived extracellular vesicles promotes diabetic wound healing. J Mater Chem B. 2023;11:837-51
102. Geng X, Qi Y, Liu X, Shi Y, Li H, Zhao L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater Adv. 2022;133:112613
103. Zhang Y, Li M, Wang Y, Han F, Shen K, Luo L. et al. Exosome/metformin-loaded self-healing conductive hydrogel rescues microvascular dysfunction and promotes chronic diabetic wound healing by inhibiting mitochondrial fission. Bioact Mater. 2023;26:323-36
104. Song Y, You Y, Xu X, Lu J, Huang X, Zhang J. et al. Adipose-Derived Mesenchymal Stem Cell-Derived Exosomes Biopotentiated Extracellular Matrix Hydrogels Accelerate Diabetic Wound Healing and Skin Regeneration. Adv Sci. 2023;10:e2304023
105. Wang Y, Zhao G, Sigen S, Xu Q, Wu X, Wang W-X. et al. In Situ Forming Hypoxia-Induced Exosome-Loaded Hydrogel for Enhanced Diabetic Wound Healing. Macromol Mater Eng. 2025 310
106. Long X, Yuan Q, Tian R, Zhang W, Liu L, Yang M. et al. Efficient healing of diabetic wounds by MSC-EV-7A composite hydrogel via suppression of inflammation and enhancement of angiogenesis. Biomater Sci. 2024;12:1750-60
107. Xu C, Cao J-F, Pei Y, Kim Y, Moon H, Fan C-Q. et al. Injectable hydrogel harnessing foreskin mesenchymal stem cell-derived extracellular vesicles for treatment of chronic diabetic skin wounds. J Control Release. 2024;370:339-53
108. Zeng J, Sun Z, Zeng F, Gu C, Chen X. M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing. Mater Today Bio. 2023;20:100649
109. Huang W, Guo Q, Wu H, Zheng Y, Xiang T, Zhou S. Engineered Exosomes Loaded in Intrinsic Immunomodulatory Hydrogels with Promoting Angiogenesis for Programmed Therapy of Diabetic Wounds. ACS Nano. 2025;19:14467-83
110. Yang F, Cai D, Kong R, Bi Y, Zhang Y, Lei Y. et al. Exosomes derived from cord blood Treg cells promote diabetic wound healing by targeting monocytes. Biochem Pharmacol. 2024;226:116413
111. Ma Y, Wang X, Huang X, He Y, Su T, Niu X. et al. Radial Egg White Hydrogel Releasing Extracellular Vesicles for Cell Fate Guidance and Accelerated Diabetic Skin Regeneration. Adv Healthc Mater. 2024;13:e2400016
112. Wang S, Wu J, Ren K, Zhang Y, Gao F, Chen Y. et al. Platelet-Rich Plasma-Derived Exosome-Encapsulated Hydrogels Accelerate Diabetic Wound Healing by Inhibiting Fibroblast Ferroptosis. Acs Appl Mater Interfaces. 2025;17:27923-36
113. Hao P-C, Burnouf T, Chiang C-W, Jheng P-R, Szunerits S, Yang J-C. et al. Enhanced diabetic wound healing using platelet-derived extracellular vesicles and reduced graphene oxide in polymer-coordinated hydrogels. J Nanobiotechnology. 2023;21:318
114. Cao Y, Chen B, Liu Q, Mao Y, He Y, Liu X. et al. Dissolvable microneedle-based wound dressing transdermally and continuously delivers anti-inflammatory and pro-angiogenic exosomes for diabetic wound treatment. Bioact Mater. 2024;42:32-51
115. Xiong Y, Lin Z, Bu P, Yu T, Endo Y, Zhou W. et al. A Whole-Course-Repair System Based on Neurogenesis-Angiogenesis Crosstalk and Macrophage Reprogramming Promotes Diabetic Wound Healing. Adv Mater. 2023;35:e2212300
116. Yang J, Zhang X, Wang G, Ma S, Yu Y, Liao C. et al. ApoSEVs-Mediated Modulation of Versatile Target Cells Promotes Diabetic Wound Healing: Unveiling a Promising Strategy. Int J Nanomedicine. 2023;18:6955-77
117. Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B. et al. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 2022;13:295
