13.3
Impact Factor
Theranostics 2026; 16(1):545-579. doi:10.7150/thno.117143 This issue Cite
Review
Engineering exosomes for targeted neurodegenerative therapy: innovations in biogenesis, drug loading, and clinical translation
1. School of Electronic and Electrical Engineering, Wuhan Textile University, Wuhan 430200, China.
2. Department of Plastic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China.
3. Institute of Chemical Biology, Shenzhen Bay Laboratory, Shenzhen 518132, China.
4. Department of Biomedical Engineering, Columbia University, New York 10027, USA.
5. Neuroregeneration and Stem Cell Programs, Institute for Cell Engineering, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
6. Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
7. Adrienne Helis Malvin Medical Research Foundation, New Orleans, LA 70130-2685, USA.
8. Institute for NanoBioTechnology, Johns Hopkins University, Baltimore, MD 21205, USA.
9. Department of Materials Science and Engineering, Johns Hopkins University, Baltimore, MD 21205, USA.
#Authors contributed equally.
Received 2025-5-8; Accepted 2025-6-10; Published 2026-1-1
Abstract
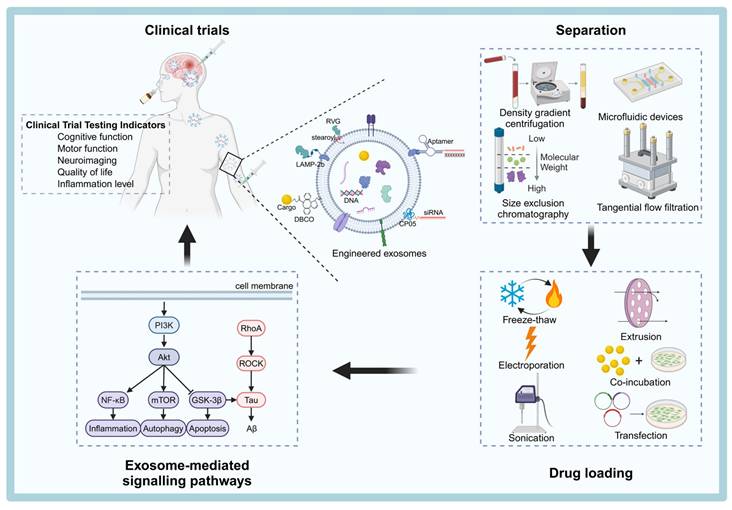
Neurodegenerative diseases (NDDs), including Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), Huntington's disease (HD) and multiple sclerosis (MS), are characterized by progressive neuronal dysfunction and limited therapeutic options, largely due to the restrictive nature of the blood-brain barrier (BBB). Exosomes, naturally occurring extracellular vesicles (EVs), have gained attention as innovative drug delivery vehicles owing to their intrinsic ability to cross the BBB, minimal immunogenicity, high biocompatibility, and capability to carry diverse therapeutic cargos such as proteins, nucleic acids, and small molecules. Furthermore, exosomes can be bioengineered to enhance drug-loading efficiency and targeting specificity, positioning them as a versatile and effective platform for treating NDDs. In this review, we summarize recent advances in exosome biogenesis, secretion, and engineering, with an emphasis on innovative strategies for exosome isolation, drug loading, and surface modification. We further explore their roles in modulating neuroinflammation, promoting neural regeneration, and enabling precise therapeutic delivery. Critical challenges associated with large-scale production, quality control, and regulatory compliance under Good Manufacturing Practices (GMP) are also discussed. Collectively, these developments underscore the transformative potential of engineered exosomes in advancing precision therapies for neurodegenerative disorders and offer strategic insights into their clinical translation.
Keywords: neurodegenerative diseases (NDDs), exosomes, blood-brain barrier (BBB), drug delivery, targeted therapy, clinical trials
Introduction
With advancements in medical technology and improvements in living standards, population aging has become an irreversible global trend, and neurodegenerative diseases (NDDs) have emerged as a significant medical challenging in modern society. According to recent research published in The Lancet Neurology, by 2021, neurological disorders affected more than one-third of the global population and have become the leading cause of illness and disability [1]. NDDs encompass a range of disorders characterized by progressive loss of neuronal structure and function in the central or peripheral nervous system, including Alzheimer's disease (AD) [2], Parkinson's disease (PD) [3], amyotrophic lateral sclerosis (ALS) [4,5], Huntington's disease (HD) [6], and multiple sclerosis (MS) [7]. These conditions are often accompanied by cognitive impairment or motor dysfunction, and the damage is frequently irreversible due to the limited regenerative capacity of the nervous system [8,9]. However, the underlying molecular and cellular mechanisms remain incompletely understood. Pathological hallmarks, including abnormal protein aggregation, mitochondrial dysfunction, neuroinflammation, and oxidative stress, provide insights into disease etiology and are central to the development of targeted therapeutic strategies [8].
However, most potential therapeutic drugs face difficulties in crossing the blood-brain barrier (BBB), and even when they do manage to cross, they often fail to reach therapeutic concentrations in the brain [10]. Clinically, this has contributed to the lack of effective treatments for NDDs, making the development of novel delivery strategies essential. The advancement of nanotechnology has led to the development of various drug delivery systems, such as liposomes [11], solid lipid nanoparticles [12], and polymer nanoparticles [13], designed to facilitate intracerebral drug delivery. However, these approaches still face considerable limitations, including difficulties in large-scale production, rapid clearance by the body's mononuclear phagocyte system, and potential toxic side effects [14,15]. This highlights the need for alternative delivery vehicles that can overcome these challenges. In this regard, natural nanovesicles, particularly exosomes, have emerged as highly promising drug carriers, providing several advantages over traditional nanoparticle systems (Table 1).
Exosomes, which range from ~40-160 nm in diameter, are naturally occurring vesicles derived from the endosomal system. These vesicles begin as early-sorting endosome formed by the invagination of the cytoplasmic membrane, which further undergo outgrowths to form multivesicular bodies (MVBs) [24-26]. MVBs then fuse with the cytoplasmic membrane to release exosomes into the extracellular space, making this a critical step in intercellular communication. The distinct biogenesis process and cargo-delivery capabilities of exosomes have increasingly positioned them as promising drug carriers in various biomedical applications.
Comparison of the performance of different nanomaterials in drug delivery
| Nanomaterials | Exosomes | Liposomes | Metal nanoparticles | Polymeric nanoparticles | Ref. | |
|---|---|---|---|---|---|---|
| Characterization | ||||||
| Biocompatibility | High biocompatibility, excellent biotolerance | Generally good, dependent on lipid composition | Lower, potential toxicity from metal ions | High, can be improved by modifying the polymer | [16] | |
| Targeting ability | Excellent targeting ability, which can be further enhanced through surface modification technologies, allowing for precise drug delivery | Moderate, can be improved through surface modifications | Moderate, requires surface modifications to enhance targeting | Good, targeting capability can be improved through surface functionalization | [17] | |
| Stability | Moderate stability, susceptible to environmental factors (e.g., pH, temperature); however, some exosomes are optimized to withstand changes in these conditions effectively | Easily affected by environmental factors (e.g., temperature, pH), especially during prolonged storage or transport, leading to rupture or leakage and reduced drug efficacy | Good, but may have issues with dissolution or oxidation | High stability, adjustable as needed, but may degrade or lose function during prolonged storage, making it unsuitable for complex drug delivery systems in brain tissue | [18-20] | |
| Drug loading capacity | High, can load a variety of drugs (proteins, RNA, etc.) | Moderate, suitable for encapsulating both water-soluble and lipophilic drugs | High, capable of loading various drug molecules | Relatively high, suitable for loading small molecules and biomacromolecules | [16] | |
| Drug release control | Adjustable, biodegradable, can achieve sustained release, can also be combined with hydrogels for controlled release | Controllable, external stimuli (e.g., pH, temperature) can be used to control release | Limited, typically not suitable for controlled release | Adjustable, especially suitable for long-term release systems | [18] | |
| Immunogenicity | Low immunogenicity, enabling better immune system evasion and ensuring the safety of long-term use | Lipid bilayer helps immune evasion | Metal nanoparticles are highly immunogenic and may provoke an excessive immune response. Their accumulation in the body can lead to organ damage, which makes clinical translation challenging | Potential for immune response, especially when polymer materials are large | [19] | |
| Production and purification difficulty | High, extraction and purification are complex | Low, production technology is mature but depends on lipid stability | Low, production is simple, but purity needs to be monitored | Moderate, synthesis and purification technologies are constantly evolving | [21] | |
| Membrane penetration ability | Excellent, able to cross the BBB, cell membranes, etc | Moderate, can be enhanced by modifications | The BBB is poorly penetrated, limiting its effectiveness in the treatment of NDDs | Moderate, can be improved by special modifications, but this challenge is often difficult to overcome in clinical translation | [22] | |
| Application advantages and scope | Suitable for the delivery of RNA, proteins and other biomolecules with natural targeting ability, which can be widely used in a variety of NDDs | Suitable for small molecule drugs, widely used in vaccine delivery, gene therapy | Suitable for metal ion transport and special treatments, currently used in antimicrobial, cancer therapy, imaging applications | Can design different carrier systems for a variety of drugs, mainly for tumor therapy and gene therapy applications | [23] |
Schematic overview of the process of engineering exosomes for therapeutic applications of NDDs. Natural exosomes are first isolated from body fluids, and then they are engineered to carry specific cargoes or express ligands on their surfaces that can be clinically therapeutic by targeting pathways. The figure was created with BioRender.
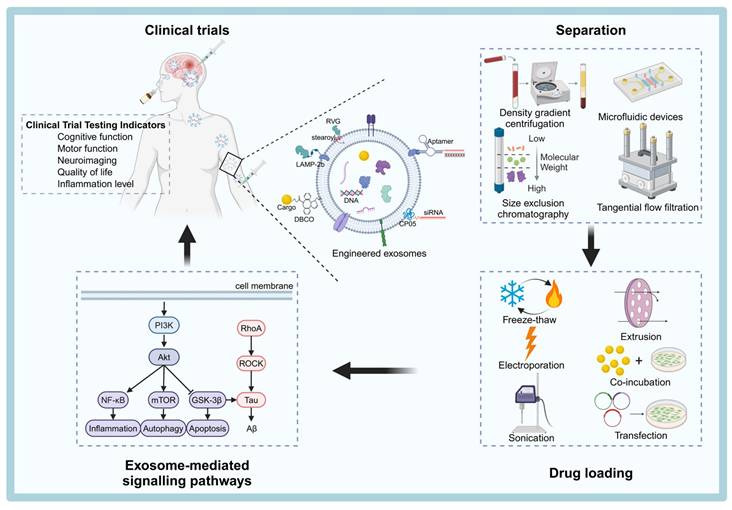
As drug carriers, exosomes offer several advantages over conventional delivery systems. Their low immunogenicity, toxicity, high biocompatibility, and stability make them ideal for treating chronic conditions like NDDs, where long-term, targeted drug delivery is crucial. Moreover, exosomes can be produced on a large scale, addressing a key challenge in traditional drug delivery. Their natural ability to target specific tissues, including the capacity to cross biological barriers such as the BBB, makes them especially promising for treating NDDs, where efficient drug delivery to the brain remains a significant challenge [26-29]. Additionally, exosomes can be engineered to carry a variety of therapeutic payloads—ranging from proteins and nucleic acids to gene-editing tools, adeno-associated viruses, and small molecule drugs—making them versatile platforms for diverse biomedical applications [26,30].
The unique structural properties of exosomes, along with their efficient secretion mechanisms, enable them to encapsulate a broad range of bioactive molecules, which can be delivered precisely to targeted sites within the nervous system. Exosomes are involved in several critical processes that regulate nervous system function. They can promote neuronal growth, differentiation, and remodeling, as well as inhibit neuroinflammation and undesired immune responses. Furthermore, exosomes contribute to the enhancement of the vascular network in the nervous system and can modulate the integrity and permeability of the BBB—a key consideration in the treatment of NDDs, where crossing the BBB is often a major obstacle [31-34]. This ability to deliver bioactive molecules—either naturally present or therapeutically loaded—enables exosomes to regulate gene expression and related signaling pathways, thereby influencing neuronal structure and function in ways that support the repair or regeneration of neural tissue [31].
This review aims to provide a comprehensive summary of the latest advances in exosome biogenesis, secretion, isolation techniques, surface modification strategies, and drug-loading techniques. Additionally, we will explore the signaling pathways targeted by exosomes in the treatment of NDDs and provide an overview of the status of exosome-based therapies. Finally, we will discuss the challenges that remain in translating exosome technologies from the laboratory to clinical applications and propose potential solutions to overcome these hurdles (Figure 1).
Exosome Biogenesis, Sources, and Therapeutic Potential in NDDs
Recent Advances in Exosome Biogenesis and Secretion Mechanisms
Exosomes are small vesicles secreted by cells that play crucial roles in intercellular signaling. They are involved in various physiological and pathological processes, such as immune response modulation, tumor progression, and the pathogenesis of NDDs. In recent years, exosomes have gained significant attention due to their potential in disease prevention, diagnosis, treatment, and prognostic assessment. A deeper understanding of the biogenesis and secretion mechanisms of exosomes, as well as the regulation of their key molecular targets, not only provides new therapeutic strategies but also lays the foundation for optimizing exosomes for clinical applications, especially in the context of NDDs. In the following, we provide an updated overview of recent advancements in the biogenesis and secretion mechanisms of exosomes, highlighting key molecular players and novel discoveries that contribute to the regulation of exosome release.
The Rab GTPase family is a critical regulator of exosome secretion. Rab GTPases influence exosome release by modulating the transport of MVBs or their docking with the plasma membrane of target cells [35]. Among the various Rab GTPases, Rab27a has been extensively studied and shown to be crucial in the fusion of MVBs with the plasma membrane, facilitating the release of exosomes [36]. Recently, Song et al. [37] revealed that knockdown of Kidney Brain Expressed Protein (KIBRA) in neuronal and podocyte cell lines led to reduced exosome secretion, despite an increase in the size and number of intracellular MVBs. Additionally, Rab27a expression was significantly diminished in KIBRA knockout mice. These findings suggest that KIBRA may facilitate exosome membrane trafficking by binding to Rab27a and regulating its degradation, thus playing a key role in exosome secretion. This mechanism could play an important role in the pathogenesis and progression of NDDs by affecting the release of neurodegeneration-associated molecules.
While the role of Rab GTPases in exosome secretion is well-established, the molecular mechanisms governing MVBs transport to the plasma membrane for exosome secretion, as opposed to lysosomal degradation, remain unclear. This gap in knowledge has sparked interest in other protein complexes involved in this process.
The Exocyst complex, a conserved octameric protein complex localized at the dynamic regions of the plasma membrane, has been implicated in exosome transport. It facilitates membrane fusion through the activation of soluble N-ethylmaleimide-sensitive factor attachment proteins [38]. Liu et al. [39] discovered that the conversion of phosphatidylinositol-3-phosphate to phosphatidylinositol-4-phosphate on MVBs is necessary for the recruitment of the Exocyst complex, which in turn promotes the outward transport and exocytosis of MVBs. Moreover, Lee et al. [40] provided further evidence that the Exocyst complex plays a critical chaperone role during SNARE complex assembly and membrane fusion, thereby regulating cytokinesis at multiple stages. These findings suggest that the Exocyst complex acts as a chaperone to facilitate the fusion of MVBs with the plasma membrane, an essential step in exosome release.
The process of exosome biogenesis is intricately linked to endocytosis, as exosomal proteins are often delivered to early endosomes via endocytic pathways. These proteins are then incorporated into nascent intraluminal vesicles (ILVs) within MVBs, which later fuse with the plasma membrane to release exosomes [24,41]. However, recent studies have shown that endocytosis can play a dual role in exosome secretion. Gao et al. [42] found that endocytosis strongly inhibited the secretion of exosomal marker proteins such as CD81, CD9, and CD63 and triggered their degradation in the presence of CD81 and CD9. The study also revealed that syntenin proteins promote the vesicular secretion of CD63 by blocking its endocytosis, shedding light on the complex relationship between endocytosis and the secretion of exosomal marker proteins. This finding introduces a new layer of complexity in our understanding of how exosomal marker proteins are regulated.
In addition, recent studies have highlighted the close relationship between exosome formation and the autophagic process, though the exact mechanisms remain incompletely understood [43]. Yanagawa et al. [44] conducted RNA interference screening of autophagy-related factors and found that Rubicon, a cysteine-rich domain-containing protein, positively regulates exosome biogenesis in an autophagy-independent manner. Further investigation showed that Rubicon regulates the activation of the endosomal sorting complexes required for transport through the Rubicon-WIPI axis, thereby precisely controlling exosome production and secretion. This discovery provides new insights into the molecular mechanisms of exosome biosynthesis and could offer novel strategies for utilizing exosomes in disease therapy.
In summary, the mechanisms underlying exosome production and secretion involve multiple key molecular and cellular processes, including Rab GTPases, the Exocyst complex, and the interplay between endocytosis and autophagy. These studies not only enhance our understanding of exosomes in intercellular communication but also open new avenues for their application in disease diagnosis and treatment. By fine-tuning the regulation of exosome biogenesis, particularly the secretion of membrane proteins, we can pave the way for breakthrough therapies in various diseases, including cancer and NDDs. The continued exploration of exosome biology will likely lead to novel strategies for using these vesicles in precision medicine and targeted drug delivery.
Exosome Sources and Their Therapeutic Specificity in NDDs
Exosomes, as nanosized EVs secreted by nearly all cell types, serve as pivotal mediators of intercellular communication by transferring diverse biological cargoes including proteins, RNAs, and lipids. In the context of NDDs, exosomes modulate key pathological processes such as neuroinflammation, neuronal survival, synaptic plasticity, and tissue repair. Consequently, therapeutic exosomes have garnered significant interest as innovative drug delivery systems and immunomodulatory agents.
Crucially, the therapeutic potential of exosomes is inherently linked to their cellular origin. Different cell types impart distinct molecular cargos, surface markers, and functional properties to their secreted exosomes. These differences not only determine the exosomes' ability to target specific cell types or tissues, but also influence their immunogenicity, safety profile, and regenerative potential. Understanding how the source of exosomes shapes their characteristics is essential for tailoring effective treatments in NDDs (Table 2).
Mesenchymal Stem Cells (MSCs)
MSCs are one of the most researched and clinically used sources of therapeutic exosomes. They can be isolated from various tissues such as umbilical cord, dental pulp, adipose tissue, and bone marrow, and are widely used in the treatment of NDDs [54] (Figure 2). MSCs-derived exosomes (MSC-Exos) are enriched with active substances such as trophic factors, anti-inflammatory cytokines (e.g., interleukin (IL)-10, transforming growth factor (TGF)-β), and regulatory miRNAs (e.g., miR-21, miR-146a), which can exert neuroprotective, immunomodulatory, and pro-angiogenic effects [55]. For instance, injection of bone marrow MSC exosomes (BMSC-Exos) into the lateral ventricle of mice significantly modulated hippocampal neuroinflammation, increased the expression of brain-derived neurotrophic factor (BDNF), improved synaptic plasticity and reduced the expression of neuroinflammatory plaques and abnormal expression of phosphorylated tau (P-Tau) [45].
Sources of exosomes
| Exosome source | Advantages | Disadvantages | Main fields of application | Ref. |
|---|---|---|---|---|
| Mesenchymal stem cells (MSCs) | Highly biocompatible; low immunogenicity; carries a variety of neuroprotective factors that can reduce neuroinflammation and promote neurogenesis; surface modifications can further improve targeting and drug-carrying capacity | Higher production costs, more challenging to control exosome purity | Used in tissue repair and regeneration, treatment of neurodegenerative diseases (e.g., AD, PD, ALS), spinal cord injury, immune modulation, cardiovascular diseases, and lung disorders, and have been extensively applied in clinical trials for NDDs | [45-47] |
| Neural stem cells (NSCs)/neurons | Naturally target the nervous system, high biological barrier penetration, low immunogenicity | Difficult to produce on a large scale, limited source of cells, long purification time, unclear dosage and mechanism of action, therefore less used in clinical trials | Mostly used for NDDs, strokes, spinal cord injuries, etc | [48,49] |
| Induced pluripotent stem cells (iPSCs) | These exosomes are capable of differentiating into various neural cell types and are rich in factors that promote cell repair and neuroprotection. With strong neurorepair potential and low immunogenicity, they can be tailored to the patient's genetic background, providing more personalized treatment options and showing considerable clinical translation potential | Technically complex, costly and potentially ethically controversial | Can be used for cardiovascular disease, nervous system, limb disease, liver disease, skin disease, etc | [50,51] |
| Endothelial cells (ECs) | Easy to obtain and culture, activates blood vessel formation | There is a risk of immune response, potential insufficient targeting of the nervous system, and the need for precise isolation and purification, which increases production complexity and costs. Additionally, their limited ability to effectively cross the BBB restricts their potential for brain-targeted therapies, making clinical translation challenging | Used in tissue regeneration, angiogenesis, cellular regeneration, and skin repair, with significant potential in the fields of regenerative medicine and tissue healing | [52,53] |
Sources of MSCs and the potential mechanisms of MSC-Exos therapy for the treatment of NDDs. MSC-Exos help reduce neuroinflammation, promote angiogenesis, enhance axon growth and myelination, support neuronal proliferation and differentiation, and maintain the integrity of the BBB, ultimately alleviating neurodegeneration and related neuropathology. The figure was created with BioRender.
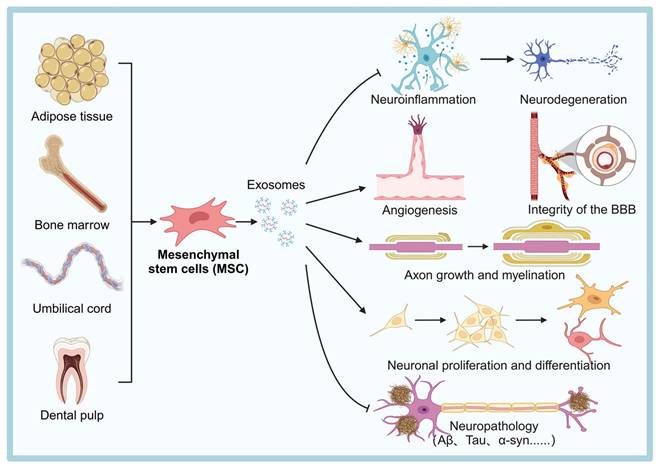
Furthermore, growth differentiation factor-15 secreted by human umbilical cord blood MSCs (hUCB-MSCs) promotes hippocampal neuronal proliferation, differentiation and synaptic activity [56]. In a mouse model of experimental autoimmune encephalomyelitis, γ-interferon (IFNγ)-stimulated MSC-Exos improved motor function, reduced neuroinflammation, and attenuated demyelination injury [57]. Additionally, miR-146a-5p-modified hUCB-MSCs exosomes reduced the toxic effects of neurotoxic astrocytes and promoted the recovery of neurological function in spinal cord injured rats [47]. These studies highlight the potential of MSC-Exos in the treatment of neuroinflammation and nerve injury. Regarding angiogenesis, MSC-Exos promote angiogenesis in human brain microvascular ECs [58]. They enhance ECs proliferation and migration by delivering microRNAs, such as miR-21-5p, which promotes angiogenesis and ameliorates cerebral ischemic injury in an ischemic stroke mouse model [59]. These effects may also help repair BBB damage and restore its function.
In terms of targeting ability, MSC-Exos express surface proteins such as integrins and tetraspanins, which help them to cross the BBB and preferentially homing to sites of injury or inflammation. For example, Perets et al. [60] using tomography with gold nanoparticle-labeled MSC-Exos, found that these exosomes specifically targeted and accumulated in the brain of a mouse model with associated pathological changes, suggesting a significant ability to migrate and home to neurons. MSC-Exos possess low immunogenicity, high biocompatibility, neuroprotective properties, and the ability to be surface-engineered for enhanced targeting. These characteristics collectively provide a strong molecular and biological foundation for their potential clinical applications in NDDs and other diseases. Although MSC-Exos show great therapeutic promise, their clinical translation still faces challenges, mainly including: relatively high production costs, difficulties in achieving consistent purity from batch to batch, and the lack of standardized dosing regimens.
Neural stem cells (NSCs) and neurons
NSCs possess self-renewal capability and can differentiate into neuronal and glial lineages. NSCs-derived exosomes (NSC-Exos) inherit abundant neuroprotective and neuroregenerative non-coding RNAs, proteins, lipids, and other active constituents from the donor cells, and exhibit excellent neuroprotective and neuroregenerative potentials, as well as immunomodulatory capabilities [61]. For example, NSC-Exos have been shown to enhance mitochondrial function, activate sirtuin 1, increase synaptic activity, reduce neuroinflammation, and ameliorate disease progression in a mouse model of AD [49]. Owing to its donor cell properties, NSC-Exos have a high degree of targeting specificity to neuronal cells and is capable of precise interactions with neurons and glial cells. Despite the significant advantages of low immunogenicity and high specificity of NSC-Exos in neurotargeting therapy, their clinical translation still faces major obstacles, such as limited donor cells and difficulties in scale-up production.
Induced Pluripotent Stem Cells (iPSCs)
iPSCs possess unlimited self-renewal capacity and the ability to differentiate into nearly all cell types. iPSCs-derived exosomes (iPSC-Exos) are rich in growth factors, developmental regulators, and miRNAs associated with pluripotency and tissue regeneration, enabling them to alleviate vascular aging, reduce neuroinflammation, and promote nerve regeneration. For example, Niu et al. [62] found that iPSC-EVs could attenuate microglia senescence and promote the shift of microglia polarization from a pro-inflammatory phenotype to an anti-inflammatory phenotype, which protects neurons from death and improves the prognosis of ischemic stroke in the elderly. In terms of targeting, undifferentiated iPSC-Exos exhibit broad tropism, relying mainly on their surface-carried adhesion molecules and integrins, to bind to receptors prevalent at the site of injury. iPSCs can be expanded indefinitely, which overcomes the problems of the limited source of progenitor cells (e.g., MSCs, neurons) and the large inter-batch variability, and provides the possibility of large-scale, standardized production of clinical-grade exosomes. However, their application to clinical practice is constrained by manufacturing complexity, high production costs, and safety issues related to their pluripotent origins. In addition, stringent quality control measures are required to ensure consistency and safety.
Endothelial cells (EC)
ECs play a critical role in vascular regeneration and tissue repair through their proliferative and migratory capabilities, contributing to new blood vessel formation and secretion of growth factors and cytokines (e.g., vascular endothelial growth factor, fibroblast growth factor) [63]. ECs-derived exosomes (EC-Exos) contain bioactive substances such as growth factors, cytokines, and pro-angiogenic microRNAs, which mimic some parental cell functions and exert protective effects on neuronal cells by promoting cell growth, migration, and inhibiting apoptosis. For example, a study by Huang et al. [52] demonstrated that EC-Exos can effectively promote and maintain the repair-related phenotype of Schwann cells, thereby significantly enhancing axonal regeneration, myelin formation, and functional recovery of damaged nerves. Although the easy cultivation of exosomes and their ability to promote vascular repair are their obvious advantages, their limited neural targeting ability and potential immunogenicity may limit their application in direct neurotherapy.
Exosomes from other sources and body fluids
Beyond cell culture-derived exosomes, exosomes isolated from body fluids such as blood, urine, bile, saliva, cerebrospinal fluid (CSF), amniotic fluid, and breast milk offer unique insights and therapeutic opportunities [26]. Studies have shown that exosomes from different body fluids vary in their ability to cross the BBB and their therapeutic potential [64]. For instance, exosomes from saliva and urine have a limited ability to cross the BBB and are generally not used for targeting the brain in drug delivery. However, CSF-derived exosomes typically exhibit higher neurospecificity and can directly reflect the pathological states within the brain, making them more suitable as carriers for delivering therapeutic cargoes to brain tissues in conditions like NDDs, brain tumors, or spinal cord injuries.
Despite their potential, direct isolation of exosomes from CSF is challenging due to the complex and painful procedures involved, which limits their widespread use. However, CSF-derived exosomes can cross the BBB and diffuse through the bloodstream into other body fluids, such as blood and urine. As a result, brain-derived exosomes can be purified from body fluids by targeting specific surface markers. For example, brain-derived exosomes have been extracted using immunoprecipitation, employing the "ExoQuick" commercial exosome precipitation kit combined with anti-human CD171 antibodies [65]. Since brain-derived exosomes are present in low quantities in other body fluids and their isolation and purification are complex and time-consuming, researchers have explored large-scale methods for isolating brain-derived exosomes by culturing neural cells. Exosomes secreted by cultured neural cells, such as neurons or NSCs, can serve as effective carriers for treating neurological diseases. For instance, Madhu et al. [50] successfully obtained exosomes derived from human iPSC (hiPSC)-derived NSCs (hiPSC-NSCs). Their experiments showed that hiPSC-NSC-EVs induced changes in the transcriptome of microglia and reactive astrocytes, which suppressed neuroinflammatory signaling in an AD model and reduced the accumulation of amyloid-β plaques and P-Tau.
In conclusion, the therapeutic efficacy and safety of exosomes in NDDs are intrinsically linked to their cellular origin, which governs their molecular cargo, targeting specificity, and immunogenic profile. Currently, MSC-Exos, NSC- Exos, and CSF-derived exosomes emerge as leading candidates for clinical translation in NDDs therapy. Future efforts should focus on optimizing large-scale production, enhancing targeting specificity via surface engineering, improving cargo loading techniques, and establishing robust quality control protocols. Such advances will pave the way for realizing the full therapeutic potential of exosomes, ultimately transforming the landscape of neurodegenerative disease treatment.
Advances in Exosome Isolation and Drug-Carrying Technologies
The low yield and purity of exosomes remain significant barriers to their widespread clinical application. The clinical production of exosomes as delivery vehicles requires removing a large number of impurities, including cellular debris, proteins, free nucleic acids, and non-exosomal EVs (e.g., apoptotic vesicles and microvesicles) [66]. Moreover, the size and physicochemical properties of exosomes often overlap with those of lipoproteins and other extracellular particles, further complicating the isolation and purification process.
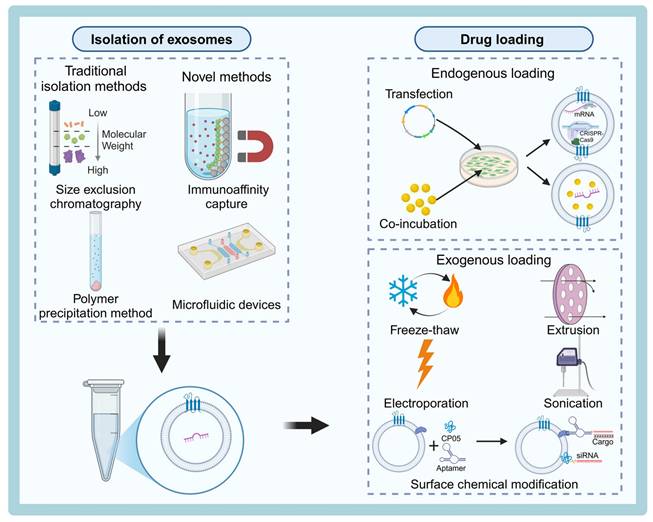
Currently, several commonly used methods for exosome isolation are available at the laboratory scale, including differential ultracentrifugation, density gradient centrifugation, ultrafiltration, polymer-based immunoprecipitation, size exclusion chromatography (SEC), immunoaffinity capture technology, and microfluidic technology (Table 3). Despite these available techniques, there is still no consensus in the field regarding the best approach for large-scale production of exosomes. Significant technical bottlenecks remain, particularly in achieving high yields and purity. Therefore, efficient isolation and purification of exosomes remains a critical area of research. Most conventional isolation methods rely on the physical properties of exosomes, such as size, density, surface charge, and immunoaffinity [67] (Figure 3).
Schematic diagram of the process for isolation and drug loading of engineered exosomes. Exosomes can be isolated using traditional methods such as size exclusion chromatography and polymer precipitation, or novel techniques like immunoaffinity capture and microfluidic devices. Drug loading approaches include endogenous methods (e.g., transfection and co-incubation) and exogenous methods (e.g., freeze-thaw, extrusion, electroporation, and sonication), along with surface chemical modifications to incorporate therapeutic cargoes such as siRNA or CRISPR components. The figure was created with BioRender.
Methods of exosome isolation
| Technique | Principle | Advantages | Disadvantages | Application scenarios | Ref. |
|---|---|---|---|---|---|
| Differential ultracentrifugation | Sedimentation coefficients for exosomes and other substances | Easy to operate, high yield, high separation purity | Time consuming, non-specific, poor reproducibility, risk of exosome rupture | High yield, suitable for exosome function studies | [68,69] |
| Density gradient ultracentrifugation | Densities | High purity | The steps are cumbersome | Typically used to study and isolate specific subpopulations of exosomes | [70,71] |
| Ultrafiltration | Volume and molecular weight | Simple and fast operation | Many steps, time-consuming, membrane holes prone to clogging | Suitable for rapid isolation of exosomes from complex mixtures when high throughput is required | [72] |
| Size exclusion chromatography | Pore size of gels | Simple operation, good reproducibility, large sample size | Low purity, requires further purification | Suitable for applications where reproducibility and large sample sizes are required but purity is secondary | [73,74] |
| Polymer-based precipitation separation | Hydrophobicity | Small sample size required, easy to operate, no need for expensive or specialized equipment | Low specificity, contaminated with free proteins | Used for small-scale separations, they are simple and inexpensive, and are best suited for screening or where specific purity is not required | [75] |
| Immunoaffinity separation | Affinity | High purity, specificity and low time consumption | Reagents are expensive and cannot be applied on a large scale | Suitable for applications requiring high purity and specificity, ideal for clinical or targeted research environments | [76,77] |
| Microfluidics-based technology | Immunoaffinity, acoustic wave, dielectrophoresis, size, magnetism | Fast, high-throughput, and low sample/reagent consumption | Requires electric heating, low resolution, low purity | Suitable for high-throughput applications with small sample sizes, best suited for lab-scale research and diagnostics | [78-80] |
Traditional Isolation Methods vs. Novel Methods
Exosome isolation based on ultracentrifugation method
Ultracentrifugation is a classical physical isolation technique that separates exosomes from other components based on their different settling velocities under centrifugal force. This technique can be broadly divided into differential ultracentrifugation and density gradient ultracentrifugation.
Differential ultracentrifugation is considered the “gold standard” for exosome extraction. In this process, exosomes, cellular debris, proteins, and other impurities are separated by centrifugal force due to differences in their size and density [81]. Teixeira-Marques et al. [82] optimized the ultracentrifugation process to reduce processing time and increase the yield of small EVs. They found that extending the centrifugation time at 200,000 × g could enhance EVs recovery. Studies have shown that factors such as centrifugation time, centrifugal force, rotor type, and other operational parameters influence both the yield and purity of the isolated exosomes. The ultrahigh-speed centrifugation involved can also damage the exosome structure, potentially affecting their function and biological activity, rendering it impractical for large-scale production.
Density gradient ultracentrifugation enhances the separation efficiency by introducing a density gradient (typically using sucrose) to isolate exosomes from particles with similar size but different densities. Exosomes typically accumulate in the density range of 1.13-1.19 g/mL, allowing them to be isolated from other particles [83]. A notable advancement in this method is the one-step sucrose-buffered ultracentrifugation technique developed by Gupta et al. [70], which improves both the purity and yield of exosomes. However, the use of sucrose as a gradient medium has limitations, including its toxicity and suboptimal separation efficiency.
Iodixanol is a highly hydrophilic, nonionic substance with low viscosity and cytotoxicity, making it less toxic to cells compared to sucrose. Additionally, iodixanol provides better separation due to its stability and finer gradient resolution. Li et al. [84] introduced a stabilized platform for buffered density gradient ultracentrifugation using a 60% iodixanol pad to concentrate exosomes and effectively remove protein contaminants and non-exosomal nanoparticles. Moreover, D'Acunzo et al. [85] successfully isolated different EV populations from mouse brain tissue using iodixanol-based high-resolution density gradients, demonstrating better resolution and efficiency in separating microvesicles, exosomes, and mitochondrial vesicles.
Despite its advantages, isodensity gradient ultracentrifugation relies solely on the density differences between the solutes in the sample. It cannot effectively separate substances with similar densities to exosomes, such as microbubbles. Furthermore, the repetitive centrifugation and high centrifugal forces involved may cause mechanical damage to exosomes, which could impact subsequent functional studies and applications in drug development.
Size-based isolation method
Size-based isolation techniques, such as ultrafiltration and SEC, leverage differences in particle size to isolate exosomes from smaller or larger contaminants. These methods provide high efficiency and lower operational complexity compared to ultracentrifugation.
Ultrafiltration is a pressurized membrane separation technique that isolates exosomes from free nucleic acids, lipoproteins, and other components based on their size. The principle of tangential flow filtration (TFF) involves the tangential flow of the sample across the membrane, which reduces damage to flexible structures like exosomes, minimizes the risk of membrane clogging, and improves filtration efficiency [86]. For example, Hou et al. [87] developed an electric field-assisted TFF device (E-TFF), which combines size-based filtration and electrophoretic migration separation techniques to achieve high throughput and high purity separation of small EVs. This method enhances separation efficiency and improves exosome integrity, making it a promising technique for both laboratory and commercial applications [88].
To further enhance the ultrafiltration process, Chen et al. [89] developed a fast and ultrafast separation system (EXODUS), which purifies exosomes from various biofluids automatically and without labels. By introducing negative-pressure oscillations and dual-coupled harmonic oscillations in a dual-membrane filter configuration, EXODUS suppresses fouling and particle aggregation, improving separation speed, yield, and purity while extending membrane lifespan. Moreover, Kim et al. [90] developed a bi-directional flow filtration system (BFF) based on direct flow filtration, integrating a backwash function. By employing 200 nm and 50 nm pore size membranes, BFF selectively isolates exosomes and prevents clogging through periodic backwashing. The BFF technique demonstrated 26-fold and 19-fold improvements in purity and recovery compared to ultracentrifugation and DFF, respectively, highlighting its potential for large-scale separations.
In comparison to ultracentrifugation, ultrafiltration offers several advantages, such as easier operation, higher enrichment efficiency, and lower cost [91]. Although ultrafiltration has shown significant success in laboratory and small-scale applications, there are still challenges to overcome in scaling up for commercial use. Key issues include selecting membranes suited for large-scale filtration, developing more efficient automated systems for membrane replacement and operation, and real-time monitoring and adjustment of operating parameters. Additionally, maintaining a consistent shear force while ensuring effective filtration efficiency during industrial-scale production remains a challenge.
SEC is a technique that utilizes particle size differences to separate substances through porous polymer gel packing. Unlike ultracentrifugation, which subjects exosomes to high g-forces that can compromise membrane integrity and degrade RNA content, SEC employs gentle gravity-driven flow, thus better preserving exosomal morphology and ensuring higher integrity of RNA and protein cargoes [92]. When compared with other isolation methods, such as ultrafiltration or polymer precipitation, SEC offers superior purity by effectively eliminating contaminating protein aggregates and soluble serum proteins. This makes it highly suitable for downstream molecular characterization or therapeutic use.
However, the efficiency of SEC is influenced by column parameters such as pore size and microsphere composition. Improper pore size selection may result in a portion of the protein cargo not being effectively separated or being trapped, affecting recovery. In addition, as the number of column uses increases, the packing may collapse, affecting column efficiency and further reducing recovery. Commonly used SEC gel polymers include crosslinked dextran, agarose, crosslinked polyacrylamide, and polyethylene-stilbene copolymer [93]. To address these limitations, researchers have focused on optimizing SEC materials and column conditions. Guo et al. [74] for instance, evaluated three types of agarose gels (Sepharose CL-6B, CL-4B, and CL-2B) and identified Sepharose CL-6B as the most effective for serum EV separation. By increasing the column bed volume of CL-6B to 20 mL, they developed a simplified, stable SEC method for rapid separation of high-purity EVs in only two elution steps, saving time and cost.
For enhanced protein cargo recovery, SEC is often combined with immunoaffinity-based approaches. In such workflows, SEC first removes bulk impurities, while subsequent immunocapture specifically isolates exosomes expressing target surface markers, thereby improving both recovery and specificity. Recent innovations such as the SmartSEC system integrate SEC and magnetic bead-based immunocapture, further reducing non-specific binding and improving protein yield.
SEC is ideal for high-resolution exosome isolation due to the small sample size required, ease of operation and compatibility. However, compared to ultracentrifugation and ultrafiltration techniques, SEC requires specialized equipment, columns and packing materials, resulting in higher equipment and consumable costs. Nonetheless, many companies have introduced commercialized exosome separation products based on SEC, which has led to widespread acceptance of the technology in scientific research and clinical applications. However, SEC still faces several challenges in commercialization and scale-up. First, selecting efficient and stable gel materials is critical for large-scale production, as the porosity and stability of the gel directly affects separation efficiency and purity. Second, reducing operating costs and optimizing the process flow are key to achieving economical large-scale production. Finally, scaling up equipment and ensuring stable throughput remain technical challenges to ensure economical and efficient large-scale production.
Polymer precipitation-based isolation technology
Polymer precipitation is a widely used technique for exosome isolation, particularly in commercial exosome extraction kits. Among the various polymers, polyethylene glycol (PEG) is the most used reagent. However, PEG-based precipitation struggles to separate exosomes from free proteins and other soluble molecules, which can negatively impact the purity and recovery of exosomes. Additionally, the elution process makes it difficult to completely remove PEG from the exosome surface, potentially compromising the activity of the exosomes and interfering with downstream experiments. A novel solution to these challenges was proposed by Khan et al. [94], who introduced a stimulus-mediated exosome enrichment and purification system. This system involves modifying a thermoresponsive, reductant-cleavable copolymer (PNN) onto the phospholipid bilayer of exosomes (PNN-Exos). When the temperature exceeds the low critical phase transition temperature of 31°C, the PNN changes from a hydrophilic to a hydrophobic state, causing the PNN-Exos to spontaneously aggregate. This method yields extremely high exosome purity and recovery, while preserving the biological activity of the exosomes, outperforming traditional methods like ultracentrifugation and PEG-based kits.