118. Shen X, Qin J, Wei Z, Liu F. Bone marrow mesenchymal stem cell exosome-derived lncRNA TUC339 influences the progression of osteoarthritis by regulating synovial macrophage polarization and chondrocyte apoptosis. Biomedecine Pharmacother. 2023;167:115488
119. Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E. et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int J Mol Sci. 2021;22:3851
120. Jiang T, Liu S, Wu Z, Li Q, Ren S, Chen J. et al. ADSC-exo@MMP-PEG smart hydrogel promotes diabetic wound healing by optimizing cellular functions and relieving oxidative stress. Mater Today Bio. 2022;16:100365
121. Wei Q, Su J, Meng S, Wang Y, Ma K, Li B. et al. MiR-17-5p-engineered sEVs Encapsulated in GelMA Hydrogel Facilitated Diabetic Wound Healing by Targeting PTEN and p21. Adv Sci. 2024;11:e2307761
122. Ma S, Hu H, Wu J, Li X, Ma X, Zhao Z. et al. Functional extracellular matrix hydrogel modified with MSC-derived small extracellular vesicles for chronic wound healing. Cell Prolif. 2022;55:e13196
123. Yu B, Shao H, Su C, Jiang Y, Chen X, Bai L. et al. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep. 2016;6:34562
124. Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733
125. Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B. et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano. 2018;12:7613-28
126. Tang S, Feng K, Yang R, Cheng Y, Shi N, Zhang H. et al. A dual-action strategy: Wound microenvironment responsive hydrogel and exosome-mediated glucose regulation enhance inside-out diabetic wound repair. J Control Release. 2025;382:113716
127. Cai S, Fan C, Xie L, Zhong H, Li A, Lv S. et al. Single-cell RNA sequencing reveals the potential mechanism of heterogeneity of immunomodulatory properties of foreskin and umbilical cord mesenchymal stromal cells. Cell Biosci. 2022;12:115
128. Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv Sci. 2019;6:1900513
129. Wang Y, Lin Q, Zhang H, Wang S, Cui J, Hu Y. et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact Mater. 2023;28:273-83
130. Asemani Y, Najafi S, Ezzatifar F, Zolbanin NM, Jafari R. Recent highlights in the immunomodulatory aspects of Treg cell-derived extracellular vesicles: special emphasis on autoimmune diseases and transplantation. Cell Biosci. 2022;12:67
131. Rojas C, Campos-Mora M, Cárcamo I, Villalón N, Elhusseiny A, Contreras-Kallens P. et al. T regulatory cells-derived extracellular vesicles and their contribution to the generation of immune tolerance. J Leukoc Biol. 2020;108:813-24
132. Yinhua Zhao MM, Yanyu Shi MM, Lin H. Extracellular vesicles from hypoxia-pretreated adipose-derived stem cells regulate hypoxia/reoxygenation-induced human dermal microvascular endothelial apoptosis and autophagy in vitro. Heliyon. 2023;9:e13315
133. Chang L-H, Wu S-C, Chen C-H, Chen J-W, Huang W-C, Wu C-W. et al. Exosomes Derived from Hypoxia-Cultured Human Adipose Stem Cells Alleviate Articular Chondrocyte Inflammaging and Post-Traumatic Osteoarthritis Progression. Int J Mol Sci. 2023;24:13414
134. Hu N, Cai Z, Jiang X, Wang C, Tang T, Xu T. et al. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023;157:175-86
135. Wang J, Wu H, Zhao Y, Qin Y, Zhang Y, Pang H. et al. Extracellular Vesicles from HIF-1α-Overexpressing Adipose-Derived Stem Cells Restore Diabetic Wounds Through Accelerated Fibroblast Proliferation and Migration. Int J Nanomedicine. 2021;16:7943-57
136. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q. et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:259
137. Zhou Z, Wang R, Wang J, Hao Y, Xie Q, Wang L. et al. Melatonin pretreatment on exosomes: Heterogeneity, therapeutic effects, and usage. Front Immunol. 2022;13:933736
138. Buikema JW, Lee S, Goodyer WR, Maas RG, Chirikian O, Li G. et al. Wnt Activation and Reduced Cell-Cell Contact Synergistically Induce Massive Expansion of Functional Human iPSC-Derived Cardiomyocytes. Cell Stem Cell. 2020;27:50-63.e5
139. Yu J, Liu D, Sun X, Yang K, Yao J, Cheng C. et al. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019;10:26
140. Wang L, Chen J, Song J, Xiang Y, Yang M, Xia L. et al. Activation of the Wnt/β-catenin signalling pathway enhances exosome production by hucMSCs and improves their capability to promote diabetic wound healing. J Nanobiotechnology. 2024;22:373
141. Xiong W, Bai X, Zhang X, Lei H, Xiao H, Zhang L. et al. Endothelial Progenitor-Cell-Derived Exosomes Induced by Astragaloside IV Accelerate Type I Diabetic-wound Healing via the PI3K/AKT/mTOR Pathway in Rats. Front Biosci (Landmark Ed). 2023;28:282
142. Xiong W, Zhang X, Zhou J, Chen J, Liu Y, Yan Y. et al. Astragaloside IV promotes exosome secretion of endothelial progenitor cells to regulate PI3KR2/SPRED1 signaling and inhibit pyroptosis of diabetic endothelial cells. Cytotherapy. 2024;26:36-50
143. Zheng Y, Xu P, Pan C, Wang Y, Liu Z, Chen Y. et al. Production and Biological Effects of Extracellular Vesicles from Adipose-Derived Stem Cells Were Markedly Increased by Low-Intensity Ultrasound Stimulation for Promoting Diabetic Wound Healing. Stem Cell Rev Rep. 2023;19:784-806
144. Zhu W, Dong Y, Xu P, Pan Q, Jia K, Jin P. et al. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022;154:212-30
145. Vezzani B, Shaw I, Lesme H, Yong L, Khan N, Tremolada C. et al. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl Med. 2018;7:876-86
146. Guo S-C, Tao S-C, Yin W-J, Qi X, Yuan T, Zhang C-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81-96
147. Xu N, Wang L, Guan J, Tang C, He N, Zhang W. et al. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol. 2018;117:102-7
148. Liu K, Gong B, Li T, Lei H, Li J, Tang J. et al. Bioactive self-healing umbilical cord blood exosomes hydrogel for promoting chronic diabetic wound healing. Biochem Biophys Res Commun. 2024;690:149241
149. Liu H, Wu B, Shi X, Cao Y, Zhao X, Liang D. et al. Aerobic exercise-induced circulating extracellular vesicle combined decellularized dermal matrix hydrogel facilitates diabetic wound healing by promoting angiogenesis. Front Bioeng Biotechnol. 2022;10:903779
150. Liao Q, Su L, Pang L, Li J, Li H, Li J. et al. Natural exosome-like nanoparticles derived from ancient medicinal insect Periplaneta americana L. as a novel diabetic wound healing accelerator. J Nanobiotechnology. 2023;21:169
151. Zaffar S, Saha S, Agrawal T, Gupta SK, Gulati P, Paul D. et al. Improved Diabetic Wound Healing via Flower Extracellular Vesicles and Carbon-Dot-Infused Alginate-Polyethylenimine Antibacterial Hydrogel. ACS Biomater Sci Eng. 2025;11:4219-30
152. Jin E, Yang Y, Cong S, Chen D, Chen R, Zhang J. et al. Lemon-derived nanoparticle-functionalized hydrogels regulate macrophage reprogramming to promote diabetic wound healing. J Nanobiotechnology. 2025;23:68
153. Yang W, Xing Z, Wang X, Xu Z, Jiang X, Xia J. et al. Microenvironment-responsive collagen hydrogel with Houttuynia Cordata Thunb vesicles for diabetic wound repair. Int J Biol Macromol. 2025;320:145840