In addition, recent studies have explored coupling antibodies or aptamers to polymers to enhance exosome isolation efficiency and purity. This approach simplifies the operational steps, making it more suitable for a wide range of applications and research. For instance, Yu et al. [95] developed a CD63 aptamer-modified thermoresponsive polymer (PNB-aptamer) for efficient exosome isolation from MSCs. The PNB-aptamer captures exosomes by introducing sequences complementary to the CD63 aptamer, and allows for gentle release. This method simplifies the separation process for more complex biological samples, supporting the clinical application of exosome-based therapeutics.
In conclusion, the polymer precipitation method, particularly the PEG-based precipitation method, is widely used for exosome isolation due to its ease of operation and low cost. However, challenges remain, including the impact of limited purity and recovery on exosome activity and the difficulty of fully removing PEG residues. Continued research into alternative polymers and modifications to existing methods will be crucial for overcoming these limitations and improving exosome isolation techniques.
Emerging Innovations in Isolation
Immunoaffinity capture-based separation technology
Exosomes are rich in transmembrane proteins (e.g., CD63, CD9 and CD81) and specific biomarker proteins (e.g., EpCAM, HER2 and CA-125) [96], which makes immunoaffinity capture a highly effective strategy for their isolation and enrichment. This method relies on the use of antibodies, aptamers, or other affinity ligands to selectively capture exosomes based on the presence of these surface markers.
Antibodies are the most commonly used recognition tools for immunoaffinity capture, owing to their high affinity and excellent specificity. For instance, Guo et al. [97] designed a novel Strep-tag II-based immunomagnetic separation system (SIMI), which modifies the surface of magnetic beads to bind anti-CD63 antibodies via the reversible binding between Strep-Tactin and Strep-tag II. This method efficiently captures exosomes from cell culture supernatants. The experimental results demonstrated that exosomes isolated using this technique maintained their structural integrity and biological activity, highlighting the SIMI system's potential for rapid isolation and enrichment of exosomes. As such, SIMI can serve as a promising new exosome separation tool for applications in disease diagnosis and drug delivery.
Magnetic bead technology, which is frequently employed in immunoaffinity capture, provides excellent superparamagnetism, simple functionalization, and compatibility with various detection strategies, making it ideal for exosome isolation [98]. However, conventional immunomagnetic beads have certain limitations, particularly when dealing with samples containing large amounts of non-target substances. These non-target substances may nonspecifically adsorb onto the surface of smooth or rigidly modified beads, which can affect the capture efficiency. To address this issue, Jia et al. [99] developed immunomagnetic spiked sphere particles (IMHPs) by introducing nanospike-like structures with redox-responsive PEG fragments and anti-CD63 antibodies at the interface of the magnetic beads. This approach not only improved the capture efficiency of target exosomes (up to 91.7%) but also enabled the negative rejection of impurities, such as cellular debris and larger-sized EVs, through the nanostructuring. The results indicated that IMHPs outperformed the conventional ultracentrifugation method, with a 10-fold increase in purity.
Although antibodies are highly specific and exhibit excellent affinity, their preparation is complex, costly, and unstable. As a result, aptamers—synthetic short-chain nucleic acids—are emerging as an alternative for exosome isolation [100,101]. Aptamers offer superior stability, are easy to synthesize, and can be modified flexibly. They also possess the ability to bind to specific target molecules with high affinity in complex samples. For example, Zhang et al. [102] constructed a Fe3O4@TiO2-CD63 aptamer with dual affinity and excellent magnetic responsiveness to rapidly isolate exosomes from human urine. In this approach, TiO2 binds specifically to the exosome lipid membrane phosphate groups, while the DNA aptamer targets the CD63 protein on the surface of exosomes. Huang et al. [103] also designed CD63 aptamers to magnetic nanoparticles Fe3O4 to achieve simple and efficient exosome isolation.
In recent years, hydrogels have been identified as an excellent carrier for encapsulating and releasing small molecule drugs, biomolecules, and exosomes in a controlled manner. This is due to their biocompatibility, environmental friendliness, and ease of production [104,105]. Tang et al. [106] designed a DNA hydrogel-based bioseparation system that uses ultra-long single-stranded DNA to recognize and bind CD63-positive exosomes. This system enables non-destructive release of exosomes, preserving their integrity and biological activity. The hydrogel method offers a nondestructive isolation technique that is not only efficient and specific but also preserves exosome integrity, making it an innovative solution for both research and clinical applications.
Moreover, recent studies have suggested that phosphatidylserine (PS), which is highly expressed on exosome surfaces, can also serve as a target for isolation [107]. For example, Yoshida et al. [108] designed an EV isolation device based on the Ca2+-dependent Tim4 protein's specific binding to PS. This method removes the Ca2+ chelator to achieve non-destructive release of captured EVs. Zhang et al. [109] developed a novel Tim4@ILI-01 immunoaffinity sheet material for exosome enrichment from serum. This strategy is simple, cost-effective, and does not require bulky instruments or sophisticated microfluidic devices, making it an efficient method for exosome capture and isolation.
While the immunoaffinity capture-based isolation method offers high specificity and purity, it is limited by its ability to selectively enrich only those exosomes that express specific surface proteins. As a result, it is difficult to comprehensively capture all exosome subpopulations. Additionally, the elution step in immunoaffinity capture may disrupt the exosome structure, affecting its biological activity and limiting its use in certain high-precision applications. Furthermore, the high cost and complexity of antibody and aptamer preparation, along with potential low yields in large-scale separations, present significant challenges. Reducing costs and improving separation efficiency remain key hurdles for the widespread application of this technology.
Microfluidic-based exosome isolation technology
With the increasing depth of exosome research, continuous innovation in separation technology has become crucial. Microfluidic technology has emerged as a cutting-edge method for isolating exosomes due to its high throughput, precise control, and ability to integrate various processes. Microfluidic-based isolation techniques are primarily divided into two categories: one based on immunoaffinity and the other combining microfluidics with acoustic waves, dielectrophoresis (DEP), and other methods to achieve label-free isolation of exosomes based on their electrical and physical properties.
Immunoaffinity-based microfluidic devices utilize specific biomarkers, such as antibodies against CD63, to capture exosomes with high specificity and purity. For example, Kang et al. [110] designed a dual-mode immunofiltration device (ExoDIF), which captures exosomes via an anti-CD63 antibody and enables efficient recovery of exosomes through cleavable junction chemistry, allowing for further analysis. Compared to the conventional ExoQuick kit, the ExoDIF device outperforms in terms of high throughput and specificity, enabling efficient capture and release of exosomes. Moreover, Hisey et al. [111] engineered an anti-EpCAM-modified herringbone microfluidic device for exosome isolation from high-grade plasma ovarian cancer serum, demonstrating the potential of immunoaffinity isolation for cancer diagnostics. While, Yu et al. [112] proposed a highly integrated exosome separation and detection chip that achieves high recovery and purity of exosomes at an injection rate of 4.8 mL/h. Furthermore, based on the high performance of the SIMI system developed by Guo's team [113], they designed a magnetic nanoparticle-based microfluidic chip (ExoCPR) that improves the purity of exosomes by integrating bubble-driven micro-mixers and surface tension-assisted immiscibility filtration technology. The ExoCPR chip demonstrated a capture efficiency of 75.8% and a release efficiency of 62.7%, with exosome purity exceeding 90%. This chip system not only simplifies the process of exosome isolation but also enhances reproducibility and efficiency.
Although these immunoaffinity-based microfluidic systems offer significant advantages in terms of purity and specificity, their complex experimental setup and dependence on specialized equipment remain limiting factors for widespread use. The immunolabeling step is often tedious, complicating the experimental process, while the coupling with equipment such as syringe pumps and fluorescence detectors restricts flexibility. Future research may focus on simplifying labeling steps, improving capture efficiency, and optimizing equipment dependence to enhance the operability and applicability of the technique.
In comparison to immunoaffinity-based methods, label-free exosome isolation techniques have gained significant attention in recent years due to their ability to avoid complex immunolabeling steps. These techniques typically combine microfluidics with acoustic waves, DEP, and other methods to separate exosomes based on their electrical and physical properties. Hua et al. [114] developed a microfluidic device based on dual tangential flow filtration to separate and purify exosomes using 200 nm and 30 nm nanoporous membranes. This device offers advantages such as ease of fabrication, low cost, and the elimination of the high pressure and clogging problems commonly encountered with traditional ultrafiltration techniques. Zhao et al. [115] introduced an automated exosome purification and assay method that combines a centrifugal microfluidic disk system, functionalized membranes (Exo-CMDS), and a novel aptamer fluorescence system (Exo-AFS). With Exo-CMDS, this platform generates purified exosomes from small blood samples in as little as 8 minutes. It offers low cost, simplicity, high purification rates, reproducibility, and broad applicability. The Exo-AFS detects enriched exosomes by recognizing proteins on the surface, enabling a one-step approach from whole blood injection to exosome isolation and purification. This method is well-suited for low-cost, non-invasive, and routine clinical workflows, making it ideal for early cancer screening.
Acoustic waves combined with microfluidics offer another innovative approach for label-free separation. Surface acoustic waves generate an acoustic radiation force that moves particles in microfluidic channels. Particles of different sizes experience different acoustic forces, enabling size-based separation. Wu et al. [116] developed an acoustic-fluidic platform that combines acoustic wave technology and microfluidics to enable label-free, contact-free separation of exosomes directly from whole blood. This platform achieves high purity and yield of exosome isolation through the synergistic action of a cell removal module and an exosome isolation module, offering advantages such as simple operation, speed, efficiency, and biocompatibility. Naquin et al. [117] introduced the ASCENDx platform, which uses microacoustic wave technology and a rotating droplet system to efficiently purify exosomes and rapidly detect miRNA biomarkers. By optimizing the disc channel geometry to enhance sample enrichment, the ASCENDx liquid biopsy platform shows great potential for liquid biopsy analysis. This platform overcomes the shortcomings of traditional exosome processing and subsequent miRNA detection, providing a promising new solution for biomedical research and diagnostic applications.
DEP is another effective label-free separation technique. It operates by applying force in a non-uniform electric field, exerting varying forces on exosomes based on the electric field strength [80,118]. Niu et al. [119] proposed a multistage continuous DEP isolation microfluidic chip that increases the lateral displacement of target particles by arranging electrodes and creating a gradient electric field. This approach improves the purity, efficiency, and stability of the isolation system. While DEP faces challenges such as low purity and resolution, advancements in chip design and optimization of electric field parameters have led to significant progress.
The application of microfluidics in exosome isolation, particularly in combination with innovative label-free technologies, demonstrates great potential for future development. Label-free separation techniques have important advantages, including simplified operation and reduced experimental errors. However, challenges remain in achieving high separation efficiency and precision. Continued advancements in microfluidic design and optimization of separation techniques will likely address these issues and expand the range of potential applications for exosome isolation.
Challenges in Exosome Isolation for Clinical Translation
The clinical translation of exosomes involves the need for large-scale production, making industrialization a critical focus. Among the various isolation techniques, some show strong potential for industrial application. For example, SEC is widely accessible, is easy to operate, and adaptable to diverse sample types. Immunoaffinity-based methods offer high specificity and precision, making them particularly promising for applications in precision medicine and disease biomarker detection. Ultrafiltration stands out as a convenient, cost-effective, and efficient approach, capable of high-throughput processing and rapid isolation of high-quality exosomes, thus suitable for large-scale clinical screening. In contrast, traditional ultracentrifugation and emerging microfluidic methods face significant hurdles in scaling for industrial production. Ultracentrifugation, though prevalent in laboratory research, requires complex operation and specialized equipment, limiting its feasibility for large-scale clinical production.
It is worth noting that although progress has been made in isolating and purifying exosomes using the methods described above, the exosomes obtained still contain many impurities, which can adversely affect exosome bioactivity and therapeutic efficacy. Regulatory agencies, including the Food and Drug Administration (FDA), have stringent requirements for the purity, potency, safety and efficacy of exosome products to ensure that they can be approved for clinical use. Therefore, comprehensive characterization and rigorous quality control to ensure high purity are essential [120]. To meet regulatory requirements, a comprehensive characterization of exosomes is necessary. Table 4 summarizes key parameters and corresponding methods for exosome characterization.
NTA is a commonly used measurement in EV characterization to estimate the overall size and concentration of particles in solution. However, as NTA analyzes all particles in suspension collectively based on light scattering, it cannot distinguish exosomes from other non-exosomal particles. Therefore, it is recommended to complement NTA with antibody-based techniques or high-resolution imaging (e.g., TEM or SEM) for accurate identification. In contrast, NanoFCM enables single-particle detection based on light scattering and multicolor fluorescence, allowing for simultaneous analysis of size, surface marker expression, and other physicochemical properties. This provides a more detailed and quantitative characterization of EVs.
It should be noted that different characterization methods and equipment may lead to differences in detection results. Therefore, for accurate characterization of individual EVs, a combination of at least two complementary methods (e.g., SEM/TEM in conjunction with NTA) is usually required for confirmation [120]. Regarding the identification of EV markers, there is currently no universal identifier for EVs due to the varied composition of EVs from different sources. The selection of markers depends on the source and type of sample.
Exosome characterization parameters
| Characterization parameter | Criteria | Method | Roles in the GMP process |
|---|---|---|---|
| Physical characteristic | Particle number, size, morphology, zeta potential | Nanoparticle tracking analysis (NTA), NanoFCM, Super resolution microscopy, Tunable resistive pulse sensing (TRPS), Dynamic light scattering (DLS), Flow cytometry, Scanning electron microscopy (SEM), Transmission electron microscopy (TEM) | Ensure the quality consistency of exosomes. Particle size directly affects the distribution, targeting and biological activity of exosomes in vivo; zeta potential provides information on the surface charge and stability of exosomes; morphological observation of whether there is a typical teacup holder structure and the integrity of the membrane structure ensures its normal functioning |
| Biochemical composition | Total Protein, lipids, nucleic acids, surface markers, non-protein markers | Western Blot, Mass Spectrometry, Colorimetry, Proteomic analysis, ELISA, Fourier transform infrared spectroscopy (FTIR), Reverse transcription real-time quantitative PCR (RT-qPCR), BCA | Accurate quantification of biomolecules in exosomes guarantees both the stability of bioactive components and exosome specificity, ensuring batch-to-batch quality consistency. This approach also enables drug-loading capacity assessment and targeting capability studies |
| Purity and integrity | Particle number/total protein, total protein/total lipid, particle number/total lipid, particle number/total nucleic acid, total protein/total nucleic acid | NTA, Nano-Flow Cytometry (NanoFCM), Triton X-100 lysis: ratio of particles before and after membrane-breaking treatment, Measurement of membrane dye, BCA, TEM | Ensure activity and function, reduce immunogenicity, and precisely control the dosage administered in clinical trials to ensure the accuracy and reproducibility of results |
| Security | Viruses, bacteria, fungi, mycoplasma, endotoxins of internal and external origin | qPCR, Gel Clot Method of Limulus Amebocyte Lysate, Culture method ELISA, Next Generation Sequencing (NGS) | Ensure exosome product quality, prevent contamination, ensure purity and clinical application safety |
| Cargo stability | miRNA integrity, protein activity, RNA fragmentation | Bioanalyzer, qPCR, enzymatic activity assays | Ensure therapeutic efficacy, maintain batch consistency, and ensure safety of exosomal products |
| Functional validation | Cellular uptake, bioactivity, BBB penetration | Cell uptake assay, fluorescence microscopy, in vitro BBB models, in vivo biodistribution | Ensure product quality and stability, guide clinical dosing, assess drug delivery efficiency, and predict clinical efficacy and safety |
Commonly used markers for exosome surface marker identification include tetratransmembrane proteins (e.g., CD9, CD63, and CD81) and late endosomal-associated proteins (e.g., Tsg101 and Alix) [121]. Following the guidelines of the International Society for Extracellular Vesicles (ISEV), exosome characterization requires the detection of at least three protein markers, typically two positive markers and one negative marker, to ensure specificity. This is most often achieved through immunoblotting techniques [122]. Finally, the purity of exosome preparations can be evaluated through various approaches, with the most widely used index being the ratio of particle number to total protein content.
In conclusion, although existing isolation and characterization techniques have provided strong support for exosome research, they still face many technical and standardization challenges during industrial production and clinical translation. Future research should focus on optimizing the existing techniques to enhance the separation efficiency and purity, as well as strengthening the standardization of separation and characterization techniques to promote the application of exosomes in clinical therapy.
Good Manufacturing Practices (GMP) Considerations and Cost Challenges
Among the biggest challenges in advancing exosome-based therapies into clinical applications, especially in the field of NDDs, is achieving scale-up production that meets the requirements of Good Manufacturing Practices (GMPs). GMPs are essential for ensuring product quality, safety, and reproducibility, but they also introduce considerable costs and technical challenges. Key issues in exosome GMP production include upstream cell culture, downstream purification, and comprehensive quality control [123].
In the upstream cell culture process, the choice of culture medium is a primary cost driver. It is also crucial for minimizing risks such as immune reactions and viral contamination. Culture media are broadly categorized into serum-containing and serum-free formulations. Although serum-free media are significantly more expensive, more complex to formulate, and yield lower initial production volumes, they offer important long-term advantages for GMP-compliant manufacturing. These advantages include improved batch-to-batch consistency, the elimination of animal-derived pathogens, and easier regulatory approval—factors that collectively reduce the likelihood of batch failures, safety concerns, and regulatory delays. Conversely, traditional serum-containing media may seem cost-effective initially but are associated with higher risks of batch variability, contamination, and regulatory hurdles that can ultimately increase overall production costs and impede scale-up. Thus, serum-free media are increasingly recognized as a more sustainable and compliant choice for large-scale, clinical-grade exosome production.
In the downstream purification process, achieving GMP-grade exosome purity involves efficient removal of host cell proteins, DNA contaminants, and non-target EV populations. Traditional manual methods such as ultracentrifugation, though widely used in research settings, are labor-intensive, low-throughput, and difficult to standardize at industrial scale. In contrast, automated purification technologies—such as TFF, SEC, and novel systems like EXODUS—require significant upfront investment but offer superior scalability, improved reproducibility, reduced labor demands, and lower contamination risks. These technologies are essential for enabling consistent and efficient large-scale production.
In conclusion, the clinical translation of exosome-based therapies must proactively address the high cost and complexity of GMP-compliant manufacturing. Establishing standardized production workflows—centered around serum-free culture systems and automated purification platforms—and continually optimizing yield and cost-efficiency are critical steps toward overcoming scalability bottlenecks. These advances are fundamental for making exosome therapeutics accessible to a broader population of patients with NDDs.
Innovations in Exosome Drug-Carrying Technologies
Exosome-based drug delivery has emerged as a promising method for targeted therapy, utilizing the unique properties of exosomes to transport a wide range of therapeutic molecules. The drug loading into exosomes can be categorized into two main techniques: endogenous loading and exogenous loading. These technologies enable the delivery of various therapeutic agents, offering a versatile platform for treating neurological disorders and other diseases.
Endogenous loading technology
Endogenous loading technology involves bioengineering donor cells to produce exosomes that contain specific drugs or molecules. In this process, target molecules are introduced into donor cells through methods such as direct transfection or co-incubation. Endogenous loading techniques can be applied to various therapeutic molecules, including RNA, proteins, and peptides. Below, we briefly outline the latest research advancements in the genetic engineering of exosome drug loading.
Currently, most studies focus on the loading of nucleic acids into exosomes via the transfection of RNA-coding plasmids. A notable breakthrough by Zickler et al. [124] developed an endogenous loading platform that significantly improves both the loading and delivery efficiency of exosomes. They engineered cells to stably express target mRNAs by fusing CD63 with the high-affinity RNA-binding protein PUF, resulting in exosomes loaded with mRNA. This platform addresses several limitations of existing endogenous loading methods, such as low mRNA loading efficiency, poor delivery rates, and interference from plasmid DNA in mRNA quantification and functional analysis. Additionally, Fu et al. [125] designed artificial genetic circuits to reprogram cells, enabling them to direct the self-assembly of exogenous small interfering RNAs (siRNAs) into secreted exosomes and facilitating systemic or targeted delivery of these self-assembled siRNAs in vivo.
Another innovative approach by Gu et al. [126] utilized an mRNA delivery nanocarrier targeting brain neuronal cells by transfecting leukocytes with DNA or RNA encoding the virus-like protein capsid Arc, along with the Arc-5'UTR motif (A5U). The Arc protein self-assembles into a virus-like capsid, encapsulating and releasing the mRNA within neuronal cells. Their study showed that incorporating Arc increased mRNA loading by over sixfold, and adding the A5U motif further stabilized the Arc capsid, enhancing the mRNA loading efficiency. This breakthrough offers new prospects for treating neurodegenerative diseases and opens fresh research directions in biomedical engineering. Despite success in chronic low-grade neuroinflammation models, further validation of the loading and delivery efficiency is necessary for acute or more severe neuroinflammatory conditions.