154. Liu D, Gao J, Wu X, Hao X, Hu W, Han L. Conductive Microneedles Loaded With Polyphenol-Engineered Exosomes Reshape Diabetic Neurovascular Niches for Chronic Wound Healing. Adv Sci. 2025 e07974
155. Weng J, Chen Y, Zeng Y, Jin W, Ji Y, Zhang W. et al. A novel hydrogel loaded with plant exosomes and stem cell exosomes as a new strategy for treating diabetic wounds. Mater Today Bio. 2025;32:101810
156. Villarreal-Leal RA, Healey GD, Corradetti B. Biomimetic immunomodulation strategies for effective tissue repair and restoration. Adv Drug Deliv Rev. 2021;179:113913
157. Xu X, Xu L, Wen C, Xia J, Zhang Y, Liang Y. Programming assembly of biomimetic exosomes: An emerging theranostic nanomedicine platform. Mater Today Bio. 2023;22:100760
158. Yu Y, Jin H, Li L, Zhang X, Zheng C, Gao X. et al. An injectable, activated neutrophil-derived exosome mimetics/extracellular matrix hybrid hydrogel with antibacterial activity and wound healing promotion effect for diabetic wound therapy. J Nanobiotechnology. 2023;21:308
159. Zhu J, Liu Z, Wang L, Jin Q, Zhao Y, Du A. et al. Exosome Mimetics-Loaded Hydrogel Accelerates Wound Repair by Transferring Functional Mitochondrial Proteins. Front Bioeng Biotechnol. 2022;10:866505
160. Li W, Zhang H, Chen L, Huang C, Jiang Z, Zhou H. et al. Cell membrane-derived nanovesicles as extracellular vesicle-mimetics in wound healing. Mater Today Bio. 2025;31:101595
161. Zhao C, Song W, Ma J, Wang N. Macrophage-derived hybrid exosome-mimic nanovesicles loaded with black phosphorus for multimodal rheumatoid arthritis therapy. Biomater Sci. 2022;10:6731-9
162. Liu J, Qiu X, Lv Y, Zheng C, Dong Y, Dou G. et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res Ther. 2020;11:507
163. Liu H, Liu S, Qiu X, Yang X, Bao L, Pu F. et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy. 2020;16:2140-55
164. Fu J, Wu H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu Rev Immunol. 2023;41:301-16
165. Teh HX, Phang SJ, Looi ML, Kuppusamy UR, Arumugam B. Molecular pathways of NF-ĸB and NLRP3 inflammasome as potential targets in the treatment of inflammation in diabetic wounds: A review. Life Sci. 2023;334:122228
166. Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63:1103-14
167. Wang Y, Jing L, Lei X, Ma Z, Li B, Shi Y. et al. Umbilical cord mesenchymal stem cell-derived apoptotic extracellular vesicles ameliorate cutaneous wound healing in type 2 diabetic mice via macrophage pyroptosis inhibition. Stem Cell Res Ther. 2023;14:257
168. Lou R, Chen J, Zhou F, Wang C, Leung C-H, Lin L. Exosome-cargoed microRNAs: Potential therapeutic molecules for diabetic wound healing. Drug Discov Today. 2022;27:103323
169. Barakat M, DiPietro LA, Chen L. Limited Treatment Options for Diabetic Wounds: Barriers to Clinical Translation Despite Therapeutic Success in Murine Models. Adv Wound Care. 2021;10:436-60
170. Ban E, Jeong S, Park M, Kwon H, Park J, Song EJ. et al. Accelerated wound healing in diabetic mice by miRNA-497 and its anti-inflammatory activity. Biomedecine Pharmacother. 2020;121:109613
171. Yan C, Chen J, Wang C, Yuan M, Kang Y, Wu Z. et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022;29:214-28
172. Rui S, Dai L, Zhang X, He M, Xu F, Wu W. et al. Exosomal miRNA-26b-5p from PRP suppresses NETs by targeting MMP-8 to promote diabetic wound healing. J Control Release. 2024;372:221-33
173. Wu J, Wu Y, Tang H, Li W, Zhao Z, Shi X. et al. Self-Adapting Biomass Hydrogel Embodied with miRNA Immunoregulation and Long-Term Bacterial Eradiation for Synergistic Chronic Wound Therapy. ACS Nano. 2024;18:18379-92
174. Xu Y, Yu T, He L, Ouyang L, Qu Y, Zhou J. et al. Inhibition of miRNA-152-3p enhances diabetic wound repair via upregulation of PTEN. Aging. 2020;12:14978-89
175. Su J, Wei Q, Ma K, Wang Y, Hu W, Meng H. et al. P-MSC-derived extracellular vesicles facilitate diabetic wound healing via miR-145-5p/ CDKN1A-mediated functional improvements of high glucose-induced senescent fibroblasts. Burns Trauma. 2023;11:tkad010
176. Li H, Jing S, Xu H. Effect and mechanism of microRNAs on various diabetic wound local cells. J Diabetes. 2023;15:955-67
177. Tang Y-B, Uwimana MMP, Zhu S-Q, Zhang L-X, Wu Q, Liang Z-X. Non-coding RNAs: Role in diabetic foot and wound healing. World J Diabetes. 2022;13:1001-13
178. Liu Y, Sun L, Li Y, Holmes C. Mesenchymal stromal/stem cell tissue source and in vitro expansion impact extracellular vesicle protein and miRNA compositions as well as angiogenic and immunomodulatory capacities. J Extracell Vesicles. 2024;13:e12472
179. Li R, Li D, Wang H, Chen K, Wang S, Xu J. et al. Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF. Stem Cell Res Ther. 2022;13:149
180. Li R, Zhao K, Ruan Q, Meng C, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. 2020;22:75
181. Yin D, Shen G. Exosomes from adipose-derived stem cells regulate macrophage polarization and accelerate diabetic wound healing via the circ-Rps5/miR-124-3p axis. Immun Inflamm Dis. 2024;12:e1274
182. Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z. et al. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814-26
183. Li B, Qian L, Pi L, Meng X. A therapeutic role of exosomal lncRNA H19 from adipose mesenchymal stem cells in cutaneous wound healing by triggering macrophage M2 polarization. Cytokine. 2023;165:156175
184. Yang H, Tu Z, Yang D, Hu M, Zhou L, Li Q. et al. Exosomes from hypoxic pre-treated ADSCs attenuate acute ischemic stroke-induced brain injury via delivery of circ-Rps5 and promote M2 microglia/macrophage polarization. Neurosci Lett. 2022;769:136389
185. Tang W, Du X, Wu Z, Nie Z, Yu C, Gao Y. circ-Erbb2ip from adipose-derived mesenchymal stem cell-derived exosomes promotes wound healing in diabetic mice by inducing the miR-670-5p/Nrf1 axis. Cell Signal. 2024;121:111245
186. J P, Jh L, Bs Y, Ek J, G L, Iy K. et al. Additive effect of bFGF and selenium on expansion and paracrine action of human amniotic fluid-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):293
187. Heo JS. Selenium-Stimulated Exosomes Enhance Wound Healing by Modulating Inflammation and Angiogenesis. Int J Mol Sci. 2022;23:11543
188. Zheng T, Li S, Zhang T, Fu W, Liu S, He Y. et al. Exosome-shuttled miR-150-5p from LPS-preconditioned mesenchymal stem cells down-regulate PI3K/Akt/mTOR pathway via Irs1 to enhance M2 macrophage polarization and confer protection against sepsis. Front Immunol. 2024;15:1397722
189. Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J. et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308
190. Zheng L, Song H, Li Y, Li H, Lin G, Cai Z. Insulin-Induced Gene 1-Enhance Secretion of BMSC Exosome Enriched in miR-132-3p Promoting Wound Healing in Diabetic Mice. Mol Pharm. 2024;21:4372-85
191. Yang K, Li D, Wang M, Xu Z, Chen X, Liu Q. et al. Exposure to blue light stimulates the proangiogenic capability of exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2019;10:358
192. Li Y, Song W, Kong L, He Y, Li H. Injectable and Microporous Microgel-Fiber Granular Hydrogel Loaded with Bioglass and siRNA for Promoting Diabetic Wound Healing. Small. 2024;20:e2309599