Protein therapeutics play a crucial role in maintaining normal body functions. Traditional protein delivery methods, such as electroporation and mechanical force-induced temporary membrane openings, are effective but complicated, costly, and can disrupt membrane integrity, potentially affecting normal cellular function. In contrast, exosome-based drug delivery systems show greater promise as efficient nanocarriers for therapeutic proteins. Han et al. [127] developed the MAPLEX system, a novel engineered exosome technology that utilizes photocleavable proteins (mMaple3) for controlled protein release. This system enables the regulation of various complex cellular events, including transcription, gene recombination, and epigenome editing. The MAPLEX exosome is created by coupling the N-terminus of the exosome scaffold protein CD9 with mMaple3. The team used this system to deliver dCas9-D3A into an AD mouse model, inducing methylation at a target CpG site, which reduced Bace1 expression, amyloid pathology, and memory impairment. Compared to Kong et al. [128] who used a different delivery method, the intranasal delivery in the MAPLEX system proved to be more efficient, safer, easier to administer, and demonstrated higher bioavailability and broader applicability. This approach offers a novel strategy to address protein delivery challenges in NDDs and opens up new possibilities for gene therapy. However, since the MAPLEX system lacks a targeting component, further research is needed to integrate appropriate targeting mechanisms and select the right exosome-producing cell types to enhance targeting specificity.
One of the main advantages of endogenous loading techniques is their ability to minimize disruption to exosome membranes, preserving their natural structure and improving biocompatibility and stability. This makes exosomes a highly promising drug delivery system. However, this method remains relatively complex and costly, requiring further optimization to enhance productivity and reduce operational costs.
Exogenous loading technology
Exogenous loading techniques involve loading drugs onto the surface or inside exosomes that are isolated and purified from cell cultures or other biological fluids, using physical or chemical methods. Common loading approaches, such as electroporation, sonication, freeze-thawing, saponification, extrusion, and co-incubation, have been widely studied (Table 5). This section will focus on recent advancements in the chemical modification of exosome surfaces.
Surface chemical modification enhances the drug-loading capacity of exosomes by attaching specific molecules (e.g., peptides, ligands, aptamers) to their surface. This approach not only increases the functionality of exosomes but also circumvents the limitations of traditional drug-loading methods (e.g., transfection, electroporation, freeze-thawing), which often result in poor loading efficiency. A common surface modification strategy involves anchoring peptides, such as the CP05 peptide. CP05 has a high affinity for the exosome surface protein CD63, and its attachment significantly improves drug-loading efficiency. For instance, Gao et al. [138] demonstrated that anchoring CP05 to the exosome surface enhanced its drug-loading capacity. Xu et al. [139] employed a similar strategy, coupling a cell-penetrating peptide (R9 peptide) to the exosome surface through an amide bond, enabling the loading of antisense oligonucleotides (ASOs). The addition of the R9 peptide simplifies the loading process, making it more suitable for large-scale production of functionalized exosomes and providing a novel design for efficient drug delivery systems.
In recent years, surface modification methods for loading nucleic acid drugs have gained traction. One common strategy involves inserting cholesterol or CP05 peptides into the membrane phospholipid bilayers to load nucleic acids [140]. Another promising method is the use of aptamers, which can load nucleic acid drugs onto exosomes through direct synthesis or annealing. This strategy bypasses the complexity and high costs associated with traditional peptide-nucleic acid coupling. Han et al. [141] used the systematic evolution of exponentially enriched ligands (SELEX) technique to identify a DNA aptamer that efficiently loads various nucleic acid drugs onto the exosome surface. This simple and scalable approach holds great potential for advancing exosome-based nucleic acid therapies, potentially making oligonucleotide therapies more effective and cost-efficient.
Additionally, the combination of metabolic engineering and click chemistry has introduced new opportunities for surface modification of exosomes. Bhatta et al. [142] designed a versatile exosome loading platform using a metabolic engineering approach. They enabled parental cells to uptake Ac4ManAz (tetraacetyl N-azidoacetylmannosamine), which added azide groups to the exosome surface. Click chemistry was then employed to efficiently load drug molecules onto the exosomes. This strategy not only simplifies the surface modification process but also improves the efficiency and diversity of drug loading, expanding the potential applications of exosomes in drug delivery.
Engineered Exosome Strategies for Enhanced Brain Targeting and Regulation of Signaling Pathways
Targeted engineering of exosomes for brain delivery
Exosomes are multifunctional nanoscale vesicles that exhibit significant diversity and are rich in surface proteins. However, due to the lack of clear tissue specificity, the targeting potential of exosomes is not inherently high. To address this, researchers frequently employ surface modification techniques to enhance exosome targeting, stability, and delivery capabilities. Below, we summarize some of the current strategies commonly used to enhance the brain-targeting ability of exosomes (Figure 4).
Exosomal drug loading technologies
| Technique | Procedure | Tested Cargo | Advantages | Disadvantages | Potential for clinical application | Ref. |
|---|---|---|---|---|---|---|
| Electroporation | Forming restorable micropores on the exosome surface through the action of an electric field | siRNAs, microRNA, DNA, mRNA, proteins | Easy to operate; less influence on the receptor-ligand on the exosome surface; suitable for macromolecules | Induces aggregation of exosomes or contents and reduces drug loading efficiency | Medium: Already used in some preclinical studies, requires a solution by adjusting the electric field strength, the perforation time or using special surface modification techniques to improve the drug-carrying efficiency | [129,130] |
| Sonication | Sonication of homogenization probes for exosomes and drug mixtures | Hydrophilic drug molecules, proteins | High drug loading efficiency; Sustainable drug release | Exosomes tend to aggregate and affect their surface protein structure | Medium: suitable for high drug loads | [131] |
| Freeze-thaw | Rapid freeze -thaw cycles at < -80°C | siRNAs, microRNA, proteins and macromolecules | Simple operation; Not easy to destroy bioactive substances | Exosomes are easily aggregated and have low encapsulation efficiency | Low: temperature and freeze-thaw cycles can be adjusted to reduce aggregation and improve encapsulation efficiency | [132] |
| Saponification | Saponins and other chemicals create pores in exosomes | Hydrophobic drugs, DNA/RNA and proteins, catalase | High drug loading efficiency | Affects exosome stability; Chemical reagents are difficult to remove; Risk of toxicity | Low: toxicity risks and stability issues hamper their clinical translation | [133] |
| Extrusion | Liposome extruder extrusion for exosomes and drug mixtures | miRNA, siRNAs, curcumin, catalase | High efficiency: Homogeneous particle size of exosomes obtained | Mechanical forces may alter the nature of the exosome membrane (e.g., membrane protein structure) and loss of contents | High: already used in several clinical trials, but there is a risk of membrane damage and further optimization of extrusion equipment is required | [134] |
| Co-incubation | Exosomes/parental cells and drug co-incubation at room temperature | Minor molecule chemicals, proteins, siRNAs, curcumin, microRNA | Easy to operate | Low loading efficiency | Medium: simple and effective for small molecules, but requires improved incubation conditions such as temperature, drug concentration and incubation time to increase drug loading efficiency | [135-137] |
Schematic representation of exosome surface modification to enhance brain targeting. Covalent modification involves stable chemical conjugation techniques, while non-covalent modification relies on reversible interactions through hydrophobic insertion. Genetic engineering approaches modify parent cells to express membrane-anchored targeting peptides. The figure was created with BioRender.
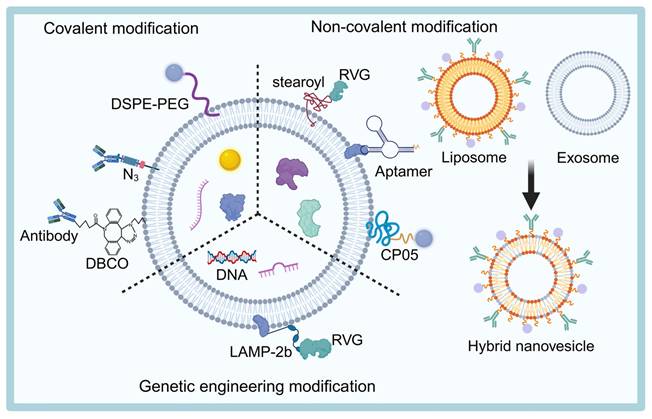
Genetic engineering modification
Genetic engineering of donor cells or exosomes is a widely used surface modification technique. The process involves constructing a fusion plasmid that links a target protein with an exosome scaffolding protein, which is then transfected into parental cells. This allows the generation of stable monoclonal cell lines that express the target protein, facilitating large-scale production of engineered exosomes [143]. The surface of these modified exosomes often expresses specific targeting sequences, peptides, or antibodies, enabling precise delivery to specific cells or tissues [144,145].
Common exosome scaffolding proteins include type I transmembrane proteins (e.g., Lamp2), tetraspanning proteins, and peripheral membrane proteins (e.g., MFGE8) [146]. Among these, Lamp2b is the most utilized exosome scaffolding protein. The N-terminal of Lamp2b is located outside the exosome membrane, allowing it to strongly fuse with specific targeting sequences or peptides to construct a targeted drug delivery system [147,148]. For example, Alvarez-Erviti et al. [129] successfully created brain-targeted exosomes (RVG-Exo) by modifying dendritic cells to express Lamp2b, fused with a peptide fragment from the neuron-specific rabies virus glycoprotein (RVG). Intravenous injection of RVG-Exo enabled drug-specific delivery to neurons, microglia, and oligodendrocytes in the brain. Similarly, Yang et al. [149] co-transfected HEK293 cells with RVG-Lamp2b and a nerve growth factor (NGF) plasmid, generating exosomes that efficiently delivered NGF to the brain. Jiang et al. [150] further optimized exosome brain targeting by modifying the surface with three BBB shuttle peptides (RVG29, TAT, or Ang2). The results indicated that RVG29-modified exosomes exhibited superior targeting efficiency to the brain and could serve as stable brain-targeted drug delivery vehicles.
In addition to RVG, other peptides have been explored to further enhance exosome brain targeting. One such peptide is angiopep-2, a Kunitz-type peptidase inhibitor with high affinity for the low-density lipoprotein receptor-related protein 1, known for its excellent brain penetration capability [151]. For instance, Zhu et al. [152] developed a bipeptide-modified small EVs drug delivery system, combining the targeting properties of angiopep-2 with the membrane-penetrating properties of TAT, enabling efficient targeting of chemotherapeutic drugs to the mouse brain. Moreover, the fusion of the T7 peptide with Lamp2b has been shown to further improve exosome brain targeting and enhance their ability to traverse the BBB [153].
However, current exosome scaffolding proteins do not fully meet the diverse requirements of engineered modifications. Therefore, identifying novel scaffolding proteins is of great importance. Recently, Zhao et al. [154] introduced a new screening criterion for exosome scaffolding proteins and identified PLXNA1 as a promising candidate with excellent sorting capability. This discovery opens new possibilities for engineering exosomes with enhanced delivery properties.
The advantages of genetically engineered exosomes include enhanced drug delivery efficiency, the ability to cross the blood-brain barrier, improved drug stability and biocompatibility, prolonged circulation time, and the capacity to carry multiple therapeutic molecules. However, there are also several drawbacks to genetic modification. These include technical complexity and high costs, which may result in a loss of bioactivity and potential immune responses. Additionally, challenges related to production efficiency and purity could impact the clinical applicability of these engineered exosomes.
Covalent modification
In addition to genetic engineering, covalent modification is a widely used technique for modifying the surface of exosomes. Through methods like click chemistry or azide-alkyne cycloaddition reactions [155], targeting ligands can be covalently attached to the exosome membrane, thereby enhancing the targeting ability of the exosome.
A notable example of this approach is the work of Sul et al. [156], who successfully bound dopamine to the surface of adipose stem-cell-derived EVs (Dopa-EVs) using a simple and direct cross-linking reaction, enabling selective targeting of dopaminergic neurons in PD. By co-culturing the exosome with neurons, it was found that dopamine enhanced the uptake of Dopa-EVs by neurons through dopamine receptor-mediated pathways. Importantly, this modification did not significantly alter the basic physical and biological properties of the exosomes, highlighting their potential for application in neurodegenerative diseases. Additionally, Zhang et al. [157] engineered a multi-targeted exosome (MP@Cur-MExo) derived from activated neutrophils. This exosome effectively targets activated neurons in AD brains by conjugating superparamagnetic iron oxide nanoparticles modified with both mitochondria-targeting and β-amyloid (Aβ)-targeting ligands via click chemistry, facilitating multi-targeted therapy.
Click chemistry offers a straightforward and efficient approach for exosome functionalization. However, there are some limitations to this method. Since exosome membranes contain various membrane proteins, covalent modifications may interfere with the function of these proteins, potentially affecting the exosome's biological properties and delivery capabilities. Therefore, precise control of the reaction conditions is essential to ensure that the targeting ligand binds to the exosome membrane without disrupting the natural function of the exosome or diminishing its biological activity.
Non-covalent modification
Non-covalent modification is another commonly used strategy for exosome surface modification. Liu et al. [158] designed a REXO-coated gene-chemical nanocomplex that efficiently crosses the BBB, targets neurons, and releases drugs in the high-reactive oxygen species (ROS) environment of dopaminergic neuronal lesions. They achieved this by embedding stearoyl-RVG within the phospholipid bilayer of exosomes through non-covalent interactions, enhancing their targeting and delivery capabilities. This method offers a gentle approach for modifying exosome surface properties, ensuring high stability and biocompatibility while preserving the exosome's natural state.
Another novel non-covalent modification method involves the hydrophobic interaction between liposomes and exosomes [159]. Sato et al. [160] introduced a fusion technique, where exosome and liposome membranes are combined via a freeze-thaw process to create engineered hybrid exosomes. These hybrid exosomes exhibit improved delivery functionality and optimized surface properties. Furthermore, Jiang et al. [161] developed ROS-responsive mimetic exosome-liposome hybrid nanovesicles by fusing angiopep-2-modified exosomes with liposomes. These hybrid exosomes effectively target the BBB and enhance drug accumulation at AD lesions, ultimately improving cognitive function.
Compared to covalent modifications, non-covalent modifications have a lesser impact on the natural structure of exosomes, offering higher stability and biocompatibility. Additionally, they are more cost-effective and provide greater flexibility. Although non-covalent modifications are milder, they may not be as stable as covalent modifications, especially during long-term storage or in vivo circulation, which could lead to the separation or failure of the modifiers.
Innovative strategies
Most existing engineering methods modify exosomes as a whole, which can compromise the integrity of the membrane structure and the activity of surface proteins or nucleic acids, resulting in impaired targeting and therapeutic efficacy [162]. Moreover, the strategy of engineering donor cells to produce specific exosomes involves cumbersome steps, high costs, and limited controllability.
To address these challenges, researchers have proposed innovative approaches. For instance, Wang et al. [163] combined nanoscale artificial modules with specific functions (nitric oxide (NO)-driven nanomotors capable of chemotaxis in PD microenvironments with high expression of iNOS/ROS) with natural exosome modules in a “one-to-one” fashion. This created engineered exosomes with “stand-alone module/cascade functions.” Thanks to the unique transmembrane ability of the natural exosome module, the artificial module drives the exosome to efficiently pass through the BBB, precisely target damaged neuronal cells and mitochondria in the disease microenvironment, and effectively treat PD through multi-step interventions. This approach reduces the non-specific binding typically seen in traditional engineered exosomes within the disease microenvironment, offering an excellent solution for enhancing targeting ability after crossing the BBB.
Surface modification technology for exosomes holds great potential in brain-targeted drug delivery. Genetically engineered modifications, covalent modifications, and non-covalent modifications each have their respective advantages and limitations. The choice of the most appropriate strategy depends on the specific goals and requirements of the application. With ongoing advancements in technology, more novel modification methods are likely to emerge, further enhancing the potential of exosomes in drug delivery and disease treatment.
Exosome-Mediated Regulation of Signaling Pathways
Exosomes play a pivotal role in regulating intracellular signaling pathways in neurons, which is crucial for the pathophysiology of NDDs. Several key signaling pathways, including PI3K/Akt/GSK-3β, PI3K/Akt/mTOR, PI3K/Akt/NF-κB, and RhoA-ROCK, are involved in neuronal survival, apoptosis, autophagy, and neuroinflammation [164-166] (Figure 5). In addition, it has been shown that human endometrial stem cells-derived small EVs can be used to treat nerve injury by activating the PI3k/AKT signaling pathway to enhance cell proliferation and migration and promote nerve growth [167]. Here we briefly describe how exosomes regulate these pathways and their potential therapeutic role in NDDs.
Schematic diagram of exosome-mediated treatment of NDDs via targeted signaling pathways. PI3K: phosphatidylinositol3 kinase; Akt: protein kinase B, PKB; GSK-3β: glycogen synthase kinase-3β; mTOR: mechanistic target of rapamycin; NF-κB: Nuclear factor kappa-B; Tau: tau protein; PTEN: phosphate and tensin homology deleted on chromosome ten; AMPK: adenosine 5'monophosphate-activated protein kinase; Bcl-2: B-cell lymphoma 2; Bax: bcl-2-associated X protein; ULK1: unc-51 like autophagy activating kinase 1; P70S6K: p70 ribosomal protein S6 kinase; IκB: Inhibitor of NF-κB; RhoA: Ras homolog gene family member A; ROCK: Rho-associated coiled-coil-containing protein kinase. The figure was created with BioRender.
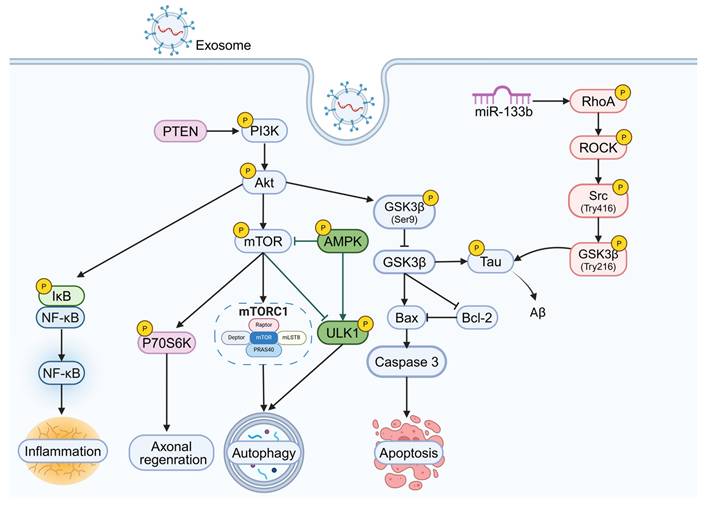
PI3K/Akt/GSK-3β signaling pathway
GSK-3β is widely expressed in the central nervous system and is a key participant in normal brain function signaling [168]. Research has shown that the PI3K/Akt/GSK-3β signaling pathway is closely linked to neuronal apoptosis [169]. In this pathway, Akt phosphorylates a specific site on GSK-3β (Ser9), inhibiting its activity and preventing further phosphorylation of β-catenin [170]. In PD, GSK-3β activation increases the expression of the pro-apoptotic protein caspase-3, promoting neuronal death [171]. The PI3K/Akt/GSK-3β pathway is not only crucial for neuronal survival but also regulates Tau protein phosphorylation, which is a key mechanism in AD [172]. Overactivation of GSK-3β leads to excessive Tau phosphorylation [173]. In AD, exosomes carrying curcumin can activate the PI3K/Akt/GSK-3β pathway, inhibiting Tau phosphorylation and preventing neuronal death, both in vitro and in vivo, thereby alleviating AD symptoms [137].
PI3K/Akt/mTOR signaling pathway
The mTOR is a key downstream target of the PI3K/Akt pathway and a critical autophagy regulator [174]. Upon Akt activation, mTORC1 is phosphorylated, enhancing protein translation and inhibiting autophagy [175]. The PI3K/Akt/mTOR pathway is essential for autophagy regulation, which is a key process in maintaining neuronal function [176].
Exosomes have been shown to modulate this pathway to enhance autophagic flux and promote neuroprotection. For example, in AD mouse models, rapamycin, a selective mTOR inhibitor delivered via exosomes, reduces mTOR activation, increases microtubule-associated protein 1 light chain 3-II (LC3-II) expression, and promotes neuronal autophagy [177]. Ebrahim et al. [178] assessed the therapeutic mechanism of MSCs-Exos in AD and analyzed the complex relationship between the PI3K/AKT/mTOR pathway and the regulation of autophagy. Liu et al. [179] found that adipose stem cell exosomes can activate mitochondrial autophagy through inhibition of the PI3K/AKT/mTOR pathway.