193. Chandra PK, Ross CL, Smith LC, Jeong SS, Kim J, Yoo JJ. et al. Peroxide-based oxygen generating topical wound dressing for enhancing healing of dermal wounds. Wound Repair Regen. 2015;23:830-41
194. Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;2015:CD004123
195. Guan Y, Niu H, Liu Z, Dang Y, Shen J, Zayed M. et al. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv. 2021;7:eabj0153
196. Han X, Saengow C, Ju L, Ren W, Ewoldt RH, Irudayaraj J. Exosome-coated oxygen nanobubble-laden hydrogel augments intracellular delivery of exosomes for enhanced wound healing. Nat Commun. 2024;15:3435
197. Ren W, Sands M, Han X, Tsipursky M, Irudayaraj J. Hydrogel-Based Oxygen and Drug Delivery Dressing for Improved Wound Healing. Acs Omega. 2024;9:24095-104
198. Jiang Y, Wu X, Wang X, Kong B, Wang J, Zhang H. Living hydrogel with blood derived elements for wound healing. Mater Today Bio. 2025;33:102002
199. Xie X-T, Gao C-H, Tan L-F, Chen L-X, Fan J-X, Xiong W. et al. Gene-engineered polypeptide hydrogels with on-demand oxygenation and ECM-cell interaction mimicry for diabetic wound healing. Biomaterials. 2025;316:122984
200. Jiang S, Zheng Y, Xia H, Liu Z, Rao S, Wang Y. et al. Oxygen-Releasing Hydrogels for Tissue Regeneration. Adv Nanobiomed Res. 2024 4
201. Li N, Lu X, Yang Y, Ning S, Tian Y, Zhou M. et al. Calcium Peroxide-Based Hydrogel Patch with Sustainable Oxygenation for Diabetic Wound Healing. Adv Healthc Mater. 2024;13:e2303314
202. Tu C, Lu H, Zhou T, Zhang W, Deng L, Cao W. et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286:121597
203. Zhao Y, Wang D, Qian T, Zhang J, Li Z, Gong Q. et al. Biomimetic Nanozyme-Decorated Hydrogels with H2O2-Activated Oxygenation for Modulating Immune Microenvironment in Diabetic Wound. ACS Nano. 2023;17:16854-69
204. Li Z, Zhao Y, Huang H, Zhang C, Liu H, Wang Z. et al. A Nanozyme-Immobilized Hydrogel with Endogenous ROS-Scavenging and Oxygen Generation Abilities for Significantly Promoting Oxidative Diabetic Wound Healing. Adv Healthc Mater. 2022;11:e2201524
205. Tolouei AE, Dülger N, Ghatee R, Kennedy S. A Magnetically Responsive Biomaterial System for Flexibly Regulating the Duration between Pro- and Anti-Inflammatory Cytokine Deliveries. Adv Healthc Mater. 2018;7:e1800227
206. Yoon DS, Lee Y, Ryu HA, Jang Y, Lee K-M, Choi Y. et al. Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater. 2016;38:59-68
207. Schirmer L, Atallah P, Werner C, Freudenberg U. StarPEG-Heparin Hydrogels to Protect and Sustainably Deliver IL-4. Adv Healthc Mater. 2016;5:3157-64
208. Kuan C-H, Chang L, Ho C-Y, Tsai C-H, Liu Y-C, Huang W-Y. et al. Immunomodulatory hydrogel orchestrates pro-regenerative response of macrophages and angiogenesis for chronic wound healing. Biomaterials. 2025;314:122848
209. Wang Z, Li W, Gou L, Zhou Y, Peng G, Zhang J. et al. Biodegradable and Antioxidant DNA Hydrogel as a Cytokine Delivery System for Diabetic Wound Healing. Adv Healthc Mater. 2022;11:e2200782
210. Li W, Xie H, Gou L, Zhou Y, Wang H, Li R. et al. DNA-Based Hydrogels with Multidrug Sequential Release for Promoting Diabetic Wound Regeneration. JACS Au. 2023;3:2597-608
211. Lohmann N, Schirmer L, Atallah P, Wandel E, Ferrer RA, Werner C. et al. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci Transl Med. 2017;9:eaai9044
212. Emiroglu DB, Singh A, Marco-Dufort B, Speck N, Rivano PG, Oakey JS. et al. Granular Biomaterials as Bioactive Sponges for the Sequestration and Release of Signaling Molecules. Adv Healthc Mater. 2024;13:e2400800
213. Shu F, Huang H, Xiao S, Xia Z, Zheng Y. Netrin-1 co-cross-linked hydrogel accelerates diabetic wound healing in situ by modulating macrophage heterogeneity and promoting angiogenesis. Bioact Mater. 2024;39:302-16
214. Deng Q-S, Gao Y, Rui B-Y, Li X-R, Liu P-L, Han Z-Y. et al. Double-network hydrogel enhanced by SS31-loaded mesoporous polydopamine nanoparticles: Symphonic collaboration of near-infrared photothermal antibacterial effect and mitochondrial maintenance for full-thickness wound healing in diabetes mellitus. Bioact Mater. 2023;27:409-28
215. Kim J, Bong KW, Cho J-K, Song S-C. Thermo-responsive hydrogel via sustained Co-delivery of TA and PDGF to modulate the diabetic microenvironment and accelerate diabetic wound healing. J Mater Chem B. 2025;13:7090-105
216. Zhang Y, Lin S, Xu X, Yao Y, Feng S, Jiang S. et al. Programmable hierarchical hydrogel dressing for sequential release of growth factor and DNase to accelerate diabetic wound healing. J Control Release. 2025;383:113825
217. Hartmeier PR, Pham NB, Velankar KY, Issa F, Giannoukakis N, Meng WS. Hydrogel Dressings for Chronic Wound Healing in Diabetes: Beyond Hydration. J Pharm Drug Deliv Res. 2021;10:1000197
218. Pang T, Shao Y, Zhou L, Wang Z, Xi P, Zhang Y. et al. rhaFGF promotes acute diabetic wound healing by suppressing chronicity of inflammation. Sci Rep. 2025;15:19085
219. Han X, Liao R, Li X, Zhang C, Huo S, Qin L. et al. Mesenchymal stem cells in treating human diseases: molecular mechanisms and clinical studies. Signal Transduct Target Ther. 2025;10:262
220. Zhou X, Ye C, Jiang L, Zhu X, Zhou F, Xia M. et al. The bone mesenchymal stem cell-derived exosomal miR-146a-5p promotes diabetic wound healing in mice via macrophage M1/M2 polarization. Mol Cell Endocrinol. 2024;579:112089
221. Zhang B, Bi Y, Wang K, Guo X, Liu Z, Li J. et al. Stem Cell-Derived Extracellular Vesicles: Promising Therapeutic Opportunities for Diabetic Wound Healing. Int J Nanomedicine. 2024;19:4357-75
222. Zhu Y, Yang H, Xue Z, Tang H, Chen X, Liao Y. Mesenchymal stem cells-derived small extracellular vesicles and apoptotic extracellular vesicles for wound healing and skin regeneration: a systematic review and meta-analysis of preclinical studies. J Transl Med. 2025;23:364
223. Tan JL, Lash B, Karami R, Nayer B, Lu Y-Z, Piotto C. et al. Restoration of the healing microenvironment in diabetic wounds with matrix-binding IL-1 receptor antagonist. Commun Biol. 2021;4:422
224. Zhang Y, Wang R, Li Y, Zhu W, Liu Y, Zhang Z. et al. Hyperglycemia-Reduced Platelet-Derived Growth Factor-BB Expression Impairs Corneal Wound Healing in Diabetic Mice. Invest Ophthalmol Vis Sci. 2025;66:61
225. Mamidi N, Mahmoudsalehi AO. Advances in three-dimensional hydrogel networks for cancer immunotherapy. J Mater Chem B. 2025;13:10440-59
226. Clegg JR, Adebowale K, Zhao Z, Mitragotri S. Hydrogels in the clinic: An update. Bioeng Transl Med. 2024 9
227. Segneanu A-E, Bejenaru LE, Bejenaru C, Blendea A, Mogoşanu GD, Biţă A. et al. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers. 2025;17:2026
228. Mohammadzadeh V, Atapour-Mashhad H, Shahvali S, Salehi B, Shaban M, Shirzad M. et al. Hydrogels as advanced drug delivery platforms for cancer immunotherapy: promising innovations and future outlook. J Nanobiotechnology. 2025;23:545