PI3K/Akt/NF-κB signaling pathway
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a transcription factor that, in its inactive state, is bound to IκB, limiting its movement in the cytoplasm [180]. Akt can phosphorylate IκB kinase, leading to IκB degradation and the release of NF-κB, which translocates to the nucleus to activate the expression of inflammatory genes such as tumor necrosis factor (TNF)-α and IL-1β [181]. In NDDs such as AD and PD, abnormal activation of the PI3K/Akt/NF-κB pathway is associated with increased inflammation [180]. Lee et al. [182] found that exosomes containing non-degradable IκB (Exo-srIκB) can inhibit NF-κB activation, thereby alleviating neuroinflammation. Exosomes carrying miR-124-3p have been shown to suppress neuroinflammation by modulating the MYH9/PI3K/Akt/NF-κB signaling cascade in neurons [183]. Additionally, MSC-Exos can regulate inflammation through the STAT3-NF-κB pathway in APP/PS1 mouse models, improving cognitive function [184].
RhoA-ROCK signaling pathway
RhoA, a small GTPase in the Rho family, is a key regulator of the cytoskeleton and cell adhesion dynamics, with its main downstream effectors being the ROCK1/2 serine/threonine kinases [185]. Activation of the RhoA-ROCK pathway promotes cell death and mediates the loss and retraction of synapses and neuronal pathways in the brain. Inhibiting RhoA-ROCK signaling is considered a promising strategy for treating CNS diseases [186]. Numerous studies have shown that inhibiting RhoA-ROCK signaling benefits the treatment of ALS, PD, and AD. For example, RVG29-Exo-133b, which targets the RhoA-ROCK pathway, reduces Tau phosphorylation and enhances motor function, mitigating neurodegeneration in PD models [150]. Schwann cell-derived exosomes targeting the RhoA-ROCK pathway have also been shown to reduce activation of protein tyrosine phosphatase sigma and promote axon regeneration following spinal cord injury [187].
Other signaling pathways
In addition to the aforementioned pathways, other signaling cascades are crucial in NDDs. For instance, the AMPK/mTOR pathway plays a significant role in regulating autophagy. Exosomes derived from human umbilical MSCs (hUMSC-Exos) have been shown to activate this pathway, enhancing autophagy and promoting spinal cord endothelial cell repair [188]. Furthermore, PTEN, a negative regulator of the mTOR pathway, is involved in axon regeneration and neuronal survival [189,190]. MSC-Exos loaded with miR-26a can activate the PTEN-AKT-mTOR pathway, promoting axon regeneration and neurogenesis [191]. Additionally, miR-146a-5p from hUMSC-Exos reduces microglia-mediated neuroinflammation through the IRAK1/TRAF6 pathway, contributing to the attenuation of neurodegenerative processes [192].
By targeting these pathways, exosomes hold significant therapeutic potential for treating neurodegenerative diseases. Despite challenges in optimizing exosome-based therapies, their ability to modulate critical signaling pathways highlights their promise as effective treatments for NDDs. Further research is needed to fully explore the therapeutic potential of exosome-based interventions in clinical applications.
Exosome-Based Therapies for NDDs
Exosomes have gained significant attention in the treatment of NDDs due to their versatility and potential to address multiple challenges in the nervous system. In particular, AD and PD are major targets for exosome-based therapeutic strategies. Researchers are investigating the use of exosomes to deliver neuroprotective factors, clear Aβ, and repair damaged neurons, with the goal of slowing disease progression and improving clinical symptoms. Additionally, exosomes have shown potential in promoting nerve regeneration and restoring cognitive function, offering new hope to patients with NDDs.
Therapeutic Potential in NDDs
Alzheimer's disease (AD)
AD is a neurodegenerative disorder primarily affecting middle-aged and older adults, characterized by cognitive decline. The pathological features of AD include the accumulation of β-amyloid plaques and neurofibrillary tangles formed by P-Tau. These hallmark features contribute to the neuronal damage observed in the disease. Despite its devastating impact, current pharmacological treatments for AD are limited to cholinesterase inhibitors and NMDA antagonists, which primarily address symptoms rather than underlying causes. The main barrier to effective treatment is the challenge of delivering drugs across the BBB and the pharmacokinetic limitations of available therapies.
Recent research has focused on targeting Aβ and P-Tau accumulation as a therapeutic strategy for AD. One promising approach involves the use of enkephalinase, an enzyme that degrades Aβ and reduces its pathological accumulation. Katsuda et al. [193] demonstrated that human adipose tissue-derived MSCs (ADSCs) secrete exosomes containing functional enkephalinase. In vitro experiments showed that ADSC-derived exosomes (ADSC-Exos) could be internalized by neuroblastoma cells, resulting in a reduction of intracellular Aβ levels. These findings suggest that ADSC-Exos could serve as a promising drug delivery vehicle for Aβ clearance in AD. Further supporting this idea, Elia et al. [194] injected MSC-Exos containing enkephalinase into the cerebral cortex of mice, which led to a reduction in Aβ plaque formation in the hippocampus and cerebral cortex, as well as a decrease in dystrophic neurite formation.
With in-depth studies on the pathogenesis of AD, researchers have found that exosomes can induce autophagy, thereby clearing misfolded proteins in NDDs, including AD [195]. In this context, Iyaswamy et al. [196] engineered exosomes by modifying Fe65, a protein that interacts with amyloid β precursor protein (APP), onto their surface to create a novel targeted drug delivery system, Fe65-Exos. This system was designed to precisely deliver the autophagy inducer Corynoxine-B (Cory-B) to neurons with high APP expression. By analyzing the fluorescence intensity and co-expression of Fe65 and APP in the mouse brain, it was demonstrated that Fe65-Exos could effectively cross the BBB and deliver Cory-B to the brains of AD mice. In vitro and in vivo experiments further showed that Fe65-Exos-Cory-B enhanced BECN1-dependent autophagy in neurons and promoted synaptic plasticity formation among hippocampal neurons in AD mice, thus improving learning and memory deficits in 5xFAD mice. This study provides an innovative exosome-based drug delivery system with AD-specific targeting, offering a new strategy for the future treatment of AD and other NDDs. However, further studies are needed to explore the potential side effects of this system and to develop an effective intranasal delivery method.
Beyond Aβ and tau pathology, AD is also associated with neuroinflammation, oxidative stress, and mitochondrial dysfunction [197]. Neuroinflammation, driven by the activation of astrocytes and microglia, is a key feature of AD and many other NDDs. Modulating neuroinflammation could therefore provide an effective therapeutic strategy. RVG-modified MSC-Exos have been shown to attenuate plaque deposition, reduce Aβ accumulation, and inhibit astrocyte activation, while also downregulating pro-inflammatory cytokines (such as TNF-α, IL-1β, and IL-6) and upregulating anti-inflammatory factors (such as IL-4 and IL-10) [198]. These effects led to improvements in spatial learning and cognitive function in AD mice.
Moreover, NSC-Exos have demonstrated the ability to reduce P-Tau levels and Aβ formation by inhibiting kinase activity, thereby downregulating the NF-κB/ERK/JNK signaling pathway in activated microglial cells. In preclinical models, NSC-Exos have been shown to effectively mitigate the pathogenesis of AD by reducing neuroinflammation and promoting neuronal health [94]. Human umbilical cord MSC-derived exosomes (hucMSC-Exos) have also been reported to modulate microglial activation and reduce Aβ deposition, improving spatial learning and memory in AD mice [199].
In addition to their anti-inflammatory and neuroprotective effects, MSC-Exos are capable of protecting against oxidative stress and synaptic damage caused by Aβ toxicity, further supporting their potential as a therapeutic tool for AD [200]. Huber et al. [201] showed that exosomes secreted by neural stem cells in response to heat shock stimulation exhibit enhanced neuroprotection against oxidative stress and Aβ-induced neurotoxicity. Mitochondrial dysfunction is another key contributor to AD, as it leads to increased ROS, oxidative damage, and synaptic defects. Targeting mitochondrial dysfunction offers a promising therapeutic avenue. NSC-Exos have been found to activate the SIRT1-PGC1α signaling pathway, enhancing mitochondrial function, restoring mitochondrial biogenesis markers (such as PGC1α, COXIV, and NRF1), and improving cognitive function in AD mice [202]. Additionally, Zhang et al. [157] designed a multi-target engineered activated neutrophil exosome (MP@Cur-MExo), which improves mitochondrial health by inhibiting extracellular Aβ deposition and aggregation, as well as mitochondrial autophagy. This approach attenuates Aβ-induced mitochondrial metabolic defects, providing a promising new direction for AD therapy.
Parkinson's disease (PD)
PD is a common neurodegenerative disorder that manifests in both motor (myotonia, resting tremor, bradykinesia) and non-motor symptoms (cognitive/psychiatric abnormalities, autonomic dysfunction, sensory deficits). Pathologically, PD is characterized by the degeneration and death of dopaminergic neurons in the substantia nigra, along with abnormal deposition of amyloid fibrils (α-syn aggregates forming Lewy bodies) [203,204].
Recent studies have revealed that the spread of α-syn between neurons accelerates the progression of PD [205]. For instance, Mao et al. [206] found that binding of α-syn preformed fibrils (PFF) to lymphocyte activation gene 3 (LAG3) can trigger endocytosis, delivery and its toxicity of α-syn PFF. As a result, targeting the propagation of pathological α-syn is emerging as a promising therapeutic strategy for PD treatment [207]. Exosomes are key mediators of the pathological progression of PD by facilitating the transfer of α-syn between neurons [208,209]. Xia et al. [210] injected exosomes derived from the plasma of PD patients into the striatum of mice and found that these exosomes were extensively taken up by microglial cells, activating them and promoting an inflammatory response. Building on these previous findings, Guo et al. [204] identified the mechanism by which microglia-derived exosomes promote the propagation and aggregation of α-syn in PD. Their findings suggest that targeting the release of microglia-derived exosomes or disrupting their propagation pathways could offer novel therapeutic targets for PD intervention. Additionally, previous studies have shown that exosomes secreted by astrocytes can induce protein aggregation in the mouse brain, suggesting that astrocytes may also play a role in the spread of misfolded proteins, such as α-syn [211]. Wang et al. [212] discovered that pathological α-syn deposition impairs the lysosomal function of astrocytes, leading to the release of more α-syn-laden exosomes. These exosomes may then interact with neighboring neurons or glial cells, further propagating α-syn and promoting neurodegeneration. Thus, targeting the production, release, or cellular interactions of α-syn-carrying exosomes holds promise for developing more effective therapeutic strategies, offering new hope for patients with PD.
The pathophysiology of PD also involves oxidative stress, mitochondrial dysfunction, and neuroinflammation. α-syn can accumulate in mitochondria, generating high levels of ROS, which in turn induce the expression of inducible nitric oxide synthase (iNOS), leading to neuroinflammation and neuronal damage [163,213]. Research has suggested that regulating oxidative stress may be promising therapeutic strategies for PD. One hypothesis is that intracerebral delivery of antioxidants could alleviate oxidative stress and protect neurons in PD patients. Catalase, a potent antioxidant, is unable to cross the BBB and is easily degraded [214].
However, studies by Haney et al. [136] demonstrated that exosomes loaded with catalase effectively degrade ROS and offer neuroprotection. Wang et al. [163] designed an engineered exosome capable of targeting damaged neurons and mitochondria in the PD microenvironment, characterized by high levels of ROS and iNOS. This exosome aims to achieve multistage therapy for PD by both inhibiting the progression of upstream factors (e.g., removal of α-syn aggregation) and repairing downstream damage (e.g., regulating ROS levels and repairing neuronal injury). Exosomes from serum also play a role in the pathogenesis of PD. For example, Jiang et al. [215] discovered that serum exosomes containing miR-137 help mitigate oxidative stress damage in PD neurons. Their experiments in vitro and in vivo showed that down-regulation of miR-137 upregulates oxidation resistance 1 (OXR1), which ameliorates oxidative stress-induced injury in PD. Additionally, exosomes derived from embryonic stem cells (T-MSC-Exos) can deliver miR-100-5p, which inhibits NADPH oxidase 4 (NOX4), reducing oxidative stress and ROS production via the Nox4-ROS-Nrf2 axis, thus improving dopaminergic neuronal damage in PD [216].
Exosomes can also be used to treat PD by inducing autophagy. By promoting autophagy, exosomes can help remove these toxic aggregates or dysfunctional mitochondria, thereby reducing cellular stress and increasing neuronal survival (Figure 6). Mitochondrial dysfunction is one of the pathological hallmarks of NDDs, and studies suggest that enhancing the mitophagy pathway may represent a novel therapeutic approach to target the potential pathogenic causes of NDDs [218].
For instance, Xu et al. [217] utilized exosomes derived from Pu-Exos to deliver therapeutic drugs to the brain, which modulated PINK1-Parkin-mediated autophagy and the activity of mitochondrial respiratory chain complexes. This approach reversed mitochondrial dysfunction in dopaminergic neurons, significantly alleviating both motor and non-motor symptoms in PD mouse models. In addition, Sul et al. [156] found that Dopa-EVs, aside from enhancing targeting efficiency, stimulate autophagy by upregulating the expression of Beclin-1, Parkin, and LC3-II, and promote the expression of tyrosine hydroxylase to alleviate dopaminergic neuron loss and motor dysfunction in a PD model. Geng et al. [219] demonstrated that MSC-Exos loaded with miR-23b-3p can modulate the Wnt/β-catenin signaling pathway, promoting autophagy in neurons and thereby mitigating the progression of PD.
In addition to modulating PD pathogenesis, researchers have also explored chemical delivery methods to improve dopamine transmission in the brain. One approach involves using exosomes loaded with dopamine or levodopa for intracerebral drug delivery. Although earlier attempts using nanocarriers to increase intracerebral drug delivery were not highly successful, Qu et al. [220] designed blood exosomes loaded with dopamine to target the brain. These exosomes cross the BBB via the transferrin-transferrin receptor interactions, resulting in more than a 15-fold increase in brain dopamine distribution. Animal studies have shown that dopamine-loaded blood exosomes improve dopaminergic neuron function, highlighting their potential for PD therapy.
Selected examples of exosomes as delivery vehicles for the treatment of PD via autophagy. Pueraria lobata (Pu-Exos) restore dopaminergic neuron function by activating PINK1-Parkin-mediated mitophagy pathway; Dopa-EVs enhance clearance of misfolded proteins by upregulating key autophagy proteins including Beclin-1/Parkin/LC3-II; and MSC-Exos deliver miR-23b-3p to regulate Wnt/β-catenin signaling pathway, thereby inhibiting neurodegeneration. These exosome therapies collectively ameliorate PD pathology by enhancing cellular autophagy to eliminate dysfunctional mitochondria and misfolded proteins. The figure was created with BioRender. The original conceptual information is derived from published work, and were adapted with permission from [217], copyright 2024, Elsevier Ltd.
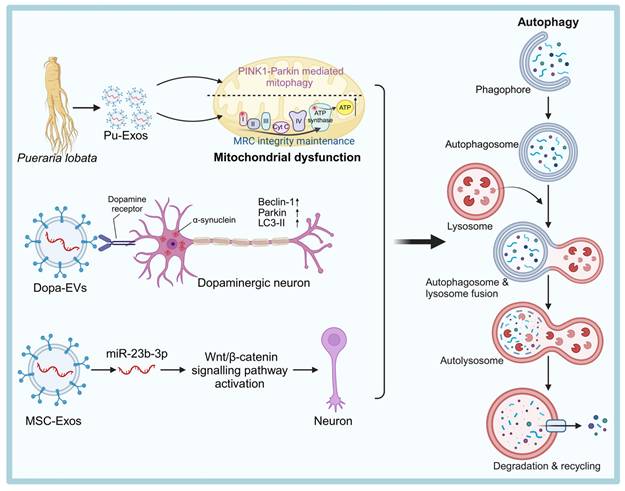
Amyotrophic lateral sclerosis (ALS)
ALS is a fatal neurodegenerative disease, characterized by progressive skeletal muscle atrophy, myofascial fibrillation, and neurological impairments [5]. The majority of patients die within 3-5 years of the first symptoms. The pathogenesis of ALS is not fully understood but involves impaired RNA metabolism, protein aggregation (such as DNA and RNA-binding protein and mutant superoxide dismutase 1 (SOD1)), autophagy defects, and mitochondrial dysfunction [221]. Exosomes are being explored as therapeutic tools for ALS. For instance, Bonafede et al. [222] showed that exosomes derived from mouse adipose-derived stromal cells (ASC-Exos) protect motor neuron-like cells from oxidative stress by targeting mutant SOD1. Therefore, ASC-Exos could be a potential therapeutic approach for ALS.
Mitochondrial dysfunction, a key feature of ALS, can be alleviated by ASC-Exos [223]. In an in vitro ALS model, ASC-Exos restored mitochondrial complex I activity, improved coupling efficiency, and membrane potential, which helped reduce mitochondrial dysfunction [224]. Furthermore, ASC-Exos have been shown to improve motor performance and protect motor neurons in ALS mouse models, indicating their therapeutic potential.
Huntington's disease (HD)
HD is an autosomal dominant neurodegenerative disorder caused by the amplification of CAG repeats in the Huntington protein (HTT) gene [225]. In normal individuals, the HTT gene contains 3-35 CAG repeats, while in individuals with HD, the HTT gene contains 36 or more CAG repeats [226]. The resulting mutant HTT (mHTT) aggregates in neurons, leading to striatal cell death through transcriptional dysregulation, activation of the intrinsic apoptotic pathway, and mitochondrial dysfunction.
Zhang et al. Current therapeutic strategies for HD mainly focus on silencing mHTT through siRNAs and ASOs. Oligonucleotide therapeutics, which target DNA or RNA to block the expression of mutant proteins, are a promising approach in gene therapy [227]. Recent studies have explored exosomes loaded with hydrophobically modified siRNAs (hsiRNAs) to specifically silence Htt mRNA [227]. Zhang et al. [228] designed genetic circuits that modify the mouse liver to transcribe and self-assemble mHTT siRNA into RVG-modified exosomes. These exosomes significantly reduced mHTT expression and alleviated symptoms in HD mouse models.
Additionally, exosomes derived from ASCs have been shown to reduce mHTT aggregation in neurons [229]. Studies have also indicated that mitochondrial dysfunction in HD is linked to the downregulation of the p-CREB-PGC1α pathway [230]. ASC-Exos protect mitochondrial function by activating the p-CREB-PGC1α pathway, providing a potential therapeutic strategy for HD.
Multiple sclerosis (MS)
MS is an autoimmune disease affecting the CNS, primarily characterized by demyelinating lesions in the brain and spinal cord, axonal damage, and neuronal loss [231]. Most treatment strategies for MS focus on preventing inflammation and promoting myelin regeneration.
Under normal physiological conditions, oligodendrocyte precursor cells (OPCs) differentiate into mature oligodendrocytes (OLs), which are responsible for forming myelin sheaths and ensuring the proper transmission of neural impulses [232]. In MS, OPC differentiation is impaired, hindering the regeneration of myelin. Research has shown that MSC-Exos can enhance myelin regeneration by directly promoting OPC differentiation and indirectly modulating neuroinflammation in the CNS, thereby improving neurological function in MS models [149]. BDNF plays a critical role in regulating the proliferation and differentiation of OPCs into OLs [233]. Unfortunately, BDNF cannot cross the BBB and thus cannot be directly administered in MS treatments. Zhai et al. [234] developed RVG-modified exosomes (RVG-Exos) as a targeted drug delivery system to overcome this challenge. Their findings showed that RVG/BDNF-Exos successfully facilitated the delivery of BDNF to the brain, promoting OPC proliferation, OL differentiation, and myelin regeneration in the corpus callosum of cuprizone-induced mice, ultimately improving motor coordination. Additionally, triiodothyronine (T3), a bioactive molecule, has been shown to enhance myelination during CNS development. Exosomes loaded with T3, and modified with platelet-derived growth factor A (PDGFA) ligands, exhibit enhanced targeting ability for OPCs and OLs, thereby further promoting myelin formation [235].
BMSC-derived exosomes (BMSC-Exos) have also been shown to reduce demyelination, minimize CNS infiltration by inflammatory cells, and significantly improve neurobehavioral outcomes [236]. After treatment with BMSC-Exos, levels of M2-associated cytokines (e.g., IL-10, TGF-β) were significantly increased, while levels of M1-associated cytokines (e.g., TNF-α, IL-12) were markedly reduced, further supporting the anti-inflammatory effects [237].
Building on previous IFN-based therapies for MS, researchers have speculated that exosomes derived from IFN-stimulated MSCs may offer enhanced therapeutic benefits. Riazifar et al. [57] assessed the efficacy of MSC-Exos and exosomes derived from IFNγ-stimulated MSCs (IFNγ-Exos) in MS treatment. Their results revealed that IFNγ-Exos significantly reduced demyelination and neuroinflammation while enhancing motor skills in experimental autoimmune encephalomyelitis mice. Moreover, IFNγ stimulation of dendritic cells (DCs) promoted the release of exosomes containing high levels of miR-219 [238]. These exosomes were effective in increasing myelin formation, reducing oxidative stress, and improving myelin regeneration following acute demyelination.