229. Yang Y, Ren Y, Song W, Yu B, Liu H. Rational design in functional hydrogels towards biotherapeutics. Mater Des. 2022;223:111086
230. Del Rio EP, Rey-Vinolas S, Santos F, Castellote-Borrell M, Merlina F, Veciana J. et al. 3D Printing as a Strategy to Scale-Up Biohybrid Hydrogels for T Cell Manufacture. Acs Appl Mater Interfaces. 2024;16:50139-46
231. Yu B, Li H, Zhang Z, Chen P, Wang L, Fan X. et al. Extracellular vesicles engineering by silicates-activated endothelial progenitor cells for myocardial infarction treatment in male mice. Nat Commun. 2023;14:2094
232. Yang C, Xue Y, Duan Y, Mao C, Wan M. Extracellular vesicles and their engineering strategies, delivery systems, and biomedical applications. J Control Release. 2024;365:1089-123
233. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748-59
234. Vo N, Tran C, Tran NHB, Nguyen NT, Nguyen T, Ho DTK. et al. A novel multi-stage enrichment workflow and comprehensive characterization for HEK293F-derived extracellular vesicles. J Extracell Vesicles. 2024;13:e12454
235. Grange C, Bussolati B. Extracellular vesicles in kidney disease. Nat Rev Nephrol. 2022;18:499-513
236. Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B. et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404
237. Zhang Q, Gong H, Gao W, Zhang L. Recent Progress in Capturing and Neutralizing Inflammatory Cytokines. CCS Chem. 2020;2:376-89
238. Kenny HM, Reynolds CM, Garcia-Vaquero M, Feeney EL. Keeping an eye on alginate: innovations and opportunities for sustainable production and diverse applications. Carbohydr Polym. 2025;366:123902
239. Kasravi M, Ahmadi A, Babajani A, Mazloomnejad R, Hatamnejad MR, Shariatzadeh S. et al. Immunogenicity of decellularized extracellular matrix scaffolds: a bottleneck in tissue engineering and regenerative medicine. Biomater Res. 2023;27:10
240. Reay SL, Marina Ferreira A, Hilkens CMU, Novakovic K. The Paradoxical Immunomodulatory Effects of Chitosan in Biomedicine. Polymers. 2025;17:19
241. Zuber J, Cascabulho PL, Piperni SG, Farias Correa do Amaral RJ, Vogt C, Carre V. et al. Fast, Easy, and Reproducible Fingerprint Methods for Endotoxin Characterization in Nanocellulose and Alginate-Based Hydrogel Scaffolds. Biomacromolecules. 2024;25:6762-72
242. Lu P, Ruan D, Huang M, Tian M, Zhu K, Gan Z. et al. Harnessing the potential of hydrogels for advanced therapeutic applications: current achievements and future directions. Signal Transduct Target Ther. 2024;9:166
243. Burtscher B, Diacci C, Makhinia A, Savvakis M, Gabrielsson EO, Veith L. et al. Functionalization of PEDOT:PSS for aptamer-based sensing of IL6 using organic electrochemical transistors. NPJ Biosensing. 2024;1:7
244. Lin C, Hu Y, Lin Z, Du L, Hu Y, Ouyang L. et al. MMP-9 responsive hydrogel promotes diabetic wound healing by suppressing ferroptosis of endothelial cells. Bioact Mater. 2025;43:240-54
245. Liu Z, Song H, Lin G, Zhong W, Zhang Y, Yang A. et al. Wireless Intelligent Patch for Closed-loop In Situ Wound Management. Adv Sci. 2024;11:e2400451
246. Turki AF, Alrafiah AR. A Bioelectrically Enabled Smart Bandage for Accelerated Wound Healing and Predictive Monitoring. Med-Lith. 2025;61:965
Author contact
![]() Corresponding authors: Bobin Mi, Email: mibobinedu.cn; Yiqiang Hu, Email: huyiqiangedu.cn; Guohui Liu, Email: liuguohuiedu.cn.
Corresponding authors: Bobin Mi, Email: mibobinedu.cn; Yiqiang Hu, Email: huyiqiangedu.cn; Guohui Liu, Email: liuguohuiedu.cn.
 Global reach, higher impact
Global reach, higher impact