Clinical Translation of Exosome Therapies: Trials and Challenges
Although basic research has demonstrated the significant therapeutic potential of exosomes in NDDs, clinical trials exploring exosomes as drug carriers remain relatively limited. A study by Ma et al. [239] showed that intranasal administration of ADSC-Exos enable rapid delivery to the brain via the olfactory nerve, alleviating memory deficits in APP/PS1 transgenic mice by reducing microglia activation, inhibiting Aβ deposition, and promoting neurogenesis and neuroprotection (Figure 7). Based on these findings, they conducted a Phase I/II clinical trial using allogeneic human ADSC-Exos (ahaMSC-Exos) in AD patients to assess its safety and efficacy [240]. The results revealed improved cognitive function at 12 weeks, with a slight reduction in hippocampal volume in the medium-dose group, despite no significant changes in amyloid or Tau deposition. This study is the first international trial to use ahaMSC-derived exosomes for nasal administration in AD treatment, validating the safety of exosomal therapy and laying the foundation for future large-scale clinical trials. Akhlaghpasand et al. [241] conducted a first-in-human, single-arm, phase I clinical trial to evaluate the safety and potential effects of intrathecal injection of allogeneic exosomes derived from HUC-MSCs-Exos in patients with fully subacute SCI. The results of this phase I trial suggest that intrathecal injection of allogeneic HUC-MSCs-Exos is both safe and well-tolerated by patients. The findings indicate the need for a larger phase II clinical trial to further evaluate the feasibility and potential therapeutic benefits of this approach.
ADSC-Exos rapidly and efficiently enter the brain via intranasal administration, accumulating primarily in neurons of the central nervous system. Its therapeutic mechanism involves reducing microglia activation, inhibiting Aβ deposition, and promoting neurogenesis and neuroprotection, which together help alleviate the pathological manifestations of AD. In clinical trials, ahaMSC-Exos were used to assess their therapeutic efficacy in AD patients, with a focus on evaluating both safety and effectiveness.
Additionally, AB126 is an unmodified neurogenic exosome with the natural ability to cross the BBB and demonstrate anti-inflammatory and neuroprotective effects. In January this year, the U.S. FDA approved an Investigational New Drug (IND) application for AB126, making it the first exosome-based drug to enter human clinical trials. This approval marks a breakthrough in exosome drug delivery and provides new therapeutic hope for patients suffering from acute ischemic stroke.
Currently, most exosome therapies are still in Phase I clinical trials, focused on testing accelerated doses in a small cohort of patients to identify potential side effects. However, several clinical studies of exosome therapies for the treatment of NDDs have been initiated, with specific information from ClinicalTrials.gov (Table 6). Early findings suggest that exosomes are effective at transporting therapeutic molecules across the BBB, reducing neuroinflammation, and promoting nerve repair.
Although challenges such as large-scale production, standardization, and clinical efficacy validation remain, the potential of exosome therapies as precise and efficient treatments is promising. As research progresses, the clinical application of exosomes in treating neurodegenerative diseases is expected to accelerate, offering more effective treatment options for patients.
The Importance of Interdisciplinary Collaboration: The Intersection of Nanotechnology, Neuroscience, and Clinical Medicine
In the rapidly advancing field of exosome-based therapies and other cutting-edge treatments for neurological and systemic diseases, interdisciplinary collaboration plays a pivotal role in fostering innovation and overcoming the complex challenges inherent in therapy development. Such collaboration has become a major trend for future advancements. For instance, Li et al. [242], addressing the current shortcomings in the treatment of neurological diseases, summarized key trends and provided in-depth insights into the innovation and development of neuromodulation technologies within the context of interdisciplinary cross-research. They identified three primary development trends and further explored the opportunities and challenges associated with applying and translating neuromodulation technologies in an interdisciplinary framework. In this context, we examine the importance of collaboration between three critical disciplines—nanotechnology, neuroscience, and clinical medicine—toward accelerating clinical trial progress and ensuring the successful translation of discoveries from the laboratory to clinical applications.
NDDs present a substantial economic and health burden worldwide. Despite ongoing research, there is still no effective strategy to cure these diseases. However, with the continuous advancement of nanotechnology, researchers have developed a variety of nanomaterials for brain tissue drug delivery, particularly the use of exosomes. Exosomes help overcome the limitations of conventional delivery systems, such as low bioavailability, instability, and rapid clearance, especially in sensitive areas like the brain. Nevertheless, focusing exclusively on nanotechnology development is insufficient to fully address the treatment of NDDs.
Therapeutic mechanisms of ADSC-Exos in AD animal experiments and clinical trials of ahaMSC-Exos. ADSC-Exos are delivered via the olfactory pathway (nasal mucosa→olfactory bulb) to target the brain, where they regulate microglial activation and Aβ production to exert neuroprotective effects. The figure also outlines the standardized clinical evaluation protocol (including cognitive tests and PET scans) for ahaMSC-Exos in clinical trials. This figure was created with BioRender.
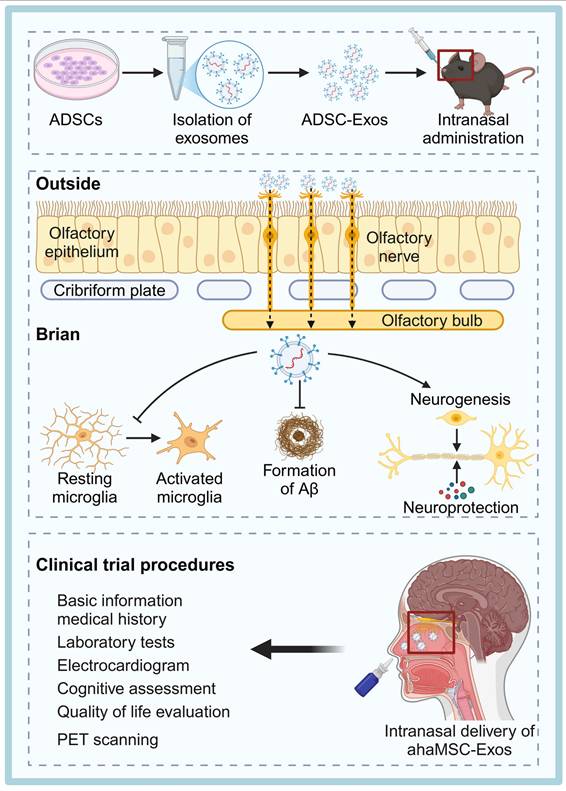
Clinical trials related to exosomes
| Disease | Trial phase and sample size | Primary objective | Experimental method | Preliminary results | Advantages of this exosome | ID |
|---|---|---|---|---|---|---|
| AD | Interventional I/II 9 | To evaluate the safety and efficacy of allogeneic human adipose-derived MSC exosomes (ahaMSCs-Exos) in patients with mild to moderate AD | Nasal administration of different doses of ahaMSCs-Exos. | Nasal administration of ahaMSCs-Exos is safe and well-tolerated, with doses of at least 4×10^8 particles considered suitable for further clinical trials | ahaMSCs-Exos exosomes have better immunomodulatory and neuroprosthetic effects, adipose tissue is easily available and less likely to cause immune rejection | NCT04388982 |
| ALS | Interventional I 38 | To assess the safety and preliminary efficacy of nasal exosomes derived from human umbilical cord MSCs (hUC-MSC-sEV-001) in ALS | Nasal administration | Ongoing | hUC-MSC-sEV possesses immune-modulatory and neuroprotective effects, and umbilical cord-derived MSCs are easier to obtain and have lower immunogenicity compared to adult-derived MSCs | NCT06598202 |
| NDDs | Interventional I 100 | To assess the safety and preliminary efficacy of nasal hUC-MSC-sEV-001 exosomes derived from human umbilical MSCs in various neurodegenerative diseases | hUC-MSC-sEV-001 intranasal administration | Ongoing | Strong neuroprotective ability to cross the blood-brain barrier and repair neurological damage | NCT06607900 |
| NDDs | Interventional Not Applicable 300 | To evaluate the safety and efficacy of exosome delivery combined with synchronized transcranial focused ultrasound for treatment-resistant depression, anxiety, and neurodegenerative dementia | Focused ultrasound delivery of intravenously infused exosomes | / | Can effectively enhance drug delivery in the brain and break the blood-brain barrier | NCT04202770 |
| PD | Observational 601 | To identify exosomal protein biomarkers related to PD susceptibility and/or progression in PD patients, and to establish targeted effect detection methods for future LRRK2 inhibitor clinical trials | / | / | The choice of exosome biomarkers as an early diagnostic tool for PD enables non-invasive detection of pathological features in PD patients, facilitating early intervention and monitoring of efficacy | NCT01860118 |
| PD | Observational 38 | To optimize the protocol for isolating exosomes from CSF to improve the detection of LRRK2 activity in human CSF | Lumbar puncture for CSF collection | / | CSF- Exos directly reflect CNS status and are suitable for clinical monitoring of PD | NCT03775447 |
| Refractoryfocal epilepsy | Interventional Early I 34 | To assess the safety, tolerability, and preliminary efficacy of nasal exosomes (GD-iEXo-002) derived from iPSC in treating refractory focal epilepsy | iPSC-derived exosomes administered nasally | Ongoing | iPSC-Exos have strong neuroprosthetic and protective properties, can promote nerve regeneration, and can be used in the clinic without rejection | NCT05886205 |
| Acute ischemic stroke | Interventional I 29 | To evaluate the safety and preliminary efficacy of intravenous exosomes derived from human iPSC (GD-iExo-003) in acute ischemic stroke | Intravenous administration of GD-iExo-003 and placebo | Ongoing | iPSC-Exos have neuroregenerative capacity to repair brain tissue damage after acute ischaemic stroke and are more immunotolerant compared to adult-derived exosomes | NCT06138210 |
Neuroscience provides the foundational understanding of the molecular and cellular mechanisms underlying neurological disorders. It has also unveiled the critical mechanisms by which exosomes can be used therapeutically—such as penetrating the BBB and facilitating targeted delivery within complex neural networks. Only by gaining a deeper understanding of the brain's workings can the delivery systems of exosomes be optimized effectively. Finally, clinical medicine plays an essential role in translating these scientific findings into real-world treatments. Clinicians possess a practical understanding of patient needs, disease heterogeneity, and the challenges inherent in large-scale clinical trials. Their involvement ensures that new therapies are not only scientifically promising but also safe, effective, and feasible for a diverse range of patient populations.
In summary, while exosomes hold great potential as therapeutic delivery vehicles for treating NDDs, their successful application hinges on the interdisciplinary collaboration of nanotechnology, neuroscience, and clinical medicine. Through the deep integration and ongoing cooperation across these disciplines, researchers can share valuable resources—such as specialized laboratory equipment, patient data, and preclinical models—accelerating the process from basic discovery to clinical application. This continued collaboration will drive progress in emerging therapies like exosome-based treatments, ultimately opening new avenues for treating patients with neurological disorders and other diseases.
Conclusion and Perspective
With an aging population, the incidence of NDDs is rising, making early intervention crucial. Recent progress in nanomedicine has deepened our understanding of exosomes, which are a promising class of EVs for drug delivery due to their excellent biocompatibility, low immunogenicity, ability to cross the BBB, prolonged circulation time, and diverse cargo-loading capacity. They can not only efficiently transport biomolecules (e.g., proteins, nucleic acids) to brain targets, but also enhance inter-neuronal communication. Notably, ISEV updated the definition of EV in the MISEV2023 guideline by deleting the expression “naturally released” [120], to avoid inadvertently excluding engineered EVs or EVs generated under different cell culture conditions, and to expand the theoretical framework for their therapeutic applications. Based on these properties, exosomes are considered as a potential game-changer for treating AD, PD, ALS, HD and MS, and several clinical trials are underway to evaluate their safety and efficacy.
However, the clinical translation of exosome therapy still faces multiple challenges. Current exosome isolation techniques can hardly meet the high requirements for yield, purity and batch consistency in clinical applications. Surface modification of exosomes, while enhancing BBB penetration and intracerebral drug delivery, may produce off-target effects and reduce reproducibility. In addition, existing cargo loading techniques, such as incubation, electroporation and sonication, still face limitations such as cost, efficiency and potential damage to exosomes. To overcome these challenges, the development and optimization of novel technologies is critical to advance exosome-based drug delivery systems. In addition, we need to deepen our understanding of exosomes' biogenesis, release mechanisms, and trans-BBB transport processes, which are still only partially understood, limiting their full therapeutic potential. Clinical trials aimed at validating the safety and efficacy of exosome therapies also need to be improved in terms of dosage selection, control group settings, efficacy assessment and safety monitoring. Standardized assessment metrics are essential for accurately determining treatment outcomes and ensuring patient safety (Table 7).
Currently, exosomes are regulated as biological products in many countries and regions. However, due to the complexity of exosomes, the regulatory environment worldwide exhibits complexity and variability. The regulatory standards in different regions are mainly based on the differences in the characteristics of exosomes: in the United States and Europe, the regulatory focus is on whether the content of exosomes affects the physiological functions of the human body; while in Japan, South Korea, and Taiwan, China, the regulatory rules are mainly based on the way of obtaining exosomes [243]. Moreover, there are also differences in the implementation of GMP quality systems in various countries, leading to differences in regulatory approaches. Current GMP (cGMP) focuses more on quality control of the final product, whereas the Pharmaceutical Inspection Agreement and Co-operation Scheme GMP (PIC/S GMP) emphasizes more detailed organization, validation, and training requirements, and differs especially in the quality assessment of cellular products. This difference in regulatory focus is a direct result of the lack of harmonization of standards for exosome manufacturing. Developers face a lack of clear guidance on product classification, evaluation criteria, and approval pathways, contributing to uncertainties in development timelines. Without a coordinated international regulatory framework, exosome therapies—despite their promising potential—may remain trapped in the bottleneck between laboratory research and clinical translation.
Beyond technical and regulatory issues, other factors such as cost, regulation, and large-scale production must be addressed for the successful clinical application of exosome therapies. Interdisciplinary collaboration between materials science and neuroscience is pivotal in overcoming these obstacles. Expertise from materials science can lead to the design of advanced materials that improve exosome stability, targeting efficiency, and cargo-loading capacity, while neuroscience can offer insights into the mechanisms governing exosome interactions with the brain. By combining knowledge from these two fields, we can develop more effective exosome therapies, reduce production costs, and meet regulatory standards, ultimately enabling widespread clinical use for treating NDDs.
Looking ahead, several areas hold promise for future technological breakthroughs in exosome-based therapies. Advances in nanomaterial design can help optimize exosome isolation and purification processes, enhancing scalability while maintaining high quality. Surface engineering could improve targeted delivery and reduce off-target effects, while cargo-loading technologies—such as synthetic biology or advanced electroporation—may increase exosome cargo capacity without compromising integrity. Furthermore, the integration of gene editing techniques could allow for precise control over exosome content, opening the door to tailored therapeutic applications.
In conclusion, short-term efforts should focus on ensuring the safety of exosome therapies through comprehensive Phase I, II, and III clinical trials to assess their tolerability and preliminary efficacy in treating NDDs. Long-term objectives should include refining production processes, enhancing cargo-loading capacities, and gaining a deeper understanding of exosome biogenesis and BBB crossing mechanisms. With sustained interdisciplinary collaboration and technological advancements in both materials science and neuroscience, exosomes hold the potential to revolutionize the treatment of NDDs.
Standardized assessment indicators for exosome treatment in NDDs
| Indicator Category | Standardized Indicators | Ref. |
|---|---|---|
| Cognitive function | Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) Mini-Mental State Examination (MMSE) Montreal Cognitive Assessment (MoCA) Neuropsychiatric Inventory (NPI) | [244-247] |
| Motor function | Movement Disorders Society Unified Parkinson Disease Rating Scale (MDS-UPDRS) Timed Up and Go Test (TUG) | [248] |
| Neuroimaging | Positron emission tomography (PET) Magnetic Resonance Imaging (MRI) Functional MRI (fMRI) | [249] |
| Quality of life | Alzheimer's Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) Instrumental Activities of Daily Living Scale (IADL) Parkinson's Disease Questionnaire (PDQ-39) | [250,251] |
| Inflammation level | TNF-α, IL-1, IL-6, Aβ, Tau | [240] |
Acknowledgements
This study was supported by financial support from the National Natural Science Foundation of China (grant number: 82202461).
Author contributions
Q. H. and X. M. provided the concept and outline of this manuscript. S. W. and Q. H. wrote this manuscript. Z. Liu., L. R., K. C. and X. M. commented and revised the entire manuscript.
AI tool use statement
The authors declare that artificial intelligence tools (e.g., ChatGPT) were used solely for limited language refinement and minor grammar corrections of a few sentences. All intellectual content, literature interpretation, and critical analysis were independently developed and verified by the authors, who take full responsibility for the content of the review.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Steinmetz JD, Seeher KM, Schiess N, Nichols E, Cao B, Servili C. et al. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23:344-81
2. Wenk GL. Neuropathologic changes in Alzheimer's disease. J Clin Psychiatry. 2003;64(Suppl 9):7-10
3. Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323:548-60
4. Jankovska N, Matej R. Molecular Pathology of ALS: What We Currently Know and What Important Information Is Still Missing. Diagnostics. 2021;11:1365
5. Costa J, De Carvalho M. Emerging molecular biomarker targets for amyotrophic lateral sclerosis. Clin Chim Acta. 2016;455:7-14
6. Jimenez-Sanchez M, Licitra F, Underwood BR, Rubinsztein DC. Huntington's Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb Perspect Med. 2017;7:a024240
7. Jakimovski D, Bittner S, Zivadinov R, Morrow SA, Benedict RH, Zipp F. et al. Multiple sclerosis. The Lancet. 2024;403:183-202
8. Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023;186:693-714
9. Xu J, Du W, Zhao Y, Lim K, Lu L, Zhang C. et al. Mitochondria targeting drugs for neurodegenerative diseases—Design, mechanism and application. Acta Pharm Sin B. 2022;12:2778-89
10. Shahjin F, Chand S, Yelamanchili SV. Extracellular Vesicles as Drug Delivery Vehicles to the Central Nervous System. J Neuroimmune Pharmacol. 2020;15:443-58
11. Qu M, Lin Q, He S, Wang L, Fu Y, Zhang Z. et al. A brain targeting functionalized liposomes of the dopamine derivative N-3,4-bis(pivaloyloxy)-dopamine for treatment of Parkinson's disease. J Controlled Release. 2018;277:173-82
12. Dal Magro R, Ornaghi F, Cambianica I, Beretta S, Re F, Musicanti C. et al. ApoE-modified solid lipid nanoparticles: A feasible strategy to cross the blood-brain barrier. J Controlled Release. 2017;249:103-10
13. Loureiro JA, Gomes B, Fricker G, Coelho MAN, Rocha S, Pereira MC. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer's disease treatment. Colloids Surf B Biointerfaces. 2016;145:8-13
14. Banskota S, Yousefpour P, Chilkoti A. Cell-Based Biohybrid Drug Delivery Systems: The Best of the Synthetic and Natural Worlds. Macromol Biosci. 2017;17:1600361
15. Martano S, De Matteis V, Cascione M, Rinaldi R. Inorganic Nanomaterials versus Polymer-Based Nanoparticles for Overcoming Neurodegeneration. Nanomaterials. 2022;12:2337
16. Bayat F, Hosseinpour-Moghadam R, Mehryab F, Fatahi Y, Shakeri N, Dinarvand R. et al. Potential application of liposomal nanodevices for non-cancer diseases: an update on design, characterization and biopharmaceutical evaluation. Adv Colloid Interface Sci. 2020;277:102121
17. Zhao W, Li A, Zhang A, Zheng Y, Liu J. Recent Advances in Functional-Polymer-Decorated Transition-Metal Nanomaterials for Bioimaging and Cancer Therapy. ChemMedChem. 2018;13:2134-49
18. Bassyouni F, ElHalwany N, Abdel Rehim M, Neyfeh M. Advances and new technologies applied in controlled drug delivery system. Res Chem Intermed. 2015;41:2165-200
19. Witwer KW, Wolfram J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 2021;6:103-6
20. Almeida JPM, Chen AL, Foster A, Drezek R. In vivo biodistribution of nanoparticles. Nanomed. 2011;6:815-35
21. Shimon MB, Shapira S, Seni J, Arber N. The Big Potential of Small Particles: Lipid-Based Nanoparticles and Exosomes in Vaccination. Vaccines. 2022;10:1119
22. Liaw K, Zhang Z, Kannan S. Neuronanotechnology for brain regeneration. Adv Drug Deliv Rev. 2019;148:3-18
23. Gao C, Xiong R, Zhang Z, Peng H, Gu Y, Xu W. et al. Hybrid nanostructures for neurodegenerative disease theranostics: the art in the combination of biomembrane and non-biomembrane nanostructures. Transl Neurodegener. 2024;13:43
24. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977
25. Mondal J, Pillarisetti S, Junnuthula V, Saha M, Hwang SR, Park I. et al. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J Controlled Release. 2023;353:1127-49
26. Rehman FU, Liu Y, Zheng M, Shi B. Exosomes based strategies for brain drug delivery. Biomaterials. 2023;293:121949
27. Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am J Physiol - Cell Physiol. 2014;306:C621-33
28. Mehryab F, Rabbani S, Shahhosseini S, Shekari F, Fatahi Y, Baharvand H. et al. Exosomes as a next-generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42-62
29. Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T. et al. Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1-14
30. Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65:357-67
31. Zappulli V, Friis KP, Fitzpatrick Z, Maguire CA, Breakefield XO. Extracellular vesicles and intercellular communication within the nervous system. J Clin Invest. 126: 1198-207.
32. Yu T, Xu Y, Ahmad MA, Javed R, Hagiwara H, Tian X. Exosomes as a Promising Therapeutic Strategy for Peripheral Nerve Injury. Curr Neuropharmacol. 2021;19:2141-51
33. Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197:591-605
34. Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 2014;11:68
35. Haas AK, Fuchs E, Kopajtich R, Barr FA. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat Cell Biol. 2005;7:887-93
36. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19-30
37. Song L, Tang S, Han X, Jiang Z, Dong L, Liu C. et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun. 2019;10:1639
38. DAmico KA, Stanton AE, Shirkey JD, Travis SM, Jeffrey PD, Hughson FM. Structure of a membrane tethering complex incorporating multiple SNAREs. Nat Struct Mol Biol. 2024;31:246-54
39. Liu D-A, Tao K, Wu B, Yu Z, Szczepaniak M, Rames M. et al. A phosphoinositide switch mediates exocyst recruitment to multivesicular endosomes for exosome secretion. Nat Commun. 2023;14:6883
40. Lee C, Lepore D, Lee S-H, Kim TG, Buwa N, Lee J. et al. Exocyst stimulates multiple steps of exocytic SNARE complex assembly and vesicle fusion. Nat Struct Mol Biol. 2025;32:150-60
41. Mathieu M, Névo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ. et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12:4389
42. Gao W, Li F, Liu L, Xu X, Zhang B, Wu Y. et al. Endothelial colony-forming cell-derived exosomes restore blood-brain barrier continuity in mice subjected to traumatic brain injury. Exp Neurol. 2018;307:99-108
43. Minakaki G, Menges S, Kittel A, Emmanouilidou E, Schaeffner I, Barkovits K. et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy. 2018;14:98-119
44. Yanagawa K, Kuma A, Hamasaki M, Kita S, Yamamuro T, Nishino K. et al. The Rubicon-WIPI axis regulates exosome biogenesis during ageing. Nat Cell Biol. 2024;26:1558-70
45. Liu S, Fan M, Xu J-X, Yang L-J, Qi C-C, Xia Q-R. et al. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation. 2022;19:35
46. Wang Q-S, Xiao R-J, Peng J, Yu Z-T, Fu J-Q, Xia Y. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal KLF4 Alleviated Ischemic Stroke Through Inhibiting N6-Methyladenosine Modification Level of Drp1 by Targeting lncRNA-ZFAS1. Mol Neurobiol. 2023;60:3945-62
47. Lai X, Wang Y, wang X, Liu B, Rong L. miR-146a-5p-modified hUCMSC-derived exosomes facilitate spinal cord function recovery by targeting neurotoxic astrocytes. Stem Cell Res Ther. 2022;13:487
48. Zhang R, Mao W, Niu L, Bao W, Wang Y, Wang Y. et al. NSC-derived exosomes enhance therapeutic effects of NSC transplantation on cerebral ischemia in mice. eLife. 2023;12:e84493
49. Gao G, Li C, Ma Y, Liang Z, Li Y, Li X. et al. Neural stem cell-derived extracellular vesicles mitigate Alzheimer's disease-like phenotypes in a preclinical mouse model. Signal Transduct Target Ther. 2023;8:1-4
50. Madhu LN, Kodali M, Upadhya R, Rao S, Somayaji Y, Attaluri S. et al. Extracellular vesicles from human-induced pluripotent stem cell-derived neural stem cells alleviate proinflammatory cascades within disease-associated microglia in Alzheimer's disease. J Extracell Vesicles. 2024;13:e12519
51. Rao S, Madhu LN, Babu RS, Nagarajan A, Narvekar E, Upadhya R. et al. Extracellular Vesicles from Human iPSC-derived NSCs Protect Human Neurons Against Aβ-42 Induced Neurodegeneration, Mitochondrial Dysfunction and Tau Phosphorylation. Alzheimers Dement. 2024;20:e093150
52. Huang J, Zhang G, Li S, Li J, Wang W, Xue J. et al. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J Nanobiotechnology. 2023;21:10
53. Huang J, Li J, Li S, Yang X, Huo N, Chen Q. et al. Netrin-1-engineered endothelial cell exosomes induce the formation of pre-regenerative niche to accelerate peripheral nerve repair. Sci Adv. 2024;10:eadm8454
54. Liao H-J, Yang Y-P, Liu Y-H, Tseng H-C, Huo T-I, Chiou S-H. et al. Harnessing the potential of mesenchymal stem cells-derived exosomes in degenerative diseases. Regen Ther. 2024;26:599-610
55. Lo Furno D, Mannino G, Giuffrida R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J Cell Physiol. 2018;233:3982-99
56. Kim DH, Lee D, Chang EH, Kim JH, Hwang JW, Kim J-Y. et al. GDF-15 Secreted from Human Umbilical Cord Blood Mesenchymal Stem Cells Delivered Through the Cerebrospinal Fluid Promotes Hippocampal Neurogenesis and Synaptic Activity in an Alzheimer's Disease Model. Stem Cells Dev. 2015;24:2378-90
57. Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lässer C, Segaliny AI. et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano. 2019;13:6670-88
58. Xue C, Li X, Ba L, Zhang M, Yang Y, Gao Y. et al. MSC-Derived Exosomes can Enhance the Angiogenesis of Human Brain MECs and Show Therapeutic Potential in a Mouse Model of Parkinson's Disease. Aging Dis. 2021;12:1211-22
59. Hu H, Hu X, Li L, Fang Y, Yang Y, Gu J. et al. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in Ischemic Stroke Mice via Upregulation of MiR-21-5p. Biomolecules. 2022;12:883
60. Perets N, Betzer O, Shapira R, Brenstein S, Angel A, Sadan T. et al. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019;19:3422-31
61. Li X, Zhu Y, Wang Y, Xia X, Zheng JC. Neural stem/progenitor cell-derived extracellular vesicles: A novel therapy for neurological diseases and beyond. MedComm. 2023;4:e214
62. Niu X, Xia Y, Luo L, Chen Y, Yuan J, Zhang J. et al. iPSC-sEVs alleviate microglia senescence to protect against ischemic stroke in aged mice. Mater Today Bio. 2023;19:100600
63. Pasut A, Lama E, Van Craenenbroeck AH, Kroon J, Carmeliet P. Endothelial cell metabolism in cardiovascular physiology and disease. Nat Rev Cardiol. 2025
64. Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic — implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18:297-312
65. Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL. et al. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85:40-7
66. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748-59
67. Kimiz-Gebologlu I, Oncel SS. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Controlled Release. 2022;347:533-43
68. Bok E-Y, Seo SY, Lee HG, Wimalasena SHMP, Kim E, Cho A. et al. Exosomes isolation from bovine serum: qualitative and quantitative comparison between ultracentrifugation, combination ultracentrifugation and size exclusion chromatography, and exoEasy methods. J Anim Sci Technol. 2024;66:1021-33
69. Chhoy P, Brown CW, Amante JJ, Mercurio AM. Protocol for the separation of extracellular vesicles by ultracentrifugation from in vitro cell culture models. STAR Protoc. 2021;2:100303
70. Gupta S, Rawat S, Arora V, Kottarath SK, Dinda AK, Vaishnav PK. et al. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res Ther. 2018;9:180
71. Chou C-Y, Chiang P-C, Li C-C, Chang J-W, Lu P-H, Hsu W-F. et al. Improving the Purity of Extracellular Vesicles by Removal of Lipoproteins from Size Exclusion Chromatography- and Ultracentrifugation-Processed Samples Using Glycosaminoglycan-Functionalized Magnetic Beads. ACS Appl Mater Interfaces. 2024;16:44386-98
72. Ko M, Kim HJ, Park J, Lee H, Lee KN, Kim K. et al. Isolation of Bovine Milk Exosome Using Electrophoretic Oscillation Assisted Tangential Flow Filtration with Antifouling of Micro-ultrafiltration Membrane Filters. ACS Appl Mater Interfaces. 2023;15:26069-80
73. Kapoor KS, Harris K, Arian KA, Ma L, Schueng Zancanela B, Church KA. et al. High throughput and rapid isolation of extracellular vesicles and exosomes with purity using size exclusion liquid chromatography. Bioact Mater. 2024;40:683-95
74. Guo J, Wu C, Lin X, Zhou J, Zhang J, Zheng W. et al. Establishment of a simplified dichotomic size-exclusion chromatography for isolating extracellular vesicles toward clinical applications. J Extracell Vesicles. 2021;10:e12145
75. Gao M, Cai J, Zitkovsky HS, Chen B, Guo L. Comparison of Yield, Purity, and Functional Properties of Large-Volume Exosome Isolation Using Ultrafiltration and Polymer-Based Precipitation. Plast Reconstr Surg. 2022;149:638
76. Pei S, Sun W, Han Q, Wang H, Liang Q. Bifunctional immunoaffinity magnetic nanoparticles for high-efficiency separation of exosomes based on host-guest interaction. Talanta. 2024;272:125790
77. Zhang K, Yue Y, Wu S, Liu W, Shi J, Zhang Z. Rapid Capture and Nondestructive Release of Extracellular Vesicles Using Aptamer-Based Magnetic Isolation. ACS Sens. 2019;4:1245-51
78. Hassanpour Tamrin S, Sanati Nezhad A, Sen A. Label-Free Isolation of Exosomes Using Microfluidic Technologies. ACS Nano. 2021;15:17047-79
79. Shen J, Ma Z, Xu J, Xue T, Lv X, Zhu G. et al. Exosome Isolation and Detection: From Microfluidic Chips to Nanoplasmonic Biosensor. ACS Appl Mater Interfaces. 2024;16:22776-93
80. Lan M, Yang F. Applications of dielectrophoresis in microfluidic-based exosome separation and detection. Chem Eng J. 2024;491:152067
81. Livshts MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE. et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319
82. Teixeira-Marques A, Monteiro-Reis S, Montezuma D, Lourenço C, Oliveira MC, Constâncio V. et al. Improved recovery of urinary small extracellular vesicles by differential ultracentrifugation. Sci Rep. 2024;14:12267
83. Chiou N-T, Ansel KM. Improved exosome isolation by sucrose gradient fractionation of ultracentrifuged crude exosome pellets. Protoc Exch. 2016
84. Li K, Wong DK, Hong KY, Raffai RL. Cushioned-Density Gradient Ultracentrifugation (C-DGUC): A Refined and High Performance Method for the Isolation, Characterization, and Use of Exosomes. Methods Mol Biol Clifton NJ. 2018;1740:69-83
85. D'Acunzo P, Kim Y, Ungania JM, Pérez-González R, Goulbourne CN, Levy E. Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain tissues. Nat Protoc. 2022;17:2517-49
86. Han Z, Peng C, Yi J, Zhang D, Xiang X, Peng X. et al. Highly efficient exosome purification from human plasma by tangential flow filtration based microfluidic chip. Sens Actuators B Chem. 2021;333:129563
87. Hou G, Li Y, Cui X, Zhao B, Liu L, Zhang Y. et al. Electric Field Assisted Tangential Flow Filtration Device for Highly Effective Isolation of Bioactive Small Extracellular Vesicles from Cell Culture Medium. Anal Chem. 2024;96:13345-51
88. Lorenzen S, Ye Y, Chen V, Christensen ML. Direct observation of fouling phenomena during cross-flow filtration: Influence of particle surface charge. J Membr Sci. 2016;510:546-58
89. Chen Y, Zhu Q, Cheng L, Wang Y, Li M, Yang Q. et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods. 2021;18:212-8
90. Kim G, Kim K-H, Seo M, Kim T, Chun H. High-throughput exosome isolation based on bidirectional flow control through nanoporous membrane. Sens Actuators B Chem. 2024;418:136233
91. Wu X, Shen J, Zhong Y, Zhao X, Zhou W, Gao P. et al. Large-Scale Isolation of Milk Exosomes for Skincare. Pharmaceutics. 2024;16:930
92. Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, Franquesa M, Beyer K, Borràs FE. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles' characteristics compared to precipitating agents. Sci Rep. 2016;6:33641
93. Sidhom K, Obi PO, Saleem A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int J Mol Sci. 2020;21:6466
94. Khan MI, Jeong ES, Khan MZ, Shin JH, Kim JD. Stem cells-derived exosomes alleviate neurodegeneration and Alzheimer's pathogenesis by ameliorating neuroinflamation, and regulating the associated molecular pathways. Sci Rep. 2023;13:15731
95. Yu J, Wei Y, Cui Z, Tian J, Cai H, Zhang W. Thermosensitive Capturer Coupled with the CD63 Aptamer for Highly Efficient Isolation of Exosomes. ACS Macro Lett. 2024;13:195-200
96. Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9:1015-28
97. Guo X, Hu F, Zhao S, Yong Z, Zhang Z, Peng N. Immunomagnetic Separation Method Integrated with the Strep-Tag II System for Rapid Enrichment and Mild Release of Exosomes. Anal Chem. 2023;95:3569-76
98. Lima Moura S, Martì M, Pividori MI. Matrix Effect in the Isolation of Breast Cancer-Derived Nanovesicles by Immunomagnetic Separation and Electrochemical Immunosensing—A Comparative Study. Sensors. 2020;20:965
99. Jia C, Zhu N, Zhang Y, Yu Y, Kang K, Yi Q. et al. Hedgehog-inspired immunomagnetic beads for high-efficient capture and release of exosomes. J Mater Chem B. 2022;10:4059-69
100. Huang R, Xi Z, He N. Applications of aptamers for chemistry analysis, medicine and food security. Sci China Chem. 2015;58:1122-30
101. Seok Kim Y, Ahmad Raston NH, Bock Gu M. Aptamer-based nanobiosensors. Biosens Bioelectron. 2016;76:2-19
102. Zhang N, Sun N, Deng C. Rapid isolation and proteome analysis of urinary exosome based on double interactions of Fe3O4@TiO2-DNA aptamer. Talanta. 2021;221:121571
103. Huang D, Yu J, Tian J, Cai H, Zhang W. Aptamer-Coupled Polymer-Grafted Fe3O4 Nanoparticles for Highly Efficient Isolation of Exosomes. Macromol Rapid Commun. 2025;46:2400819
104. Peng Y, Liang S, Meng Q-F, Liu D, Ma K, Zhou M. et al. Engineered Bio-Based Hydrogels for Cancer Immunotherapy. Adv Mater. 2024;36:2313188
105. Tan D, Zhu W, Liu L, Pan Y, Xu Y, Huang Q. et al. In situ formed scaffold with royal jelly-derived extracellular vesicles for wound healing. Theranostics. 2023;13:2811-24
106. Tang J, Jia X, Li Q, Cui Z, Liang A, Ke B. et al. A DNA-based hydrogel for exosome separation and biomedical applications. Proc Natl Acad Sci. 2023;120:e2303822120
107. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog Lipid Res. 2017;66:30-41
108. Yoshida T, Ishidome T, Hanayama R. High Purity Isolation and Sensitive Quantification of Extracellular Vesicles Using Affinity to TIM4. Curr Protoc Cell Biol. 2017;77:3.45.1-3.45.18
109. Zhang L, Wang H, Zhao G, Li N, Wang X, Li Y. et al. Anti-Tim4 Grafting Strongly Hydrophilic Metal-Organic Frameworks Immunoaffinity Flake for High-Efficiency Capture and Separation of Exosomes. Anal Chem. 2021;93:6534-43
110. Kang Y-T, Kim YJ, Bu J, Cho Y-H, Han S-W, Moon B-I. High-purity capture and release of circulating exosomes using an exosome-specific dual-patterned immunofiltration (ExoDIF) device. Nanoscale. 2017;9:13495-505
111. Hisey CL, Dorayappan KDP, Cohn DE, Selvendiran K, Hansford DJ. Microfluidic affinity separation chip for selective capture and release of label-free ovarian cancer exosomes. Lab Chip. 2018;18:3144-53
112. Yu Z, Lin S, Xia F, Liu Y, Zhang D, Wang F. et al. ExoSD chips for high-purity immunomagnetic separation and high-sensitivity detection of gastric cancer cell-derived exosomes. Biosens Bioelectron. 2021;194:113594
113. Guo X, Hu F, Yong Z, Zhao S, Wan Y, Wang B. et al. Magnetic Nanoparticle-Based Microfluidic Platform for Automated Enrichment of High-Purity Extracellular Vesicles. Anal Chem. 2024;96:7212-9
114. Hua X, Zhu Q, Liu Y, Zhou S, Huang P, Li Q. et al. A double tangential flow filtration-based microfluidic device for highly efficient separation and enrichment of exosomes. Anal Chim Acta. 2023;1258:341160
115. Zhao L, Wang H, Fu J, Wu X, Liang X, Liu X. et al. Microfluidic-based exosome isolation and highly sensitive aptamer exosome membrane protein detection for lung cancer diagnosis. Biosens Bioelectron. 2022;214:114487
116. Wu M, Ouyang Y, Wang Z, Zhang R, Huang P-H, Chen C. et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci. 2017;114:10584-9
117. Naquin TD, Canning AJ, Gu Y, Chen J, Naquin CM, Xia J. et al. Acoustic separation and concentration of exosomes for nucleotide detection: ASCENDx. Sci Adv. 2024;10:eadm8597
118. Liu L, Chen K, Xiang N, Ni Z. Dielectrophoretic manipulation of nanomaterials: A review. ELECTROPHORESIS. 2019;40:873-89
119. Niu J, Lin S, Xu Y, Tong S, Wang Z, Cui S. et al. A stepwise multi-stage continuous dielectrophoresis separation microfluidic chip with microfilter structures. Talanta. 2024;279:126585
120. Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B. et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404
121. Takakura Y, Hanayama R, Akiyoshi K, Futaki S, Hida K, Ichiki T. et al. Quality and Safety Considerations for Therapeutic Products Based on Extracellular Vesicles. Pharm Res. 2024;41:1573-94
122. Théry C, Kenneth W W, Elena A, Maria Jose A, Johnathon D A, Ramaroson A. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750
123. Chen Y-S, Lin E-Y, Chiou T-W, Harn H-J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med J. 2020;32:113
124. Zickler AM, Liang X, Gupta D, Mamand DR, De Luca M, Corso G. et al. Novel Endogenous Engineering Platform for Robust Loading and Delivery of Functional mRNA by Extracellular Vesicles. Adv Sci. 2024;11:2407619
125. Fu Z, Zhang X, Zhou X, Ur-Rehman U, Yu M, Liang H. et al. In vivo self-assembled small RNAs as a new generation of RNAi therapeutics. Cell Res. 2021;31:631-48
126. Gu W, Luozhong S, Cai S, Londhe K, Elkasri N, Hawkins R. et al. Extracellular vesicles incorporating retrovirus-like capsids for the enhanced packaging and systemic delivery of mRNA into neurons. Nat Biomed Eng. 2024;8:415-26
127. Han J, Sul JH, Lee J, Kim E, Kim HK, Chae M. et al. Engineered exosomes with a photoinducible protein delivery system enable CRISPR-Cas-based epigenome editing in Alzheimer's disease. Sci Transl Med. 2024;16:eadi4830
128. Kong W, Li X, Guo X, Sun Y, Chai W, Chang Y. et al. Ultrasound-Assisted CRISPRi-Exosome for Epigenetic Modification of α-Synuclein Gene in a Mouse Model of Parkinson's Disease. ACS Nano. 2024;18:7837-51
129. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-5
130. Izco M, Blesa J, Schleef M, Schmeer M, Porcari R, Al-Shawi R. et al. Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol Ther. 2019;27:2111-22
131. Sridharan B, Lim HG. Exosomes and ultrasound: The future of theranostic applications. Mater Today Bio. 2023;19:100556
132. Xu X, Xu L, Wen C, Xia J, Zhang Y, Liang Y. Programming assembly of biomimetic exosomes: An emerging theranostic nanomedicine platform. Mater Today Bio. 2023;22:100760
133. Tian J, Han Z, Song D, Peng Y, Xiong M, Chen Z. et al. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int J Nanomedicine. 2023;18:7923-40
134. Guo P, Busatto S, Huang J, Morad G, Moses MA. A Facile Magnetic Extrusion Method for Preparing Endosome-Derived Vesicles for Cancer Drug Delivery. Adv Funct Mater. 2021;31:2008326
135. Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E. et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1-12
136. Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z. et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Controlled Release. 2015;207:18-30
137. Wang H, Sui H, Zheng Y, Jiang Y, Shi Y, Liang J. et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale. 2019;11:7481-96
138. Gao X, Ran N, Dong X, Zuo B, Yang R, Zhou Q. et al. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med. 2018;10:eaat0195
139. Xu H, Liao C, Liang S, Ye B-C. A Novel Peptide-Equipped Exosomes Platform for Delivery of Antisense Oligonucleotides. ACS Appl Mater Interfaces. 2021;13:10760-7
140. Haraszti RA, Miller R, Didiot M-C, Biscans A, Alterman JF, Hassler MR. et al. Optimized Cholesterol-siRNA Chemistry Improves Productive Loading onto Extracellular Vesicles. Mol Ther. 2018;26:1973-82
141. Han G, Zhang Y, Zhong L, Wang B, Qiu S, Song J. et al. Generalizable anchor aptamer strategy for loading nucleic acid therapeutics on exosomes. EMBO Mol Med. 2024;16:1027-45
142. Bhatta R, Han J, Liu Y, Bo Y, Lee D, Zhou J. et al. Metabolic tagging of extracellular vesicles and development of enhanced extracellular vesicle based cancer vaccines. Nat Commun. 2023;14:8047
143. Salunkhe S, Dheeraj, Basak M, Chitkara D, Mittal A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J Controlled Release. 2020;326:599-614
144. Wu J, Lu H, Xu X, Rao L, Ge Y. Engineered Cellular Vesicles Displaying Glycosylated Nanobodies for Cancer Immunotherapy. Angew Chem Int Ed. 2024;63:e202404889
145. Lai J, Huang C, Guo Y, Rao L. Engineered extracellular vesicles and their mimics in cardiovascular diseases. J Controlled Release. 2022;347:27-43
146. Mentkowski KI, Snitzer JD, Rusnak S, Lang JK. Therapeutic Potential of Engineered Extracellular Vesicles. AAPS J. 2018;20:50
147. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-79
148. Eskelinen E-L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med. 2006;27:495-502
149. Yang J, Wu S, Hou L, Zhu D, Yin S, Yang G. et al. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol Ther - Nucleic Acids. 2020;21:512-22
150. Jiang P, Xiao Y, Hu X, Wang C, Gao H, Huang H. et al. RVG29 Peptide-Modified Exosomes Loaded with Mir-133b Mediate the RhoA-ROCK Pathway to Improve Motor and Neurological Symptoms in Parkinson's Disease. ACS Biomater Sci Eng. 2024;10:3069-85
151. Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. 2012;33:8167-76
152. Zhu Z, Zhai Y, Hao Y, Wang Q, Han F, Zheng W. et al. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J Extracell Vesicles. 2022;11:e12255
153. Kim G, Kim M, Lee Y, Byun JW, Hwang DW, Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J Controlled Release. 2020;317:273-81
154. Zhao H, Li Z, Liu D, Zhang J, You Z, Shao Y. et al. PlexinA1 (PLXNA1) as a novel scaffold protein for the engineering of extracellular vesicles. J Extracell Vesicles. 2024;13:e70012
155. Wu D, Yang K, Zhang Z, Feng Y, Rao L, Chen X. et al. Metal-free bioorthogonal click chemistry in cancer theranostics. Chem Soc Rev. 2022;51:1336-76
156. Sul JH, Shin S, Kim HK, Han J, Kim J, Son S. et al. Dopamine-conjugated extracellular vesicles induce autophagy in Parkinson's disease. J Extracell Vesicles. 2024;13:e70018
157. Zhang L, Lin J, Xiang K, Shi T, Guo B. Omnidirectional improvement of mitochondrial health in Alzheimer's disease by multi-targeting engineered activated neutrophil exosomes. J Controlled Release. 2024;376:470-87
158. Liu L, Li Y, Peng H, Liu R, Ji W, Shi Z. et al. Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing α-synuclein and immune activation of Parkinson's disease. Sci Adv. 2020;6:eaba3967
159. Rao L, Wu L, Liu Z, Tian R, Yu G, Zhou Z. et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun. 2020;11:4909
160. Sato YT, Umezaki K, Sawada S, Mukai S, Sasaki Y, Harada N. et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933
161. Jiang S, Cai G, Yang Z, Shi H, Zeng H, Ye Q. et al. Biomimetic Nanovesicles as a Dual Gene Delivery System for the Synergistic Gene Therapy of Alzheimer's Disease. ACS Nano. 2024;18:11753-68
162. Sancho-Albero M, Encabo-Berzosa M del M, Beltrán-Visiedo M, Fernández-Messina L, Sebastián V, Sánchez-Madrid F. et al. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale. 2019;11:18825-36
163. Wang Q, Li T, Yang J, Zhao Z, Tan K, Tang S. et al. Engineered Exosomes with Independent Module/Cascading Function for Therapy of Parkinson's Disease by Multistep Targeting and Multistage Intervention Method. Adv Mater. 2022;34:2201406
164. Pan J, Yao Q, Wang Y, Chang S, Li C, Wu Y. et al. The role of PI3K signaling pathway in Alzheimer's disease. Front Aging Neurosci. 2024;16:1459025
165. Razani E, Pourbagheri-Sigaroodi A, Safaroghli-Azar A, Zoghi A, Shanaki-Bavarsad M, Bashash D. The PI3K/Akt signaling axis in Alzheimer's disease: a valuable target to stimulate or suppress? Cell Stress Chaperones. 2021;26:871-87
166. Zhang R, Deng X, Liu Q, Zhang X, Bai X, Weng S. et al. Global research trends and hotspots of PI3K/Akt signaling pathway in the field of osteoarthritis: A bibliometric study. Medicine (Baltimore). 2023;102:e33489
167. Namini MS, Beheshtizadeh N, Ebrahimi-Barough S, Ai J. Human endometrial stem cell-derived small extracellular vesicles enhance neurite outgrowth and peripheral nerve regeneration through activating the PI3K/AKT signaling pathway. J Transl Med. 2025;23:6
168. Wadhwa P, Jain P, Jadhav HR. Glycogen Synthase Kinase 3 (GSK3): Its Role and Inhibitors. Curr Top Med Chem. 20: 1522-34.
169. Gupta R, Kumari S, Tripathi R, Ambasta RK, Kumar P. Unwinding the modalities of necrosome activation and necroptosis machinery in neurological diseases. Ageing Res Rev. 2023;86:101855
170. Zhang J, Yang S-G, Zhou F-Q. Glycogen synthase kinase 3 signaling in neural regeneration in vivo. J Mol Cell Biol. 2023;15:mjad075
171. Chen L, Cheng L, Wei X, Yuan Z, Wu Y, Wang S. et al. Tetramethylpyrazine Analogue CXC195 Protects Against Dopaminergic Neuronal Apoptosis via Activation of PI3K/Akt/GSK3β Signaling Pathway in 6-OHDA-Induced Parkinson's Disease Mice. Neurochem Res. 2017;42:1141-50
172. Sen T, Saha P, Jiang T, Sen N. Sulfhydration of AKT triggers Tau-phosphorylation by activating glycogen synthase kinase 3β in Alzheimer's disease. Proc Natl Acad Sci U S A. 2020;117:4418-27
173. Terwel D, Muyllaert D, Dewachter I, Borghgraef P, Croes S, Devijver H. et al. Amyloid Activates GSK-3β to Aggravate Neuronal Tauopathy in Bigenic Mice. Am J Pathol. 2008;172:786-98
174. Cuyàs E, Corominas-Faja B, Joven J, Menendez JA. Cell cycle regulation by the nutrient-sensing mammalian target of rapamycin (mTOR) pathway. Methods Mol Biol Clifton NJ. 2014;1170:113-44
175. Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81-94
176. Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26:2694-701
177. Tramutola A, Lanzillotta C, Barone E, Arena A, Zuliani I, Mosca L. et al. Intranasal rapamycin ameliorates Alzheimer-like cognitive decline in a mouse model of Down syndrome. Transl Neurodegener. 2018;7:28
178. Ebrahim N, Al Saihati HA, Alali Z, Aleniz FQ, Mahmoud SYM, Badr OA. et al. Exploring the molecular mechanisms of MSC-derived exosomes in Alzheimer's disease: Autophagy, insulin and the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2024;176:116836
179. Liu C, Khairullina L, Qin Y, Zhang Y, Xiao Z. Adipose stem cell exosomes promote mitochondrial autophagy through the PI3K/AKT/mTOR pathway to alleviate keloids. Stem Cell Res Ther. 2024;15:305
180. Guo Q, Jin Y, Chen X, Ye X, Shen X, Lin M. et al. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. 2024;9:1-37
181. Zabel U, Baeuerle PA. Purified human IκB can rapidly dissociate the complex of the NF-κB transcription factor with its cognate DNA. Cell. 1990;61:255-65
182. Lee C-J, Jang SH, Lim J, Park H, Ahn S-H, Park SY. et al. Exosome-based targeted delivery of NF-κB ameliorates age-related neuroinflammation in the aged mouse brain. Exp Mol Med. 2025;57:235-48
183. Jiang D, Gong F, Ge X, Lv C, Huang C, Feng S. et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnology. 2020;18:105
184. Cui G-H, Wu J, Mou F-F, Xie W-H, Wang F-B, Wang Q-L. et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32:654-68
185. Iyer M, Subramaniam MD, Venkatesan D, Cho S-G, Ryding M, Meyer M. et al. Role of RhoA-ROCK signaling in Parkinson's disease. Eur J Pharmacol. 2021;894:173815
186. Ravichandran N, Iyer M, Uvarajan D, Kirola L, Kumra SM, Babu H winster S. et al. New insights on the regulators and inhibitors of RhoA-ROCK signalling in Parkinson's disease. Metab Brain Dis. 2025;40:90
187. Zhu S, Ma H, Hou M, Li H, Ning G. Schwann Cell-Derived Exosomes Induced Axon Growth after Spinal Cord Injury by Decreasing PTP-σ Activation on CSPGs via the Rho/ROCK Pathway. Neurochem Res. 2024;49:2120-30
188. Chen Z, Wu S, Sheng S, Wang S, Qian Y, Wang X. et al. hUCMSC-derived exosomes mitigate blood-spinal cord barrier disruption by activating AMPK/mTOR-mediated autophagic flux after acute spinal cord injury. Compos Part B Eng. 2025;289:111944
189. Geoffroy CG, Lorenzana AO, Kwan JP, Lin K, Ghassemi O, Ma A. et al. Effects of PTEN and Nogo Codeletion on Corticospinal Axon Sprouting and Regeneration in Mice. J Neurosci. 2015;35:6413-28
190. Fan L, Liu C, Chen X, Zheng L, Zou Y, Wen H. et al. Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv Sci. 2022;9:2105586
191. Chen Y, Tian Z, He L, Liu C, Wang N, Rong L. et al. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res Ther. 2021;12:224
192. Zhang Z, Zou X, Zhang R, Xie Y, Feng Z, Li F. et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging. 2021;13:3060-79
193. Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K. et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197
194. Elia CA, Tamborini M, Rasile M, Desiato G, Marchetti S, Swuec P. et al. Intracerebral Injection of Extracellular Vesicles from Mesenchymal Stem Cells Exerts Reduced Aβ Plaque Burden in Early Stages of a Preclinical Model of Alzheimer's Disease. Cells. 2019;8:1059
195. Wei Y, Zhou J, Wu J, Huang J. ERβ promotes Aβ degradation via the modulation of autophagy. Cell Death Dis. 2019;10:565
196. Iyaswamy A, Thakur A, Guan X-J, Krishnamoorthi S, Fung TY, Lu K. et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer's disease. Signal Transduct Target Ther. 2023;8:1-14
197. Cano A, Turowski P, Ettcheto M, Duskey JT, Tosi G, Sánchez-López E. et al. Nanomedicine-based technologies and novel biomarkers for the diagnosis and treatment of Alzheimer's disease: from current to future challenges. J Nanobiotechnology. 2021;19:122
198. Cui G, Guo H, Li H, Zhai Y, Gong Z, Wu J. et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease. Immun Ageing. 2019;16:10
199. Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z. et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer's Disease. Neurochem Res. 2018;43:2165-77
200. Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL. et al. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regen Res. 2019;14:1626
201. Huber CC, Callegari EA, Paez MD, Romanova S, Wang H. Heat Shock-Induced Extracellular Vesicles Derived from Neural Stem Cells Confer Marked Neuroprotection Against Oxidative Stress and Amyloid-β-Caused Neurotoxicity. Mol Neurobiol. 2022;59:7404-12
202. Li B, Chen Y, Zhou Y, Feng X, Gu G, Han S. et al. Neural stem cell-derived exosomes promote mitochondrial biogenesis and restore abnormal protein distribution in a mouse model of Alzheimer's disease. Neural Regen Res. 2024;19:1593
203. Morris HR, Spillantini MG, Sue CM, Williams-Gray CH. The pathogenesis of Parkinson's disease. The Lancet. 2024;403:293-304
204. Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q. et al. Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease. Brain. 2020;143:1476-97
205. Zhang S, Liu Y-Q, Jia C, Lim Y-J, Feng G, Xu E. et al. Mechanistic basis for receptor-mediated pathological α-synuclein fibril cell-to-cell transmission in Parkinson's disease. Proc Natl Acad Sci. 2021;118:e2011196118
206. Mao X, Ou MT, Karuppagounder SS, Kam T-I, Yin X, Xiong Y. et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353:aah3374
207. Butler YR, Liu Y, Kumbhar R, Zhao P, Gadhave K, Wang N. et al. α-Synuclein fibril-specific nanobody reduces prion-like α-synuclein spreading in mice. Nat Commun. 2022;13:4060
208. Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L. et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42
209. Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T. et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584-93
210. Xia Y, Zhang G, Han C, Ma K, Guo X, Wan F. et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis. 2019;10:1-15
211. Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome Reduction In Vivo Is Associated with Lower Amyloid Plaque Load in the 5XFAD Mouse Model of Alzheimer's Disease. Neurobiol Aging. 2014;35:1792-800
212. Wang P, Lan G, Xu B, Yu Z, Tian C, Lei X. et al. α-Synuclein-carrying astrocytic extracellular vesicles in Parkinson pathogenesis and diagnosis. Transl Neurodegener. 2023;12:40
213. Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in Parkinson's disease. Prog Neurobiol. 1996;48:1-19
214. Haney MJ, Zhao Y, Li S, Higginbotham SM, Booth SL, Han H-Y. et al. Cell-mediated transfer of catalase nanoparticles from macrophages to brain endothelial, glial and neuronal cells. Nanomed. 2011;6:1215-30
215. Jiang Y, Liu J, Chen L, Jin Y, Zhang G, Lin Z. et al. Serum secreted miR-137-containing exosomes affects oxidative stress of neurons by regulating OXR1 in Parkinson's disease. Brain Res. 2019;1722:146331
216. He S, Wang Q, Chen L, He YJ, Wang X, Qu S. miR-100a-5p-enriched exosomes derived from mesenchymal stem cells enhance the anti-oxidant effect in a Parkinson's disease model via regulation of Nox4/ROS/Nrf2 signaling. J Transl Med. 2023;21:747
217. Xu Y, Yan G, Zhao J, Ren Y, Xiao Q, Tan M. et al. Plant-derived exosomes as cell homogeneous nanoplatforms for brain biomacromolecules delivery ameliorate mitochondrial dysfunction against Parkinson's disease. Nano Today. 2024;58:102438
218. Antico O, Thompson PW, Hertz NT, Muqit MMK, Parton LE. Targeting mitophagy in neurodegenerative diseases. Nat Rev Drug Discov. 2025;24:276-99
219. Geng X, Zou Y, Li J, Li S, Qi R, Zhong L. et al. Mesenchymal stem cell exosomes rich in miR-23b-3p affect the Wnt signaling pathway and promote neuronal autophagy to alleviate PD symptoms. Neurosci Lett. 2023;814:137437
220. Qu M, Lin Q, Huang L, Fu Y, Wang L, He S. et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease. J Controlled Release. 2018;287:156-66
221. Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ. et al. Amyotrophic lateral sclerosis. The Lancet. 2022;400:1363-80
222. Bonafede R, Scambi I, Peroni D, Potrich V, Boschi F, Benati D. et al. Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res. 2016;340:150-8
223. Cozzolino M, Carrì MT. Mitochondrial dysfunction in ALS. Prog Neurobiol. 2012;97:54-66
224. Calabria E, Scambi I, Bonafede R, Schiaffino L, Peroni D, Potrich V. et al. ASCs-Exosomes Recover Coupling Efficiency and Mitochondrial Membrane Potential in an in vitro Model of ALS. Front Neurosci. 2019;13:1070
225. Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83-98
226. Yadav M, Harding RJ, Li T, Xu X, Gall-Duncan T, Khan M. et al. Huntingtin is an RNA binding protein and participates in NEAT1-mediated paraspeckles. Sci Adv. 2024;10:eado5264
227. Didiot M-C, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K. et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol Ther. 2016;24:1836-47
228. Zhang L, Wu T, Shan Y, Li G, Ni X, Chen X. et al. Therapeutic reversal of Huntington's disease by in vivo self-assembled siRNAs. Brain. 2021;144:3421-35
229. Lee M, Liu T, Im W, Kim M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington's disease in vitro model. Eur J Neurosci. 2016;44:2114-9
230. St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S. et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397-408
231. Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S. et al. Multiple sclerosis. Nat Rev Dis Primer. 2018;4:1-27
232. Franklin RJM, Ffrench-Constant C. Regenerating CNS myelin - From mechanisms to experimental medicines. Nat Rev Neurosci. 2017;18:753-69
233. Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-Derived Neurotrophic Factor Promotes Central Nervous System Myelination via a Direct Effect upon Oligodendrocytes. Neurosignals. 2011;18:186-202
234. Zhai Y, Wang Q, Zhu Z, Hao Y, Han F, Hong J. et al. High-efficiency brain-targeted intranasal delivery of BDNF mediated by engineered exosomes to promote remyelination. Biomater Sci. 2022;10:5707-18
235. Wang L-B, Liao B-Y, Li Y-J, Wang Z-H, Yu Y, Li X. et al. Engineered PDGFA-ligand-modified exosomes delivery T3 for demyelinating disease targeted therapy. Exp Neurol. 2024;375:114730
236. Zheng X, Sun K, Liu Y, Yin X, Zhu H, Yu F. et al. Resveratrol-loaded macrophage exosomes alleviate multiple sclerosis through targeting microglia. J Controlled Release. 2023;353:675-84
237. Li Z, Liu F, He X, Yang X, Shan F, Feng J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol. 2019;67:268-80
238. Pusic AD, Pusic KM, Clayton BLL, Kraig RP. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neuroimmunol. 2014;266:12-23
239. Ma X, Huang M, Zheng M, Dai C, Song Q, Zhang Q. et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer's disease. J Controlled Release. 2020;327:688-702
240. Xie X, Song Q, Dai C, Cui S, Tang R, Li S. et al. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer's disease: a phase I/II clinical trial. Gen Psychiatry. 2023;36:e101143
241. Akhlaghpasand M, Tavanaei R, Hosseinpoor M, Yazdani KO, Soleimani A, Zoshk MY. et al. Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: a first-in-human, single-arm, open-label, phase I clinical trial. Stem Cell Res Ther. 2024;15:264
242. Li Z-J, Zhang L-B, Chen Y-X, Hu L. Advancements and challenges in neuromodulation technology: interdisciplinary opportunities and collaborative endeavors. Sci Bull. 2023;68:1978-82
243. Wang C-K, Tsai T-H, Lee C-H. Regulation of exosomes as biologic medicines: Regulatory challenges faced in exosome development and manufacturing processes. Clin Transl Sci. 2024;17:e13904
244. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-98
245. Julayanont P, Tangwongchai S, Hemrungrojn S, Tunvirachaisakul C, Phanthumchinda K, Hongsawat J. et al. The Montreal Cognitive Assessment—Basic: A Screening Tool for Mild Cognitive Impairment in Illiterate and Low-Educated Elderly Adults. J Am Geriatr Soc. 2015;63:2550-4
246. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi D, Gornbein J. The Neuropsychiatric Inventory. Neurology. 1994;44:2308-2308
247. Verma N, Beretvas SN, Pascual B, Masdeu JC, Markey MK, The Alzheimer's Disease Neuroimaging Initiative. New scoring methodology improves the sensitivity of the Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog) in clinical trials. Alzheimers Res Ther. 2015;7:64
248. Liu W, Lin X, Chen X, Wang Q, Wang X, Yang B. et al. Vision-based estimation of MDS-UPDRS scores for quantifying Parkinson's disease tremor severity. Med Image Anal. 2023;85:102754
249. Schwarz AJ. The Use, Standardization, and Interpretation of Brain Imaging Data in Clinical Trials of Neurodegenerative Disorders. Neurotherapeutics. 2021;18:686-708
250. Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M. et al. An Inventory to Assess Activities of Daily Living for Clinical Trials in Alzheimer's Disease. Alzheimer Dis Assoc Disord. 1997;11:33
251. Ferrea E, Negahbani F, Cebi I, Weiss D, Gharabaghi A. Machine learning explains response variability of deep brain stimulation on Parkinson's disease quality of life. Npj Digit Med. 2024;7:1-11
Author contact
![]() Corresponding authors: xmao4edu, kc3727edu, lraoac.cn.
Corresponding authors: xmao4edu, kc3727edu, lraoac.cn.
 Global reach, higher impact
Global reach, higher impact